Abstract
Objectives:
To examine the ability of a removable thermoplastic appliance (RTA) to adsorb hygienic solutions and inhibit bacterial growth and to examine the efficacy of three hygiene protocols in reducing bacterial biofilm adherence to RTA.
Materials and Methods:
Solution adsorption and bacterial growth inhibition were examined in vitro using paper vs RTA discs. Subsequently, 11 patients treated with RTA (mean age, 29.1 ± 4.7 years) were assigned into a sequence of three hygiene protocols: regular RTA brushing (baseline), immersion RTAs in chlorhexidine mouthwash (CHX), and using a vibrating bath with cleaning solution (VBC). For each patient, 12 upper RTAs were examined (2 baseline RTAs, 5 CHX RTAs, and 5 VBC RTAs), for a total of 132 RTAs. All RTAs were stained with gentian violet, and biofilm presence was measured using a photodensitometer.
Results:
The RTA discs did not adsorb CHX or cleaning solution. The later agent did not show antibacterial features. Baseline RTAs showed significant biofilm adherence (P < .001) on the posterior palatal side of the aligner and on the anterior incisal edge. CHX and VBC hygienic protocols significantly (P < .001) reduced baseline biofilm adherence by 16% and 50%, respectively. Hygienic improvement was maintained over 140 days when CHX and VBC were used. However, VBC was three times more efficient than CHX.
Conclusions:
This study highly recommends the use of a VBC protocol. Biofilm deposits on the RTA, especially on incisal edges and attachment dimples, could lead to inadequate tooth/RTA and attachment/RTA overlap and consequently impair tooth alignment.
Keywords: Thermoplastic appliance, Biofilm, Oral hygiene
INTRODUCTION
A removable thermoplastic appliance (RTA) is made from transparent plastic material such as polyurethane from methylene diphenyl diisocyanate and 1,6-hexanediol (Align Technology, Santa Clara, Calif), which hardens when cool and has desirable properties of springback, stiffness, and formability.1
However, Schuster et al.2 have demonstrated that significant surface modifications of the RTA might contribute to an alteration in the surface profile of the material3 and thus may facilitate bacterial adhesion.4 Salivary film (the acquired pellicle) can coat any solid surface present in the mouth,5 followed by dental plaque (oral biofilm), which can accumulate on artificial surfaces such as acrylic appliances in the mouth.6,7 Following adherence, bacteria can multiply and attach to each other, forming bacterial biofilm and aggregates.4 Adherence of Streptococcus mutans and Lactobacillus colonies to RTA was demonstrated, with increase in colonies over time.8 It was previously observed that some subjects required a longer period of time to accumulate plaque (referred to as slower plaque forming) than others who developed plaque more rapidly.9 Low et al.10 have demonstrated bacterial colonization on the raised edges of the aligner in both slow- and fast-plaque–forming groups. This biofilm accumulation was enhanced by physical changes such as microcracks and abraded and delaminated areas, which appeared during 14 days of aligner wear.11 Since RTAs are worn for several weeks (aligners) or for years (orthodontic retainers, occlusal stabilization splints),12 it is of major clinical importance to define a reliable protocol to control bacterial adherence to the RTA.
Chlorhexidine (CHX) is a well-known, widely used antimicrobial agent for plaque and gingival health control, which may be suitable for cleaning removable orthodontic appliances in order to reduce the plaque buildup on their surfaces.13,14 CHX mouthwash is composed of chlorhexidine digluconate (antimicrobial agent) 0.12 g, xylitol (sweetener) 1.00 g, and excipient q.s. 100 mL. Cleaning-Crystals solutions (CS) are widely used in conjunction with vibrating baths to remove dental plaque from patients' dentures.15 For this study, Invisalign Cleaning-Crystals were dissolved in water according to the manufacturer's instructions (Align Technology) to obtain cleaning CS. The Cleaning-Crystals are composed of sodium sulfate (60%), sodium carbonate (30%), sodium tripolyphosphate (7.5%), sodium dichloroisocyanurate (2%), and sodium lauryl sulfate (0.15%). These agents hold chemical properties such as pH neutralizer (eg, sodium sulfate), disinfectant (eg, sodium dichloroisocyanurate), and detergent (eg, sodium lauryl sulfate). Regardless of published evidence showing adherence of bacterial biofilm to the aligner within 2 weeks of wear, no study evaluated quantitatively the efficacy of removing biofilm deposits from the aligner.
The aims of the present study were to evaluate the in vitro ability of the RTA material to adsorb cleaning solutions and to inhibit bacterial growth in culture, and to examine the in vivo efficacy of three hygiene protocols in reducing the amount of oral biofilm adhesion to the aligners over time.
