Abstract
Background
Chronic liver disease (CLD) is among the strongest risk factors for adverse prescription opioid-related events. Yet, the current prevalence and factors associated with high-risk opioid prescribing in patients with chronic liver disease (CLD) remain unclear, making it challenging to address opioid safety in this population. Therefore, we aimed to characterize opioid prescribing patterns among patients with CLD.
Methods
This retrospective cohort study included patients with CLD identified at a single medical center and followed for one year from 10/1/2015-9/30/2016. Multivariable, multinomial regression was used identify the patient characteristics, including demographics, medical conditions, and liver-related factors, that were associated with opioid prescriptions and high-risk prescriptions (≥90mg morphine equivalents per day [MME/day] or co-prescribed with benzodiazepines).
Results
Nearly half (47%) of 12,425 patients with CLD were prescribed opioids over a one-year period, with 17% of these receiving high-risk prescriptions. The baseline factors significantly associated with high-risk opioid prescriptions included female gender (adjusted incident rate ratio, AIRR = 1.32, 95% CI = 1.14–1.53), Medicaid insurance (AIRR = 1.68, 95% CI = 1.36–2.06), cirrhosis (AIRR = 1.22, 95% CI = 1.04–1.43) and baseline chronic pain (AIRR = 3.40, 95% CI = 2.94–4.01), depression (AIRR = 1.93, 95% CI = 1.60–2.32), anxiety (AIRR = 1.84, 95% CI = 1.53–2.22), substance use disorder (AIRR = 2.16, 95% CI = 1.67–2.79), and Charlson comorbidity score (AIRR = 1.27, 95% CI = 1.22–1.32). Non-alcoholic fatty liver disease was associated with decreased high-risk opioid prescriptions (AIRR = 0.56, 95% CI = 0.47–0.66).
Conclusion
Opioid medications continue to be prescribed to nearly half of patients with CLD, despite efforts to curtail opioid prescribing due to known adverse events in this population.
Introduction
Although the opioid prescribing rate in the United States has been declining since 2012 [1], in 2017, opioids accounted for 69% of drug overdose deaths, many of which are due to prescription opioids [2]. In fact, nearly half of patients starting treatment for opioid use disorder report their first exposure to opioids through an opioid prescription for pain [3]. Ongoing high rates of opioid prescribing are particularly problematic among high-risk subgroups, such as people with chronic liver diseases (CLD).
Frequent high-risk opioid prescriptions, defined as doses above 90 mg morphine equivalents (MME) or co-prescriptions with benzodiazepines, and opioid-related complications have been well-documented in patients with cirrhosis [4–11]. Moreover, chronic opioid prescriptions may present a barrier to liver transplantation, the only cure for cirrhosis [12]. Moreover, patients with cirrhosis have increased opioid toxicity compared to other populations [13]. These well-documented adverse consequences are concerning because opioid prescribing is extremely common for patients with cirrhosis. In fact, up to half of people with cirrhosis are prescribed an opioid each year [4].
While opioids are commonly prescribed to patients with cirrhosis, the degree to which this affects persons with less advanced CLD remains unclear. Yet, opioid prescribing is likely particularly problematic in this population. CLD itself is among the highest risk factors for opioid-related adverse events in the general population [14, 15]. Adverse consequences of opioids in this population include increased healthcare utilization [10]. Opioids have been associated with increased liver injury in murine models and in humans [16–22]. This may be in part due to opioid-related Inflammation and changes in the microbiome leading to accumulation of toxins [23–25]. Opioids are often co-prescribed with acetaminophen, risking unintentional liver injury for this population with underlying liver disease [4, 14]. Thus, to intervene to prevent adverse opioid-related outcomes in this population, it is critical to understand when in the course of CLD patients are prescribed opioids. Furthermore, with the rising burden of fatty liver disease, which affects one-third of the population in the US, it is increasingly important to understand how to safety manage pain in this growing population of persons with non-cirrhotic CLD [26–28].
Currently, because the risks and frequency of opioid prescribing to patients with CLD are unknown, it is challenging to combat this issue. Therefore, this retrospective cohort study aimed to investigate the medical and demographic characteristics associated with opioid prescribing to a population of outpatients with CLD and to further characterize high risk prescriptions, defined as defined as ≥90 MME per day or opioids prescribed with benzodiazepines [29]. We hypothesized the patients with non-cirrhotic CLD would have high rates of opioid prescriptions, similar to those with cirrhosis, suggesting that this issue starts earlier in disease progression than previously appreciated.
Materials and methods
Ethics approval
This study was approved by the University of Pittsburgh Institutional Review Board, protocol number PRO16120217, with a waiver of informed consent due to the retrospective nature of the study.
