Abstract
Background
Novel therapies have dramatically changed cystic fibrosis (CF) and innovative care delivery systems are needed to meet future patient needs. Telehealth has been shown to be an efficient and desirable form of care delivery. The COVID-19 pandemic caused a rapid shift to telehealth, and this presented a unique opportunity to study facilitators, barriers, and satisfaction with this mode of care delivery. We aim to report survey methods, demographics and telehealth use among CF care programs, patients, and families during the pandemic.
Methods
CF programs completed two surveys between July 29 and September 18, 2020, and between April 19 and May 19, 2021. Patients and families completed a similar survey between August 31 and October 30, 2020. The surveys addressed topics assessing the pandemic's financial impact, telehealth modes and experiences, licensure and reimbursement issues, health screening, and remote monitoring. Quantitative data were analyzed with descriptive statistics and were compared to the CF Foundation Patient Registry.
Results
Most programs (278 at timepoint one and 274 at timepoint two) provided telehealth during the pandemic. The percent of visits containing either telephone or video components changed from 45% to 25% over the time periods. Additionally, 424 patients and families from various ages and backgrounds responded to the survey and 81% reported having a telehealth visit.
Conclusions
The pandemic accelerated telehealth adoption and these datasets are a valuable source for exploring telehealth barriers and facilitators, the quality-of-care experience, financial and workforce implications, the impact on underrepresented populations, and implications for coverage and reimbursement.
Keywords: Cystic fibrosis, Telehealth, Methods
Abbreviations: CF, Cystic Fibrosis; CFF, Cystic Fibrosis Foundation; CFFPR, Cystic Fibrosis Foundation Patient Registry; CFTR, Cystic fibrosis transmembrane conductance regulator; PFSoC, Patient and Family CF State of Care Survey; PwCF, People with CF; SoC1, CF Care Program State of Care Survey Version 1; SoC2, CF Care Program State of Care Survey Version 2; TDI, The Dartmouth Institute for Health Policy & Clinical Practice
1. Background
Cystic fibrosis (CF) is an autosomal recessive, multisystem disease that affects over 34,000 children and adults in the US [1]. Approximately 80 to 85% of U.S. CF patients receive their care at one of 287 CF Foundation (CFF)-accredited care programs [1]. These programs, led by a physician program director and program coordinator, deliver highly specialized interdisciplinary care, enable basic and clinical research, and educate and train the next generation of clinicians [2].
Scientific advances, novel therapies, and the systematic practice of quality improvement have contributed to increases in quality of life and predicted median survival, dramatically shifting the US CF population to a greater number of adults than children [3]. The advent of modulator therapies that address the basic defect of CF have led to further improvements in outcomes with the belief and evidence that long-term sequelae are minimized [4,5]. This represents a momentous change in the approach to CF treatment. CF is also a costly and burdensome disease [6], and this coupled with people with CF (PwCF) living longer and fuller lives has led the CF community to acknowledge that the approach to CF care must change [7]. As individuals experience the disease differently, CF care needs to adapt to the future needs of PwCF while at the same time optimizing health [6]. Innovative care delivery systems, like telehealth, will be necessary to achieve more efficient and effective care.
Prior to the COVID-19 pandemic, the US health system, even for those delivering complex care, had begun to slowly pivot to telehealth [8]. Patients and clinicians have found that efficiencies of reduced travel and wait times and increases in real-time care coordination have outweighed technological challenges [9]. In U.S. CF care however, telehealth has not been used much and insufficient evidence exists that it improves clinical outcomes [10]. Internationally, telehealth use in CF has been more broadly adopted. In Australia, telehealth usage for rural and remote communities has been shown to have high patient satisfaction, to increase clinic attendance, and to increase the detection and treatment of pulmonary exacerbations [11].
The COVID-19 pandemic affected people of all age groups, but risk factors for more severe disease have been well documented in older patients and those with certain comorbidities, including CF [12], [13]. These factors caused most institutions to rapidly reorganize personnel and technology to accommodate telehealth to ensure care continuity [14], [15], [16]. This timely solution also provided institutions the opportunity to test hybrid care approaches as a possible longer-term solution for CF disease management [17]. Patients and families have embraced telehealth, particularly for its convenience and effectiveness in meeting their healthcare needs [18]. However, despite the accelerated uptake, many recommend exercising caution towards a permanent use of telehealth without first carefully considering the effects and adequacy of these changes [19].
