Abstract
Histopathological growth patterns (HGPs) are a reliable, reproducible, and strong prognostic biomarker that can be assessed on haematoxylin and eosin‐stained sections of resected colorectal liver metastases (CRLM). Assessment estimates the relative fraction of the tumour–liver interface for each of the three growth patterns; the desmoplastic HGP reflects good prognosis. Whether preoperative chemotherapy affects the HGP is currently unclear. The present international multicentre study evaluates this in an original cohort of 877 consecutive patients treated in the Netherlands, an external validation cohort of 1,203 consecutive patients treated in the USA, and a post hoc analysis from the phase III randomised controlled European Organization for Research and Treatment of Cancer (EORTC) 40983 trial (n = 70). All patients underwent resection of CRLM with or without preoperative systemic chemotherapy. Trial patients were randomised between perioperative chemotherapy and resection or resection alone. HGPs were determined according to consensus guidelines and compared for preoperative treatment status. Data from three separate tumour regression grading systems were available for the trial cohort. These were correlated with HGP stratified for treatment arm. In the original cohort, the average presence of desmoplastic HGP was 43% for chemo‐naïve versus 67% for preoperatively treated patients (p < 0.001). A significant association between chemotherapy and desmoplastic HGP was found on multivariable analysis (β [95% confidence interval, CI]: 24.57 [18.28–30.87], p < 0.001). In the validation cohort, the average presence of desmoplastic HGP was 40% for chemo‐naïve versus 63% for preoperatively treated patients (p < 0.001). This association remained on multivariable analysis (β [95% CI]: 24.18 [18.70–29.66], p < 0.001). In the EORTC 40983 trial, the average desmoplastic HGP presence was 33% in the resection arm versus 61% in the chemotherapy arm (p = 0.005). Chemotherapy was independently associated with an increase in desmoplastic HGP (β [95% CI]: 23.29 [1.78–44.79], p = 0.022). All three tumour regression gradings were significantly associated with the desmoplastic HGP in the chemotherapy arm (all p < 0.04). None were associated in the resection arm (all p > 0.11). Preoperative chemotherapy induces histopathological changes that alter the HGP of CRLM.
Keywords: colorectal cancer, colorectal liver metastases, histopathological growth patterns, systemic chemotherapy
Introduction
Histopathological growth patterns (HGPs) describe distinct phenotypes of tumour growth at the transition zone between pre‐existing liver parenchyma and colorectal liver metastases (CRLM) [1]. HGPs have been associated with prognosis in patients undergoing resection of CRLM [1, 2, 3, 4, 5, 6, 7, 8, 9]. The determination of HGPs has been standardised in international guidelines [1]. Three main HGP phenotypes are recognised: the replacement, the pushing, and the desmoplastic type HGP (Figure 1) [1, 10]. Based on prognosis, a dichotomy can be made. Patients with any observed non‐desmoplastic HGP (i.e. any pushing or replacement HGP) have worse survival outcomes compared to patients with pure desmoplastic HGP [5]. This difference in survival was less apparent for patients treated with preoperative systemic chemotherapy [5]. Furthermore, higher proportions of the desmoplastic HGP were observed in pre‐treated patients. These results suggest that preoperative chemotherapy may affect the HGP and raises questions regarding the assessment and value of this biomarker after preoperative systemic treatment. These results require external validation.
Figure 1.
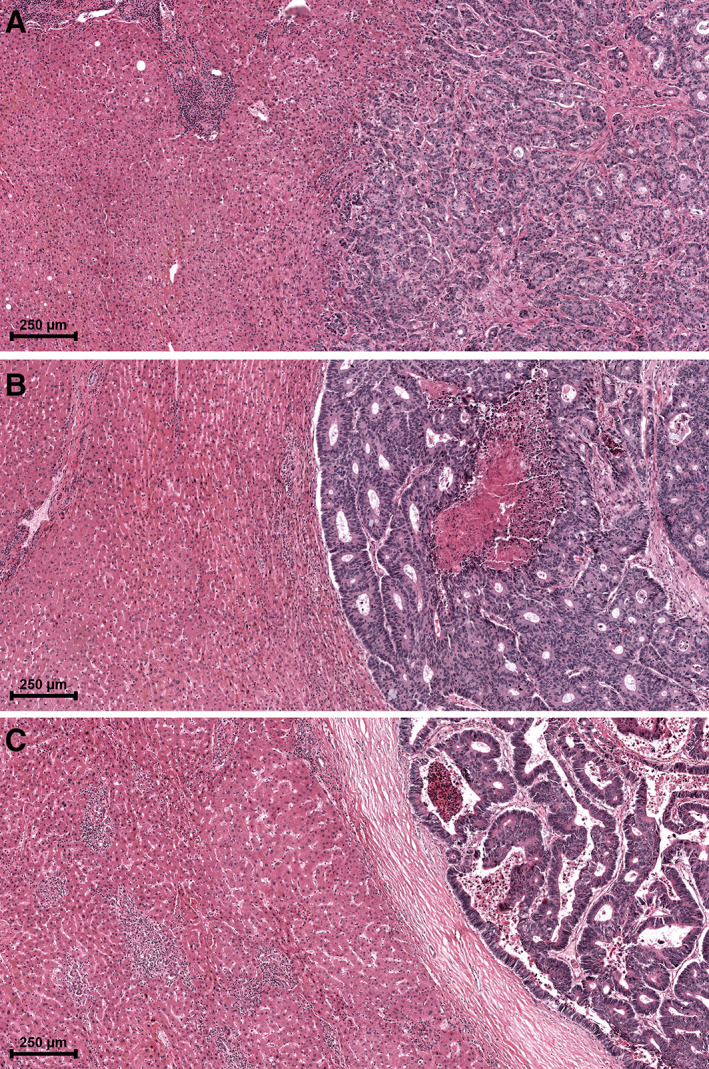
Examples of the distinct HGPs. (A) Example of replacement type HGP in which tumour cells ‘replace’ hepatocytes and infiltrate the liver parenchyma with direct tumour–liver cell contact. (B) Example of pushing type HGP in which the liver parenchyma is ‘pushed’ aside but is not infiltrated. No direct tumour–liver cell contact is present. (C) Example of desmoplastic type HGP, in which the tumour is separated from the liver parenchyma by a desmoplastic capsule. No direct tumour–liver cell contact is present.
The European Organization for Research and Treatment of Cancer (EORTC) intergroup study 40983 randomised controlled phase III trial compared surgery alone to surgery combined with perioperative systemic chemotherapy in patients with resectable CRLM [11, 12].
This study evaluates the effect of preoperative systemic chemotherapy on the HGPs of CRLM in an original cohort of consecutive patients undergoing resection in the Netherlands, a similar external validation cohort of patients treated in the USA, and in a post hoc analysis of a subset from the EORTC 40983 randomised controlled clinical trial.
Materials and methods
The current study was performed according to the STROBE guidelines for cohort studies and approved by the medical ethics committee of the Erasmus University Medical Centre Rotterdam (MEC 2018‐1743) [13]. A waiver for renewed written informed consent was granted.
Original cohort
All consecutive patients undergoing first resection of CRLM between January 2000 and February 2019 at the Erasmus MC Cancer Institute (Rotterdam, The Netherlands) were evaluated for eligibility. Part of this cohort was previously described by Galjart et al [5]. In accordance with the previous study, patients with incomplete resection, treated by ablation only, or in whom the HGP could not be determined were excluded. Patient characteristics, primary tumour and CRLM characteristics, treatment details, follow‐up, and disease recurrence were extracted from a prospectively maintained database.
External validation cohort
All consecutive patients undergoing first resection of CRLM between January 2000 and January 2019 at the Memorial Sloan Kettering Cancer Center (MSKCC) (New York City, NY, USA) were considered for inclusion in the external validation cohort. Similar exclusion criteria were applied. In addition, patients receiving preoperative hepatic arterial infusion pump (HAIP) chemotherapy were excluded as this study evaluates the relationship between HGPs and preoperative systemic chemotherapy. Data regarding patient characteristics, primary tumour and CRLM characteristics, treatment details, follow‐up, and disease recurrence were also extracted from a prospectively maintained database.
Randomised patient cohort
A subset of patients from the EORTC 40983 trial (NCT00006479), from whom digitalised haematoxylin and eosin (H&E)‐stained tissue sections were available, were included for post hoc analysis. This subset of patients has been described previously [14]. The details of the original trial including its short‐ and long‐term results are reported elsewhere [11, 12]. In summary, the EORTC 40983 trial randomised 364 patients with up to four resectable CRLM between either perioperative chemotherapy and resection (CTx arm) or resection only (Rx arm). Perioperative chemotherapy consisted of the FOLFOX4 regimen with six planned preoperative and six planned postoperative cycles [15].
