Summary:
Absence of the proximal upper limb, whether congenital or acquired, has a profound impact on quality of life. Targeted muscle reinnervation (TMR) was originally developed to improve functional control over myoelectric prostheses; however, it has also been shown to decrease phantom limb pain and neuroma pain as well as prevent neuroma formation. In children, whose rates of prosthetic use are considerably lower than adults, the effects of amputation on limb function can be devastating. To date, there is very little literature regarding the use of TMR in children. In this case report, we review the current literature and present the case of a 9-year-old boy with a transhumeral amputation secondary to a traumatic injury who underwent acute TMR at the time of wound closure. At 22 months follow-up, the patient is doing well with minimal pain, no evidence of neuroma formation, and signs of muscle reinnervation.
Absence of the proximal upper limb in childhood has a profound impact on patient quality of life. Congenital deficiency accounts for most limb loss in patients younger than 10 years old, and acquired deficiency is the leading cause in those older than 10.1,2 The rate of prosthetic rejection in children is high,3,4 especially among upper arm amputees.4 Introduction of targeted muscle reinnervation5 offers new possibilities for these patients. TMR involves surgical transfer of terminal peripheral nerves to motor nerves within the residual limb for amplification of myoelectric signals for more intuitive control of myoelectric prosthetics.3,5 Although targeted muscle reinnervation has been successfully utilized in adults,5,6 to our knowledge, no published reports exist about its use in children. We present herein a case of a traumatic transhumeral amputation and acute TMR in a 9-year-old boy.
CASE REPORT
The patient is a 9-year-old boy who suffered a gunshot wound to his right distal humerus (Fig. 1). At an outside hospital, he immediately underwent a transhumeral guillotine amputation without closure for hemorrhage control. He was then transferred to our pediatric hospital for multidisciplinary management.
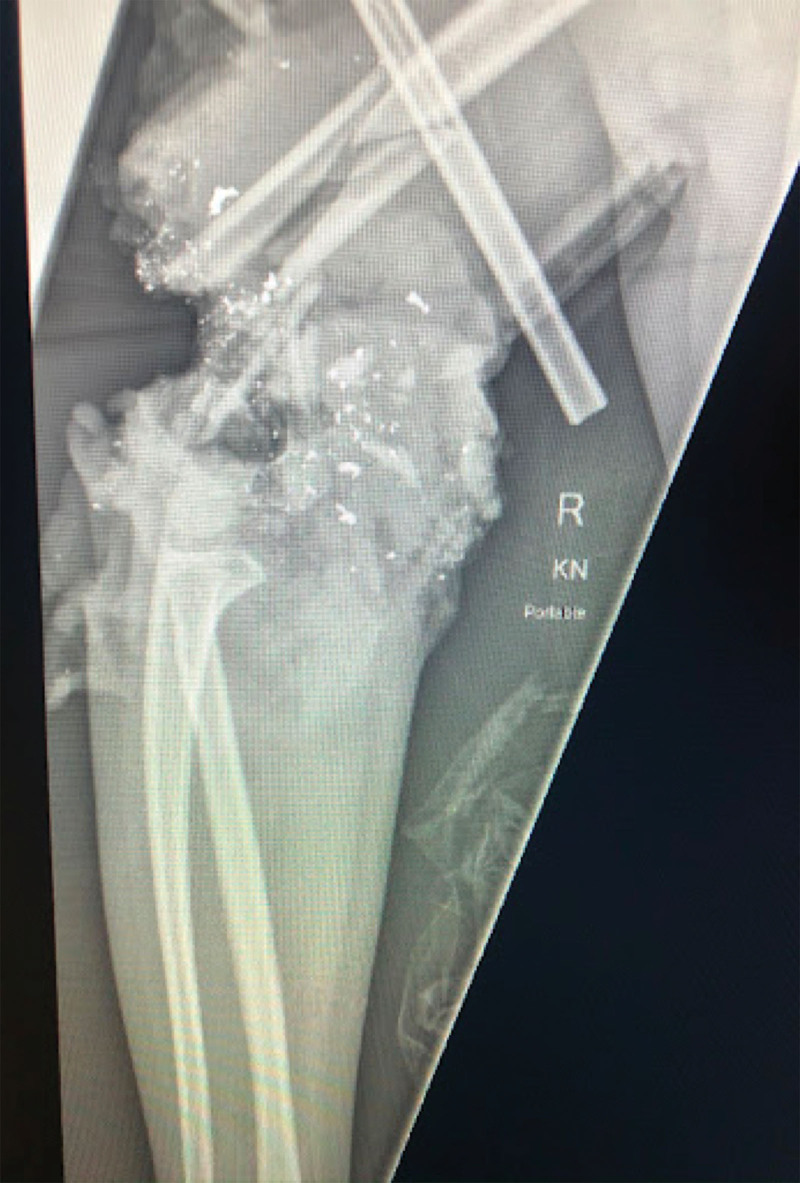
Fig. 1.