The CFF and The Dartmouth Institute for Health Policy & Clinical Practice (TDI) developed a series of surveys to learn how telehealth was being implemented during this monumental shift in health care delivery. We aimed to characterize care team and patient and family perspectives on this new state of care as we looked to establish a new baseline with respect to telehealth.
Here we present the methods used for data collection and summarize key respondent demographics, and telehealth uptake during the survey period. Subsequent in-depth analyses are presented elsewhere in this supplement addressing such topics as facilitators and barriers to telehealth [20], the patient telehealth experience [21], the financial impact of telehealth and the COVID-19 pandemic [22], the experience of under-represented populations [23], and perspectives of remote monitoring [24].
2. Methods
2.1. Survey design
The CFF formed a cross-departmental team made up of clinical and policy staff to design three 15-minute surveys to ask clinical care teams and patients and their families about the state of CF care since the onset of the COVID-19 pandemic. The survey design went through an iterative process and sought input from members of the CF community including adult and pediatric pulmonologists, program directors, a program coordinator, a respiratory therapist, patients, and parents of people with CF. The Dartmouth Institute was engaged through a CFF grant to leverage their experience in evaluation science and to perform analyses. The CF Care Program State of Care Surveys (SoC1 and SoC2) and the Patient and Family CF State of Care Survey (PFSoC) had topics tailored to their specific stakeholders and included questions assessing the financial impact of the pandemic, modes and experiences with virtual care delivery, licensure and reimbursement issues, health screening, and remote monitoring. Surveys included field-defined and free-text response options. The surveys are available in Appendix 1.
2.2. Survey dissemination
SoC1 was distributed to 286 CF care programs between July 29 and September 18, 2020. All programs were required to complete this survey as a condition of their CFF accreditation. A second version of the SoC (SoC2) was released to CF care programs between April 19 and May 19, 2021, but participation was considered voluntary. SoC2’s primary focus was to assess significant changes since SoC1. We were interested in objective responses and due to the voluntary nature of SoC2, all open-ended questions were removed. Questions whose answers were not likely to have changed or were considered to be of lesser importance were also removed. Questions were added to collect details of the state of care in the context of COVID-19 and telehealth. Questions within each survey that did not change are highlighted and can be seen in Appendix 1.
Both surveys were hosted using a customized platform developed by the CFF and were available only to CF program leadership (i.e., program directors and program coordinators). The surveys required an electronic signature by the physician program director and were designed to only allow one response per program. Programs received several reminders during the intervals to complete the surveys.
The PFSoC survey was fielded between August 31 and October 30, 2020. Between August 31 and September 30, the survey was hosted on the same customized platform used to deploy the care program surveys. The CFF asked CF care programs to share the survey link with their patients and families. The CFF also directly emailed members of its patient and family advisory listserv and a group of patient and family volunteers that have expressed interest in shaping CFF programs. Between October 1 and October 30, the same survey was recreated and launched through SurveyMonkey to increase the response rate by removing the log-in requirements. The survey only permitted one response per user. The CFF announced the release of this second survey through its national newsletter and a paid Facebook advertisement. This study was approved by the Advarra Institutional Review Board for the Protection of Human Subjects (B. Marshall, Pro00045302).
2.3. Data Validation
The CF Foundation Patient Registry (CFFPR) is a large, national, observational, disease registry that captures longitudinal clinical and treatment data from approximately 80-85% of CF patients in the US [1]. The 2019 and 2020 CFFPR data were analyzed to complement the CF program (SoC1) and patient and family survey samples. The 2021 CFFPR database will not lock until March 2022. Given these data are incomplete, they have been excluded from the analysis.