MATERIALS AND METHODS
The study was composed of in vitro and in vivo studies.
In Vitro Antibacterial Assay
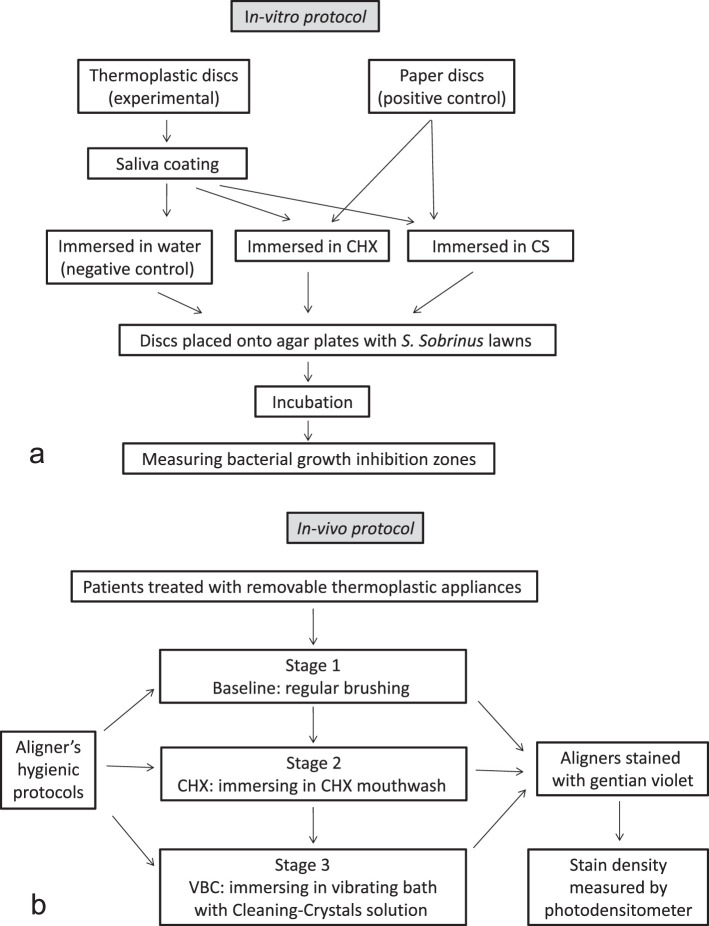
To evaluate the feasibility of the RTA to adsorb hygienic solution and inhibit bacterial growth in culture, sixty 5-mm-diameter discs were punched from sheets of thermoplastic material (Align Technology) and sterilized by immersing discs in 70% ethanol for 10 minutes followed by overnight alcohol evaporation in a sterile covered 10-cm disposable polystyrene Petri dish (Miniplast, Ein Shemer, Israel) at 37°C.16 The sterile discs were initially immersed in sterile saliva followed by immersion in each of the hygienic protocol solutions (Figure 1a).
Figure 1.
(a) Schematic presentation of the in vitro part of the study. (b) Schematic presentation of the in vivo part of the study.
Disc Immersion Protocol
Saliva coating
Saliva coating was performed to simulate intraoral pellicle formation on the plastic discs. It has been shown that CHX binds to saliva-coated substrates rather than onto bare substrate.17 Unstimulated whole saliva was collected from one donor and sterilized by filtration through a 45-µ sterile filter. Sterile aligner-material discs (N = 60) (Cameo 25GAS, 0.45 pore size, Micron Separations, Inc. MSI, Westboro, MA) were placed in the filtered saliva and vortexed for 3 minutes and then dried at 37°C for 2 hours to enable adsorption of the different cleaning solutions.16
CHX mouthwash
Sterilized saliva-coated aligner discs (N = 25), were immersed in sterile CHX mouthwash (Lacer Co, Barcelona, Spain) for 15 minutes, washed in sterile distilled water at room temperature for 2 minutes, and dried at 37°C for 2 hours.16
For positive control, 10 µL of sterile CHX mouthwash was placed onto 5-mm discs of filter paper (N = 5) following drying at 37°C for 2 hours.
Cleaning solution
Aligner discs (N = 25) were immersed and shaken for 15 minutes in cleaning CS (Align Technology), which were dissolved in sterile distilled water at room temperature; they were then rinsed in sterile distilled water for 2 minutes and dried at 37°C for 2 hours.
For positive controls, 5-mm filter paper discs (N = 5) were each adsorbed with 10 µL of the CS and dried for 2 hours at 37°C. For negative control, saliva-coated sterile aligner material discs, prepared as described above (N = 10), were immersed in sterile distilled water at room temperature and dried for 2 hours at 37°C.
