Abstract
Objective:
To investigate the relative predictive value of circulating immune cell markers for cardiovascular mortality in ambulatory adults without cardiovascular disease.
Patient and methods:
We analyzed data of participants enrolled in National Health and Nutrition Examination Survey from January 1, 1999, to December 31, 2010, with total leukocyte count within a normal range (4000-11,000 cells/μL) and without cardiovascular disease. The relative predictive value of circulating immune cell markers measured at enrollment including total leukocyte count, absolute neutrophil, lymphocyte and monocyte count, monocyte-lymphocyte ratio (MLR), neutrophil-lymphocyte ratio, and CRP to predict cardiovascular mortality was evaluated. The marker with the best predictive value was added to the 10-year atherosclerotic cardiovascular disease (ASCVD) risk score to estimate net risk reclassification indices for 10-year cardiovascular mortality.
Results:
Among 21,599 participants eligible for this analysis, the median age was 47 years (interquartile range 34, 63), 49.2% were women and 49.5% were self-reported non-Hispanic Whites. During a median follow-up of 9.6 years (interquartile range 6.8 to 13.1 years), there were 627 cardiovascular deaths. MLR had the best predictive value for cardiovascular mortality. The addition of elevated MLR (≥ 0.3) to the 10-year ASCVD risk score improved the classification by 2.7±1.4%, P=.04. Elevated MLR had better predictive value than CRP and several components of the 10-year ASCVD risk score.
Conclusions:
Among ambulatory US adults without pre-existing cardiovascular disease, we found that MLR had the best predictive value for cardiovascular mortality among circulating immune markers. The addition of MLR to the 10-year risk score significantly improved the risk-classification of participants.
Introduction
Activation of the immune system has been described in cardiovascular diseases such as atherosclerosis, acute coronary syndrome, and heart failure.1-4 The associated immune markers may have prognostic significance to detect long-term cardiovascular events. But the widely used cardiovascular risk prediction models do not include any immune markers.5,6
Circulating immuno-inflammatory markers such as C-reactive protein (CRP), total leukocyte count, and differential leukocyte count are widely available and studies report their independent association with adverse cardiovascular outcomes.1-3,7,8 However, their ability to predict the long-term risk of cardiovascular mortality in a population without cardiovascular disease remains unknown. Further, no previous study has compared the relative predictive values of these circulating immune markers. Finally, it remains unclear how the predictive value of circulating immune markers compares to traditional cardiovascular risk factors such as age, hypertension, and dyslipidemia.
We investigated the role of widely available circulating immune cell markers to predict cardiovascular mortality in a representative ambulatory US adult population without cardiovascular disease. We hypothesized that circulating immune cell markers in the normal range are differentially associated with cardiovascular risk. We aimed to rank their relative predictive value and explore how the addition of these circulating immune cell markers to current prediction models modifies cardiovascular risk classification.
Methods
The study was conducted following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (eTable 1 in the supplement).
Study Design and Participants
The National Health and Nutrition Examination Survey (NHANES) is a serial cross-sectional survey designed to gather data about the health status of the resident civilian non-institutionalized US population. The details of the interview, laboratory, and physical examination have been published previously9 and are given in eMethods in the supplement. The data is linked to the National Death Index through December 31, 2011. We used publicly available de-identified data. Therefore, approval from the University of Alabama at Birmingham Institutional Review Board was not required. Data from participants aged ≥18 years from six NHANES cycles from 1999-2010 were used for the current analysis (Figure 1).
Figure 1.
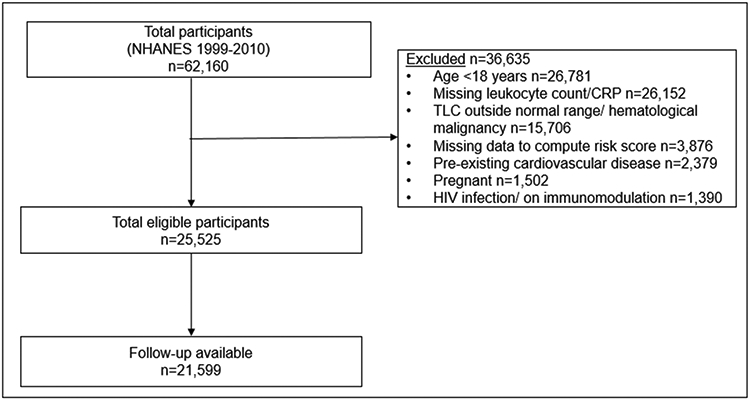
Flow diagram for study selection. Normal range of TLC defined as 4,000-11,000 cells/μL. Cardiovascular disease defined as self-reported coronary artery disease, heart failure, stroke or on pacemaker. CRP, C-reactive protein; HIV, Human Immunodeficiency Virus; NHANES, National Health, and Nutrition Examination Survey; TLC, Total leukocyte Count.
