Abstract
Objectives:
To investigate differences in the gingival crevicular fluid (GCF) composition between adolescent and adult patients undergoing orthodontic treatment with fixed appliances.
Materials and Methods:
Ten adolescents (14.4 ± 1.43) and 10 adults (28.5 ± 7.83) with Class I malocclusions and minor upper incisor crowding were allocated to two different age groups. Brackets were bonded only in the upper arch over the 20-week period of the experiment. Samples of GCF were collected from the labial sides of the upper incisors (experimental sites) and lower incisors (control sites) of each subject at five time points. Aliquots from diluted GCF were screened for the presence of receptor activator of nuclear factor kappa B ligand (RANKL), osteoprotegerin (OPG), interleukin-1 (IL-1), interleukin-1 receptor antagonist (IL-1RA), and metalloproteinase-9 (MMP-9) using a microarray technique. The values were statistically analyzed.
Results:
In adults, the ratio of IL-1 to IL-1RA decreased significantly (P = .033) in experimental sites 3 weeks after appliance placement and first archwire activation. In adolescents, the ratio of RANKL to OPG peaked 6 weeks after the insertion of the first rectangular archwire. This ratio peak found in adolescents was a consequence of a decrease in the mean concentration of OPG. No significant changes over time were observed in the concentration of MMP-9.
Conclusion:
This study demonstrates age trends in the GCF levels of IL-1, IL-1RA, RANKL, and OPG that may be used to track differences in tissue response between adults and adolescents undergoing orthodontic treatment.
Keywords: GCF, Orthodontic treatment, Age, Biomarkers
INTRODUCTION
As the number of adults seeking orthodontic treatment continues to increase, the biological differences between young and adult periodontal tissues need to be better understood. The literature supports the idea that chronological age is a relevant risk factor for root resorption, bone loss, and periodontal problems because adults tend to have a more quiescent periodontal ligament.1,2 The mechanical stress from orthodontic appliances induces cells in the periodontium to form biologically active substances responsible for connective tissue remodeling and osteoclast activation. These substances can be monitored noninvasively in humans by following changes in the composition of the gingival crevicular fluid (GCF) during tooth movement.1,3,4
Interleukins can be particularly important for consequent tooth movement in that these cytokines stimulate osteoclast formation and bone-resorbing activities of preformed osteoclasts.5,6 Interleukins act synergistically or antagonistically on each other; thus, they are classified as pro-inflammatory or anti-inflammatory, respectively.5 Pro-inflammatory interleukins are interleukin-1 (IL-1), interleukin-2, interleukin-6, and interleukin-8. Anti-inflammatory cytokines are interleukin-1 receptor antagonist (IL-1RA), interleukin-4, interleukin-10, and interleukin-13. Tooth movement also requires the binding of receptor activator of nuclear factor kappa B ligand (RANKL), a protein produced by osteoblasts, to receptor activator of nuclear factor kappa (RANK), a cell membrane protein found on osteoclast precursor cells. On the other hand, osteoprotegerin (OPG) acts as a decoy receptor that binds to RANKL and blocks osteoclastogenesis.7
Several studies7–9 have shown that the RANK–RANKL–OPG system plays an important role in osteoclast differentiation during orthodontic tooth movement, where the levels of RANKL in GCF have been observed to increase during tooth movement, while the levels of OPG decreased. Finally, it is important to emphasize that tooth movement also demands extensive tissue changes within the periodontal ligament. An in vitro study indicated that matrix metalloproteinase-9 (MMP-9) is important for the degradation of collagen matrices, facilitating the migration of osteoclast precursors to sites of bone resorption.10 Bildt el al.11 found higher MMP-9 expression in human GCF collected from pressure sites. Therefore, crevicular MMP-9 may also serve as biomarker to monitor remodeling of the periodontal tissues during tooth movement.
The specific aim of this study was to investigate differences in the GCF composition between adults and adolescents undergoing orthodontic treatment. The ultimate goal would be to give clinicians a better understanding of what biomarkers can be quantified and how age may affect the response of the periodontium to an applied force. In addition, the determination of biomarker profiles may further enhance individual risk assessment and pharmacologic modulation of tooth movement in a clinical setting.
