Abstract
Benincasa hispida (Thunb.) Cogn. (Cucurbitaceae) is an annual climbing plant, native to Asia with multiple therapeutic uses in traditional medicine. This updated review is aimed at discussing the ethnopharmacological, phytochemical, pharmacological properties, and molecular mechanisms highlighted in preclinical experimental studies and toxicological safety to evaluate the therapeutic potential of this genus. The literature from PubMed, Google Scholar, Elsevier, Springer, Science Direct, and database was analyzed using the basic keyword “Benincasa hispida.” Other searching strategies, including online resources, books, and journals, were used. The taxonomy of the plant has been made by consulting “The Plant List”. The results showed that B. hispida has been used in traditional medicine to treat neurological diseases, kidney disease, fever, and cough accompanied by thick mucus and to fight intestinal worms. The main bioactive compounds contained in Benincasa hispida have cytotoxic, anti-inflammatory, and anticancer properties. Further safety and efficacy investigations are needed to confirm these beneficial therapeutic effects and also future human clinical studies.
1. Introduction
Food and food products are being used as medicines over centuries worldwide. Many species from the family Cucurbitaceae have been used as medicaments in various diseases in Ayurveda and ancient Chinese medicine. This family is also known as the gourd family. It provides approximately 5 to 6% of the total vegetables in the world. To date, 825 species from under 118 genera have been reported growing in temperate regions of the world [1]. It should be mentioned that the Cucurbit species can grow in diverse climatic conditions, including arid deserts, tropical, subtropical, and temperate regions. These various types of species are included in food systems and Indian traditional medicines. Generally, the gourd family vegetables provide vitamins, essential minerals, antioxidants, and soluble fibres [2].
The word “herb” derived from the Latin word “herba” and an old French word “herbe” refers to any part of the plant like fruit, seed, stem, bark, flower, leaf, stigma, or a root, as well as a non-woody plant. Many herbs are currently under-using as a source of foods, flavonoids, medicines, or perfumes as well as in certain spiritual activities. Ancient era literature including Unani manuscripts, Chinese writings, and Egyptian papyrus also depicted the use of herbs in various diseases. The Indian Vaids, Unani Hakims, and European and Mediterranean cultures are using herbs for more than 4000 years as medicines. Native people of Iran, Rome, Egypt, Africa, and America used medicinal herbs in healing habits. The Unani, Ayurveda, and Chinese Medicine are using herbal remedies systematically. These all are the potential sources of medicinal plant-based modern medicines. According to World Health Organization (WHO), about 80% of people in the world depend on herbal medicines to fulfil their basic health care needs, and around 21,000 species of plants have been identified as potential medicinal plants. In developed countries, around 25% of the total drugs come from plant origin, while in fast-developing countries as much as 80% [3].
Benincasa hispida (Thunb.) Cogn. (synonym: Benincasa cerifera Savi) (Cucurbitaceae) especially in Asian countries is considered as one of the famous crops under the Cucurbitaceae family that grows mainly for its fruits and well renowned for its nutritional and medicinal properties [4, 5]. Scientific reports suggest that B. hispida possesses many important nutritious substances, including vitamins, natural sugars, amino acids, organic acids, and mineral elements [4, 6, 7]. This review is aimed at sketching an up-to-date scenario on the indigenous uses, nutraceutical, and phytochemical composition along with the pharmacological activities of B. hispida based on database reports.
2. Review Methodology
Using the PubMed database and the search engines Google scholar, Elsevier, Springer, Science Direct, research articles, and reviews related to B. hispida were analyzed. Abstracts and papers peer reviewed were selected according to the objectives of the research: the molecular pharmacological mechanisms of action proven by preclinical experimental studies [8] and which scientifically justify the traditional uses of B. hispida.
Other sources of “grey literature” information such as Web pages, book chapters, and specialized monographs were also analyzed to obtain maximum updated information on the biological properties of this plant. The keywords used were “Benincasa hispida” or “traditional uses” or “phytochemistry” of “pharmacological properties” or “biological activities” or “toxicology” or “safety” or “side effects.” The scientific names of the plants were verified according to PlantList, and the chemical formulas were revised by consulting the PubChem database (https://scholar.google.com).
Inclusion criteria: the most relevant articles written in English on taxonomy, ethnopharmacology, phytochemistry, pharmacology, various biological activities, and toxicity of this plant were included and analyzed.
Exclusion criteria: the papers containing homoeopathic preparations, papers written in languages other than English, publications without pharmacological mechanisms of action.
3. Botany and Traditional Uses
3.1. Botany (Plant Profile)
B. hispida (Figure 1), also known as kundur fruit, chalkumra, wax gourd, winter gourd, ash gourd, winter melon, white gourd, tallow gourd, Chinese preserving melon, ash pumpkin, and (alu) puhul, a creeper grown for its very big size fruit, is eaten as a green mature vegetable or greens [9–11].
Figure 1.
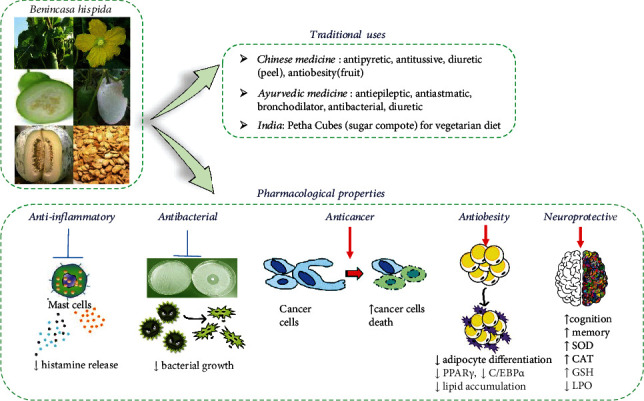
Diagram with different parts (aerial parts, flower, fruit pulp unripe, fruits, fruit pulp mature, and seeds) of Benincasa hispida (Thunb.) Cogn, traditional uses, and its most important pharmacological properties. Abbreviations: PPARγ: peroxisome proliferator-activated receptor gamma; C/EBPα: CCAAT enhancer-binding protein alpha; CAT: catalase; SOD: superoxidase dismutase; GSH: reduced glutathione; LPO: lipid peroxidations.
There is a fine hairs fuzzy coating outer side of the young fruit and has solid thick white flesh of sweet in tastes. The mature fruit sheds its hairs and forms a waxy white coating, giving the name of “wax gourd.” The gourd wax coating increases the storage facilities of it. It can grow of a length up to 80 cm and also have broad leaves and yellow flowers. The taste is rather bland. B. hispida is a native of South and Southeast Asia. However, it is commonly grown all over Asia, including Japan, Burma, Ceylon, Sri Lanka, Java, and Australia [10].
Taxonomy
Kingdom: Plantae
Phylum: Tracheophyta
Class: Magnoliopsida
Oder: Cucurbitales
Family: Cucurbitaceae
Genus: Benincasa
Species: Benincasa hispida (Thunb.) Cogn.
3.2. Traditional Uses and Ethnopharmacology
In India, B. hispida is used as a winter season vegetable for a wide variety of diseases. Its medicinal properties have been also recognized in the Ayurvedic system of medicine, spiritual traditions of India and Yoga. In Vietnam, its soup (cooked with pork short ribs) is traditionally used by breastfeeding mothers. In north India and almost all regions in Bangladesh, it is added with pulses like as moong which usually crushed, along with wax gourd, makes a dish locally called bori, which after sun drying is used in curry dishes and eaten with rice or chapati [12]. To make wax gourd soup in China, it is used in stir-fries or added into pork or pork/beef bones, which often served in the scooped-out gourd, carved by scraping off the waxy coating. It is also cut into pieces, candied and normally eaten during the time of New Year festivals, or used as filling in Sweetheart cake. For the Moon Festival, the Chinese and Taiwanese also used it in moon cakes as a base filling. It is candied by the people of the Philippines and is used as a pastry filling for bakpia. In some savoury soups and stir-fries, it also acts as an ingredient. In Nepal, India, and Bangladesh, the tendrils, shoots, and leaves of the plant are consumed as green vegetables [6].
B. hispida is widely used in Chinese medicine, in the treatment of fever, cough accompanied by thick mucus and urinary disorders, it is used especially in bark with a very good diuretic effect. The fruit is recommended for overweight people who want to follow diets. In Ayurvedic medicine, it is used in the treatment of epilepsy, cough, lung disease, hiccups, asthma, internal bleeding, and urinary retention. In India, a fruit compote called Petha Cubes is made from the pulp of the fruit, which is recommended for vegetarians [13].
The fruit is also used in peptic ulcer, and it is also used in diabetes mellitus, urinary infection, haemorrhages from internal organs, insanity, epilepsy, and other nervous disorders in Ayurveda [14]. The fruit is sweet and traditionally used as a cooling, styptic, antiperiodic, laxative, diuretic, tonic, aphrodisiac, and cardiotonic, and also in jaundice, dyspepsia, urinary calculi, blood disease (e.g., haemorrhages from internal organs), insanity, epilepsy, asthma, diabetes, vitiated conditions of pitta, fever, menstrual disorders, and balancing the body heat [15] (Figure 1).
3.3. Phytochemical Profile
3.3.1. Nutritional Composition
The edible portion of B. hispida contains moisture (93.80-96.80/100 g), proteins (0.30-0.70/100 g), carbohydrates (1.10-4.00/100 g), fat (0.02-0.20/100 g), fibre (0.50-2.10/100 g), and ash (0.27-0.70/100 g).
Vitamins present in the edible portion (per 100 g) of this plant are vitamin C (1.35-68.00), thiamin (0.02-0.04), riboflavin (0.02-0.31), niacin (0.20-0.46), and vitamin E. Major minerals in the edible portion (per 100 g) include sodium (0.14-6.00), potassium (77.00-131.00), calcium (5.00-23.32), iron (0.20-0.49), and phosphorus (225.39-234.61) [6, 16, 17]. The fruit contains water-soluble polysaccharides [18], such as arabinogalactans [19].
The fruit pulp contains homogalacturonan, β-(1 → 4)-D-galactan, acidic arabinan [20], and natural sugars (e.g., glucose and fructose) [21]. The mature fruit also contains organic acids such as malic and citric acid.
3.3.2. Chemical Phytoconstituents
The leaf contains alkaloids, flavonoids, steroids [22], and the fruit amino acids, pectic polysaccharides [20], hemicellulose polysaccharides [18], terpenes and terpenoids, flavonoid C–glycosides, sterols [23], proteins [24], phenols, alkaloids, glycosides, tannins, saponins [25], hydroxybenzoic acids, flavonols, hydrocinnamic acids, and triterpenes [9] (Figure 2).
Figure 2.
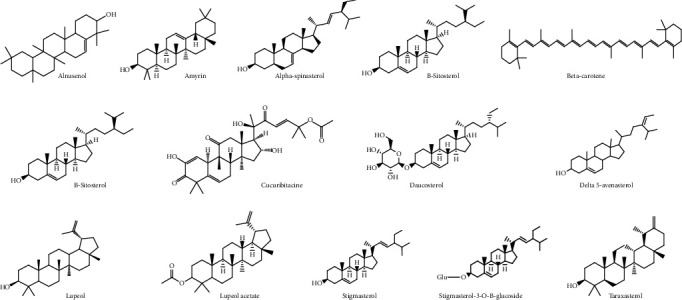
The chemical formulas of most important representative sterols and terpenes from Benincasa hispida.
