Abstract
Objective:
To evaluate the release of metal ions from fixed orthodontic appliances.
Materials and Methods:
A new system for in vitro testing of dental materials was constructed and consisted of a thermostatic glass reactor that enabled immersion of the studied material. Experimental conditions reflected the human oral cavity, with a temperature of 37°C and a saliva flow rate of 0.5mL/min. The simulated fixed orthodontic appliance made of stainless steel was evaluated. Sampling was performed at several time points during the 28-day study, and the metal ion concentration was determined by inductively coupled plasma optical emission spectrometry.
Results:
The total mass of released metal ions from the appliance during 4 weeks of the experiment was as follows nickel 18.7 μg, chromium 5.47 μg, copper 31.3 μg.
Conclusions:
The estimated doses of nickel, chromium, and copper determined by extrapolation of experimental data released during the treatment period were far below the toxic dose to humans. This shows that orthodontic treatment might not be a significant source of exposure to these metal ions.
Keywords: Metal ions, Orthodontic appliance, Artificial saliva
INTRODUCTION
Recent decades have seen great interest in the biocompatibility aspects of biomaterials, whether internally or externally applied. Materials applied in dentistry have to pass specific biocompatibility tests to meet regulatory standards.1 Some metals (eg, nickel [Ni], chromium [Cr], cobalt (Co)) that are components of orthodontic alloys are well known to be allergenic, cytotoxic, and mutagenic.2,3 Appliances and additional devices used during orthodontic treatment are exposed to different factors, such as temperature, pH, mechanical stress (corrosion), and microflora (biocorrosion).4 All these factors may lead to release of toxic metal ions from alloys.
The biocompatibility of orthodontic fixed appliances (brackets, bands, wires) might be determined by evaluating the amount of metal ions that are released during the process of dissolution as the consequence of corrosion in in vitro and in vivo studies.5 In vivo studies provide information on the behavior of a material under the conditions for which the material is designated. However, sample collection of invasive matrices (blood) is difficult because of the need for patient consent. Noninvasive matrices such as urine or saliva are easier to collect, but it must be emphasized that all matrices (blood, urine, saliva) reflect only momentary exposure. Thus, the methodology of sample collection and the results obtained to simulate the average orthodontic treatment time of 2 years are not as meaningful as that which occurs in chronic exposure.5–7 On the other hand, in vitro studies are performed in a controlled laboratory environment but do not reflect the conditions prevailing in the oral cavity.8 Many studies of the corrosion behavior of orthodontic devices in artificial saliva did not take into account the equilibrium reached in batch experiments; that is, during the experiment, the saliva was not replaced or allowed to flow through the system, as in the human oral cavity.9–11 This may lead to results that do not accurately reflect the actual degree of ion release from the evaluated material, and thus it is also difficult to assess the dose to which a patient is exposed during orthodontic treatment.
The aim of the present study was to evaluate the release of metal ions from fixed orthodontic appliances in vitro in a continuous-flow system. Another goal was to elaborate a new, more reliable, and more meaningful technique for evaluation of the release of potentially toxic chemicals from dental materials.
MATERIALS AND METHODS
In Vitro System
The experimental conditions were selected to reflect as closely as possible (in an in vitro system) the environment of the human oral cavity with an orthodontic appliance. The experiments were carried out in a specially designed thermostatic glass reactor (Figure 1), in which the orthodontic appliance was submerged in artificial saliva, which was flowing through the system at a rate reflecting the flow of saliva in humans (0.5 mL/min).12 The duration of the experiment was 4 weeks (28 days). The samples were collected over time, which enabled us to investigate the kinetics of metal ion dissolution from parts of the fixed orthodontic appliance. The experiment duration enabled an attempt to extrapolate the data and estimate (after subtraction of the results from the control group) the overall dose to which a patient is exposed during orthodontic treatment.
