Abstract
PURPOSE
The randomized PANAMA trial investigated the efficacy of panitumumab (Pmab) when added to maintenance therapy with fluorouracil and folinic acid (FU/FA) in patients with RAS wild-type metastatic colorectal cancer.
METHODS
Following first-line induction therapy with six cycles of FU/FA and oxaliplatin plus Pmab, responding patients (stable disease or partial or complete remission) were randomly assigned (1:1, open-label) to maintenance treatment with either FU/FA plus Pmab or FU/FA alone. The primary objective was to demonstrate superiority of progression-free survival (PFS, time from random assignment until progression or death) in favor of FU/FA plus Pmab with a hazard ratio (HR) of 0.75, a power of 80%, and a significance level of 10%. Secondary end points included overall survival, objective response rate of maintenance therapy, and toxicity. Survival end points were analyzed by the Kaplan-Meier method and compared by log-rank test and Cox regressions. Dichotomous variables were compared by Fisher's exact test; odds ratios were indicated when appropriate. The trial is registered with ClinicalTrials.gov (NCT01991873).
RESULTS
Overall, 248 patients were randomly assigned and received maintenance therapy with either FU/FA plus Pmab (125 patients) or FU/FA alone (123 patients). At data cutoff, with 218 events (of 218 needed), PFS of maintenance therapy was significantly improved with FU/FA plus Pmab (8.8 months v 5.7 months; HR, 0.72; 80% CI, 0.60 to 0.85; P = .014). Overall survival (event rate 54%) numerically favored the FU/FA plus Pmab arm (28.7 months v 25.7 months; HR, 0.84; 95% CI, 0.60 to 1.18; P = .32). Objective response rates were 40.8% in patients receiving FU/FA plus Pmab versus 26.0% in patients receiving FU/FA alone (odds ratio, 1.96; 95% CI, 1.14 to 3.36; P = .02). The most frequent Common Terminology Criteria for Adverse Event grade ≥ 3 event during maintenance therapy was skin rash (7.2%).
CONCLUSION
In RAS wild-type metastatic colorectal cancer, maintenance therapy with FU/FA plus Pmab induced a significantly superior PFS compared with FU/FA alone. If active maintenance therapy is aspired following induction therapy with FU/FA and oxaliplatin plus Pmab, FU/FA plus Pmab appears to be the most favorable option.
INTRODUCTION
Previously untreated patients with microsatellite-stable metastatic colorectal cancer (mCRC) are typically treated with combinations of fluorouracil and folinic acid (FU/FA) plus either oxaliplatin (FOLFOX) or irinotecan or with all three agents.1-8 Additionally, monoclonal antibodies targeting either the epidermal growth factor receptor (EGFR) or the vascular endothelial growth factor are added to these chemotherapy backbones. Whereas anti-EGFR antibodies are typically used in patients with RAS wild-type (WT) mCRC and primaries located between the splenic flexure and rectum, all other patients are candidates for anti–vascular endothelial growth factor therapy with bevacizumab.1,2,6,7,9-16 Importantly, chemotherapeutic regimens (notably oxaliplatin-based regimens because of neurotoxicity) are associated with toxicities that frequently impair the tolerability and the continuation of treatment.17-21
CONTEXT
Key Objective
This trial aims to evaluate the efficacy of panitumumab (Pmab) in combination with fluorouracil and folinic acid (FU/FA) versus FU/FA alone as maintenance therapy following six cycles of induction therapy with FU/FA, oxaliplatin, and Pmab in patients with RAS wild-type metastatic colorectal cancer.
Knowledge Generated
Pmab adds efficacy to FU/FA as maintenance therapy in terms of progression-free survival. Moreover, overall survival numerically favors this combination over FU/FA alone without reaching statistical significance. More patients achieved objective tumor responses during maintenance with FU/FA plus Pmab as compared with FU/FA alone.
Relevance
To the best of our knowledge, this is the first randomized trial evaluating the addition of an epidermal growth factor receptor antibody to FU/FA maintenance therapy in patients with RAS wild-type metastatic colorectal cancer. The trial suggests that if active maintenance therapy is aspired, FU/FA plus Pmab appears to be the most favorable option.