Bacterial lawns
Streptococcus sobrinus 27351 was grown in 2 ml of brain heart infusion (BHI) broth at 37°C and then transferred onto BHI-agar plates (Hy Labs, Rehovot, Israel). Seven disks were placed onto each of two sets of five agar plates: one positive control (filter paper) either CHX or CS, one negative control (sterile aligner disk), and five aligner discs immersed in either of the two tested solutions (CHX and CS). Plates were incubated anaerobically for 24 hours at 37°C, and zones of bacterial growth inhibition were measured with a Vernier digital caliper.
In Vivo Clinical Study
Eleven orthodontic patients treated with Invisalign RTA (8 females and 3 males), treated at the Department of Orthodontics at Tel-Aviv University, Israel) aged 29.1 ± 4.7 years, were each assigned to a consecutive three-stage examination (Figure 1b). In the present crossover study, only upper aligners with no attachments were examined, and three hygienic protocols were compared in each patient. A total of 132 aligners were examined (ie, 12 aligners from each patient). The rights of the human subjects were protected, and approval was obtained from the Ethics Committee of Tel Aviv University.
Stage 1
Patients received two aligners with regular instructions of tooth/aligner brushing using any toothpaste containing 1400 ppm fluoride. These aligners served as baseline control. A total of 22 baseline aligners were examined in the first 28 days.
Stage 2
Patients were requested to brush the next five consecutive aligners with a toothbrush every evening, then immerse it in a CHX mouthwash for 15 minutes, rinse with water, and place it back in the mouth. The second stage was assigned as the chlorhexidine hygienic protocol (CHX). A total of 55 CHX aligners were examined every 2 weeks for the next 70 days.
Stage 3
Patients received a vibrating bath with Cleaning-Crystals solution (VBC; Align Technology) for the next five aligners. The VBC is a compact electric vibrating bath filled with the dissolved cleaning crystals to be used every evening. The aligner was immersed in the bath and shaken in the solution for 15 minutes, then rinsed with water and placed back in the mouth. A total of 55 VBC aligners were examined every 2 weeks for the next 70 days.
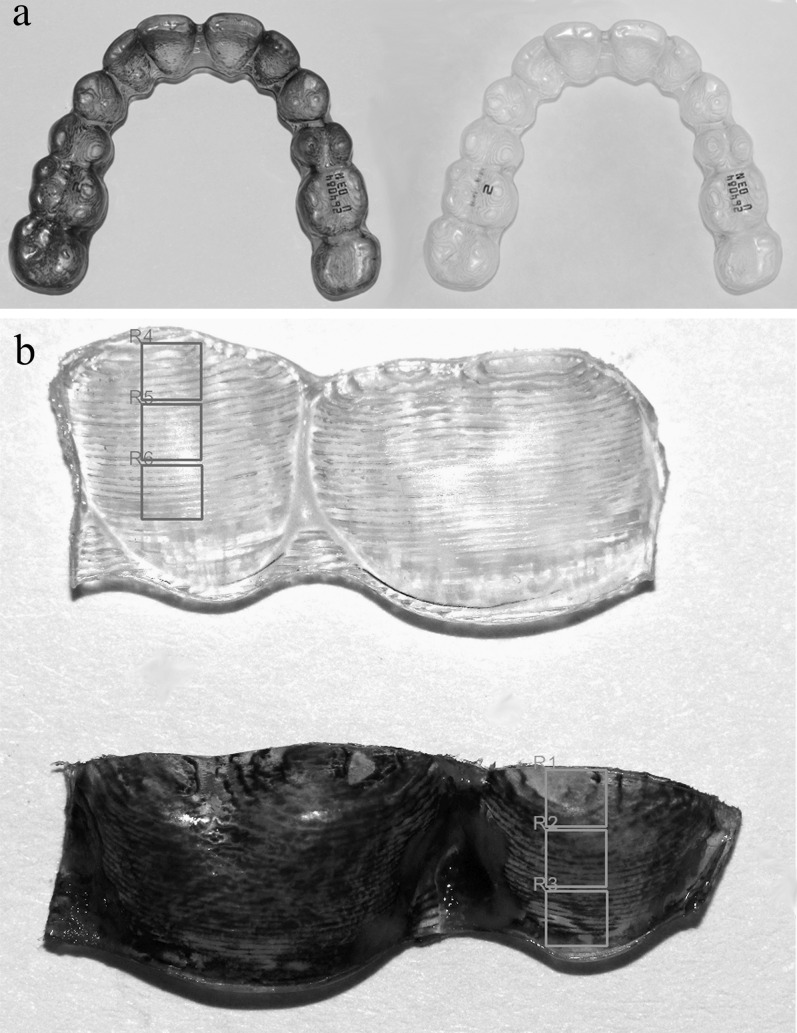
In a preliminary study, we found that bacterial biofilm adhesion to RTA can be measured using gentian violet staining, the intensity of which can be measured by a photodensitometer (Universal Hood II, BIO-RAD Laboratories, Segrate, Milan, Italy). Gentian violet stains the bacterial cell wall18 and leaves no residual color on a new, clean, unused aligner. Each of the 132 collected aligners was rinsed with water, air dried, and stained with 1% gentian violet for 5 minutes. An unstained, unused aligner served as reference to calibrate the background, which was subtracted from the photodensitometric measurements of the used stained aligner (Figure 2a).