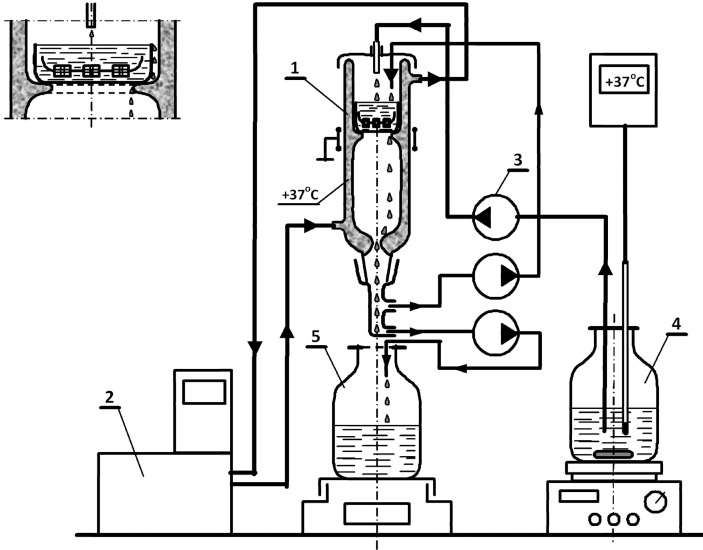
Figure 1.
Schematic diagram of the system for the evaluation of ion release from dental materials. The installation consisted of: (1) a glass reactor with a water jacket, (2) a water bath with an external circuit to enable operation of the reactor under temperature-controlled conditions, (3) a multi-channel peristaltic pump to feed the solution into the reactor, (4) a glass tank placed on a thermostatic magnetic stirrer, and (5) the collecting vessel. The receiving solution flowed down gravitationally. The various streams of solutions were distributed through the system by flexible, chemically resistant hoses made of Norprene tubes (Masterflex, Vernon Hills, IL, USA) for use with the peristaltic pumps. Adequate flow rate was achieved by the diameter of the tubing. The material was placed in a glass tank, which was immersed in the solution, which flowed into the system.
The system consisted of a magnetic stirrer with heating and a contact thermometer with a glass-coated sensor (Heidolph MR Hei-Tec and Heidolph EKT Hei-Con G, (Heidolph: Schwabach, Germany), a multiple-channel peristaltic pump (Heidolph Peristaltic Pumps PD 5201 MC, (Heidolph: Schwabach, Germany), a water bath with an external circuit (LAUDA ECO E 10 S, Lauda, Lauda-Koenigschofen, Germany), and a thermostatic glass reactor with a cover (Figure 1).
Tested Material
The evaluated materials were brand new parts of the orthodontic appliance. Wires, brackets, and bands were all made of stainless steel, whereas elastic ligatures were fabricated of a polymer. The following elements of the orthodontic appliance were tested: 0.017 × 0.025-inch archwires (American Orthodontics, ref no. 300-021, 300-022), size 37+ bands, Sheboygan, Wis, USA). (3M Unitek, Victory Series brackets (3M Unitek, Monrovia, Calif, USA), and elastic ligatures (American Orthodontics, Sheboygan, Wis, USA). The orthodontic fixed appliance consisted of two wires, four bands, 20 brackets, and 20 elastic ligatures to correspond to the upper and lower dental arches during particular phases of orthodontic treatment.
Experimental Setup
The appliance was placed in the thermostatic glass reactor with a cover and incubated (immersed) in the modified artificial saliva solution13 at 37°C for 28 days. The solution of artificial saliva was spiked with Ni2+ to ensure a physiological concentration similar to that of human saliva. The concentration was calculated as the average from the values found in the available literature.14–21
The feed solution of artificial saliva was pumped into the glassware in a continuous manner. The average flow rate was 0.4825 mL/min (0.4808 g/min, d = 0.9966 g/mL). The recycle ratio was 1∶1. The samples (50 mL) were collected directly from the outlet vessel. At the beginning of the experiment, sampling was done more frequently to investigate the initially (according to literature reports) more intense metal ion dissolution during the preliminary phase (first 12 hours: every 50 minutes; first week: every 12 hours; second week: every 24 hours; third week: every 3.5 days; fourth week: at the end of the experiment).
As a control, artificial saliva was made to flow through the system under the same conditions as the experimental set, although in the absence of the orthodontic appliance.