HGP determination
Determination of HGPs was done in accordance with international consensus guidelines [1]. Assessment was performed by light microscopy on all available H&E‐stained tissue sections from all resected CRLM and blinded for outcome, preoperative treatment status, and all other clinicopathological patient characteristics. Assessment was performed by trained observers (PMHN, DJH, EPvdS, and BG) together/in consultation with a dedicated HGP pathologist (PBV) [1]. For the EORTC 40983 trial, patient assessment was performed on digitalised H&E‐stained tissue sections [14] by trained observers (PMHN, DJH, and BG) and a dedicated HGP pathologist (PBV) separately. Discordant cases were subsequently reviewed by all observers together (PMHN, DJH, BG, and PBV) to achieve consensus. As multiple HGPs can be present in a single tumour, the entire tumour–liver interface on each slide was examined. During assessment, the relative fraction of the total length of the interface of desmoplastic, replacement, and/or pushing HGP was estimated and expressed as percentage. Herein, each proportion of the interface representing 5% or more was taken into account. Metastasis level estimates were calculated with equal weights assigned to individual tissue sections. The final patient level HGP scores were subsequently calculated with equal weights assigned to individual metastases. The average presence of each distinct HGP observed was determined in each of the three cohorts and stratified for preoperative treatment status. The proportional distribution of distinct HGPs was displayed graphically and stratified for preoperative treatment status, in which the horizontal axis represented individual patients and the vertical axis the corresponding observed proportion of each distinct HGP at the tumour–liver interface. The average presence of each distinct HGP was represented by its surface area. HGP determination was not performed if no viable tumour was present, in cases with inadequate tissue preservation of H&E‐stained tissue section(s), or if less than 20% of the tumour–liver interface was assessable [1]. In accordance with previous findings, patients were classified as either pure desmoplastic HGP (i.e. 100% desmoplastic HGP) or non‐desmoplastic HGP (any replacement and/or pushing HGP) [5, 16]. A simplified decision tree to determine the HGP on a patient level based on this clinically relevant distinction, adapted with permission from van Dam et al [1], is provided in supplementary material, Figure S1. With regard to preoperative treatment stratification, patients in the original cohort and the external validation cohort who received any systemic chemotherapy within 6 months prior to CRLM resection – with the exception of capecitabine as radiosensitiser in the treatment for rectal cancer – were considered preoperatively treated. In addition, several examples of the desmoplastic HGP with and without preoperative systemic chemotherapy were selected and were evaluated in a descriptive manner.
Tumour regression grading
For the subset of the EORTC 40983 trial, three separate tumour regression gradings were available: the Mandard tumour regression grade (TRG) [17], the mean percentage of tumour cells according to Blazer et al [18], and the histological tumour regression according to Rubbia‐Brandt [19]. These three tumour regression gradings were all determined prior to the conception of this study, by an independent senior pathologist (CJ) not involved in HGP assessment and blinded for treatment arm and patient outcome. The Mandard TRG recognises five grades: 1 – absence of cancer cells replaced by abundant fibrosis; 2 – rare residual cancer cells scattered throughout abundant fibrosis; 3 – increase in the number of cancer cells but fibrosis remains predominant; 4 – residual cancer outgrowing fibrosis; and 5 – absence of regressive changes [17]. The method described by Blazer et al assesses pathological response to preoperative chemotherapy in patients with CRLM by semi‐quantitatively estimating the percentage of viable tumour in relation to tumour surface area [18]. The histological tumour regression according to Rubbia‐Brandt is an adaptation of the Mandard TRG and recognises three grades of tumour regression in CRLM: no histological tumour regressive or response changes (NHR), partial histological tumour response (PHR), and major or complete histological tumour response (MjHR) [19]. Tumour regression according to all three grading systems was correlated with HGP stratified for treatment arm.
Statistical analysis
Categorical data are reported using absolute numbers and corresponding percentages and continuous data using medians with corresponding interquartile ranges (IQR). Proportional differences were evaluated with the chi‐squared test. Differences in medians between two groups were assessed using the Mann–Whitney U‐test. The average presence of distinct HGPs was compared across preoperative treatment status by means of a parametric t‐test. To evaluate whether preoperative chemotherapy was associated with the observed proportion of the desmoplastic HGP at the interface, uni‐ and multi‐variable linear regression analyses were performed and expressed using the β coefficient with corresponding 95% confidence intervals (CIs). Additional uni‐ and multi‐variable linear regression models were computed in a combined cohort of all patients with available data on APC, KRAS, NRAS, and BRAF mutational status, as well as on microsatellite instability (MSI) status. The association between tumour regression and the desmoplastic HGP was assessed in the trial cohort for each of the three gradings and in each treatment arm separately by multivariable logistic regression. Results are graphically displayed using scatter plots with corresponding regression line and are reported using the β coefficient with corresponding 95% CI. The reversed Kaplan–Meier method was applied to estimate the median follow‐up time for survivors. Overall survival (OS) was defined as the time in months from the date of resection until the date of death. When alive, patients were censored at the date of last follow‐up. Kaplan–Meier analysis was used to determine survival estimates which were compared by means of the log‐rank test. Uni‐ and multi‐variable Cox regression analyses for OS were performed in the original and the external validation cohort to correct for potential confounding. In these cohorts, survival analyses on the HGP stratified by preoperative chemotherapy have previously been performed and were therefore not repeated [5, 16, 20]. Results of the Cox regression analyses were expressed using hazard ratios (HRs) and corresponding 95% CIs. For the EORTC 40983 trial subset, the OS difference between treatment arms was estimated and compared to the long‐term results of the entire trial (expressed as HR with corresponding 95% CI) [12]. In an attempt to assess differences between pre‐treated and chemo‐naïve patients with a desmoplastic HGP (i.e. 100% desmoplastic), clinicopathological factors and OS were compared between these subgroups in a combined cohort of all available patients. All analyses were performed using R version 4.1.0 (http://www.r-project.org).
Results
Original cohort
At the Erasmus MC Cancer Institute, 1,257 patients were treated surgically for CRLM between January 2000 and February 2019. Patients were excluded due to incomplete resection of CRLM (n = 133), ablative therapy only (n = 33), and unsuitable or unavailable H&E‐stained tissue sections for HGP determination (n = 214). The remaining 877 (70%) patients were included for analysis. Preoperative systemic chemotherapy was administered to 462 patients (53%). Baseline patient characteristics stratified by preoperative treatment are presented in Table 1. A graphical display of the distinct HGPs stratified for preoperative treatment status is shown in Figure 2. The average presence of desmoplastic HGP observed at the interface was 43% in chemo‐naïve versus 67% in preoperatively treated patients (p < 0.001; Figure 2D). Preoperative systemic chemotherapy was independently associated with a higher proportion of desmoplastic HGP observed (adjusted β [95% CI]: 24.57 [18.28–30.87], p < 0.001; Table 2). On multivariable analysis, a non‐desmoplastic HGP was associated with an adjusted HR (95% CI) for OS of 1.56 (1.23–1.98) (p < 0.001; see supplementary material, Table S1) [5, 16].
Table 1.
Baseline characteristics of all three cohorts stratified by preoperative treatment status.