We excluded participants with pre-existing cardiovascular disease (self-reported angina, heart attack, coronary artery disease, heart failure, stroke or pacemaker), or total leukocyte count beyond the normal range (<4,000 cells/μL or >11,000 cells/μL). Other exclusion criteria are given in eMethods in the supplement.
Study Variables
Several circulating immune markers such as total and differential leukocyte count and CRP used in this investigation were available from the blood sample collected at the time of the visit to the mobile examination center during NHANES. Details are given in eMethods and eTable 2 in the supplement. We computed the neutrophil-lymphocyte ratio by diving absolute neutrophil count by absolute lymphocyte count and the monocyte-lymphocyte ratio (MLR) by diving absolute monocyte count by absolute lymphocyte count. Both these ratios are independently associated with worse cardiovascular prognosis.10,11
Demographic and Clinical Information
Data on other demographic and clinical characteristics were collected either during the interview or visit to the mobile examination center. The variable codes and diagnostic criteria to define co-morbidities are given in eMethods and eTable 2 in the supplement.
Study Outcome
Cardiovascular mortality during follow-up was the outcome for all survival analyses. Cardiovascular mortality that occurred within 10 years of the visit to the mobile examination center was the outcome for all logistic models. The cause of death was derived from linked death certificate records from the National Death Index.12 Details are given in eMethods and eTable 2 in the supplement. Follow-up time was taken as time from mobile examination center date to the date of death or end of mortality period.
Statistical Analysis
Continuous variables were reported as medians with interquartile ranges (IQRs) and categorical variables were represented as counts with proportions. The Wilcoxon rank-sum test and Chi-squared tests were used to identify the differences in baseline characteristics in continuous and categorical variables, respectively. We estimated the 10-year ASCVD and Framingham risk scores for all participants.5,6 For calculation, participants aged <40 years or >79 years were considered to be 40 and 79 years old, respectively. The baseline characteristics of the participants with age<40, 40-79, and >79 years are given in eTable 3. The 10-year ASCVD and Framingham risk score varied across the NHANES cycle (eTable 4 in the supplement).
We assessed the relative predictive value of circulating immune markers using the Cox proportional hazards model and the logistic regression model. Details are given in eMethods in the supplement. The optimal cutoff of the circulating immune marker with the best predictive value for 10-year cardiovascular mortality was estimated using the Youden and Liu index.13,14 We then evaluated the independent association of the marker with the best predictive value at a categorical cut off ascertained in the previous step. We used both the Cox proportional hazards model and competing-risk regression analysis with non-cardiovascular mortality as a competing-risk for cardiovascular mortality in the adjusted model. The adjusted Cox proportional hazards model included the following covariates: age, gender, race, hypertension, hemoglobin, diabetes mellitus, chronic obstructive pulmonary disease, current smoking, dyslipidemia, estimated glomerular filtration rate, malignancy, obesity, and NHANES cycle. The variables in multivariate models were included if they were established risk factors for CV events or associated with inflammation and CV mortality. We also included the NHANES cycle as a covariate as sampling strategy changed in NHANES over the years which likely lead to variation in 10-year ASCVD risk varied across the NHANES cycles. The proportionality assumption was verified using the Schöenfeld residuals.15 We determined if there was a dose-response relationship of cardiovascular mortality with the marker with the best predictive value. Poisson regression analyses were to estimate the incident rate (per 100 person-years) of cardiovascular mortality. Missing data for obesity (n=291, 1.4%) and estimated glomerular filtration rate (n=54, 0.3%) were imputed using multivariate chained equations with age, gender, and race as predictors.16 There was no difference in the central tendency, spread, and predictive ability in the Cox model between imputed and the un-imputed variables (eTable 5 in the supplement).
We also determined the independent prognostic importance of all circulating immune markers using the Cox proportional hazards model to predict cardiovascular mortality.
Reclassification and Comparison with CRP, and components of ASCVD and Framingham risk scores
We then estimated the net reclassification index (NRI) after adding the circulating immune marker with the best predictive value in the same model as the 10-year ASCVD risk score and the Framingham Risk Score with a pre-specified cutoff of 5% (low-risk).17 NRI helps compare the discriminative ability of two risk prediction models by logistic regression.17 The reclassification indices for both scores were also estimated after the addition of CRP, given that it is the most commonly used inflammatory marker to predict cardiovascular outcomes.18 Furthermore, we ranked the predictive value of the components in the 10-year ASCVD risk score, Framingham risk score, and the circulating immune marker for 10-year cardiovascular mortality. These factors were ranked using the likelihood and Wald χ2 statistics for the Cox proportional hazards analyses and the area-under-the-curve (AUC) and standardized domination statistic for the logistic regression analyses.11,12 All statistical analyses were performed in Stata/SE version 15.1 (StataCorp, College Station, TX, U.S.A.). All p-values were 2-sided with <0.05 considered statistically significant.