Analytical Methods
A sample (50 g) was purified from organic matter with concentrated nitric acid (69% m/m; 5 mL), which was spectrally pure (Suprapur, Darmstadt, Germany), in Teflon bombs in a microwave oven (Milestone Start D, Sorisole, Italy). The selected parameters of the process ensured complete digestion of the samples. After mineralization, the samples were diluted with redemineralized water (Millipore Simplicity, Molsheim, France) to 50 g. Mineralized samples underwent multi-elemental analysis. The concentrations of Ni, Cr, cadmium (Cd), copper (Cu), iron (Fe), manganese (Mn), molybdenum (Mo), and silicon (Si) were determined with an inductively coupled plasma optical emission spectrometry device (ICP-OES Varian-Vista MPX, Brisbane, Australia) equipped with an ultrasonic nebulizer (CETAC U5000AT+, Omaha, Neb, USA). Also, the presence of any additional elements was ascertained. The analyses were carried out in a quality management system according to PN-EN ISO/IEC 17025:2005. The samples were analyzed three times, and the arithmetic mean and relative standard deviation (SD) (<5%) were calculated.
Statistical Methods
Statistical analysis of the results was performed by the software Statistica (version 9.0, Tulsa, Okla, USA). Descriptive statistics (mean, median, SD, minimum [min], and maximum [max]) were reported. Statistical differences between the experimental and control groups were assessed by the nonparametric Wilcoxon test for paired samples. The statistical significance of differences between the groups was accepted at the α < .05 level. Analysis of interelement correlations was carried out by determination of the Pearson coefficient. A correlation was considered statistically significant at α < .05.
RESULTS
In the present work, metal ion release in the environment of artificial saliva was investigated in a continuous mode with recycling (the ratio was 1∶1). The artificial saliva was collected from the outlet of the system, and the concentrations of Cr, Ni, Cd, Cu, Mn, Mo, Fe, and Si ions were determined. In all, 19.4 L of artificial saliva flowed through the system during the 28 days of the experiment.
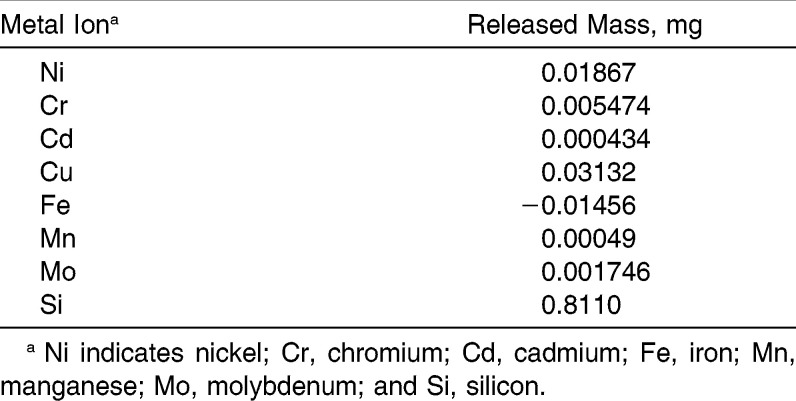
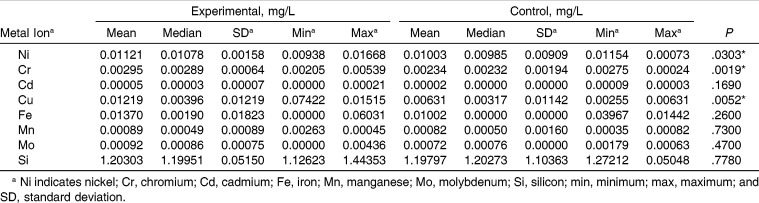
Table 1 reports the total quantity of metal ions (expressed in milligrams) that was released from the orthodontic appliance during the whole experiment. The reported data are the result of subtraction of the control samples from the experimental samples. The ions were released in the following order: Si>Cu>Ni>Cr>Mo>Mn>Cd. Fe ions were not released from the appliance. There was no correlation between the content of a given metal in an alloy and the released quantity.
Table 1.
