Abstract
The most relevant information on the clinical uses of tedizolid from studies published in the last 18 months is presented in this brief review. The most important data indicate better tolerance and safety profile of long-term therapeutic regimes in off-label indications, such as osteoarticular infections and those caused by mycobacteria. Its lower risk of hazardous interactions compared to linezolid should be emphasized. Furthermore, tedizolid in its combination with rifampicin shows a more favourable way of acting as demonstrated in vitro and in vivo studies. A recent trial also opens the door for its potential use in nosocomial pneumonia caused by Gram-positive bacteria.
Keywords: Tedizolid phosphate, Gram-positive, osteoarticular infections, pneumonia, safety
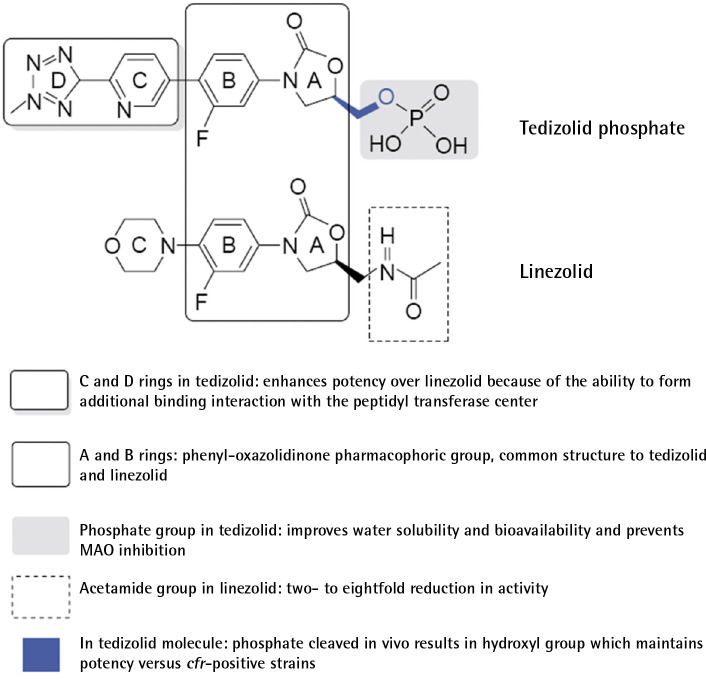
Tedizolid phosphate is an expanded-spectrum oxazolidinone with activity against Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus and cfr-mediated linezolid-resistant S. aureus. Currently, it is indicated for the treatment of acute bacterial skin and skin structure infections (ABSSSI) in adults (Figure 1). Two phase III randomized, double-blind clinical trials -ESTABLISH-1 and ESTABLISH-2-demonstrated the non-inferiority of tedizolid for 6 days (200 mg per day) versus linezolid for 10 days (600 mg every 12 h) in patients with ABSSSI. Gastrointestinal disorders (nausea, diarrhoea, and vomiting) and myelotoxicity were less frequent in the tedizolid group than in the linezolid group [1]. A recent meta-analysis of four randomized clinical trials involving 2,056 patients, comparing the efficacy of linezolid versus tedizolid for the treatment of ABSSSI, reconfirmed the previous results [2]. Its potent activity against Gram-positive cocci, high oral bioavailability, improved dosage profile (once daily), as well as the expected lower risk of drug-drug interactions with better safety profile at 6 days of treatment compared to linezolid makes tedizolid an attractive alternative for infections requiring long therapeutic regimens, such as bone and joint infections or mycobacterial infections, among others. New interesting evidence on tedizolid has appeared in the last year and a half, somewhat hidden by the overwhelming COVID19 pandemic we are still suffering, which will be summarized in this brief review.
Figure 1.
Structure–activity differences between tedizolid and linezolid
Drug reference Company; MSD
Despite there is scarce information about the tolerability of tedizolid for treatments lasting more than 6 days, new data have recently come to light. A few years ago, Kim et al. evaluated the safety and tolerability of tedizolid in 25 patients with nontuberculous mycobacterial infections who received tedizolid for a median of 91 days [3]. They suggested that long-term tedizolid therapy might have a safety profile comparable to linezolid. Tedizolid was approved in Spain in 2015, and it has been used off-label for more than 6 days in different clinical situations. A recent study carried out by a Spanish group has evaluated these indications and described the long-term safety profile of tedizolid. A multicentric retrospective study of patients who received tedizolid for more than 6 days was conducted [4]. Eighty-one patients, treated with tedizolid 200 mg once daily for a median duration of 28 (14 to 59) days, were included; 36 (44.4%) had previously received linezolid. The main reasons for choosing tedizolid were to avoid potential linezolid toxicity or interactions (53.1%) or previously documented linezolid-related adverse effects (27.2%). The most common indications were off-label, such as osteomyelitis, prosthetic joint infections (PJI), and respiratory infections (77.8%). A favourable clinical outcome was documented in 75.3% of the patients, with clinical or microbiological failure in 19.8% during the follow-up. Overall, only 9/81 patients (11.1%) experienced a probably associated adverse event: 2 patients (2.5%) developed gastrointestinal disorders, 1 (1.2%) developed anaemia, and 6 developed thrombocytopenia (7.4%) after a median duration of treatment of 26.5 (17 to 58.5) days. Four (5%) patients discontinued tedizolid due to adverse events. The rate of myelotoxicity among 23 patients with chronic renal failure (CRF) was 17.4%. Only 8.7% had to stop tedizolid and 20 out of 22 patients with previous linezolid-associated toxicity had no adverse events. Long-term tedizolid treatments showed good tolerance, with lower rates of gastrointestinal and haemato-logical toxicity than those reported with linezolid, especially in patients with CRF or a history of linezolid-associated toxicity.