Cohort definition
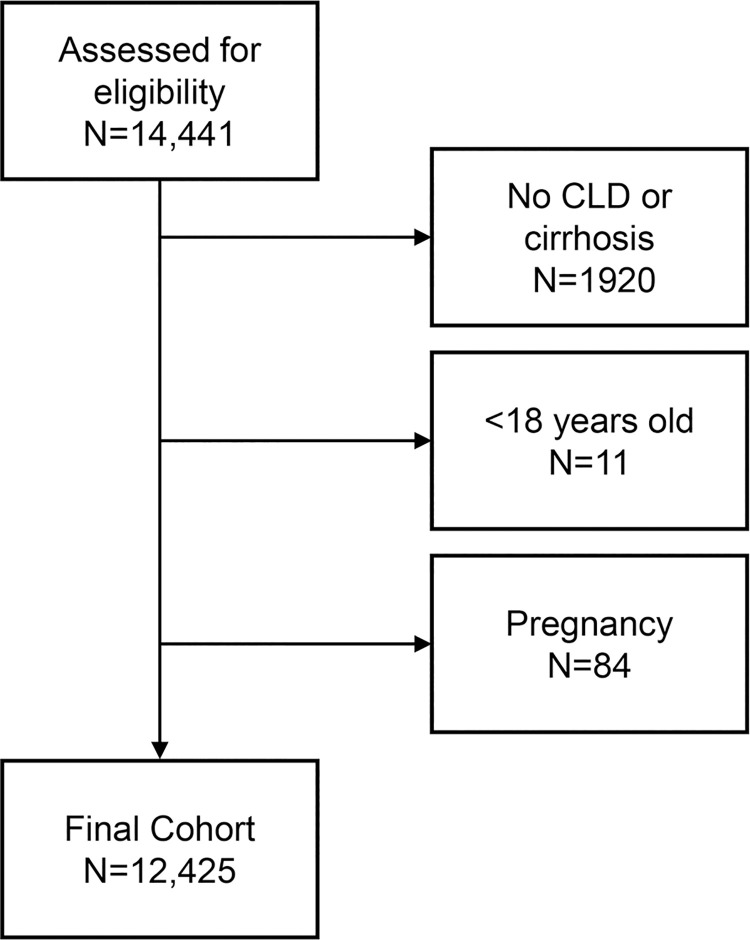
We identified a cohort of patients with CLD who had clinical encounters at the University of Pittsburgh Medical Center between October 1, 2015 and September 30, 2016. CLD was defined by the presence of one corresponding outpatient or inpatient ICD code for cirrhosis, alcohol-related liver disease (ALD), non-alcoholic fatty liver disease (NAFLD), viral hepatitis, hemochromatosis, autoimmune hepatitis, primary biliary cholangitis, or other CLD (S1 Table). Chart review was conducted for patients with codes for “unspecified viral hepatitis” to confirm that they had CLD. Patients were excluded if they were found to not have CLD, were not 18 years of age, or were pregnant. Each patient’s index visit was defined as the first outpatient, in-person visit between October 2015 and September 2016 with an ICD-10 code for CLD within the timeframe.
Baseline characteristics
We extracted baseline demographic factors including age, sex, race/ethnicity (Hispanic/LatinX vs. non-Hispanic Black vs. non-Hispanic white vs. not specified vs. other specified race/ethnicity), and marital status. Insurance status (Medicaid vs. other) was used as a proxy for socioeconomic status, following examples from the literature [30, 31]. We defined comorbidities using two outpatient ICD-9 or ICD-10 codes within one year prior to the index visit and used previously published ICD coding strategies to define Charlson Comorbidity Index [32], mental health (i.e., anxiety and depression), alcohol use disorder, substance use disorders (SUDs), pain-related diagnoses [4], and hepatocellular carcinoma (ICD-10 C22.0, C22.9 and Z85.05). Etiology of liver disease was re-categorized as NAFLD vs. other since this was the most common etiology. Cirrhosis was defined by either an ICD-10 code at baseline for cirrhosis, a recognized complication of cirrhosis, or fibrosis-4 (FIB-4) score >3.25 [33].
Opioid prescribing
Starting with an index clinical encounter, patients were followed for one year to assess opioid prescribing. All prescribed opioids (e.g., tramadol, morphine, fentanyl) were included in the definition of opioids, with the exception of cough syrups or medications used for opioid use disorder (e.g., buprenorphine), following previously-published methods and conventions [4, 34, 35]. We collected the dose and frequency of opioids and calculated milligrams of morphine equivalents using an “as-prescribed” approach, which assumes that patients take their prescribed opioids at the maximum dose and on the schedule recommended by their clinicians [36]. Patients were divided into three groups based on those prescribed no opioids vs. moderate-risk opioids vs. high-risk opioids. High-risk prescriptions were defined as an average dose ≥90 morphine milligram equivalents (MME) per day or co-prescription with a benzodiazepine, based on the Centers for Disease Control and Prevention guidelines [29]. We defined “moderate-risk” opioid prescriptions as <90 MME/day and with no benzodiazepine prescription.
