Supplemental Digital Content is available in the text.
Keywords: Environmental phenols, Parabens, Diabetes, Midlife women, Mixture
Abstract
Background:
Environmental phenols have been suggested as diabetogens but evidence from prospective cohort studies is limited. We examined associations between urinary concentrations of phenols and parabens, assessed at two time-points, and incident diabetes in the Study of Women’s Health Across the Nation (SWAN).
Methods:
We examined 1,299 women, aged 45–56 years, who were diabetes-free at baseline of the SWAN Multi-Pollutant Study (MPS) (1999–2000) and were followed through January 2017. Urinary concentrations of bisphenol-A, bisphenol-F, triclosan, 2,4-dichlorophenol, 2,5-dichlorophenol, benzophenone-3, methyl-paraben, ethyl-paraben, propyl-paraben, and butyl-paraben were measured twice at MPS baseline and 3 years later (2002–2003), and the two average concentrations were used as exposure variables. Associations of incident diabetes with individual phenols and parabens were examined using Cox regression. We evaluated the overall joint effects using quantile-based g-computation.
Results:
Adjusted hazard ratios (HRs) for incident diabetes of the third tertile compared with the first tertile of urinary concentrations were 0.40 (95% confidence interval [CI] = 0.29, 0.56) for methyl-paraben; 0.42 (0.30, 0.58) for propyl-paraben; 0.53 (0.38, 0.75) for 2,5-diclrorophenol; and 0.55 (0.39, 0.80) for benzophenone-3. Nonlinear associations were found for bisphenol-A and 2,4-dichlorophenol (significant positive associations in the second tertile but no associations in the third tertile compared with the first tertile). No significant associations were observed for the other individual chemicals or the joint effect of mixtures.
Conclusions:
Our findings do not support diabetogenic effects of urinary parabens which were inversely associated with incident diabetes among mid-life women. Epidemiologic findings for biomarkers with short half-lives and high within-person variability need to be interpreted with caution.
What this study adds
We investigated the association between urinary concentrations of phenols and parabens and incident diabetes using a large prospective cohort study of midlife women. Urinary phenols and parabens were assessed at two time-points, which allows us to evaluate reliability of the associations and account for within-person variability. We found inverse associations between average concentrations of urinary parabens and incident diabetes. We found nonlinear associations for average concentrations of urinary bisphenol-A and 2,4-dichlorophenols and incident diabetes (significant positive associations in the second tertile but no associations in the third tertile compared with the first tertile). Additionally, we found no significant association for the overall joint effect of phenols and parabens as mixtures using quantile-based g-computation.
Introduction
Diabetes is one of the fastest growing public health problems worldwide. In 2019, the International Diabetes Federation estimated that 463 million people (9.3% of adults with 20–79 years of age) suffered from diabetes globally with the number expected to increase to 700 million by 2045 (type 1 and type 2 combined of which approximately 90% are type 2).1 Type 2 diabetes mellitus (T2DM), characterized by high blood sugar caused by insulin resistance and relative insulin deficiency, increases the risk of developing cardiovascular disease, renal disease, neurological disorders, and certain cancers.2 Recent studies suggest that endocrine-disrupting chemicals (EDCs) including phenols and parabens, may play a role in the etiology of T2DM.3
Environmental phenols and parabens are synthetic chemicals extensively used in personal care and consumer products. For example, bisphenol A (BPA) and its analogues, such as bisphenol S (BPS) and bisphenol F (BPF), are used in polycarbonate plastics and epoxy resins to coat the interior of food cans and food and beverage storage containers.4 Triclosan are broad spectrum antibacterial agents added to soaps, toothpastes, and underarm deodorants.5 Parabens are mainly used as antimicrobial preservatives in cosmetics.6 Benzophenone-3, an ultraviolet (UV) filter, is widely used in sunscreen products.7 2,5-dichlorophenol is a major metabolite of 1,4-dichlorobenzene, which has been used for mothballs and space deodorants.8 2,4-dichlorophenol is used in the production of phenoxy acid herbicides such as 2,4-diphenoxyacetic acid.8
Previous experimental studies suggest several potential underlying mechanisms between BPA exposure and diabetes. BPA may increase the risk of diabetes through insulin resistance, disrupted gluconeogenesis, pancreatic beta-cell dysfunction, or impaired insulin secretion.9 Furthermore, recent studies have found that BPS and BPF, BPA alternatives, can be as hormonally active as BPA.10 Experimental evidence for other phenols and parabens and diabetes is very limited. Phenols and parabens interfere with estrogens and androgens by binding to the extracellular face of membrane-bound receptors or by diffusing across cell membranes and binding to intracellular receptors.11,12 Phenols and parabens also activate the expression of peroxisome proliferator–activated receptor-γ (PPARγ) and promote adipogenesis which can affect glucose homeostasis.13,14
Previous epidemiological studies, conducted mostly in cross-sectional settings, reported that higher urinary bisphenols and 2,5-dichlorophenol concentrations were associated with a higher risk of diabetes,15,16 whereas higher urinary parabens and benzophenone-3 concentrations were associated with lower risk of diabetes.17,18 However, information based on prospective designs is limited, raising concerns related to causal inference and problems of reverse causality. One longitudinal study reported that urinary concentrations of BPA were associated with higher risk of T2DM in the Nurses Health cohorts.19 One French study20 reported that urinary concentrations of BPA and BPS were associated with incident T2DM. No previous prospective study has investigated the effects of other phenols and parabens on incident diabetes. In addition, most studies used phenols and paraben concentrations evaluated at only one point in time, raising concerns of measurement error.
Herein, we examined the associations between urinary concentrations of phenols and parabens and incident diabetes in a multi-site, multiethnic prospective cohort of middle-aged women in the Study of Women’s Health Across the Nation (SWAN). Although the types of diabetes were not determined in our study, incident diabetes cases during this life stage are overwhelmingly T2DM and truly identifying type 1 cases is difficult due to the co-occurrence of many T2DM-related risk factors.21 Urinary phenols and parabens were assessed at two time points, three years apart, to evaluate consistency of the findings. We conducted an exploratory analysis to evaluate the overall joint effects of phenols and parabens as mixtures given that no standard approach for mixture effects in relation to time-to-event data is available yet.
Methods
Study population
SWAN is a multisite, multiethnic, community-based longitudinal study designed to evaluate the effect of menopausal transition on women’s health and to identify risk factors for age-related chronic diseases.22 Between 1996 and 1997, 3,302 women were recruited from seven study sites, namely Boston, MA; Chicago, IL; southeast Michigan, MI; Los Angeles, CA; Oakland, CA; Newark, NJ; and Pittsburgh, PA. White women from all sites and women from one specified minority group in each site were enrolled (Black women from Boston, Chicago, Southeast Michigan, and Pittsburgh; Chinese women from Oakland; Japanese women from Los Angeles; Hispanic women from Newark). Eligibility criteria for enrollment into the SWAN cohort included: age 42–52 years, intact uterus and at least one ovary, no use of exogenous hormones affecting ovarian function in the past 3 months, at least one menstrual period in the previous three months, and self-identification with the site’s designated racial/ethnic groups. Participants have undergone clinical examinations and laboratory tests near annually. Institutional Review Board approval was obtained at each study site, and all participants provided signed informed consent at each study visit.