Results
Baseline Characteristics
Among 62,160 participants in six NHANES cycles spanning 1999-2010, 21,599 participants (34.7%) were included in the analyses (Figure 1). The baseline characteristics of the study population are given in Table 1.
Table 1:
Baseline Demographic, Clinical and Laboratory parameters in the assembled NHANES Cohort (1999-2010)
| Factor | Overall (n=21,599) |
|---|---|
| Demographic Parameters | |
| Age (years) | 47.0 (34.0, 63.0) |
| Women | 10651 (49.2%) |
| Race | |
| Non-Hispanic White | 10713 (49.5%) |
| Non-Hispanic Black | 3887 (18.0%) |
| Mexican American | 4660 (21.5%) |
| Other Hispanic | 1544 (7.1%) |
| Other Race - Including Multi-Racial | 845 (3.9%) |
| Anthropometry | |
| Weight (kg) | 77.8 (66.4, 91.0) |
| Height (m) | 1.7 (1.6, 1.8) |
| Body mass index (kg/m2) | 27.6 (24.2, 31.6) |
| Comorbidities | |
| Diabetes mellitus | 2977 (13.8%) |
| Dyslipidemia | 14082 (65.0%) |
| Hypertension | 11441 (52.8%) |
| Systolic blood pressure (mmHg) | 122.0 (112.0, 135.0) |
| Diastolic blood pressure (mmHg) | 72.0 (64.0, 79.0) |
| Smoking | 10143 (46.9%) |
| Chronic obstructive pulmonary disease | 122 (0.6%) |
| Malignancy | 750 (5.7%) |
| Obesity | 7104 (33.3%) |
| Laboratory Parameters | |
| Hemoglobin (g/dL) | 14.4 (13.4, 15.4) |
| Platelet count (*106 cells/μL) | 260.0 (223.0, 305.0) |
| Estimated GFR (mL/min/1.73m2)a | 99.7 (82.1, 122.0) |
| Circulating immune markers | |
| C-reactive protein (mg/dL) | 0.2 (0.1, 0.4) |
| Total leukocyte count (*103 cells/μL) | 6.9 (5.7, 8.1) |
| Absolute Lymphocyte count (*103 cells/μL) | 2.0 (1.7, 2.5) |
| Absolute Neutrophil count (*103 cells/μL) | 4.0 (3.1, 4.9) |
| Absolute Monocyte count (*103 cells/μL) | 0.5 (0.4, 0.6) |
| Absolute Eosinophil count (*103 cells/μL) | 0.2 (0.1, 0.2) |
| Absolute Basophil count (*103 cells/μL) | 0.0 (0.0, 0.1) |
| Neutrophil-Lymphocyte Ratio | 2.0 (1.5, 2.6) |
| Monocyte-Lymphocyte Ratio | 0.3 (0.2, 0.3) |
| Risk Score | |
| 10-year ASCVD Riskb | 5.0 (1.6, 16.7) |
| 10-year Framingham Risk | 7.4 (2.0, 22.0) |
Data are represented as median (25th to 75th percentile), number (percentage).
GFR estimated by the modification of diet in renal disease (MDRD) 4-component study equation.
For calculation of 10-year ASCVD risk, participants aged <40 years (n=7,547; 34.9%) or >79 years (n=1,315; 6%) were considered to be 40 and 79 years old, respectively. Neutrophil-Lymphocyte and Monocyte-Lymphocyte Ratio derived by dividing absolute neutrophil and monocyte count by absolute lymphocyte count, respectively. ASCVD, atherosclerotic cardiovascular disease, GFR, glomerular filtration rate; NHANES, National Health, and Nutrition Examination Survey; mmHg = millimeters of mercury; μl=microliter; kg/m2= kilogram per-squared meter; g/dl=grams per deciliter; ml/min=milliliters per minute; mmol/L= millimoles per liter; mg/dl=milligrams per deciliter.
The median age of the participants was 47 years (IQR 34 to 63) with 49.2% women and 49.5% as self-identified non-Hispanic White. The prevalence of diabetes mellitus, hypertension, and dyslipidemia was 13.7%, 52.8%, and 65.0%, respectively (Table 1). The median leukocyte count was 6,900 cells/μL (IQR 5,700 to 8,100 cells/μL). The median absolute neutrophil and monocyte counts were 4000 cells/μL (IQR 3,100 to 4,900 cells/μL) and 500 cells/μL (IQR 400 to 600 cells/μL), respectively. The median 10-year ASCVD risk score and Framingham Risk score was 5% (IQR 1.6, 16.7) and 7.4% (IQR 2.0, 21.9), respectively.
