Abstract
Migration of cells requires interactions with the extracellular matrix mediated, in part, by integrins, proteases, and their receptors. Previous studies have shown that β3-integrin interacts with the urokinase-type plasminogen activator receptor (u-PAR) at the cell surface. Since integrins mediate signaling into the cell, the current study was undertaken to determine if in addition β3-integrin regulates u-PAR expression. Overexpression of β3-integrin in CHO cells, which are avid expressers of the receptor, downregulated u-PAR protein and mRNA expression. The u-PAR promoter (−1,469 bp) that is normally constitutively active in CHO cells was downregulated by induced β3-integrin expression. A region between −398 and −197 bp of the u-PAR promoter was critical for β3-integrin-induced downregulation of u-PAR promoter activity. Deletion of the PEA3/ets motif at −248 bp substantially impaired the ability of β3-integrin to downregulate the u-PAR promoter, suggesting that the PEA3/ets site acts as a silencing element. An expression vector encoding the transcription factor PEA3 caused inhibition of the wild-type but not the PEA3/ets-deleted u-PAR promoter. The PEA3/ets site bound nuclear factors from CHO cells specifically, but binding was enhanced when β3-integrin was overexpressed. A PEA3 antibody inhibited DNA-protein complex formation, indicating the presence of PEA3. Downregulation of the u-PAR promoter was achieved by the β3A-integrin isoform but not by other β3-integrin isoforms and required the cytoplasmic membrane NITY759 motif. Moreover, overexpression of the short but not the long isoform of the β3-integrin adapter protein β3-endonexin blocked u-PAR promoter activity through the PEA3/ets binding site. Thus, besides the physical interaction of β3-integrin and u-PAR at the cell surface, β3 signaling is implicated in the regulation of u-PAR gene transcription, suggesting a mutual regulation of adhesion and proteolysis receptors.
Invasion and metastasis of tumor cells require a complex regulation of different cell surface-associated proteins (4, 53, 55) that facilitate proteolysis and adhesion of cells to the basement membrane and the extracellular matrix (ECM). The invasive process starts with the attachment of cells to the ECM, followed by degradation of various ECM components through proteases, subsequent cell detachment, and migration (5). One important protease involved in these processes is the serine protease urokinase-type plasminogen activator (urokinase) which is bound to a specific receptor (u-PAR). Urokinase converts plasminogen to plasmin, a serine protease with broad substrate specificity for several components of the ECM, including vitronectin, laminin, and fibronectin (5, 27, 43). Together, these adhesive and proteolytic functions of tumor cells facilitate their migration through the basement membrane and the ECM.
Urokinase binds with high affinity to its heavily glycosylated receptor, u-PAR (33, 35), which is composed of three similar protein domains and is linked through a glycosylphosphatidylinositol anchor to the plasma membrane. The amino-terminal domain I of u-PAR interacts with urokinase while the other two domains bind vitronectin (43), a major component of the ECM and an integrin-binding ligand. Binding of urokinase to u-PAR increases the rate of plasmin formation at the plasma membrane (13) and focuses the proteolytic activity onto the leading edge of tumor cells (5). Besides its role in proteolysis, u-PAR is a multifunctional receptor that is involved in chemotaxis, angiogenesis, signal transduction, migration, and adhesion of cells (5, 36).
Expression of u-PAR is controlled mainly at the transcriptional level (26, 29, 48), but posttranscriptional regulation (46) and recycling of u-PAR to the cell membrane (10) represent additional levels of regulation. Transcription of the u-PAR gene gives rise to a 1.4-kb mRNA or an alternatively spliced variant that lacks the carboxy-terminal membrane attachment peptide sequence (37). The importance of transcriptional regulation of the u-PAR promoter by activator protein 1 (AP-1), AP-2, and Sp-1 transcription factors and their corresponding binding sites in the 5′-flanking site of the u-PAR gene have been recently defined (2, 26, 48). However, the aforementioned transcription factor binding sites mediate constitutive and inducible activation of the u-PAR gene, and a negative regulatory element has so far not been characterized. In consideration of the importance of u-PAR for several biological processes, the existence of such a site could be hypothesized.
Recently, proteolysis and adhesion were functionally linked after it had been found that u-PAR binds to vitronectin and is associated with different members of the integrin family (7, 36, 52). Integrins are cell surface heterodimeric transmembrane glycoproteins consisting of α and β subunits that mediate cell-cell and cell-ECM interactions. Each subunit encompasses a large extracellular domain, a membrane-spanning domain, and a short cytoplasmic tail. Most integrins interact with components of the ECM (e.g., vitronectin, fibronectin, and collagen) or blood (e.g., fibrinogen). These integrin ligands cross-link or cluster integrins on the cell surface by binding to adjacent integrin molecules, leading to the formation of focal adhesion contacts, thus activating intracellular signaling pathways (“outside-in signaling”) (9). Integrins are implicated in various biological processes including angiogenesis, wound healing, tumor cell invasion, and metastasis (30, 53), but the specificity and mechanisms by which all these functions are regulated are not fully understood. However, there is increasing evidence that the cytoplasmic domain of the integrin β subunits plays a major regulatory role in these processes (24, 40, 44). u-PAR physically interacts on leukocytes with β2-integrin as shown by colocalization and immunoprecipitation studies (6) and on fibrosarcoma cells with β1- and β3-integrins (58). The direct interaction between u-PAR and integrins plays a role in the binding affinity of integrins toward their ligands and might be important for urokinase-mediated signal transduction (47). Although these studies demonstrated that u-PAR physically interacts with β-integrins, they do not address the consequence of β3-integrin expression for u-PAR regulation. Taking into consideration that u-PAR expression is regulated mainly at the transcriptional level (26) and that integrins regulate gene expression via outside-in signaling (39), we undertook a study with the following objectives: (i) to determine if β3-integrin regulates u-PAR gene activity and (ii) to elucidate the molecular mechanisms by which this occurs. We show for the first time that overexpression of β3A-integrin downregulates u-PAR transcription via a PEA3/ets binding site in the u-PAR promoter, involving the β3A-integrin cytoplasmic domain and the adapter protein β3-endonexin short.
(This study was performed in partial fulfillment of the Ph.D. thesis of S. Hapke.)
MATERIALS AND METHODS
Cell culture.
CHO cells were obtained from the American Type Culture Collection (CRL 9096). The stable CHO cell clone A5 expresses a high level of αIIbβ3-integrin (15) and was generously supplied by Mark Ginsberg, The Scripps Research Institute, La Jolla, Calif. The cells were cultivated in MEM alpha medium supplemented with l-glutamine and 10% fetal calf serum (FCS; all from GIBCO, Life Technologies, Grand Island, N.Y.).
Vectors.
The αv- and β3-integrin expression vector constructs (28), a friendly gift from J. Loftus, Mayo Clinic, Scottsdale, Ariz., were cloned into the SalI site of the pBabe Puro plasmid (31). To study the role of the three known β3-integrin cytoplasmic isoforms on the u-PAR promoter, we used single-chain chimeric receptors which bear CH2 and CH3 portions of human immunoglobulin G1 (IgG1) at the cell surface, the transmembrane domain of CD7, and the full-length cytoplasmic domains of β3-A-, β3-B-, and β3-C-integrins (see Fig. 7A). All constructs were based on previously described chimeric receptors and are in the context of the P5C7 vector, cloned into the MluI/NotI site (23). β1-integrin NITY and β3-integrin NPKY are a swap of the cytoplasmic domain of β1-integrin with the cytoplasmic domain of β3 and vice versa (see Fig. 7A). A full-length polyomavirus enhancer activator 3 (PEA3) cDNA (56) was inserted between the HindIII and BamHI cloning sites of the cytomegalovirus promoter-driven pcDNA3.1 expression vector (Invitrogen, Leek, The Netherlands).
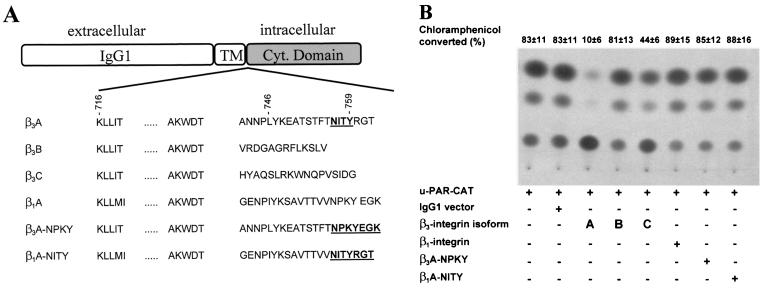
FIG. 7.
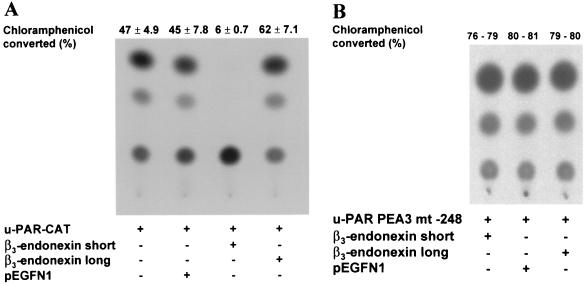
Regulation of the u-PAR promoter by the cytoplasmic domain of β3-integrin. (A) Schematic outline of the chimeric receptors used in this study. The amino acid sequences of the cytoplasmic domains of integrin isoforms β3A, β3B, β3C, and β1A and the mutated β3A- and β1A- integrins are shown. Wild-type and mutant intracellular domains of β-integrins were expressed in the context of single-chain chimeric receptors, fused to heterologous extracellular and transmembrane domains corresponding to the CH2 and CH3 domains of human IgG1 and the CD7 antigen, respectively. Mutated amino sequences are shown in boldface and underlined. (B) CHO cells were transfected with the indicated wt or mutated integrin isoforms or the appropriate control vector (IgG1). After 3 h, the medium was changed, and the cells were cultivated for a further 45 h. The cells were harvested, lysed, and assayed for CAT activity. The conversion of [14C]chloramphenicol to acetylated derivatives was determined by using a PhosphorImager. The data shown represent the average values and the standard deviations for three independent experiments.