The SWAN Multi-Pollutant Study (MPS) was designed to evaluate exposure to environmental chemicals, including environmental phenols and parabens, and metabolic diseases. The third SWAN follow-up visit was used as the MPS baseline with 1,400 women sampled for the SWAN MPS including 4 racial/ethnic groups (White, Black, Chinese, and Japanese) and 5 study sites (Boston, Pittsburgh, Southeast Michigan, Los Angeles, Oakland). For assessment of environmental phenols and parabens, we analyzed urine samples collected at MPS baseline and the third follow-up visits (1999–2000 [n = 1,400] and 2002–2003 [n = 1,386], respectively) to evaluate consistency of association for nonpersistent chemicals. For the present study, we excluded prevalent diabetes cases at the MPS baseline, missing data for urinary phenols, parabens, and creatinine concentrations at the two exposure measurements, yielding 1,299 participants for data analysis (eFigure 1; http://links.lww.com/EE/A154).
Assessment of urinary phenol and paraben concentrations
All specimens were collected and stored in the SWAN Repository (http://swanrepository.com/) using a systematic protocol: urine specimens were collected in a 50-mL sterile cup made from clear polypropylene before 9 am but were not the first voided urine. Although the urine did not need to be refrigerated between the time of collection and delivery to the study center that same morning, it was protected from direct sunlight by wrapping in brown paper bag to protect it from sunlight. After receiving the urine from the participant, it was immediately aliquoted into 0.5 mL vials, which were frozen and stored in ultra-low freezers at –80°C without thawing until they were analyzed for the phenol and paraben contents. A total of eight phenols (BPA, BPS, BPF, 2,4-dichlorophenol, 2,5-dichlorophenol, triclosan, triclocarban, and benzophenone-3) and four parabens (methyld-paraben, ethyl-paraben, propyl-paraben, an butyl-paraben) were measured using online solid phase extraction coupled to high-performance liquid chromatography–isotope dilution tandem mass spectrometry (MS/MS) by the Applied Research Center of NSF International (Ann Arbor, Michigan), a part of the Michigan Children’s Health Exposure Analysis Resource (M-CHEAR) Laboratory Hub. We used the laboratory procedure manual published by the Centers for Disease Control and Prevention (CDC) (Method 6301.01). The allowed percent deviation from nominal quality controls (QCs) values at all QC level is +2 standard deviations of the mean of each QC level determined from historical QC data for each of the phenols and parabens in the method. The limits of detections (LODs) of urinary phenols, and parabens concentrations, ranged from 5.2% to 99.8% (Table 1 and eTable 1; http://links.lww.com/EE/A154) at both time points of exposure assessment. We excluded BPS and triclocarban because of low detection rate (5.2–17.3% for BPS and 9.5–11.4% for triclocarban), thus six phenols and four parabens were included in this study. Urinary phenols and parabens concentrations below LODs were not imputed with constants because of potential bias.23
Table 1.
Summary statistics of creatinine-adjusted urinary concentrations of phenols and parabens at MPS baseline (1999–2000) and MPS follow-up visit 3 (2002–2003)
| Detection rate (%) | LOD | GMa | GSDa | Percentiles | ||||
|---|---|---|---|---|---|---|---|---|
| Min | P33 | P66 | Max | |||||
| MPS baseline | ||||||||
| BPA | 83.0 | 0.4 | 1.39 | 2.84 | <LOD | 0.83 | 2.10 | 76.81 |
| BPF | 73.7 | 0.4 | 0.99 | 2.86 | <LOD | 0.53 | 1.41 | 56.87 |
| 2,4-diclorophenosl | 98.6 | 0.2 | 3.68 | 4.23 | <LOD | 1.75 | 5.35 | 1,168.05 |
| 2,5-diclorophenosl | 99.8 | 0.2 | 12.79 | 5.63 | <LOD | 4.73 | 18.52 | 6,942.46 |
| Triclosan | 81.1 | 2.0 | 17.23 | 5.94 | <LOD | 5.97 | 35.40 | 2,468.05 |
| Methyl-paraben | 99.8 | 1.0 | 130.47 | 4.66 | <LOD | 79.64 | 297.26 | 4,440.59 |
| Ethyl-paraben | 60.5 | 1.0 | 3.91 | 6.23 | <LOD | <LOD | 5.98 | 1,218.74 |
| Propyl-paraben | 97.8 | 0.2 | 22.64 | 6.88 | <LOD | 10.29 | 60.53 | 1,895.33 |
| Butyl -paraben | 54.4 | 0.2 | 0.79 | 6.83 | <LOD | <LOD | 1.22 | 274.76 |
| Benzophenone-3 | 97.7 | 0.4 | 24.01 | 12.18 | <LOD | 4.57 | 69.42 | 21,493.4 |
| MPS follow-up visit 3 | ||||||||
| BPA | 93.4 | 0.4 | 1.77 | 2.40 | <LOD | 1.21 | 2.40 | 333.35 |
| BPF | 80.6 | 0.4 | 1.11 | 2.64 | <LOD | 0.71 | 1.47 | 86.32 |
| 2,4-diclorophenosl | 99.0 | 0.2 | 2.01 | 3.53 | <LOD | 1.06 | 2.35 | 902.32 |
| 2,5-diclorophenosl | 99.3 | 0.2 | 7.92 | 5.50 | <LOD | 3.06 | 11.37 | 4,447.77 |
| Triclosan | 82.9 | 2.0 | 16.04 | 5.57 | <LOD | 6.41 | 25.99 | 13,315.6 |
| Methyl-paraben | 99.6 | 1.0 | 128.93 | 4.61 | <LOD | 81.02 | 269.30 | 11,504.7 |
| Ethyl-paraben | 60.9 | 1.0 | 4.37 | 5.78 | <LOD | <LOD | 6.97 | 2,054.10 |
| Propyl-paraben | 98.4 | 0.2 | 19.17 | 6.74 | <LOD | 8.67 | 55.86 | 2,760.12 |
| Butyl-paraben | 59.3 | 0.2 | 0.95 | 6.91 | <LOD | <LOD | 1.70 | 423.29 |
| Benzophenone-3 | 97.7 | 0.4 | 33.28 | 11.82 | <LOD | 7.72 | 92.09 | 41,715.0 |
aGeometric mean and geometric standard deviation was calculated after substituting values below LOD with LOD/√2.
GM, geometric mean; GSD, geometric standard deviation; LOD, limit of detection; MPS, multipollutant study.
Incident diabetes ascertainment
We used conservative criteria to ascertain incident diabetes to minimize outcome misclassification. A participant was considered to have incident diabetes if she met any of the following criteria during follow-up24–26: (1) use of an antidiabetic medication at any visit, (2) fasting glucose ≥126 mg/dL on at least half of 3 attended visits or two consecutive visits, or (3) any two visits with self-reported diabetes and at least one visit with fasting blood glucose ≥126 mg/dL. Diabetes cases were not distinguished between type 1 and type 2 in the SWAN, but the majority of diabetes cases after age 40 are T2DM.