Figure 2.
(a) Right, an unused unstained aligner. Left, used aligner after staining with gentian violet. R1–R6 are the sites that were scanned and measured in the TINA software. (b) Top, unused, unstained aligner's segment used to calibrate background noises. Bottom, a used aligner's segment. In each tooth, the three sites were delineated in the TINA software for photodensitometric measurements.
The stained aligners were cut into buccal and palatal flat segments of one to two dental units (Figure 2b). For each dental unit, six sites were measured by the photodensitometer. These sites were assigned as gingival, mid, and incisal sites, for the palatal or buccal sides.
The photodensitometer images of the buccal and palatal sites were analyzed using computerized software (TINA version 2.07d, PCBAS, Raytest Isotopenmellgerate, GmbH, Germany, 1993). Five representative teeth were examined: central incisor (1), lateral incisor (2), canine (3), second premolar (5), and first molar (6). The intensity of the stain was expressed as optical density per mm2 (arbitrary units; Figure 2b).
Statistical Analysis
Statistical analysis was carried out with SPSS software V16.0 (SPSS Inc, Chicago, Ill). The in vitro part of the study was examined with the Mann-Whitney test. The in vivo data were analyzed by analysis of variance with repeated measures for the five within-subject factors (treatment, tooth type, aligner side, tooth site, and time).
RESULTS
In Vitro Antibacterial Assay
For CHX, all positive control paper discs displayed zones of bacterial growth inhibition (Table 1). Only 4 of 25 aligner discs (16%) showed bacterial inhibition zones; however, they were more than twofold smaller than the positive control. No inhibition zones appeared around negative control discs.
Table 1.
Diameter of Bacterial Inhibition Zones (mm) for Paper Discs (Positive Controls) (a, c) and Saliva-Coated Aligner Discs Soaked in CHX (b) or CC (d)a
For the CS solution, no bacterial inhibition zones were obtained around any of the paper or aligner discs (Table 1).
Clinical Study
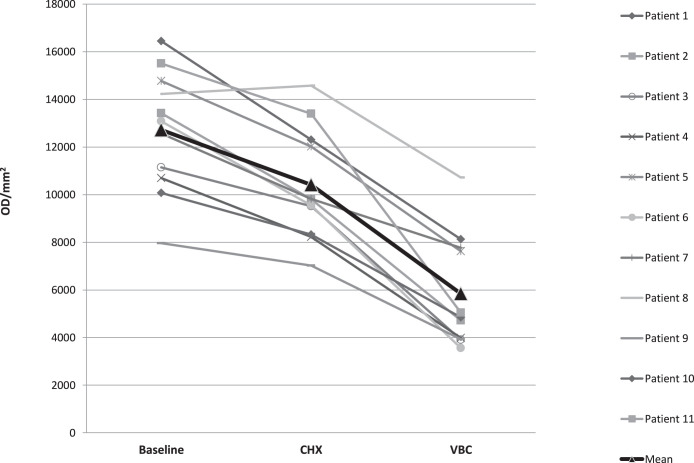
The mean optical density measurements for the baseline, CHX, and VBC aligners for each patient are presented in Figure 3.
Figure 3.
Mean adherent biofilm level in optical density per mm2 (OD/mm2) per patient, for each of the three treatment protocols: baseline (B), chlorhexidine (CHX), and vibrating bath with Cleaning-Crystals solution (VBC). The triangle bold curve represents the mean of all 11 patients.