Total Mass (mg) of Metal Ions Released During the Experiment
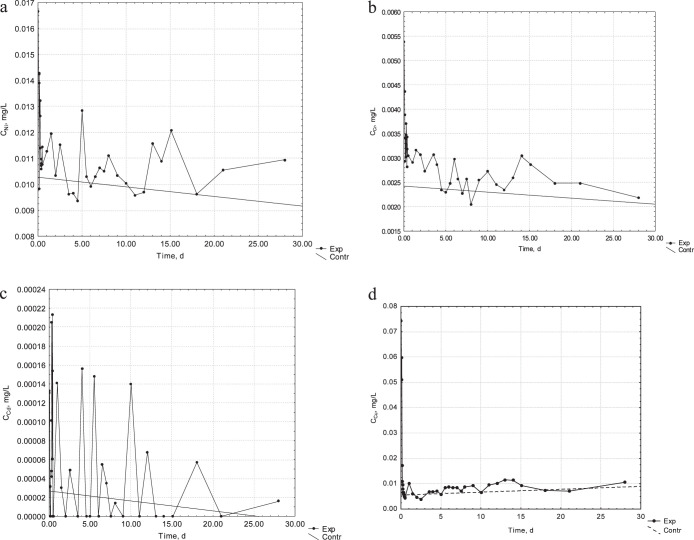
The concentration of metal ions in the experimental and control samples is presented in Figure 2a–h. In the case of Ni, Cr, and Cu ions, a more intense release was observed during the first 2 hours of the process, in comparison to the concentration in the control samples.
Figure 2.
(a) Kinetics of release of Ni ions from the orthodontic appliance into the artificial saliva. (b) Kinetics of release of Cr ions from the orthodontic appliance into the artificial saliva. (c) Kinetics of release of Cd ions from the orthodontic appliance into the artificial saliva. (d) Kinetics of release of Cu ions from the orthodontic appliance into the artificial saliva.
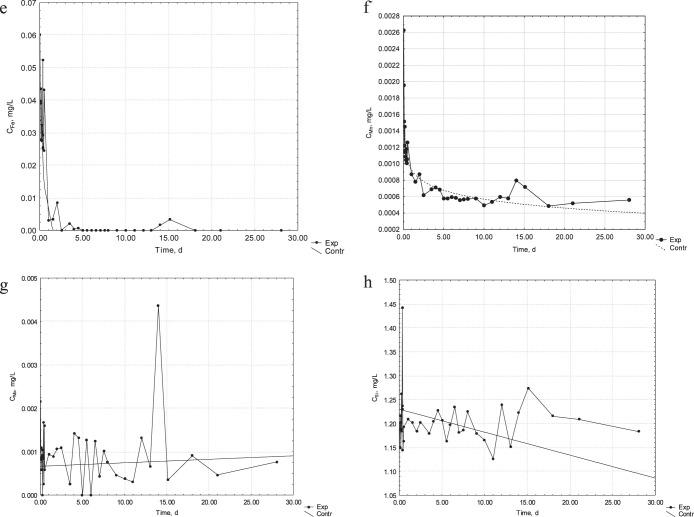
Figure 2.
Continued. (e) Kinetics of release of Fe ions from the orthodontic appliance into the artificial saliva. (f) Kinetics of release of Mn ions from the orthodontic appliance into the artificial saliva. (g) Kinetics of release of Mo ions from the orthodontic appliance into the artificial saliva. (h) Kinetics of release of Si ions from the orthodontic appliance into the artificial saliva.
Table 2 presents a statistical elaboration of results from the experimental and control groups (n = 38 samples drawn during the study in each group) for each evaluated released metal ion. The concentration of ions was in the following order: Si>Fe>Cu>Ni>Cr>Mo>Mn>Cd. The level of Cd was close to the lower limit of detection. The concentrations of the remaining ions were detectable in the experimental samples.
Table 2.
Results and Statistical Analysis of the Experimental and Control Groups (n = 38)
The differences between the experimental and control groups were assessed by the nonparametric Wilcoxon test for paired samples. Statistically significant differences were found for Ni, Cr, and Cu. The mean concentrations were 12%, 26%, and 93% higher, respectively, in the experimental group vs the control group.