MATERIALS AND METHODS
Twenty patients were recruited to participate in this study. To be included, subjects needed to have good general health, no evidence of periodontal disease, Class I malocclusion, permanent dentition, incisor irregularity index12 between 3 and 6 mm in the upper dental arch, and no extraction of any permanent teeth included in the treatment plan. Smokers and drug users were excluded from the study. Written informed consent was obtained from the participants, and ethical approval was granted from the Health Research Ethics Board at the University of Manitoba (reference number H2010:093).
Subjects were divided into two groups based on age. The first group consisted of 10 adolescents (three boys and seven girls) with an age range of 13 to 15 years (14.4 ± 1.43). The second group consisted of 10 adults (four men and six women) between the ages of 21 to 39 years old (28.5 ± 7.83). Two weeks before fixed appliance placement, the participants underwent tooth-cleaning sessions and assessment of their periodontal health to ensure that no disease-active periodontal sites were present. The subjects received treatment with a conventional fixed edgewise bracket system (Mini Master Series, American Orthodontics, Sheboygan, Wis) with an MBT 0.022-in slot prescription. Brackets were bonded only in the upper arch over the 20-week period of the experiment, whereas the mandibular incisors, free from any orthodontic appliance, were used as controls. The subjects were seen every 6 weeks for archwire progression/appliance activation. The archwire sequence during the alignment phase was standardized as follows: 0.014-in, 0.018-in, 0.016 × 0.022-in and 0.019 × 0.025-in nickel-titanium.
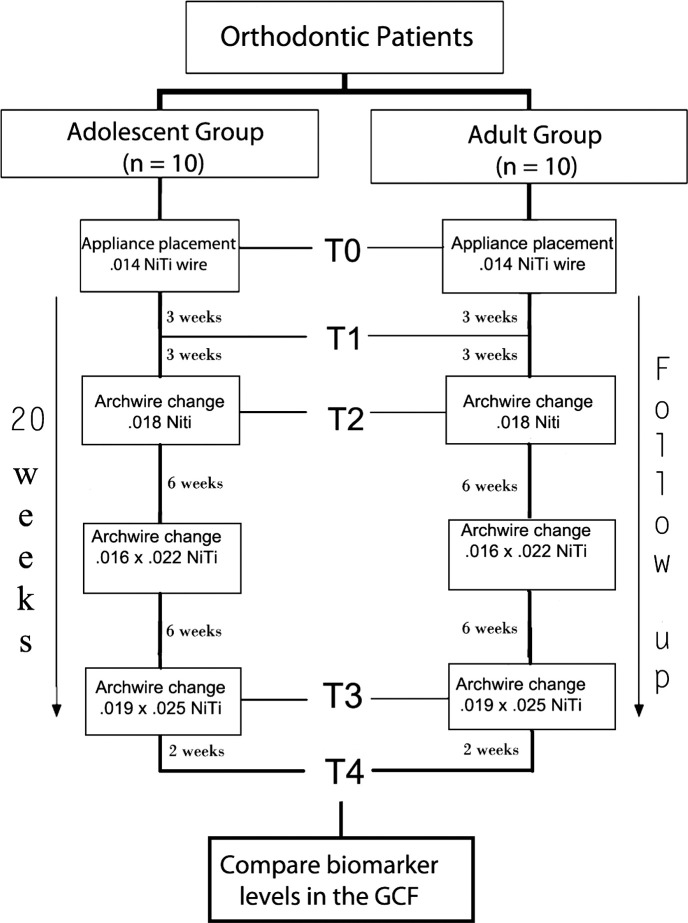
A schematic of the experiment is shown in Figure 1. Samples of GCF were collected from the labial sides of the upper and lower incisors of each subject at five time points. GCF sampling was performed according to the following schedule: T0 (baseline), immediately before bonding; T1, 3 weeks after appliance placement and insertion of the first aligning archwire (0.014-in NiTi); T2, 6 weeks after insertion of the first archwire (0.014-in NiTi); T3, 6 weeks after insertion of the third archwire (0.016 × 0.022-in NiTi); and T4, 2 weeks after the last aligning archwire (0.019 × 0.025-in NiTi) was fully engaged into the brackets slots.
Figure 1.
Study design.
GCF Sampling
Before GCF collection, each site was gently dried and isolated from saliva with a cotton roll. GCF collection was performed using paper strips (Periopaper, Oraflow Inc, Plainview, NY). One strip was carefully inserted for 60 seconds into the gingival sulcus at the distolabial side of each incisor. Paper strips of upper incisors (experimental sites) and lower incisors (control sites) were then pooled into separate sealed tubes and immediately frozen at −80°C.