Analysis
Data were summarized using descriptive statistics, describing baseline covariates for the entire cohort, and then for patients with no opioid use, moderate-risk opioid use, and high-risk opioid use. Differences between groups were assessed using chi-square tests for categorical variables, Fisher’s exact test for sparse categorical variables (n<5), and ANOVA for comparison of groups greater than two, in order to assess the factors significantly associated with opioid status in univariate analyses. We subsequently assessed the factors associated with opioid prescriptions within one year after the index visit using multivariable multinomial regression with automated AIC optimization with the R MASS package [37].
Results
Baseline cohort characteristics
After excluding 108 patients for pregnancy (n = 84), being under 18 years old (n = 11), and having no CLD (n = 1,920), the final cohort included 12,425 patients with CLD, including 3,980 with cirrhosis (Fig 1). The mean age was 58±13 and 51% were women. The most common etiology of liver disease was NAFLD (34%) (Table 1).
Fig 1. Cohort of patients with chronic liver disease.
Table 1. Baseline characteristics of 12,425 patients with chronic liver disease, overall and by opioid prescriptions over follow-up.
| Patient Characteristics | Total Cohort (N = 12,425) | No opioids (N = 6,625) | Moderate-risk opioid (N = 4,809) | High-risk opioid (N = 991) | P value |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (mean, sd) | 58±13 | 57±14 | 59±12 | 58±12 | <0.001 |
| Female (n,%) | 6,357 (51) | 3,195 (48) | 2,582 (54) | 581 (59) | <0.001 |
| Medicaid (n,%) | 1,456 (12) | 654 (10) | 634 (13) | 168 (17) | <0.001 |
| Race/ethnicity (n,%) | <0.001 | ||||
| Hispanic/LatinX | 96 (1) | 51 (1) | 39 (1) | 6 (1) | |
| Non-Hispanic Black | 781 (6) | 349 (5) | 382 (8) | 50 (5) | |
| Non-Hispanic White | 11051 (89) | 5854 (88) | 4277 (89) | 920 (91) | |
| Not specified | 334 (3) | 258 (4) | 64 (1) | 12 (5) | |
| Other race/ethnicity | 163 (1) | 113 (2) | 47 (1) | 3 (0) | |
| Married (n,%) | 7,138 (58) | 3,890 (59) | 2,716 (56) | 532 (54) | 0.003 |
| Medical diagnoses | |||||
| Cirrhosis (n,%) | 3,980 (32) | 2,064 (31) | 1,557 (33) | 359 (36) | 0.005 |
| HCC (n,%) | 103 (1) | 37 (0.6) | 54 (1) | 12 (1) | 0.002 |
| NAFLD (n,%) | 4,190 (34) | 2,307 (35) | 1,627 (34) | 256 (26) | <0.001 |
| Chronic Pain (n,%) | 5,420 (44) | 2,135 (32) | 2,637 (55) | 648 (65) | <0.001 |
| Depression (n,%) | 1,887 (15) | 667 (10) | 950 (20) | 270 (27) | <0.001 |
| Anxiety (n,%) | 1,690 (14) | 683 (10) | 753 (16) | 254 (26) | <0.001 |
| Substance Use Disorder (n,%) | 826 (7) | 297 (4) | 412 (9) | 117 (12) | <0.001 |
| Alcohol use disorder | 576 (5) | 308 (5) | 229 (5) | 39 (4) | 0.53 |
| Charlson Index (median, IQR) | 0 (0,2) | 0 (0,1) | 1 (0,2) | 1 (0, 3) | <0.001 |
Abbreviations: HCC, hepatocellular carcinoma; IQR, interquartile range; NAFLD, nonalcoholic fatty liver disease.
High risk opioids are defined as >90MME/day or co-prescription with benzodiazepines; moderate risk are all other prescriptions opioids.
Baseline characteristics associated with opioid prescriptions
After one year of follow-up, 47% of patients had been prescribed an opioid medication. Of those patients who were prescribed opioids, 17% received high-risk prescriptions, defined as ≥90 MME per day or opioids prescribed with benzodiazepines. Factors associated with high-risk opioid prescriptions in bivariate analyses included being female, unmarried, or white and having Medicaid insurance, cirrhosis, non-NAFLD-related CLD and chronic pain, depression, and SUDs (Table 1).
In the multivariate model, baseline factors significantly associated with “moderate-risk” opioid prescriptions included female sex (Adjusted Incidence Rate Ratio [AIRR] = 1.12, 95% CI = 1.03–1.21), Medicaid insurance (AIRR = 1.41, 95% CI = 1.24–1.60), baseline chronic pain (AIRR = 2.24, 95% CI = 2.07–2.43), depression (AIRR = 1.63, 95% CI = 1.45–1.84), SUDs (AIRR = 1.66, 95% CI = 1.41–1.96), and Charlson comorbidity score (AIRR = 1.17, 95% CI = 1.14–1.20). Factors associated with reduced risk of receiving moderate-risk opioid prescriptions included NAFLD (AIRR = 0.90, 95% CI = 0.82–0.98) and having unspecified race (AIRR 0.44, CI = 0.26–0.74) (Table 2).