Covariates
Sociodemographic factors including age, site, race/ethnicity, and educational attainment were assessed during the SWAN baseline examination (1996–1997). Since only one minority group was recruited from each site, a single combined site and race/ethnicity variable was created and used in data analysis. Education was categorized as high school diploma or less, some college, a four-year college, and graduate school or higher. Lifestyle variables included physical activity and smoking. Self-reported physical activity was collected at the MPS baseline and follow-up visit 3 and smoking status was collected at every visit using a self-administered questionnaire. Total physical activity score was based on the sum of each physical activity score in three domains (sports/exercise, household/caregiving, and daily routine), with scores in each domain ranging from 1 to 5 (total physical activity score ranged from 3 to 15 with 15 indicating the highest level of activity). Cigarette smoking was categorized as never, former, or current smoking. Menopause status and objectively measured body mass index (BMI) were also collected at every visit. Menopause status was determined from questions on bleeding patterns and categorized into premenopause, perimenopause, postmenopause, or current hormone use. Total caloric intake was collected at the SWAN baseline and visit 5, using a detailed semiquantitative food frequency questionnaire (FFQ) adopted from the Block FFQ. Urine creatinine, a maker of urine dilution, was determined using the Cobas Mira analyzer (Horiba ABX, Montpellier, France).
Statistical analysis
Urinary phenol and paraben concentrations were divided by urinary creatinine concentration to adjust the urine volume.27 Spearman correlations coefficients between urinary creatinine-adjusted phenol and paraben concentrations and between the two measurement times were calculated and presented via. correlation-matrix heat-maps. Creatinine-adjusted intraclass correlations (ICCs) of log-transformed urinary concentrations between two time-points (MPS baseline and three years later) were calculated using linear-mixed models. Based on the ICC, reliability of exposure marker was determined as poor (ICC < 0.40), fair (0.40≤ ICC < 0.60), good (0.6≤ ICC < 0.75), and excellent (ICC ≥ 0.75).28 This criterion was used as the reliability indicator of the observed associations.
As participants in the SWAN-MPS cohort may differ from the original SWAN cohort, we assigned weights to participants based on inverse probability weighting (IPW) to create a pseudo population representative of the original cohort, to address potential selection bias (see eMethods; http://links.lww.com/EE/A154; for details of the inverse probability weighting method).
To examine the associations of incident diabetes with phenols and parabens individually, we used Cox proportional hazards models. Follow-up time was computed from MPS baseline to the date of first diabetes event, loss to follow-up, or the date of the final SWAN visit (16th follow-up visit in 2016–2017). The average concentrations of two exposure measurements were used as the primary exposure variables. As secondary analyses, we also used exposure concentrations measured at MPS baseline and at MPS follow-up visit 3 each as exposure variables. Visit-specific tertile cutoffs were used. We fitted exposure variables as tertiles instead of continuous because agreement of the rank orders across tertiles is relatively high-despite high within-person variations of these nonpersistent chemicals. All models were adjusted for age at baseline, site and ethnicity, education levels, physical activity score, menopausal status, smoking status, and total caloric intake. Education and menopausal status were treated as strata because these variables did not hold the proportional hazards assumption. All covariates were collected at the baseline of each exposure time point. Hazard ratios (HRs) and 95% confidence intervals (CIs) were computed comparing the second and the third tertiles with the first tertile. For the chemicals with detection rates < 2/3 (ethyl and butyl parabens), we dichotomized them at the LOD and compared women who had concentrations >LOD versus ≤LOD.
To evaluate the overall joint effects of phenols and parabens as mixtures, we used a quantile based g-computation approach, which was recently proposed for addressing the effects of chemical mixture.29 Quantile based g-computation has certain advantages over other mixture approaches such as weighted quantile sum regression.30 Quantile based g-computation does not require the directional homogeneity assumption and therefore, it can estimate the mixture effect of chemicals that are either positively or negatively associated with a health outcome. Further, this approach can capture nonlinearity because it allows the inclusion of interaction terms and quadratic terms in the model for estimating mixture effect. Tertile was used as the quantile. We also included quadratic and pairwise interaction terms to capture nonlinear associations. The same confounders listed earlier were included in the base model. Cox proportional hazards model was utilized as the underlying model. The overall mixture effect was estimated as the sum of estimated regression coefficients (i.e., log HR) of all phenol and paraben variables. The overall mixture effect can be interpreted as the hazard ratio of incident diabetes per one tertile of change in all urinary phenol and paraben concentrations although controlling for covariates. For this analysis, we utilized the R package “qgcomp” version 2.6.0.31
We conducted sensitivity analyses to evaluate robustness of the associations. First, we evaluated whether additional adjustment for BMI substantially reduced HRs because BMI could be a mediator in the associations between phenols/parabens and incident diabetes. Likewise, we also evaluated the mediating role of menopausal status after excluding it from the model. Second, we evaluated the association of urinary phenols and parabens with incident diabetes without incorporating inverse probability weighting. Third, we also examined the associations of creatinine-unadjusted urinary phenols and parabens with incident diabetes to evaluate the impact of creatinine adjustment. Fourth, we restricted the follow-up time up to 6 years with the assumption that exposure assessment was conducted at baseline and in the middle of follow-up. Finally, we examined the associations with continuous scales of urinary phenol and paraben concentrations. All analyses were conducted using SAS, version 9.3 (SAS Institute Inc.) and R, version 3.6.1 (R Core Team 2019).
Results
Study population
The characteristics of the study population at the MPS baseline stratified by incident diabetes are described in Table 2. During the SWAN follow-up after the MPS baseline (median = 16 years), 132 incident cases of diabetes were identified (incidence rate = 7.07 per 1,000 person-years; 95% CI = 5.93, 8.35). The average age of participants was 49.03 years (SD = 2.65); it was significantly higher in women with subsequent incident diabetes than those without incident diabetes (P < 0.001). Study site, race/ethnicity, total physical activity score, total caloric intake, and BMI differed significantly by subsequent incident diabetes status (P < 0.01). Generally, women with incident diabetes were more likely to be physically inactive, consume more total caloric intake per day, and have higher BMI (characteristics at MPS follow-up visit 3 are presented in eTable 2; http://links.lww.com/EE/A154).
Table 2.