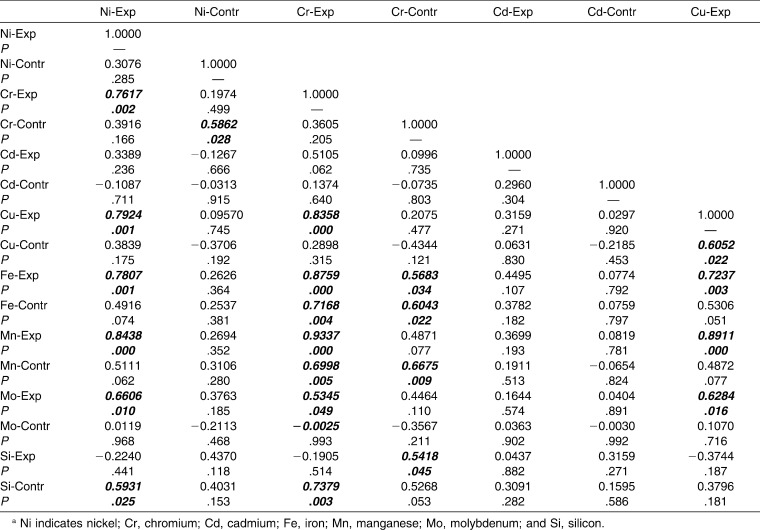
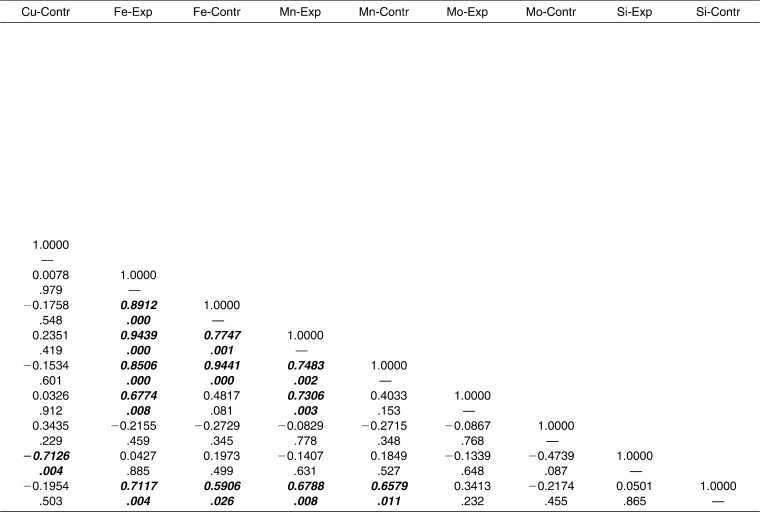
Interelement interactions were investigated in the experimental and control groups separately. Strong and statistically significant correlations were found between the following elements in the experimental group: Ni-Cr, Ni-Cu, Ni-Fe, Ni-Mn, Cr-Cu, Cr-Fe, Cr-Mn, Cu-Mn, Fe-Mn (Table 3). It can be concluded that Ni, Cr, Cu, Fe, and Mn were interrelated. The concentrations of Cd, Mo, and Si ions were not correlated with those of the other ions of metals in the alloys of the orthodontic appliance.
Table 3.
Interelement Correlation Matrix for Experimental (Exp) vs Control (Contr) Groups
Table 3.
Extended.
DISCUSSION
Many in vitro studies that evaluated ion release from fixed orthodontic appliances used a different methodological/material approach from that employed in the present study. Various types of immersion liquids, different analytical methods, and materials obtained from several manufacturers make a comparison of studies more difficult and sometimes even impossible. It is hard to draw meaningful conclusions from a comparison of the degree of released ions from various materials.
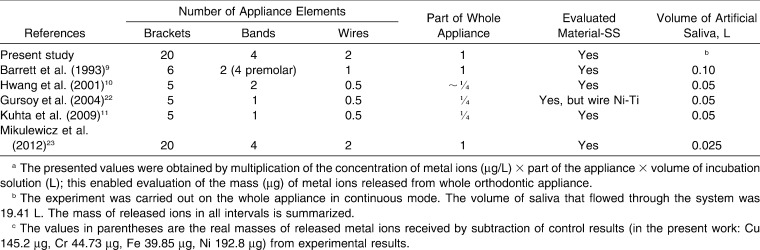
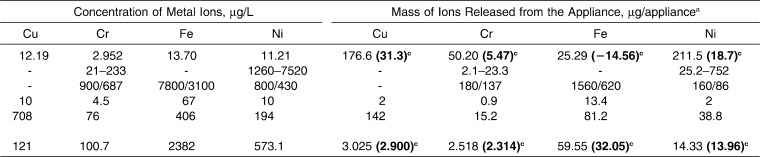
All available studies reported the results of in vitro experiments in artificial saliva in batch mode. Table 4 shows the results of those studies alongside those of the present investigation.