A similar experience of more than 6 days tedizolid therapy and other indications beyond ABSSSI, has been reported by a research group from the United Kingdom [5]. Sixty patients received tedizolid (from May 2016 to November 2018) mainly after documenting adverse effects with linezolid. Bone and joint infections were the most frequent indications. Despite the mean length of tedizolid therapy was 27 days, haemato-logical adverse effects were infrequent. Most patients (72%) finished the antibiotic course and their clinical condition improved during treatment (72%). Adverse events were common, but often not thought to be tedizolid-related. The authors concluded that tedizolid appears to be safe in prolonged regimes. Hence, it could be suitable as long-term antibiotic therapy in the context of complex outpatient oral and parenteral antibiotic treatments. Patients who do not tolerate linezolid can be safely switched to tedizolid if appropriate.
The experience of long-term use of tedizolid in osteoarticular infections has been described by a research group from Barcelona (Catalonia, Spain) in a multicentric retrospective study [6]. Cases (n = 51) included patients with osteoarthritis (53%), prosthetic joint infection (33.3%), and diabetic foot infections (18%), 59% of which were orthopaedic device-related. The most frequent isolates were Staphylococcus spp. (65%, n = 47; S. aureus, 48%). The reasons for choosing tedizolid were potential drug-drug interactions (63%) and cytopenia (55%). The median treatment duration was 29 days. Twenty-four per cent received rifampicin concomitantly, with scarce adverse effects (3 cases). Long-term use of tedizolid was effective, showing a better safety profile with less myelotoxicity and lower drug-drug interactions than linezolid. For the authors, further confirmation of these advantages could make tedizolid the oxazolidinone of choice for most osteoarticular infections. The debate on the adequacy of combining oxazolidinones with rifampicin has progressed after a study in an in vitro model of S. aureus mature biofilm showed that the combination of tedizolid-rifampicin prevented the appearance of rifampicin resistance [7]. These effects were similar to those obtained with the well-known and widely used combination of daptomycin plus rifampicin.
Also recently, the results of a French prospective multi-centre study reassured the good tolerance of prolonged oral tedizolid therapy for PJI. This study included patients with PJI who were treated for at least 6 weeks but not more than 12 weeks [8]. Thirty-three adult patients with PJI [hip (n = 19), knee (n = 13) and shoulder (n = 1)] were included. All patients underwent surgery, with retention of the infected implants and one/two stage replacements in 11 (33.3%) and 17/5 (51.5/15.2%), respectively. Staphylococci and enterococci were the most prevalent identified bacteria. The mean duration of tedizolid therapy was 8.0 ± 3.27 weeks. Tedizolid was associated with another antibiotic in 18 patients (54.5%), including rifampicin in 16 cases (48.5). Six patients (18.2%) had to prematurely stop tedizolid therapy because of potentially tedizolid-attributable intolerance (n = 2), early failure of PJI treatment (n = 2) or severe anaemia due to bleeding (n = 2). Regarding therapeutic compliance with tedizolid, two or more omissions of drug intake were not recorded during the whole treatment duration. These results suggest good compliance and a favourable safety profile of tedizolid, providing evidence of the potential benefit of its use in PJI. A summary of the main data and most relevant results of the reviewed studies are presented in Table 1.
Table 1.