Characteristics of the study population at MPS-baseline, overall and by incident diabetes status (1999–2000)
| Characteristic | Total(N = 1,299) | No incident diabetes (N = 1,167) | Incident diabetic (N = 132) | P |
|---|---|---|---|---|
| Age (years) | 49.03 ± 2.65 | 49.00 ± 2.65 | 49.29 ± 2.73 | <0.001 |
| Site | <0.001 | |||
| Los Angeles | 349 (26.87) | 325 (27.85) | 24 (18.18) | |
| Oakland | 285 (21.94) | 260 (22.28) | 25 (18.94) | |
| Michigan | 234 (18.01) | 191 (16.37) | 43 (32.58) | |
| Pittsburgh | 218 (16.78) | 191 (16.37) | 27 (20.45) | |
| Boston | 213 (16.40) | 200 (17.14) | 13 (9.85) | |
| Race/ethnicity | <0.001 | |||
| White | 672 (51.73) | 620 (53.13) | 52 (39.39) | |
| Black | 270 (20.79) | 222 (19.02) | 48 (36.36) | |
| Chinese | 161 (12.39) | 146 (12.51) | 15 (11.36) | |
| Japanese | 196 (15.09) | 179 (15.34) | 17 (12.88) | |
| Education levels | 0.067 | |||
| High schools or less | 223 (17.17) | 199 (17.05) | 24 (18.18) | |
| Some college | 409 (31.49) | 354 (30.33) | 55 (41.67) | |
| College | 325 (25.02) | 300 (25.71) | 25 (18.94) | |
| Postcollege | 335 (25.79) | 308 (26.39) | 27 (20.45) | |
| Missing | 7 (0.53) | 6 (0.51) | 1 (0.76) | |
| Physical activity score | 7.86 ± 1.74 | 7.93 ± 1.75 | 7.32 ± 1.61 | <0.001 |
| Menopause status | 0.127 | |||
| Premenopause | 155 (11.93) | 147 (12.60) | 79 (59.85) | |
| Perimenopause | 761 (58.58) | 682 (58.44) | 20 (15.15) | |
| Postmenopause | 179 (13.78) | 159 (13.62) | 8 (6.06) | |
| Hormone therapy | 204 (15.70) | 179 (15.34) | 25 (18.94) | |
| Smoking status | 0.214 | |||
| Never | 819 (63.05) | 743 (63.67) | 76 (57.58) | |
| Former | 346 (26.64) | 310 (26.52) | 36 (27.27) | |
| Current | 133 (10.24) | 113 (9.68) | 20 (15.15) | |
| Missing | 1 (0.08) | 1 (0.99) | 0 (0.00) | |
| Total caloric intake (kcal/d) | 1,808 ± 692 | 1,789 ± 687 | 2,001 ± 0.709 | <0.001 |
| Body mass index (kg/m2) | 27.48 ± 6.87 | 26.86 ± 6.48 | 33.07 ± 7.69 | <0.001 |
Data are expressed as mean ± standard deviation (SD) or n (%).
Urinary phenols and parabens
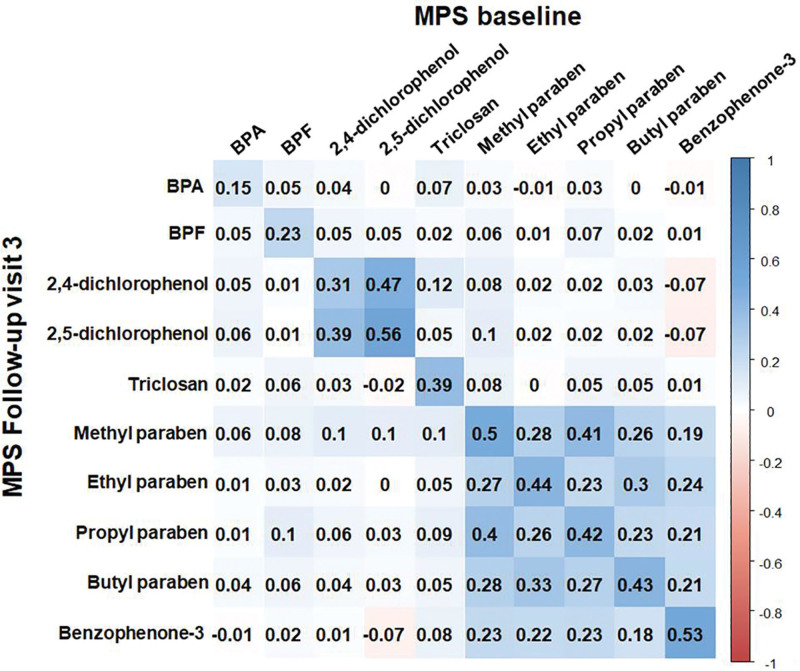
The distribution of urinary phenol and paraben concentrations and creatinine-adjusted phenol and paraben concentrations at MPS baseline and the follow-up visit 3 are provided in Table 1 and eTable 1; http://links.lww.com/EE/A154, respectively. In general, the phenol and paraben concentrations at the MPS follow-up visit 3 were lower than those at MPS baseline (eFigure 2; http://links.lww.com/EE/A154). Figure 1 shows the Spearman correlation matrix of the log-transformed creatinine-adjusted phenol and paraben concentrations. Parabens and benzophenone-3 were modestly and positively correlated with each other at both visits but bisphenols were not correlated with each other. Creatinine-adjusted ICCs of urinary BPA between visits were less than 0.2, whereas those of the other chemicals ranged from 0.30 to 0.59 (eFigure 2; http://links.lww.com/EE/A154). Reliability of exposure marker based on ICC was poor for bisphenols, 2,4-diclorophenol, triclosan; fair for 2,5-diclorophenol, methyl-paraben, ethyl-paraben, propyl-paraben, butyl-paraben, and benzophenone-3.
Figure 1.
Correlation coefficients matrix between log-transformed creatinine-adjusted urinary phenol and paraben concentrations at MPS baseline (1999–2000) and MPS follow-up visit 3 (2002–2003). The color intensity of boxes indicates the magnitude of correlation. Blue and red colors represent positive and negative correlations, respectively. BPA, bisphenol A; BPF, bisphenol F; MPS, multipollutant study.
Associations of urinary phenol and paraben concentrations with incident diabetes
The ranges of each tertile of creatinine-adjusted urinary phenols and parabens concentrations are presented in Table 1. The associations between the average concentrations of urinary phenol and paraben across the two time points and incident diabetes are presented in Table 3. Adjusted HRs (95% CIs) for incident diabetes comparing the second and third tertiles with the first tertile of urinary paraben concentrations were 0.67 (0.50, 0.89) and 0.40 (0.29, 0.56) for methyl paraben (p-for-trend: <0.001) and 0.60 (0.44, 0.80) and 0.42 (0.30, 0.58) for propyl-paraben (p-for-trend: <0.001), respectively. Adjusted HRs (95% CIs) comparing the second and third tertiles with the first tertile of 2,5-diclorophenol were 0.82 (0.60, 1.13) and 0.53 (0.38, 0.75) (p-for-trend: <0.001). Additionally, an inverse association was observed with benzohenone-3 [HR = 0.80 (95% CI = 0.60, 1.07) for the second tertile and HR=0.55 (95% CI = 0.39, 0.80) for the third tertile]. For BPA that had a poor reliability, a significant positive association was observed in the second tertile [HR=1.67 (95% CI = 1.22, 2.28)] but not in third tertile [HR=1.31 (95% CI = 0.94, 1.83)]. The significant positive association remained after adjustment for BMI [HR=1.77 (95% CI = 1.28, 2.46) for second tertile; HR=1.53 (95% CI = 1.08, 2.16) for third tertile] (eTable 3; http://links.lww.com/EE/A154). Also, the association was robust after excluding menopausal status [HR=1.68 (95% CI = 1.23, 2.30) for second tertile; HR=1.37 (95% CI = 0.98, 1.90) for third tertile] (eTable 4; http://links.lww.com/EE/A154). A positive association was also found for 2,4-dichlorophenol [HR=1.63 (95% CI = 1.18, 2.23) for second tertile; HR=1.28 (95% CI = 0.91, 1.80) for third tertile]. No statistically significant associations were found for other chemicals. Similar associations were found for parabens when concentrations at MPS baseline or the follow-up visit 3 were used as exposure variables but the associations for other chemicals were inconsistent depending on the exposure measurement timing (Table 3).
Table 3.
