Abstract
Purpose
Describe the differences in characteristics and outcomes between COVID-19 and other viral pneumonia patients admitted to Dutch ICUs.
Materials and methods
Data from the National-Intensive-Care-Evaluation-registry of COVID-19 patients admitted between February 15th and January 1th 2021 and other viral pneumonia patients admitted between January 1st 2017 and January 1st 2020 were used. Patients' characteristics, the unadjusted, and adjusted in-hospital mortality were compared.
Results
6343 COVID-19 and 2256 other viral pneumonia patients from 79 ICUs were included. The COVID-19 patients included more male (71.3 vs 49.8%), had a higher Body-Mass-Index (28.1 vs 25.5), less comorbidities (42.2 vs 72.7%), and a prolonged hospital length of stay (19 vs 9 days). The COVID-19 patients had a significantly higher crude in-hospital mortality rate (Odds ratio (OR) = 1.80), after adjustment for patient characteristics and ICU occupancy rate the OR was respectively 3.62 and 3.58.
Conclusion
Higher mortality among COVID-19 patients could not be explained by patient characteristics and higher ICU occupancy rates, indicating that COVID-19 is more severe compared to other viral pneumonia. Our findings confirm earlier warnings of a high need of ICU capacity and high mortality rates among relatively healthy COVID-19 patients as this may lead to a higher mental workload for the staff.
Keywords: COVID-19, Coronavirus, Pneumonia, Mortality, Outcome, Intensive care, Critical care
1. Introduction
Since the outbreak of the COVID-19 pandemic, healthcare professionals warned for the rapid spread and severity of this disease. Their main concerns included the higher need of hospital and ICU capacity and excess mortality rates [1,2]. During the pandemic we saw that the COVID-19 patients share symptoms with other viral pneumonia patients occurring in seasonal influenza [3]. Higgins TL et al. showed that the severity-adjusted mortality and length of stay are higher for coronavirus disease 2019 patients than for viral pneumonia patients admitted to ICUs in the USA [4]. Richards-Belle et al. described the COVID-19 patients across England, Wales and Northern Ireland and showed that the mortality of COVID-19 patients was 43.0%, which exceeded the mortality of other viral pneumonia patients (24.7%) [5]. Similarly, a German study of Ludwig et al. reported a higher in-hospital mortality and worse clinical outcomes among COVID-19 patients compared to those admitted due to influenza. This difference was not attributable to demographic characteristics and pre-existing comorbidities. They also stated that this difference was not attributable to patient triage due to lack of ICU capacity since the German healthcare system had not reached its limits in the pandemic [6]. Wilde et al. showed that an increasing occupancy of beds compatible with mechanical ventilation is associated with a higher mortality risk for individuals admitted to ICU [7], thus the higher bed utilization during the COVID-19 pandemic and associated higher workload for the medical staff may have an unfavorable influence on the outcome. It is hard to compare international studies due to the considerably differences in sample size, included patient population, and follow-up period. Furthermore, there is no standardized reporting across countries; some experiencing stress on available (ICU) beds, others do not. As a consequence, different mortality rates ranging from 24% to 67% among COVID-19 patients admitted to the ICU were reported [2,[4], [5], [6],[8], [9], [10]].
Since the first confirmed case in the Netherlands on February 15th 2020 the National Intensive Care Evaluation (NICE) registry collected data on all COVID-19 cases admitted to Dutch ICUs. This made it possible to analyze all COVID-19 patients admitted to the ICU from an entire healthcare system including its stress on bed occupancy, which would make international comparisons more meaningful. The aim of this study is to describe and compare the clinical characteristics and outcomes between COVID-19 patients and patients with other community-acquired viral pneumonias admitted to Dutch ICUs. As COVID-19 also shares symptoms with non-COVID-19 acute respiratory distress syndrome (ARDS) [11,12] and pulmonary sepsis [13], an additional analysis compared the clinical characteristics and outcomes of COVID-19 patients with those of ARDS and pulmonary sepsis patients.
2. Methods
2.1. Study design
This study uses prospectively collected data from the Dutch National Intensive Care Evaluation (NICE) registry which contains, among others, demographic, physiological, clinical, ICU-, and in-hospital mortality data of all consecutive patients admitted to all Dutch ICUs [14]. ICUs extract routinely collected data from their electronic health records (EHR) on a monthly basis and upload this to the NICE database (NICE-DB). During the COVID-19 pandemic all COVID-19 patients admitted to the ICU were online registered in the COVID-Database (COVID-DB) of NICE on a daily basis to monitor the course of the outbreak in real-time [15]. Patients were considered to have COVID-19 when the RT-PCR of their respiratory secretions was positive for SARS-CoV-2 or when their CT-scan was consistent with COVID-19 (i.e. a COVID-19 Reporting and Data System (CO-RADS) score of ≥4 indicating a high suspicion on COVID-19 in combination with the absence of an alternative diagnosis) [16,17]. To enrich the COVID-DB with more comprehensive clinical data this COVID-DB was linked to the NICE-DB, to enable analyses of all Dutch critically ill COVID-19 patients. In this study we used the linked data of the patients with confirmed COVID-19 admitted to the ICU between February 15th 2020 and January 1st 2021. All these COVID-19 patients were followed until hospital discharge. COVID-19 patients who were transferred between hospitals were followed across the different hospitals and the information of the consecutive ICU admissions in all hospitals were combined leading to one record with the in-hospital mortality of the last hospital in which the patient was admitted.