Microarray Assay
The paper strips in each tube were eluted with 100 µL of phosphate-buffered saline (Invitrogen, Burlington, ON, Canada) and shaken at room temperature for 15 minutes. Subsequently, the strips were carefully removed from the Eppendorf tube and the eluate was centrifuged (5 min, 3000 g) to remove bacterial biofilms and cellular elements. Supernatant samples were screened for the presence of IL-1, IL-1RA, MMP-9, RANKL, and OPG with a customized Quantibody Array kit (RayBiotech, Norcross, Ga). The immunoassay was performed according to the manufacturer's instructions and is described in more detail elsewhere.13 The concentration of biomarker levels expressed in picograms per milliliter (pg/mL) was calculated with Q Analyzer software V8.6.4 (Norcross, Ga) against a standard curve set for each biomarker.
Statistical Analysis
Basic statistics were calculated with means, medians, and standard deviations. When analyzing the data longitudinally, all outcomes and their ratios were analyzed with mixed-effects repeated measures models, which were necessary to account for the correlation between measurements on the same subject over time. Each model imposed covariance structures that accounted for the repeated measures on a given tooth as well as the correlation between experimental and control teeth within the same subject. The specific form of the covariance structure was allowed to vary between outcomes and was motivated by the behavior of the data. Group membership, measurement occasion (time), and their interaction were the only covariates used. The interaction term is of chief interest because when significant, it indicates that groups have different trajectories over the course of the measurement period. Outcomes were log-transformed to achieve conditional normality whenever deemed necessary after inspecting histograms of residuals. The ratio of RANLK to OPG was analyzed on the original scale (not log transformed).
Biomarker concentration ratios were compared using a modified ratio given by the equations IL-1/(IL-1 + IL-1RA) and RANKL/(RANKL + OPG). This is in contrast to the simpler ratios usually seen in the literature, given by IL-1/IL-1RA and RANKL/OPG. Although these ratios are intuitive, they are plagued by the problem that they are mathematically undefined whenever the denominator is zero, which may occur as a result of a lack of biomarker expression or detection