Adjusted hazard ratios for incident diabetes comparing the second and the third tertiles with the first tertile of creatinine-adjusted urinary phen and paraben concentrations
| Chemical | Reliability of exposure markera | Tertile | Averageb | MPS baselinec | MPS follow-up visit 3d | |||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P trend | HR (95% CI) | P trend | HR (95% CI) | P trend | |||
| BPA | Poor | 0.10 | 0.94 | 0.17 | ||||
| T2 | 1.67 (1.22, 2.28) | 1.26 (0.94, 1.69) | 1.08 (0.74, 1.58) | |||||
| T3 | 1.31 (0.94, 1.83) | 0.96 (0.69, 1.33) | 1.29 (0.89, 1.87) | |||||
| BPF | Poor | 0.91 | 0.06 | 0.45 | ||||
| T2 | 1.27 (0.93, 1.74) | 0.61 (0.44, 0.84) | 1.35 (0.94, 1.94) | |||||
| T3 | 1.01 (0.74, 1.37) | 0.77 (0.57, 1.03) | 1.14 (0.79, 1.65) | |||||
| 2,4-diclorophenol | Poor | 0.20 | 0.10 | 0.67 | ||||
| T2 | 1.63 (1.18, 2.23) | 1.65 (1.19, 2.29) | 0.94 (0.64, 1.36) | |||||
| T3 | 1.28 (0.91, 1.80) | 1.36 (0.98, 1.90) | 0.92 (0.64, 1.33) | |||||
| 2,5-diclorophenol | Fair | <0.001 | <0.001 | 0.12 | ||||
| T2 | 0.82 (0.60, 1.13) | 0.83 (0.60, 1.13) | 0.80 (0.54, 1.18) | |||||
| T3 | 0.53 (0.38, 0.75) | 0.63 (0.45, 0.89) | 0.72 (0.48, 1.08) | |||||
| Triclosan | Poor | 0.38 | 0.69 | 0.69 | ||||
| T2 | 0.99 (0.72, 1.36) | 1.31 (0.96, 1.79) | 0.70 (0.48, 1.01) | |||||
| T3 | 1.14 (0.84, 1.56) | 1.07 (0.78, 1.47) | 0.89 (0.62, 1.28) | |||||
| Methyl-paraben | Fair | <0.001 | <0.001 | 0.002 | ||||
| T2 | 0.67 (0.50, 0.89) | 0.61 (0.45, 0.81) | 1.09 (0.77, 1.55) | |||||
| T3 | 0.40 (0.29, 0.56) | 0.38 (0.27, 0.53) | 0.52 (0.34, 0.78) | |||||
| Ethyl-paraben | Fair | <0.001 | <0.001 | <0.001 | ||||
| ≥LODe | 0.49 (0.38, 0.64) | 0.52 (0.40, 0.67) | 0.59 (0.44, 0.81) | |||||
| Propyl-paraben | Fair | <0.001 | .001 | 0.006 | ||||
| T2 | 0.60 (0.44, 0.80) | 0.67 (0.50, 0.88) | 0.73 (0.52, 1.04) | |||||
| T3 | 0.42 (0.30, 0.58) | 0.36 (0.25, 0.51) | 0.60 (0.41, 0.87) | |||||
| Butyl-paraben | Fair | <0.001 | <0.001 | <0.001 | ||||
| ≥LODe | 0.61 (0.47, 0.79) | 0.82 (0.63, 1.06) | 0.65 (0.48, 0.88) | |||||
| Benzophenone-3 | Fair | 0.001 | 0.65 | 0.13 | ||||
| T2 | 0.80 (0.60, 1.07) | 1.17 (0.87, 1.57) | 0.88 (0.62, 1.24) | |||||
| T3 | 0.55 (0.39, 0.80) | 0.81 (0.57, 1.15) | 0.74 (0.49, 1.10) | |||||
All models were adjusted for age at baseline, site and ethnicity, education levels, physical activity score, menopausal status, smoking status, and total caloric intake. Statistically significant (P < 0.05) HRs (95% CIs) were in bold.
aReliability of exposure marker was determined by intraclass correlation (ICC); poor (ICC < 0.40), fair (0.40 ≤ ICC < 0.60), good (0.60 ≤ ICC < 0.75), and excellent (ICC ≥ 0.75).
bThe models with average of exposures at MPS baseline and follow-up visit 3 were followed from MPS baseline to final SWAN visit.
cThe models with exposures at MPS baseline were followed from MPS baseline to final SWAN visit.
dThe models with exposures at MPS follow-up visit 3 were followed from MPS follow-up visit 3 to final SWAN visit.
eThese chemicals were dichotomized at the limit of detections because of low detections.
BPF, bisphenol F; BPA, bisphenol A.
In sensitivity analyses, the results were essentially unchanged when inverse probability weighting was not used (eTable 5; http://links.lww.com/EE/A154); creatinine-unadjusted urinary phenols and parabens were used (eTable 6; http://links.lww.com/EE/A154); and continuous urinary concentrations of phenols and parabens instead of tertiles were used (eTable 7; http://links.lww.com/EE/A154). Results for BPA, 2,4 dicholorophenol, parabens, and benzophenone-3 remained unchanged when follow-ups were restricted through up to 6 years but the associations for 2,5-dichlorophenol and triclosan were changed [HR = 1.76 (95% CI = 1.06, 2.93) for second tertile and 0.77 (95% CI = 0.41, 1.45) for third tertile of 2,5-dichlorophenol; HR = 1.80 (95% CI = 1.14, 2.84) for second tertile and 0.96 (95% CI = 0.57, 1.62) for third tertile of triclosan] (eTable 8; http://links.lww.com/EE/A154).
Overall joint effect of urinary phenol and paraben mixture on incident diabetes
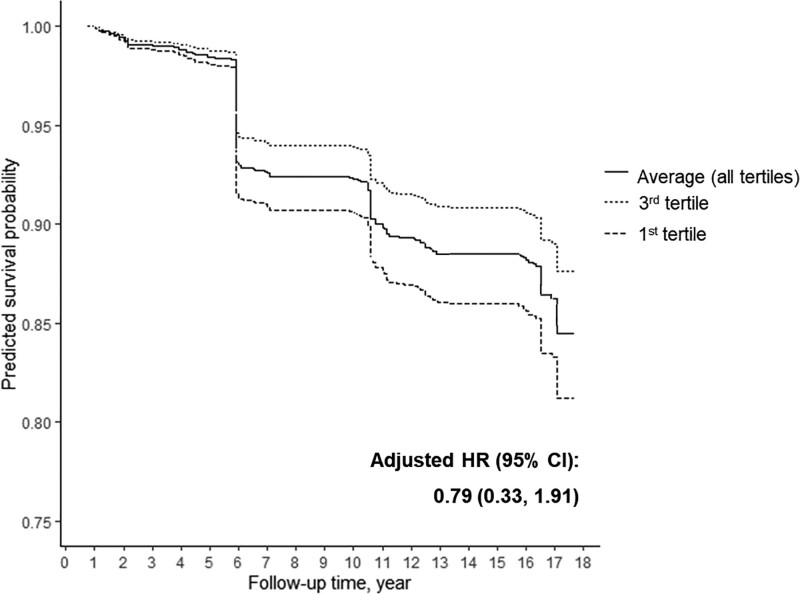
Individual log HRs for incident diabetes per one tertile increase in each linear, quadratic, and pairwise interaction terms of phenol and paraben tertiles (treated as continuous) from the quantile-based g-computation approach are presented in eFigure 3; http://links.lww.com/EE/A154. The directions of the associations with incident diabetes were in line with those of single pollutant models. The overall joint effect was not statistically significant (Figure 2) (HR = 0.79 [95% CI = 0.33, 1.91]). The predicted survival probability when all phenol and paraben concentrations were set at the first tertiles more rapidly decreased over the follow-up than the predicted survival probability when all phenol and paraben concentrations were set at the third tertiles (Figure 2).