The patient characteristics and outcomes of the linked COVID-19 patients admitted to the ICU were compared with other community-acquired viral pneumonia patients admitted to the ICU between January 1st 2017 and January 1st 2020. To select these patients from the NICE-DB, we used the Acute Physiology and Chronic Health Evaluation (APACHE)-IV diagnosis category “viral pneumonia” [18]. This reason for ICU admission includes patients with influenza, other respiratory viruses but also patients with opportunistic viral infections (e.g. CMV pneumonitis in neutropenic patients). In order to exclude these opportunistic or hospital acquired viral pneumonias we restricted the control group to patients with a maximum length of stay in the hospital of three calendar days prior to ICU admission. For readability reasons we call this group ‘other viral pneumonia patients’. In additional analyses the patient characteristics and outcome of COVID-19 patients were also compared with pulmonary sepsis (APACHE-IV diagnosis: “pulmonary sepsis”) and acute respiratory distress syndrome (ARDS, APACHE-IV diagnosis: “non-cardiogenic pulmonary edema ARDS-adult respiratory distress syndrome”) patients admitted to the ICU between January 1st 2017 and January 1st 2020.
The Institutional Research Board of the Amsterdam University Medical Centre reviewed the research proposal and waived the need for informed consent (IRB protocol W20_260 # 20·295). The full study protocol has been published on the NICE website on forehand [19].
2.2. Statistical analyses
The COVID-19 patients from the COVID-DB that could be linked to the NICE-DB and those who could not be linked were compared regarding hospital characteristics (university, teaching, or non-teaching), age, and mortality rates to evaluate the possibility of biased results. Thereafter, patient characteristics of the linked COVID-19 patients and of the other viral pneumonia patients admitted to the ICU were compared. Differences between the cohorts were tested with a Chi-square test for categorical variables, and a non-parametric Wilcoxon test for continuous variables.
The crude in-hospital mortality among COVID-19 patients was compared to the in-hospital mortality among other viral pneumonia patients using the odds ratio estimated in a logistic regression model. To correct for important clinical differences between the two cohorts that may confound the association between the cohort and observed in-hospital mortality, the logistic regression model was expanded with adjustment for important patient characteristics: age (categorized in 11 age groups), gender, Body Mass Index (BMI) (categorized in the six internationally defined BMI groups), comorbidities present before hospitalization (immunological insufficiency, chronic renal failure, chronic respirator insufficiency or COPD, chronic cardiovascular insufficiency, cirrhosis, malignancy, and diabetes), the acute physiology score of the APACHE-III prognostic model (APACHE III APS) to describe the severity of physiological disturbance in the first 24 h of ICU admission (categorized in five groups based on quintiles of the APACHE III APS), and mechanical ventilation in the first 24 h of ICU admission). During adjustment for these patient characteristics, a separate category was made for patients with missing information on the particular characteristic. In additional exploratory analysis the model with adjustment for patient characteristics was extended with adjustment for ICU occupancy rate, first by using the occupancy rate at the day of ICU admission (categorized in five groups based on quintiles) and second by using the mean occupancy rate during all days of ICU admission (categorized in five groups based on quintiles). To estimate the ICU occupancy rate on a daily basis we first calculated a reference level for each ICU per calendar year (2017, 2018, and 2019) based on the average daily ICU occupancy rate during that year. For the year 2020 the average daily ICU occupation during 2019 was used as reference. For each ICU the number of patients present per day was calculated and this number was compared with the reference level of that ICU and expressed as percentage for that particular day. For example, an ICU with a yearly average ICU occupancy rate of 12 patients per day that had 15 patients present at a certain day had an ICU occupancy rate of 125% on that specific day. To better understand the differences in characteristics between the two cohorts and their effect on outcome, case-mix adjustment was performed in steps. The odds ratio (OR) for in-hospital mortality among COVID-19 patients compared to other viral pneumonia patients and associated 95%-confidence interval are presented at first without adjustment, second with adjustment for patient characteristics and third with additional adjustment for ICU occupancy. A P value of <0.05 is regarded as statistically significant. All analyses were performed using the R statistical environment (version 3.6.1) (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
From February 15th, 2020 to January 1st 2021, 6624 COVID-19 patients were admitted to all 81 ICUs in the Netherlands. Seventy-nine of these Dutch ICUs uploaded the data for the (NICE-DB), therewith the 6343 (95.8%) COVID-19 patients of these 79 ICUs could be linked to the NICE-DB and were included in the analyses. The non-linked COVID-19 patients were comparable to the linked COVID-19 patients except that non-linked patients were admitted less frequently in a teaching hospital (32.4 vs. 53.8%, see Table 1 ).