The u-PAR chloramphenicol acetyltransferase (CAT) reporter (25) consists of 449 bp of sequence, stretching from −398 to + 51 bp, cloned into the XbaI site of the pCAT-basic vector (Promega, Mannheim, Germany); it was a kind gift of D. Boyd, M. D. Anderson Cancer Center, Houston, Tex. Site-directed mutagenesis of the u-PAR promoter (26) was performed, using the Transformer kit from Clontech (Heidelberg, Germany). The u-PAR firefly luciferase reporter (−1,469, −398, and −197 bp) was generated by cloning the human u-PAR promoter into the SmaI site of pGL3 (Promega). Four different deletion constructs (see Fig. 4D) were made within the full-length −1,469-bp u-PAR luciferase promoter between −402 and −203 bp using the QuickChange site-directed mutagenesis kit from Stratagene (Amsterdam, The Netherlands). u-PAR del 1 (−402/−350) was made using the primer 5′-TTTCAGGATGCATCT|AAATCCTGTTAGCCA-3′, u-PAR del 2 (−349/−300) was made using the primer 5′-TGACAAAACTAACAA|TTTATCCTCATTTTA-3′, u-PAR del 3 (−299/−260) was made using the primer 5′-TTTACCGTCAAAGTT|GTCCCACTTTAGGAA-3′, and u-PAR del 4 (−237/−203) was made using the primer 5′-TTTAGGAAGAGAGAG|GCTGTGATCACAACT-3′.
FIG. 4.
β3-integrin downregulates u-PAR promoter activity. (A) CHO cells, at 70% confluency, were transiently transfected with 1 μg of a CAT reporter driven by the u-PAR promoter (u-PAR-CAT), a firefly luciferase vector, and the indicated amounts of αv and β3-integrin expression vectors. After 3 h, the medium was changed and the cells were cultured for a further 45 h. The cells were lysed, harvested, and assayed for luciferase activity. Cell extracts corrected for differences in transfection efficiency were incubated with [14C]chloramphenicol, and after extraction with ethyl acetate, they were subjected to thin-layer chromatography. The conversion of [14C]chloramphenicol to acetylated derivatives was determined, using a PhosphorImager. The data shown represent the average values and standard deviations for five independent experiments. (B) CHO cells were transiently transfected with 1 μg of bp −398 u-PAR promoter luciferase construct and the indicated amounts of β3-integrin. Luciferase activity was analyzed 48 h after transfection and standardized for Renilla luciferase activity. The data shown represent the average values and the standard deviations for five independent experiments.
Both isoforms of β3-endonexin were cloned from a natural killer cell cDNA library through amplification by PCR, using the primer 5′-GGGGCGACGCGTATGATGCCTGTTAAAAGATCACTGAAGTTGGATGGTCTG-3′ (fw)/5′- GGGGCGGCGGCCGCTTCACAGAGGTTGTGACATCTGAGGCTGACC TTTGTG-3′ (rev) (β3-endonexin long) or 5′-GGGGCGACGCGTATGATGCCTGTTAAAAGATCACTGAAGTTGGATGGTCTG-3′ (fw)/5′-GGGGCGG CGGCCGCTTCACTGTATACTACTTAAATTTTGCATTATCTCCAT-3′ (rev) (β3-endonexin short). The resulting PCR fragments were fused to the respective 3′ termini of an enhanced green fluorescent protein cloning cassette (Clontech).
All constructs were identified by restriction digestion and verified by DNA sequencing, and at least two different DNA preparations were used for transient transfections.
Confocal laser scanning microscopy.
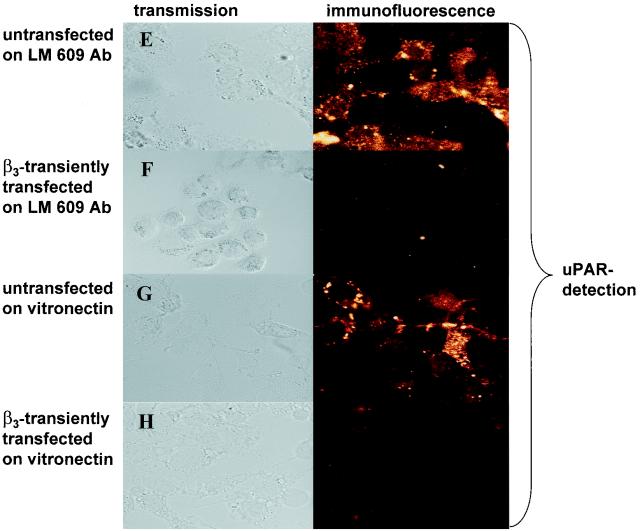
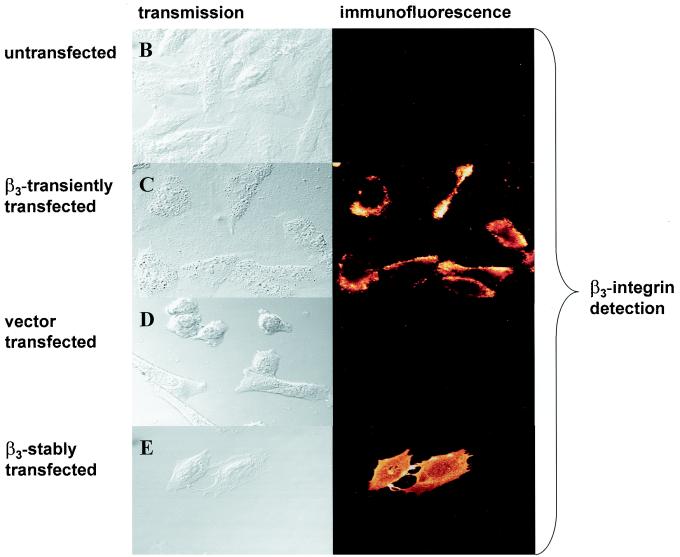
For immunofluorescence, 50,000 untransfected CHO cells or stable β3-expressing A5 cells were seeded into each well of a four-well chamber slide (Nunc Lab-Tek, Naperville, Ill.). CHO cells were transfected with β3-integrin or the vector (pBabe Puro) control (31) with TransFast transfection reagent (Promega). At 48 h posttransfection, viable cells were collected, washed briefly in phosphate-buffered saline (PBS), and incubated with a mouse polyclonal antibody against u-PAR (0.75 μg/ml; HD 13.1; a kind gift of M. Kramer, University of Heidelberg, Heidelberg, Germany) or monoclonal antibody against β3-integrin (0.1 μg/ml; CD61-UNLB; Southern Biotechnology Associates, Birmingham, United Kingdom) in PBS at 4°C for 2 h. After three washes in PBS–2% bovine serum albumin (5 min each), the cells were incubated with 50 ng of Alexa 488-conjugated goat anti-mouse IgG (Molecular Probes, Eugene, Oreg.) secondary antibody/ml for 1 h at room temperature in PBS. Cells were washed three times with PBS (10 min each), and coverslips were examined with a Zeiss Axiovert 35 microscope (Zeiss, Heidelberg, Germany) attached to a laser scanning detection unit (Leica, Bensheim, Germany). For β3-integrin and u-PAR double staining (see Fig. 2), a β3-integrin antibody that was coupled with Alexa 488 was used and u-PAR was detected with HD 13.1 followed by the secondary antibody Alexa 568 (goat anti-mouse IgG; Molecular Probes). The monoclonal antibody LM609 against αvβv-integrin was supplied by Chemicon (Hofheim, Germany).
FIG. 2.
Transfection of β3-integrin reduces u-PAR protein levels. Immunofluorescence assays were performed. Untransfected CHO cells (A), CHO cells transiently transfected with β3-integrin (B) or the β3-integrin vector (C), or a stable β3-integrin-expressing CHO cell clone, A5 (D), was plated on chamber slides. For panels E and F, first chamber slides were coated with antibody LM609 and then untransfected CHO cells (E) or CHO cells transiently transfected with β3-integrin (F) were plated. For panels G and H, untransfected CHO cells (G) or CHO cells transiently transfected with β3-integrin (H) were plated on chamber slides coated with vitronectin. For panels A to D, cells were double stained: integrin was detected with a monoclonal β3-integrin antibody coupled with Alexa 488, and u-PAR was detected with a polyclonal antibody followed by an Alexa 568-conjugated secondary antibody. For panels E to H, cells were stained by incubating them with an antibody against u-PAR, followed by an Alexa 488-conjugated secondary antibody. The experiments were repeated three to five times.
Northern blotting.
The levels of steady-state u-PAR transcripts were determined by Northern blot analysis (42). Total RNA was extracted from cells with TRIzol (GIBCO), and 15 μg of RNA was electrophoresed in a 1.5% agarose formaldehyde gel and transferred to a positively charged nylon membrane by capillary action, using 10× SSC (1× SSC is 0.15 M NaCl plus 15 mM sodium citrate, pH 7.4). The Northern blot was probed at 65°C with a randomly primed, 32P-labeled cDNA specific for u-PAR mRNA (14) and subsequently washed at 62°C, using 2× SSC in the presence of 1.0% sodium dodecyl sulfate. Loading efficiencies were checked by reprobing the blot with a radioactive cDNA which hybridizes with the 18S rRNA.
Western blot analysis.
Cells were lysed in a buffer containing 50 mM HEPES (pH 7.5), 150 mM NaCl, 1 mM EDTA, 10% glycerol, 1% Triton X-100, 10 mM sodium fluoride, 1 mM sodium orthovanadate, 10 μg of aprotinin/ml, and 1 mM phenylmethylsulfonyl fluoride; cleared by centrifugation; and electrophoresed by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis under reducing conditions (38). The resolved proteins were transferred to a nitrocellulose membrane (BA-S85; Schleicher & Schuell, Dassel, Germany); the filter was subjected for 1 h to a buffer containing 150 mM NaCl, 5 mM EDTA, 50 mM Tris HCl, 0.25% (wt/vol) gelatin, and 0.5% (vol/vol) Triton X-100; and the filter was incubated sequentially with a rabbit polyclonal antibody directed against u-PAR (40 ng/ml; American Diagnostica no. 3931; Greenwich, Conn.), followed by a mouse horseradish peroxidase-conjugated anti-rabbit IgG. Reactive proteins were visualized by ECL as directed by the manufacturer (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom).
Transfections.