2.4. Analysis
We used descriptive statistics (frequency distributions) to characterize SoC1 and SoC2 respondents, including CF program size, type, and geographic region. We used median and inter-quartile ranges to characterize percentages of visit by care delivery mode (in-person, phone-based telehealth, and video-based telehealth). We used a Wilcoxon Signed Rank Test to identify differences between SoC1 and SoC2 responses.
We used CFFPR data from October 2019 through December 2020 to complement survey responses on key variables within SoC1. Before May 8, 2020, the CFFPR captured the location of non-clinic encounters (i.e., telehealth visits) as “other.” After May 8, 2020, the location options were expanded to include “by phone” and “by phone/computer with video.” To compare pre- and post-pandemic encounters, all visits not listed as either a clinic encounter or hospitalization were grouped as non-clinic visits. Hospitalizations were excluded from this analysis as telehealth is not a standard-of-care option for these visit types.
We used descriptive statistics to characterize PFSoC respondent characteristics between survey distribution modes (CFF customized platform; Survey Monkey) and with the 2020 CFFPR database. Chi square tests were used to compare differences in geographic distribution, age, gender, race/ethnicity, insurance type, and those taking a cystic fibrosis transmembrane conductance regulator (CFTR) modulator. We used a two-sided significance test and a P-value threshold of <0.05 to identify significant differences. Quantitative analyses were conducted using SPSS version 26. Missing data were excluded on an analysis-by-analysis basis.
3. Results
3.1. CF Care Program State of Care Survey
Except for one pediatric program, all accredited programs completed SoC1 survey (n=286). All but seven programs completed SoC2 (n=280). CFF accredited programs are located throughout the US. Table 1 shows the breakdown by geographic distribution, program type and program size. The median size of a CF care program is 80 patients (IQR: 53-134).
Table 1.
Characteristics of SoC1 Respondents.
| CF | n | % |
|---|---|---|
| Program Type | ||
| Adult | 118 | 41.3 |
| Pediatric | 128 | 44.8 |
| Affiliate | 40 | 14.0 |
| Geographic Distribution | ||
| Midwest1 | 71 | 24.8 |
| Northeast2 | 69 | 24.1 |
| Southeast3 | 69 | 24.1 |
| Southwest4 | 27 | 9.4 |
| West5 | 50 | 17.5 |
| Program Size | ||
| Small: 0-70 patients | 113 | 39.5 |
| Medium: 71-140 patients | 105 | 36.7 |
| Large: 141+ patients | 68 | 23.8 |
IA, IL, IN, KS, MI, MN, MO, ND, NE, OH, SD, WI
CT, DC, DE, MA, MD, ME, NH, NJ, NY, PA, RI, VT
AL, AR, FL, GA, KY, LA, MS, NC, SC, TN, VA, WV
AZ, NM, OK, TX
AK, CA, CO, HI, ID, MT, NV, OR, UT, WA, WY
At the time of SoC1, almost all CF programs were actively providing telehealth services (Table 2 ). All programs in the West and Southwest were actively providing telehealth while four programs in the Midwest and one in the Southeast provided telehealth earlier in the pandemic but switched back to solely in-person care. Two programs in the Midwest and one program in the Northeast did not offer telehealth services to its patients and families. The proportions were similar in SoC2.
Table 2.
State of telehealth delivery by region at two timepoints (August/September 2020 and May 2021)
| State of Care 11 (July 29 - September 18, 2020) | State of Care 22 (April 19 - May 19, 2021) | |||
|---|---|---|---|---|
| Region3 | Currently providing telehealth services | Not currently providing telehealth services | Currently providing telehealth services | Not currently providing telehealth services |
| Midwest | 65 (91.5%) | 6 (8.4%) | 66 (95.7%) | 3 (4.3%) |
| Northeast | 68 (98.6%) | 1 (1.4%) | 65 (97.0%) | 2 (3.0%) |
| Southeast | 68 (98.6) | 1 (1.4%) | 68 (100%) | 0 (0%) |
| Southwest | 27 (100%) | 0 (0%) | 27 (100%) | 0 (0%) |
| West | 50 (100%) | 0 (0%) | 48 (98.0%) | 1 (2.0%) |
| Total | 278 (97.2%) | 8 (2.8%) | 274 (97.9%) | 6 (2.1%) |
Survey completed by 286 programs.