limits. Thus, the modified ratio proposed in this study may be more robust in this regard. By adding both biomarkers to the denominator, the interpretation changes from a multiplicative scale without measurement to a percent of total expression. For instance, if the RANKL/(RANKL + OPG) ratio happens to equal 0.75, we would conclude that RANKL was responsible for 75% of the overall expression within the individual. All analyses were performed with SAS version 9.2 (SAS Institute, Cary, NC). The repeated measures models were calculated with the proc mixed subroutine. Differences less than .05 (P < .05) were regarded as significant.
RESULTS
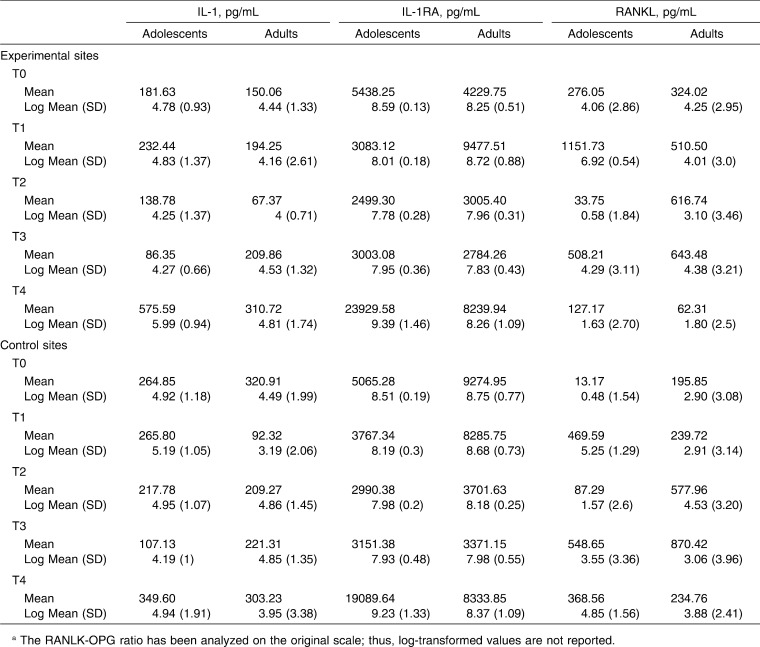
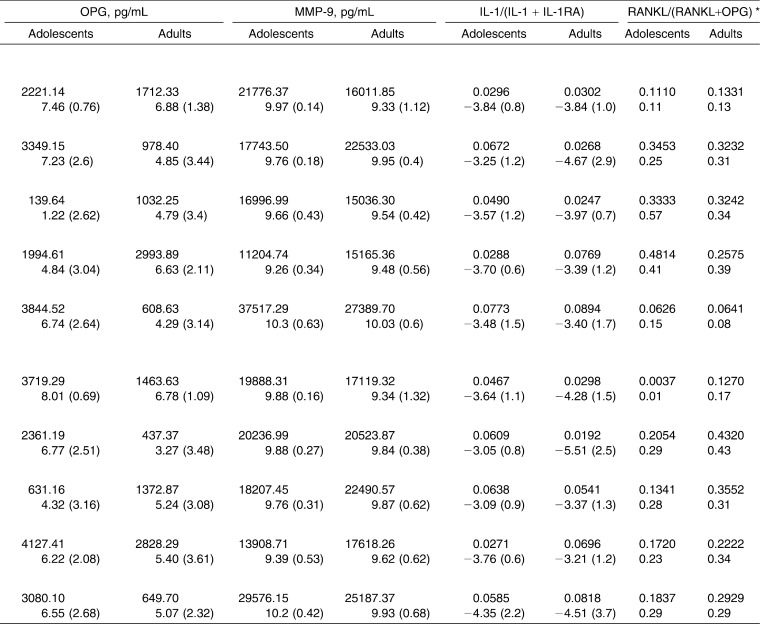
The mean (SD) and the log-transformed mean (SD) of each biomarker and ratio at the different time points are given in Table 1. Interaction P-values are cited throughout the results when comparing group trends over time.
Table 1.
Mean and Log-Transformed Mean (SD) of Each Biomarker and Ratio at the Different Time Pointsa
Table 1.
Extended
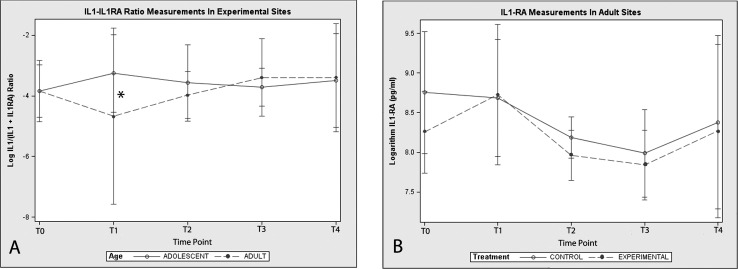
When IL-1 concentrations were compared between experimental and control teeth at various time points, there were no statistically significant differences (P > .05) in adults or adolescents. On the other hand, the ratio of IL-1 to IL-1RA (IL-1/[IL-1 + IL-1RA]) was significantly decreased (P = .033) in the GCF collected from experimental sites in adult patients at T1, that is, 3 weeks after appliance placement and the first archwire activation (Figure 2A). This seems to be a direct consequence of an increase of IL-1RA values in GCF collected from the upper incisors (experimental sites) of adult patients at the same time point. Indeed, the concentration of IL-1RA in adults' experimental sites suddenly increased and became higher than control sites at T1, although this difference was not statistically significant (Figure 2B). The same trend between experimental and control teeth was not noticeable in the adolescent group, where the IL-1RA log mean values were very close to each other at any time point.
Figure 2.
(A) IL1-IL1RA ratio [IL-1/(IL-1 + IL-1RA)] fluctuation in experimental sites throughout the study in adolescents and adults; (B) IL-1RA concentration in experimental and control sites at different time points of the orthodontic treatment in adults. * Statistically significant difference (P < .05).