The participants in the assembled cohort were followed for a median of 9.6 years (IQR 6.8 to 13.1 years). Cause of death was available in 99.8% of participants. There were 627 deaths due to cardiovascular causes at an incident rate of 0.29 per 100-person years (95% confidence interval [CI] 0.27, 0.32). Out of these, 509 (81.2%) cardiovascular deaths were within 10 years of follow-up.
Relative Predictive Value of Circulating Immune Markers
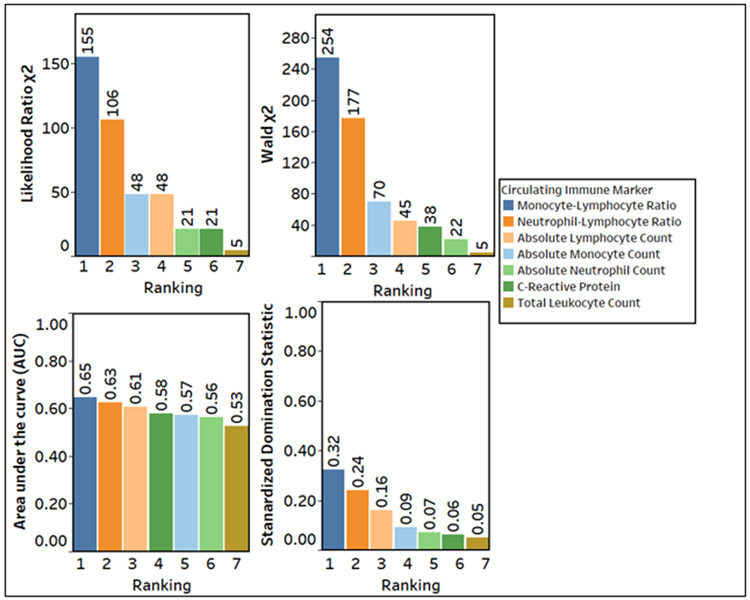
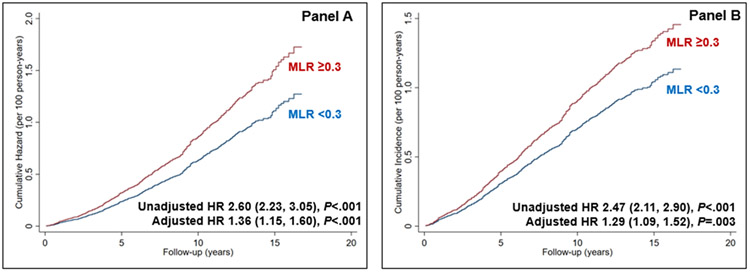
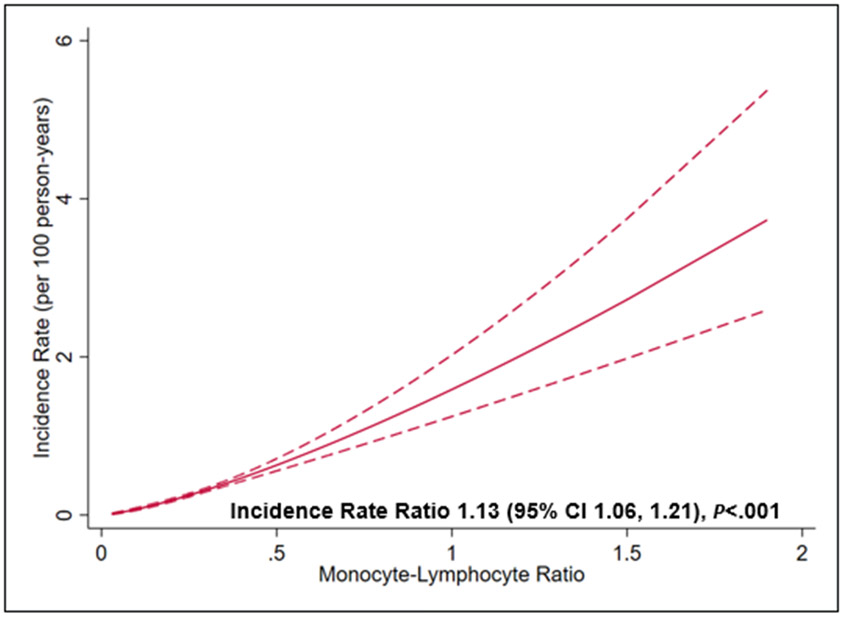
The MLR had the best predictive value for 10-year cardiovascular mortality (Figure 2) in both time-to-event (using likelihood and Wald χ2 statistics) and logistic regression analyses (AUC and standardized domination statistic). The risk of CV mortality was higher with 1 standard deviation rise of MLR (β=.34) as compared to 1 standard deviation rise of CRP (β=.08) (Wald χ2 for comparison of β in survival model = 71.30, P<.001). The optimal cutoff of the MLR to classify patients with the highest accuracy for the risk of 10-year cardiovascular mortality using the Youden Index was 0.30 (eFigure 1 in the supplement). This cutoff had a sensitivity and specificity of 55% and 69%. Participants with an elevated MLR (≥0.3) had a higher risk of cardiovascular mortality in the adjusted (Hazard ratio 1.36, 95% CI 1.15, 1.60, P<.001) and competing-risk analyses with non-cardiovascular mortality as a competing risk (Hazard ratio 1.29, 95% CI 1.09, 1.52, P=.003) (Figure 3 Panel A and B, respectively) (all p<0.05). The effect estimates remained robust in the adjusted model with un-imputed data (Hazard ratio 1.37, 95% CI 1.15, 1.63, P<.001). There was a dose-response relationship such that the incidence of cardiovascular mortality increased by 13% (95% CI 6, 21) for every 1 unit increase in the MLR in the adjusted model Figure 4 in the supplement).