Reporter plasmids were transfected with SuperFect transfection reagent (Qiagen, Hilden, Germany) with 3 × 105 CHO cells seeded overnight into six-well plates. The transfection efficiency for CHO cells was about 75% as determined by β-galactosidase staining. For the CAT assays, all transient transfections were performed in the presence of 1 μg of u-PAR–CAT reporter constructs, 1 μg of a luciferase expression vector, and, where indicated, 3 μg of an expression plasmid coding for β3-integrin or equimolar amounts of the control vector. After 3 h, the cells were rinsed twice with PBS, changed to 10% FCS-containing medium, and cultured for another 45 h. The cells were harvested and then lysed by repeated freeze-thaw cycles in 0.25 M Tris-HCl, pH 7.8. Transfection efficiencies were determined by the luciferase activity assay. After normalization for transfection efficiency, CAT activity was measured by incubation of cell lysates at 37°C with 4 μM [14C]chloramphenicol and 1 mg of acetyl coenzyme A/ml. The mixture was separated by extraction with ethyl acetate, and acetylated products were separated on thin-layer chromatography plates using chloroform-methanol as the mobile phase. The radioactive dots were visualized by autoradiography, and radioactivity was quantified using a Molecular Dynamics 445 SI PhosphorImager (26, 38).
For luciferase assays, transient transfections were performed in the presence of 1 μg of the u-PAR luciferase reporter construct and 10 ng of a Renilla luciferase pRL-SV40 plasmid (Promega) as an internal control together with 3 μg of an expression plasmid coding for β3-integrin or the control vector. After transfection, cells were cultured for 3 h in serum-free medium, followed by cultivation in 10% FCS for a further 45 h. Firefly luciferase activities were standardized for Renilla luciferase activity, which was used as an internal control.
Mobility shift assays.
Nuclear extracts were prepared from CHO cells at 80% confluency as described elsewhere (49). Nuclear extracts (7.5 μg) were incubated in a buffer containing 25 mM HEPES (pH 7.5), 0.5 mM EDTA, 0.2 mM dithiothreitol, 100 mM NaCl, 4% glycerol, and 2 μg of poly(dI/dC). Five femtomoles of a Klenow end-labeled ([α-32P]ATP) oligonucleotide (u-PAR PEA3, 5′-TTGGGTCCCACGTTAGGAAGAGAGAGAACTGGG-3′) was added to each reaction mixture in the absence or presence of a 100-fold excess of the wild-type (wt) or mutated (mt) competitor sequence (underlined), and binding was allowed at room temperature for 25 min. Subsequently, 1 μg of PEA3 antibody (sc-113X; Santa Cruz Inc., Santa Cruz, Calif.) was added, and incubation was continued at 4°C for 2 h. The reaction mixture was electrophoresed in a 5% polyacrylamide gel, using 0.5× TBE (89 mM Tris, 89 mM boric acid, 1 mM EDTA) running buffer. The gel was dried and exposed to X-ray film overnight at −80°C (26).
RESULTS
β3-integrin downregulates u-PAR.
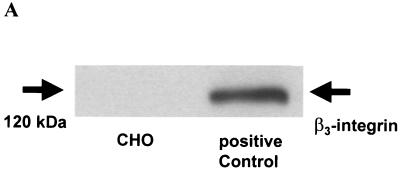
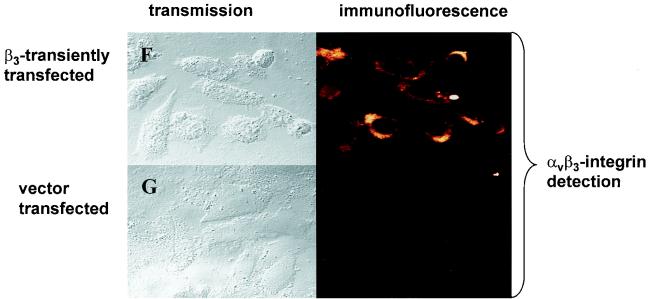
To define the role of β3- integrin for u-PAR regulation, we utilized a CHO cell line that does not express the endogenous β3-integrin subunit (40). As shown in Fig. 1A by Western blot analysis and in Fig. 1B and D by immunofluorescent staining with an antibody directed to the β3-integrin subunit, CHO cells express no detectable β3- integrin. However, upon transient (Fig. 1C) and stable (Fig. 1E) transfection of a β3-integrin expression construct into CHO cells, the β3-integrin subunit can be readily detected by immunofluorescence. As parent CHO cells have been reported to express endogenous αv-integrin (40), we were interested to know if this endogenous αv-integrin subunit is recruited by exogenous β3-integrin into a correctly assembled αvβ3-integrin heterodimer complex. Indeed, immunofluorescence studies with the monoclonal antibody LM609, which recognizes the complexed integrin subunits (αvβ3), showed that expression of β3-integrin leads to the surface expression of an αvβ3-integrin heterodimer (Fig. 1F) in CHO cells. In contrast, no αvβ3-integrin could be detected in the vector-transfected CHO cells (Fig. 1G).
FIG. 1.
Detection of β3-integrin in CHO cells. (A) Western blot. Cell lysates were resolved on a 10% polyacrylamide gel, and β3-integrin was detected by Western blot analysis using an antibody to β3- integrin. The band at ∼120 kDa represents β3-integrin. The β3-integrin-expressing ovarian cancer cell line OVMZ-6 served as a positive control. The experiment was repeated twice. (B to G) Immunofluorescence. Untransfected CHO cells (B), CHO cells transiently transfected with β3-integrin (C and F) or the β3-integrin vector (D and G), or a stable β3-integrin-expressing CHO cell clone, A5 (E), was plated on chamber slides and stained by indirect immunofluorescence. CHO cells were incubated with either a monoclonal antibody against β3- integrin (B to E) or an antibody against αvβ3-integrin (F and G) followed by an Alexa 488-conjugated goat anti-mouse secondary antibody. The experiment was repeated twice.
Untransfected CHO cells display a high level of u-PAR as shown by immunofluorescence (Fig. 2A). Upon transient overexpression of β3-integrin in CHO cells, u-PAR expression is downregulated. As exemplified in Fig. 2B, the cell overexpressing β3-integrin is not expressing u-PAR while the adjacent non-β3-transfected cell (the transient-transfection efficiency of CHO cells is about 75%) is still expressing u-PAR. The reduction of u-PAR could not be accounted for by a vector effect, since the same amount of the pBabe Puro expression vector did not reduce u-PAR protein expression (Fig. 2C). In summarizing five transient transfections, we found that 89% of untransfected CHO cells showed u-PAR expression, in contrast to 15% when β3-integrin was transiently overexpressed. Eighty-five percent of β3-integrin-expressing CHO cells showed no u-PAR expression. This indicates that in transient transfections u-PAR is downregulated in most β3-integrin-expressing cells. In contrast, in the stable β3-integrin-expressing A5 CHO cell clone all cells showed β3-integrin expression and a complete absence of u-PAR protein (Fig. 2D).
αvβ3-integrin occupancy by ligand is important for several αvβ3-integrin functions, including adhesion, migration, and outside-in signal transduction (61). In order to establish if this also is important for β3-integrin-mediated u-PAR regulation, we studied the effect of antibody-mediated (LM609) clustering of αvβ3-integrin and integrin ligation (immobilized vitronectin) on u-PAR expression. Plating of β3-integrin-transfected CHO cells on vitronectin or on LM609 completely abolished u-PAR expression in the cells (Fig. 2E to H). This repression was reproducibly stronger than that in cells which had been transfected with β3-integrin but then plated on plastic alone. These data indicate that downregulation of u-PAR expression requires the intact assembled αvβ3-complex and can be increased by integrin clustering or ligand occupancy.
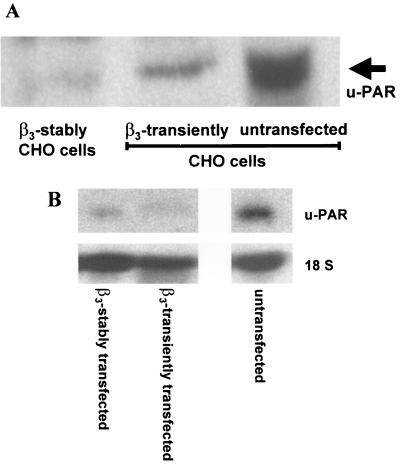
The immunofluorescence data were corroborated by Western blotting with a u-PAR antibody (Fig. 3A) revealing high u-PAR levels in the parental cell and significantly lower u-PAR antigen concentrations in the transiently and the stably β3-integrin-expressing CHO A5 cell clone. The transient β3-integrin-transfected CHO cells still showed residual u-PAR protein expression, probably because their transfection efficiency was only about 75%. Therefore, their u-PAR protein expression was probably due to u-PAR expression of the untransfected CHO cells.
FIG. 3.
Downregulation of u-PAR by β3-integrin on the protein and mRNA levels (A) Western blot. Cell lysates from untransfected and transiently and stably β3-integrin-transfected CHO cells were electrophoresed in a 10% acrylamide gel, and u-PAR was detected by Western blot analysis with a polyclonal antibody to u-PAR. (B) Northern blot analysis of u-PAR mRNA. mRNA was extracted from untransfected and transiently and stably β3-integrin-transfected CHO cells, electrophoresed in a 1.5% formaldehyde-agarose gel, and subsequently transferred to a nylon filter. The filter was probed with cDNAs specific for u-PAR and 18S rRNA transcripts.
For determining if u-PAR protein data are mirrored by a decrease in steady-state u-PAR mRNA, Northern blotting was performed after RNA extraction and purification (Fig. 3B). Untransfected CHO cells contained significantly more u-PAR mRNA than did cells transiently overexpressing β3-integrin or the A5 cell clone, which stably expresses β3-integrin. Altogether, the data shown in Fig. 2 and 3 demonstrate that overexpression of β3-integrin in CHO cells downregulates u-PAR protein and mRNA expression.
Analysis of u-PAR gene transcription by β3-integrin.