Table 1.
Patient characteristics of linked and non-linked COVID-19 patients.
| Linked COVID-19 patients (N = 6343) | Non-linked COVID-19 patients (N = 281) | |
|---|---|---|
| Type of hospital | ||
| University hospitals N (%)/number of ICUs | 788 (12.4)/8 | 50 (17.8)/7 |
| Teaching hospitals N (%)/number of ICUs | 3415 (53.8)/32 | 91 (32.4)/20 |
| Non-teaching hospitals N (%)/number of ICUs | 2140 (33.7)/39 | 140 (49.8)/21 |
| Mean age (SD) | 63.8 (11.6) | 65.0 (11.1) |
| In-hospital mortality N (%) | 1960 (30.9) | 92 (32.7) |
| Confirmation method | ||
| Lab confirmation N (%) | 6235 (98.3) | 279 (99.3) |
| CT confirmation N (%) | 108 (1.7) | 2 (0.7) |
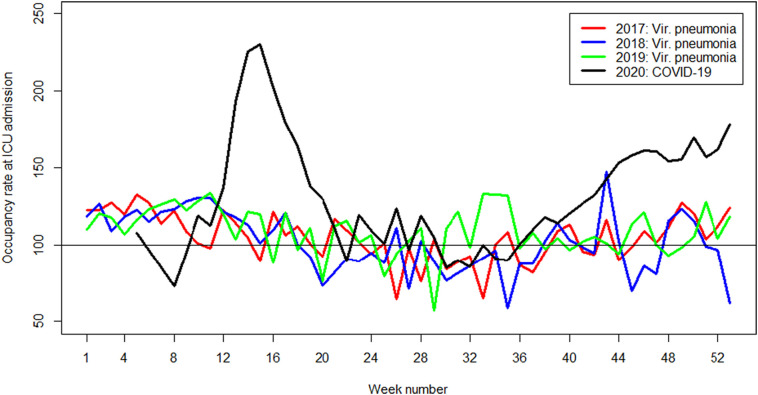
Of the 6343 linked COVID-19 patients, 1831 (28.9%) patients were admitted directly to the ICU from home or the emergency department. The other 4512 (71.1%) were admitted from a general ward of the same or other hospital and had a mean length of hospital stay of 2.6 calendar days prior to ICU admission. Among the COVID-19 patient, only information on the BMI and age was missing for respectively 2.6% and 1.6% of the patients. Among the other viral pneumonia patients only information on the BMI was missing for 6.4% of the patients Table 2 shows the patient characteristics and ICU occupancy rate of the included COVID-19 and other viral pneumonia patients. The COVID-19 patients were more often men (71.3% versus 49.8%), were slightly younger (median age 65 versus 67 year), had an higher BMI (median BMI 28.1 versus 25.5), had less often one or more comorbidities (42.2% versus 72.7%), and had a prolonged hospital length of stay (19 versus 9 days) compared to the patients with other viral pneumonia. In Fig. 1 the occupancy rate at ICU admission of COVID-19 and pneumonia patients during the year is shown. The ICU occupancy rate at ICU admission of COVID-19 patients was significant higher compared to the other viral pneumonia patients (median occupancy rate at ICU admission 150% versus 120%).
Table 2.
Patient characteristics of COVID-19 and viral pneumonia patients.
| COVID-19 | Other viral pneumonia | |
|---|---|---|
| Number of admissions | 6343 | 2256 |
| Median age (IQR) | 65 (57–72) | 67 (58–75) |
| Number of patient with unknown age | 0 | 37 |
| Number of males (%) | 4524 (71.3) | 1123 (49.8) |
| Median BMI (IQR) | 28.1 (25.5–31.7) | 25.5 (22.5–29.7) |
| Number of patient with unknown BMI | 162 | 145 |
| Comorbidities⁎ | ||
| Malignancy | 187 (2.9) | 133 (5.9) |
| Immunological insufficiency | 574 (9) | 359 (15.9) |
| COPD | 614 (9.7) | 1069 (47.4) |
| Chronic respiratory insufficiency | 270 (4.3) | 417 (18.5) |
| Chronic renal failure | 285 (4.5) | 157 (7) |
| Chronic cardiovascular insufficiency | 103 (1.6) | 85 (3.8) |
| Cirrhosis | 22 (0.3) | 10 (0.4) |
| Diabetes | 1506 (23.7) | 451 (20) |
| Vasoactive medication in first 24 h of ICU admission | 3584 (56.5) | 908 (40.2) |
| Mechanical ventilation at ICU admission | 2205 (34.8) | 1201 (53.2) |
| Mechanical ventilation in first 24 h of ICU admission | 4243 (66.9) | 1542 (68.4) |
| Median APACHE III APS (IQR) | 47 (38–59) | 48 (37–61) |
| Median APACHE III score (IQR) | 60 (48–73) | 62 (49–77) |
| Median LOS ICU in days (IQR) | 13 (6–25) | 3.6 (1.7–7.6) |
| Median LOS hospital in days (IQR) | 19 (11–34) | 9 (5–16) |
| Median ICU occupancy rate at ICU admission (IQR) | 150 (120−200) | 120 (100−130) |
| Median ICU occupancy during ICU treatment (IQR) | 160 (130−200) | 120 (100–130) |
| ICU mortality N(%) | 1709 (26.9) | 336 (14.9) |
| Hospital mortality N(%) | 1960 (30.9) | 444 (19.7) |
*Malignancy: Encompasses malignant lymphoma, acute leukaemia, multiple myeloma, metastases which have been diagnosed by clinical examination or confirmed by a pathology report OR if there is Stage IV cancer.