Figure 2:
The relative predictive value of circulating immune markers to predict CV mortality. The circulating immune markers are arranged in the decreasing order of importance. Likelihood ratio χ2 using Cox Model to predict CV mortality (Panel A). Wald χ2 using Cox Model to predict CV mortality (Panel B). The area under the curve (AUC) using a logistic model to predict 10-year CV mortality (Panel C). Standardized Domination Statistic using a logistic model to predict the relative importance of immune markers for 10-year CV mortality (Panel D).
Figure 3.
Risk of cardiovascular mortality stratified by monocyte-lymphocyte ratio in the Cox proportional hazards model (Panel A) and competing-risk regression model with non-cardiovascular mortality as competing risk (Panel B) adjusted for age, gender, race, hypertension, hemoglobin, diabetes mellitus, chronic obstructive pulmonary disease, current smoking, dyslipidemia, estimated glomerular filtration rate, malignancy, obesity, and NHANES cycle. MLR, monocyte-lymphocyte ratio, HR, hazards ratio, CI, confidence interval, NHANES, National Health, and Nutrition Examination Survey
All immune markers except absolute lymphocyte count were independently associated with cardiovascular mortality (eTable 6 in the supplement).
Reclassification and Comparison with CRP, and components of ASCVD and Framingham risk scores
Baseline demographic, clinical, and laboratory characteristics of participants by low (<0.3) or elevated (≥0.3) MLR are given in eTable 7 in the supplement. The participants with elevated MLR were older and included a higher representation of men and non-Hispanic Whites, and participants with hypertension, smoking, and malignancy. Participants in the elevated MLR group had a significantly higher 10-year ASCVD (8.3% vs. 4.2%, P<.001) and Framingham Risk Score (12.1% vs. 6.0%, p<0.001).
Addition of elevated MLR to the categorical 10-year ASCVD risk score correctly up classified 3.2% participants with cardiovascular mortality (49.3% to 52.2%) and incorrectly up classified 0.4% participants with no cardiovascular mortality (8.8% to 9.2%). There was a significant improvement in risk classification (NRI 2.7±1.4%, P=.04, eFigure 2 Panel A in the supplement). Adding elevated CRP to the categorical 10-year ASCVD risk score had no impact on the risk classification (NRI −0.2±0.0%, P=.83, eFigure 2 Panel B in the supplement). Risk reclassification with addition of other circulating immune markers to the 10-year ASCVD risk score with different statistical models is given in eTable 8.
Similarly, adding elevated MLR to the 10-year Framingham Risk Score significantly improved the reclassification by correctly up classifying 4% of participants with cardiovascular mortality (NRI 4.0±1.2, P=.001). Adding elevated CRP had no impact on the risk classification (NRI 0.3±0.7, P=.67).
Comparing the Relative Predictive Value of MLR with components of ASCVD and Framingham Risk Score
We ranked the predictive value of the nine components of the 10-year ASCVD risk score and elevated MLR for cardiovascular mortality. Age had the highest predictive value in all models. The MLR ranked fifth and had a higher predictive value than race, smoking status, high-density lipoprotein, and total cholesterol in both the time-to-event and logistic regression models (eFigure 3 in the supplement). Similarly, it ranked fifth when compared to the components of the Framingham risk score
Discussion
We found that multiple circulating immune markers were independently associated with cardiovascular mortality over a median follow-up of 9.6 years among ambulatory US adults without pre-existing cardiovascular disease and a total leukocyte count within the normal range. Among the available circulating immune markers, the MLR had the best predictive value. The MLR significantly improved the classification power of the 10-year ASCVD risk score and the Framingham risk score to predict cardiovascular mortality and performed better than CRP as a predictor. Additionally, it had greater prognostic importance than traditional cardiovascular risk factors such as race, smoking status, high-density lipoprotein, and total cholesterol.