The β3-integrin subfamily includes αIIbβ3- and αvβ3-integrin. While the expression of αIIbβ3-integrin is largely confined to cells of the megakaryocytic lineage and is required for platelet aggregation (12, 60), αvβ3-integrin is expressed more widely, including in fibroblasts, smooth muscle cells, and endothelial cells. To study the effect of αv- and β3-integrin on u-PAR promoter activity, we transiently cotransfected CHO cells with a CAT reporter driven by 398 bp of 5′-flanking sequence of the u-PAR promoter. Transient expression of both αv- and β3-integrin subunits together with the u-PAR promoter resulted in a reduction of u-PAR promoter activity (Fig. 4A). This reduction was brought about by the β3-integrin expression construct because transfection of αv-integrin alone did not show any effect on the u-PAR promoter. Lysates of CHO cells transfected with the u-PAR promoter–CAT reporter and the β3-integrin expression vector resulted in 18% [14C]chloramphenicol conversion, compared to 87% in the absence of β3-integrin. By contrast, expression of the same amount of αv-integrin showed no effect on the high constitutive activity of the u-PAR promoter. This indicates that the u-PAR promoter contains a transcription factor binding site that might account for the β3-integrin-mediated repression of u-PAR gene transcription in CHO cells. Dose-response studies demonstrated that β3-integrin expression resulted in a potent inhibition of u-PAR promoter activity (Fig. 4B). A significant reduction of promoter activity was observed upon transfection with 0.5 to 5 μg of β3-integrin expression vector, whereas an equimolar amount of the empty expression construct (pBabe Puro) had a minimal effect on reporter activity. Cotransfection of 2 μg of β3-integrin caused an 80% reduction of u-PAR promoter activity, which, however, could not be further decreased, not even with 5 μg of β3-expression plasmid. To exclude the possibility that the effect of β3-integrin is due to a general inhibition of transcriptional activity, we performed a control experiment with the β-actin promoter. Compared with the effect of β3-integrin transfection on a luciferase promoter regulated either by u-PAR or by β-actin, the activity of β-actin was not affected by the expression of β3-integrin while the u-PAR promoter activity was inhibited (data not shown).
Inhibition of the u-PAR promoter activity by β3-integrin requires a binding site for PEA3/ets.
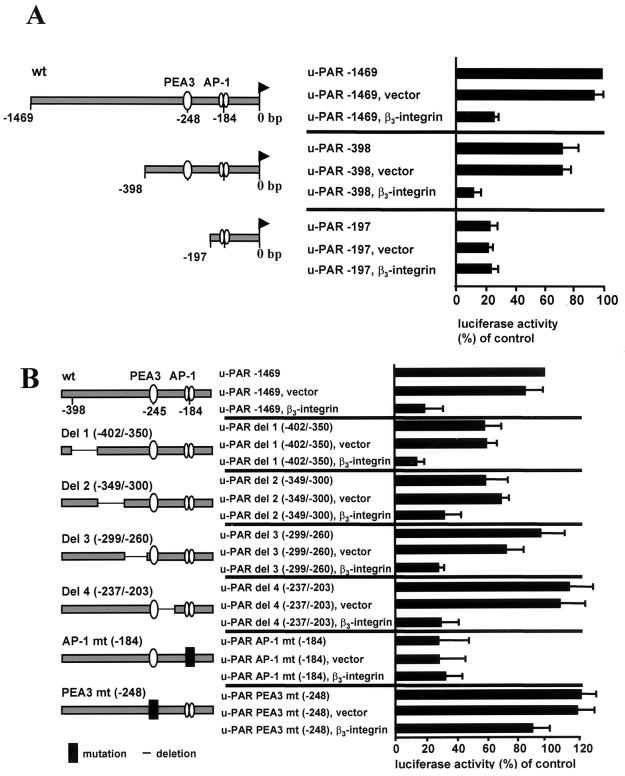
For localization of the region of the u-PAR promoter responsible for β3-integrin-mediated inhibition of u-PAR promoter activity, CHO cells were cotransfected with a luciferase reporter driven by 5′-deletion fragments of the u-PAR promoter and the β3-integrin expression vector (Fig. 5A). A dramatic inhibition of the u-PAR promoter was evident with the −1,469-bp and −398-bp u-PAR promoter construct when cotransfected with β3-integrin while the −197-bp luciferase reporter was not affected by the β3-integrin. The u-PAR −197 promoter had a low constitutive activity that could not be further downregulated by β3- integrin. These data suggest that a sequence residing between −398 and −197 bp is critical for the reducing effect of β3- integrin on u-PAR promoter activity.
FIG. 5.
The inhibition of the u-PAR promoter by β3-integrin requires a PEA3/ets binding site in the 5′-flanking sequence of the u-PAR gene. (A) CHO cells were transiently transfected with 1 μg of u-PAR promoter luciferase constructs of different lengths and were indicated with 3 μg of β3-integrin or the control vector. Luciferase activity was analyzed 48 h after transfection and standardized for Renilla luciferase activity. The data shown represent the average values and standard deviations for five independent experiments. (B) CHO cells were transiently cotransfected with 1 μg of a luciferase reporter driven by the −1469 u-PAR promoter or the indicated deletions and mutations (in the context of −1469 u-PAR) with or without 3 μg of β3-integrin or the empty expression vector alone. The data shown represent the average values and the standard deviations for four independent experiments.
Since the u-PAR promoter region from −398 to −197 bp was associated with a reduction of u-PAR by β3-integrin, we reasoned that transcription factor binding sites in this part of the promoter were required for silencing of the u-PAR transcription by β3-integrin. A computer search of this part of the sequence (48) indicated the presence of a previously uncharacterized PEA3/ets binding site (−248 bp) identical with the canonical binding sequence (AGGAAG). Since this motif has been shown elsewhere to be responsible for the basal and inducible regulation of many genes, including those for several matrix metalloproteinases (MMPs) as well as urokinase (34, 54), we investigated the possible role of this motif in the regulation of the u-PAR promoter by β3-integrin. Mutation of the PEA3/ets binding site at −248 bp induced the constitutive activity of the u-PAR promoter by 20% compared to the wt promoter, which indicates that this sites acts as a (weak) silencing element in the u-PAR promoter. Cotransfection of the −1469 u-PAR PEA3/ets mutant with β3-integrin did not further lower u-PAR promoter activity, which implies that the PEA3/ets transcription factor binding site is important for downregulation of the u-PAR promoter by β3-integrin (Fig. 5B). To investigate if other transcription factor binding sites in the 200-bp region of the u-PAR promoter between −398 bp and −197 bp are involved in repression of u-PAR by β3-integrin, we performed further deletion analyses. Deletion of four different regions around the PEA3/ets site of the u-PAR promoter in the −1469 u-PAR promoter did not affect the reduction of the u-PAR promoter by β3-integrin (Fig. 5B). However, both deletion 1 (u-PAR del 1 −402/−350) and deletion 2 (u-PAR del 2 −349/−300) showed only 60% of the constitutive activity of the wt −1469 u-PAR luciferase promoter, which implies positive regulatory elements that are important for the basal promoter activity between −402 and −300 bp of the u-PAR promoter.
Because the constitutive activity of the u-PAR promoter is driven at least in part through an AP-1 binding site (26) and transcription factors of the AP-1 family have been shown to cooperate with Ets family members, we were interested in the role of the AP-1 site in the regulation of the u-PAR promoter. Mutation of the AP-1 binding site at −184 bp in the −1,469-bp u-PAR luciferase construct reduced the high constitutive promoter activity in CHO cells by 75% (Fig. 5B). Cotransfection of the β3-integrin expression plasmid with the mutated u-PAR promoter had no further impact on the already low u-PAR promoter activity, implying that the AP-1 site is not involved in the regulation of the u-PAR promoter by β3-integrin.
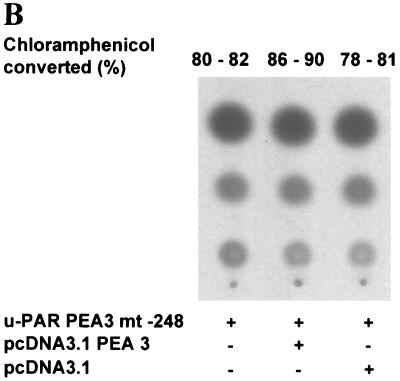
The observation that a deletion of the PEA3-ets site at −248 bp impaired the reduction of the u-PAR promoter activity by β3-integrin suggests that this transcription factor binding site mediates, at least in part, the reduction by the β3-integrin. To further investigate this possibility, we determined whether expression of the PEA3 transcription factor itself could downregulate the u-PAR promoter (Fig. 6). CHO cells were cotransfected with the u-PAR promoter-driven CAT reporter (u-PAR–CAT) and an expression vector that encoded the PEA3 transcription factor (pcDNA3 PEA3). Expression of 3 μg of PEA3 reduced the constitutive activity of the u-PAR promoter from an average conversion of [14C]chloramphenicol of 89% to 15%. By contrast, expression of the vector lacking the coding sequence for PEA3 (pcDNA3) had no effect on the u-PAR promoter activity. Deletion of the PEA3/ets site at −248 bp (u-PAR PEA3 mt −248) in the u-PAR promoter prevented a reduction of u-PAR promoter activity by the Ets family member (Fig. 6B). These data indicate that PEA3 is a transrepressor of the u-PAR promoter.
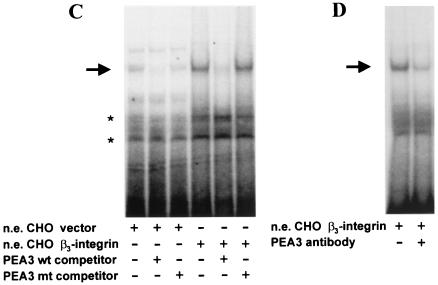
FIG. 6.