| Original cohort Erasmus MC Cancer Institute | External validation cohort MSKCC | Randomised patient cohort EORTC 40983 trial | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Preoperative chemotherapy | Preoperative chemotherapy | Treatment arm | ||||||||
| No | Yes | No | Yes | Rx arm | CTx arm | |||||
| n = 462 (%) | n = 415 (%) | P value* | n = 410 (%) | n = 793 (%) | P value* | n = 40 (%) | n = 30 (%) | P value* | ||
| Age at resection CRLM (median [IQR]) | 66.0 [59.2–73.0] | 63.0 [56.0–69.0] | <0.001 | 62.0 [52.0–72.0] | 57.0 [48.0–66.0] | <0.001 | 67.5 [59.8–72.0] | 65.0 [58.5–71.8] | 0.536 | |
| Gender | Male | 298 (65) | 270 (65) | 0.863 | 228 (56) | 452 (57) | 0.645 | 22 (55) | 19 (63) | 0.484 |
| Female | 164 (35) | 145 (35) | 182 (44) | 341 (43) | 18 (45) | 11 (37) | ||||
| Primary tumour location | Right‐sided | 80 (18) | 65 (16) | 0.655 | 141 (36) | 204 (27) | 0.003 | 8 (20) | 8 (27) | 0.768 |
| Left‐sided | 195 (43) | 175 (43) | 175 (44) | 331 (45) | 15 (38) | 11 (37) | ||||
| Rectal | 175 (39) | 170 (41) | 80 (20) | 207 (28) | 16 (40) | 11 (37) | ||||
| Missing | 12 (3) | 5 (1) | 14 (3) | 51 (6) | 1 † (2) | 0 (0) | ||||
| Adjuvant CTx for primary | No | 369 (80) | 383 (93) | <0.001 | 140 (56) | 256 (56) | 0.967 | 30 (75) | 23 (77) | 0.872 |
| Yes | 92 (20) | 28 (7) | 109 (44) | 198 (44) | 10 (25) | 7 (23) | ||||
| Missing | 1 (0) | 4 (1) | 161 (39) | 339 (43) | — | — | ||||
| (y)pT‐stage | 0 | 6 (1) | 15 (4) | 0.004 | 0 (0) | 7 (1) | <0.001 | 0 (0) | 0 (0) | 0.823 |
| 1 | 11 (2) | 4 (1) | 21 (5) | 10 (1) | 1 (3) | 0 (0) | ||||
| 2 | 74 (16) | 47 (12) | 46 (12) | 65 (9) | 4 (10) | 4 (13) | ||||
| 3 | 327 (72) | 259 (69) | 263 (67) | 462 (66) | 30 (77) | 23 (77) | ||||
| 4 | 38 (8) | 51 (14) | 60 (15) | 153 (22) | 4 (10) | 3 (10) | ||||
| Missing | 6 (1) | 39 (9) | 20 (5) | 96 (12) | 1 (2) | 0 (0) | ||||
| (y)pN‐stage | 0 | 196 (43) | 139 (37) | 0.202 | 185 (46) | 246 (32) | <0.001 | 17 (44) | 13 (43) | 0.893 |
| 1 | 170 (38) | 151 (40) | 155 (38) | 307 (40) | 17 (44) | 12 (40) | ||||
| 2 | 87 (19) | 83 (22) | 64 (16) | 213 (28) | 5 (13) | 5 (17) | ||||
| Missing | 9 (2) | 42 (10) | 6 (1) | 27 (3) | 1 (2) | 0 (0) | ||||
| Differentiation grade | pCR | 5 (2) | 13 (4) | 0.189 | — | — | 0 (0) | 0 (0) | 0.733 | |
| G1 | 6 (2) | 6 (2) | — | — | 9 (23) | 7 (23) | ||||
| G2 | 267 (93) | 266 (89) | — | — | 28 (72) | 20 (67) | ||||
| G3 | 9 (3) | 15 (5) | — | — | 2 (5) | 3 (10) | ||||
| Missing | 175 (38) | 115 (28) | 410 (100) | 793 (100) | 1 (2) | 0 (0) | ||||
| Disease‐free interval in months (median [IQR]) | 11.0 [0.0–22.8] | 0.0 [0.0–0.5] | <0.001 | 12.0 [0.0–24.8] | 0.0 [0.0–5.0] | <0.001 | 5.8 [0.0–14.5] | 0.0 [0.0–14.8] | 0.334 | |
| Number of CRLM (median [IQR]) | 1.0 [1.0–2.0] | 3.0 [1.0–5.0] | <0.001 | 1.0 [1.0–2.0] | 2.0 [1.0–4.0] | <0.001 | 1.0 [1.0–2.0] | 2.0 [1.0–3.0] | 0.096 | |
| Diameter of largest CRLM in cm (median [IQR]) | 3.2 [2.1–4.8] | 2.2 [1.3–3.7] | <0.001 | 3.0 [2.1–5.0] | 2.5 [1.6–4.2] | <0.001 | 3.0 [2.5–4.6] | 2.5 [2.0–3.6] | 0.100 | |
| Preoperative CEA in μg/l (median [IQR]) | 11.0 [4.1–29.0] | 19.0 [5.3–74.0] | <0.001 | 9.8 [3.8–31.2] | 9.5 [3.9–35.3] | 0.577 | 7.4 [2.3–23.9] | 17.2 [4.8–58.5] | 0.078 | |
| Clinical risk score | Low risk (0–2) | 331 (75) | 177 (48) | <0.001 | 286 (74) | 346 (47) | <0.001 | 27 (69) | 15 (52) | 0.142 |
| High risk (3–5) | 111 (25) | 190 (52) | 100 (26) | 393 (53) | 12 (31) | 14 (48) | ||||
| Missing | 20 (4) | 48 (12) | 24 (6) | 54 (7) | 1 (2) | 1 (3) | ||||
| Extrahepatic disease | No | 429 (93) | 350 (84) | <0.001 | 362 (88) | 651 (82) | 0.005 | 38 (95) | 27 (90) | 0.421 |
| Yes | 33 (7) | 65 (16) | 48 (12) | 142 (18) | 2 (5) | 3 (10) | ||||
| Resection margin status | R0 | 410 (90) | 329 (80) | <0.001 | 368 (91) | 675 (86) | 0.009 | 37 (92) | 29 (97) | 0.457 |
| R1 | 48 (10) | 84 (20) | 35 (9) | 109 (14) | 3 (8) | 1 (3) | ||||
| Missing | 4 (1) | 2 (0) | 7 (2) | 9 (1) | — | — | ||||
| HGP | Desmoplastic | 85 (18) | 125 (30) | <0.001 | 53 (13) | 185 (23) | <0.001 | 7 (18) | 8 (27) | 0.355 |
| Non‐desmoplastic | 377 (82) | 290 (70) | 357 (87) | 608 (77) | 33 (82) | 22 (73) | ||||
| APC | Wildtype | 11 (46) | 8 (24) | 0.075 | 21 (15) | 53 (16) | 0.737 | — | — | |
| Mutant | 13 (54) | 26 (76) | 118 (85) | 271 (84) | — | — | ||||
| Missing | 438 (95) | 381 (92) | 271 (66) | 469 (59) | 40 (100) | 30 (100) | ||||
| KRAS | Wildtype | 42 (63) | 65 (56) | 0.345 | 151 (58) | 360 (56) | 0.633 | — | — | |
| Mutant | 25 (37) | 52 (44) | 109 (42) | 279 (44) | — | — | ||||
| Missing | 395 (85) | 298 (72) | 150 (37) | 154 (19) | 40 (100) | 30 (100) | ||||
| NRAS | Wildtype | 36 (95) | 65 (97) | 0.558 | 204 (96) | 510 (95) | 0.791 | — | — | |
| Mutant | 2 (5) | 2 (3) | 9 (4) | 25 (5) | — | — | ||||
| Missing | 424 (92) | 348 (84) | 197 (48) | 258 (33) | 40 (100) | 30 (100) | ||||
| BRAF | Wildtype | 56 (95) | 102 (100) | 0.022 | 226 (96) | 563 (96) | 0.663 | — | — | |
| Mutant | 3 (5) | 0 (0) | 10 (4) | 21 (4) | — | — | ||||
| Missing | 403 (87) | 313 (75) | 174 (42) | 209 (26) | 40 (100) | 30 (100) | ||||
| MSI status | MSS | 62 (97) | 71 (95) | 0.523 | 116 (97) | 284 (98) | 0.314 | — | — | |
| MSI | 2 (3) | 4 (5) | 4 (3) | 5 (2) | — | — | ||||
| Missing | 398 (86) | 340 (82) | 290 (71) | 504 (64) | 40 (100) | 30 (100) | ||||
Percentages of categorical variables are reported across valid cases only (i.e. missing are not considered).
CTx, chemotherapy; G, grade; MSS, microsatellite stable; pCR, pathological complete response; R0, negative resection margin (>0 mm margin); R1, positive resection margin; Rx, resection.
P values are calculated across valid cases only (i.e. missing are not considered).
Multiple primary tumours.
Figure 2.
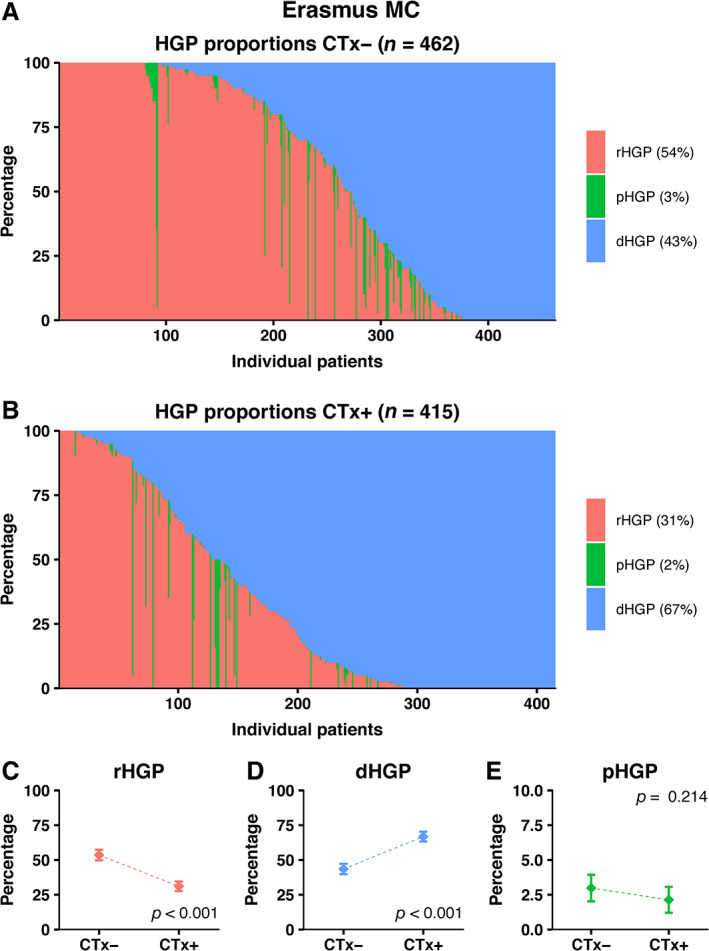
Distribution of HGPs in the original cohort of the Erasmus MC Cancer Institute stratified for preoperative treatment status. (A) Distribution of HGPs in the chemo‐naïve cohort. (B) Distribution of HGPs in the preoperatively treated cohort. (C–E) Average observed proportion of replacement type HGP (C), desmoplastic type HGP (D), and pushing type HGP (E) in chemo‐naïve patients compared to preoperatively treated patients.