Table 2. Final multivariate multinomial regression model of baseline factors associated with opioid prescriptions*.
| Patient Characteristics | Moderate risk opioids | High risk opioids | ||||
|---|---|---|---|---|---|---|
| Demographics | IRR | CI | IRR | CI | ||
| Age | 1.00 | 1.00 | 1.00 | 0.99* | 0.98 | 1.00 |
| Female | 1.12* | 1.03 | 1.21 | 1.32* | 1.14 1.53 | |
| Medicaid | 1.41* | 1.24 | 1.60 | 1.68* | 1.36 | 2.06 |
| Race/ethnicity (vs. white) | ||||||
| Hispanic/LatinX | 1.29 | 0.81 | 2.05 | 1.05 | 0.42 | 2.67 |
| Non-Hispanic | ||||||
| Black | 1.02 | 0.66 | 1.58 | 1.56 | 0.65 | 3.76 |
| Unspecified | 0.44* | 0.26 | 0.74 | 0.69 | 0.24 | 1.98 |
| Other specified | 0.64 | 0.37 | 1.13 | 0.30 | 0.07 | 1.30 |
| Medical Conditions | ||||||
| Cirrhosis | 1.04 | 0.95 | 1.14 | 1.22* | 1.04 | 1.43 |
| NAFLD | 0.90* | 0.82 | 0.98 | 0.58* | 0.49 | 0.68 |
| HCC | 1.69* | 1.08 | 2.63 | 1.69 | 0.85 | 3.38 |
| Substance use disorder | 1.66* | 1.41 | 1.96 | 2.00* | 1.56 | 2.56 |
| Chronic pain | 2.24* | 2.07 | 2.43 | 3.40* | 2.92 | 3.94 |
| Depression | 1.63* | 1.45 | 1.84 | 1.87* | 1.56 | 2.24 |
| Anxiety | 1.16* | 1.03 | 1.31 | 1.85* | 1.54 | 2.21 |
| Charlson index | 1.17* | 1.14 | 1.20 | 1.27* | 1.22 | 1.32 |
*Indicates statistically significant association, p<0.05.
High risk opioids are defined as >90MME/day or co-prescription with benzodiazepines; moderate risk are all other prescriptions opioids.
Abbreviations: IRR, incidence rate ratio; CI, confidence interval; NAFLD, nonalcoholic fatty liver disease; HCC, hepatocellular carcinoma.
The baseline factors significantly associated with high-risk opioid prescriptions (Table 2) included female gender (AIRR = 1.32, 95% CI = 1.14–1.53), Medicaid insurance (AIRR = 1.68, 95% CI = 1.36–2.06), cirrhosis (AIRR = 1.22, 95% CI = 1.04–1.43) and baseline chronic pain (AIRR = 3.40, 95% CI = 2.94–4.01), depression (AIRR = 1.93, 95% CI = 1.60–2.32), anxiety (AIRR = 1.84, 95% CI = 1.53–2.22), SUDs (AIRR = 2.16, 95% CI = 1.67–2.79), and Charlson comorbidity score (AIRR = 1.27, 95% CI = 1.22–1.32). NAFLD (AIRR = 0.56, 95% CI = 0.47–0.66) was associated with reduced risk of receiving high-risk opioid prescriptions.
Discussion
Despite a reduction in opioid prescribing in the general US population, prescribing to individuals with CLD remains high. In this cohort of patients with CLD, nearly half of patients received opioid prescriptions in a single year, with nearly one fifth of those prescriptions being high-risk, despite extensive national opioid safety efforts [38]. High-risk opioid prescribing was more common to patients with CLD who were women and those with Medicaid insurance, depression, chronic pain, SUD, and higher comorbidity scores. This study expands upon work finding high rates of opioid prescribing to patients with cirrhosis by evaluating outpatients with all stages and types of CLD, suggesting that efforts to curtail opioid use for pain management should be considered earlier in the natural history of liver disease.
It is notable that rates of opioid prescribing were high among individuals without cirrhosis, suggesting that opioid prescribing starts early in the course of liver disease and must be addressed early. Other cohort studies have examined Veterans with cirrhosis, inpatients with decompensated cirrhosis, exclusively one etiology of CLD (e.g., hepatitis C), or cohorts established prior to efforts to curtail opioid use in the general population [9, 39–42]. This study is unique in including a general population with a diversity of disease etiologies and stages. The finding that opioid prescribing starts in this population prior to disease progression suggests a unique target for opioid de-implementation. Once individuals begin chronic opioids for chronic pain, it becomes more challenging to stop, or deprescribe, these medications [43, 44]. Deprescribing requires a careful, personalized approach [45]. Therefore, finding alternative solutions to manage pain early in the course of CLD may be key to avoiding initiation of opioids.