Immune cells and inflammatory markers have an important pathophysiological role in cardiovascular diseases. They are influenced by myocardial stressors such as ischemia, volume overload or pressure overload, and co-morbidities commonly associated with cardiovascular diseases, such as dyslipidemia and obesity.19,20 At a cellular level, there is an increasing appreciation of the interplay between cardiac myocytes and immune cells during physiological and pathological states. Extant literature suggests that there is an initial infiltration of blood neutrophils and then pro-inflammatory monocytes and monocyte-derived macrophages to clear necrotic debris after myocardial injury.21,22 Thereafter, tissue macrophages transition to a phenotype of inflammation resolution and tissue repair. This complex inflammatory response, initially directed at myocardial repair, may become dysregulated and cause adverse cardiac remodeling and worsen prognosis.23 These observations lend biological credence to our findings where both neutrophils and monocytes were independent risk factors for increased cardiovascular mortality.
The critical role of monocytes in the pathophysiology of cardiovascular diseases such as atherosclerosis, heart failure, and atrial fibrillation is well described.24,25 Distinct subsets of circulating monocytes with a pro-inflammatory or reparative phenotype have been identified in humans and mouse models.21,26 The elevated risk of cardiovascular death with elevated neutrophil and monocyte count in our study may reflect ongoing myocardial and vascular damage and/or dysregulated inflammation in ambulatory adults without pre-existing cardiovascular disease.
We also found that a low lymphocyte count is not an independent risk factor for cardiovascular mortality, in contradistinction to previous reports in advanced heart failure.27 The association of a lower lymphocyte count with cardiovascular mortality was not observed in the fully adjusted model, suggesting that the adverse prognostic role of decreased lymphocytes is likely mediated (or modulated) by other co-variates. Increased cortisol production during stress shifts the leukocyte production in favor of neutrophils and monocytes over lymphocytes.28 This might explain why the neutrophil-lymphocyte ratio and MLR may be a better marker of inflammation than absolute neutrophil or monocyte count.
Elevated CRP is a widely used marker for adverse cardiovascular outcomes, both in the general population and in patients with cardiovascular disease. Further, CRP is the most widely used inflammatory marker in cardiovascular risk prediction models.7,8 The exact role of CRP in the pathogenesis of cardiovascular diseases is not clear. In atherosclerosis, it has been proposed that some isoforms of CRP drive increased expression of proinflammatory adhesion molecules that mediate the migration of monocytes to atheromatous plaques.29 Further, activated monocytes in the arterial wall have been reported to be critical for hypertension-induced vascular remodeling in humans.30 The above observations, together with a weaker association between CRP and CV mortality in our study, lead us to speculate that CRP may not reliably indicate tissue-level immune cell recruitment and dysregulated responses as compared to circulating immune cells. Another possibility is that CRP may not be as reliable as MLR as a marker of non-atherosclerotic (such as heart failure and cardiometabolic disease) myocardial insult. Further, the monocytes recruited to the myocardium may be the final pathway after myocardial damage leading to adverse myocardial remodeling. Thus, monocytes have a higher predictive value than lipoproteins that drive atherosclerosis and may associate with the severity of atherosclerosis at the time of measurement in those without established CV disease.31,32 Our study shows that MLR may be a better risk prognosticator than CRP in those without prevalent CVD while validating the association of CRP with CV events. Collectively, these conceivable but hypothesis-generating observations may explain the higher predictive value of the MLR than CRP and lipoproteins to predict cardiovascular mortality in our study.
The adverse prognostic implications of readily available circulating immune cell markers for adverse cardiovascular outcomes in healthy participants have been previously reported in separate studies.1,3,7,8,10,11 Despite their independent adverse prognostic role, traditional risk prediction models such as the 10-year ASCVD risk score do not include any immune markers.6 To the best of our knowledge, this is the first time that the relative contribution of total and differential leukocyte counts, neutrophil-lymphocyte ratio and MLR, and CRP have been compared in a single risk prediction model with an established risk score to predict 10-year cardiovascular mortality. Randomized control trials have suggested a beneficial role of immunomodulation in secondary prevention to reduce adverse cardiovascular events.33,34 Future studies in other prospective cohorts are needed to validate these findings and investigate if immunomodulation has a role in the primary prevention of cardiovascular disease in subjects with a cardio-inflammatory phenotype. Immune markers, such as MLR, can help identify a subset of patients without cardiovascular disease who may benefit from immunomodulation.