Figure 2.
Predicted survival probabilities of incident diabetes estimated by quantile-based g-computation. The predicted survival probabilities were calculated from marginal structure Cox models, assuming that all creatinine-adjusted urinary phenol and paraben concentrations were fixed at first tertiles, third tertiles, or the averages of all tertiles of chemical concentrations. All models were adjusted for age at baseline, site and ethnicity, education levels, physical activity score, menopausal status, smoking status, and total caloric intake. HR, hazard ratio; CI, confidence interval.
Discussion
This study examined the associations between urinary concentrations of six phenols and four parabens with incident diabetes in a multiracial/ethnic, community-based cohort of midlife women in the United States, using repeated phenol and paraben concentrations measured three years apart. We observed inverse associations of parabens, 2,5-dichlorophenol, and benzophenone-3 with incident diabetes. In contrast, we observed nonlinear associations of BPA and 2,4-dichlorophenol with incident diabetes (significant positive associations in the second tertile but no associations in the third tertile compared with the first tertile). The sensitivity analyses suggested that the observed associations for parabens were robust whereas those for other chemicals were inconsistent. These findings suggest that exposure to parabens may be associated with lower risk of incident diabetes. Epidemiologic findings for biomarkers with short half-lives and high within-person variability need to be interpreted with caution.
Phenols and parabens are nonpersistent compounds with short half-lives that are excreted from the human body within 24–48 hours.32–35 Urinary concentrations of these chemicals have high within-person variability over time and the ICCs of urinary phenols and parabens generally range from 0.2 to 0.7.36–38 A study reported that estimations obtained from an epidemiological study using chemicals with an ICC of 0.6 or less may be biased and weakly powered if chemical concentrations are determined from a single spot urine.39 To address this problem, we measured the urinary phenol and paraben concentrations at two time-points and used the average concentrations across the two-time points as exposure variables. Although our exposure assessment at two time-points is a strength, it was insufficient for some chemicals, such as bisphenols and 2,4-dichlorophenol, to make valid causal inference. However, the results were relatively stable when we restricted the follow-up up to 6 years.
We found inverse associations between parabens and incident diabetes. Previous studies in adults found ICCs of parabens ranged from 0.4 to 0.7,37,38 and ICCs for parabens were generally higher than that for BPA, which is also in line with our findings. Only a few studies have examined the association between parabens and diabetes. A case-control study of phenolic chemicals and T2DM in Saudi Arabia (N = 101) showed that the odds ratios (ORs) for diabetes diagnosis were significantly higher for the fourth quartile of urinary concentrations of parabens compared with the first quartile [OR = 9.21 (95% CI = 1.60, 53.2) for methyl-paraben; 104 (10.6, 1.0e3) for ethyl-paraben; and 9.48 (1.37, 65.5) for propyl-paraben].40 However, the confidence intervals were very wide and the power of that study is likely inadequate. Consistent with our study, the National Health and Nutrition Examination Survey (NHANES) using data from 2005 to 2014 (n = 8,498) reported that parabens were inversely associated with the prevalence of diabetes [OR of diabetes comparing the 75th to 25th percentiles = 0.71 (95% CI = 0.61, 0.83) for propyl paraben; 0.66 (0.54, 0.80) for butyl paraben; 0.60 (0.51, 0.71) for ethyl paraben; and 0.79 (0.68, 0.91) for methyl paraben].17 Similarly, a study using data from cycle 4 (2014–2015) of the Canadian Health Measure Survey found that urinary ethyl paraben concentration was significantly associated with a 63% (95% CI = 2, 86) lower prevalence of metabolic syndrome among women.18 These inverse associations are biologically plausible. Antiandrogenic and estrogenic properties of parabens have been reported.12 Estrogens are important for maintaining body weight and metabolic health, can promote healthier types of fat, and protect against insulin resistance.41,42 Furthermore, parabens also act as an agonist for PPARγ.13 PPARγ agonists improve glucose homeostasis through systemic insulin sensitization or the direct action of PPARγ on the transcription of genes involved in glucose disposal.43 Some animal and in vitro studies have observed that high dose paraben exposure (50–200 µM) made an adipogenic effect by activating glucocortcoid receptors (GR) and PPARγ.13,44 However, the concentrations capable of inducing the adipogenic effect was high compared with the concentrations observed in the human studies (10–80 nM).45 Thus, paraben concentrations at levels measured in human tissue may activate PPARγ and estrogen receptors leading to antiobesogenic and antidiabetogenic effects, while higher concentrations may activate GR and suppress PPARγ leading to adipogenic effects.
Previous epidemiologic studies of the association between BPA and diabetes have yielded inconsistent results. Numerous cross-sectional studies have reported a positive association between BPA and prevalence of diabetes,46–48 whereas others have failed to detect an association.49,50 Findings from the few prospective studies have not been consistent. Two studies of incident T2DM with BPA measured at a single point also reported nonsignificant associations.19,50 A third longitudinal study (N = 755) with BPA concentrations measured at two time points, similar to our study, reported a positive association between the BPA concentrations measured at the later time point and incident T2DM, similar to our findings.20 Urinary BPA concentrations are known to have within-person variability with the range of ICCs of approximately 0.04–0.2,20,38 in line with our results. This low reliability indicates that a single BPA measurement is not adequate to approximate typical exposure, resulting in attenuation bias with significant loss of powers. According to a simulation study, the bias in the effect estimate is over 60% when a chemical with an ICC of 0.2 is measured twice.39 Despite having two urine samples measured 3 years apart in our study, inconsistent findings suggest that two samples may be insufficient to account for exposure measurement error.39 Additionally, the discordant associations between 2,4- (inconsistent) and 2,5-dichlorophenol (consistent associations between two time points) may be due to the poor reliability of urinary 2,4-diclorophenol concentrations (ICC = 0.3) compared with 2,5-diclorophenol concentrations (ICC = 0.59).
To the best of our knowledge, this is the first study to examine the overall joint effect associated with phenols and parabens mixtures. We found no significant overall joint effects utilizing the quantile-based g-computation approach. This may be because the associations for individual phenols and parabens were in opposite directions and offset each other. The major strengths of quantile based g-computation is that this approach can estimate the overall mixture effect of chemicals having either positive or negative association with health outcome as well as reflect the nonlinear effect of chemical exposure on health outcome.29 However, this approach also has a limitation: loss of information because continuous chemical concentration variables are categorized into quantiles.29 Other statistical approaches for time-to-event outcome, such as Cox penalized regression model and random survival forests,51,52 were not used in the present study because those approaches were not designed to evaluate the overall joint effects of the mixture.