The seeds contain proteins [24], carbohydrates, phenolic compounds, amino acids, flavonoids, sterols [26], glycosides, alkaloids, fixed oils and fats, phenolic compounds, steroids [27], and unsaturated fatty acids [28]. The peel contains alkaloids, saponins, steroids, carbohydrates, flavonoids [29], tannins, carotenoids, oxalates, and phytate [17].
The root contains proteins [24]. The fruit contains many volatile compounds, including (E,E)-2,4-nonadienal, (E)-2-hexenal, n-hexanal, n-hexyl formate, (E,E)-2,4-heptadienal, (Z)-3-hexenal, (E)-2-heptenal, 1-octen-3-ol [30], 2,5-dimethylpyrazine, 2-methyl pyrazine, 2-ethyl-5-methyl pyrazine, and 2,6-dimethylpyrazine, 2,3,5-trimethylpyrazine [30].
B. hispida is rich in phenolic compounds. Several other bioactive compounds present in it are isomultiflorenyl acetate, isovitexin, 1-sinapoylglucose, multiflorenol, 5-gluten-3-β-ylacetate, alnusenol, and benzylalcolcohol-O-α-l-arabinopyranosyl-(1-6)-β-d-glucopyranoside [31]. The most representative phytochemicals present in B. hispida has been shown in Figures 2–5 and Table 1.
Figure 3.
The chemical formulas of most important representative carbohydrates from Benincasa hispida.
Figure 4.
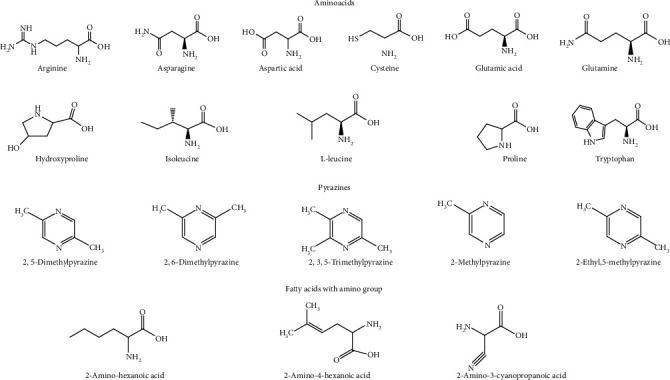
The chemical formulas of most important representative aminoacids, pyrazines, and fatty acids with amino group from Benincasa hispida.
Figure 5.
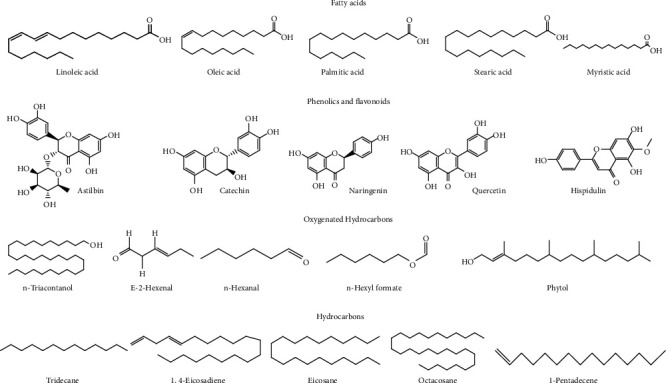
The chemical formulas of most important representative fatty acids, phenolic and flavomoids, oxygenated hydrocarbons, and hydrocarbons from Benincasa hispida.
Table 1.
Chemical phytoconstituents of Benincasa hispida (Thunb.) Cogn.
| Compounds | Plant parts | Locality/country | References |
|---|---|---|---|
| E-2-hexenal, n-hexanal and n-hexyl formate; however, 2,5-dimethylpyrazine, 2,6-dimethylpyrazine, 2,3,5-trimethylprazine, 2-methylpyrazine, 2-ethyl-5-methylpyrazine | Fruit | Taipei, Taiwan/China | [30] |
| Cucumisin-like protease | Sarcocarp | Kagoshima/Japan | [32] |
| Triterpenes, sterols, flavonoid C-glycoside, benzyl glycoside, alnusenol, multiflorenol | Fruit | Kyoto/Japan | [33] |
| Osmotin-like protein | Seeds | New York/USA | [34] |
| Chitinase | Seeds | New York/USA | [35] |
| Astilbin, catechin, naringenin | Fruit | Hainan/China | [31] |
| Di-2-ethylhexyl phthalate | Fruit | Hainan/China | [36] |
| W-sitosterol, V-amyrin, quercetin | Stem | Visakhapatnam/India | [37] |
| β-Carotene | Fruit | Faisalabad/Pakistan | [16] |
| Tryptophan | Fruit | Gwalior/India | [38] |
| Linoleic, palmitic, oleic, and stearic acids | Fruit | Temerloh, Pahang/Malaysia | [39] |
| Acetoin, octanal, nonanal | — | Mumbai/India | [40] |
| α-Tocopherol, δ-tocopherol, linoleic acid, β-sitosterol, campesterol, stigmasterol, Δ5-avenasterol | Fruit | Serdang, Selangor/Malaysia | [41] |
| Galactose, glucose, xylose, sorbose | Peel | Karnataka/India | [42] |
| Linoleic acid, linolenic acid | Seeds | Serdang, Selangor/Malaysia | [43] |
| Myristic acid, palmitoleic acid, oleic acid, linoleic acid, stearic acid, α-linolenic acid, palmitic acid, other saturated and unsaturated fatty acids | Seed oil | Serdang, Selangor/Malaysia | [44] |
| 3α,29-O-di-trans-cinnamoyl-D:C-friedooleana-7,9(11)-diene, oleanolic acid 28-O-β-D-xylopyranosyl-[β-D-xylopyranosyl-(1 → 4)]-(1 → 3)-α-L-rhamnopyranosyl (1 → 2)-α-L-arabinopyranoside, oleanolic acid 28-O-β-D-glucopyranosyl-(1 → 3)-β-D-xylopyranosyl-[β-D-xylopyranosyl-(1 → 4)]-(1 → 3)-α-L-rhamnopyranosyl-(1 → 2)-α-L-arabinopyranoside, multiflorenol, isomultiflorenyl acetate, stigmasterol, stigmasterol 3-O-β-D-glucopyranoside, α-spinasterol, α-spinasterol 3-O-β-D-glucopyranoside, β-sitosterol, daucosterol, arbutin, nicotinic acid, (+)-pinonesinol, ethyl β-D-glucopyranoside | Fruit | Jinghong/China | [45] |
| Phloem lectin-like protein | Exudate | Fukuoka/Japan | [46] |
| Linoleic acid, palmitic acid, oleic acid, stearic acid | Seeds | Rambagh, Allahabad/India | [47] |
| Gallic acid | Fruit | Kota Bharu/Malaysia | [15] |
| Lupeol | Seeds | Mumbai/India | [27] |
| Gallic acid, linoleic acid | Seeds | Serdang, Selangor/Malaysia | [28] |
| β-Sitosterol | Seeds | Mumbai/India | [48] |
| Ascorbic acid | Fruit | Kubang Kerian, Kelantan/Malysia | [49] |
| β-Carotein, ascorbic acid | Peel | Mysore/India | [17] |
| Polysaccharides | Fruit | Guangzhou/China | [50] |
| Gallic acid, catechin, epicatechin, rutin, quercetin, quercetin-3-D-galactoside, trans-ferrulic acid, oleanolic acid, ursolic acid | Fruit | Buzau/Romania | [9] |
4. Pharmacological Activities
4.1. Antioxidant Effects
Oxidative stress is a term used for free radical diseases [51, 52]. It is defined as the imbalance between free radicals and antioxidants, given that oxidants (free radicals) are more and have a destructive potential on the human body [53, 54].
The methanolic seed extract showed a concentration-dependent (25-200 μg/mL) 2,2-diphenyl-1-picrylhydrazyl (DPPH) and hydrogen peroxide radical scavenging effects [55]. Another study revealed that the ethanolic seed extract shows better DPPH and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical scavenging along with total phenolic content (TPC) than its ethyl acetate and n-hexane extracts [43]. The seed oil (0.1 mg/mL) also showed significant DPPH and ABTS radical scavenging capacity [56]. This study also determined the TPC in seed oil. The aqueous extract of this plant reduced reactive oxygen species (ROS) in human umbilical vein endothelial cells (HUVECs) [57].
Polysaccharides of fruit extract showed DPPH free radicals scavenging activity with an EC50 value of 0.98 mg/mL [50]. The seed oil also showed DPPH and ABTS radical scavenging capacity. However, the antioxidant activity was lower than the catechin and BHT at the same concentration (0.1 mg/mL) [44]. Petroleum ether and methanol fruit extracts increased in catalase (CAT) levels in gastric ulcer rats [58]. Hispidalin isolated from this herb also showed DPPH radical scavenging and inhibition of lipid peroxidation capacity [59]. The aqueous fruit extract significantly increased the antioxidant status as well as levels of vitamin C concentration in gastric juice or rats [60].
Antioxidant effects of various parts of B. hispida on various test models have been also observed by several authors [17, 28, 29, 56, 61]. Table 2 shows the antioxidant effects of various parts of B. hispida.
Table 2.
Antioxidant properties of different parts or their extracts/fractions of isolated compounds.
| Extract/isolated compounds | Test system | Results | References |
|---|---|---|---|
| Crude oil from seeds | DPPH ABTS TPC |
DPPH: EC50 = 0.1 mg/mL ABTS: EC50 = 0.1 mg/mL Significant antioxidant effect Standards: methyl ether, fatty acids |
[56] |
| Seeds extract | DPPH, ABTS, total phenolic content | EC50 = 10 − 100 μg/mL Significant antioxidant effect Standards: methyl ether, fatty acids |
|
| Methanolic and aqueous peel extracts | DPPH | EC50 = 10 − 100 μg/mL Concentration-dependent radical scavenging activity The methanolic extract exhibited a better antioxidant effect Standard: DPPH |
[29] |
| Aqueous seeds extract | TPC, TFC DPPH, ABTS, H2O2, linoleic acid oxidation nitrite scavenging assay |
TPC: EC50 = 81.3 ± 1.4 μg gallic acid/g TFC: EC50 = 486.8 ± 4.1 μg catechin/g dry mass DPPH: EC50 = 0.6 − 3 mg/mL Concentration-dependent antioxidant activity Standards: catechin 0.05-0.5 mg/mL, BHT, ascorbic acid 10 mg/mL |
[61] |
| Seed oil | DPPH ABTS radical scavenging assay |
DPPH: EC50 = 0.1 mg/mL The antioxidant activity of the seed oil was lower than the catechin and BHT at the same concentration. Standard: FAME |
[44] |
| Hispidalin | DPPH Lipid peroxidation assay |
DPPH: EC50 = 2 − 40 μg/mL EC50 = 40 μg/mL Significant DPPH radical scavenging and inhibition of lipid peroxidation capacity Standard: methyl ether |
[59] |
| Methanol, ethanol, aqueous peel extracts | DPPH Reducing power assay |
Significant antioxidant effect Standard: acarbose 20, 40, 60, 80, 100 μg.mL−1 |
[17] |
| Seed extract | DPPH ABTS |
Significant antioxidant effect Standard: FAME |
[28] |
| Polysaccharides of fruit extract | DPPH | EC50 = 0.98 mg/mL Significant antioxidant effect Standard: glucose |
[50] |
Abbreviations: TPC: total phenolic contents; TFC: total flavonoid contents (TFC); ABTS: 2, 2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid); DPPH: 2,2-diphenyl-1-picrylhydrazyl free radical-scavenging ability; BHT: antioxidant butylated hydroxytoluene; FAME: fatty acid methyl ester; EC50: the half-maximal effective concentration.