Our study has important limitations. The baseline values of circulating immune markers were used in the prediction model. We could not correlate temporal trends of immune markers with cardiovascular mortality due to a lack of serial measurements in NHANES. Even though we excluded those with leukocyte counts outside the normal range, the measures of circulating immune markers could have been confounded by an acute illness at the time of enrollment. We used a self-reported history of heart attack, angina, coronary heart disease, heart failure, and stroke at baseline to exclude patients with pre-existing cardiovascular disease which may have resulted in bias. However, prior data shows good reliability and validity of self-reported comorbidities in the NHANES data.35 In the study population, 41% of participants were outside the age range of ASCVD (<40 years or >79 years); the 10-year ASCVD risk score estimation in these participants may have over- or under-estimated the cardiovascular risk. The 10-year ASCVD risk score predicts future risk of non-fatal myocardial infarction or coronary heart disease death or fatal or nonfatal stroke over 10 years.6 However, due to the lack of other outcomes in the NHANES, we used CV mortality as an outcome and it is plausible that the 10-year ASCVD risk score overestimated the risk. But the fact that up the classification of risk of CV mortality was better than the ASCVD score indicates that it is unlikely that the results observed in our study were simply due to overestimation of risk.
Conclusion
Several circulating immune markers are independently associated with cardiovascular mortality in ambulatory US adults. The MLR has the best predictive value for cardiovascular mortality among readily available circulating immune markers. Further study is needed to confirm our findings and understand the pathobiology linking immune cells and cardiovascular risk.
Supplementary Material
Figure 4:
Dose-response relationship between monocyte-lymphocyte ratio and cardiovascular mortality. The solid red line represents the incidence rate, and the dashed red lines represent the 95% confidence interval (CI). There was a linear increase in the risk of cardiovascular mortality with increase in the monocyte-lymphocyte ratio.
Funding:
Dr. Bajaj is supported by Walter B. Frommeyer, Jr. Fellowship in Investigative Medicine awarded by the University of Alabama at Birmingham, the American College of Cardiology Presidential Career Development Award and the National Center for Advancing Translational Research of the National Institutes of Health under award number UL1TR001417. Dr. Prabhu is or was supported by NIH R01 grants HL125735 and HL147549 and a VA Merit Award I01 BX002706.
Abbreviations
- ASCVD
Atherosclerotic cardiovascular disease
- AUC
Area under the curve
- CI
Confidence interval
- CRP
C-reactive protein
- IQR
Interquartile range
- MLR
Monocyte-lymphocyte ratio
- NHANES
National Health and Nutrition Examination Survey
- NRI
Net-reclassification index
Footnotes
Conflicts of Interest: None of the authors had any conflicts of interest or financial disclosures to declare directly related to this investigation.
References:
- 1.Friedman GD, Klatsky AL, Siegelaub AB. The Leukocyte Count as a Predictor of Myocardial Infarction. New England Journal of Medicine. 1974;290(23):1275–1278. [DOI] [PubMed] [Google Scholar]
- 2.Ernst E, Hammerschmidt DE, Bagge U, Matrai A, Dormandy JA. Leukocytes and the risk of ischemic diseases. Jama. 1987;257(17):2318–2324. [PubMed] [Google Scholar]
- 3.Sweetnam PM, Thomas HF, Yarnell JW, Baker IA, Elwood PC. Total and differential leukocyte counts as predictors of ischemic heart disease: the Caerphilly and Speedwell studies. American journal of epidemiology. 1997;145(5):416–421. [DOI] [PubMed] [Google Scholar]
- 4.Bajaj NS, Kalra R, Gupta K, et al. Leucocyte count predicts cardiovascular risk in heart failure with preserved ejection fraction: insights from TOPCAT Americas. ESC Heart Fail. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Agostino RB Sr., Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–753. [DOI] [PubMed] [Google Scholar]
- 6.Goff DC Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–73. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98(8):731–733. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Glynn RJ, Hennekens CH. C-Reactive Protein Adds to the Predictive Value of Total and HDL Cholesterol in Determining Risk of First Myocardial Infarction. Circulation. 1998;97(20):2007–2011. [DOI] [PubMed] [Google Scholar]
- 9.National Health and Nutrition Examination Survey: Plan and Operations 1999-2010. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/data/series/sr_01/sr01_056.pdf. Accessed 05/07/2020. [Google Scholar]