There are several challenges with analgesia that are unique to populations with CLD that may explain the high rates of opioid prescribing. In addition to contraindications to common analgesics, patients with CLD commonly have chronic pain, and this pain is often complex and complicated by prior SUDs and AUD, There is confusion, even among experienced clinicians, about the safety of the over-the-counter medications in this population [40, 46, 47]. Non-steroidal anti-inflammatory drugs (NSAIDs) are contraindicated, specifically in patients with cirrhosis, due to potential nephrotoxicity, exacerbations of ascites, and increased bleeding risk [47]. However, they are often prescribed due to the misperception that NSAIDs are safer than acetaminophen in this population. In fact, up to 2 grams of acetaminophen is allowable and first-line in this population, though higher doses can lead to hepatotoxicity [39, 46, 47]. Other potential opioid alternatives may include topical preparations (e.g., capsaicin) or local treatments (e.g., joint injections). However, perhaps the most effective and underutilized treatments are behavioral in nature.
Efforts to curtail opioid prescriptions are due to the key role that opioids to play in overdose-related deaths and the high numbers of opioid-related adverse events in general populations [35, 48, 49]. In fact, opioid-related deaths have contributed to a declining life expectancy in the US [50]. Moreover, opioid prescriptions are not effective for the treatment of chronic pain [51, 52]. As such, opioid prescribing has been a priority for US governmental agencies and regulatory bodies, resulting in a general decline in overall opioid prescribing over the last 10 years [53]. This reduction in prescribing in the general population highlights the striking nature of the ongoing crisis for patients with CLD, a population with a number of risk factors for increased adverse events from opioids.
Within this cohort of patients with CLD, high-risk opioid prescribing was more common among patients who had concurrent anxiety, depression, and SUDs, similar to prior findings in patients with cirrhosis [42]. In the general population, long-term prescriptions are also more common in these subpopulations, despite increased risk of medication-related overdose death in patients with mental health and substance use disorders [38, 54]. Because prescription opioids for chronic, non-malignant conditions are associated with increased depressive symptoms among individuals free of depression upon opioid treatment initiation, it is also concerning that opioids could worsen pre-existing mental health concerns [55]. Patients with cirrhosis were more likely to receive high-risk, but not any, opioids, even though this group is more likely to have complications from opioids vs. those with less advanced CLD. Thus, the patients most likely to receive higher risk prescriptions are also those at the highest risk of adverse opioid-related events.
Given the challenges of pain management in patients with liver disease, the strong associations between pain, substance use, and psychiatric symptoms in this population, and emerging evidence that opioids are not effective in treating chronic pain, a more comprehensive approach to pain and symptom management in this population is needed [42, 52]. It therefore is reasonable to pursue therapeutic options that address other possible underlying etiologies of pain, as well as offer a multidisciplinary and multidimensional treatment approach, including physical, behavioral, procedural, and pharmacologic interventions. Alternative analgesia may include topical preparations (e.g., capsaicin), which have a lower potential for systemic side effects. It is sometimes appropriate to prescribe opioids, after engaging in a careful shared decision-making process with patients. For example, at the end of life, when pain management is prioritized and short-term analgesia is needed, opioids are a reasonable option, even in high-risk patients. When opioids are prescribed, the safest formulations include hydromorphone and oxycodone without acetaminophen [13, 39, 46]. While tramadol is perceived to be safer and is frequently used, it is indeed more unsafe due to its first and second pass hepatic metabolism causing unpredictable metabolism [4, 13, 39, 46]. Providers prescribing opioids to patients with cirrhosis should follow general opioid safety guidelines, evaluate and follow for HE and consider prophylactic lactulose [5, 38]. In addition, clinicians should monitor the use of prescription opioids in individuals with mental health or SUDs, as well as screen for depression and SUDs when on opioid medications [38].
Despite the important finding that high rates of opioid prescribing and high-risk opioid prescribing persist in patients with CLD, there were notable limitations of this study. This was a single-center study of mostly white patients, precluding our ability to assess racial disparities in detail. Furthermore, comorbidities and psychiatric diagnoses were based on retrospective code-based determinations and not standardized, prospective instruments. The retrospective nature of the study also precluded our ability to discern the indication for the opioids, given the lack of sensitivity and specificity of ICD codes for this purpose (e.g., only 60% carried a diagnostic code for a painful condition). Opioids are often co-prescribed with acetaminophen, which has been previously assessed; however, this study was not designed to evaluate specific formulations of opioids [4, 56]. Limitations include the lack of clarity around indication for opioids in this population; this is a limit of most electronic health record studies in this area. We also used a conservative of definition of high-risk, including very high doses and benzodiazepines, while the “safer” doses of opioids in this population are likely much lower. Regardless of these limitations, these findings point to the importance of considering opioid-sparing pain management strategies for patients with cirrhosis and earlier stages of CLD.