4.2. Anti-Inflammatory Effect
The methanolic seed extract (100-300 mg/kg, p.o.) showed dose-dependent anti-inflammatory effects on carrageenan-induced paw oedema rat (n = 6) model [55]. The fruit peel methanolic extract showed an anti-inflammatory effect on egg albumin-induced inflammation in rats [62]. The petroleum ether and methanolic fruit extract of B. hispida (300 mg/kg, p.o.) showed a dose-dependent anti-inflammatory effect on cotton pellet-induced granuloma models in rats, carrageenan-induced paw oedema, and histamine-induced paw oedema [58].
4.3. Antimicrobial, Antihelmintic, and Larvicidal Effects
Due to the excessive use of antibiotics that can lead to the development of antibiotic resistance of various strains of bacteria [63–65], attempts have been made to use natural antibiotic alternatives [66, 67]. Most of these options include plants with antiviral and antibacterial properties that can be effective against gram-negative and gram-negative germs, which are often difficult to eradicate [68, 69]. The methanolic whole plant extract (500 μg/disc) was found to act against Pseudomonas aeruginosa and Vibrio parahaemolyticus [70]. In the latter case, the zone of inhibition was 6 mm only. Hispidalin, an isolated compound from this herb, was found to act against several bacteria (e.g., Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Salmonella enterica) and fungi (e.g., Aspergillus flavus, Penicillium chrysogenum, Fusarium Solani, and Colletotrichum gloeosporioides) [59]. In this case, the minimum inhibitory concentrations (MIC) were 30-120 and 100-200 μg/mL for bacterial and fungal strains, respectively. Moreover, the aqueous, methanol, and petroleum extracts of seeds showed significant therapeutic efficacy with methanol extract being the best comparable to the antibiotic ciprofloxacin. In other study, the aqueous peel extract showed strong antibacterial activity against S. aureus (MIC = 14.5 μg/mL), Micrococcus luteus (MIC = 8.6 μg/mL), E. coli (MIC = 6.1 μg/mL), and Klebsiella pneumoniae (MIC = 13.4 μg/mL) [11]. The herb shows prebiotic activity [71]. Table 3 shows the antimicrobial effects of various parts of B. hispida.
Table 3.
Antimicrobial, anthelmintic, and larvicidal effects of different parts or their extracts/fractions or isolated compounds.
| Extract/isolated compounds | Dose/concentration model (in vitro/in vivo) | Results/mechanisms | References |
|---|---|---|---|
| Antimicrobial effects | |||
| Methanolic whole plant extract |
Pseudomonas aeruginosa
Vibrio parahaemolyticus In vitro Standard: DMSO |
IC50 = 500 μg/disc Zone of inhibition = 6 mm |
[70] |
| Hispidalin |
Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Salmonella enterica; Fungi: Penicillium chrysogenum, Fusarium solani, Aspergillus flavus, Colletotrichum gloeosporioides In vitro Standard: acetoin (0.01–20 μg/μl) |
Antibacterial: MIC = 30 − 120 μg/mL, Antifungal: MIC = 100 − 200 μg/mL |
[59] |
| Aqueous peel extract |
Staphylococcus aureus, Micrococcus luteus, Escherichia coli, Klebsiella pneumoniae In vitro Standard: DMSO 150 μL |
Antibacterial: MIC = 6.1 − 14.5 μg/mL | [11] |
| Anthelmintic effect | |||
| Ethanolic seed extract |
Pheretima posthuman/in vitro
Standard: phenytoin sodium |
IC50 = 20, 40, and 60 mg Dose-dependent anthelmintic effect |
[72] |
| Larvicidal effect | |||
| Phloem lectin-like protein from the exudate |
Samia ricini larvae/in vitro Standards: Precision Plus Protein™, serum albumin |
↑ inhibitory activity against the larvae Dose: 70 μg/g |
[46] |
Abbreviations: IC50: value concentration that inhibits cell growth by 50%; MIC: minimum inhibitory concentration.
Ethanolic seed extract (20, 40 and 60 mg) showed a dose-dependent anthelmintic in anthelmintic activity on Pheretima posthuma [72]. The phloem lectin-like protein from the exudate of the herb exerted an inhibitory effect on the Samia ricini larvae [46] (Table 3).
4.4. Cytotoxic and Anticancer Effects
Cancer is a term used to define malignancies in which abnormal cells multiply in an uncontrolled and continuous manner and can invade the surrounding healthy tissues [73, 74]. Abnormal cells come from any tissue in the human body and can occur anywhere in the body [75–77]. Natural anticancer alternatives can have a direct effect on malignant cells, as well as by stimulating the body's immune capacity in the fight against the aggression of carcinogenic factors, internal or external [78, 79]. The favourable effects of some medicinal plants are due to the main biochemical components: flavonoids—which inhibit the activity of carcinogens and prevent the metastasis of malignant cells; carotenoids—which protect the body against colon cancer; terpenes in essential oils—block the action of carcinogens, having a strong antioxidant action; β-carotene, a powerful antioxidant with anticancer protection and a recognized inhibitor of malignant cells; antioxidant vitamins C, E, and A, destroy free radicals, prevent cancer, and block the metastasis process [80–83].
The fruit, seed, and root proteins (10-1000 μg/mL) exerted a concentration-dependent cytotoxic effect on Artemia salina. The median lethal concentration (LC50) values of fruit, seed, and root extract were 44, 41, and 50 μg/mL, respectively [24]. In this study, the root proteins inhibited the proliferation of HeLa and K-562 cells by 28.50 and 36.60%, respectively. Another study reveals that the whole plant methanolic extract (5-50 μg/mL) exerted a cytotoxic effect on A. salina (LC50: 45.187 μg/mL) [70]. Moreover, the aqueous seed extract (20-800 μg/mL) did not exert cytotoxic effects on HUVECs and normal fibroblast (NIH/3T3) cells. On male C57BL/6 mice, the extract showed a potent inhibitory effect on basic fibroblast growth factor- (bFGF-) induced angiogenesis [84]. The aqueous extract (1–20 μg/mL) also reduced cell adhesion molecules activation by inhibiting monocyte adhesion, ROS, and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) on high glucose (25 mM) induced HUVECs cells [57] (Figure 6).
Figure 6.
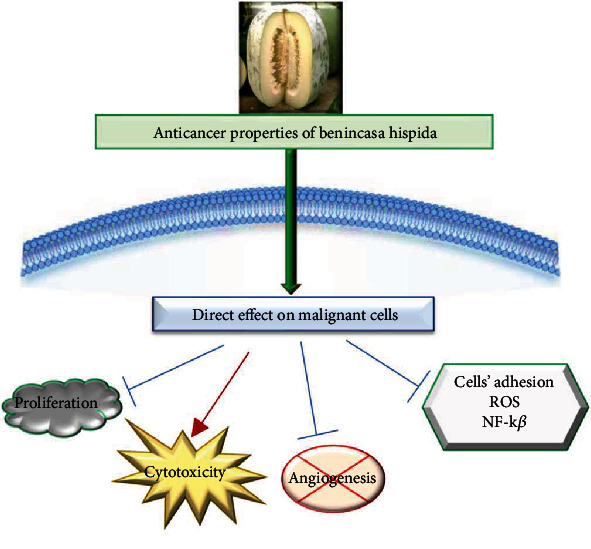
A schematic diagram with anticancer mechanisms of natural compounds from Benincasa hispida. Legend: blue arrow: inhibition, reduction; red arrow: increase, stimulation, ROS reactive oxygen species, NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells).
Table 4 shows the cytotoxic and anti-cancer effects of various parts of B. hispida, and Figure 1 summarizes the most important anticancer mechanism.
Table 4.
Cytotoxic and anticancer effects of various parts of B. hispida extracts/fractions.
| Extract/isolated compounds | Model dose/concentration | Results/mechanisms | References |
|---|---|---|---|
| Aqueous seed extract | HUVECs, NIH/3T3 cells/in vitro Male C57BL/6 mice/in vivo IC50 = 20 − 800 μg/mL Standard: NNGH |
No cytotoxicity on HUVECs, NIH/3T3 cells decrease bFGF-induced angiogenesis in mice |
[84] |
| Fruit, seed, root proteins | HeLa, K-562 cells/in vitro IC50 of fruit, seeds root extract = 44, 40-50 μg/mL IC50 = 10 − 1000 μg/mL in Artemia salina IC50 = 10–50 μg/mL on HeLa, K-562 cells Standards: lysozime, tyrosine, carbonic anhydrase, ovalbumin, albumin |
Decrease cell proliferation by 28.50-36.80% | [24] |
| Aqueous extract | HUVECs cells/in vitro IC50 = 1–20 μg/mL on high glucose (25 mM) Standards: glucose 25 mM, glucose and ABH 5 μg/ml, 20 μg/ml |
Decrease cell adhesion molecules activation, Decrease ROS, NF-κB Decrease inhibiting monocyte adhesion |
[57] |
| Methanolic/whole plant extract |
Artemia salina/in vitro
IC50 = 45.186 μg/mL Standard: DMSO, vincristine sulphate 0.91 μg/mL |
Increase cytotoxic effect concentration-dependent | [70] |
Abbreviations: IC50: value concentration that inhibits cell growth by 50%; bFGF: basic fibroblast growth factor; ROS: reactive oxygen species; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; NNGH: N-isobutyl-N-(4-methoxyphenylsulfonyl)-glycylhydroxamic acid.
4.5. Gastrointestinal Protective Effects
4.5.1. Gastroprotective Effect
Fresh juice (1-4 mL/animal, p.o.), ethanol (12, 24 and 48 mg/kg, p.o.), and pet ether extract (0.75, 1.5 and 3 mg/kg, p.o.) in swimming stress, aspirin plus restraint, serotonin-induced ulcers, and indomethacin plus histamine displayed a dose-dependent antiulcerogenic effect in rats and mice [13].
The petroleum ether and methanol fruit extracts (300 mg/kg, p.o.) significantly (P < 0.05) reduced ulcer index, vascular permeability, and malondialdehyde (MDA) content, while an increase in CAT levels in comparison to the control group in pylorus ligated (PL) gastric ulcers, ethanol-induced gastric mucosal damage, and cold restraint stress- (CRS-) induced gastric ulcer rat models [58]. The fruit extract (1 mL/kg, p.o.) also decreased ulcer index as well as MDA, superoxide dismutase (SOD), and vitamin C levels in indomethacin-induced gastric ulcer in rats [23].
The hydromethanol, ethyl acetate, and aqueous ripe fruit extracts (20 mg/kg, p.o./alternative days) were treated for 14 days in ranitidine (5 mg/kg, p.o.) induced hypochlorhydria in rats. The aqueous extract showed better effects on the test animals. It increased the antioxidant status as well as levels of pepsin, vitamin C, and gastric juice chloride concentration than the other extracts [60]. On the other hand, the extract of fruits with the whole plant of Fumaria vaillantii Loisel (1 : 1) (20 mg/kg, p.o.) was administrated in ranitidine (5 mg/kg) induced hypochlorhydria in rats as pre-and cotreatment manners. The extract significantly (P < 0.05) enhanced the concentration of pepsin, iron levels in serum, chloride level in gastric juice, and liver along with blood haemoglobin level in experimental animals [85].