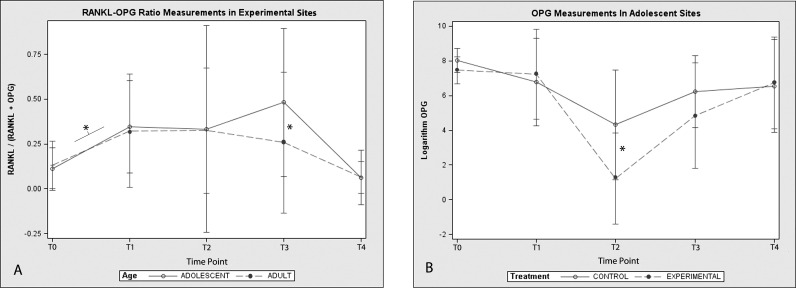
A statistically significant increase (P < .05) in the ratio of RANKL to OPG (RANKL/[RANKL + OPG]) coincident with the commencement of orthodontic treatment was observed in this study (Figure 3A). The mean ratios at T1 were significantly higher than the mean ratios at baseline (T0) for both adolescents and adults (P < .05). In adolescents, a peak in the ratio of RANKL to OPG was observed at T3, that is, 6 weeks after the insertion of the 0.016 × 0.022-in nickel-titanium archwire. Thereafter, the ratio returned to a baseline level. The same trend over time was not observed in the adult group, where the ratio of RANKL to OPG was significantly higher than baseline values at T1 only (Figure 2A). The ratio peak found in adolescents at T3 was a consequence of a decrease in the mean concentration of OPG in experimental sites, which remained below adult values at T2 and T3 (P = .017) (Table 1). When OPG concentrations were compared between experimental and control teeth at corresponding time points, there was no statistically significant difference in adult patients. In adolescents, OPG concentration levels in experimental teeth proved to be significantly lower than those of control teeth at T2 (P = .029) (Figure 3B).
Figure 3.
(A) RANKL-OPG ratio [RANKL/(RANKL + OPG)] fluctuation in experimental sites throughout the study in adolescents and adults; (B) OPG concentration in experimental and control sites at different time points of the orthodontic treatment in adolescents. * Statistically significant difference (P < .05).
There were no statistically significant changes over time in the concentration of MMP-9 between experimental and control teeth (P > .05). Also, there were no statistically significant differences in the concentration of MMP-9 collected from experimental teeth between adults and adolescents.
DISCUSSION
The present research was designed to evaluate age-related changes in GCF during the leveling and alignment phase of orthodontic treatment. Time points in our experiment were chosen in an attempt to capture different biological mechanisms underlying tooth movement, as demonstrated in previous animal studies.14,15 Ideally, the relationship between biomarker expression in GCF and histologic changes of the periodontium should have been confirmed to substantiate the discussion; however, the invasive nature of histomorphometric techniques limits their use in human beings.
The bone resorptive portion of the human remodeling cycle lasts about 28–40 days, whereas in the rats this seems to last 7–10 days.14,15 Although it is difficult to extrapolate from a rodent model of bone remodeling directly to humans, it seems reasonable to speculate that the timeframe for humans would be at least four times longer. Thus, T1 may represent the transition between the lag phase and the linear phase of tooth movement, a time point where a dramatic increase in osteoclast activity is observed within the periodontal ligament.15 Indeed, our results showed an elevation of the ratio of RANKL to OPG in experimental sites compared with baseline levels (Figure 3A). On the other hand, it was intriguing to discover the higher concentration of IL-1RA and subsequent decrease of the ratio of IL-1 to IL-1RA in adult patients at T1, that is, 3 weeks after appliance activation (Figure 2A). Human and animal studies16,17 indicate that the ratio of IL-1 to IL-1RA ratio plays an important role in the speed and amount of tooth movement. In this context, our findings suggest that the distinctive behavior of IL-1RA expression in experimental sites may explain the slower speed of tooth movement observed in some adult patients during the alignment phase.
Forces generated by the fixed appliance should have decayed significantly 6 weeks after the insertion of the 0.014 nickel-titanium archwire; thus, little compression of the periodontal ligament (PDL) is observed at T2. Osteoclast activity should be steady and concentrated in the PDL because the alveolar bone is undergoing the process of frontal resorption. Although OPG levels decreased significantly in adolescents at T2, significant differences in the ratio of RANKL to OPG could not be detected in experimental sites at the same time point for both age groups (Figure 3). Indeed, the ratio of RANKL to OPG remained at the same level observed at T1. The ratio of IL-1 to IL-1RA ratio returned to baseline levels, and a significant difference between adults and adolescents was no longer detectable.
In the present study, the ratio of RANKL to OPG peaked in adolescents at T3, that is, 6 weeks after the first rectangular archwire was tied in. The significant elevation of the ratio of RANKL to OPG at T3 may reflect a higher rate of alveolar bone turnover in the periodontium of adolescents compared with adults.