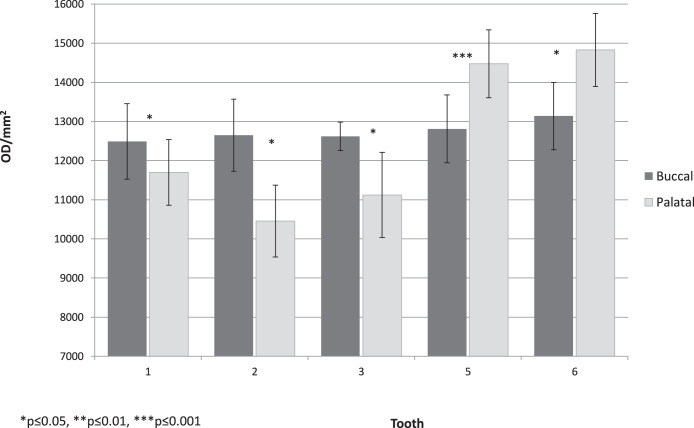
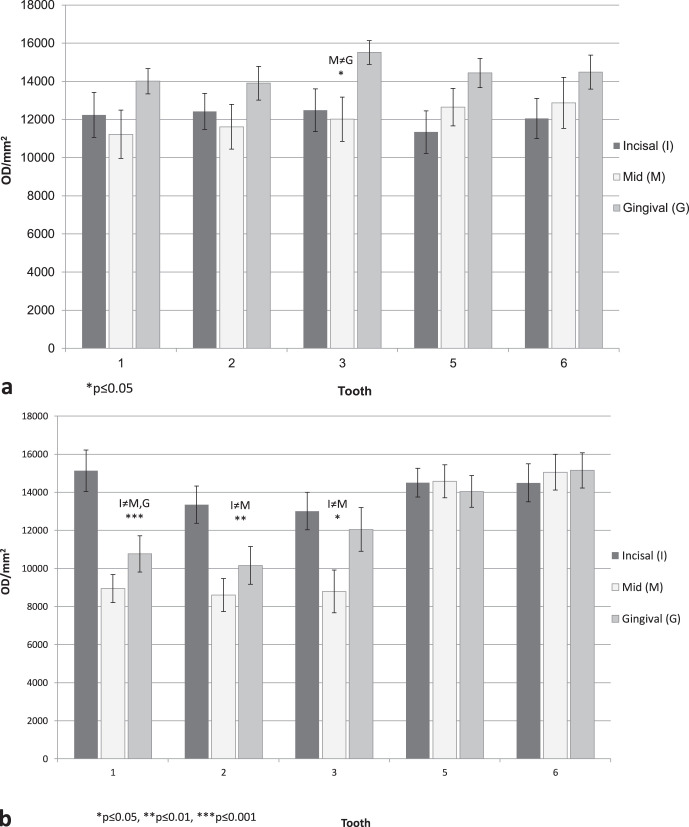
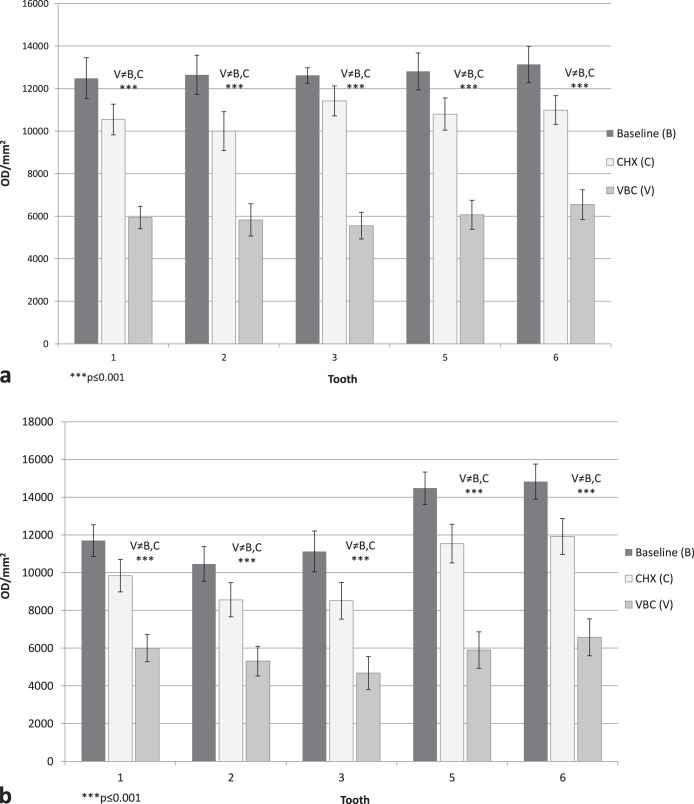
Per tooth, under regular oral hygiene (baseline aligners), there were statistically significant differences in optical density between the buccal and palatal sides (P < .001; Figure 4), with cleaner surfaces in the palatal sides. Statistically significant differences were also found between the three sites (incisal, middle, and gingival; P < .001; Figure 5), with much cleaner teeth in the middle site. Statistically significant differences can be seen between the three hygienic protocol groups at both sides of the aligners (P < .001; Figure 6).
Figure 4.
Baseline group, mean adherent biofilm level per tooth in optical density per mm2 (OD/mm2), buccal vs palatal side.