Table 2.
Uni‐ and multi‐variable linear regression analyses for association with the desmoplastic HGP.
| Original cohort – Erasmus MC Cancer Institute | ||||
|---|---|---|---|---|
| Univariable | Multivariable (n = 725) | |||
| β (95% CI) | P value | β (95% CI) | P value | |
| Primary tumour location, right‐ versus left‐sided or rectal | 1.26 (−5.92–8.44) | 0.731 | 0.74 (−6.59–8.06) | 0.843 |
| Primary tumour T‐stage, (y)pT 0–4* | −2.65 (−6.45–1.14) | 0.170 | −1.05 (−5.12–3.01) | 0.611 |
| Primary tumour nodal status, (y)pN 0–2* | −5.51 (−9.15–−1.87) | 0.003 | −5.49 (−9.42–−1.56) | 0.006 |
| Disease‐free interval, months* | −0.24 (−0.40–−0.09) | 0.002 | −0.04 (−0.21–0.14) | 0.664 |
| Number of CRLM* | 1.33 (0.28–2.38) | 0.013 | −0.15 (−1.38–1.07) | 0.806 |
| Diameter of largest CRLM, cm* | −2.48 (−3.54–−1.41) | <0.001 | −1.23 (−2.37–−0.09) | 0.035 |
| Preoperative CEA level, 100 μg/l* | −0.24 (−1.10–0.61) | 0.577 | −0.75 (−1.60–0.11) | 0.087 |
| Preoperative chemotherapy, yes versus no | 23.30 (18.18–28.42) | <0.001 | 24.57 (18.28–30.87) | <0.001 |
| External validation cohort – MSKCC | ||||
|---|---|---|---|---|
| Univariable | Multivariable (n = 899) | |||
| β (95% CI) | P value | β (95% CI) | P value | |
| Primary tumour location, right‐ versus left‐sided or rectal | −1.51 (−6.46–3.45) | 0.550 | 2.67 (−2.68–8.03) | 0.328 |
| Primary tumour T‐stage, (y)pT 0–4* | −1.36 (−4.75–2.04) | 0.433 | −2.21 (−5.95–1.54) | 0.247 |
| Primary tumour nodal status, (y)pN 0–2* | −2.48 (−5.42–0.45) | 0.097 | −6.19 (−9.50–−2.87) | <0.001 |
| Disease‐free interval, months* | −0.27 (−0.39–−0.15) | <0.001 | −0.12 (−0.28–0.04) | 0.149 |
| Number of CRLM* | 1.12 (0.20–2.04) | 0.017 | 0.02 (−1.08–1.12) | 0.977 |
| Diameter of largest CRLM, cm* | −2.11 (−2.87–−1.35) | <0.001 | −1.53 (−2.38–−0.68) | <0.001 |
| Preoperative CEA level, 100 μg/l* | −0.08 (−0.35–0.19) | 0.556 | −0.09 (−0.35–0.18) | 0.511 |
| Preoperative chemotherapy, yes versus no | 22.19 (17.69–26.69) | <0.001 | 24.18 (18.70–29.66) | <0.001 |
| Randomised patient cohort – EORTC 40983 trial | ||||
|---|---|---|---|---|
| Univariable | Multivariable (n = 68) | |||
| β (95% CI) | P value | β (95% CI) | P value | |
| Primary tumour location, right‐ versus left‐sided or rectal | 3.85 (−19.91–27.62) | 0.747 | 3.56 (−21.14–28.26) | 0.774 |
| Primary tumour T‐stage, (y)pT 0–4* | −4.69 (−23.85–14.47) | 0.627 | −0.86 (−21.15–19.43) | 0.933 |
| Primary tumour nodal status, (y)pN 0–2* | −4.84 (−19.05–9.37) | 0.499 | −5.71 (−20.83–9.42) | 0.453 |
| Disease‐free interval, months* | 0.15 (−0.48–0.77) | 0.645 | 0.09 (−0.56–0.73) | 0.785 |
| Number of CRLM* | 5.86 (−2.46–14.18) | 0.165 | 4.12 (−4.65–12.89) | 0.351 |
| Diameter of largest CRLM, cm* | −2.13 (−4.86–0.61) | 0.126 | −1.66 (−4.74–1.43) | 0.288 |
| Preoperative CEA level, 100 μg/l* | 0.03 (−0.05–0.11) | 0.425 | 0.02 (−0.06–0.11) | 0.597 |
| Treatment arm, CTx versus Rx arm | 27.97 (8.95–46.98) | 0.005 | 23.29 (1.78–44.79) | 0.034 |
CTx, chemotherapy; Rx, resection.
Entered as continuous variable.
External validation
During the study period, 2,550 patients were treated surgically for CRLM at the MSKCC and were potentially eligible for inclusion. Patients were excluded due to any preoperative HAIP chemotherapy (n = 202), incomplete resection of CRLM (n = 84), ablative therapy only (n = 14), unsuitable or unavailable H&E‐stained tissue sections for HGP determination (n = 1042), and missing clinical information on inclusion and exclusion criteria (n = 5). In total, 1,203 (47%) patients were included for analysis. Preoperative systemic chemotherapy was administered to 793 patients (66%). Baseline characteristics compared for preoperative treatment are presented in Table 1. A graphical display of the distinct HGPs stratified for preoperative treatment status is shown in Figure 3. The average presence of desmoplastic HGP observed at the interface was 40% in chemo‐naïve patients versus 63% in preoperatively treated patients (p < 0.001; Figure 3D). On multivariable analysis, preoperative chemotherapy was significantly associated with a higher proportion of desmoplastic HGP (adjusted β [95% CI]: 24.18 [18.70–29.66], p < 0.001; Table 2). A non‐desmoplastic HGP was associated with an adjusted HR (95% CI) for OS of 1.75 (1.29–2.37) (p < 0.001; see supplementary material, Table S2) [16, 20].
Figure 3.
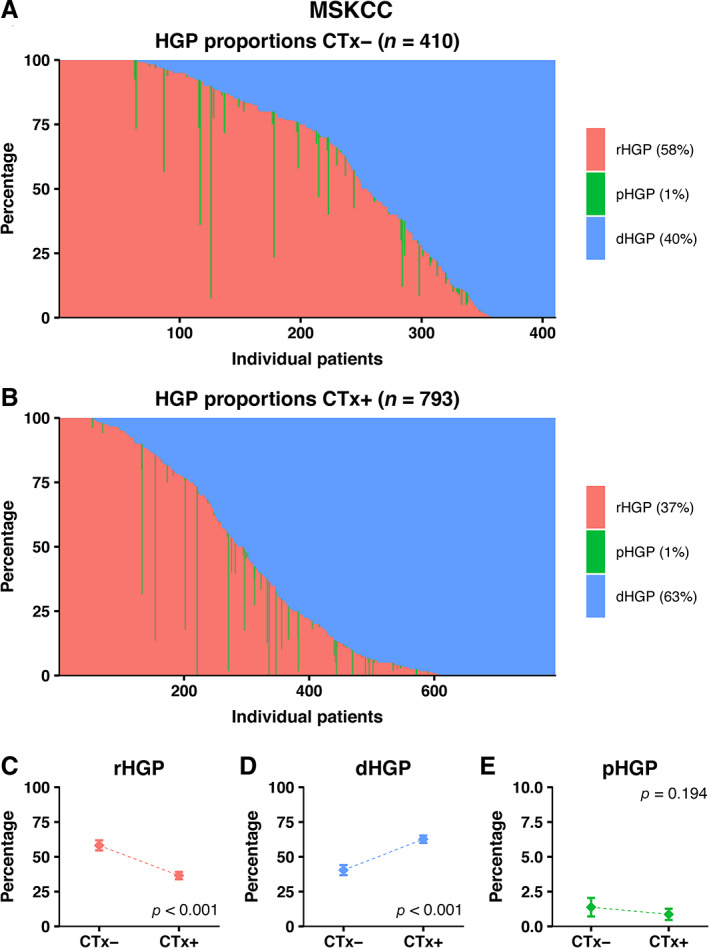
Distribution of HGPs in the external validation cohort of the MSKCC stratified for preoperative treatment status. (A) Distribution of HGPs in the chemo‐naïve cohort. (B) Distribution of HGPs in the preoperatively treated cohort. (C–E) Average observed proportion of replacement type HGP (C), desmoplastic type HGP (D), and pushing type HGP (E) in chemo‐naïve patients compared to preoperatively treated patients.