Conclusion
In this retrospective study, prescription opioid use was common in patients with CLD over a one-year period. Future research should evaluate the benefits and risks of alternative opioid-sparing and non-pharmacologic analgesia in this population.
Supporting information
Abbreviations: CLD, chronic liver disease.
(DOCX)
Abbreviations
- CLD
chronic liver disease
- HE
hepatic encephalopathy
- ALD
alcohol-related liver disease
- NAFLD
non-alcoholic fatty liver disease
- SUD
substance use disorder
- FIB-4
fibrosis-4
- MME
morphine milligram equivalents
- AIRR
Adjusted Incidence Rate Ratio
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
SSR’s time was supported by AHRQ K12HS019461 (PI: Kapoor) and NIDA K23DA048182 (PI Rogal) funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Centers for Disease Control and Prevention. Prescribing Practices | Drug Overdose | CDC Injury Center. November 18, 2020]; Available from: https://www.cdc.gov/drugoverdose/data/prescribing/prescribing-practices.html.
- 2.National Center for Health Statistics (US). Health, United States, 2018 –Data Finder. November 18, 2020]; Available from: https://www.cdc.gov/nchs/hus/contents2018.htm. [PubMed]
- 3.Cicero T.J., Ellis M.S., and Kasper Z.A., Psychoactive substance use prior to the development of iatrogenic opioid abuse: A descriptive analysis of treatment-seeking opioid abusers. Addict Behav, 2017. 65: p. 242–244. doi: 10.1016/j.addbeh.2016.08.024 [DOI] [PubMed] [Google Scholar]
- 4.Rogal S.S., et al., Characteristics of Opioid Prescriptions to Veterans With Cirrhosis. Clin Gastroenterol Hepatol, 2019. 17(6): p. 1165–1174 e3. doi: 10.1016/j.cgh.2018.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moon A.M., et al., Opioid prescriptions are associated with hepatic encephalopathy in a national cohort of patients with compensated cirrhosis. Aliment Pharmacol Ther, 2020. 51(6): p. 652–660. doi: 10.1111/apt.15639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moon A.M., et al., Opioid Use is More Common in Non-Alcoholic Fatty Liver Disease Patients with Cirrhosis, Higher Body Mass Index and Psychiatric Disease. Dig Dis, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubin J.B., et al., Patterns of Inpatient Opioid Use and Related Adverse Events Among Patients With Cirrhosis: A Propensity-Matched Analysis. Hepatol Commun, 2021. 5(6): p. 1081–1094. doi: 10.1002/hep4.1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konerman M.A., et al., Opioid and benzodiazepine prescription among patients with cirrhosis compared to other forms of chronic disease. BMJ Open Gastroenterol, 2019. 6(1): p. e000271. doi: 10.1136/bmjgast-2018-000271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogal S.S., et al., Inflammation, psychiatric symptoms, and opioid use are associated with pain and disability in patients with cirrhosis. Clin Gastroenterol Hepatol, 2015. 13(5): p. 1009–16. doi: 10.1016/j.cgh.2014.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogal S.S., et al., Healthcare utilization in chronic liver disease: the importance of pain and prescription opioid use. Liver Int, 2013. 33(10): p. 1497–503. doi: 10.1111/liv.12215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleming K.M., et al., The rate of decompensation and clinical progression of disease in people with cirrhosis: a cohort study. Aliment Pharmacol Ther, 2010. 32(11–12): p. 1343–50. doi: 10.1111/j.1365-2036.2010.04473.x [DOI] [PubMed] [Google Scholar]
- 12.Fleming J.N., et al., Opioid and opioid substitution therapy in liver transplant candidates: A survey of center policies and practices. Clin Transplant, 2017. 31(12). doi: 10.1111/ctr.13119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dwyer J.P., Jayasekera C., and Nicoll A., Analgesia for the cirrhotic patient: a literature review and recommendations. J Gastroenterol Hepatol, 2014. 29(7): p. 1356–60. doi: 10.1111/jgh.12560 [DOI] [PubMed] [Google Scholar]
- 14.Zedler B., et al., Development of a Risk Index for Serious Prescription Opioid-Induced Respiratory Depression or Overdose in Veterans’ Health Administration Patients. Pain Med, 2015. 16(8): p. 1566–79. doi: 10.1111/pme.12777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zedler B., et al., Risk factors for serious prescription opioid-related toxicity or overdose among Veterans Health Administration patients. Pain Med, 2014. 15(11): p. 1911–29. doi: 10.1111/pme.12480 [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y.T., et al., Oxidative damage of biomolecules in mouse liver induced by morphine and protected by antioxidants. Basic Clin Pharmacol Toxicol, 2004. 95(2): p. 53–8. doi: 10.1111/j.1742-7843.2004.950202.x [DOI] [PubMed] [Google Scholar]