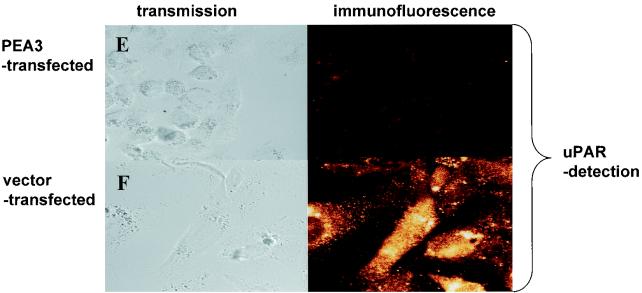
Downregulation of the u-PAR promoter by β3-integrin is mediated by the transcription factor PEA3. (A and B) CHO cells were transiently transfected with the −398 u-PAR promoter (u-PAR CAT) (A) or the same promoter with a deletion of the PEA3/ets site at −248 (u-PAR PEA3 mt −248), a luciferase vector for normalization, and expression vectors encoding PEA3 (3 μg) or β3-integrin (3 μg) in equimolar concentrations (B). For panels A and B, the medium was changed 3 h after transfection, and the cells were cultured for a further 45 h. The cells were harvested and assayed for luciferase activity. Cell extracts corrected for differences in transfection efficiency were incubated with [14C]chloramphenicol and, after extraction with ethyl acetate, subjected to thin-layer chromatography. The conversion of [14C]chloramphenicol to acetylated derivatives was determined by using a PhosphorImager. The data shown represent the average values for two to four independent experiments. (C) Induction of PEA3 DNA-binding activity by β3-integrin. Gel shift assays were performed. Nuclear extracts (n.e.) from CHO cells transfected with β3-integrin or the empty expression vector were incubated with 2 × 104 cpm of a Klenow α-32P-end-labeled oligonucleotide spanning the PEA3 site of the u-PAR promoter at −248 bp in the absence or presence of a 100-fold excess of the indicated competitors and electrophoresed in a 5% polyacrylamide gel. The PEA3 mt competitor oligonucleotide has point mutations in the PEA3 site. The arrow indicates a retarded complex of CHO β3-integrin-transfected cells; the asterisks indicate unspecific binding. (D) Nuclear extracts (n.e.) were prepared from the CHO β3-integrin-transfected cells and incubated with a Klenow α-32P-end-labeled oligonucleotide spanning the PEA3 site of the urokinase receptor promoter. After 15 min, antibody to PEA3 was added, and the reaction mixture was subsequently subjected to gel electrophoresis. The data are representative of three experiments. (E and F) Immunofluorescence. CHO cells transiently transfected with PEA3 (E) or untransfected (F) were plated on chamber slides. For panels E and F, CHO cells were incubated with a polyclonal antibody against u-PAR, followed by an Alexa 488-conjugated goat anti-mouse secondary antibody. The experiment was repeated three times. (G) Transfection. CHO cells were transiently transfected with 1 μg of the −398 u-PAR promoter (u-PAR-CAT), a firefly luciferase vector, 1 μg of β3-integrin, and the indicated amounts of PEA3 expression vector. The conversion of [14C]chloramphenicol to acetylated derivatives was determined by using a PhosphorImager. The data shown represent the values for two independent experiments.
In consideration of the presence of the PEA3/ets site at −248 bp, which is required for the reduction of u-PAR by β3-integrin, we carried out electrophoretic mobility shift assays. Nuclear extracts from parental and β3-integrin-transfected CHO cells were prepared and incubated with an end-labeled 31-bp oligonucleotide spanning the PEA3 site at −248 bp (see Materials and Methods for sequence). A retarded complex (Fig. 6C, arrow) was apparent with nuclear extract from parental and β3-integrin-transfected cells. However, the intensity of the retarded band was higher in β3-integrin-transfected cells than it was in the parental cell line. The binding of the factor(s) to the oligonucleotide was specific, since a 100-fold excess of wt oligonucleotide, but not the oligonucleotide which had been mutated in the PEA3/ets motif, competed for the binding. A PEA3-specific antibody substantially counteracted the binding of transcription factors to the PEA3 binding site, which indicates that PEA3 is a major component of the DNA-binding complex (Fig. 6D). These data suggest that the downregulation of the u-PAR promoter by β3-integrin is mediated, at least in part, through a previously unrecognized PEA3/ets site located at −248 bp upstream of the transcriptional start site of the u-PAR gene. The data in Fig. 5A to D characterize only the transcriptional regulation of the u-PAR promoter by PEA3. To strengthen the notion that PEA3 can also downregulate u-PAR protein expression, we transfected PEA3 in the CHO parental cell line (Fig. 6E and F). Indeed, overexpression of PEA3 alone without β3-integrin almost completely abolished u-PAR protein expression, as is shown by immunofluorescence, which implies that the transcriptional downregulation of the u-PAR promoter is paralleled by a reduction in u-PAR protein.
To investigate if β3-integrin and PEA3 cooperate to regulate u-PAR, β3-integrin and PEA3 were cotransfected. For this experiment, a β3-integrin expression vector was expressed in a concentration (1 μg) that leads to only a partial reduction of u-PAR promoter activity and, where indicated, PEA3 was cotransfected (Fig. 6G). In this case, a 1-μg concentration of PEA3 expression vector is sufficient to downregulate the u-PAR promoter to a level that is achieved only with 3 μg of PEA3 when no β3-integrin is cotransfected (cf. Fig. 6A and G). Thus, the ability of PEA3 to inhibit the u-PAR promoter is enhanced in β3-integrin-overexpressing cells. Cotransfection of 2 and 3 μg of PEA3 with β3-integrin abolished u-PAR promoter activity completely, but this level of downregulation could never be achieved with PEA3 alone. This suggests that transcription factors other than PEA3 are necessary to mediate the effect of β3-integrin.
Regulation of the u-PAR promoter by the cytoplasmic domain of β3-integrin.
The analysis of the regulatory role of β3-integrin cytoplasmic domains in multiple cellular functions has recently acquired a new level of complexity with the identification of variants of β3-integrin (50). There are three β3-integrin isoforms (β3A-, β3B-, and β3C-integrin) which result from alternative splicing and differ from one another by their cytoplasmic sequence (Fig. 7A) (21, 60). For a further evaluation of the mechanism of integrin-dependent u-PAR regulation, we created single-chain chimeric receptors (15a) which bear the full-length cytoplasmic domain of β3-integrin fused to an Ig tag (23) (Fig. 7A). CHO cells were transiently cotransfected with the u-PAR–CAT reporter and the different β3-integrin isoforms (Fig. 7B). The high constitutive activity of the u-PAR promoter in CHO cells could be downregulated almost completely by the β3A-integrin while the β3B-integrin and the IgG1 vector had no effect on the u-PAR promoter activity. Conversion of [14C]chloramphenicol was reduced from 83%, which mirrors the constitutive activity of the u-PAR promoter, to 10% in cells cotransfected with the β3A-integrin. The β3C-integrin isoform reduced the u-PAR promoter activity by 39%.
The cytoplasmic domain of β3-integrin interacts with certain proteins whose binding is crucial for the signaling function of β3-integrin (21, 60). One motif which has been shown to be important for the β3A-integrin function and is present only in this isoform is the NITY759 motif in the cytoplasmic region. Therefore, we asked whether a mutation of this motif affects the u-PAR promoter regulation by β3A-integrin. CHO cells were cotransfected with the u-PAR promoter and a β3A-integrin expression plasmid which has a substitution in the distal cytoplasmic membrane NITY759 motif. The NITYRGT762 domain in β3A-integrin was replaced with the NPKYEGK778 of the β1A-integrin, yielding a chimeric construct (Fig. 7A). The chimeric β3A-integrin (β3A NPKY) could not reduce u-PAR promoter activity, compared with its wt form (Fig. 7B). In the opposite experiment, we replaced seven amino acids in the cytoplasmic domain of a β1-integrin expression plasmid with the NITYRGT762 motif of β3A-integrin (Fig. 7A) and cotransfected it with the u-PAR promoter (Fig. 7B). This construct did not show any effect on u-PAR promoter activity, which indicates that the NITYRGT762 motif of β3A-integrin is necessary but not sufficient for the downregulation of u-PAR promoter activity by β3A-integrin.
β3-endonexin short downregulates u-PAR promoter activity.
Previous studies have shown that the short form of a cytoplasmic protein called β3-endonexin binds to the NITY759 motif within the cytoplasmic domain of the β3A-integrin (12, 45). This interaction is specific because β1- and β2-integrins do not interact with β3-endonexin. Two isoforms of β3-endonexin have been discovered, but only the short form interacts with the cytoplasmic domain of β3A-integrin (22). Since we have shown that the NITY759 motif in the cytoplasmic domain of β3A-integrin is necessary for regulating the u-PAR promoter, we tested whether β3-endonexin short or its longer isoform is involved in this regulation. CHO cells were cotransfected with the u-PAR promoter-driven CAT reporter along with various amounts of an expression vector that encoded β3-endonexin short or β3-endonexin long (Fig. 8A). CAT activity was inhibited almost completely by the short form of β3-endonexin while the empty expression vector had no effect. An input of the short form of β3-endonexin diminished the u-PAR promoter activity by 87%. By contrast, the β3-endonexin long form induced u-PAR promoter activity by 32%. When both β3-endonexin isoforms were cotransfected with the u-PAR promoter mutated in the PEA3/ets site (u-PAR PEA3 mt −248 bp), the expression of the short form of β3-endonexin did not reduce the u-PAR promoter activity any further (Fig. 8B). Evidently, the short isoform of β3-endonexin downregulates the u-PAR gene at least in part through the PEA3/ets site in the u-PAR promoter.
FIG. 8.
β3-Endonexin short downregulates the u-PAR promoter. CHO cells were transiently transfected, using 1 μg of a CAT reporter driven either by the wt u-PAR promoter (u-PAR CAT) (A) or by the same promoter with a deletion of the PEA3/ets site at −248 (u-PAR PEA3 mt −248), expression vectors encoding the short or long forms of β3-endonexin short, or the empty expression vector (pEGFN1) (B) . Cell extracts normalized for luciferase activity were incubated with [14C]chloramphenicol, extracted with ethyl acetate, and subjected to thin-layer chromatography. The conversion of [14C]chloramphenicol to acetylated derivatives was determined by using a PhosphorImager. The data shown represent the average values and the standard deviations for three (A) or two (B) independent experiments.
DISCUSSION
Cell migration depends on the coordinated regulation of proteolysis and adhesion at the cell surface and is tightly regulated in several physiologic and pathological conditions. Thus, it is critical to understand how these processes affect each other. In the present study, we provide evidence that overexpression of the adhesion receptor β3-integrin downregulates the protease receptor, u-PAR. Furthermore, we have demonstrated that this downregulation is mediated, at least in part, through a PEA3/ets site in the u-PAR promoter and involves a NITY759 motif in the cytoplasmic domain of the β3-integrin subunit and the adapter protein β3-endonexin.