Randomised patient cohort
Digital H&E‐stained tissue sections of 70 patients, of whom 40 were treated in the Rx only arm, were obtained and the HGP was subsequently scored. In total, 112 digitalised H&E‐stained tissue sections were reviewed. Baseline characteristics compared for treatment arm are displayed in Table 1. No significant differences were found and baseline characteristics were comparable to those of the original trial cohort [11, 12]. In addition, OS did not differ between treatment arms (HR [95% CI]: 0.79 [0.42–1.48], p = 0.46), and was similar to the published long‐term results of the original trial (HR [95% CI]: 0.88 [0.68–1.14], p = 0.34) [12].
A graphical display of the distinct HGPs stratified for treatment arm is shown in Figure 4. The average presence of desmoplastic HGP observed at the interface was 33% in the Rx only arm versus 61% in the CTx arm (p = 0.005; Figure 4D). Preoperative systemic chemotherapy was independently associated with a higher proportion of desmoplastic HGP (adjusted β [95% CI]: 23.29 [1.78–44.79], p = 0.034; Table 2).
Figure 4.
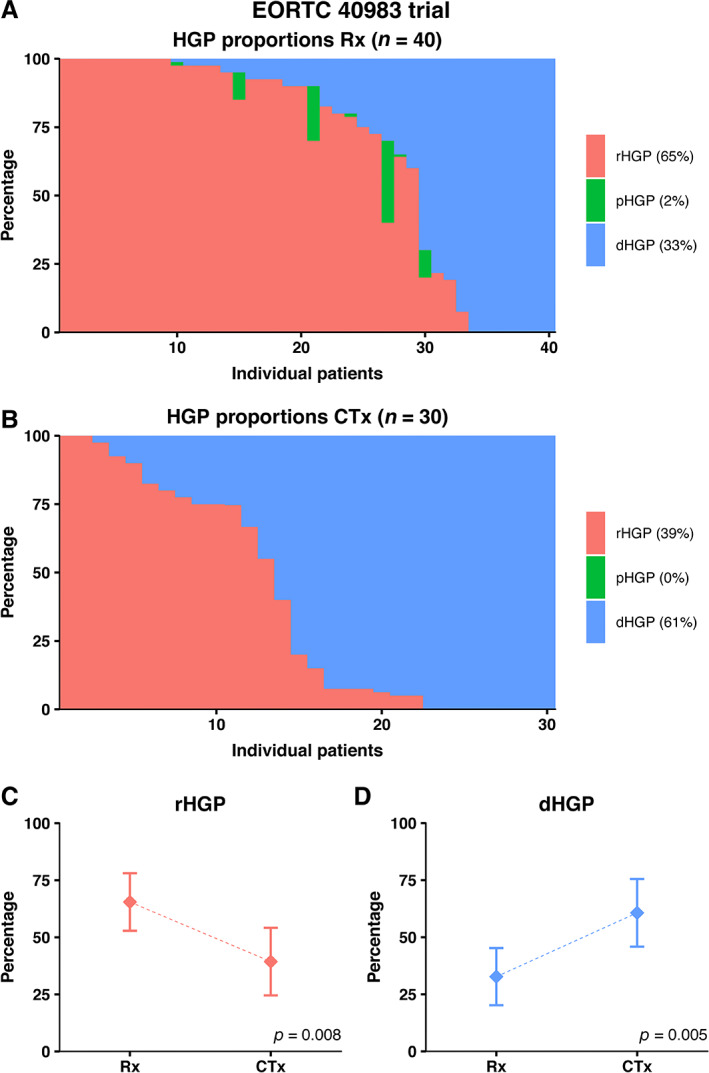
Distribution of HGPs in the EORTC 40983 trial stratified for preoperative treatment status. (A) Distribution of HGPs in the resection only arm. (B) Distribution of HGPs in the preoperatively treated arm. (C, D) Average observed proportion of replacement type HGP (C) and desmoplastic type HGP (D) in chemo‐naïve patients compared to preoperatively treated patients.
Within the Rx only arm, no associations were found between either the Mandard TRG, mean percentage of tumour cells, or Rubbia‐Brandt TRG and the observed percentage of desmoplastic HGP (all p > 0.11; Figure 5A–C and supplementary material, Table S3). In the CTx only arm, increased levels of tumour regression based on either the Mandard TRG, mean percentage of tumour cells, or Rubbia‐Brandt TRG were all significantly associated with an increase in desmoplastic HGP (all p < 0.04; Figure 5D–F and supplementary material, Table S3).
Figure 5.
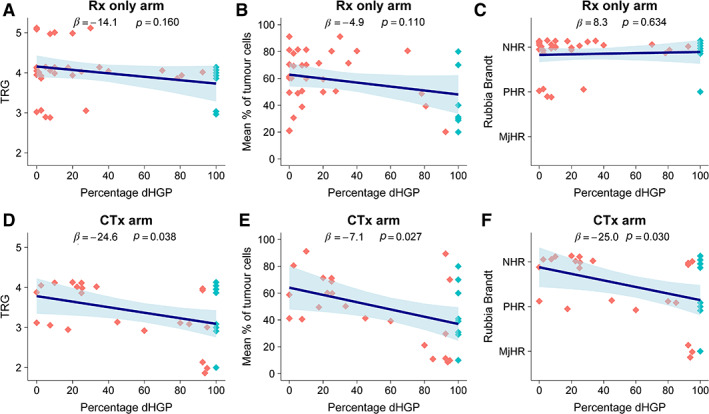
(A–F) Results of the multivariable linear regression models investigating three separate gradings of tumour regression in patients randomised to either resection only (A–C) or perioperative chemotherapy with resection (D–F) within the EORTC 40983 phase III trial. The dots resemble individual patients; dark grey dots represent a non‐desmoplastic and light grey dots a desmoplastic phenotype, respectively. The regression line represents the association for one of the three tumour regression gradings with the dHGP on multivariable analysis, with the ribbon representing the 95% CI of the estimate. dHGP, desmoplastic HGP; NHR, no histological response; PHR, partial histological response; MjHR, major histological response.
The median follow‐up for survivors (IQR) was 103 months (93–120) during which 41 patients (59%) died. The reported 5‐year OS rates in the Rx only arm were 83% versus 51% for patients with a pure desmoplastic HGP compared to patients with a non‐desmoplastic HGP (overall log‐rank: p = 0.16; see supplementary material, Figure S2). In the CTx arm, the 5‐year OS rates were 63% versus 59% for patients with a pure desmoplastic HGP compared to patients with a non‐desmoplastic HGP (overall log‐rank: p = 0.99; see supplementary material, Figure S2).
Genetics data
The uni‐ and multi‐variable linear regression models within the combined cohort of all patients with available genetics data showed that MSI was significantly associated with an increased proportion of desmoplastic HGP at the interface (adjusted β [95% CI]: 39.97 [13.59–66.34], p = 0.003; Table 3). There were no significant associations between APC, KRAS, NRAS, or BRAF mutational status and the proportion of desmoplastic HGP (all p > 0.6; Table 3). When correcting for genetic risk factors, preoperative chemotherapy remained independently associated with a higher proportion of desmoplastic HGP (adjusted β [95% CI]: 19.83 [10.85–28.82], p < 0.001).
Table 3.
Uni‐ and multi‐variable linear regression analyses for the association with the desmoplastic HGP in a combined cohort of patients with available genetics data.
| Univariable | Multivariable (n = 333) | |||
|---|---|---|---|---|
| β (95% CI) | P value | β (95% CI) | P value | |
| Primary tumour location, right‐ versus left‐sided or rectal | −0.61 (−4.64–3.43) | 0.767 | 2.46 (−6.57–11.49) | 0.593 |
| Primary tumour T‐stage, (y)pT 0–4* | −1.91 (−4.41–0.60) | 0.136 | −1.77 (−6.88–3.33) | 0.495 |
| Primary tumour nodal status, (y)pN 0–2* | −3.71 (−5.99–−1.43) | 0.001 | −8.59 (−13.76–−3.42) | 0.001 |
| APC, mutant versus wildtype | 0.82 (−7.40–9.03) | 0.845 | 0.81 (−10.14–11.75) | 0.885 |
| KRAS, mutant versus wildtype | 3.70 (−1.02–8.41) | 0.124 | −0.55 (−8.38–7.28) | 0.890 |
| NRAS, mutant versus wildtype | 8.83 (−3.65–21.31) | 0.165 | 5.33 (−15.06–25.73) | 0.607 |
| BRAF, mutant versus wildtype | 3.31 (−10.09–16.71) | 0.628 | 5.45 (−15.41–26.31) | 0.608 |
| MSI, MSI versus MSS | 20.82 (1.89–39.75) | 0.031 | 39.97 (13.59–66.34) | 0.003 |
| Disease‐free interval, months* | −0.26 (−0.35–−0.17) | <0.001 | −0.26 (−0.54–0.03) | 0.076 |
| Number of CRLM* | 1.21 (0.52–1.91) | <0.001 | 1.66 (0.15–3.17) | 0.031 |
| Diameter of largest CRLM, cm* | −2.23 (−2.85–−1.61) | <0.001 | −1.34 (−2.96–0.27) | 0.103 |
| Preoperative CEA level, 100 μg/l* | −0.09 (−0.35–0.17) | 0.479 | 0.31 (−0.15–0.77) | 0.191 |
| Preoperative chemotherapy, yes versus no | 22.00 (18.68–25.32) | <0.001 | 19.83 (10.85–28.82) | <0.001 |
MSS, microsatellite stable.