The present study has several limitations. First, although our exposure assessment for urinary biomarkers of short half-life chemicals at two time-points is a strength, some chemicals with low ICCs, such as bisphenols and 2,4-dichlorophenol, were still subject to exposure measurement errors. Second, since urine samples were collected up to 20 years ago, which allowed us to evaluate incident diabetes prospectively, the urinary concentrations used in the present study reflect exposure levels at the time of collection (from 1999 to 2003) but do not reflect current population exposure levels. Finally, although we adjusted for many potential confounding factors in this study, residual and unmeasured confounding cannot be ruled out, especially for the inverse association between parabens and incident diabetes. Parabens are commonly used as preservatives in medications and food supplements,53 which may reduce the risk of developing diabetes.
In conclusion, our findings do not support diabetogenic effects of parabens, which were inversely associated with incident diabetes among mid-life women. Epidemiologic findings for biomarkers with short half-lives and high within-person variability need to be interpreted with caution. Proper exposure assessment for chemicals with low reliability needs to be considered during the design of future studies to evaluate diabetogenic effects of phenols and parabens.
Conflicts of interest statement
The authors declare that they have no conflicts of interest with regard to the content of this report.
Acknowledgments
Clinical Centers: University of Michigan, Ann Arbor—Siobán Harlow, PI 2011–present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA—Joel Finkelstein, PI 1999–present; Robert Neer, PI 1994–1999; Rush University, Rush University Medical Center, Chicago, IL—Howard Kravitz, PI 2009–present; Lynda Powell, PI 1994–2009; University of California, Davis/Kaiser—Ellen Gold, PI; University of California, Los Angeles—Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY—Carol Derby, PI 2011–present, Rachel Wildman, PI 2010–2011; Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry—New Jersey Medical School, Newark—Gerson Weiss, PI 1994–2004; and the University of Pittsburgh, Pittsburgh, PA—Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD—Chhanda Dutta 2016–present; Winifred Rossi 2012–2016; Sherry Sherman 1994–2012; Marcia Ory 1994–2001; National Institute of Nursing Research, Bethesda, MD—Program Officers.
Central Laboratory: University of Michigan, Ann Arbor—Daniel McConnell (Central Ligand Assay Satellite Services).
SWAN Repository: University of Michigan, Ann Arbor—Siobán Harlow 2013–Present; Dan McConnell 2011–2013; MaryFran Sowers 2000–2011.
Coordinating Center: University of Pittsburgh, Pittsburgh, PA—Maria Mori Brooks, PI 2012–present; Kim Sutton-Tyrrell, PI 2001–2012; New England Research Institutes, Watertown, MA—Sonja McKinlay, PI 1995–2001.
Steering Committee: Susan Johnson, Current Chair; Chris Gallagher, Former Chair.
We thank the study staff at each site and all the women who participated in SWAN.
Supplementary Material
Footnotes
Published online 1 October 2021
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). The SWAN Repository was supported by U01AG017719. This study also was supported by grants from the National Institute of Environmental Health Sciences (NIEHS) R01-ES026578, R01-ES026964 and P30-ES017885, and by the Center for Disease Control and Prevention (CDC)/National Institute for Occupational Safety and Health (NIOSH) grant T42-OH008455, and by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 RR024131.
SWAN provides access to public use datasets that include data from SWAN screening, the baseline visit and follow-up visits (https://agingresearchbiobank.nia.nih.gov/). To preserve participant confidentiality, some, but not all, of the data used for this manuscript are contained in the public use datasets. A link to the public use datasets is also located on the SWAN web site: http://www.swanstudy.org/swan-research/data-access/. Investigators who require assistance accessing the public use dataset may contact the SWAN Coordinating Center at the following email address: swanaccess@edc.pitt.edu. Computing code can be shared through our GitHub (https://github.com/um-mpeg) upon request.
SDC Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.environepidem.com).
References
- 1.Saeedi P, Petersohn I, Salpea P, et al. ; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. [DOI] [PubMed] [Google Scholar]
- 2.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88–98. [DOI] [PubMed] [Google Scholar]
- 3.Neel BA, Sargis RM. The paradox of progress: environmental disruption of metabolism and the diabetes epidemic. Diabetes. 2011;60:1838–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen D, Kannan K, Tan H, et al. Bisphenol analogues other than BPA: environmental occurrence, human exposure, and toxicity-A review. Environ Sci Technol. 2016;50:5438–5453. [DOI] [PubMed] [Google Scholar]
- 5.Bhargava HN, Leonard PA. Triclosan: applications and safety. Am J Infect Control. 1996;24:209–218. [DOI] [PubMed] [Google Scholar]
- 6.Darbre PD, Harvey PW. Paraben esters: review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J Appl Toxicol. 2008;28:561–578. [DOI] [PubMed] [Google Scholar]
- 7.Gustavsson Gonzalez H, Farbrot A, Larkö O. Percutaneous absorption of benzophenone-3, a common component of topical sunscreens. Clin Exp Dermatol. 2002;27:691–694. [DOI] [PubMed] [Google Scholar]
- 8.Ye X, Wong LY, Zhou X, Calafat AM. Urinary concentrations of 2,4-dichlorophenol and 2,5-dichlorophenol in the U.S. population (National Health and Nutrition Examination Survey, 2003-2010): trends and predictors. Environ Health Perspect. 2014;122:351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alonso-Magdalena P, Quesada I, Nadal A. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat Rev Endocrinol. 2011;7:346–353. [DOI] [PubMed] [Google Scholar]
- 10.Rochester JR, Bolden AL. Bisphenol S and F: a systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ Health Perspect. 2015;123:643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemini C, Jaimez R, Avila ME, Franco Y, Larrea F, Lemus AE. In vivo and in vitro estrogen bioactivities of alkyl parabens. Toxicol Ind Health. 2003;19:69–79. [DOI] [PubMed] [Google Scholar]
- 12.Nowak K, Ratajczak-Wrona W, Górska M, Jabłońska E. Parabens and their effects on the endocrine system. Mol Cell Endocrinol. 2018;474:238–251. [DOI] [PubMed] [Google Scholar]
- 13.Taxvig C, Dreisig K, Boberg J, et al. Differential effects of environmental chemicals and food contaminants on adipogenesis, biomarker release and PPARγ activation. Mol Cell Endocrinol. 2012;361:106–115. [DOI] [PubMed] [Google Scholar]
- 14.González-Casanova JE, Pertuz-Cruz SL, Caicedo-Ortega NH, Rojas-Gomez DM. Adipogenesis regulation and endocrine disruptors: emerging insights in obesity. Biomed Res Int. 2020;2020:7453786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei Y, Zhu J. Urinary concentrations of 2,5-dichlorophenol and diabetes in US adults. J Expo Sci Environ Epidemiol. 2016;26:329–333. [DOI] [PubMed] [Google Scholar]
- 16.Duan Y, Yao Y, Wang B, et al. Association of urinary concentrations of bisphenols with type 2 diabetes mellitus: a case-control study. Environ Pollut. 2018;243(pt B):1719–1726. [DOI] [PubMed] [Google Scholar]
- 17.Ward JB, Casagrande SS, Cowie CC. Urinary phenols and parabens and diabetes among US adults, NHANES 2005-2014. Nutr Metab Cardiovasc Dis. 2020;30:768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J, Chevrier J. Exposure to parabens and prevalence of obesity and metabolic syndrome: an analysis of the Canadian Health Measures Survey. Sci Total Environ. 2020;713:135116. [DOI] [PubMed] [Google Scholar]