- 10.Kim S, Eliot M, Koestler DC, Wu W-C, Kelsey KT. Association of Neutrophil-to-Lymphocyte Ratio With Mortality and Cardiovascular Disease in the Jackson Heart Study and Modification by the Duffy Antigen Variant. JAMA Cardiology. 2018;3(6):455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gijsberts CM, Ellenbroek GHJM, ten Berg MJ, et al. Effect of Monocyte-to-Lymphocyte Ratio on Heart Failure Characteristics and Hospitalizations in a Coronary Angiography Cohort. American Journal of Cardiology. 2017;120(6):911–916. [DOI] [PubMed] [Google Scholar]
- 12.National Center for Health Statistics. Office of Analysis and Epidemiology, Public-use Linked Mortality File. 2015. https://www.cdc.gov/nchs/data-linkage/mortality-public.htm. Accessed February 27, 2020. [Google Scholar]
- 13.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. [DOI] [PubMed] [Google Scholar]
- 14.Liu X Classification accuracy and cut point selection. Stat Med. 2012;31(23):2676–2686. [DOI] [PubMed] [Google Scholar]
- 15.Schoenfeld D Partial residuals for the proportional hazards regression model. Biometrika. 1982;69(1):239–241. [Google Scholar]
- 16.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Statistics in Medicine. 2011;30(4):377–399. [DOI] [PubMed] [Google Scholar]
- 17.Pencina MJ, D'Agostino RB Sr., D'Agostino RB Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Statistics in medicine. 2008;27(2):157–212. [DOI] [PubMed] [Google Scholar]
- 18.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. The New England journal of medicine. 1997;336(14):973–979. [DOI] [PubMed] [Google Scholar]
- 19.Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res. 2015;116(7):1254–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adamo L, Rocha-Resende C, Prabhu SD, Mann DL. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol. 2020;17(5):269–285. [DOI] [PubMed] [Google Scholar]
- 21.Nahrendorf M, Swirski FK, Aikawa E, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. The Journal of Experimental Medicine. 2007;204(12):3037–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prabhu SD, Frangogiannis NG. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ Res. 2016;119(1):91–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Libby P, Swirski FK, Nahrendorf M. The Myocardium. Journal of the American College of Cardiology. 2019;74(25):3136. [DOI] [PubMed] [Google Scholar]
- 24.Libby P Inflammation in atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2012;32(9):2045–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shahid F, Lip GYH, Shantsila E. Role of Monocytes in Heart Failure and Atrial Fibrillation. Journal of the American Heart Association. 2018;7(3):e007849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong KL, Yeap WH, Tai JJY, Ong SM, Dang TM, Wong SC. The three human monocyte subsets: implications for health and disease. Immunologic Research. 2012;53(1):41–57. [DOI] [PubMed] [Google Scholar]
- 27.Ommen SR, Hodge DO, Rodeheffer RJ, McGregor CG, Thomson SP, Gibbons RJ. Predictive power of the relative lymphocyte concentration in patients with advanced heart failure. Circulation. 1998;97(1):19–22. [DOI] [PubMed] [Google Scholar]
- 28.Thomson SP, McMahon LJ, Nugent CA. Endogenous cortisol: a regulator of the number of lymphocytes in peripheral blood. Clin Immunol Immunopathol. 1980;17(4):506–514. [DOI] [PubMed] [Google Scholar]
- 29.Thiele JR, Habersberger J, Braig D, et al. Dissociation of pentameric to monomeric C-reactive protein localizes and aggravates inflammation: in vivo proof of a powerful proinflammatory mechanism and a new anti-inflammatory strategy. Circulation. 2014;130(1):35–50. [DOI] [PubMed] [Google Scholar]
- 30.Ishibashi M, Hiasa K-i, Zhao Q, et al. Critical Role of Monocyte Chemoattractant Protein-1 Receptor CCR2 on Monocytes in Hypertension-Induced Vascular Inflammation and Remodeling. Circulation Research. 2004;94(9):1203–1210. [DOI] [PubMed] [Google Scholar]
- 31.Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109(21 Suppl 1):II2–II10. [DOI] [PubMed] [Google Scholar]
- 32.Hashimoto K, Kataoka N, Nakamura E, Tsujioka K, Kajiya F. Oxidized LDL specifically promotes the initiation of monocyte invasion during transendothelial migration with upregulated PECAM-1 and downregulated VE-cadherin on endothelial junctions. Atherosclerosis. 2007;194(2):e9–e17. [DOI] [PubMed] [Google Scholar]
- 33.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. New England Journal of Medicine. 2017;377(12):1119–1131. [DOI] [PubMed] [Google Scholar]
- 34.Tardif J-C, Kouz S, Waters DD, et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. New England Journal of Medicine. 2019;381(26):2497–2505. [DOI] [PubMed] [Google Scholar]
- 35.Bergmann MM, Byers T, Freedman DS, Mokdad A. Validity of self-reported diagnoses leading to hospitalization: a comparison of self-reports with hospital records in a prospective study of American adults. Am J Epidemiol. 1998;147(10):969–977. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.