Immunological insufficiency: HIV-positive with clinical complications, long-term immunosuppressive therapy, corticosteroid use, active chemotherapy, radiotherapy in the past year, chemotherapy or radiotherapy for Hodgkin's or non-Hodgkins lymphoma at any time for IC admission OR documented humoral/cellular deficiencies.
COPD: chronic condition in which pulmonary function swiftly deteriorates.
Chronic respiratory insufficiency: Chronic restrictive, obstructive or vascular conditions in the lungs resulting in very severe restriction of mobility (GOLD IV), registered chronic hypoxia, secondary polycythaemia, severe pulmonary hypertension (PAPsys > 40 mmHg) OR respiratory dependence.
Chronic renal failure: evidence of raised serum creatinine > 177 umol/L (2.0 mg/dl) and renal insufficiency in the medical history OR long-term haemodialysis/peritoneal dialysis prior to the current hospital admission.
Chronic cardiovascular insufficiency: Angina or symptoms at rest or during minimal effort (New York Heart Association class IV).
Cirrhosis: positive biopsy and documented portal hypertension, previous periods of high gastrointestinal bleeding as a result of portal hypertension, previous periods of hepatic failure, coma OR encephalopathy.
Diabetes: medication-dependent form of diabetes diagnosed before the current IC admission.
Fig. 1.
Mean occupancy rate at ICU admission of COVID-19 (2020) and other viral pneumonia (2017–2019) patients.
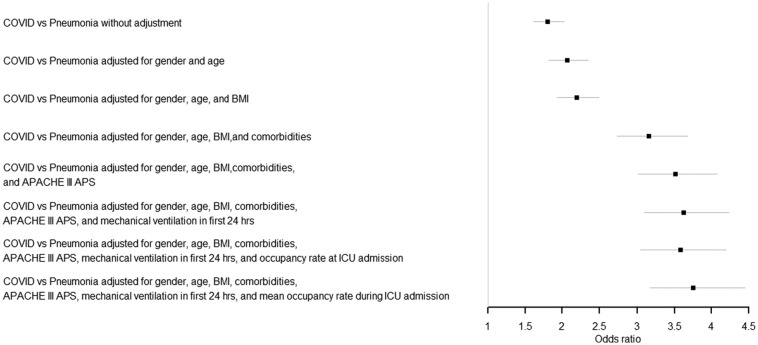
Of the 6343 COVID-19 patients, 1709 (26.9%) patients died in the ICU and another 251 (4.0%) died in the hospital after ICU discharge, resulting in a total of 1960 (30.9%) in-hospital deaths at the end of the study period. All other COVID-19 patient were discharged alive from the hospital. The in-hospital mortality rate among the other viral pneumonia patients was 19.7%. In Fig. 2 the unadjusted and adjusted odds ratios for COVID-19 patients compared to the other viral pneumonia patients are presented. This figure shows that without case-mix adjustment, the COVID-19 patients have a significantly higher mortality rate compared to other viral pneumonia patients (OR = 1.80 (1.61–2.02)). After adjustment for gender and age the odds ratio increased to 2.06 (1.82–2.34), and after adjustment for all available patient characteristics the odds ratio even increased up to 3.62 (3.10–4.23). The exploratory analysis with further adjustment for ICU occupancy rate showed similar OR's; 3.58 (3.05–4.20) when ICU occupancy at ICU admission was used and 3.75 (3.17–4.45) when the mean ICU occupancy during ICU treatment was used. Table 3 shows that a higher age, higher APACHE III APS and the presence of a comorbidity contributed to a significantly higher in-hospital mortality. The supplementary analyses in which the COVID-19 patients were compared with pulmonary sepsis and ARDS patients showed similar results, see supplementary tables.
Fig. 2.
Odds ratio COVID-19 compared to other viral pneumonia patients.
Table 3.