Therefore, in the preantibody era, maintenance therapy with fluoropyrimidines was established following induction therapy with fluoropyrimidines and oxaliplatin on the basis of the results of the OPTIMOX trial.19 The evaluation of maintenance strategies following induction therapy including an anti-EGFR antibody has not lead to a standard of care yet. Previous studies have demonstrated the efficacy of anti-EGFR antibodies alone during maintenance therapy.22-24 However, a recent study suggested that maintenance therapy with panitumumab (Pmab), a fully humanized anti-EGFR antibody, plus FU/FA was superior in terms of progression-free survival (PFS) compared with Pmab alone.25
The PANAMA trial was designed to evaluate the efficacy of Pmab during maintenance therapy with FU/FA in patients with RAS WT mCRC in a randomized, controlled, open-label, phase II trial. Patients with complete or partial remission or stable disease after six cycles of FOLFOX plus Pmab were randomly assigned to either continuation of therapy with FU/FA plus Pmab or FU/FA alone. PFS of maintenance therapy was analyzed as the primary end point.
METHODS
Patients
Main inclusion criteria included the following: RAS WT mCRC (KRAS and NRAS exons 2-4), Eastern Cooperative Oncology Group performance status 0-1, no previous chemotherapy for metastatic disease with the exception of one application of FOLFOX in patients in need of treatment while waiting for the result of RAS testing, measurable disease (on the basis of RECIST version 1.1 criteria), and adequate organ function. Key exclusion criteria included the following: untreated central nervous system lesions and < 6 months interval after end of adjuvant treatment for colorectal cancer.
Design of the Trial and End Points
The trial was designed by T.T., D.P.M., S.K., S.S., and U.G. within the Arbeitsgemeinschaft Internistische Onkologie working group of colorectal cancer. The trial started with induction therapy with six cycles of FOLFOX plus Pmab (see below, “Treatment” section) for all patients. Following induction therapy, patients with stable disease versus partial or complete remission were randomly assigned in a 1:1 ratio to FU/FA plus Pmab versus FU/FA alone. After failure of these maintenance therapies, the study scheduled reintroduction of FOLFOX plus Pmab in both arms of the trial (Fig 1).
FIG 1.
Study design. aDropouts include death, progression, adverse events, and investigator's decision. CR, complete remission; ECOG, Eastern Cooperative Oncology Group; FOLFOX, fluorouracil, folinic acid, and oxaliplatin; FU/FA, fluorouracil and folinic acid; mCRC, metastatic colorectal cancer; PD, progressive disease; PFS, progression-free survival; Pmab, panitumumab; PR, partial remission; R, random assignment; SD, stable disease; WT, wild type.
Random assignment into maintenance therapy arms was organized centrally by electronic case report form using permuted block randomization, sizes 4 and 6. Random assignment was stratified by objective response to therapy after induction therapy (complete or partial remission v stable disease), prior adjuvant therapy with oxaliplatin (yes v no), and planned full dose of Pmab during maintenance therapy versus reduced dose in case of random assignment into the Pmab arm.
The primary end point was PFS of maintenance therapy, defined as time from random assignment to progression (according to RECIST 1.1, assessed by the local investigator) or death from any cause, whichever occurred first.
The statistical hypothesis was based on the median PFS of 8.6 months in the OPTIMOX trial with planned de-escalation of oxaliplatin.19 With the exclusion of the induction therapy interval but selection for patients with favorable prognostic factors (no early progressions or deaths, tolerated therapy, and RAS WT tumors), we hypothesized a median PFS of 7.5 months in the FU/FA arm. On the basis of PFS improvements in phase III trials adding Pmab to chemotherapy with hazard ratios (HRs) between 0.73 and 0.80,17,26 the PANAMA trial aimed to improve the PFS of maintenance therapy to 10.0 months with FU/FA plus Pmab, corresponding to an HR of 0.75. With 218 events for PFS, the trial had 80% power and a (one-sided) α-error of .1 to detect the aforementioned improvement with Pmab. Secondary end points of the trial included PFS of reinduction therapy (progression during maintenance until progression or death during reinduction), objective response rate after 12 weeks of induction chemotherapy according to RECIST version 1.1, objective best response during maintenance and reinduction (according to RECIST 1.1. with baseline at respective start of treatment phase), overall survival (OS) measured from time of random assignment and from start of induction therapy until death from any cause, safety, health, and skin-related quality of life. Treatment duration of maintenance therapy and reinduction therapy was defined as time from first to last application of maintenance or reinduction therapy, respectively.