Summary of new evidence for long-term treatments with tedizolid
| Author (year, N) | Age (median, in years) | Linezolid (previous use,%) | BJI (%) | Duration of tedizolid therapy (days, interval) | Adverse events (%) | Discontinuation (%) | Cure or improvement (%) |
|---|---|---|---|---|---|---|---|
| Mensa et al., 2020; N=81 | 66 | 44% | 47% | 28 (14-59) | 11% | 5% | 80% |
| York et al., 2020; N=60 | 62 | 82% | 85% | 27 (22-32) | GI: 15% fatigue: 12% anaemia: 2% |
18% | 72% |
| Benavent et al., 2021; N=51 | 65 | 16% | 100% | 29 (15-44) | 5.8% (only GI) |
0 | 83% |
| Senneville et al., 2020; N=33 | 73 | 9% | 100% (PJI) | 56 (42-84) | 60% anaemia: 12% pruritus:12% |
12% | 82% |
BJI: bone and joint infections; GI: gastrointestinal; N: number of patients /cases; PJI: prosthetic joint infections
Lastly, important information has been provided in the field of the treatment of Gram-positive nosocomial pneumonia. A recent randomized, non-inferiority, double-blind, phase 3 clinical trial has evaluated the efficacy and safety of tedizolid for the treatment of Gram-positive hospital-acquired bacterial pneumonia (HABP) and ventilator-associated bacterial pneumonia (VABP) [9]. Patients were randomized 1:1 to receive intravenous tedizolid phosphate 200 mg once daily for 7 days or intravenous linezolid 600 mg every 12 hours for 10 days. Treatment duration was 14 days in patients with concurrent Gram-positive bacteraemia. Overall, 726 patients were randomized. Their baseline characteristics, including the incidence of methicillin-resistant Staphylococcus aureus (31.3% overall), were well balanced. Tedizolid was non-inferior to linezolid for day 28 all-cause mortality rate (28.1% and 26.4%, respectively) in the treatment of Gram-positive VABP. Non-inferiority of tedizolid was not demonstrated for investigator-assessed clinical cure at test of cure (TOC) in the intention-to-treat population. The difference in the clinical response of both groups was not determined by any single factor according to the post hoc analyses. Approximately 12% and 8% of the patients presented adverse effects with linezolid and tedizolid, respectively. Regardless of whether this trial would allow expanding the indications in the technical data sheet for tedizolid, it represents a great advance in reinforcing the potential clinical use of this safer drug in the treatment of nosocomial pneumonia involving Gram-positive microorganisms.
CONFLICTS OF INTEREST
The authors declare no conflict of interests.
References
- 1.Shorr AF, Lodise TP, Corey GR, De Anda C, Fang E, Das AF, et al. Analysis of the phase 3 ESTABLISH trials of tedizolid versus linezolid in acute bacterial skin and skin structure infections. Antimicrob Agents Chemother. 2015; 59(2):864-71. doi: 10.1128/AAC.03688-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lan SH, Lin WT, Chang SP, Lu LC, Chao CM, Lai CC, et al. Tedizolid Versus Linezolid for the Treatment of Acute Bacterial Skin and Skin Structure Infection: A Systematic Review and Meta-Analysis. Antibiotics (Basel). 2019; 8(3):137. doi: 10.3390/antibiotics8030137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim T, Wills AB, Markus A, Daniel-Wayman S, Prevots DR, Olivier KN. Safety and tolerability of long term use of tedizolid for treatment of nontuberculous mycobacterial infections. Open Forum Infect Dis. 2016; 3:577. doi: 10.1093/ofid/ofw172.440. [DOI] [Google Scholar]
- 4.Mensa Vendrell M, Tasias Pitarch M, Salavert Lletí M, Calabuig Muñoz E, Morata Ruiz L, Castells Lao G, et al. Safety and Tolerability of More than Six Days of Tedizolid Treatment. Antimicrob Agents Chemother. 2020; 64(7): e00356-20. doi: 10.1128/AAC.00356-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.York JA, Adams K, Cullen L, Delahay J, Ivan M, Lillie PJ, et al. Tedizolid: a service evaluation in a large UK teaching hospital. Eur J Clin Microbiol Infect Dis. 2021; 40(2): 397-405. doi: 10.1007/s10096-020-04015-2. [DOI] [PubMed] [Google Scholar]
- 6.Benavent E, Morata L, Escrihuela-Vidal F, Reynaga EA, Soldevila L, Albiach L, et al. Long-Term Use of Tedizolid in Osteoarticular Infections: Benefits among Oxazolidinone Drugs. Antibiotics (Basel). 2021; 10(1): 53. doi: 10.3390/antibiotics10010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gidari A, Sabbatini S, Schiaroli E, Perito S, Francisci D, Baldelli F, et al. Tedizolid-Rifampicin Combination Prevents Rifampicin-Resistance on in vitro Model of Staphylococcus aureus Mature Biofilm. Front Microbiol. 2020; 11:2085. doi: 10.3389/fmicb.2020.02085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Senneville E, Dinh A, Ferry T, Beltrand E, Blondiaux N, Robineau O. Tolerance of Prolonged Oral Tedizolid for Prosthetic Joint Infections: Results of a Multicentre Prospective Study. Antibiotics (Basel). 2020; 10(1): 4. doi: 10.3390/antibiotics10010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wunderink RG, Roquilly A, Croce M, Rodriguez Gonzalez D, Fujimi S, Butterton JR, et al. A Phase 3, Randomized, Double-Blind Study Comparing Tedizolid Phosphate and Linezolid for Treatment of Ventilated Gram-Positive Hospital-Acquired or Ventilator-Associated Bacterial Pneumonia. Clin Infect Dis. 2021:ciab032. doi: 10.1093/cid/ciab032. [DOI] [PMC free article] [PubMed] [Google Scholar]