Table 4.
Release of Metal Ions from an Orthodontic Appliance in the Present Study and Reported in the Literature in an Artificial Saliva Environment (37°C, pH 7)
Table 4.
Extended.
A literature review retrieved five studies9–11,22,23 that reported on the release of metal ions from fixed orthodontic appliances (brackets, bands, and wires manufactured from stainless steel) in controlled in vitro conditions, ie, artificial saliva (25–100 mL) at 37°C and pH 7 with the experiment lasting 28–90 days. Barrett et al.9 conducted a study on a simulated fixed appliance that represented a complete maxillary arch. The experiment lasted 28 days; samples were collected at 1, 7, 14, 21, and 28 days; and the analytical technique was flame atomic absorption specrometry.9 Hwang et al.10 performed a study on a simulated fixed appliance, which represented half of the maxillary arch; the experiment lasted 90 days and samples were collected at 1, 3, 7, 14, 21, 28, 56, and 84 days. The analytical technique was inductively coupled plasma mass spectrometry. Two types of stainless steel wires were investigated. Brackets and bands were the same in the experimental groups. The elements of the appliance were soldered together.10 Gursoy et al.22 conducted a study on a half-arch of a fixed appliance. The experiment was carried out for 45 days, and the samples were collected on the last day. The analyses were carried out by inductively coupled plasma optical emission spectrometry.22 Kuhta et al.11 also used a maxillary half-arch. The duration of the experiment was 28 days, and sampling times were 1, 7, 14, and 28 days. The analytical technique was high-resolution inductively coupled plasma mass spectrometry.11 Mikulewicz et al.23 conducted a study on a complete appliance (representing upper and lower arches) in 25 mL of artificial saliva, in which the appliance was incubated for 30 days (sampling was done on the last day). The analytical technique was inductively coupled plasma mass spectrometry.23
The different concentrations and masses of released metal ions from the appliances are shown in Table 4. Differences can be explained by the slightly different experimental methodologies and materials: proportions of the elements in the appliance, ratio of appliance to solution, manufacturers of orthodontic parts and sometimes materials (Ni-Ti wire, solder), composition of artificial saliva (which may have contained traces of the studied metal ions), and analytical techniques with different lower detection limits, interferences, and matrix effects. Also, the studies were carried out between years 1993 and 2012. The sensitivity of applied analytical techniques has improved significantly in recent years. Finally, in three of the studies, no control group was used. The knowledge of the composition of the control solution enables one to subtract the quantity of the studied ions in the matrix (solution of artificial saliva, metal ions released from the system, glass, tubes). Consequently, if one is attempting to calculate the mass of released ions, the ions present in the solution from other sources should be taken into consideration. Knowledge of the level of ions in the control group enabled us to report the ions released from the orthodontic appliance only.
In in vitro experiments, the influence of single factors can be considered. In contrast, in in vivo studies, many parameters influence the obtained results and make it difficult to interpret the data; however, they reflect actual conditions.24–26
Based on the mass of released metal ions in the continuous flow experiment, an attempt to calculate the average mass of metal ions released during a potential 2-year treatment period was undertaken. The masses of metal ions were as follows: Cr 131 µg, Ni 444 µg, and Cu 751 µg. The World Health Organization's recommended daily doses are 50–200 µg for Cr and 25–35 µg for Ni.27 The average daily doses released from the appliance in this study were calculated as 0.195 µg for Cr, 0.661 µg for Ni, and 1.12 µg for Cu; thus, all values are far below the recommended daily doses. It must be emphasized that ion release from metal orthodontic devices in vivo is affected by various factors, such as saliva composition, pH, diet, and microflora. Also, there is a wide range of applied materials with different compositions (which can affect ion release). It is nearly impossible to take into account all those factors in an in vitro study. On the other hand, the results of an in vivo study—which are typically based on noninvasive matrices (saliva, urine, hair)—are affected by methodological aspects: sampling regimen, other sources of exposure, and a multitude of other factors related to living habits.