Survey completed by 280 programs.
Midwest - IA, IL, IN, KS, MI, MN, MO, ND, NE, OH, SD, WI
Northeast - CT, DC, DE, MA, MD, ME, NH, NJ, NY, PA, RI, T VT
Southeast - AL, AR, FL, GA, KY, LA, MS, NC, SC, TN, VA, WV
Southwest - AZ, NM, OK, TX.
West - AK, CA, CO, HI, ID, MT, NV, OR, UT, WA, WY.
Table 3 shows the percentage of visits by care delivery mode for programs that were actively providing telehealth services at the time of each survey administration. The proportion of visits offered by telehealth (video or phone only) was substantially greater during August/September 2020, compared to May 2021. By May 2021, programs providing telehealth estimated that a median of 80% of visits were held in person, whereas only 57% of visits were held in person in August/September 2020.
Table 3.
Percentage of visits by mode of care delivery by survey timepoint, among those actively providing telehealth at the time of the survey.
| SoC1 (n=278) Median (IQR) | SoC2 (n=274) Median (IQR) | Wilcoxon Signed Rank Test | |
|---|---|---|---|
| Phone | 0% (0-10%) | 0% (0-1%) | < 0.001 |
| Phone/computer with video | 30% (10-60%) | 15% (5-30%) | < 0.001 |
| In-person | 57% (25-86.25%) | 80% (64.75-95%) | < 0.001 |
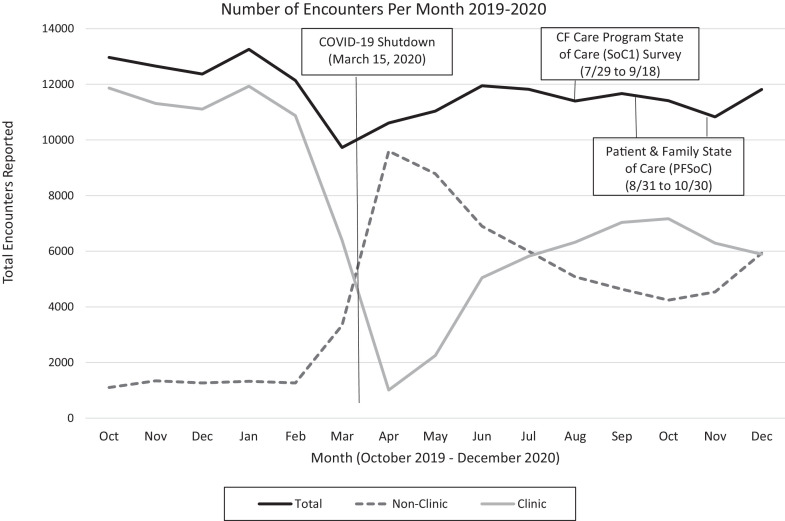
Data derived from the SoC surveys aligns with those captured in the CFFPR. Figure 1 shows the rapid uptake of telehealth coinciding with the COVID-19 shutdown and telehealth visits exceeded in-person visits between mid-March and July. In August, 55% of CFFPR encounters were in-person which matches the mean self-reported data from SoC1.
Fig. 1.
Number of Encounters by Visit Type Prior to the COVID-19 Pandemic (October 2019-March 2020) and During the Pandemic (March 2020 – December 2020)
Data source: CF Foundation Patient Registry, CF Foundation, Bethesda, MD, USA
1Non-Clinic: Visits coded as “other” prior to May 8, 2020, and “by phone” or “by phone/computer with video” after May 8, 2020.
2Clinic: Non-hospitalization, in-office visits.
3.2. Patient and Family CF State of Care Survey
The 2020 PFSoC survey was completed by 424 respondents, including 222 adults with CF (52%), 201 parents of a child with CF (47%), and one unidentified individual. Parents were asked to provide responses on behalf of their child. Nearly half (46%, n = 197) of respondents completed the survey using the CFF's customized platform, while 54% (n = 227) used SurveyMonkey. There were no significant differences in any demographic category between survey modes.