Patient’s x-ray upon initial presentation to an outside hospital.
Upon arrival, the patient’s wound was managed with serial debridements and wound vac changes. The wound was deemed appropriate for closure with no further ongoing tissue necrosis at hospital day 13 (Fig. 2). At this time, he underwent TMR with the goal of leaving one head of the triceps and biceps natively innervated and re-innervating the other heads as well as the brachialis to achieve five potential myoelectric signals in contrast to only two possible signals without TMR. A second reason for the TMR was to decrease the chances of painful neuromas and phantom limb pain.
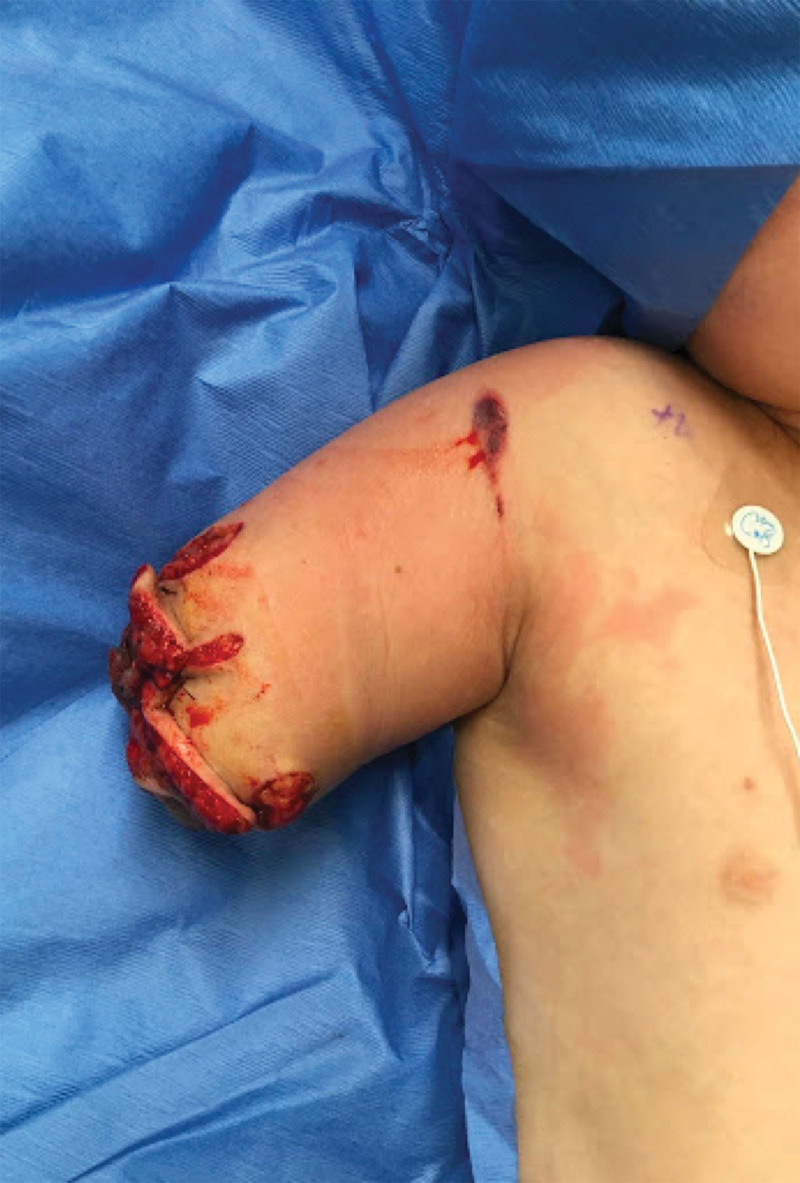
Fig. 2.