Several of our observations suggest a pivotal role for β3- integrin in regulating u-PAR. The introduction of β3-integrin into CHO cells which have a high constitutive u-PAR expression blocks u-PAR protein expression, as is shown by Western blot analysis and immunofluorescence. Equally important, overexpression of β3-integrin also downregulates u-PAR mRNA and, in both transient and stable transfections, also downregulates u-PAR promoter activity. In contrast, αv-integrin and β1- integrin alone have no influence on u-PAR regulation. However, upon overexpression of β3-integrin, αv-integrin is recruited into an αvβ3-integrin complex, as is evidenced by our immunofluorescence results with an antibody recognizing the ECM ligand binding site of the heterodimer αvβ3. Clustering of αvβ3-integrin, which was achieved after ligation with immobilized vitronectin or the antibody LM609, even enhanced the repression of u-PAR in CHO cells. This indicates that the αvβ3-integrin complex and its ligation (outside-in signaling) and not the single β3-integrin subunit is required for u-PAR regulation. Thus, besides its involvement in adhesion, migration, and signal transduction (61), αvβ3-integrin can also inhibit u-PAR transcription.
u-PAR is a multifunctional receptor and not only focuses urokinase enzymatic activity onto the cell surface but is also associated with and modulates the function of β1-, β2-, and β3- integrins (16, 52, 58, 59). It is presently known that u-PAR and integrins coassemble within the plasma membrane and modulate the adhesive and migratory functions of cells. Wei et al. (52) showed that a complex of u-PAR, β1-integrin, and caveolin affects the adhesive properties of embryonic kidney 293 cells. u-PAR inhibited cell adhesion on fibronectin but promoted cell adhesion to vitronectin, which implies that one effect of the association between u-PAR and integrins is to affect the ligand-binding function of that integrin (7). In addition to the already-described physical interaction between u-PAR and β3-integrin at the cell surface, we now report that an increased level of β3-integrin expression results in a reduced transcription of the u-PAR promoter and thus reduced u-PAR protein expression. This helps us to understand how integrins counteract u-PAR action at the cell surface and identifies a new regulatory mechanism between β3-integrin and the u-PAR. Our findings may explain the observations by Danen et al. (11), who showed that stable overexpression of β3-integrin in the β3-negative human melanoma cell line MV3 strongly reduces invasion into Matrigel and also lung colonization but not proliferation in nude mice. Since u-PAR is involved in tumor cell invasion and metastasis, we speculate from our data that the β3-mediated reduced expression of u-PAR modulates the proteolytic potential of tumor cells required for invasion and metastasis.
The cytoplasmic domain of integrins is fundamental for gene expression, cell proliferation, and cell cycle regulation (9, 39). The membrane-distal NXXY motif within the cytoplasmic domain is highly conserved in β1-, β2-, β3-, β5-, β6-, and β7-integrins and plays a crucial role in integrin-mediated cell functions. For β3-integrin, this motif (NITY759) is important for different functions, including cell adhesion, focal contact formation, and FAK/paxillin tyrosine phosphorylation (40). Our present results show that the transfection of the cytoplasmic domain of β3-integrin is sufficient to reduce u-PAR gene transcription. This effect was evident when the β3A-integrin isoform was used, but it was not manifest with the β3B isoform. Because the NITY759 motif is found exclusively within the β3A-isoform sequence and not in other integrins, we evaluated the importance of the NITY759 motif for u-PAR gene regulation and found that deletion and substitution of the NITY759 motif through the cytoplasmic NPKY775 of β1-integrin abolish the inhibitory effect of β3A-integrin on u-PAR expression. However, the NITY759 motif itself is necessary but not sufficient to mediate the reduction of the u-PAR promoter activity, because its integration into the β1-integrin cytoplasmic domain did not inhibit the u-PAR transcription. Probably, other parts of the β3-integrin cytoplasmic domain in addition to this motif are involved in u-PAR downregulation. This is also suggested by the ability of β3C-integrin to downregulate the u-PAR promoter, albeit to a lower degree than β3A-integrin. The β3A-integrin isoform plays a role in signal transduction and inside-out signaling (1), and now our results point to an additional role as a regulator of u-PAR expression. Because expression of β3-integrin isoforms might be differentially regulated depending on the functional state of the cell, a change in the pattern of β3-integrin isoforms might have an impact on β3-integrin-dependent u-PAR regulation.
The importance of the NITY759 motif for u-PAR regulation is also emphasized by the fact that β3-endonexin, the binding of which relies on a structurally intact NITY759 motif (12) in the cytoplasmic domain of β3A-integrin, downregulates the u-PAR promoter. The NITY759 motif is the binding site of β3A-integrin for its specific cytoplasmic protein β3-endonexin, which exists in a short and a long isoform, the latter of which does not bind to the cytoplasmic domain of β3-integrin (45). We established that the short isoform of β3-endonexin downregulates u-PAR promoter activity, indicating a role for β3-endonexin short in u-PAR regulation by binding to the cytoplasmic tail of β3-integrin. The specificity of this regulation is emphasized by the inability of the long form of β3-endonexin to regulate u-PAR promoter activity. Kashiwagi et al. (22) presented evidence of the interaction of the short form of β3-endonexin with the β3 cytoplasmic tail modulating the affinity state of αIIbβ3- integrin and enhancing fibrinogen-dependent cell aggregation. β3-endonexin short increases the affinity of individual αIIbβ3- heterodimers for specific ligands, which suggests inside-out signaling with β3-endonexin affecting the affinity of αIIbβ3 for certain ligands (22). Besides these functions, β3-endonexin also participates in the integrin-mediated gene regulation that starts with activation-binding of β3-integrin, results in β3-endonexin recruitment, and eventually affects u-PAR gene regulation via a PEA3/ets binding site in the u-PAR promoter.
Cytoplasmic domains of β-integrins mediate downstream signaling events and affect gene regulation. In untransformed cells, integrins induce cell cycle progression via the Ras–mitogen-activated protein kinase (MAPK) pathway (8, 24). Our previous studies indicated that the u-PAR promoter is regulated via a MAPK-dependent signal transduction pathway (25), raising the possibility that β3-integrin uses this signal transduction pathway to regulate u-PAR (19). However, we did not find any involvement of MAPK in u-PAR gene regulation by β3-integrin, neither by testing synthetic inhibitors nor with dominant-negative expression vectors for Erk-, Jnk-, or p38-MAPK (data not shown). This implies that integrin-dependent u-PAR gene regulation does not take place via well-documented signaling pathways. Therefore, further studies will be necessary to elucidate the signal transduction pathway(s) involved in the regulation of u-PAR by β3-integrin and β3- endonexin.
The involvement of the transcription factor PEA3 in mediating the reduction of the u-PAR promoter by β3-integrin is supported by several experiments. First, the ability of β3-integrin to downregulate u-PAR promoter activity is abolished by deletion of the PEA3/ets site at −248 bp. Second, an expression vector encoding the Ets family member PEA3 was sufficient to reduce wt u-PAR promoter activity but not the promoter with a mutation in this consensus sequence. Third, overexpression of PEA3 reduced u-PAR protein expression. Fourth, nuclear extracts of β3-integrin-overexpressing cells showed enhanced binding activity for the PEA3/ets site, and the Ets family member PEA3 was identified within the DNA-protein complex. Fifth, β3-endonexin short downregulates only the wild type and not the PEA3/ets-mutated u-PAR promoter. Thus, downregulation of the u-PAR promoter by β3-integrin and the short form of β3-endonexin is mediated at least in part through a previously undescribed PEA3/ets silencer region at −248 bp in the u-PAR promoter by PEA3.
Several positive regulatory elements, including AP-1, AP-2, and Sp-1, have been identified in the u-PAR promoter (2, 26, 48), most of them contained within the first 200 bp 5′ of the transcriptional start site. We found now that additional positive regulatory elements are located between −400 and −300 bp of the u-PAR promoter, because two deletions in this region (u-PAR del 1 −402/−350 and u-PAR del 2 −349/−300) reduced the constitutive activity significantly. Within this region are putative transcription factor binding sites for the transcription factors Sp-1, GATA-2, and NF-1, and further analysis will be necessary to investigate their role in the constitutive and inducible expression of the u-PAR promoter. Deletion of this region had no effect on the regulation of u-PAR by β3-integrin. Even though the basal promoter activity was lower than the wt u-PAR promoter activity, it could be further reduced by β3- integrin.
Identification of the PEA3/ets motif represents the first report on a silencing element in the u-PAR promoter. The PEA3/ets site is an oncogene-regulated element and an important positive regulator of gene expression for several matrix-degrading proteolytic enzymes. The Ets family member PEA3, in cooperation with an AP-1 family member, induces the transcription of several MMPs and the serine protease urokinase (3, 20, 38, 51). While all these genes have juxtaposed PEA3/ets–AP-1 elements in their promoters, the PEA3/ets binding site in the u-PAR promoter has no adjacent AP-1 in its vicinity. Our data indicate that PEA3 is not only a transcriptional activator but can also act as a transcriptional repressor, a feature that has not been reported for PEA3 before. However, while this paper was in review, Xing et al. (57) showed that PEA3 can repress HER-2/neu transcription through a PEA3/ets site in the HER-2/neu promoter identical to the one in the u-PAR promoter (5′-TTAGGAAG-3′). The PEA3/ets site in the HER-2/neu promoter functions as a positive regulatory element, because its mutation abrogates constitutive HER-2/neu promoter activity. Therefore, the authors speculate that overexpression of PEA3 displaces a transactivating factor(s) that binds to the PEA3/ets site and then represses HER-2/neu transcription. In contrast, the PEA3/ets site in the u-PAR promoter acts as a weak negative regulatory element inducing constitutive promoter activity when mutated but is crucial for the inhibition of promoter activity by β3-integrin. The same PEA3/ets site is a positive regulatory element in the HER-2/neu promoter and a negative regulatory element in the u-PAR promoter. Probably, the promoter context is important to define the role of this PEA3/ets motif in a given promoter. PEA3 alone is not the only transcription factor mediating the inhibitory effect of β3-integrin at the u-PAR PEA3/ets site. While β3-integrin can abrogate u-PAR promoter activity completely, even overexpression of a large amount of PEA3 could not achieve this, which suggests that another corepressor(s) is also required for this regulation. It seems to be a feature of Ets proteins to form complexes with other unrelated transcription factors, thereby determining whether they have an activating or an inhibitory effect on gene regulation.