Demographic information was provided by a subset of respondents (Table 4 ). Respondents were distributed throughout the United States, with roughly one-fifth in each of the Midwest, Northeast, and Southeast, 28% in the West, and 10% in the Southwest. Patient age was widely distributed and nearly three-fifths (58%) were female. Nearly 88% of PwCF were white, with 6% Hispanic / Latinx and 2% African American / Black. In aggregate, 10% (n=35) identified as being a member of any racial/ethnic minority group. Private insurance was the most common form of insurance (62%), followed by Medicaid or state programs (20%), and Medicare (11%). Most PwCF were taking a CFTR modulator (71%) although adult PwCF were more likely to report taking a CFTR modulator than parents of children with CF (80% vs. 61%). Four-fifths (81%) of survey respondents reported having a telehealth visit at some point during the pandemic.
Table 4.
Demographics of the patient/family state of care survey.
| PFSoC (n=424) n2 (%) | PFSoC Had a telehealth visit n3 | CFFPR 4 (n=32,095) n (%) | Comparison of PFSoC to CFFPR (p-value) | |
|---|---|---|---|---|
| Geographic distribution (n=340)1 | ||||
| Midwest | 68 (20.0%) | 52 | 8,413 (26.2%) | 0.01 |
| Northeast | 70 (20.6%) | 62 | 6,598 (20.6%) | |
| Southeast | 75 (22.1%) | 65 | 8,011 (25.0%) | |
| Southwest | 33 (9.7%) | 31 | 3,209 (10.0%) | |
| West | 94 (27.6%) | 73 | 5,864 (18.3%) | <.001 |
| Age (n=342)1 | ||||
| Less than 2 years | 16 (4.7%) | 15 | 1,142 (3.6%) | |
| 2-5 years | 30 (8.8%) | 26 | 3,164 (9.9%) | |
| 6-10 years | 47 (13.7%) | 35 | 4,166 (13.0%) | |
| 11-17 years | 55 (16.1%) | 45 | 5,837 (18.2%) | |
| 18-24 years | 31 (9.1%) | 27 | 5,346 (16.7%) | <.001 |
| 25-34 years | 54 (15.8%) | 45 | 5,892 (18.4%) | |
| 35-44 years | 37 (10.9%) | 32 | 3,453 (10.8%) | |
| 45+ years | 72 (21.1%) | 59 | 3,095 (9.6%) | <.001 |
| Gender (% Female) (n=334)1 | 196 (58.7%) | 164 | 15,486 (48.3%) | <.001 |
| Race / ethnicity (n=347)1 | ||||
| White | 307 (88.5%) | 261 | 27,354 (85.2%) | |
| Hispanic/Latinx | 22 (6.3%) | 12 | 3,049 (9.5%) | |
| Prefer not to answer | 9 (2.6%) | 8 | N/A | |
| African-American/Black | 8 (2.3%) | 7 | 1,102 (3.4%) | |
| Other | 5 (1.4%) | 5 | 590 (1.8%) | |
| Insurance type (n=347)1 | ||||
| Private health insurance policy | 247 (71.4%) | 212 | 18,838 (58.7%) | <.001 |
| Medicaid/state programs | 82 (23.7%) | 66 | 13,318 (41.5%) | <.001 |
| Medicare | 46 (13.3%) | 38 | 3,285 (10.2%) | |
| Other | 12 (3.5%) | 10 | 491 (1.5%) | <.001 |
| TriCare / other military health plan | 10 (2.9%) | 10 | 768 (2.4%) | |
| Prefer not to answer | 4 (1.2%) | 3 | N/A | |
| No insurance | 1 (0.3%) | 1 | 275 (0.9%) | |
| Taking a CFTR-modulator (% Yes) (n=347)1 | 248 (71.5%) | 205 | 20,897 (66.0%) | 0.032 |
| Missing data | - | - | 438 |
Within each demographic variable, the rows sum to 100% of the population with available data.
Demographic survey responses were not required fields. Some respondents chose not to answer these questions and therefore, demographic categories do not always add up the total number of survey responses due to missing data. Data specifically refers to the characteristics of the PwCF regardless of whether the respondent was an adult with CF or a parent of a child with CF.