Figure 5.
(a) Baseline group, mean adherent biofilm level (OD/mm2) per site (incisal, middle, gingival) for the buccal side. (b) Baseline group, mean adherent biofilm level (OD/mm2) per site (incisal, middle, gingival) for the palatal side.
Figure 6.
(a) Mean adherent biofilm level in the three hygienic protocol groups (baseline [B], chlorhexidine [CHX], and vibrating bath with cleaning solution [VBC]) for the buccal side. (b) Mean adherent biofilm level in the three hygienic protocol groups (baseline [B], chlorhexidine [CHX], and vibrating bath with cleaning solution [VBC]) for the palatal side.
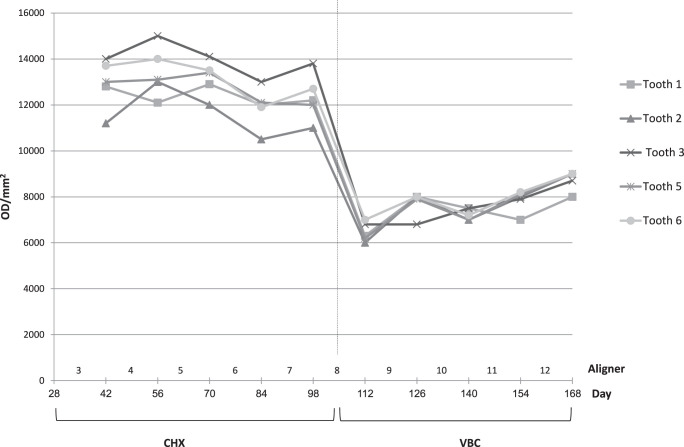
The effect of treatment progression (time) for the CHX and VBC protocols was examined in the buccogingival site in all teeth of all patients for 10 sequential aligners (5 CHX and 5 VBC; Figure 7). A significant difference in optical density was found between the two hygienic protocols (P < .001).
Figure 7.
Change over time in mean adherent biofilm level during sequential wear of 10 aligners (ie, 5 aligners treated with the CHX protocol, followed by 5 aligners treated with the VBC protocol).
DISCUSSION
In Vitro Antibacterial Assay
Despite bacterial growth inhibition found in paper discs with CHX, CHX demonstrated no such effect with aligner-material discs (Table 1). It was documented that the CHX molecule reacts with negatively charged groups on the cell surface, causing an irreversible loss of cytoplasmic constituents, membrane damage, and enzyme inhibition.19 It could be speculated that this effect was neutralized by the positive charge of the thermoplastic material. However, we could not find any evidence for the electric charge of thermoplastic material in the literature, suggesting that the main cause is due to no absorption of the CHX by the thermoplastic material.
For the CS solution, no bacterial inhibition zones were obtained around any of the paper positive control and the aligner discs (Table 1). Thus, it seems that this solution has no antibacterial effect against the tested bacteria.
In Vitro Clinical Study
Since the results of the in vitro study showed that the Cleaning-Crystals solution protocol did not have any inhibition effect (Table 1), it was not examined in the clinical trial per se. Therefore, the clinical trial included the CHX and the VBC hygiene protocols. Under regular tooth/aligner brushing, maximal bacterial biofilm adherence was recorded (Figure 3), which was more prone on the posterior region (premolar/molar) of the aligner than the aligner's anterior region (central and lateral incisors; Figure 4). This is in accordance with plaque accumulation on teeth per se.20 However, the aligner biofilm buildup differed from that of the dental arch by being more pronounced on the palatal side (Figure 5), in contrast to dental biofilm, which accumulated four to five times more on the buccal than the palatal side.20 This can be explained by the reduction in self-cleansing provided by the tongue and by the increased contour at the palatal gingival margin, leading to undercuts that are prone to contamination, as occurs in artificial crowns.21 Additional disparity is the extensive biofilm accumulation at the incisal edge of the anterior teeth on the aligner (Figure 6), in contrast to the dental arch, where the incisal edge is a self-cleansing area. Because of the funnel shape of the aligner, the incisal edge tends to collect and preserve all food debris remnants from the buccal and palatal crown surfaces.
VBC reduction of biofilm level was threefold higher than CHX in comparison to baseline (Figures 3 and 6), demonstrating the advantage in using the vibrating bath. This improvement is related to teeth, sides (palatal/buccal), and sites (especially the incisal). Although the aligner's biofilm was not investigated, we assume that during a 2-week period, it was partially transformed from softened plaque to calcified deposits,2,11 and for this reason, only the vibrating bath could remove the deposits. This effect was also demonstrated in the field of full dentures.22–24
The time effect on biofilm accumulation over the study period (Figure 7) demonstrated that each of the CHX or VBC hygienic protocols provided a fairly constant level of biofilm prevention over the 70 days of measurement (five consecutive aligners). That is, gradual deterioration in aligner maintenance over time did not occur. Moreover, the total maintenance time for CHX and VBC (140 days) suggests full patient compliance. Namely, patients are willing to spend extra hygiene time in order to preserve aligner transparency and prevent biofilm accumulation. It can be deduced from the results of Low et al.10 that the application of the VBC is highly recommended, especially in patients predisposed to fast plaque formation.