Patient’s wound when deemed appropriate for TMR and wound closure.
In the prone position, a posterior triceps splitting approach was made and a proximally based adipofascial flap was raised to isolate the future long head and lateral head myoelectric signals. Transfer of the distal radial nerve stump to a motor branch of the lateral head of the triceps (Fig. 3) was then performed. The adipofascial flap was then interposed between the long and lateral heads of the muscle. After closure, he was turned to the supine position and an anterior biceps splitting approach was made, raising an adipofascial flap in a similar fashion as above. Transfer of the median nerve to a motor branch of the long head of the biceps, and transfer of the ulnar nerve to a motor branch of the brachialis was then performed (Fig. 4). The medial and lateral antebrachial cutaneous nerves were buried into the short head of the biceps and the brachialis respectively, and the epineurium was secured with 4.0 monocryl. The skin incisions were closed, and a split-thickness skin graft was placed distally over muscle to preserve as much length as possible for prosthetic fitting. After surgery, the patient participated in physical therapy and was trained to use a body-powered cable prosthesis.
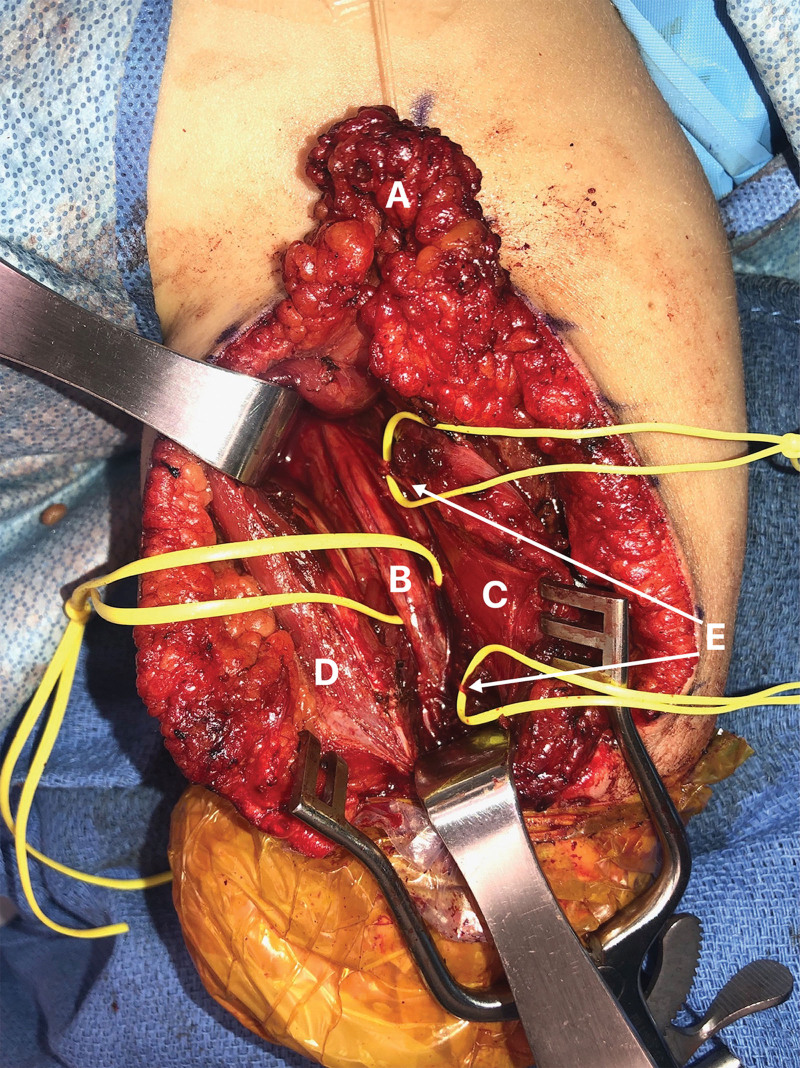
Fig. 3.
Posterior view intraoperatively during the TMR procedure showcasing transfer of the distal radial nerve stump to a motor branch of the lateral head of the triceps.
A: adipofascial flap; B: radial nerve; C: lateral head of the triceps; D: long head of the triceps; E: motor branches to the lateral head of the triceps.