A prospective pilot study on dyspeptic patients (n = 20) (baseline between 30 days and 45 days) aged between 18 and 45 years with only single dose of 200 mL fruit juice every morning in empty stomach for thirty days suggests that a significant improvement of pain, nausea, belching, retrosternal burning, and bowel habits among the patients [86]. Table 5 shows the gastrointestinal -protective effects of various parts of B. hispida.
Table 5.
Gastrointestinal protective effects of different parts or their extracts/fractions of B. hispida.
| Gastroprotective | Model/dose/concentration | Mechanisms | References |
|---|---|---|---|
| Antiulcer effect | |||
| Fresh juice, petroleum ether, alcoholic/fruits extract | Aspirin plus restraint, serotonin-induced ulcers, indomethacin plus histamine Swimming stress Mice/in vivo Dose: 1 ml/mouse |
↓ulcer index formed by several ulcerogenic | [13] |
| Fresh juice, ethanol, petroleum ether extracts (5% v/v) | Aspirin plus restraint, swimming stress, indomethacin plus histamine, and serotonin-induced ulcers Rats and mice/in vivo Fresh juice (1-4 mL/animal, p.o.), Dose: ethanol extract 12, 24, and 48 mg/kg, p.o. Dose: petroleum ether extract 0.75, 1.5, 3 mg/kg, p.o. |
Dose-dependent anti-ulcerogenic effect The fresh juice treatment for 3 months did not change the indices (i.e., WBC, RBC counts HCT, HB, MCV, MCH urea, and sugar) No behavioural changes in experimental animals. |
[13] |
| Petroleum ether, methanol/fruits extract | Pylorus ligated (PL) gastric ulcers, ethanol-induced gastric mucosal damage, cold restraint-stress- (CRS-) induced gastric ulcer Rats/in vivo Dose: 300 mg/kg, p.o. |
↓ulcer index ↓MDA ↓vascular permeability ↑ CAT |
[58] |
| Fruit extract | Indomethacin-induced gastric ulcer Rats/in vivo Dose: 1 mL/kg, p.o. |
↓ulcer index, ↓MDA ↓SOD, ↓vitamin C |
[23] |
| Hydromethanol, aqueous ripe fruit, ethyl acetate extracts | Ranitidine (5 mg/kg) induced hypochlorhydria Rats/in vivo Dose: 20 mg/kg, p.o./alternative days for 14 days |
The aqueous extract showed better effects: ↑antioxidant status, ↑pepsin, ↑vitamin C, ↑chloride in gastric juice | [60] |
| Fruit extract with the whole plant of Fumaria vaillantii Loisel (1 : 1) | Ranitidine (5 mg/kg) induced hypochlorhydria Rats/in vivo Dose: 20 mg/kg, p.o. |
↑iron levels in serum, ↑pepsin, ↑gastric juice chloride level and liver ↑blood haemoglobin level | [85] |
| Fruit juice | Prospective pilot study Dyspeptic patients (n = 20) (baseline between 30-45 days); age 18-45 years; 200 mL single-dose every morning in empty stomach for thirty days |
↓pain ↓belching retrosternal burning ↓nausea ↓bowel habits |
[86] |
| Antidiarrheal effect | |||
| Methanolic fruit extract | Castor oil-induced diarrheal, PGE2-induced, enter pooling and charcoal meal models Rats/in vivo Dose: 200, 400, and 600 mg/kg, orally by gavage |
Dose-dependent antidiarrheal effect ↓PGE2- induced, enter pooling ↓gastrointestinal motility |
[90] |
| Methanolic fruit extract | Castor oil, charcoal meal, and antienter pooling models in rats/in vivo Dose: 200, 400, 600 mg/kg, p.o. |
↓ activity against castor oil-induced diarrhoea; ↓PGE2 induced enter pooling ↓gastrointestinal motility | [90] |
Abbreviations and symbols:↑(increased); ↓(decreased); WBC: white blood cells; RBC: red blood cells; HCT: hematocrit; HB: haemoglobin; MCV: mean corpuscular volume; MCH: mean corpuscular haemoglobin concentration; MDA: malondialdehyde; CAT: catalase; SOD: superoxide dismutase; PGE2: prostaglandin E2.
4.5.2. Antidiarrheal Effect
Diarrhoea is a condition characterized by frequent watery stools, and usually, diarrhoea persists for a few days and is treated with diet [87]. But there are also more serious situations, in which diarrhoea requires drug/complementary treatment and is more difficult to cure [88, 89].
The B. hispida fruit methanolic extract displayed potential antidiarrheal activity on the castor oil-induced diarrheal rat model. It was also seen to inhibit induced PGE2, enter pooling, and reduce in the motility of gastro-intestine in charcoal meal rats [90]. The same extract also possessed a significant inhibitory activity against castor oil-induced diarrhoea and induced PGE2, enter pooling and gastrointestinal motility at 200, 400, and 600 mg/kg (p.o.) in castor oil, charcoal meal, and antienter pooling models in rats [90] (Table 5).
4.6. Effects on Metabolic Diseases
4.6.1. Antidiabetic Effects
The methanolic stem extract (50,100, 200 mg/kg, p.o.) dose-dependently lowered the blood glucose level in alloxan-induced diabetic rats [37]. The chloroform fruit extract (250 and 500 mg/kg, p.o.) dose-dependently ameliorated the derangements in lipid metabolism in alloxan-induced diabetic albino rats after 14 days of treatment [91]. The study reveals that the methanol, ethanol, and aqueous peel extracts showed significant α-amylase inhibition activity [17]. The ethanol and ethyl ethanoate leaf extracts lowered the blood glucose level of the diabetic mice in a dose-dependent manner [22]. Antidiabetic effects of various parts of B. hispida have been shown in Table 6.
Table 6.
Other pharmacological activities of Benincasa hispida (Thunb.) Cogn.
| Extract/isolated compounds | Model dose/concentration | Results/potential mechanisms | References |
|---|---|---|---|
| Lipid-lowering effect | |||
| Hexane, chloroform, ethyl acetate/aqueous fruit extract | 3T3-L1 cells/in vitro | Hexane extract: ↓adipocyte differentiation, ↓PPARγ, ↓C/EBPα, ↓leptin gene expression, ↓lipids accumulation, ↑releasing of glycerol, ↑ triglycerides | [97] |
| Antidiabetic effect | |||
| Methanolic/stem extract | Alloxan-induced diabetes Rats/in vivo Dose: 50,100, 200 mg/kg p.o. |
↓blood glucose level dose-dependent | [37] |
| Chloroform/fruits extract | Alloxan-induced diabetes Rats/in vivo Dose: 250, 500 mg/kg p.o. |
Dose-dependently ameliorated the disorders in the metabolism of lipids in diabetic mice | [91] |
| Ethanol, hexane, ethyl ethanoate/leaf extract | STZ-induced diabetes Mice/in vivo Dose: 0.2-1 g/kg, i.p |
Ethanol, ethyl ethanoate extracts: ↓blood glucose level | [22] |
| Antiobesity effect | |||
| Methanolic fruit extract | Mice/in vivo Dose: 0.2-1 g/kg, i.p. |
Anorexic activity ↓food intake |
[96] |
| Antiageing of skin | |||
| Petroleum ether, chloroform, ethyl acetate, methanol/dried fruit pulp extract | Stratum corneum of human skin and dansyl chloride fluorescence models In vitro |
Cream prepared from the fruit extract showed the significant antiageing effect | [12] |
| Effects on other diseases | |||
| Fruit methanol extract | Antigen-antibody induced reaction in rats exudate cells/in vitro | ↓histamine release, anti-inflammatory effect Triterpenes, sterols, multiflorenol, alnusenol exerted better inhibitory effects |
[33] |
| Methanolic fruits extract | Histamine and acetylcholine-induced bronchospasm Guinea pigs/in vivo Dose: 50, 200, 400 mg/kg, p.o. |
Bronchodilator effect: dose-dependent protection against histamine and acetylcholine-induced bronchospasm | [115] |
| Petroleum ether, methanolic/fruits extract | Histamine stimulated paw oedema carrageenan- stimulated paw oedema cotton pellet stimulated granuloma Rats/in vivo Dose: 300 mg/kg, p.o. |
↓histamine release Anti-inflammatory effect |
[58] |
| Juice | Isolated rat aortic ring/in vitro Cultured porcine endothelial cells/in vitro Rats/in vivo Dose: 0.4–1.6 mL/kg, i.v. |
Antihypertensive effect dose-dependent ↓blood pressure ↑relaxation, ↓contraction of isolated rat aortic ring ↑NO in cultured porcine aortic endothelial cells |
[116] |
| Methanolic fruit extract | Renal ischemia/reperfusion injury model Rats/in vivo Dose: 500 mg/kg/day, p.o. for 5 days |
Nephroprotective ↓MDA, ↑SOD, CAT, ↑GSH |
[117] |
| Ethanolic seeds extract | Ethylene glycol induced chronic Hyperoxaluria model Rats/in vivo Dose:250, 500 mg/kg, p.o. for 35 days |
Nephroprotective ↓ urinary oxalate, ↓endogenous oxalate synthesis; ↓urinary protein excretion, ↓kidney oxalate and calcium; ↓elevated serum levels of sodium, creatinine, calcium, phosphorus |
[118] |
| Neuroprotective effects | |||
| Fruit juice | Morphine addiction model Mice/in vivo Dose: 1 mL/mouse, p.o. |
The development of morphine addiction prevented along with the suppression of opioid withdrawal symptoms | [113] |
| Methanolic fruit extract | Spontaneous motor, muscle relaxant, antihistaminic effect and barbiturate induced hypnosis models Mice, rats, and guinea pigs/in vivo Dose: 200-3000 mg/kg, p.o. |
↑ barbiturate induced hypnosis ↑ antihistaminic activity |
[114] |
| Fruit methanol extract | Pentylenetetrazole, strychnine, picrotoxin, and maximal electro seizures model Rats/in vivo Dose: 0.2-1 g/kg, p.o. |
Dose-dependent anticonvulsant activity | [98] |
| Methanolic fruit extract | Acetic acid-induced writhing and hot plate Model Mice/in vivo Dose: 200, 400, 600 mg/kg, p.o. |
Dose-dependent analgesic effect | [111] |
| Aqueous pulp extract | Colchicine-induced Alzheimer's model Rats/in vivo Dose: 100-450 mg/kg, p.o. |
↑SOD, ↑CAT, ↑GSH, ↓LPO dose-dependent | [16] |
| Ethanolic seed extract | Rats/in vivo Dose: 250, 500 mg/kg, p.o. |
Dose-dependent analgesic and antipyretic effects | [26] |
| Methanolic fruit extract | Marble-burying and motor coordination tests Mice/in vivo Dose: 200, 400, 600 mg/kg, p.o. |
Significant dose-dependent anticompulsive effect | [38] |
| Methanolic leaf extract | Acetic acid-induced writhing Mice/in vivo Dose: 50, 100, 200, 400 mg/kg, p.o. |
Dose-dependent analgesic effect | [112] |
| Fruit peel methanolic extract | Egg albumin-induced inflammation in rats; acetic acid-induced writhing, formalin-induced pain, hot plate-induced, and pentylenetetrazol-induced convulsions Mice/in vivo Dose: 50, 100, 200, 400 mg/kg, p.o. |
Dose-dependently (0.25-1.5 g/kg) inhibited acetic acid-induced writhing, formalin-induced pain licking, and hot plate-induced pain in mice. Significantly inhibition of egg albumin-induced inflammation in rats and pentylenetetrazol-induced convulsion in mice |
[62] |
| Ethanolic seed extract | Anticonvulsant activity Mice/in vivo Dose: 250, 500 mg/kg, p.o. |
Dose-dependent anticonvulsant effects | [72] |
| Methanolic fruit extract | TST and FST model Mice/in vivo Dose: 50, 100, 200 mg/kg, p.o. |
Dose-dependent antidepressant effect possibly through GABAergic involvement. | [110] |
| Petroleum ether, methanolic, aqueous/fruit extracts | Motor coordination, locomotor, cognitive behaviour, anxiolytic, haloperidol-induced catalepsy, and anticonvulsant models Mice/in vivo Dose: 100, 200, 400 mg/kg, p.o. |
Dose-dependent anxiolytic, analgesic, and nootropic activity | [107] |
4.6.2. Antiobesity and Lipid-Lowering Effect
Lipids are fatty organic substances that are the largest source of energy for the body. The vast majority of fats are stored in solid form in various organs or skin, and a small part circulates in the blood in liquid form [92, 93]. Imbalances in lipid metabolism lead to pathophysiological changes and the appearance of chronic diseases such as cardiovascular disease, fatty liver, endocrine disorders, and diabetes [94, 95]. Methanolic fruit extract (0.2-1 g/kg, i.p.) reduced food intake, suggesting anorectic activity in mice [96]. Hexane fraction from the aqueous fruit extract inhibited adipocyte differentiation by blocking leptin gene expression, peroxisome proliferator-activated receptor gamma (PPARγ), and CCAAT enhancer-binding protein alpha (C/EBPα), resulting in the reduction of lipid accumulation, increased releasing of glycerol and intracellular triglycerides in 3T3-L1 cells [97].