CONCLUSIONS
The highest concentration of metal ions was released at the beginning of the experiment. After this, passivation probably occurred, which hindered further intense release. It was found that the ions were released in doses that are not toxic to humans.
Comparison with batch-testing data reported in the literature showed that, in fact, more ions were released from the material than had been previously reported. In the present paper, in contrast to other data available in the literature, the conditions did not reach equilibrium.
ACKNOWLEDGMENT
This research was financially supported by The National Centre for Research and Development in Poland (N R130006 10).
REFERENCES
- 1.Brantley WA, Eliades T. Orthodontic Materials Scientific and Clinical Aspects. Stuttgart, Germany: Georg Thieme Verlag; 2001. [Google Scholar]
- 2.Kusy RP. Orthodontic biomaterials: from the past to the present. Angle Orthod. 2002;72:501–512. doi: 10.1043/0003-3219(2002)072<0501:OBFTPT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Eliades T, Bourauel C. Intraoral aging of orthodontic materials: the picture we miss and its clinical relevance. Am J Orthod Dentofacial Orthop. 2005;127:403–412. doi: 10.1016/j.ajodo.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Eliades T, Athanasiou AE. In vivo aging of orthodontic alloys: implications for corrosion potential, nickel release, and biocompatibility. Angle Orthod. 2002;72:222–237. doi: 10.1043/0003-3219(2002)072<0222:IVAOOA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 5.Schmaltz G, Arenholt-Bindslev D. Biocompatibility of Dental Materials. Heidelberg, Germany: Springer-Verlag Berlin; 2008. [Google Scholar]
- 6.Amini F, Rakhshan V, Sadeghi P. Effect of fixed orthodontic therapy on urinary nickel levels: a long-term retrospective cohort study. Biol Trace Elem Res. 2012 Dec;150(1–3):31–36. doi: 10.1007/s12011-012-9478-6. DOI: 10.1007/s12011-012-9478-6. [DOI] [PubMed] [Google Scholar]
- 7.Chojnacka K, Mikulewicz M. Hair mineral analysis in the assessment of human exposure to metals. In: Preedy V, editor. Handbook of Hair in Health and Disease. Wageningen, The Netherlands: Wageningen Academic Publishers; 2012. [Google Scholar]
- 8.Mikulewicz M, Chojnacka K. Trace metal release from orthodontic appliances by in vitro studies: a systematic literature review. Biol Trace Elem Res. 2010;139:241–256. doi: 10.1007/s12011-010-8670-9. [DOI] [PubMed] [Google Scholar]
- 9.Barrett RD, Bishara SE, Quinn JK. Biodegradation of orthodontic appliances. Part I. Biodegradation of nickel and chromium in vitro. Am J Orthod Dentofacial Orthop. 1993;103:8–14. doi: 10.1016/0889-5406(93)70098-9. [DOI] [PubMed] [Google Scholar]
- 10.Hwang CJ, Shin JS, Cha JY. Metal release from simulated fixed orthodontic appliances. Am J Orthod Dentofacial Orthop. 2001;120:383–391. doi: 10.1067/mod.2001.117911. [DOI] [PubMed] [Google Scholar]
- 11.Kuhta M, Pavlin D, Slaj M, Varga S, Lapter-Varga M, Slaj M. Type of archwire and level of acidity: effects on the release of metal ions from orthodontic appliances. Angle Orthod. 2009;79:102–110. doi: 10.2319/083007-401.1. [DOI] [PubMed] [Google Scholar]
- 12.Dawes C. The unstimulated salivary flow rate after prolonged gum chewing. Arch Oral Biol. 2005;50:561–563. doi: 10.1016/j.archoralbio.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Jensen CS, Lisby S, Baadsgaard O, Byrialsen K, Menné T. Release of nickel ions from stainless steel alloys used in dental braces and their patch test reactivity in nickel sensitive individuals. Contact Dermatitis. 2003;48:300–304. doi: 10.1034/j.1600-0536.2003.00118.x. [DOI] [PubMed] [Google Scholar]