- 17.Payabvash S., et al., Chronic morphine treatment induces oxidant and apoptotic damage in the mice liver. Life Sci, 2006. 79(10): p. 972–80. doi: 10.1016/j.lfs.2006.05.008 [DOI] [PubMed] [Google Scholar]
- 18.De Minicis S., et al., Role of endogenous opioids in modulating HSC activity in vitro and liver fibrosis in vivo. Gut, 2008. 57(3): p. 352–364. doi: 10.1136/gut.2007.120303 [DOI] [PubMed] [Google Scholar]
- 19.Ilic G., et al., Ultrastructural changes in the liver of intravenous heroin addicts. Bosn J Basic Med Sci, 2010. 10(1): p. 38–43. doi: 10.17305/bjbms.2010.2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trigueiro de Araújo M.S., et al., Cellular and matrix changes in drug abuser liver sinusoids: a semiquantitative and morphometric ultrastructural study. Virchows Arch A Pathol Anat Histopathol, 1993. 422(2): p. 145–52. doi: 10.1007/BF01607166 [DOI] [PubMed] [Google Scholar]
- 21.Moore K. and Dusheiko G., Opiate abuse and viral replication in hepatitis C. Am J Pathol, 2005. 167(5): p. 1189–91. doi: 10.1016/S0002-9440(10)61207-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imbernon M., et al., Hypothalamic kappa opioid receptor mediates both diet-induced and melanin concentrating hormone-induced liver damage through inflammation and endoplasmic reticulum stress. Hepatology, 2016. 64(4): p. 1086–104. doi: 10.1002/hep.28716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tapper E.B., Jiang Z.G., and Patwardhan V.R., Refining the Ammonia Hypothesis: A Physiology-Driven Approach to the Treatment of Hepatic Encephalopathy. Mayo Clin Proc, 2015. 90(5): p. 646–658. doi: 10.1016/j.mayocp.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 24.Ghelardini C., Di Cesare Mannelli L., and Bianchi E., The pharmacological basis of opioids. Clin Cases Miner Bone Metab, 2015. 12(3): p. 219–21. doi: 10.11138/ccmbm/2015.12.3.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acharya C., et al., Chronic opioid use is associated with altered gut microbiota and predicts readmissions in patients with cirrhosis. Aliment Pharmacol Ther, 2017. 45(2): p. 319–331. doi: 10.1111/apt.13858 [DOI] [PubMed] [Google Scholar]
- 26.Beste L.A., et al., Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001–2013. Gastroenterology, 2015. 149(6): p. 1471–1482 e5; quiz e17-8. doi: 10.1053/j.gastro.2015.07.056 [DOI] [PubMed] [Google Scholar]
- 27.Estes C., et al., Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology, 2018. 67(1): p. 123–133. doi: 10.1002/hep.29466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong R.J., Liu B., and Bhuket T., Significant burden of nonalcoholic fatty liver disease with advanced fibrosis in the US: a cross-sectional analysis of 2011–2014 National Health and Nutrition Examination Survey. Aliment Pharmacol Ther, 2017. 46(10): p. 974–980. doi: 10.1111/apt.14327 [DOI] [PubMed] [Google Scholar]
- 29.Strickler G.K., et al., Opioid Prescribing Behaviors—Prescription Behavior Surveillance System, 11 States, 2010–2016. MMWR Surveill Summ, 2020. 69(1): p. 1–14. doi: 10.15585/mmwr.ss6901a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcin J.P., et al., A population-based analysis of socioeconomic status and insurance status and their relationship with pediatric trauma hospitalization and mortality rates. Am J Public Health, 2003. 93(3): p. 461–6. doi: 10.2105/ajph.93.3.461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casey J.A., et al., Measures of SES for Electronic Health Record-based Research. Am J Prev Med, 2018. 54(3): p. 430–439. doi: 10.1016/j.amepre.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charlson M., et al., Validation of a combined comorbidity index. J Clin Epidemiol, 1994. 47(11): p. 1245–51. doi: 10.1016/0895-4356(94)90129-5 [DOI] [PubMed] [Google Scholar]
- 33.Kim B.K., et al., Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int, 2010. 30(4): p. 546–553. doi: 10.1111/j.1478-3231.2009.02192.x [DOI] [PubMed] [Google Scholar]
- 34.Gellad W.F., Good C.B., and Shulkin D.J., Addressing the Opioid Epidemic in the United States: Lessons From the Department of Veterans Affairs. JAMA Intern Med, 2017. 177(5): p. 611–612. doi: 10.1001/jamainternmed.2017.0147 [DOI] [PubMed] [Google Scholar]
- 35.Gellad W.F., et al., Impact of Dual Use of Department of Veterans Affairs and Medicare Part D Drug Benefits on Potentially Unsafe Opioid Use. Am J Public Health, 2018. 108(2): p. 248–255. doi: 10.2105/AJPH.2017.304174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bohnert A.S.B., et al., A Detailed Exploration Into the Association of Prescribed Opioid Dosage and Overdose Deaths Among Patients With Chronic Pain. Med Care, 2016. 54(5): p. 435–441. doi: 10.1097/MLR.0000000000000505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Team, R.C. R: A language and environment for statistical computing. 2020; Available from: https://www.R-project.org/.