4.7. Neuroprotective Properties
4.7.1. Anticonvulsant Effects
The fruit methanol extract (0.2-1 g/kg, p.o.) showed a dose-dependent anticonvulsant activity in pentylenetetrazole, strychnine and picrotoxin, and maximal electro seizures model [98]. On the other hand, the fruit peel methanolic extract exerted a dose-dependent (0.25-1.5 g/kg) anticonvulsant effect on pentylenetetrazol-induced convulsion in mouse models [62]. Ethanolic seed extract (250 and 500 mg/kg, p.o.) showed a dose-dependent anticonvulsant effect in anticonvulsant activity in Swiss albino mice [72].
4.7.2. Effects on Alzheimer's Disease
Neurodegenerative diseases such as Alzheimer's disease are characterized by the presence of the central nervous system, protein aggregates, inflammation, and oxidative stress [99, 100]. Several factors are involved in triggering neurodegenerative diseases, including the lifestyle that leads to the gradual deterioration of the health of the nervous system, with serious consequences on the quality of life of the patient with such a disease [101]. Although there are still no treatment solutions to restore nerve function in neurodegenerative diseases, more and more studies insist on several natural formulas that have been shown to have the effect of reducing symptoms and improving the quality of life of patients with neurodegenerative diseases [102, 103].
The fruit extract at a dose of 400 mg/kg (p.o.) showed a protective effect on colchicine-induced Alzheimer's disease rats, possibly through the presence of both vitamin E and β-carotene protecting rat neurons against oxidative stress. On the other hand, the aqueous fruit pulp extract (100-450 mg/kg, p.o.) dose-dependently increased SOD, CAT, and GSH, while reduced in LPO levels in the colchicine-induced Alzheimer's rat model [16].
4.7.3. Effects on Memory and Cognitive Behaviour
Cognitive disorders are characterized by changes in brain structure and function that affect learning, orientation, judgment, memory, and intellectual abilities [104–106]. The methanolic fruit extract (200, 400, or 600 mg/kg, p.o.) showed a significant dose-dependent anticompulsive effect in marble-burying and motor coordination test models in mice [38]. The petroleum ether, methanolic, and aqueous fruit extracts (100, 200, and 400 mg/kg, p.o.) showed a dose-dependent nootropic activity in the cognitive behaviour mouse model [107]. Kumar and Nirmala [108] also studied the possible nootropic effects of the fruit on experimental animals.
4.7.4. Antidepressant and Anxiolytic Effects
Anxiety is defined as a diffuse fear, without a well-defined cause regarding various events of daily life [109]. Methanolic fruit extract (50, 100, and 200 mg/kg, p.o.) showed a dose-dependent antidepressant-like effect in TST and FST models possibly through GABAergic involvement in mice in Swiss mice [110].
Petroleum ether, methanolic, and aqueous fruit extracts (100, 200, and 400 mg/kg, p.o.) confirmed a dose-dependent anxiolytic activity in mice [107]. Effects of various parts of B. hispida on the nervous system have been shown in Table 6.
4.8. Analgesic and Antipyretic Effects
The methanolic fruit extract (200, 400, and 600 mg/kg, p.o.) showed a dose-dependent analgesic effect in acetic acid-induced writhing and hot plate model in mice [111]. The ethanolic seed extract (250 and 500 mg/kg, p.o.) exerted a dose-dependent analgesic effect in rats [26]. The fruit peel methanolic extract also dose-dependently (0.25-1.5 g/kg) inhibited acetic acid-induced writhing, formalin-induced pain licking, and hot plate-induced pain in mice [62].
In another study, the methanolic seed extract (100-300 mg/kg, p.o.) also showed a dose-dependent analgesic effect on the rats (n = 6) model [55]. The methanolic leaf extract (50-400 mg/kg, p.o.) exerted a dose-dependent analgesic effect in an acetic acid-induced writhing mouse model [112]. Petroleum ether, methanolic, and aqueous fruit extracts (100, 200 and 400 mg/kg, p.o.) showed a dose-dependent analgesic effect in the mouse model [107]. The fruit juice (1 mL, p.o.) prevents morphine addiction development along with the suppression of opioid withdrawal symptoms [113]. In experimental animals such as rats, mice, and guinea pigs, the methanolic fruit extract (200-3000 mg/kg, p.o.) significantly potentiated the barbiturate stimulated hypnosis [114].
Qadrie et al. [26] reported that the ethanolic seed extract (250 and 500 mg/kg, p.o.) displayed a dose-dependent antipyretic effect in rats.
4.9. Other Potential Biological Activities
4.9.1. Bronchodilatator Effect
Fruit methanol extract inhibited histamine release. In this study, two triterpenes, the triterpenes and sterols, multiflorenol and alnusenol exerted better inhibitory effects [33]. The methanolic extract (50, 200, and 400 mg/kg, p.o.) of B. hispida exhibited significant protection in guinea pigs against the histamine and acetylcholine-induced bronchospasm [115]. The methanolic fruit extract (200-3000 mg/kg, p.o.) showed significant antihistaminic activity on experimental animals (e.g., rats, mice, and guinea pigs) [114].
4.9.2. Antihypertensive Effect
The ACE inhibitory effect of the plant may show the pharmacological basis in the treatment of high blood pressure for its long time uses in traditional Chinese medicine. The fruit juice (0.4 – 1.6 mL/kg, i.v.) dose-dependently lowered blood pressure, concentration-dependently showed relaxation of isolated rat aortic rings and produced nitric oxide (NO) from the cultured porcine aortic endothelial cells [116]. Polysaccharides of fruit extract showed an antiglycation effect [50].
4.9.3. Nephroprotective Effects
Methanolic fruit extract (500 mg/kg/day, p.o.) for five days reduced the MDA content, while the increase in SOD, CAT, and GSH levels in renal ischemia/reperfusion injury in female Wistar albino rats [117]. The seed ethanolic extract (250 and 500 mg/kg, p.o.) for 35 days significantly lowered the increased urinary oxalate, presenting a regulatory action on endogenous oxalate synthesis; decreased in the urinary excretion and kidney retention levels of protein, oxalate, and calcium; and reduced the increased serum levels of sodium, calcium, phosphorus, and creatinine levels in ethylene glycol induced chronic hyperoxaluria in Wistar albino rat [118].
Effects of various parts of B. hispida on the kidney have been shown in Table 6.
4.9.4. Antiageing of Skin
A cream prepared from the dried fruit pulp extract (petroleum ether, chloroform, ethyl acetate, and methanol) showed a significant antiageing effect on the stratum corneum of human skin and dansyl chloride fluorescence models [12].
5. Toxicological Profile: Safety and Adverse Effects
The fresh juice (5% v/v) treatment for 3 months did not change the total white blood cells (WBC), red blood cells (RBC), haemoglobin (HB), mean corpuscular haemoglobin (MCH), hematocrit (HCT), mean corpuscular volume (MCV), sugar, and urea levels in rats and mice. The treatment also caused no behavioural changes in experimental animals [13]. The methanolic extract of fruit was nontoxic and did not cause the death of mice, rats, and guinea pigs in doses up to 3.0 g/kg [114]. Other studies, performed in female and male rats, concluded that the standardized hydroalcoholic (70% ethanol) extract of the fruit pulp of B. hispida administered orally was relatively safe when to female and male rats [119]. Up to oral dose (1000 mg/kg body weight/day) level, no-observe-adverse-effect-level (NOAEL) was obtained for the extract in the 90-days toxicity study. The ethanolic seed extract up to 5000 mg/kg (p.o.) did not exert toxicity in rats [26]. Di-2-Ethylhexyl phthalate (18.3-75.5 mg/kg), isolated from the fruit of this herb, is a popularly used plasticizer and is harmful to human health [36].
6. Conclusions and Future Perspectives
Benincasa hispida (Cucurbitaceae) is an annual plant, originating in Indonesia. The Chinese have been cultivating it for over 2000 years; its medicinal uses first appeared in the medical field of the Tang Dynasty. In Chinese medicine, the crust is used to treat urinary dysfunction, and the fruits are used to treat fever. In Ayurveda, the fruits are also used to treat epilepsy, lung diseases, asthma, cough, and urinary retention. Starting from these traditional uses, the present paper evaluated the latest in vivo and in vitro pharmacological studies that demonstrated the molecular mechanisms which confirmed ethnopharmacological uses. However, a limiting aspect of this paper is the lack of clinical trials in human subjects. In the future, they are needed to complete the pharmacological properties and to pave the way for new pharmaceutical forms based on natural compounds with proven therapeutic effects. Improvements in control standards are also needed for future pharmacological studies that include B. hispida. In our work, they are relative, phytochemical compounds being identified only by high-performance liquid chromatography (HPLC). Another limiting aspect is represented by the antioxidant action of this plant which has been researched only in vitro, which does not guarantee the same effect on in vivo experimental models. Also, in future studies, the bioavailability, pharmacokinetics, mechanism of action, and study of the activity relationship of the identified and isolated pure phytochemicals should be analyzed, to better understand the reported biological actions.
Although experimental toxicological studies in animals have not shown any adverse effects, no human clinical trials have been performed to demonstrate pharmacological properties or to systematically assess toxicity and safety in humans. These studies are very important for the evaluation of short- and long-term toxicity as well as clinical therapeutic efficacy. However, the results of the present study support the clinical use of B. hispida in modern medicine and can serve as a basis for further studies based on this plant.