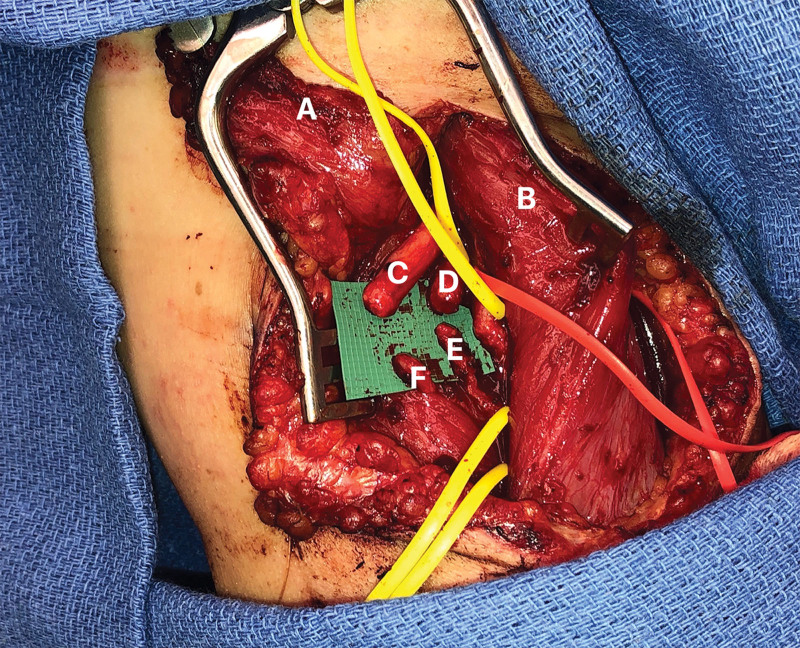
Fig. 4.
Anterior view intraoperatively during the TMR procedure showcasing nerve transfers. A: adipofascial flap; B: short head of the biceps; C: median nerve; D: Ulnar nerve. E, Motor branch of the brachialis. F, Motor branch of the long head of the biceps.
At 21 months postoperative, the patient underwent a revision amputation due to development of a sharp bone spur which deterred his prosthetic use. At 6 weeks postrevision, he had healed well and could fire his residual limb when attempting to flex and extend the elbow, make a fist, and abduct his fingers. (See Video 1 [online], which shows the patient’s physical examination at 22.5 months post-TMR operation.) Overall, he reported no pain and retained some sensation to the distal residual limb. On rare occasions he experiences tingling in the phantom arm after exertion of the residual limb. Mild, nonpainful Tinel’s sign was present along the median and ulnar nerves. He had full active and passive range of motion of the residual shoulder. The patient performs informal physical therapy exercises three to four times per day. On surface electrode testing, the patient was found to have at least three distinct signals in his residual limb. (See Video 2 [online], which shows the surface electrode testing with prosthetist at 23 months post-TMR operation.) The patient scored 40.91 on the qDASH questionnaire, which is an 11-item disability survey that translates to a score of 0–100, with higher scores indicating greater disability.
Video 1. Patient’s physical exam at 22.5 months post-TMR operation. Video 1 from “ Targeted Muscle Reinnervation in Children: A Case Report and Brief Overview of the Literature.”.
Video 2. Surface electrode testing with prosthetist at 23 months post-TMR operation. Video 2 from “ Targeted Muscle Reinnervation in Children: A Case Report and Brief Overview of the Literature.”.
DISCUSSION
When contemplating the use of TMR in children, numerous factors must be considered. Studies of adults have shown evidence of cortical remodeling following amputation and TMR.7 Thus, the enhanced rate of cortical plasticity in the pediatric population8 provides promising opportunities for substantial functional improvement post-TMR in children.
The decision of when to pursue TMR in children is complex. To mitigate the rates of prosthetic rejection in this population, there are accepted timing standards for introduction of prosthetics3,4; however, there is no consensus for when to consider TMR. Particularly in the pediatric population, determining whether TMR should be prioritized as initial treatment rather than awaiting development of pain or prosthetic failure will need to be investigated. Since its introduction into clinical practice, TMR has also proved to be an effective method for prevention and treatment of painful neuromas and phantom limb pain in adults.9 Although literature on neuromas in pediatric amputees is sparse, children do form neuromas that sometimes necessitate surgical intervention.10 Therefore, performing TMR at the time of amputation in children may be beneficial to prevent neuroma development and to set them up for more intuitive control of myoelectric prosthetics.