- 19.Sun Q, Cornelis MC, Townsend MK, et al. Association of urinary concentrations of bisphenol A and phthalate metabolites with risk of type 2 diabetes: a prospective investigation in the Nurses’ Health Study (NHS) and NHSII cohorts. Environ Health Perspect. 2014;122:616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rancière F, Botton J, Slama R, et al. ; D.E.S.I.R. Study Group. Exposure to bisphenol A and bisphenol S and incident type 2 diabetes: a case-cohort study in the French Cohort D.E.S.I.R. Environ Health Perspect. 2019;127:107013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas NJ, Jones SE, Weedon MN, Shields BM, Oram RA, Hattersley AT. Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol. 2018;6:122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sowers M, Crawford S, Sternfeld B, et al. SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, eds. Menopause: Biology and Pathobiology. Academic Press; 2000:175–188. [Google Scholar]
- 23.Nie L, Chu H, Liu C, Cole SR, Vexler A, Schisterman EF. Linear regression with an independent variable subject to a detection limit. Epidemiology. 2010;21 (suppl 4):S17–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Karvonen-Gutierrez CA, Herman WH, Mukherjee B, Harlow SD, Park SK. Urinary metals and incident diabetes in midlife women: study of Women’s Health Across the Nation (SWAN). BMJ Open Diabetes Res Care. 2020;8:e001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reeves AN, Elliott MR, Brooks MM, et al. Symptom clusters predict risk of metabolic-syndrome and diabetes in midlife: the Study of Women’s Health Across the Nation. Ann Epidemiol. 2021;58:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karvonen-Gutierrez CA, Peng Q, Peterson M, Duchowny K, Nan B, Harlow S. Low grip strength predicts incident diabetes among mid-life women: the Michigan Study of Women’s Health Across the Nation. Age Ageing. 2018;47:685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113:192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cicchetti DV. The precision of reliability and validity estimates re-visited: distinguishing between clinical and statistical significance of sample size requirements. J Clin Exp Neuropsychol. 2001;23:695–700. [DOI] [PubMed] [Google Scholar]
- 29.Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ. A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ Health Perspect. 2020;128:47004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrico C, Gennings C, Wheeler DC, Factor-Litvak P. Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. J Agric Biol Environ Stat. 2015;20:100–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Dries NNM, Werder E, Buckley J, O’Brien K. The qgcomp package: g-computation on exposure quantiles. Available at: https://cran.r-project.org/web/packages/qgcomp/vignettes/qgcomp-vignette.html. Accessed 5 October 2020.
- 32.Janjua NR, Frederiksen H, Skakkebaek NE, Wulf HC, Andersson AM. Urinary excretion of phthalates and paraben after repeated whole-body topical application in humans. Int J Androl. 2008;31:118–130. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez H, Farbrot A, Larkö O, Wennberg AM. Percutaneous absorption of the sunscreen benzophenone-3 after repeated whole-body applications, with and without ultraviolet irradiation. Br J Dermatol. 2006;154:337–340. [DOI] [PubMed] [Google Scholar]
- 34.Thayer KA, Doerge DR, Hunt D, et al. Pharmacokinetics of bisphenol A in humans following a single oral administration. Environ Int. 2015;83:107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandborgh-Englund G, Adolfsson-Erici M, Odham G, Ekstrand J. Pharmacokinetics of triclosan following oral ingestion in humans. J Toxicol Environ Health A. 2006;69:1861–1873. [DOI] [PubMed] [Google Scholar]
- 36.Yazdy MM, Coull BA, Gardiner JC, et al. A possible approach to improving the reproducibility of urinary concentrations of phthalate metabolites and phenols during pregnancy. J Expo Sci Environ Epidemiol. 2018;28:448–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meeker JD, Cantonwine DE, Rivera-González LO, et al. Distribution, variability, and predictors of urinary concentrations of phenols and parabens among pregnant women in Puerto Rico. Environ Sci Technol. 2013;47:3439–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollack AZ, Perkins NJ, Sjaarda L, et al. Variability and exposure classification of urinary phenol and paraben metabolite concentrations in reproductive-aged women. Environ Res. 2016;151:513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perrier F, Giorgis-Allemand L, Slama R, Philippat C. Within-subject pooling of biological samples to reduce exposure misclassification in biomarker-based studies. Epidemiology. 2016;27:378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li AJ, Xue J, Lin S, et al. Urinary concentrations of environmental phenols and their association with type 2 diabetes in a population in Jeddah, Saudi Arabia. Environ Res. 2018;166:544–552. [DOI] [PubMed] [Google Scholar]
- 41.Palmisano BT, Zhu L, Stafford JM. Role of estrogens in the regulation of liver lipid metabolism. Adv Exp Med Biol. 2017;1043:227–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol. 2015;402:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim HI, Ahn YH. Role of peroxisome proliferator-activated receptor-gamma in the glucose-sensing apparatus of liver and beta-cells. Diabetes. 2004;53(suppl 1):S60–S65. [DOI] [PubMed] [Google Scholar]
- 44.Pereira-Fernandes A, Demaegdt H, Vandermeiren K, et al. Evaluation of a screening system for obesogenic compounds: screening of endocrine disrupting compounds and evaluation of the PPAR dependency of the effect. PLoS One. 2013;8:e77481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Meeuwen JA, van Son O, Piersma AH, de Jong PC, van den Berg M. Aromatase inhibiting and combined estrogenic effects of parabens and estrogenic effects of other additives in cosmetics. Toxicol Appl Pharmacol. 2008;230:372–382. [DOI] [PubMed] [Google Scholar]
- 46.Ahmadkhaniha R, Mansouri M, Yunesian M, et al. Association of urinary bisphenol a concentration with type-2 diabetes mellitus. J Environ Health Sci Eng. 2014;12:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hwang S, Lim JE, Choi Y, Jee SH. Bisphenol A exposure and type 2 diabetes mellitus risk: a meta-analysis. BMC Endocr Disord. 2018;18:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silver MK, O’Neill MS, Sowers MR, Park SK. Urinary bisphenol A and type-2 diabetes in US adults: data from NHANES 2003-2008. PLoS One. 2011;6:e26868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim K, Park H. Association between urinary concentrations of bisphenol A and type 2 diabetes in Korean adults: a population-based cross-sectional study. Int J Hyg Environ Health. 2013;216:467–471. [DOI] [PubMed] [Google Scholar]
- 50.Shu X, Tang S, Peng C, et al. Bisphenol A is not associated with a 5-year incidence of type 2 diabetes: a prospective nested case-control study. Acta Diabetol. 2018;55:369–375. [DOI] [PubMed] [Google Scholar]
- 51.Park SK, Tao Y, Meeker JD, Harlow SD, Mukherjee B. Environmental risk score as a new tool to examine multi-pollutants in epidemiologic research: an example from the NHANES study using serum lipid levels. PLoS One. 2014;9:e98632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park SK, Zhao Z, Mukherjee B. Construction of environmental risk score beyond standard linear models using machine learning methods: application to metal mixtures, oxidative stress and cardiovascular disease in NHANES. Environ Health. 2017;16:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dodge LE, Kelley KE, Williams PL, et al. Medications as a source of paraben exposure. Reprod Toxicol. 2015;52:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.