Abbreviations
- ABTS:
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid
- ALP:
Alkaline phosphatase
- bFGF:
Basic fibroblast growth factor
- CAT:
Catalase
- C/EBPα:
CCAAT enhancer-binding protein alpha
- DPPH:
2,2-diphenyl-1-picrylhydrazyl
- HPLC:
High-performance liquid chromatography
- HUVECs:
Human umbilical vein endothelial cells
- GSH:
Reduced glutathione
- ICAM-1:
Intercellular adhesion molecule
- IL-6:
Interleukin 6
- LC50:
Median lethal concentration
- LPO:
Lipid peroxidation
- MCV:
Mean corpuscular volume
- MDA:
Malondialdehyde
- NIH/3 T3:
Normal fibroblast cells
- NO:
Nitric oxide
- PGE2:
Prostaglandin E2
- PPARγ:
Peroxisome proliferator-activated receptor-gamma
- ROS:
Reactive oxygen species
- SGOT:
Serum glutamic oxaloacetic transaminase
- SGPT:
Serum glutamic pyruvic transaminase
- SOD:
Superoxidase dismutase
- STZ:
Streptozotocin
- TPC:
Total phenolic content
- TNF-α:
Tumour necrosis factor-alpha.
Contributor Information
Javad Sharifi-Rad, Email: javad.sharifirad@gmail.com.
Monica Butnariu, Email: monicabutnariu@yahoo.com.
Daniela Calina, Email: calinadaniela@gmail.com.
William C. Cho, Email: chocs@ha.org.hk.
Data Availability
The data supporting this review are from previously reported studies and datasets, which have been cited. The processed data are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Ghebretinsae A. G., Thulin M., Barber J. C. Relationships of cucumbers and melons unraveled: molecular phylogenetics ofCucumisand related genera (Benincaseae, Cucurbitaceae) American Journal of Botany . 2007;94(7):1256–1266. doi: 10.3732/ajb.94.7.1256. [DOI] [PubMed] [Google Scholar]
- 2.Palamthodi S., Lele S. S. Nutraceutical applications of gourd family vegetables: Benincasa hispida, Lagenaria siceraria and Momordica charantia. Biomedicine & Preventive Nutrition . 2014;4:15–21. doi: 10.1016/j.bionut.2013.03.004. [DOI] [Google Scholar]
- 3.WHO. WHO monograps on selected medicinal plants . World Health Organization (WHO); 2014. [Google Scholar]
- 4.Purohit P., Palamthodi S., Lele S. S. Effect of karwanda (Carissa congesta Wight) and sugar addition on physicochemical characteristics of ash gourd (Benincasa hispida) and bottle gourd (Langenaria siceraria) based beverages. Journal of Food Science and Technology . 2019;56:1037–1045. doi: 10.1007/s13197-019-03570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palamthodi S., Kadam D., Lele S. S. Physicochemical and functional properties of ash gourd/bottle gourd beverages blended with jamun. Journal of Food Science and Technology . 2019;56:473–482. doi: 10.1007/s13197-018-3509-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaini N. A. M., Anwar F., Hamid A. A., Saari N. Kundur [Benincasa hispida (Thunb.) Cogn.]: a potential source for valuable nutrients and functional foods. Food Research International . 2011;44:2368–2376. doi: 10.1016/j.foodres.2010.10.024. [DOI] [Google Scholar]
- 7.Andrias D. R., Fahmida U., Adi A. C. Nutritional potential of underutilized food crops to improve diet quality of young children in food insecure prone areas of Madura Island, Indonesia. Asia Pacific Journal of Clinical Nutrition . 2019;28:826–836. doi: 10.6133/apjcn.201912_28(4).0020. [DOI] [PubMed] [Google Scholar]
- 8.Heinrich M., Appendino G., Efferth T., et al. Best practice in research - Overcoming common challenges in phytopharmacological research. Journal of Ethnopharmacology . 2020;246 doi: 10.1016/j.jep.2019.112230. [DOI] [PubMed] [Google Scholar]
- 9.Busuioc A. C., Botezatu A.-V. D., Furdui B., et al. Comparative study of the chemical compositions and antioxidant activities of fresh juices from Romanian Cucurbitaceae varieties. Molecules . 2020;25 doi: 10.3390/molecules25225468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patil J. K., Patel M. R. Pharmacognostic and phytochemical investigation of Benincasa hispida (Thunb.) Cogn. fruit. Pharma Science Monitor . 2012;3:146–156. [Google Scholar]
- 11.Soliman W. E., Khan S., Rizvi S. M. D., et al. Therapeutic applications of biostable silver nanoparticles synthesized using peel extract of Benincasa hispida: antibacterial and anticancer activities. Nanomaterials . 2020;10(10):p. 1954. doi: 10.3390/nano10101954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabale V., Kunjwani H., Sabale P. Formulation and in vitro evaluation of the topical antiageing preparation of the fruit of Benincasa hispida. Journal of Ayurveda and Integrative Medicine . 2011;2(3):124–128. doi: 10.4103/0975-9476.85550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grover J. K., Adiga G., Vats V., Rathi S. S. Extracts of Benincasa hispida prevent development of experimental ulcers. Journal of Ethnopharmacology . 2001;78:159–164. doi: 10.1016/S0378-8741(01)00334-8. [DOI] [PubMed] [Google Scholar]
- 14.Ramesh M., Gayathri V., Rao A. V. N. A., Prabhakar M. C., Rao C. S. Pharmacological actions of fruit juice of Benincasa hispida. Fitoterapia . 1989;60:241–247. [Google Scholar]
- 15.Fatariah Z., Zulkhairuazha T. Y. T., Wan Rosli W. I. Quantitative HPLC analysis of gallic acid in Benincasa hispida prepared with different extraction techniques. Sains Malaysiana . 2014;43:1181–1187. [Google Scholar]
- 16.Roy C., Ghosh T. K., Guha D. Dose dependent activity of Benincasa hispida on colchicine induced experimental rat model of Alzheimer’s disease. International Journal of Pharmacology . 2008;4(4):237–244. doi: 10.3923/ijp.2008.237.244. [DOI] [Google Scholar]
- 17.Nagarajaiah S. B., Prakash J. Chemical composition and bioactive potential of dehydrated peels of Benincasa hispida, Luffa acutangula, and Sechium edule. Journal of Herbs Spices & Medicinal Plants . 2015;21:193–202. doi: 10.1080/10496475.2014.940437. [DOI] [Google Scholar]
- 18.Mazumder S., Lerouge P., Loutelier-Bourhis C., Driouich A., Ray B. Structural characterisation of hemicellulosic polysaccharides from Benincasa hispida using specific enzyme hydrolysis, ion exchange chromatography and MALDI-TOF mass spectroscopy. Carbohydrate Polymers . 2005;59:231–238. doi: 10.1016/j.carbpol.2004.09.014. [DOI] [Google Scholar]
- 19.Mazumder S., Ray B., Ghosal P. K. Chemical investigation on the polysaccharides present in the mesocarp of chalkumra (Benincasa hispida) fruit. Asian Journal of Chemistry . 2001;13:1389–1395. [Google Scholar]
- 20.Mazumder S., Morvan C., Thakur S., Ray B. Cell wall polysaccharides from chalkumra (Benincasa hispida) fruit. Part I. Isolation and characterization of pectins. Journal of Agricultural and Food Chemistry . 2004;52:3556–3562. doi: 10.1021/jf0343130. [DOI] [PubMed] [Google Scholar]
- 21.Wills R. B. H., Wong A. W. K., Scriven F. M., Greenfield H. Nutrient composition of Chinese vegetables. Journal of Agricultural and Food Chemistry . 1984;32(2):413–416. doi: 10.1021/jf00122a059. [DOI] [Google Scholar]
- 22.Arbotante C., Arriola E. Investigation of the bioactive properties and hypoglycemic effects of ethanol, hexane and ethyl ethanoate extracts from kondol leaves (Benincasa hispida Cogniaux) American Journal of Clinical Pathology . 2016;146(Supplement 1):S33–S38. doi: 10.1093/ajcp/aqw163.008. [DOI] [Google Scholar]
- 23.Shetty B. V., Arjuman A., Jorapur A., et al. Effect of extract of Benincasa hispida on oxidative stress in rats with indomethacin induced gastric ulcers. Indian Journal of Physiology and Pharmacology . 2008;52:178–182. [PubMed] [Google Scholar]
- 24.Churiyah, Darusman L. K. Bioactive Proteins from Benincasa hispida (Thunb.) Cogn. Journal of Biosciences . 2009;16(4):161–164. doi: 10.4308/hjb.16.4.161. [DOI] [Google Scholar]
- 25.Nadhiya K., Haripriya D., Vijayalakshmi K. Pharmacognostic and preliminary phytochemical analysis on Benincasa hispida fruit. Asian Journal of Pharmaceutical and Clinical Research . 2014;7:98–101. [Google Scholar]
- 26.Qadrie Z. L., Hawisa N. T., Khan M. W., Samuel M., Anandan R. Antinociceptive and anti-pyretic activity of Benincasa hispida (thunb.) cogn. in Wistar albino rats. Pakistan Journal of Pharmaceutical Sciences . 2009;22:287–290. [PubMed] [Google Scholar]
- 27.Hemant D. U., Doshi G. M. Chromatographic studies on Benincasa hispida (thunb.) Cogn. seed extract scrutinized by HPLC and HPTLC. Pharmacognosy Journal . 2014;6:42–48. [Google Scholar]
- 28.Bimakr M., Rahman R. A., Ganjloo A., Taip F. S., Adzahan N. M., Sarker M. Z. I. Characterization of valuable compounds from winter melon (Benincasa hispida (Thunb.) Cogn.) seeds using supercritical carbon dioxide extraction combined with pressure swing technique. Food and Bioprocess Technology . 2016;9(3):396–406. doi: 10.1007/s11947-015-1636-3. [DOI] [Google Scholar]
- 29.Rana S., Suttee D. A. Phytochemical investigation and evaluation of free radical scavenging potential of Benincasa hispida peel extracts. International Journal of Current Pharmaceutical Review and Research . 2012;3:43–46. [Google Scholar]
- 30.Wu C. M., Liou S. E., Chang Y.-H., Chiang W. Volatile compounds of the wax gourd (Benincasa hispida, Cogn) and a wax gourd beverage. Journal of Food Science . 1987;52(1):132–134. doi: 10.1111/j.1365-2621.1987.tb13988.x. [DOI] [Google Scholar]
- 31.Du Q., Zhang Q., Ito Y. Isolation and identification of phenolic compounds in the fruit ofBenincasa hispidaby HSCCC. Journal of Liquid Chromatography and Related Technologies . 2005;28(1):137–144. doi: 10.1081/JLC-200038620. [DOI] [Google Scholar]
- 32.Uchikoba T., Yonezawa H., Kaneda M. Cucumisin like protease from the sarcocarp of Benincasa hispida var. ryukyu. Phytochemistry . 1998;49(8):2215–2219. doi: 10.1016/S0031-9422(98)00135-6. [DOI] [PubMed] [Google Scholar]
- 33.Yoshizumi S., Murakami T., Kadoya M., Matsuda H., Yamahara J., Yoshikawa M. Medicinal Foodstuffs. XI. Histamine release inhibitors from wax gourd, the fruits of Benincasa hispida Cogn. Yakugaku Zasshi . 1998;118(5):188–192. doi: 10.1248/yakushi1947.118.5_188. [DOI] [PubMed] [Google Scholar]
- 34.Shih C. T., Wu J., Jia S., Khan A. A., Ting K. H., Shih D. S. Purification of an osmotin-like protein from the seeds of Benincasa hispida and cloning of the gene encoding this protein. Plant Science . 2001;160:817–826. doi: 10.1016/s0168-9452(00)00450-7. [DOI] [PubMed] [Google Scholar]
- 35.Shih C.-Y. T., Khan A. A., Jia S., Wu J., Shih D. S. Purification, characterization, and molecular cloning of a Chitinase from the seeds of Benincasa hispida. Bioscience, Biotechnology, and Biochemistry . 2001;65:501–509. doi: 10.1271/bbb.65.501. [DOI] [PubMed] [Google Scholar]
- 36.Du Q., Shen L., Xiu L., Jerz G., Winterhalter P. Di-2-ethylhexyl phthalate in the fruits of Benincasa hispida. Food Additives & Contaminants . 2006;23:552–555. doi: 10.1080/02652030500539758. [DOI] [PubMed] [Google Scholar]