The number of patients by each demographic variable that responded to the PFSoC question that they had experienced a telehealth visit.
The CFFPR sample includes all active patients (n=32,095) that had an annual case report form completed in 2020.
Compared to participants in the CFFPR (Table 4), survey responses reflected a greater percentage of female PwCF (59% vs. 48%); were less likely to be age 18-24 (9% vs. 17%) and more likely to be over 45 years of age (21% vs 10%). PwCF were less likely to be from the Midwest (20% vs. 26%) and more likely to be from the West (28% vs. 18%); and less likely to have Medicaid / state insurance (24% vs. 42%) and more likely to have private insurance (71% vs. 59%) or other insurance (3.5% vs. 1.5%).
4. Discussion
We described the demographic data derived from three large, national datasets (SoC1, SoC2, PFSoC) that inform us about telehealth uptake during the COVID-19 pandemic. Two datasets, eight months apart, show that almost all care programs provided some level of telehealth services throughout the pandemic. While a few programs discontinued these services as local pandemic conditions changed, most continued to provide telehealth throughout the survey periods. Early on, there was a large proportion of patient visits provided via telehealth (44.5%) that decreased (24.7%) just eight months later. Data from the CFFPR confirm the rapid uptake of telehealth across the care center network and closely matches the self-reported data from SoC1.
Perkins et. al, collected similar survey data from 80 clinicians at seven US programs aimed at characterizing their telehealth usage, experiences, and preferences. They showed that almost all those surveyed had no prior experience with telehealth prior to the pandemic and that by June 2020 (the survey period), 65 percent of respondents had completed more than 10 visits via telehealth [25]. While not a direct comparison to our data, the results are consistent with the rapid uptake reflected in the CFFPR and the relatively high percentage of telehealth visits reported in SoC1. To our knowledge, this is the first national study assessing the uptake and clinician experiences with telehealth during the COVID-19 pandemic.
Our third dataset, collected from 424 patients and families in October 2020, showed that over 81 percent of respondents reported at least one telehealth visit since the start of the COVID-19 pandemic. The respondents were well distributed across the United States and included patients from various backgrounds. To our knowledge, Davis et al. is the only other similar study to capture patient and family perceptions of telehealth during the pandemic [18]. While our study is only slightly larger, we collected granular demographic data and information from patients that did not experience telehealth which will allow for sub-analyses, like those by Albon et. al, in this supplement.
All three datasets have their limitations. The PFSoC survey is a relatively small sample size. When compared to a more generalizable dataset, the PFSoC respondents were biased towards females, slightly older (45+ years of age) patients, and those with private insurance. Additionally, the PFSoC survey was a voluntary sample which may select for individuals with a particularly strong opinion or who had more time to devote to this topic. SoC1 is the most comprehensive dataset due to the requirements to complete the survey. SoC2 on the other hand, was not required and as such, seven programs are missing from the dataset. While both surveys required the physician program director to sign off prior to submission, we are unable to differentiate which responses were provided by the physician director, the program coordinator, or both. Each individual may bring different perspectives.
While telehealth visits showed a substantial decrease during our survey periods, the great majority of CF care programs are still providing some level of telehealth services. This may indicate that telehealth is likely to become a more mainstream care delivery option, particularly for preventative and chronic care disease management and for those on modulator therapies. The survey data presented is the first comprehensive characterization of the rapid uptake of telehealth from both a clinician and patient and family perspective. Despite the noted limitations, these datasets are a valuable source to explore barriers and facilitators to telehealth care, financial issues associated with telehealth, and the impact telehealth might have on underrepresented populations.
Funding sources
This work was supported by the Cystic Fibrosis Foundation. A grant was also provided by the Cystic Fibrosis Foundation to Eugene Nelson at The Dartmouth Institute for Health Policy and Clinical Practice (NELSON20QI0) that supported Aricca D. Van Citters.
Declaration of Competing Interest
None.