The results of the present study highly recommend using the vibrating bath with Cleaning-Crystal solution (VBC) for 15 minutes prior to bedtime as an aligner-cleansing protocol. Clean aligners comply with esthetic requirements and prevent gingivitis, enamel demineralization, and bad breath. VBC should be applied not only for a short-term RTA-wearing regimen (2-week replacement) but also for a long-term wearing regimen such as retainers, in which an increase in bacterial colonies is a serious issue.8 The accumulation of biofilm on the incisal edge of the aligner implies that failure in removing this deposit will result in inadequate three-dimensional coverage of the tooth by the aligner with the consequence of unaccomplished tooth movement. Moreover, with the latest introduction of sophisticated optimized attachments, prevention of biofilm deposition in the attachments' dimples is of major concern. Neglecting this requirement implies that intricate tooth movements such as root uprighting could be doomed to failure. In summary, the use of VBC counteracts oral hygiene deterioration, increases treatment performance, and improves esthetic requirements.
CONCLUSIONS
Thermoplastic material does not possess an adsorbing capacity of any of the tested hygienic solutions.
In contrast to biofilm buildup in the dental arch, in the aligner the posterior palatal side and the incisal edge of anterior teeth are more plaque accumulative under regular brushing with fluoride paste (1400 ppm).
Maximal reduction in biofilm adherence and accumulation to the posterior aligner (50%) was obtained when the vibrating bath was used in combination with the Cleaning-Crystals solution.
ACKNOWLEDGMENTS
This study is based on a thesis submitted as part of the requirements toward a Master's degree in orthodontics at Tel Aviv University. We wish to acknowledge the significant contributions of Ms Ilana Gelernter from the Department of Statistics, Tel Aviv University. This study was supported in part by a grant from Align Technology.
REFERENCES
- 1.Zhang N, Bai Y, Ding X, Zhang Y. Preparation and characterization of thermoplastic materials for invisible orthodontics. Dent Mater J. 2011;30:954–959. doi: 10.4012/dmj.2011-120. [DOI] [PubMed] [Google Scholar]
- 2.Schuster S, Eliades G, Zinelis S, Eliades T, Bradley TG. Structural conformation and leaching from in vitro aged and retrieved Invisalign appliances. Am J Orthod Dentofacial Orthop. 2004;126:725–728. doi: 10.1016/j.ajodo.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 3.Eliades T, Bourauel C. Intraoral aging of orthodontic materials: the picture we miss and its clinical relevance. Am J Orthod Dentofacial Orthop. 2005;127:403–412. doi: 10.1016/j.ajodo.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Kolenbrander PE, London J. Adhere today, here tomorrow: oral bacterial adherence. J Bacteriol. 1993;175:3247–3252. doi: 10.1128/jb.175.11.3247-3252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jendresen MD, Glantz PO. Clinical adhesiveness of selected dental materials: an in-vivo study. Acta Odontol Scand. 1981;39:39–45. doi: 10.3109/00016358109162257. [DOI] [PubMed] [Google Scholar]
- 6.Taylor R, Maryan C, Verran J. Retention of oral microorganisms on cobalt-chromium alloy and dental acrylic resin with different surface finishes. J Prosthet Dent. 1998;80:592–597. doi: 10.1016/s0022-3913(98)70037-x. [DOI] [PubMed] [Google Scholar]
- 7.Keevil CW, Bradshaw DJ, Dowsett AB, Feary TW. Microbial film formation: dental plaque deposition on acrylic tiles using continuous culture techniques. J Appl Bacteriol. 1987;62:129–138. doi: 10.1111/j.1365-2672.1987.tb02390.x. [DOI] [PubMed] [Google Scholar]