- 37.Battu G. R., Mamidipalli S. N., Parimi R., Viriyala R. K., Patchula R. P., Mood L. R. Hypoglycemic and anti-hyperglycemic effect of alcoholic extract of Benincasa hispida in normal and in alloxan induced diabetic rats. Pharmacognosy Magazine . 2007;3:101–105. [Google Scholar]
- 38.Girdhar S., Wanjari M. M., Prajapati S. K., Girdhar A. Evaluation of anti-compulsive effect of methanolic extract of Benincasa hispida Cogn. fruit in mice. Acta Poloniae Pharmaceutica . 2010;67:417–421. [PubMed] [Google Scholar]
- 39.Sew C., Zaini N., Anwar F., Hamid A., Saari N. Nutritional composition and oil fatty acids of kundur [Benincasa hispida (Thunb.) Cogn.] seed. Pakistan Journal of Botany . 2010;42:3247–3255. [Google Scholar]
- 40.Sharma J., Chatterjee S., Kumar V., Variyar P. S., Sharma A. Analysis of free and glycosidically bound compounds of ash gourd (Benincasa hispida): Identification of key odorants. Food Chemistry . 2010;122(4):1327–1332. doi: 10.1016/j.foodchem.2010.03.099. [DOI] [Google Scholar]
- 41.Anwar F., Mohammad N., Othman F., Saari N. Inter-varietal variation in the composition of seeds and seed oils from winter melon [Benincasa hispida (Thunb.) Cogn.] fruit. Pakistan Journal of Botany . 2011;43:2029–2037. [Google Scholar]
- 42.Kumar C. C., Mythily R., Chandraju S. Extraction and mass characterization of sugars from ash gourd peels (Benincasa hispida) Rasayan Journal of Chemistry . 2012;5:280–285. [Google Scholar]
- 43.Mandana B., Russly A. R., Farah S. T., Noranizan M. A., Zaidul I. S., Ali G. Antioxidant activity of winter melon (Benincasa hispida) seeds using conventional soxhlet extraction technique. International Food Research Journal . 2012;19:229–234. [Google Scholar]
- 44.Bimakr M., Rahman R. A., Taip F. S., Adzahan N. M., Sarker M. Z., Ganjloo A. Supercritical carbon dioxide extraction of seed oil from winter melon (Benincasa hispida) and its antioxidant activity and fatty acid composition. Molecules . 2013;18(1):997–1014. doi: 10.3390/molecules18010997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han X. N., Liu C. Y., Liu Y. L., Xu Q. M., Li X. R., Yang S. L. New triterpenoids and other constituents from the fruits of Benincasa hispida (Thunb.) Cogn. Journal of Agricultural and Food Chemistry . 2013;61(51):12692–12699. doi: 10.1021/jf405384r. [DOI] [PubMed] [Google Scholar]
- 46.Ota E., Tsuchiya W., Yamazaki T., Nakamura M., Hirayama C., Konno K. Purification, cDNA cloning and recombinant protein expression of a phloem lectin-like anti-insect defense protein BPLP from the phloem exudate of the wax gourd, Benincasa hispida. Phytochemistry . 2013;89:15–25. doi: 10.1016/j.phytochem.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 47.Rayees B., Dorcus M., Chitra S. Nutritional composition and oil fatty acids of Indian winter melon Benincasa hispida (Thunb.) seeds. International Food Research Journal . 2013;20:151–155. [Google Scholar]
- 48.Doshi G., Chaskar P., Une H. Elucidation of β-sitosterol from Benincasa hispida seeds, Carissa congesta roots and Polyalthia longifolia leaves by high performance thin layer chromatography. The Pharmacogenomics Journal . 2015;7:221–227. [Google Scholar]
- 49.Fatariah Z., Zulkhairuazha T. T. Y., Rosli W. W. I. Ascorbic acid quantification in Benincasa hispida fruit extracted using different solvents. International Food Research Journal . 2015;22:208–212. [Google Scholar]
- 50.Jiang X., Kuang F., Kong F., Yan C. Prediction of the antiglycation activity of polysaccharides from Benincasa hispida using a response surface methodology. Carbohydrate Polymers . 2016;151:358–363. doi: 10.1016/j.carbpol.2016.05.079. [DOI] [PubMed] [Google Scholar]
- 51.Sharifi-Rad J., Dey A., Koirala N., et al. Cinnamomum species: bridging phytochemistry knowledge, pharmacological properties and toxicological safety for health benefits. Frontiers in Pharmacology . 2021;12 doi: 10.3389/fphar.2021.600139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chouikh A. Phytochemical profile, antioxidant, analgesic and hypolipidaemic effects of ephedra alata decne. female cones extract. Farmacia . 2020;68:1011–1020. doi: 10.31925/farmacia.2020.6.7. [DOI] [Google Scholar]
- 53.Sharifi-Rad J., Rodrigues C. F., Sharopov F., et al. Diet, lifestyle and cardiovascular diseases: linking pathophysiology to cardioprotective effects of natural bioactive compounds. International Journal of Environmental Research and Public Health . 2020;17(7):p. 2326. doi: 10.3390/ijerph17072326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsoukalas D., Zlatian O., Mitroi M., et al. A novel nutraceutical formulation can improve motor activity and decrease the stress level in a murine model of middle-age animals. Journal of Clinical Medicine . 2021;10(4):p. 624. doi: 10.3390/jcm10040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gill N., Dhiman K., Bajwa J., Sharma P., Sood S. Evaluation of free radical scavenging, antiinflammatory and analgesic potential of Benincasa hispida seed extract. International Journal of Pharmacology . 2010;6(5):652–657. doi: 10.3923/ijp.2010.652.657. [DOI] [Google Scholar]
- 56.Bimakr M., Rahman R. A., Taip F. S., Adzahan N. M., Sarker M. Z., Ganjloo A. Optimization of ultrasound-assisted extraction of crude oil from winter melon (Benincasa hispida) seed using response surface methodology and evaluation of its antioxidant activity, total phenolic content and fatty acid composition. Molecules . 2012;17:11748–11762. doi: 10.3390/molecules171011748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moon M. K., Kang D. G., Lee Y. J., Kim J. S., Lee H. S. Effect of Benincasa hispida Cogniaux on high glucose-induced vascular inflammation of human umbilical vein endothelial cells. Vascular Pharmacology . 2009;50:116–122. doi: 10.1016/j.vph.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 58.Rachchh M. A., Jain S. M. Gastroprotective effect of Benincasa hispida fruit extract. Indian journal of pharmacology . 2008;40:271–275. doi: 10.4103/0253-7613.45154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharma S., Verma H. N., Sharma N. K. Cationic Bioactive Peptide from the Seeds of Benincasa hispida. International Journal of Peptides . 2014;2014:12. doi: 10.1155/2014/156060.156060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mandal U., De D., Ali K. M., Biswas A., Ghosh D. Effect of different solvent extracts of Benincasa hispida T. on experimental hypochlorhydria in rat. Journal of Advanced Pharmaceutical Technology & Research . 2012;3:41–46. doi: 10.4103/2231-4040.93563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Samad N. B., Debnath T., Jin H. L., et al. Antioxidant activity of benincasa hispidaseeds. Journal of Food Biochemistry . 2013;37(4):388–395. doi: 10.1111/j.1745-4514.2011.00643.x. [DOI] [Google Scholar]
- 62.Parida N. K., Sahu M. R., Debata P. C., Panda P. K. Antinociceptive and antiinflammatory effects of methanolic extract of Benincasa hispida fruit peel in rodents. Asian Journal of Chemistry . 2010;22:7573–7579. [Google Scholar]
- 63.Zlatian O., Balasoiu A. T., Balasoiu M., et al. Antimicrobial resistance in bacterial pathogens among hospitalised patients with severe invasive infections. Experimental and Therapeutic Medicine . 2018;16:4499–4510. doi: 10.3892/etm.2018.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taheri Y., Joković N., Vitorović J., Grundmann O., Maroyi A., Calina D. The burden of the serious and difficult-to-treat infections and a new antibiotic available: cefiderocol. Frontiers in Pharmacology . 2021;11 doi: 10.3389/fphar.2020.578823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ghenea A. E., Cioboată R., Drocaş A. I., et al. Prevalence and antimicrobial resistance of Klebsiella strains isolated from a county hospital in Romania. Antibiotics . 2021;10(7):p. 868. doi: 10.3390/antibiotics10070868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ungureanu A., Zlatian O., Mitroi G., et al. Staphylococcus aureus colonisation in patients from a primary regional hospital. Molecular Medicine Reports . 2017;16(6):8771–8780. doi: 10.3892/mmr.2017.7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salehi B., Capanoglu E., Adrar N., et al. Cucurbits plants: a key emphasis to its pharmacological potential. Molecules . 2019;24(10):p. 1854. doi: 10.3390/molecules24101854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Islam M. T., Salehi B., Karampelas O., et al. High skin melanin content, vitamin d deficiency and immunity: potential interference for severity of COVID-19. Farmácia . 2020;68:970–983. doi: 10.31925/farmacia.2020.6.3. [DOI] [Google Scholar]
- 69.Sharifi-Rad J., Quispe C., Rahavian A., et al. Bioactive compounds as potential agents for sexually transmitted diseases management: a review to explore molecular mechanisms of action. Frontiers in Pharmacology . 2021;12 doi: 10.3389/fphar.2021.674682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ullah M. O., Haque M., Urmi K. F., et al. Anti-bacterial activity and brine shrimp lethality bioassay of methanolic extracts of fourteen different edible vegetables from Bangladesh. Asian Pacific Journal of Tropical Biomedicine . 2013;3:1–7. doi: 10.1016/s2221-1691(13)60015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sreenivas K. M., Lele S. S. Prebiotic activity of gourd family vegetable fibres using in vitro fermentation. Food Bioscience . 2013;1:26–30. doi: 10.1016/j.fbio.2013.01.002. [DOI] [Google Scholar]
- 72.Qadrie Z., Anandan R., Mushtaque M., Kumar K., Ashraf H. Anthelmintic and anticonvulsant studies of ethanolic extract of Benincasa hispida seeds. Pharmacology . 2011;2:1298–12302. [Google Scholar]
- 73.Drocaş A. I., Tomescu P. I., Mitroi G., et al. The cadherin switch assessment in the epithelial-mesenchymal transition of urothelial bladder carcinomas. Romanian Journal of Morphology and Embryology . 2016;57:1037–1044. [PubMed] [Google Scholar]
- 74.Docea A. O., Mitrut P., Grigore D., Pirici D., Calina D. C., Gofita E. Immunohistochemical expression of TGF beta (TGF-beta), TGF beta receptor 1 (TGFBR1), and Ki67 in intestinal variant of gastric adenocarcinomas. Romanian Journal of Morphology and Embryology . 2012;53:683–692. [PubMed] [Google Scholar]
- 75.Zlatian O. M., Comanescu M. V., Rosu A. F., et al. Histochemical and immunohistochemical evidence of tumor heterogeneity in colorectal cancer. Romanian Journal of Morphology and Embryology . 2015;56:175–181. [PubMed] [Google Scholar]