Statistical Analysis
The preplanned analysis of the primary end point (PFS of maintenance therapy) was conducted in the full analysis population, a modified intent-to-treat population comprising all randomly assigned patients with at least one dose of maintenance treatment. For confirmatory testing, the difference in PFS of maintenance therapy between the two treatment arms was evaluated by a stratified log-rank test. Furthermore, a stratified Cox regression model was used to estimate the HR. For sensitivity analysis, a multivariate Cox proportional hazards model was fitted using the stratification factors at random assignment as covariates in addition to the treatment parameter. Dichotomous variables were compared by Fisher's exact test; additionally, odds ratios with 95% CIs were indicated when appropriate. Survival end points were analyzed by the Kaplan-Meier method, expressed as median, and compared with log-rank tests and Cox regressions (HRs with 80%/95% CI were indicated). The two-sided significance level for secondary and exploratory end points was set to .05. Further differences between study arms were described and evaluated on an exploratory level—respective statistical methods included the inverse Kaplan-Meier method (follow-up). All statistical analyses were done using SAS 9.4 (SAS Institute Inc, Cary, NC) and SPSS 27 (IBM, Armonk, NY). Data cutoff was March 3, 2021.
Trial Conduct
The trial recruited patients in 70 centers in Germany in accordance with the Protocol (online only) and in compliance with the Declaration of Helsinki. The Protocol was approved by the responsible ethics committees of the participating centers. Patients provided written informed consent before trial entry. A contract research organization (ClinAssess GmbH, Leverkusen, Germany) was responsible for random assignment, data management, monitoring, and primary data analysis. The trial is registered with ClinicalTrials.gov (NCT01991873).
Treatment
FOLFOX plus Pmab was given once every two weeks for six cycles with oxaliplatin 85 mg/m2, folinic acid 400 mg/m2, fluorouracil 2,400 mg/m2 (one dose administered over 48 hours) plus Pmab 6 mg per kg body weight. After random assignment, the frequency of application and dosing was maintained with continued application of FU/FA with or without Pmab until progression or occurrence of unacceptable toxicity. To prevent patients from Pmab-induced acneiform rash, it was recommended to use doxycycline as prophylaxis for the first 6 weeks and reactively if needed beyond this interval.
Assessments
The study Protocol defined tumor assessment as computed tomography or magnetic resonance imaging of the thorax and the abdomen. Following initial imaging (within 21 days before study start), reassessments were scheduled after six cycles of induction therapy and every 8 weeks during maintenance therapy. For the evaluation of maintenance therapy, the assessment after six cycles of induction therapy served as baseline. Assessments were performed according to RECIST version 1.1. After study participation, further assessments were scheduled for a maximum of 5 years. Adverse events were documented according to the grading of the National Cancer Institute Common Terminology Criteria for Adverse Events from registration into the trial until the final study visit.