- 38.Dowell D., Haegerich T.M., and Chou R., CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. JAMA, 2016. 315(15): p. 1624–45. doi: 10.1001/jama.2016.1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klinge M., et al., The assessment and management of pain in cirrhosis. Curr Hepatol Rep, 2018. 17(1): p. 42–51. doi: 10.1007/s11901-018-0389-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moon A.M., et al., Opioid Use Is More Common in Nonalcoholic Fatty Liver Disease Patients with Cirrhosis, Higher BMI, and Psychiatric Disease. Dig Dis, 2021. 39(3): p. 247–257. doi: 10.1159/000511074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rogal S.S., et al., Fibromyalgia symptoms and cirrhosis. Dig Dis Sci, 2015. 60(5): p. 1482–9. doi: 10.1007/s10620-014-3453-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogal S.S., et al., Pain and opioid use in chronic liver disease. Dig Dis Sci, 2013. 58(10): p. 2976–85. doi: 10.1007/s10620-013-2638-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gastala N., Denial: The Greatest Barrier to the Opioid Epidemic. Ann Fam Med, 2017. 15(4): p. 372–374. doi: 10.1370/afm.2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mathieson S., et al., Deprescribing Opioids in Chronic Non-cancer Pain: Systematic Review of Randomised Trials. Drugs, 2020. 80(15): p. 1563–1576. doi: 10.1007/s40265-020-01368-y [DOI] [PubMed] [Google Scholar]
- 45.Glare P., et al., Deprescribing long-term opioid therapy in patients with chronic pain. Intern Med J, 2020. 50(10): p. 1185–1191. doi: 10.1111/imj.15023 [DOI] [PubMed] [Google Scholar]
- 46.Chandok N. and Watt K.D., Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin Proc, 2010. 85(5): p. 451–8. doi: 10.4065/mcp.2009.0534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lewis J.H. and Stine J.G., Review article: prescribing medications in patients with cirrhosis—a practical guide. Aliment Pharmacol Ther, 2013. 37(12): p. 1132–56. doi: 10.1111/apt.12324 [DOI] [PubMed] [Google Scholar]
- 48.Lin L.A., et al., Impact of the Opioid Safety Initiative on opioid-related prescribing in veterans. Pain, 2017. 158(5): p. 833–839. doi: 10.1097/j.pain.0000000000000837 [DOI] [PubMed] [Google Scholar]
- 49.Midboe A.M., et al., Academic Detailing to Improve Opioid Safety: Implementation Lessons from a Qualitative Evaluation. Pain Med, 2018. 19(suppl_1): p. S46–S53. doi: 10.1093/pm/pny085 [DOI] [PubMed] [Google Scholar]
- 50.Dowell D., et al., Contribution of Opioid-Involved Poisoning to the Change in Life Expectancy in the United States, 2000–2015. JAMA, 2017. 318(11): p. 1065–1067. doi: 10.1001/jama.2017.9308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frank J.W., et al., Patient Outcomes in Dose Reduction or Discontinuation of Long-Term Opioid Therapy: A Systematic Review. Ann Intern Med, 2017. 167(3): p. 181–191. doi: 10.7326/M17-0598 [DOI] [PubMed] [Google Scholar]
- 52.Krebs E.E., et al., Effect of Opioid vs Nonopioid Medications on Pain-Related Function in Patients With Chronic Back Pain or Hip or Knee Osteoarthritis Pain: The SPACE Randomized Clinical Trial. JAMA, 2018. 319(9): p. 872–882. doi: 10.1001/jama.2018.0899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schieber L.Z., et al., Trends and Patterns of Geographic Variation in Opioid Prescribing Practices by State, United States, 2006–2017. JAMA Netw Open, 2019. 2(3): p. e190665. doi: 10.1001/jamanetworkopen.2019.0665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dobscha S.K., et al., Correlates of Prescription Opioid Initiation and Long-term Opioid Use in Veterans With Persistent Pain. Clin J Pain, 2013. 29(2): p. 102–108. doi: 10.1097/AJP.0b013e3182490bdb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Semenkovich K., et al., Prescription Opioid Analgesics Increase Risk of Major Depression: New Evidence, Plausible Neurobiological Mechanisms and Management to Achieve Depression Prophylaxis. Mo Med, 2014. 111(2): p. 148–154. [PMC free article] [PubMed] [Google Scholar]
- 56.Serper M., et al., Risk Factors, Clinical Presentation, and Outcomes in Overdose With Acetaminophen Alone or With Combination Products: Results From the Acute Liver Failure Study Group. J Clin Gastroenterol, 2016. 50(1): p. 85–91. doi: 10.1097/MCG.0000000000000378 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Abbreviations: CLD, chronic liver disease.
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.