Until recently, only a few Ets family members had been described as transcriptional repressors, while an activating function had been outlined in detail for many different Ets proteins (18). Schneikert et al. demonstrated that association of the androgen receptor with ERM, an Ets family member within the PEA3 group, downregulates the MMP-1 promoter activity (41). Goldberg et al. (17) reported that c-Ets-1 can inhibit tetradecanoyl phorbol acetate induction of a PEA3/ets–AP-1 element as shown by in vitro transcription and transient transfections. An inhibitory role for an Ets transcription factor has also been described for Drosophila melanogaster (32). In response to the activation of the epidermal growth factor (EGF) receptor pathway, the Ets transcription factor homologue pointed is overexpressed and an inhibitory signal is generated. pointed reduces the expression of a dorsal follicle marker, rhomboid, that controls patterning in the dorsal region. Our data together with the study by Xing et al. (57) now show that PEA3 can also act as a transcriptional repressor.
In conclusion, we have shown that overexpression of β3A- integrin downregulates u-PAR at the transcriptional level through a PEA3/ets motif in the u-PAR promoter. The β3-integrin-dependent downregulation is mediated through the NITY759 motif in the β3A-integrin and through the short form of β3-endonexin. The negative regulatory role of β3A-integrin in u-PAR transcription is important in view of the poorly understood interaction and regulation between adhesion and proteolysis. Besides the known physical interaction between β3A-integrin and u-PAR, data from this study support an additional mechanism in which the activation of β3A-integrin downregulates the receptor for urokinase at the transcriptional level. This additional regulatory mechanism might be important for coordinating extracellular proteolysis, adhesion, and cell migration at the right time and place. The mutual regulation of both adhesion and proteolysis receptors at different regulation levels may help to achieve this.
ACKNOWLEDGMENTS
We express our appreciation to Douglas Boyd (M. D. Anderson Cancer Center, Department of Cancer Biology, Houston, Tex.) for his critical appraisal of the manuscript. We are very grateful to M. Kramer (Laboratory of Immunopathology, University of Heidelberg, Heidelberg, Germany) for the u-PAR antibody HD 13.1. We thank L. Miles, The Scripps Research Institute, La Jolla, Calif., for the u-PAR hamster cDNA; J. Loftus, Mayo Clinic, Scottsdale, Ariz., for αv and β3 expression constructs; and Mark Ginsberg, The Scripps Research Institute, for the αIIbβ3-integrin-expressing A5 CHO cell clone. We thank A. Kopp for critical reading of the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft (DFG Le 889-4/1 to E.L., M.G., and M.S.), Sanders Stiftung (M.G.), and the Medical Faculty of the Technische Universität München (KKF-8756155 to U.R., E.L., and M.S.).
REFERENCES
- 1.Akiyama S K, Yamada S S, Yamada K M, La Flamme S E. Transmembrane signal transduction by integrin cytoplasmic domains expressed in single-subunit chimeras. J Biol Chem. 1999;269:15961–15964. [PubMed] [Google Scholar]
- 2.Allgayer H, Wang H, Wang Y, Heiss M M, Bauer R, Nyormoi O, Boyd D. Transactivation of the urokinase-type plasminogen activator receptor gene through a novel promoter motif bound with an activator protein-2α-related factor. J Biol Chem. 1999;274:4702–4714. doi: 10.1074/jbc.274.8.4702. [DOI] [PubMed] [Google Scholar]
- 3.Benbow U, Brinckerhoff C E. The Ap-1 site and MMP gene regulation: what is all the fuss about? Matrix Biol. 1998;15:519–526. doi: 10.1016/s0945-053x(97)90026-3. [DOI] [PubMed] [Google Scholar]
- 4.Bergers G, Coussens L. Extrinsic regulators of epithelial tumor progression: metalloproteinases. Curr Opin Genet Dev. 2000;10:120–127. doi: 10.1016/s0959-437x(99)00043-x. [DOI] [PubMed] [Google Scholar]
- 5.Blasi F. Proteolysis, cell adhesion, chemotaxis, and invasiveness are regulated by the u-PA-u-PAR-PAI-1 system. Thromb Haemostasis. 1999;82:298–304. [PubMed] [Google Scholar]
- 6.Bohuslav J, Horejsi V, Hansmann C, Stockl J, Weidle U H, Majdic O, Bartke I, Knapp W, Stockinger H. Urokinase plasminogen activator receptor, β2-integrins, and src-kinases within a single receptor complex of human monocytes. J Exp Med. 1995;181:1381–1390. doi: 10.1084/jem.181.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapman H. Plasminogen activators, integrins, and the coordinated regulation of cell adhesion and migration. Curr Opin Cell Biol. 1997;9:714–724. doi: 10.1016/s0955-0674(97)80126-3. [DOI] [PubMed] [Google Scholar]
- 8.Chen Q, Kinch M S, Lin T H, Burridge K, Juliano R L. Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J Biol Chem. 1994;269:26602–26605. [PubMed] [Google Scholar]
- 9.Clark E A, Brugge J S. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 10.Conese M, Nykjaer A, Petersen C M, Andreasen P A, Gliemann J, Christensen E I, Blasi F. α-2 Macroglobulin receptor/LDL receptor-related protein (Lrp)-dependent internalization of the urokinase receptor. J Cell Biol. 1995;131:1609–1622. doi: 10.1083/jcb.131.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danen E H J, van Kraats A A, Cornelissen I M H A, Ruiter D J, van Muijen G N P. Integrin β3 cDNA transfection into a highly metastatic αvβ3-negative human melanoma cell line inhibits invasion and experimental metastasis. Biochem Biophys Res Commun. 1996;226:75–81. doi: 10.1006/bbrc.1996.1313. [DOI] [PubMed] [Google Scholar]
- 12.Eigenthaler M, Höfferer L, Shattil S J, Ginsberg M H. A conserved sequence motif in the integrin β3 cytoplasmic domain is required for its specific interaction with β3-endonexin. J Biol Chem. 1997;272:7693–7698. doi: 10.1074/jbc.272.12.7693. [DOI] [PubMed] [Google Scholar]
- 13.Ellis V, Behrendt N, Dano K. Plasminogen activation by receptor-bound urokinase. J Biol Chem. 1991;266:12752–12758. [PubMed] [Google Scholar]
- 14.Fowler B, Mackman N, Parmer R J, Miles L A. Binding of human single chain urokinase to chinese hamster ovary cells and cloning of hamster u-PAR. Thromb Haemostasis. 1998;80:148–154. [PubMed] [Google Scholar]
- 15.Gawaz M, Loftus B, Bajt M L, Frojmovic M M, Plow E F, Ginsberg M H. Ligand bridging mediates integrin αIIbβ3 (platelet GPIIB-IIIA) dependent homotypic and heterotypic cell-cell interactions. J Clin Investig. 1991;88:1128–1134. doi: 10.1172/JCI115412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a.Gawaz, M., F. Besta, T. Knorr, H. Dierks, and W. Kolanus. The NITY motif of the beta-chain cytoplasmic domain is involved in stimulated internalization of the β3-integrin A form. J. Cell Sci., in press. [DOI] [PubMed]
- 16.Ghiso J A, Kovalski K, Ossowski L. Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J Cell Biol. 1999;147:89–103. doi: 10.1083/jcb.147.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg Y, Treier M, Ghysdael J, Bohmann D. Repression of AP-1 stimulated transcription by c-Ets-1. J Biol Chem. 1994;269:16566–16573. [PubMed] [Google Scholar]
- 18.Graves B, Petersen J. Specificity within the ets family of transcription factors. Adv Cancer Res. 1998;75:1–55. doi: 10.1016/s0065-230x(08)60738-1. [DOI] [PubMed] [Google Scholar]
- 19.Gum R, Juarez C, Allgayer H, Mazar A, Wang Y, Boyd D. Stimulation of urokinase-type plasminogen activator receptor expression by PMA requires JNK1-dependent and -independent signaling modules. Oncogene. 1998;17:213–225. doi: 10.1038/sj.onc.1201917. [DOI] [PubMed] [Google Scholar]
- 20.Gum R, Lengyel E, Juarez J, Chen J H, Sato H, Seiki M, Boyd D. Stimulation of 92-kDA gelatinase B promoter activity by ras is mitogen-activated protein kinase kinase 1-independent and requires multiple transcription factor binding sites including closely spaced PEA3/ets and AP-1 sequences. J Biol Chem. 1996;271:10672–10680. doi: 10.1074/jbc.271.18.10672. [DOI] [PubMed] [Google Scholar]
- 21.Hemler M E. Integrin associated proteins. Curr Opin Cell Biol. 1998;10:578–585. doi: 10.1016/s0955-0674(98)80032-x. [DOI] [PubMed] [Google Scholar]
- 22.Kashiwagi H, Schwartz M A, Eigenthaler M, Davis K A, Ginsberg M H, Shattil S J. Affinity modulation of platelet integrin αIIbβ3 by β3-endonexin, a selective binding partner of the β3 integrin cytoplasmic tail. J Cell Biol. 1997;137:1433–1443. doi: 10.1083/jcb.137.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolanus W, Romeo C, Seed B. T cell activation by clustered tyrosine kinases. Cell. 1993;74:171–183. doi: 10.1016/0092-8674(93)90304-9. [DOI] [PubMed] [Google Scholar]
- 24.LaFlamme S E, Homan S M, Bodeau A L, Mastrangelo M. Integrin cytoplasmic domains as connectors to the cell's signal transduction apparatus. Matrix Biol. 1997;16:153–163. doi: 10.1016/s0945-053x(97)90003-2. [DOI] [PubMed] [Google Scholar]
- 25.Lengyel E, Wang H, Gum R, Simon C, Wang Y, Boyd D. Elevated urokinase-type plasminogen activator receptor expression in a colon cancer cell line is due to a constitutively activated extracellular-signal regulated kinase 1 dependent signaling cascade. Oncogene. 1997;14:2563–2574. doi: 10.1038/sj.onc.1201098. [DOI] [PubMed] [Google Scholar]