Entered as continuous variable.
Comparing chemo‐naïve and pre‐treated desmoplastic patients
Comparison of clinicopathological characteristics between all chemo‐naïve and pre‐treated desmoplastic patients (i.e. 100% desmoplastic HGP) is provided in Table 4 (combined cohort). In comparison to chemo‐naïve patients, pre‐treated desmoplastic patients were younger, had more advanced (y)pT&N stage, a shorter disease‐free interval, more CRLM, and a higher preoperative serum carcinoembryonic antigen (CEA) (Table 4). The size of the largest CRLM measured at pathological examination was however significantly smaller for the pre‐treated desmoplastic patients (Table 4). Concerning genetic risk factors, no significant differences were observed.
Table 4.
Clinicopathological characteristics of desmoplastic patients only (i.e. 100% desmoplastic HGP) stratified by preoperative treatment status (combined cohort).
| Desmoplastic patients only | |||||
|---|---|---|---|---|---|
| Preoperative chemotherapy | |||||
| No | Yes | ||||
| Missing (%) | n = 145 (%) | n = 318 (%) | P value | ||
| Age at resection CRLM (median [IQR]) | 67.0 [56.0–75.0] | 61.0 [51.0–68.0] | <0.001 | ||
| Gender | Female | 87 (60) | 206 (65) | 0.322 | |
| Male | 58 (40) | 112 (35) | |||
| Primary tumour location | Right‐sided | 21 (5) | 31 (22) | 82 (27) | 0.560 |
| Left‐sided | 64 (46) | 129 (43) | |||
| Rectal | 44 (32) | 92 (30) | |||
| (y)pT‐stage | 0 | 31 (7) | 1 (1) | 15 (5) | <0.001 |
| 1 | 5 (4) | 2 (1) | |||
| 2 | 29 (20) | 25 (9) | |||
| 3 | 97 (68) | 190 (66) | |||
| 4 | 10 (7) | 58 (20) | |||
| (y)pN‐stage | 0 | 13 (3) | 84 (58) | 119 (39) | <0.001 |
| 1 | 46 (32) | 116 (38) | |||
| 2 | 14 (10) | 71 (23) | |||
| Disease‐free interval in months (median [IQR]) | 5 (1) | 9.0 [0.0–22.0] | 0.0 [0.0–1.0] | <0.001 | |
| Number of CRLM (median [IQR]) | 3 (1) | 1.0 [1.0–2.0] | 2.0 [1.0–4.0] | <0.001 | |
| Diameter of largest CRLM in cm (median [IQR]) | 15 (3) | 2.3 [1.5–3.5] | 1.8 [1.1–3.0] | 0.002 | |
| Preoperative CEA in μg/l (median [IQR]) | 37 (8) | 4.8 [2.6–11.5] | 8.0 [3.1–29.9] | <0.001 | |
| APC | Wildtype | 364 (79) | 3 (18) | 15 (18) | 0.950 |
| Mutant | 14 (82) | 67 (82) | |||
| KRAS | Wildtype | 256 (55) | 17 (52) | 94 (54) | 0.791 |
| Mutant | 16 (48) | 80 (46) | |||
| NRAS | Wildtype | 293 (63) | 25 (93) | 135 (94) | 0.713 |
| Mutant | 2 (7) | 8 (6) | |||
| BRAF | Wildtype | 277 (60) | 28 (97) | 149 (95) | 0.704 |
| Mutant | 1 (3) | 8 (5) | |||
| MSI status | MSS | 343 (74) | 27 (93) | 86 (95) | 0.779 |
| MSI | 2 (7) | 5 (5) | |||
MSS–microsatellite stable.
Chemo‐naïve patients with desmoplastic lesions had a significantly longer OS compared to the pre‐treated desmoplastic patients, with 5‐year OS (95% CI) of 74% (67–83%) compared to 60% (54–66%) (p = 0.004; see supplementary material, Figure S3). This difference remained on multivariable analysis, with an adjusted HR for OS of 1.78 (1.16–2.74); p = 0.008 (see supplementary material, Table S4) for pre‐treated desmoplastic versus chemo‐naïve desmoplastic patients.
Desmoplastic HGP examples with and without preoperative chemotherapy
Several examples of CRLM with a desmoplastic HGP are presented in panels A–J of Figure 6. Panels A–E pertain to resected CRLM of chemo‐naïve patients, and in panels F–J CRLM resected from preoperatively treated patients are displayed. As delineated by international consensus guidelines, the desmoplastic HGP can exhibit several distinguishing features like the presence of a rim of desmoplastic stroma separating the metastasis from the liver parenchyma (prerequisite), which is often accompanied by a (dense) lymphocytic infiltrate around this stroma [1, 21]. Moreover, the composition of the tumour cells does not mimic the architectural pattern of the liver parenchyma. Almost all of the aforementioned histopathological features apply to all panels in Figure 6. Despite these general similarities, closer observation reveals some apparent morphological differences. Varying degrees of tumour regression are present in the preoperatively treated CRLM. For example, in panels F and G, Mandard TRGs of 2 and 3, respectively, are seen. While these examples formally meet the conditions to be classified as desmoplastic HGP (i.e. separation of the metastasis from the liver tissue by a desmoplastic rim), few vital tumour cells are present. Therefore, it is unknown whether the currently observed morphology represents the ‘original’ (prior to systemic chemotherapy) metastasis morphology as such an assessment (HGP pre‐chemotherapy) is currently impossible. In panel H, we observe a metastasis that is formally classified as desmoplastic (i.e. separation of the liver parenchyma and the metastasis by desmoplasia) but the tumour cells in the periphery of the metastases are organised in a trabecular‐like (plate‐like) pattern resembling liver parenchyma, albeit accompanied by relatively obvious inter‐plate fibrosis. This plate‐like pattern is often observed in the replacement HGP type. Again, as pre‐chemotherapy morphology of this metastasis is unknown, it is not possible to assess with certainty whether chemotherapy induced this increased intercellular fibrosis and desmoplasia surrounding the metastasis. Panels I and J display CRLM with a ‘classic’ desmoplastic phenotype, although the higher amount of necrosis in panel I might be regarded as the impact of the preoperative chemotherapy.
Figure 6.
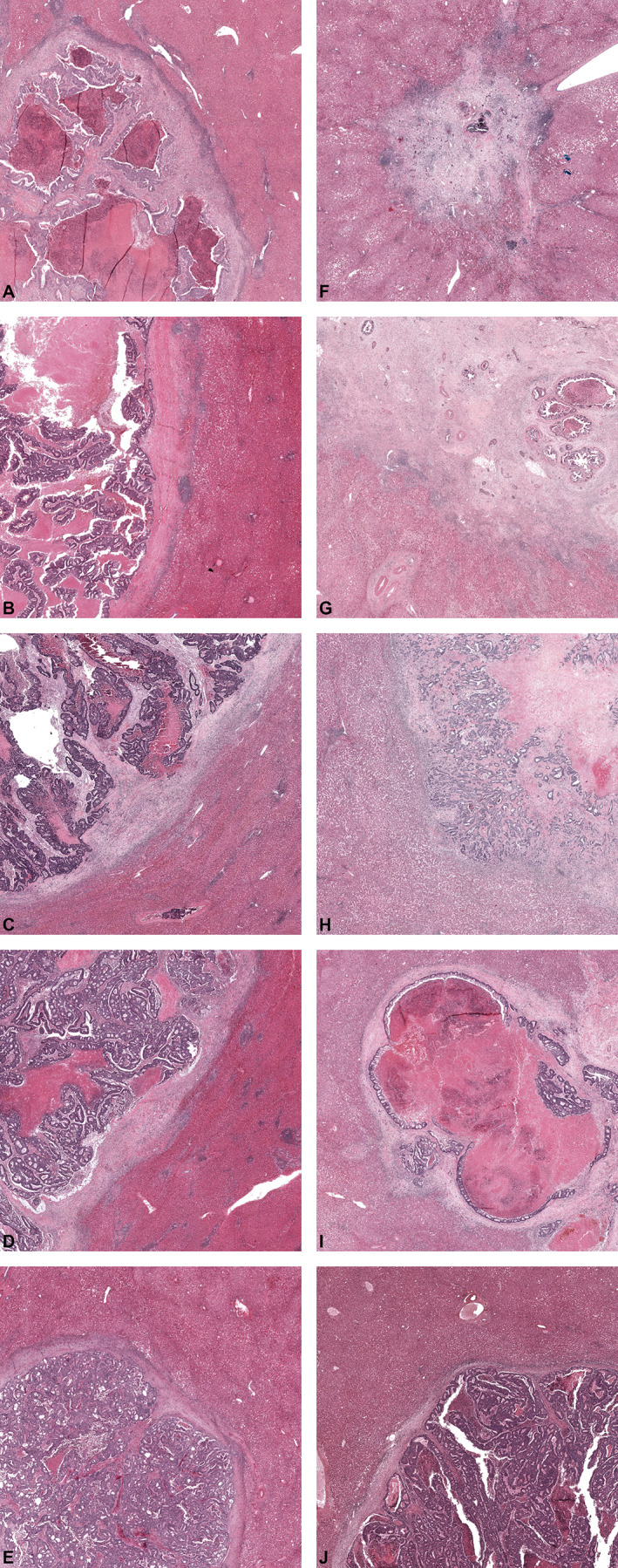
(A–J) Representative examples of resected CRLM exhibiting a desmoplastic HGP in chemo‐naïve (A–E) and pre‐treated (D–J) patients.