Multivariable logistic regression model with adjustment for age, BMI, gender, comorbidities, APACHE-III APS, and occupancy rate at ICU admission.
| Odds ratio (95% CI) | |
|---|---|
| COVID-19 vs other viral pneumonia | 3.58 (3.05–4.20) |
| Age | |
| 40–45 vs <40 | 0.95 (0.43–2.10) |
| 45–50 vs <40 | 1.97 (1.12–3.47) |
| 50–55 vs <40 | 2.21 (1.30–3.74) |
| 55–60 vs <40 | 2.46 (1.48–4.08) |
| 60–65 vs <40 | 3.74 (2.28–6.12) |
| 65–70 vs <40 | 5.63 (3.45–9.19) |
| 70–75 vs <40 | 8.28 (5.09–13.47) |
| 75–80 vs <40 | 12.01 (7.34–19.65) |
| 80–85 vs <40 | 15.35 (9.14–25.76) |
| ≥85 vs <40 | 17.09 (9.35–31.22) |
| Unknown vs <40 | 0.46 (0.06–3.62) |
| Gender: Female vs Male | 0.84 (0.75–0.95) |
| Body Mass Index | |
| <18·5 vs 18·5–25 | 0.66 (0.43–1.01) |
| 25–30 vs 18·5–25 | 0.59 (0.38–0.90) |
| 30–35 vs 18·5–25 | 0.59 (0.38–0.91) |
| 35–40 vs 18·5–25 | 0.60 (0.38–0.96) |
| ≥40 vs 18·5–25 | 0.70 (0.42–1.16) |
| Unknown vs 18·5–25 | 0.76 (0.45–1.27) |
| Immunological insufficiency | 1.57 (1.33–1.86) |
| Chronic renal failure | 1.53 (1.22–1.91) |
| COPD | 1.57 (1.35–1.82) |
| Chronic respiratory insufficiency | 2.09 (1.72–2.54) |
| Chronic cardiovascular insufficiency | 1.69 (1.22–2.35) |
| Cirrhosis | 2.39 (1.06–5.36) |
| Malignancy | 2.13 (1.63–2.78) |
| Diabetes | 1.14 (1.00–1.28) |
| Acute Physiology Score (APACHE III APS score) | |
| 34–43 vs <34 | 1.20 (1.01–1.42) |
| 43–52 vs <34 | 1.49 (1.27–1.75) |
| 52–64 vs <34 | 2.45 (2.08–2.90 |
| ≥64 vs <34 | 4.53 (3.73–5.51) |
| Mechanical ventilation in first 24 h of ICU admission | 1.73 (1.53–1.95) |
| Occupancy rate at ICU admission (based on quintiles) | |
| 105–125 vs <105 | 0.99 (0.84–1.18) |
| 125–150 vs <105 | 0.95 (0.80–1.13) |
| 150–195 vs <105 | 1.01 (0.85–1.19) |
| ≥195 vs <105 | 1.02 (0.86–1.21) |
4. Discussion
In the beginning of the COVID-19 pandemic it was suggested that COVID-19 might be just another influenza-like illness or pneumonia. However, in our study we showed that the crude in-hospital mortality rate in COVID-19 patients admitted to the ICU in the Netherlands was significantly higher compared to other viral pneumonia patients (OR = 1.80). After adjustment for age, gender, BMI, comorbidities, APACHE III APS, mechanical ventilation in the first 24 h this odds ratio became even higher (OR = 3.62). The effect of adjustment is in accordance with our expectation as COVID-19 patients admitted to the ICU are younger and have less comorbidities compared to other viral pneumonia patients. Further adjustment for occupancy rate at ICU admission and during the entire ICU treatment showed an OR of respectively 3.58 and 3.75, indicating that the higher ICU occupancy rate during the COVID-19 pandemic could not explain the higher mortality rate among the COVID-19 patients compared to other viral pneumonia patients in former years. Our exploratory analysis with further adjustment for occupancy rate gives an important first impression of the effect of occupancy rate on quality of care and associated mortality risk, at least for the patient groups included in our analysis. No important and statistical significant differences in mortality between the quintiles for occupancy rate were found, indicating that serious threats for the quality of care associated with overcrowding during the COVID-19 pandemic were adequately dealt with. Future research should focus on the examination of the effect of occupancy rate on the quality of care for ICU patients in general, other ICU subgroups like trauma and other non-COVID-19 patients, but especially for the patients that were denied admission to the ICU during the COVID-19 pandemic.