- 14.Kerosuo H, Moe G, Hensten-Pettersen A. Salivary nickel and chromium in subjects with different types of fixed orthodontic appliances. Am J Orthod Dentofacial Orthop. 1997;111:595–598. doi: 10.1016/s0889-5406(97)70310-x. [DOI] [PubMed] [Google Scholar]
- 15.Kocadereli L, Ataç PA, Kale PS, Ozer D. Salivary nickel and chromium in patients with fixed orthodontic appliances. Angle Orthod. 2000;70:431–434. doi: 10.1043/0003-3219(2000)070<0431:SNACIP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Ağaoğlu G, Arun T, Izgi B, Yarat A. Nickel and chromium levels in the saliva and serum of patients with fixed orthodontic appliances. Angle Orthod. 2001;71:375–379. doi: 10.1043/0003-3219(2001)071<0375:NACLIT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Eliades T, Trapalis C, Eliades G, Katsavrias E. Salivary metal levels of orthodontic patients: a novel methodological and analytical approach. Eur J Orthod. 2003;25:103–106. doi: 10.1093/ejo/25.1.103. [DOI] [PubMed] [Google Scholar]
- 18.Faccioni F, Franceschetti P, Cerpelloni M, Fracasso ME. In vivo study on metal release from fixed orthodontic appliances and DNA damage in oral mucosa cells. Am J Orthod Dentofacial Orthop. 2003;124:687–694. doi: 10.1016/j.ajodo.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Amini F, Borzabadi Farahani A, Jafari A, Rabbani M. In vivo study of metal content of oral mucosa cells in patients with and without fixed orthodontic appliances. Orthod Craniofac Res. 2008;11:51–56. doi: 10.1111/j.1601-6343.2008.00414.x. [DOI] [PubMed] [Google Scholar]
- 20.Matos de Souza R, Macedo de Menezes L. Nickel, chromium and iron levels in the saliva of patients with simulated fixed orthodontic appliances. Angle Orthod. 2008;78:345–350. doi: 10.2319/111806-466.1. [DOI] [PubMed] [Google Scholar]
- 21.Petoumeno E, Kislyuk M, Hoederath H, et al. Corrosion susceptibility and nickel release of nickel titanium wires during clinical application. J Orofac Orthop. 2008;69:411–423. doi: 10.1007/s00056-008-0808-4. [DOI] [PubMed] [Google Scholar]
- 22.Gürsoy S, Acar AG, Seşen C. Comparison of metal release from new and recycled bracket-archwire combinations. Angle Orthod. 2004;75:92–94. doi: 10.1043/0003-3219(2005)075<0092:COMRFN>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 23.Mikulewicz M, Chojnacka K, Woźniak B, Downarowicz P. Release of metal ions from orthodontic appliances: an in vitro study. Biol Trace Elem Res. 2012;146:272–280. doi: 10.1007/s12011-011-9233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ousehal L, Lazrak L. Change in nickel levels in the saliva of patients with fixed orthodontic appliances. Int Orthod. 2012;10:190–197. doi: 10.1016/j.ortho.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Natarajan M, Padmanabhan S, Chitharanjan A, Narasimhan M. Evaluation of the genotoxic effects of fixed appliances on oral mucosal cells and the relationship to nickel and chromium concentrations: an in-vivo study. Am J Orthod Dentofacial Orthop. 2011;140:383–388. doi: 10.1016/j.ajodo.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 26.Sahoo N, Kailasam V, Padmanabhan S, Chitharanjan AB. In-vivo evaluation of salivary nickel and chromium levels in conventional and self-ligating brackets. Am J Orthod Dentofacial Orthop. 2011;140:340–345. doi: 10.1016/j.ajodo.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 27.Kabata-Pendias A, Pendias H. Biogeochemistry of Trace Elements [in Polish] Warsaw: PWN; 1993. pp. 53–66. [Google Scholar]