Discussion
In all three cohorts described in this study, preoperative chemotherapy was associated with a higher proportion of desmoplastic HGP observed at the interface. These results were obtained in an original cohort and validated in both an independent retrospective external validation cohort, as well as in a post hoc analysis of the prospective randomised controlled EORTC 40983 clinical trial.
We previously demonstrated a significant difference in HGPs between chemo‐naïve and preoperatively treated patients undergoing surgical treatment of CRLM [5]. The value of those findings was limited at the time, as it was the only study describing a difference and it was based on retrospective data from a single centre. One other study has described a modest difference in the observed percentage of distinct HGPs after preoperative treatment with chemotherapy and bevacizumab [4]. Similar to the results of the current study, a higher percentage of desmoplastic HGP was reported after preoperative treatment, albeit not significant, but this can likely be attributed to the limited sample size. The current study has addressed both shortcomings of these two previous papers, large sample sizes and multiple external validation cohorts, including a subset of a randomised controlled trial. And indeed, in the current study, we were able to confirm an increase in desmoplastic HGP observed after preoperative chemotherapy in three independent cohorts. In addition, we demonstrate within the randomised controlled trial that pathology gradings designed for quantifying tumour regression as a result of therapy were associated with higher proportions of the desmoplastic HGP for the pre‐treated patients only. Herein, it is important to note that these gradings were determined prior to the conception of the current study, and that determination was performed blinded for treatment arm by an independent senior pathologist not involved in the HGP scoring presented. Therefore, this presents the optimal method to assess such an association, for if we now were to determine these gradings this cannot be done independent of HGP as they are determined – and therefore visible – on the same H&E‐stained slides. These results strongly suggest HGP phenotype alteration by chemotherapy. This should be taken into account in future studies and/or guidelines regarding HGPs of CRLM as desmoplastic growth induced by chemotherapy may be a distinct phenotype, with considerable biological and clinical differences with the naturally occurring desmoplastic growth pattern.
This alteration in growth pattern phenotype after preoperative treatment could occur in at least two ways: either the chemotherapy agents induce desmoplasia in a proportion of non‐desmoplastic liver metastasis, or relatively more desmoplastic lesions remain after chemotherapy. In an attempt to determine which explanation is more likely, we compared the clinicopathological factors between chemo‐naïve and preoperatively treated desmoplastic patients only. In this comparison, the pre‐treated desmoplastic patients had more advanced (y)pT&N stage, as well as more metastases in general, and a higher preoperative serum CEA, all traits that have previously been associated with non‐desmoplastic metastases [5, 16]. It has to be noted however that these findings are at significant risk of selection bias, as patients with more advanced disease are more likely to receive preoperative systemic chemotherapy. Nevertheless, comparing OS between these two groups showed that the pre‐treated patients with desmoplastic lesions had worse survival compared to the chemo‐naïve patients with desmoplastic lesions, even after correction for these baseline differences. This is in line with previous reports, which either demonstrate a marginally prognostic value for HGPs in pre‐treated patients [5], or a prognostic value that is less pronounced compared to that in chemo‐naïve patients [16]. To address selection bias, we attempted to validate these findings in the randomised cohort presented, as the EORTC 40983 trial randomised between perioperative chemotherapy and upfront resection. While the current post hoc analysis was severely underpowered to find significant survival differences, the observed survival estimates did resemble those reported previously [5, 20]. In addition to this difference in prognosis, a recent study demonstrated that adjuvant systemic chemotherapy resulted in a survival benefit for the chemo‐naïve patients with non‐desmoplastic lesions only, hinting at differences in chemo‐sensitivity between the untreated growth patterns [20]. Our results and the literature are therefore more suggestive of dilution of the preoperatively treated ‘pure desmoplastic’ population by a more aggressive former non‐desmoplastic tumour type component.
Systemic chemotherapy has indeed been associated with alterations in gene expression in CRLM [22], the immune infiltrate of CRLM [14, 22], the immune response in malignancies in general [23, 24], and tumoural fibrosis or necrosis in CRLM [18, 19]. The explanation for the conversion of non‐desmoplastic to desmoplastic HGP as a consequence of preoperative systemic chemotherapy might therefore also (partially) lie in these associations. However, the problem currently faced is that the assessment of HGPs is only possible after systemic therapy and subsequent resection. There is currently no way to assess whether a patient's lesion(s) was/were desmoplastic prior to the start of chemotherapy, or that a ‘desmoplastic‐like’ pattern was induced, and consequently also which mechanism may have induced this change. Future attempts should therefore focus on the pre‐treatment or preoperative determination of the HGPs, with recent reports showing promise for a medical imaging approach [25].
There is as yet no clear consensus on the biology behind the prognostic value of HGPs itself. Some potential explanatory factors are the differences in vascular architecture of CRLM [26, 27], the variation of immune infiltrate in and around CRLM [2, 21], and the up‐regulation of signalling pathways of cell motility and invasiveness of cancer cells in the replacement HGP [4]. Concerning genetics, our recent external validation study did associate the desmoplastic HGP with MSI, but not with the known genetic risk factors KRAS and BRAF mutation [16]. Here, we again report similar results. MSI was associated with an increased proportion of the desmoplastic HGP, while no such associations existed for APC, KRAS, NRAS, or BRAF mutations. A potential explanation for the association between MSI tumours and the desmoplastic HGP might be that MSI tumours are hypermutated and therefore present more potential neoantigens to target T cells, resulting in a higher probability for a (partially) successful anti‐cancer T‐cell response [28, 29]. It has been demonstrated by us and others that the microenvironment of desmoplastic HGP is indeed enriched with T cells [2, 21]. This association between the desmoplastic HGP and MSI is especially of interest as MSI tumours represent an actionable target for immunotherapy in metastatic colorectal cancer [30]. Future investigations aimed at validating these findings, and to identify other potential genetic associations related to the HGPs, therefore seem warranted.
Limitations of the current study should be taken into account. HGP determination was performed retrospectively. Furthermore, a complete pathological response to preoperative chemotherapy makes HGP assessment impossible. This means that the patients with the most favourable response to chemotherapy (i.e. Mandard TRG 1), albeit rare, were excluded. Complete pathological response to chemotherapy is associated with fibrosis on histopathological examination; excluding these patients therefore makes it likely that conversion to desmoplastic HGP may be underestimated in the current study. Only a subset of patients from the randomised EORTC 40983 trial were available for post hoc analysis, which may have introduced selection bias. Baseline characteristics and survival outcomes of the currently presented subset were however comparable to those found in the original trial [11, 12]. This suggests that this subset is a proper representation of the EORTC 40983 trial population. Rather, the risk of selection bias could apply to the external validation cohort, as it represents a retrospective, non‐randomised cohort.
The results of the current study strongly suggest that systemic chemotherapy induces histopathological changes that lead to an increase of desmoplastic HGP as recognised by international consensus guidelines. As it is currently impossible to assess the HGP prior to chemotherapy treatment, we can at present not determine whether this increase reflects actual change of underlying biology, or is a limitation of the current HGP assessment algorithm after systemic preoperative chemotherapy. The limited evidence currently available may however favour the latter. This should be taken into account in future studies and/or guidelines regarding HGPs of CRLM.
Disclaimer
The contents of this publication and methods used are solely the responsibility of the authors and do not necessarily represent the official views of the EORTC.