The COVID-19 patients in this study had a prolonged ICU length of stay and received more often mechanical ventilation during the first 24 h of ICU admission compared to other viral pneumonia patients, leading to a higher need of ICU capacity during the COVID-19 outbreak. The impending shortage of ICU capacity gave rise to a stricter selection for hospital and ICU admission by care-givers [20,21], which lead to a lower percentage of older patients with known comorbidity and frailty admitted to the Dutch ICUs. This could have led to a lower in-hospital mortality among the COVID-19 patients that were admitted, which reinforces our conclusion that the mortality among COVID-19 patient is high compared to pneumonia patients. Differences in available ICU capacity across counties and in cultural aspects such as the estimated benefit and burden of an ICU admission for very old and frail people probably have influenced the ICU admission and discharge policies between countries. For instance, the number of patients with one or more comorbidities in Italy was higher compared to the Netherlands (60.5% versus 42.2%) [2]. The differences in case-mix characteristics of the included patients between studies can partially explain the differences in mortality rates among studies. The crude in-hospital mortality of 30.9% for the COVID-19 patients in our study is lower than the mortality rates reported in some of the studies published in the beginning of the COVID-19 pandemic (i.e. mortality rates of 37.4 to 67.0%) [2,5,6,[8], [9], [10]]. Besides the differences in ICU admission policies, another explanation for the variation in reported mortality rates could be the small sample size of some of these studies [8,9]. For example, Arentz et al. reported an in-hospital mortality rate of 67% among the only 21 included COVID-19 ICU patients, with an average age of 70 years of whom 86% had one or more comorbidities [8]. However, Richards-Belle et al. had a large sample size (n = 10,834) and also found a higher mortality rate (42.0%) among COVID-19 patients which were slightly younger (mean age of 60 versus 65) and had less comorbidities (patient with at least one comorbidity was 8.2% versus 35.7%) than the patients in our study population [5]. A possible explanation for the large difference in the number of COVID-19 patients with at least one comorbidity could be the difference in the included comorbidities and/or in the definition of the included comorbidities. We showed a 11.2% significantly higher crude in-hospital mortality rate in COVID-19 patients compared to other viral pneumonia patients (30.9% versus 19.7%), in the UK this difference was 17.3% (42.0% versus 24.7%) mainly because of the higher crude in-hospital mortality among the COVID-19 patients [5], in the US this difference was higher namely 13.4% (24.3% versus 10.9%) mainly because of the lower crude in-hospital mortality among the viral pneumonia patients [4]. Future research could help us to identify possible explanations for these differences.
A strength of this study is that it includes almost all COVID-19 patients (91.6%) admitted to the ICU from an entire country. As the NICE registry contains clinical information on the admitted ICU patients we were able to correct for important differences in patients characteristics between COVID-19 and other viral pneumonia patients. We also adjusted for bed occupancy rate taking the average daily ICU occupancy rate during 2019 as a reference for 2020. As limitation of this study it should be mentioned that we could not adjust for the number of available (ICU certified) health workers and the perceived workload of the health workers during the COVID-19 pandemic which could have had a negative influence on the outcome of patients. However, as episodes of high stress, sickness leave and other unfavorable organizational factors coincide with episodes of high occupancy rate, our somewhat simplistic estimate is probably a valid indicator of these potential threats for the quality of care. As we found no association between occupancy rate and mortality risk, the episodes of high occupancy rate during the COVID-19 pandemic waves in the Netherlands were probably not associated with serious shortage of personal and medical equipment or that this shortage was adequately managed. As a large part of non-urgent care was postponed, in many instances non-ICU personal co-operated with ICU nurses to deliver care and to overcome potential shortage of ICU nurses.
Our data add to disease understanding and disease management on different levels. From an organizational point of view, we were able to show that the time course and mortality risk of COVID-19 differs from other viral pneumonias requiring ICU admission. In governmental, regional, and local planning it has to be recognized that in terms of resource use (e.g. ICU and hospital length of stay and necessity for mechanical ventilation) COVID-19 patients surpass other viral pneumonia patients. When ICUs get overwhelmed (again) by a new outbreaks of COVID-19 they should be warned for the high mortality risk which also might affect the mental workload of care-givers [22,23].
5. Conclusion
Compared to other viral pneumonia patients, the COVID-19 patients were more often male, had a higher BMI, less comorbidities, a prolonged hospital length of stay, and received more often mechanical ventilation. Almost one third of COVID-19 patients admitted to the Dutch ICUs died in the hospital. The in-hospital mortality was higher compared to ICU patients with other viral pneumonias, irrespective of adjustment for important clinical characteristics. The higher ICU occupancy rate during the COVID-19 pandemic did not explain the higher mortality among COVID-19 patients compared to viral pneumonia patients in former years. Based on these results, we may conclude that COVID-19 and other viral pneumonia at the ICU are not similar. These findings confirm earlier warnings of a high need of hospital and ICU capacity and high mortality rates among previously relatively healthy and younger patients with COVID-19 infections as this may lead to a higher physical and mental workload for the staff. ICUs and policy makers should be aware of the high need of care capacity to manage the prolonged hospital length of stay and high need for mechanical ventilation in case of a new COVID-19 outbreak. Future research should focus on long-term outcome (i.e. 6 or 12 months after COVID-19) in terms of mortality and quality of life.
Funding
This research was funded by the The Netherlands Organisation for Health Research and Development (ZonMw) COVID-19 Programme in the bottom-up focus area 1 “Predictive diagnostics and treatment” for theme 3 “Risk analysis and prognostics” (project number 10430 01 201 0011: IRIS). The funder had no role in the design of the study or writing the manuscript.
Availability of data and material
Access to data of the NICE registry is regulated and described at https://www.stichting-nice.nl/extractieverzoek_procedure.jsp (in Dutch).
Code availability
Requests should be submitted to the corresponding author.
Authors contributions
Conceptualization – Ideas; formulation or evolution of overarching research goals and aims: S. Brinkman, F. Termorshuizen, D.A. Dongelmans, F. Bakhshi-Raiez, M.S. Arbous, D.W. de Lange, and N.F. de Keizer.