Two weeks after a heavy rectangular archwire is fully engaged into the bracket slot may reflect a time point of maximal hyalinization production in periodontal tissues; therefore, minimal tooth movement is expected at T4. Perhaps at this stage of tooth movement it is not possible to detect more subtle alterations in GCF mediator levels because the PDL is necrotic and osteoclast activity is primarily occurring in adjacent alveolar bone marrow spaces.15 Indeed, both the ratio of RANKL to OPG and the ratio of IL-1 to IL-1RA returned to a baseline level in experimental sites at this point.
Recent work18 has reported that MMP-9 levels in GCF fluctuated with an 80-day cycle when a continuous orthodontic force was applied; however, a significant decrease compared with baseline values was observed during the first 24 hours after appliance activation. In our study, MMP-9 levels in GCF did not show a consistent behavior in adults or adolescents as the values fall and rise in the experimental and control sites in a time-dependent manner.
Although promising, human GCF studies have not provided conclusive answers. It is becoming increasingly clear that the development of accurate diagnostic assays will be dependent on the identification of a panel of biomarkers that together can discriminate between physiological and pathological tissue-remodeling processes. The physical hurdles of component complexity, the tremendous range in individual protein concentration, and the dynamic nature of the proteome are still major barriers to overcome. Perinetti et al.19 asserted that the low repeatability found in GCF studies can result from individual variability or collection procedures. The authors reported method errors in biomarker activity ranging from 40% to 58%, a level that can have an unequivocal impact on data interpretation. Thus, optimal threshold values for each biomarker need to be determined in order for a variation in activity to be considered indicative of tissue changes during tooth movement.
Among the limitations of this study, the lack of control of biomechanics and the absence of clinical measurements need to be stressed. For this reason, more research in this area is needed to confirm the predictive value of those biomarkers. Additionally, the sample size was not very large given the complexity of the study design. Future studies should seek to gather more information by recruiting more subjects or increasing the number of measurement occasions.
CONCLUSIONS
There are age trends in the levels of GCF biomarker during orthodontic treatment, which invite further investigation.
There was an upregulation of IL-1RA, and consequently, a decrease in the ration of IL-1 to IL-1RA in adult samples taken 3 weeks after the initial archwire was placed.
In adolescents, a peak in the RANKL-OPG ratio was observed in GCF samples taken 6 weeks after the first rectangular archwire was placed.
The confirmation of these results in a larger cohort of patients may reveal the RANKL-OPG and IL1-IL1RA pathways as suitable targets for monitoring tooth movement.
ACKNOWLEDGMENT
This study was supported by the Manitoba Medical Service Foundation (MMSF), grant number 810-11, Winnipeg, Manitoba, Canada.
REFERENCES
- 1.Ren Y, Maltha JC, Van't Hof MA, Von Den Hoff JW, Kuijpers-Jagtman AM, Zhang D. Cytokine levels in crevicular fluid are less responsive to orthodontic force in adults than in juveniles. J Clin Periodontol. 2002;29:757–762. doi: 10.1034/j.1600-051x.2002.290813.x. [DOI] [PubMed] [Google Scholar]
- 2.Kyomen S, Tanne K. Influences of aging changes in proliferative rate of PDL cells during experimental tooth movement in rats. Angle Orthod. 1997;67:67–72. doi: 10.1043/0003-3219(1997)067<0067:IOACIP>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Iwasaki LR, Crouch LD, Tutor A, et al. Tooth movement and cytokines in gingival crevicular fluid and whole blood in growing and adult subjects. Am J Orthod Dentofacial Orthop. 2005;128:483–491. doi: 10.1016/j.ajodo.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 4.Rody WJ, Jr, Iwasaki LR, Krokhin O. Oral fluid-based diagnostics and applications in orthodontics. In: McNamara J, editor. Taking Advantage of Emerging Technologies in Clinical Practice. Ann Arbor: University of Michigan; 2012. pp. 223–261. [Google Scholar]