CRediT author statement
Christopher Dowd: Conceptualization, Methodology, Validation, Writing – original draft. Aricca D. Van Citters: Methodology, Formal analysis, Data curation, Visualization, Writing – review & editing. Olivia Dieni: Conceptualization, Methodology, Writing review & editing. Anne Willis: Conceptualization, Methodology, Writing – review & editing. Leslie Powell: Conceptualization, Methodology. Kathryn A. Sabadosa: Conceptualization, Methodology, Writing – review & editing.
Acknowledgement
We are grateful to the entire Care Center Network and to all the people with CF and their families that took the time to respond to our survey. We would also like to thank the CF Foundation Communications and Community Partnership departments for assisting us in the outreach to the patient and family community.
Footnotes
This paper is part of a Supplement supported by the Cystic Fibrosis Foundation.
Supplementary data associated with this article can be found, in the online version, at 10.1016/j.jcf.2021.08.028.
Appendix A. Supplementary materials
References
- 1.Knapp E.A., Fink A.K., Goss C.H., Sewall A., Ostrenga J., Dowd C., et al. The Cystic Fibrosis Foundation Patient Registry. Design and Methods of a National Observational Disease Registry. Ann Am Thorac Soc. 2016;13(7):1173–1179. doi: 10.1513/AnnalsATS.201511-781OC. [DOI] [PubMed] [Google Scholar]
- 2.Mogayzel P.J., Jr., Dunitz J., Marrow L.C., Hazle L.A. Improving chronic care delivery and outcomes: the impact of the cystic fibrosis Care Center Network. BMJ Qual Saf. 2014;23(Suppl 1):i3–i8. doi: 10.1136/bmjqs-2013-002363. [DOI] [PubMed] [Google Scholar]
- 3.Marshall B.C., Nelson EC. Accelerating implementation of biomedical research advances: critical elements of a successful 10 year Cystic Fibrosis Foundation healthcare delivery improvement initiative. BMJ Qual Saf. 2014;23(Suppl 1):i95–i103. doi: 10.1136/bmjqs-2013-002790. [DOI] [PubMed] [Google Scholar]
- 4.Rowe S.M., Heltshe S.L., Gonska T., Donaldson S.H., Borowitz D., Gelfond D., et al. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med. 2014;190(2):175–184. doi: 10.1164/rccm.201404-0703OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. Food and Drug Administration. FDA approves new breakthrough therapy for cystic fibrosis: U.S. Food and Drug Administration; 2019 Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-new-breakthrough-therapy-cystic-fibrosis.
- 6.Grosse S.D., Do T.Q.N., Vu M., Feng L.B., Berry J.G., Sawicki GS. Healthcare expenditures for privately insured US patients with cystic fibrosis, 2010-2016. Pediatr Pulmonol. 2018;53(12):1611–1618. doi: 10.1002/ppul.24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cystic Fibrosis Foundation. Our Strategic Plan: Care [cited 2021 May]. Available from: https://www.cff.org/About-Us/Reports-and-Financials/Our-Strategic-Plan/Care/].
- 8.Smith A.C., Thomas E., Snoswell C.L., Haydon H., Mehrotra A., Clemensen J., et al. Telehealth for global emergencies: Implications for coronavirus disease 2019 (COVID-19) J Telemed Telecare. 2020;26(5):309–313. doi: 10.1177/1357633X20916567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Appleman E.R., O'Connor M.K., Rockefeller W., Morin P., Moo L.R. Using Video Telehealth to Deliver Patient-Centered Collaborative Care: The G-IMPACT Pilot. Clin Gerontol. 2020:1–10. doi: 10.1080/07317115.2020.1738000. [DOI] [PubMed] [Google Scholar]
- 10.Cox N.S., Alison J.A., Rasekaba T., Holland AE. Telehealth in cystic fibrosis: a systematic review. J Telemed Telecare. 2012;18(2):72–78. doi: 10.1258/jtt.2011.110705. [DOI] [PubMed] [Google Scholar]
- 11.Wood J., Mulrennan S., Hill K., Cecins N., Morey S., Jenkins S. Telehealth clinics increase access to care for adults with cystic fibrosis living in rural and remote Western Australia. J Telemed Telecare. 2017;23(7):673–679. doi: 10.1177/1357633X16660646. [DOI] [PubMed] [Google Scholar]
- 12.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA internal medicine. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Coronavirus [Available from: https://www.who.int/health-topics/coronavirus#tab=tab_1.