In addition to timing, it is also crucial to have appropriate patient selection. Zuo et al hypothesize that the pediatric patients most likely to benefit from TMR include those with bilateral proximal upper limb absence and adolescents with acquired unilateral proximal limb absence. They argue that in patients with bilateral limb loss, any function granted by TMR will increase patient independence and quality of life. In patients with acquired unilateral limb absence, Zuo contends these patients will be acutely aware of the functional and psychological impact of limb loss and thus will be more likely to be compliant in postoperative rehabilitation.3
Although there are many potential benefits of using TMR in children, several limitations must be considered. Pediatric patients are dependent on caregivers for activities of daily living, adherence to treatment plans, and dedication to postoperative rehabilitation; thus, robust familial support is essential.3 Financial considerations are also important, including the cost of the procedure, the myoelectric prosthetic, and subsequent replacement parts as the patient outgrows and/or damages their prosthesis.3 Furthermore, myoelectric prosthetic devices are not routinely manufactured in pediatric sizes, thereby increasing costs.3 Even if all cost burdens are met, access to a specialized team who performs TMR is difficult for many families, especially those living in rural areas.
CONCLUSIONS
As TMR is further integrated into amputee care, it is important to consider the benefits and challenges of TMR specific to the pediatric population. These topics should be components of informed consent discussions with patients and their families before proceeding with TMR.
ACKNOWLEDGMENTS
This case report was conducted in accordance with the Declaration of Helsinki. The collection and evaluation of all protected patient health information was performed in a Health Insurance Portability and Accountability Act (HIPAA)-compliant manner.
Footnotes
Published online 17 December 2021.
Disclosure: Dr. Mendenhall receives unrelated research funding from CoNextions Medical Inc. and previously was a consultant for PolyNovo. All the other authors have no financial interest to declare in relation to the content of this article. No funding was received for this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Huizing K, Reinders-Messelink H, Maathuis C, et al. Age at first prosthetic fitting and later functional outcome in children and young adults with unilateral congenital below-elbow deficiency: A cross-sectional study. Prosthet Orthot Int. 2010;34:166–174. [DOI] [PubMed] [Google Scholar]
- 2.Jain S. Rehabilitation in limb deficiency. 2. The pediatric amputee. Arch Phys Med Rehabil. 1996;77(3 suppl):S9–S13. [DOI] [PubMed] [Google Scholar]
- 3.Zuo KJ, Willand MP, Ho ES, et al. Targeted muscle reinnervation: Considerations for future implementation in adolescents and younger children. Plast Reconstr Surg. 2018;141:1447–1458. [DOI] [PubMed] [Google Scholar]
- 4.Meurs M, Maathuis CG, Lucas C, et al. Prescription of the first prosthesis and later use in children with congenital unilateral upper limb deficiency: A systematic review. Prosthet Orthot Int. 2006;30:165–173. [DOI] [PubMed] [Google Scholar]
- 5.Kuiken TA, Dumanian GA, Lipschutz RD, et al. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthet Orthot Int. 2004;28:245–253. [DOI] [PubMed] [Google Scholar]
- 6.Myers H, Lu D, Gray SJ, et al. Targeted muscle reinnervation to improve electromyography signals for advanced myoelectric prosthetic limbs: A series of seven patients. ANZ J Surg. 2020;90:591–596. [DOI] [PubMed] [Google Scholar]
- 7.Chen A, Yao J, Kuiken T, et al. Cortical motor activity and reorganization following upper-limb amputation and subsequent targeted reinnervation. Neuroimage Clin. 2013;3:498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenroot RK, Giedd JN. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–729. [DOI] [PubMed] [Google Scholar]
- 9.Janes LE, Fracol ME, Dumanian GA, et al. Targeted muscle reinnervation for the treatment of neuroma. Hand Clin. 2021;37:345–359. [DOI] [PubMed] [Google Scholar]
- 10.Hanna SA, Catapano J, Borschel GH. Painful pediatric traumatic neuroma: Surgical management and clinical outcomes. Childs Nerv Syst. 2016;32:1191–1194. [DOI] [PubMed] [Google Scholar]