Data curation – Management activities to annotate (produce metadata), scrub data and maintain research data (including software code, where it is necessary for interpreting the data itself) for initial use and later re-use: S. Brinkman, F. Termorshuizen, and N.F. de Keizer.
Formal analysis – Application of statistical, mathematical, computational, or other formal techniques to analyze or synthesize study data: S. Brinkman and F. Termorshuizen.
Investigation – Conducting a research and investigation process, specifically performing the experiments, or data/evidence collection: S. Brinkman and F. Termorshuizen.
Methodology – Development or design of methodology; creation of models: F. Termorshuizen, S. Brinkman, and N.F. de Keizer.
Resources – Provision of study materials, reagents, materials, patients, laboratory samples, animals, instrumentation, computing resources, data, or other analysis tools: D.A. Dongelmans, M.S. Arbous, D.W. de Lange, and the Dutch COVID-19 Research Consortium.
Software – Programming, software development; designing computer programs; implementation of the computer code and supporting algorithms; testing of existing code components: S. Brinkman and F. Termorshuizen.
Validation – Verification, whether as a part of the activity or separate, of the overall replication/reproducibility of results/experiments and other research outputs: S. Brinkman and F. Termorshuizen.
Writing – original draft – Preparation, creation and/or presentation of the published work, specifically writing the initial draft (including substantive translation). S. Brinkman, F. Termorshuizen, and N.F. de Keizer.
Writing – review & editing – Preparation, creation and/or presentation of the published work by those from the original research group, specifically critical review, commentary or revision – including pre- or post-publication stages: D.A. Dongelmans, M.S. Arbous, F. Bakhshi-Raiez, D.W. de Lange, and the Dutch COVID-19 Research Consortium.
Visualization – Preparation, creation and/or presentation of the published work, specifically visualization/data presentation. S. Brinkman and F. Termorshuizen.
Supervision – Oversight and leadership responsibility for the research activity planning and execution, including mentorship external to the core team. N.F. de Keizer and D.A. Dongelmans,
Project administration – Management and coordination responsibility for the research activity planning and execution. N.F. de Keizer.
Funding acquisition - Acquisition of the financial support for the project leading to this publication. N.F. de Keizer.
Declaration of Competing Interest
All authors state that there were no conflict of interest. The NICE foundation pays the department of Medical Informatics to process and analyze data for the registry. This institution is not paid specifically for this study but it is part of the task agreement to perform analyses like this on data from the NICE registry.
Acknowledgements
We would like to acknowledge the effort of all Dutch hospitals to record their COVID-19 patients three times a day and additionally all the ICUs that also collected the comprehensive clinical data of the COVID-19 patients to NICE-DB. Without their effort this study would not have been possible. We thank The Netherlands Organisation for Health Research and Development (ZonMw) for funding this project (project number 10430012010011). We also like to thank all employees of NICE Research & Support for the technical development of the COVID-19 Online Module, processing the data, and support under high work pressure.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcrc.2021.12.007.
Contributor Information
the Dutch COVID-19 Research Consortium:
D.P. Verbiest, L.F. te Velde, E.M. van Driel, T. Rijpstra, P.W.G. Elbers, A.P.I. Houwink, L. Georgieva, E. Verweij, R.M. de Jong, F.M. van Iersel, T.J.J. Koning, E. Rengers, N. Kusadasi, M.L. Erkamp, R. van den Berg, C.J.M.G. Jacobs, J.L. Epker, A.A. Rijkeboer, M.T. de Bruin, P. Spronk, A. Draisma, D.J. Versluis, A.E. van den Berg, M. Vrolijk-de Mos, J.A. Lens, R.V. Pruijsten, H. Kieft, J. Rozendaal, F. Nooteboom, D.P. Boer, I.T.A. Janssen, L. van Gulik, M.P. Koetsier, V.M. Silderhuis, R.M. Schnabel, I. Drogt, W. de Ruijter, R.J. Bosman, T. Frenzel, L.C. Urlings-Strop, A. Dijkhuizen, I.Z. Hené, A.R. de Meijer, J.W.M. Holtkamp, N. Postma, A.J.G.H. Bindels, R.M.J. Wesselink, E.R. van Slobbe-Bijlsma, P.H.J. van der Voort, B.J.W. Eikemans, D.J. Mehagnoul-Schipper, D. Gommers, J.G. Lutisan, M. Hoeksema, M.G.W. Barnas, B. Festen-Spanjer, M. van Lieshout, N.C. Gritters, M. van Tellingen, G.B. Brunnekreef, J. Vandeputte, T.P.J. Dormans, M.E. Hoogendoorn, M. de Graaff, D. Moolenaar, A.C. Reidinga, J.J. Spijkstra, and R. de Waal
Appendix A. Supplementary data
Supplementary material
References