- 14.Brody A.A., Sadarangani T., Jones T.M., Convery K., Groom L., Bristol A.A., et al. Family- and Person-Centered Interdisciplinary Telehealth: Policy and Practice Implications Following Onset of the COVID-19 Pandemic. J Gerontol Nurs. 2020;46(9):9–13. doi: 10.3928/00989134-20200811-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paterson C., Bacon R., Dwyer R., Morrison K.S., Toohey K., O'Dea A., et al. The Role of Telehealth During the COVID-19 Pandemic Across the Interdisciplinary Cancer Team: Implications for Practice. Semin Oncol Nurs. 2020;36(6) doi: 10.1016/j.soncn.2020.151090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasan S., Lansang M.C., Salman Khan M., Dasenbrook E. Managing Cystic Fibrosis related diabetes via telehealth during COVID-19 pandemic. J Clin Transl Endocrinol. 2021;23 doi: 10.1016/j.jcte.2021.100253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.List R., Compton M., Soper M., Bruschwein H., Gettle L., Bailey M., et al. Preserving Multidisciplinary Care Model and Patient Safety During Reopening of Ambulatory Cystic Fibrosis Clinic for Nonurgent Care: A Hybrid Telehealth Model. Telemed J E Health. 2021;27(2):193–199. doi: 10.1089/tmj.2020.0247. [DOI] [PubMed] [Google Scholar]
- 18.Jaclyn D., Andrew N., Ryan P., Julianna B., Christopher S., Nauman C., et al. Patient and family perceptions of telehealth as part of the cystic fibrosis care model during COVID-19. J Cyst Fibros. 2021 doi: 10.1016/j.jcf.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies J. The coronavirus pandemic has forced rapid changes in care protocols for cystic fibrosis. Nature. 2020;583(7818):S15. doi: 10.1038/d41586-020-02112-y. [DOI] [PubMed] [Google Scholar]
- 20.Gifford A.H., Ong T., Dowd C., Van Citters A.D., Scalia P., Sabadosa K.A., et al. Evaluating barriers and promotors of telehealth during the COVID-19 pandemic at cystic fibrosis programs to inform new models of CF care. J Cyst Fibros. 2021;20(S3):S14–S15. doi: 10.1016/j.jcf.2021.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solomon G.M., Bailey J., Lawlor J., Scalia P., Sawicki G.S., Dowd C., et al. Patientand family experience of telehealth care delivery as part of the CF chronic caremodel early in the COVID-19 pandemic. J Cyst Fibros. 2021;20(S3):S41–S46. doi: 10.1016/j.jcf.2021.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawicki G.S., Van Citters A.D., Dieni O., Powell L., Sabadosa K.A., Willis A., et al. Financial impacts of the COVID-19 pandemic on cystic fibrosis care: lessons forthe future. J Cyst Fibros. 2021;20(S3):S16–S20. doi: 10.1016/j.jcf.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albon D., Van Citters A.D., Ong T., Dieni O., Dowd C., Willis A., et al. Telehealthuse in the cystic fibrosis community during the COVID-19 pandemic: association with race, ethnicity, and socioeconomic factors. J Cyst Fibros. 2021;20(S3):S49–S54. doi: 10.1016/j.jcf.2021.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ong T., Van Citters A.D., Dowd C., Fullmer J., List R., Pai S.A., et al. Remote monitoring in telehealth care delivery across the U.S. cystic fibrosis care network. J Cyst Fibros. 2021;20(S3):S57–S63. doi: 10.1016/j.jcf.2021.08.035. [DOI] [PubMed] [Google Scholar]
- 25.Perkins R.C., Davis J., NeSmith A., Bailey J., Powers M.R., Chaudary N., et al. Favorable Clinician Acceptability of Telehealth as Part of the Cystic Fibrosis Care Model during the COVID-19 Pandemic. Ann Am Thorac Soc. 2021 doi: 10.1513/AnnalsATS.202012-1484RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.