- 5.Ren Y, Hazemeijer H, de Haan B, Qu N, de Vos P. Cytokine profiles in crevicular fluid during orthodontic tooth movement of short and long durations. J Periodontol. 2007;78:453–458. doi: 10.1902/jop.2007.060261. [DOI] [PubMed] [Google Scholar]
- 6.Basaran G, Ozer T, Kaya FA, Kaplan A, Hamamci O. Interleukine-1beta and tumor necrosis factor-alpha levels in the human gingival sulcus during orthodontic treatment. Angle Orthod. 2006;76:830–836. doi: 10.1043/0003-3219(2006)076[0830:IATNFL]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi M. RANK/RANKL/OPG during orthodontic tooth movement. Orthod Craniofac Res. 2009;12:113–119. doi: 10.1111/j.1601-6343.2009.01444.x. [DOI] [PubMed] [Google Scholar]
- 8.Nishijima Y, Yamaguchi M, Kojima T, Aihara N, Nakajima R, Kasai K. Levels of RANKL and OPG in gingival crevicular fluid during orthodontic tooth movement and effect of compression force on releases from periodontal ligament cells in vitro. Orthod Craniofac Res. 2006;9:63–70. doi: 10.1111/j.1601-6343.2006.00340.x. [DOI] [PubMed] [Google Scholar]
- 9.Kawasaki K, Takahashi T, Yamaguchi M, Kasai K. Effects of aging on RANKL and OPG levels in gingival crevicular fluid during orthodontic tooth movement. Orthod Craniofac Res. 2006;9:137–142. doi: 10.1111/j.1601-6343.2006.00368.x. [DOI] [PubMed] [Google Scholar]
- 10.Ishibashi O, Niwa S, Kadoyama K, Inui T. MMP-9 antisense oligodeoxynucleotide exerts an inhibitory effect on osteoclastic bone resorption by suppressing cell migration. Life Sci. 2006;79:1657–1660. doi: 10.1016/j.lfs.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 11.Bildt MM, Bloemen M, Kuijpers-Jagtman AM, Von den Hoff JW. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in gingival crevicular fluid during orthodontic tooth movement. Eur J Orthod. 2009;31:529–535. doi: 10.1093/ejo/cjn127. [DOI] [PubMed] [Google Scholar]
- 12.Little RM, Wallen TR, Riedel RA. Stability and relapse of mandibular anterior alignment-first premolar extraction cases treated by traditional edgewise orthodontics. Am J Orthod. 1981;80:349–365. doi: 10.1016/0002-9416(81)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Rody WJ, Jr, Akhlaghi H, Akyalcin S, Wiltshire WA, Wijegunasinghe M, Filho GN. Impact of orthodontic retainers on periodontal health status assessed by biomarkers in gingival crevicular fluid. Angle Orthod. 2011;81:1083–1089. doi: 10.2319/011011-15.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King GJ, Keeling SD, Wronski TJ. Histomorphometric study of alveolar bone turnover in orthodontic tooth movement. Bone. 1991;12:401–409. doi: 10.1016/8756-3282(91)90029-i. [DOI] [PubMed] [Google Scholar]
- 15.Rody WJ, Jr, King GJ, Gu G. Osteoclast recruitment to sites of compression in orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2001;120:477–489. doi: 10.1067/mod.2001.118623. [DOI] [PubMed] [Google Scholar]
- 16.Iwasaki LR, Haack JE, Nickel JC, Reinhardt RA, Petro TM. Human interleukin-1 beta and interleukin-1 receptor antagonist secretion and velocity of tooth movement. Arch Oral Biol. 2001;46:185–189. doi: 10.1016/s0003-9969(00)00088-1. [DOI] [PubMed] [Google Scholar]
- 17.Jager A, Zhang D, Kawarizadeh A, et al. Soluble cytokine receptor treatment in experimental orthodontic tooth movement in the rat. Eur J Orthod. 2005;27:1–11. doi: 10.1093/ejo/cjh089. [DOI] [PubMed] [Google Scholar]
- 18.Capelli J, Jr, Kantarci A, Haffajee A, Teles RP, Fidel R, Jr, Figueredo CM. Matrix metalloproteinases and chemokines in the gingival crevicular fluid during orthodontic tooth movement. Eur J Orthod. 2011;33:705–711. doi: 10.1093/ejo/cjq148. [DOI] [PubMed] [Google Scholar]
- 19.Perinetti G, Di Leonardo B, Di Lenarda R, Contardo L. Repeatability of gingival crevicular fluid collection and quantification, as determined through its alkaline phosphatase activity: implications for diagnostic use. J Periodontal Res. 2013;48:98–104. doi: 10.1111/j.1600-0765.2012.01508.x. [DOI] [PubMed] [Google Scholar]