Author contributions statement
PMHN and DJH had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. PMHN, DJH, DJG, MID'A and CV conceived and designed the study. PMHN, DJH, FEB, EPvdS, BG, VPB, WRJ, TPK, JS, MM, BN, CJ, BGK, MD, PBV, DJG, MID'A and CV acquired, analysed, or interpreted data. PMHN, DJG and CV drafted the manuscript. FEB, EPvdS, BG, VPB, WRJ, TPK, JS, MM, BN, CJ, BGK, MD, PBV and MID'A critically revised the manuscript. PMHN and DJH carried out the statistical analysis. DJG, MID'A and CV supervised the study.
Supporting information
Figure S1. Simplified decision tree to determine the growth patterns of liver metastases based on the key histopathological characteristics
Figure S2. Kaplan–Meier OS curves stratified by HGP in the resection only (Rx) arm and perioperative chemotherapy (CTx) arm of the EORTC 40983 trial
Figure S3. Kaplan–Meier OS curves stratified by preoperative treatment status in a combined cohort of patients with only desmoplastic lesions
Table S1. Erasmus MC uni‐ and multi‐variable Cox regression analyses for OS
Table S2. MSKCC uni‐ and multi‐variable Cox regression analyses for OS
Table S3. Multivariable linear regression analysis of the relationship between tumour regression gradings and the percentage of desmoplastic HGP within the EORTC 40983 treatment arms
Table S4. Uni‐ and multi‐variable Cox regression analyses for OS in patients with only desmoplastic lesions (combined cohort)
Acknowledgements
The data of the EORTC 40983 trial were kindly provided for the current study by the EORTC. We thank the investigators and all participating patients of the EORTC 40983 trial. All funding for this study was institutional. The EORTC 40983 trial was supported by grants from the Swedish Cancer Society (Sweden), Cancer Research UK (United Kingdom), the Ligue Nationale Contre le Cancer (France), and the National Cancer Institute (Bethesda, MD, USA; grants 5U10‐CA11488‐28 through 5U10 CA11488‐37). The respective institutions and the funder of the EORTC 40983 trial had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
No conflicts of interest were declared.
References
- 1. van Dam PJ, van der Stok EP, Teuwen LA, et al. International consensus guidelines for scoring the histopathological growth patterns of liver metastasis. Br J Cancer 2017; 117: 1427–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brunner SM, Kesselring R, Rubner C, et al. Prognosis according to histochemical analysis of liver metastases removed at liver resection. Br J Surg 2014; 101: 1681–1691. [DOI] [PubMed] [Google Scholar]
- 3. Eefsen RL, Vermeulen PB, Christensen IJ, et al. Growth pattern of colorectal liver metastasis as a marker of recurrence risk. Clin Exp Metastasis 2015; 32: 369–381. [DOI] [PubMed] [Google Scholar]
- 4. Frentzas S, Simoneau E, Bridgeman VL, et al. Vessel co‐option mediates resistance to anti‐angiogenic therapy in liver metastases. Nat Med 2016; 22: 1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Galjart B, Nierop PMH, van der Stok EP, et al. Angiogenic desmoplastic histopathological growth pattern as a prognostic marker of good outcome in patients with colorectal liver metastases. Angiogenesis 2019; 22: 355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nielsen K, Rolff HC, Eefsen RL, et al. The morphological growth patterns of colorectal liver metastases are prognostic for overall survival. Mod Pathol 2014; 27: 1641–1648. [DOI] [PubMed] [Google Scholar]
- 7. Siriwardana PN, Luong TV, Watkins J, et al. Biological and prognostic significance of the morphological types and vascular patterns in colorectal liver metastases (CRLM): looking beyond the tumor margin. Medicine (Baltimore) 2016; 95: e2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van den Eynden GG, Bird NC, Majeed AW, et al. The histological growth pattern of colorectal cancer liver metastases has prognostic value. Clin Exp Metastasis 2012; 29: 541–549. [DOI] [PubMed] [Google Scholar]
- 9. Nierop PMH, Galjart B, Höppener DJ, et al. Salvage treatment for recurrences after first resection of colorectal liver metastases: the impact of histopathological growth patterns. Clin Exp Metastasis 2019; 36: 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vermeulen PB, Colpaert C, Salgado R, et al. Liver metastases from colorectal adenocarcinomas grow in three patterns with different angiogenesis and desmoplasia. J Pathol 2001; 195: 336–342. [DOI] [PubMed] [Google Scholar]
- 11. Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 2008; 371: 1007–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long‐term results of a randomised, controlled, phase 3 trial. Lancet Oncol 2013; 14: 1208–1215. [DOI] [PubMed] [Google Scholar]
- 13. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology 2007; 18: 800–804. [DOI] [PubMed] [Google Scholar]
- 14. Tanis E, Julié C, Emile JF, et al. Prognostic impact of immune response in resectable colorectal liver metastases treated by surgery alone or surgery with perioperative FOLFOX in the randomised EORTC study 40983. Eur J Cancer 2015; 51: 2708–2717. [DOI] [PubMed] [Google Scholar]
- 15. de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first‐line treatment in advanced colorectal cancer. J Clin Oncol 2000; 18: 2938–2947. [DOI] [PubMed] [Google Scholar]
- 16. Höppener DJ, Galjart B, Nierop PMH, et al. Histopathological growth patterns and survival after resection of colorectal liver metastasis: an external validation study. JNCI Cancer Spectrum 2021; 5: pkab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994; 73: 2680–2686. [DOI] [PubMed] [Google Scholar]
- 18. Blazer DG 3rd, Kishi Y, Maru DM, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol 2008; 26: 5344–5351. [DOI] [PubMed] [Google Scholar]
- 19. Rubbia‐Brandt L, Giostra E, Brezault C, et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo‐adjuvant chemotherapy followed by liver surgery. Ann Oncol 2007; 18: 299–304. [DOI] [PubMed] [Google Scholar]
- 20. Buisman FE, van der Stok EP, Galjart B, et al. Histopathological growth patterns as biomarker for adjuvant systemic chemotherapy in patients with resected colorectal liver metastases. Clin Exp Metastasis 2020; 37: 593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Höppener DJ, Nierop PMH, Hof J, et al. Enrichment of the tumour immune microenvironment in patients with desmoplastic colorectal liver metastasis. Br J Cancer 2020; 123: 196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van den Eynde M, Mlecnik B, Bindea G, et al. The link between the multiverse of immune microenvironments in metastases and the survival of colorectal cancer patients. Cancer Cell 2018; 34: 1012–1026.e3. [DOI] [PubMed] [Google Scholar]
- 23. Zitvogel L, Apetoh L, Ghiringhelli F, et al. The anticancer immune response: indispensable for therapeutic success? J Clin Invest 2008; 118: 1991–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zitvogel L, Apetoh L, Ghiringhelli F, et al. Immunological aspects of cancer chemotherapy. Nat Rev Immunol 2008; 8: 59–73. [DOI] [PubMed] [Google Scholar]
- 25. Cheng J, Wei J, Tong T, et al. Prediction of histopathologic growth patterns of colorectal liver metastases with a noninvasive imaging method. Ann Surg Oncol 2019; 26: 4587–4598. [DOI] [PubMed] [Google Scholar]
- 26. Nowak‐Sliwinska P, Alitalo K, Allen E, et al. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis 2018; 21: 425–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stessels F, Van den Eynden G, Van der Auwera I, et al. Breast adenocarcinoma liver metastases, in contrast to colorectal cancer liver metastases, display a non‐angiogenic growth pattern that preserves the stroma and lacks hypoxia. Br J Cancer 2004; 90: 1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology 2010; 138: 2073–2087.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen DS, Mellman I. Elements of cancer immunity and the cancer‐immune set point. Nature 2017; 541: 321–330. [DOI] [PubMed] [Google Scholar]
- 30. André T, Shiu K‐K, Kim TW, et al. Pembrolizumab in microsatellite‐instability–high advanced colorectal cancer. N Engl J Med 2020; 383: 2207–2218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Simplified decision tree to determine the growth patterns of liver metastases based on the key histopathological characteristics
Figure S2. Kaplan–Meier OS curves stratified by HGP in the resection only (Rx) arm and perioperative chemotherapy (CTx) arm of the EORTC 40983 trial
Figure S3. Kaplan–Meier OS curves stratified by preoperative treatment status in a combined cohort of patients with only desmoplastic lesions
Table S1. Erasmus MC uni‐ and multi‐variable Cox regression analyses for OS
Table S2. MSKCC uni‐ and multi‐variable Cox regression analyses for OS
Table S3. Multivariable linear regression analysis of the relationship between tumour regression gradings and the percentage of desmoplastic HGP within the EORTC 40983 treatment arms
Table S4. Uni‐ and multi‐variable Cox regression analyses for OS in patients with only desmoplastic lesions (combined cohort)