- 26.Lengyel E, Wang H, Stepp E, Juarez J, Wang Y, Doe W F, Pfarr C M, Boyd D. Requirement of an upstream AP-1 motif for the constitutive and phorbol ester-inducible expression of the urokinase-type plasminogen activator receptor gene. J Biol Chem. 1996;271:23176–23184. doi: 10.1074/jbc.271.38.23176. [DOI] [PubMed] [Google Scholar]
- 27.Liotta L, Goldfarb R, Brundage R, Siegel G, Terranova V, Garbisa S. Effect of plasminogen activator (urokinase), plasmin, and thrombin on glycoprotein and collagenous components of basement membrane. Cancer Res. 1981;41:4629–4636. [PubMed] [Google Scholar]
- 28.Loftus J C, O'Toole T E, Plow E, Glass A, Frelinger A L, Ginsberg M H. A β3 integrin mutation abolishes ligand binding and alters divalent cation-dependent conformation. Science. 1990;249:915–918. doi: 10.1126/science.2392682. [DOI] [PubMed] [Google Scholar]
- 29.Lund L R, Ellis V, Ronne E, Pyke C, Dano K. Transcriptional and post-transcriptional regulation of the receptor for urokinase-type plasminogen activator by cytokines and tumor promoters in the human lung carcinoma cell line A549. Biochem J. 1995;310:345–352. doi: 10.1042/bj3100345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marshall J F, Hart I R. The role of αv-integrins in tumour progression and metastasis. Semin Cancer Biol. 1996;7:129–138. doi: 10.1006/scbi.1996.0018. [DOI] [PubMed] [Google Scholar]
- 31.Morgenstern J P, Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morimoto A M, Jordan K C, Tietze K, Britton J S, O'Neill E M, Ruohola-Baker H. Pointed, an ETS domain transcription factor, negatively regulates the EGF receptor pathway in Drosophila oogenesis. Development. 1995;122:3745–3754. doi: 10.1242/dev.122.12.3745. [DOI] [PubMed] [Google Scholar]
- 33.Needham G K, Sherbet G V, Farndon J R, Harris A L. Binding of urokinase to specific receptor sites on human breast cancer membranes. Br J Cancer. 1986;55:13–16. doi: 10.1038/bjc.1987.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nerlov C, Rorth P, Blasi F, Johnsen M. Essential AP-1 and PEA3 binding elements in the human urokinase enhancer display cell type-specific activity. Oncogene. 1991;6:1583–1593. [PubMed] [Google Scholar]
- 35.Ploug M, Rahbeck-Nielsen H, Nielsen P, Roepstorff P, Dano K. Glycosylation profile of a recombinant urokinase-type plasminogen activator expressed in chinese hamster ovary cells. J Biol Chem. 1998;273:13933–13943. doi: 10.1074/jbc.273.22.13933. [DOI] [PubMed] [Google Scholar]
- 36.Preissner K T, Kanse S M, Chavakis T, May A E. The dual role of the urokinase receptor system in pericellular proteolysis and cell adhesion: implications for cardiovascular function. Basic Res Cardiol. 1999;94:315–319. doi: 10.1007/s003950050157. [DOI] [PubMed] [Google Scholar]
- 37.Pyke C, Eriksen J, Solberg H, Schnack B, Nielsen S, Kristensen P, Lund L R, Dano K. An alternatively spliced variant of mRNA of the human receptor for urokinase plasminogen activator. FEBS Lett. 1993;326:69–74. doi: 10.1016/0014-5793(93)81763-p. [DOI] [PubMed] [Google Scholar]
- 38.Ried S, Jäger C, Vande Woude G F, Graeff H, Schmitt M, Lengyel E. Activation mechanisms of the urokinase-type plasminogen activator promoter by hepatocyte growth factor/scatter factor (HGF/SF) J Biol Chem. 1999;274:16377–16386. doi: 10.1074/jbc.274.23.16377. [DOI] [PubMed] [Google Scholar]
- 39.Riikonen T, Westermarck J, Koivisto L, Broberg A, Kähäri V-M, Heino J. Integrin α2β1 is a positive regulator of collagenase (MMP-1) and collagen α1(I) gene expression. J Biol Chem. 1995;270:13548–13552. doi: 10.1074/jbc.270.22.13548. [DOI] [PubMed] [Google Scholar]
- 40.Schaffner-Reckinger E, Gouon V, Melchior C, Plançon S, Kieffer N. Distinct involvement of β3 integrin cytoplasmic domain tyrosine residues 747 and 759 in integrin-mediated cytoskeletal assembly and phosphotyrosine signaling. J Biol Chem. 1998;278:12623–12632. doi: 10.1074/jbc.273.20.12623. [DOI] [PubMed] [Google Scholar]
- 41.Schneikert J, Peterziel H, Defossez P A, Klocker H, Launoit Y, Cato A C. Androgen receptor-Ets protein interaction is a novel mechanism for steroid hormone-mediated down-modulation of matrix metalloproteinase expression. J Biol Chem. 1996;271:23907–23913. doi: 10.1074/jbc.271.39.23907. [DOI] [PubMed] [Google Scholar]
- 42.Schnelzer A, Prechtel D, Knaus U, Dehne K, Gerhard M, Graeff H, Harbeck N, Schmitt M, Lengyel E. Rac1 in human breast cancer: overexpression, mutation analysis, and characterization of a new isoform, Rac1b. Oncogene. 2000;19:3013–3020. doi: 10.1038/sj.onc.1203621. [DOI] [PubMed] [Google Scholar]
- 43.Schvartz I, Seger D, Shaltiel S. Vitronectin. Int J Biochem Cell Biol. 1999;31:539–544. doi: 10.1016/s1357-2725(99)00005-9. [DOI] [PubMed] [Google Scholar]
- 44.Seftor E A. Role of the β3 integrin subunit in human primary melanoma progression. Am J Pathol. 1999;153:1347–1351. doi: 10.1016/s0002-9440(10)65719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shattil S J, O'Toole T, Eigenthaler M, Thon V, Williams M, Babior B M, Ginsberg M H. β3-endonexin, a novel polypeptide that interacts specifically with the cytoplasmic tail of the integrin β3 subunit. J Cell Biol. 1995;131:807–816. doi: 10.1083/jcb.131.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheety S, Kumar A, Idell S. Posttranscriptional regulation of urokinase receptor mRNA: identification of a novel urokinase receptor mRNA binding protein in human mesothelioma cells. Mol Cell Biol. 1997;17:1075–1083. doi: 10.1128/mcb.17.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simon D I, Rao N K, Xu H, Wei Y, Majdic O, Ronne E, Kobzik L, Chapman H. Mac-1 (CD11b/CD18) and the urokinase receptor (CD 87) form a functional unit on monocytic cells. Blood. 1996;88:3185–3194. [PubMed] [Google Scholar]
- 48.Soravia E, Grebe A, De Luca P, Helin K, Suh T T, Degen J L, Blasi F. A conserved TATA-less proximal promoter drives basal transcription from the urokinase-type plasminogen activator receptor gene. Blood. 1995;86:624–635. [PubMed] [Google Scholar]
- 49.Turkson J, Bowman T, Garcia R, Caldenhoven E, de Groot R P, Jove R. Stat3 activation by Src induces specific gene regulation and is required for cell transformation. Mol Cell Biol. 1998;18:2545–2552. doi: 10.1128/mcb.18.5.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Kuppevelt T H S M M, Languino L R, Gailit J, Suzuki S, Ruoslahti E. An alternative cytoplasmic domain of the integrin β3 subunit. Proc Natl Acad Sci USA. 1989;86:5415–5418. doi: 10.1073/pnas.86.14.5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wasylyk B, Hahn S, Giovane A. The Ets family of transcription factors. Eur J Biochem. 1993;211:7–18. doi: 10.1007/978-3-642-78757-7_2. [DOI] [PubMed] [Google Scholar]
- 52.Wei Y, Lukashev M, Simon D I, Bodary S C, Rosenberg S, Doyle M V, Chapman H. Regulation of integrin function by the urokinase receptor. Science. 1996;273:1551–1555. doi: 10.1126/science.273.5281.1551. [DOI] [PubMed] [Google Scholar]
- 53.Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- 54.Westermarck J, Kähäri V-M. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781–792. [PubMed] [Google Scholar]
- 55.Woodhouse E C, Chuaqui R F, Liotta L. General mechanisms of metastasis. Cancer. 1997;80:1529–1537. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1529::aid-cncr2>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- 56.Xin J, Cowie A, Lachance P, Hassell J A. Molecular cloning and characterization of PEA3, a new member of the Ets oncogene family that is differentially expressed in mouse embryonic cells. Genes Dev. 1992;6:481–496. doi: 10.1101/gad.6.3.481. [DOI] [PubMed] [Google Scholar]
- 57.Xing X, Wang S W, Xia W, et al. The Ets protein PEA3 suppresses Her2/neu overexpression and inhibits tumorigenesis. Nat Med. 2000;6:189–195. doi: 10.1038/72294. [DOI] [PubMed] [Google Scholar]
- 58.Xue W, Mizukami I, Todd R F, Petty H R. Urokinase-type plasminogen activator receptors associate with β1 and β3 integrins of fibrosarcoma cells: dependence on extracellular matrix components. Cancer Res. 1997;57:1682–1689. [PubMed] [Google Scholar]
- 59.Yebra M, Goretzki L, Pfeifer M, Mueller B. Urokinase-type plasminogen activator binding to its receptor stimulates tumor cell migration by enhancing integrin-mediated signal transduction. Exp Cell Res. 1999;250:231–240. doi: 10.1006/excr.1999.4510. [DOI] [PubMed] [Google Scholar]
- 60.Ylänne J. Conserved functions of the cytoplasmic domains of integrin beta subunits. Front Biosci. 1999;3:877–886. doi: 10.2741/a329. [DOI] [PubMed] [Google Scholar]
- 61.Zheng B, Clemmons R D. Blocking ligand occupancy of the αvβ3 integrin inhibits insulin-like growth factor I signaling in vascular smooth muscle cells. Proc Natl Acad Sci USA. 1998;95:11217–11222. doi: 10.1073/pnas.95.19.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]