- 8.Türköz C, Canigür Bavbek N, Kale Varlik S, Akça G. Influence of thermoplastic retainers on Streptococcus mutans and Lactobacillus adhesion. Am J Orthod Dentofac Orthop. 2012;141:598–603. doi: 10.1016/j.ajodo.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 9.Zee KY, Samaranayake LP, Attstrom R. Predominant cultivable supragingival plaque in Chinese “rapid” and “slow” plaque formers. J Clin Periodontol. 1996;23:1025–1031. doi: 10.1111/j.1600-051x.1996.tb00532.x. [DOI] [PubMed] [Google Scholar]
- 10.Low B, Lee W, Seneviratne CJ, Samaranayake LP, Hägg U. Ultrastructure and morphology of biofilms on thermoplastic orthodontic appliances in ‘fast’ and ‘slow’ plaque formers. Eur J Orthod. 2011;33:577–583. doi: 10.1093/ejo/cjq126. [DOI] [PubMed] [Google Scholar]
- 11.Gracco A, Mazzoli A, Favoni O, et al. Short-term chemical and physical changes in Invisalign appliances. Aust Orthod J. 2009;25:34–40. [PubMed] [Google Scholar]
- 12.Ommerborn MA, Schneider C, Giraki M, et al. Effects of an occlusal splint compared with cognitive-behavioral treatment on sleep bruxism activity. Eur J Oral Sci. 2007;115:7–14. doi: 10.1111/j.1600-0722.2007.00417.x. [DOI] [PubMed] [Google Scholar]
- 13.Lang NP, Brecx MC. Chlorhexidine digluconate: an agent for chemical plaque control and prevention of gingival inflammation. J Periodontal Res Suppl. 1986;21(suppl S16):74–89. [Google Scholar]
- 14.Brecx MC, Macdonald LL, Legary K, Cheang M, Forgay MGE. Long-term effects of Meridol® and chlorhexidine mouthrinses on plaque, gingivitis, staining, and bacterial vitality. J Dent Res. 1993;72:1194–1197. doi: 10.1177/00220345930720080601. [DOI] [PubMed] [Google Scholar]
- 15.Nishi Y, Seto K, Kamashita Y, Kaji A, Kurono A, Nagaoka E. Survival of microorganisms on complete dentures following ultrasonic cleaning combined with immersion in peroxide-based cleanser solution. Gerodontology. doi: 10.1111/ger.12027. Published online: December 5, 2012 (doi:10.1111/ger.12027) [DOI] [PubMed] [Google Scholar]
- 16.Kozlovsky A, Artzi Z, Moses O, Kamin-Belsky N, Bar-Ness Greenstein R. Interaction of chlorhexidine with smooth and rough types of titanium surfaces. J Periodontol. 2006;77:1194–1200. doi: 10.1902/jop.2006.050401. [DOI] [PubMed] [Google Scholar]
- 17.Freitas LB, Vassilakos N, Arnebrant T. Interactions of chlorhexidine with salivary films adsorbed at solid/liquid and air/fluid interfaces. J Periodont Res. 1993;28:92–97. doi: 10.1111/j.1600-0765.1993.tb01055.x. [DOI] [PubMed] [Google Scholar]
- 18.Popescu A, Doyle RJ. The Gram stain after more than a century. Biotech Histochem. 1996;71:145–151. doi: 10.3109/10520299609117151. [DOI] [PubMed] [Google Scholar]
- 19.Hope CK, Wilson M. Analysis of the effects of chlorhexidine on oral biofilm vitality and structure based on viability profiling and an indicator of membrane integrity. Antimicrob Agents Chemother. 2004;48:1461–1468. doi: 10.1128/AAC.48.5.1461-1468.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohan N, Mahesh MR, Varghese VI, Pretty IA, Taylor AM, Ellwood RP. Evaluation of the sensitivity of a digital plaque imaging system on different tooth surfaces. J Clin Dent. 2012;23:11–16. [PubMed] [Google Scholar]
- 21.Parkinson CF. Excessive crown contours facilitate endemic plaque niches. J Prosthet Dent. 1976;35:424–429. doi: 10.1016/0022-3913(76)90010-x. [DOI] [PubMed] [Google Scholar]
- 22.Murata H, Chimori H, Hong G, Hamada T, Nikawa H. Compatibility of tissue conditioners and denture cleansers: influence on surface conditions. Dent Mater J. 2010;29:446–453. doi: 10.4012/dmj.2009-135. [DOI] [PubMed] [Google Scholar]
- 23.Zanatta FB, Antoniazzi RP, Rösing CK. The effect of 0.12% chlorhexidine gluconate rinsing on previously plaque-free and plaque-covered surfaces: a randomized, controlled clinical trial. J Periodontol. 2007;78:2127–2134. doi: 10.1902/jop.2007.070090. [DOI] [PubMed] [Google Scholar]
- 24.Tritten CB, Armitage GC. Comparison of a sonic and a manual toothbrush for efficacy in supragingival plaque removal and reduction of gingivitis. J Clin Periodontol. 1996;23:641–648. doi: 10.1111/j.1600-051x.1996.tb00588.x. [DOI] [PubMed] [Google Scholar]