- 76.Buga A. M., Docea A. O., Albu C., et al. Molecular and cellular stratagem of brain metastases associated with melanoma. Oncology Letters . 2019;17:4170–4175. doi: 10.3892/ol.2019.9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sharifi-Rad J., Quispe C., Butnariu M., et al. Chitosan nanoparticles as a promising tool in nanomedicine with particular emphasis on oncological treatment. Cancer Cell International . 2021;21(1) doi: 10.1186/s12935-021-02025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sharifi-Rad J., Kamiloglu S., Yeskaliyeva B., et al. Pharmacological activities of psoralidin: a comprehensive review of the molecular mechanisms of action. Frontiers in Pharmacology . 2020;11 doi: 10.3389/fphar.2020.571459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Salehi B., Prakash Mishra A., Nigam M., et al. Ficus plants: state of the art from a phytochemical, pharmacological, and toxicological perspective. Phytotherapy Research . 2020;35 doi: 10.1002/ptr.6884. [DOI] [PubMed] [Google Scholar]
- 80.Salehi B., Rescigno A., Dettori T., et al. Avocado-soybean unsaponifiables: a panoply of potentialities to be exploited. Biomolecules . 2020;10 doi: 10.3390/biom10010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salehi B., Sharifi-Rad J., Capanoglu E., et al. Cucurbita plants: from farm to industry. Applied Sciences . 2019;9(16):p. 3387. doi: 10.3390/app9163387. [DOI] [Google Scholar]
- 82.Salehi B., Lopez-Jornet P., Pons-Fuster López E., et al. Plant-derived bioactives in oral mucosal lesions: a key emphasis to curcumin, lycopene, chamomile, aloe vera, Green Tea and Coffee Properties. Biomolecules . 2019;9(3):p. 106. doi: 10.3390/biom9030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Akev N., Candoken E., Kuruca S. E. Evaluation of aloe vera leaf extracts and aloe emodin on several cancer cell lines. Farmácia . 2020;68(6):1155–1165. doi: 10.31925/farmacia.2020.6.24. [DOI] [Google Scholar]
- 84.Lee K. H., Choi H. R., Kim C. H. Anti-angiogenic effect of the seed extract of Benincasa hispida Cogniaux. Journal of Ethnopharmacology . 2005;97(3):509–513. doi: 10.1016/j.jep.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 85.Mandal U., Ali K. M., Chatterjee K., De D., Biswas A., Ghosh D. Management of experimental hypochlorhydria with iron deficiency by the composite extract of Fumaria vaillantii L. and Benincasa hispida T. in rat. Journal of Natural Science, Biology, and Medicine . 2014;5:397–403. doi: 10.4103/0976-9668.136202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vinaya T. M., Aravind B. S., Sibbritt D., Tapasbrata T., Shivakumar S. The use of _Benincasa hispida_ for the treatment of uninvestigated dyspepsia: Preliminary results of a non-randomised open label pilot clinical trial. Advances in Integrative Medicine . 2015;2(3):130–134. doi: 10.1016/j.aimed.2015.09.003. [DOI] [Google Scholar]
- 87.Scheau C., Caruntu C., Badarau I. A., et al. Cannabinoids and inflammations of the gut-lung-skin barrier. Journal of Personalized Medicine . 2021;11(6):p. 494. doi: 10.3390/jpm11060494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sharifi-Rad J., Rodrigues C. F., Stojanović-Radić Z., et al. Probiotics: versatile bioactive components in promoting human health. Medicina . 2020;56(9) doi: 10.3390/medicina56090433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mitrut P., Docea A. O., Kamal A. M., et al. Colorectal Cancer: From Pathogenesis to Treatment . Rijeka: Intech Europe; 2016. Colorectal cancer and inflammatory bowel disease; pp. 185–199. [DOI] [Google Scholar]
- 90.Vrushabendra Swamy B., Rao T., Dhanapal R., Balamuralidhar V., Ashoka Babu V. Antidiarrheal evaluation of Benincasa hispida (Thunb.) Cogn. fruit extracts. Iranian Journal of Pharmacology and Therapeutics (IJPT) . 2005;4:24–27. [Google Scholar]
- 91.Patil R. N., Patil R. Y., Ahirwar B., Ahirwar D. Evaluation of antidiabetic and related actions of some Indian medicinal plants in diabetic rats. Asian Pacific Journal of Tropical Medicine . 2011;4(1):20–23. doi: 10.1016/s1995-7645(11)60025-4. [DOI] [PubMed] [Google Scholar]
- 92.Tsatsakis A., Docea A. O., Calina D., et al. A mechanistic and pathophysiological approach for stroke associated with drugs of abuse. Journal of Clinical Medicine . 2019;8(9):p. 1295. doi: 10.3390/jcm8091295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Amir S., Shah S. T. A., Mamoulakis C., et al. Endocrine disruptors acting on estrogen and androgen pathways cause reproductive disorders through multiple mechanisms: a review. International Journal of Environmental Research and Public Health . 2021;18(4):p. 1464. doi: 10.3390/ijerph18041464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Găman A. E., Ungureanu A. M., Turculeanu A., et al. The impact of liver steatosis on early and sustained treatment response in chronic hepatitis C patients. Romanian Journal of Morphology and Embryology . 2017;58:107–113. [PubMed] [Google Scholar]
- 95.Ţieranu E. N., Donoiu I., Istrătoaie O., et al. Rare case of single coronary artery in a patient with liver cirrhosis. Romanian Journal of Morphology and Embryology . 2017;58:1505–1508. [PubMed] [Google Scholar]
- 96.Kumar A., Vimalavathini R. Possible anorectic effect of methanol extract of Benincasa hispida (Thunb). Cogn, fruit. Indian Journal of Pharmacology . 2004;36:348–350. [Google Scholar]
- 97.You Y., Jun W. Effects of fractions from Benincasa hispida on inhibition of adipogenesis in 3T3-L1 Preadipocytes. Journal of the Korean Society of Food Science and Nutrition . 2012;41(7):895–900. doi: 10.3746/jkfn.2012.41.7.895. [DOI] [Google Scholar]
- 98.Kumar A., Ramu P. Anti-convulsant activity of Benincasa hispida fruit, methanol extract. Journal of Natural Remedies . 2004;4(2):195–198. [Google Scholar]
- 99.Salehi B., Calina D., Docea A., et al. Curcumin's nanomedicine formulations for therapeutic application in neurological diseases. Journal of Clinical Medicine . 2020;9(2):p. 430. doi: 10.3390/jcm9020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Calina D., Buga A. M., Mitroi M., et al. The treatment of cognitive, behavioural and motor impairments from brain injury and neurodegenerative diseases through cannabinoid system modulation-evidence from in vivo studies. Journal of Clinical Medicine . 2020;9(8) doi: 10.3390/jcm9082395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sharifi-Rad M., Anil Kumar N. V., Zucca P., et al. Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Frontiers in Physiology . 2020;11 doi: 10.3389/fphys.2020.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Salehi B., Sharifi-Rad J., Cappellini F., et al. The therapeutic potential of anthocyanins: current approaches based on their molecular mechanism of action. Frontiers in Pharmacology . 2020;11 doi: 10.3389/fphar.2020.01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Siokas V., Aloizou A. M., Tsouris Z., et al. ADORA2A rs5760423 and CYP1A2 rs762551 polymorphisms as risk factors for Parkinson's disease. Journal of Clinical Medicine . 2021;10(3):p. 381. doi: 10.3390/jcm10030381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sharifi-Rad M., Lankatillake C., Dias D. A., et al. Impact of natural compounds on neurodegenerative disorders: from preclinical to pharmacotherapeutics. Journal of Clinical Medicine . 2020;9(4):p. 1061. doi: 10.3390/jcm9041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Salehi B., Sestito S., Rapposelli S., et al. Epibatidine: a promising natural alkaloid in health. Biomolecules . 2019;9(1):p. 6. doi: 10.3390/biom9010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Aloizou A. M., Siokas V., Pateraki G., et al. Thinking outside the ischemia box: advancements in the use of multiple sclerosis drugs in ischemic stroke. Journal of Clinical Medicine . 2021;10(4) doi: 10.3390/jcm10040630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ambikar D., Mohanta G. Effect of dried fruit extract of Benincasa hispida on brain behaviour in laboratory animals. Journal of Cell and Tissue Research . 2013;13:3519–3524. [Google Scholar]
- 108.Kumar A., Nirmala V. Nootropic activity of methanol extract of Benincasa hispida fruit. Indian Journal of Pharmacology . 2003;35:p. 130. [Google Scholar]
- 109.Islam M. S., Quispe C., Hossain R., et al. Neuropharmacological effects of quercetin: a literature-based review. Frontiers in Pharmacology . 2021;12 doi: 10.3389/fphar.2021.665031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dhingra D., Joshi P. Antidepressant-like activity of Benincasa hispida fruits in mice: possible involvement of monoaminergic and GABAergic systems. Journal of Pharmacology and Pharmacotherapeutics . 2012;3(1):60–62. doi: 10.4103/0976-500x.92521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hemamalini K., Varma M. V. M. Antinociceptive effects of methanolic extract of Benincasa hispida (Thunb.) Cong. fruit. Pharmacologyonline . 2007;3:327–332. [Google Scholar]
- 112.Jahan F., Hossain M., Mamun A., et al. An evaluation of antinociceptive effect of methanol extracts of Desmodium gangeticum (L.) Dc. stems and Benincasa hispida (Thunb.) Cogn. leaves on acetic acid-induced gastric pain in mice. Advances in Natural and Applied Science . 2010;4:365–369. [Google Scholar]
- 113.Grover J. K., Rathi S. S., Vats V. Preliminary study of fresh juice of Benincasa hispida on morphine addiction in mice. Fitoterapia . 2000;71(6):707–709. doi: 10.1016/S0367-326X(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 114.Babu S. C., Ilavarasan R., Refai M. S., Thameemul-Ansari L. H., Kumar D. A. Preliminary pharmacological screening of Benincasa hispida Cogn. Journal of Natural Remedies . 2003;3 doi: 10.18311/jnr/2003/154. [DOI] [Google Scholar]
- 115.Kumar A., Ramu P. Effect of methanolic extract of Benincasa hispida againsthistamine and acetylcholine induced bronchospasm in guinea pigs. Indian Journal of Pharmacology . 2002;34:365–366. [Google Scholar]
- 116.Nakashima M., Shigekuni Y., Obi T., et al. Nitric oxide-dependent hypotensive effects of wax gourd juice. Journal of Ethnopharmacology . 2011;138(2):404–407. doi: 10.1016/j.jep.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 117.Yagnik B., Jitendra V., Nurudin J., Nilesh K., Rameshvar P., Natavarlal P. Antioxidant activity of Benincasa hispida on renal ischemia/reperfusion injury. Pharmacology . 2009;1:44–49. [Google Scholar]
- 118.Patel R., Patel S., Shah J. Anti-urolithiatic activity of ethanolic extract of seeds of Benincasa hispida (thumb) Pharmacology . 2011;3:586–591. [Google Scholar]
- 119.Shakya A., Chaudhary S. K., Bhat H. R., Ghosh S. K. Acute and sub-chronic toxicity studies of Benincasa hispida (Thunb.) cogniaux fruit extract in rodents. Regulatory Toxicology and Pharmacology . 2020;118, article 104785 doi: 10.1016/j.yrtph.2020.104785. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this review are from previously reported studies and datasets, which have been cited. The processed data are available from the corresponding author upon request.