- 1.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G., Greco M., Zanella A., Albano G., Antonelli M., Bellani G., et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Int Med. 2020;180(10):1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu L., Wang B., Yuan T., Chen X., Ao Y., Fitzpatrick T., et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J Infect. 2020;80(6):656–665. doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Higgins T.L., Stark M.M., Henson K.N., Freeseman-Freeman L. Coronavirus disease 2019 ICU patients have higher-than-expected acute physiology and chronic health evaluation-adjusted mortality and length of stay than viral pneumonia ICU patients. Crit Care Med. 2021;49(7) doi: 10.1097/CCM.0000000000005012. (e701-e6) [DOI] [PubMed] [Google Scholar]
- 5.Richards-Belle A., Orzechowska I., Gould D.W., Thomas K., Doidge J.C., Mouncey P.R., et al. COVID-19 in critical care: epidemiology of the first epidemic wave across England, Wales and Northern Ireland. Intensive Care Med. 2020;46(11):2035–2047. doi: 10.1007/s00134-020-06267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ludwig M., Jacob J., Basedow F., Andersohn F., Walker J. Clinical outcomes and characteristics of patients hospitalized for Influenza or COVID-19 in Germany. Int J Infect Dis. 2021;103:316–322. doi: 10.1016/j.ijid.2020.11.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilde H., Mellan T., Hawryluk I., Dennis J.M., Denaxas S., Pagel C., et al. The association between mechanical ventilator availability and mortality risk in intensive care patients with COVID-19: a national retrospective cohort study. medRxiv. 2021;19:213. doi: 10.1186/s12916-021-02096-0. 2021.01.11.21249461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M., et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. Jama. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bain W., Yang H., Shah F.A., Suber T., Drohan C., Al-Yousif N., et al. COVID-19 versus non-COVID-19 acute respiratory distress syndrome: comparison of demographics, physiologic parameters, inflammatory biomarkers, and clinical outcomes. Ann Am Thorac Soc. 2021;18(7):1202–1210. doi: 10.1513/AnnalsATS.202008-1026OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X., Ma X. Acute respiratory failure in COVID-19: is it “typical” ARDS? Crit Care. 2020;24(1):198. doi: 10.1186/s13054-020-02911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olwal C.O., Nghochuzie N.N., Tapela K., LAZ Djomkam, Owoicho O., Bediako Y., et al. Parallels in Sepsis and COVID-19 conditions: implications for managing severe COVID-19 patients. Front Immunol. 2021;12:91. doi: 10.3389/fimmu.2021.602848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van de Klundert N., Holman R., Dongelmans D.A., de Keizer N.F. Data resource profile: the Dutch National Intensive Care Evaluation (NICE) registry of admissions to adult intensive care units. Int J Epidemiol. 2015;44(6) doi: 10.1093/ije/dyv291. (1850-h) [DOI] [PubMed] [Google Scholar]
- 15.NICE National intensive care evaluation (NICE) 2020. https://www.stichting-nice.nl/watweregistreren.jsp cited 2020. Available from:
- 16.Specialisten F.M. Guide to diagnostics (PCR and CT) in patients with recording indication and suspected COVID-19 infection. 2020. https://www.demedischspecialist.nl/sites/default/files/handreiking_%20diagnostiek_bij%20verdenking_COVID%20-19.pdf Available from:
- 17.Prokop M., Everdingen W., Vellinga T., Ufford J., Stöger L., Beenen L., et al. CO-RADS – a categorical CT assessment scheme for patients with suspected COVID-19: definition and evaluation. Radiology. 2020;296(2):E97–E104. doi: 10.1148/radiol.2020201473. 201473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zimmerman J.E., Kramer A.A., McNair D.S., Malila F.M. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Crit Care Med. 2006;34(5):1297–1310. doi: 10.1097/01.CCM.0000215112.84523.F0. [DOI] [PubMed] [Google Scholar]
- 19.NICE NICE; study protocol. 2020. https://www.stichting-nice.nl/extractieverzoek.jsp Available from:
- 20.Sprung C.L., Joynt G.M., Christian M.D., Truog R.D., Rello J., Nates J.L. Adult ICU triage during the coronavirus disease 2019 pandemic: who will live and who will die? Recommendations to improve survival. Crit Care Med. 2020;48(8):1196–1202. doi: 10.1097/CCM.0000000000004410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phua J., Weng L., Ling L., Egi M., Lim C.M., Divatia J.V., et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8(5):506–517. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenberg N., Docherty M., Gnanapragasam S., Wessely S. Managing mental health challenges faced by healthcare workers during covid-19 pandemic. BMJ (Clinical research ed) 2020;368 doi: 10.1136/bmj.m1211. [DOI] [PubMed] [Google Scholar]
- 23.Bohlken J., Schömig F., Lemke M.R., Pumberger M., Riedel-Heller S.G. COVID-19 pandemic: stress experience of healthcare workers - a short current review. Psychiatr Prax. 2020;47(4):190–197. doi: 10.1055/a-1159-5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Access to data of the NICE registry is regulated and described at https://www.stichting-nice.nl/extractieverzoek_procedure.jsp (in Dutch).