RESULTS
Patients and Treatment
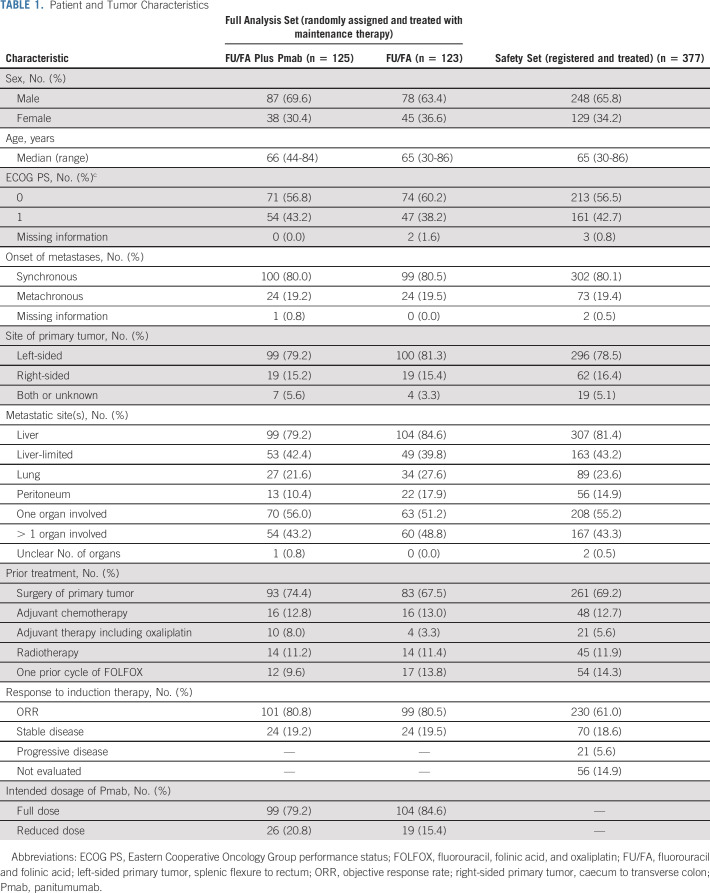
The PANAMA trial included 387 patients between May 2014 and February 2021. Of those, 377 patients received induction therapy (safety set), 265 underwent random assignment, and 248 received maintenance therapy (125 patients FU/FA plus Pmab; 123 patients FU/FA alone). The latter population served as the full analysis set for the confirmatory analysis of the primary end point. Reinduction therapy with FOLFOX plus Pmab was performed in 45 patients (36%) of the FU/FA plus Pmab group and 75 patients (61%) of the FU/FA maintenance group (Fig 2). Characteristics of patients and tumors were evaluated for the safety set and for the full analysis set. Relevant characteristics were generally balanced between the study arms with a slightly higher frequency of peritoneal lesions and > 1 organ disease in the FU/FA arm (Table 1). Median follow-up of the full analysis set was 35.8 months (95% CI, 32.8 to 43.1) and 36.3 months (95% CI, 28.8 to 42.8) in the FU/FA plus Pmab and FU/FA alone group, respectively.
FIG 2.

CONSORT diagram. FOLFOX, fluorouracil, folinic acid, and oxaliplatin; FU/FA, fluorouracil and folinic acid; PFS, progression-free survival; Pmab, panitumumab; secondary resections, resection of metastases.
TABLE 1.
Patient and Tumor Characteristics
Study Treatment Duration and Treatment Beyond Study
Median treatment duration with maintenance therapy was 5.2 months (range, 0.0-32.5) in the FU/FA plus Pmab arm and 4.4 months (range, 0.0-32.9) in the FU/FA alone arm. More than six cycles of maintenance therapy were observed in 96 of 125 patients of the FU/FA plus Pmab arm (76.8%) and 81 of 123 patients of the FU/FA alone arm (65.9%). Dose intensities are summarized in Appendix Figure A1 (online only). Median treatment duration of reinduction therapy was 1.9 months (range, 0.0-9.9) in patients pretreated with FU/FA plus Pmab and 3.3 months (range, 0.0-33.9) in patients following FU/FA maintenance therapy. Progression was the most frequent reason for the end of study treatment during maintenance therapy (Appendix Table A1, online only). At data cutoff, 63 patients of the FU/FA plus Pmab arm (50.4%) and 50 patients of the FU/FA arm (40.7%) had documented second-line therapies (Appendix Table A2, online only).
Efficacy of Maintenance Therapy
The primary end point of the trial (analyzed with 218 events) was met with a significant improvement of PFS of maintenance therapy (HR, 0.72; 80% CI, 0.60 to 0.85; P = .014) by the addition of Pmab to FU/FA (8.8 months; 80% CI, 7.6 to 10.2) as compared with FU/FA alone (5.7 months; 80% CI, 5.6 to 6.0; refer to Figure 3A). The multivariate sensitivity analysis confirmed the treatment effect (HR, 0.71; 80% CI, 0.60 to 0.85; P = .014). In an exploratory analysis of PFS, a relatively uniform magnitude of benefit from the addition of Pmab was observed in all analyzed subgroups (Fig 4).
FIG 3.

Kaplan-Meier estimates of the full analysis set for PFS and OS. Indicated HRs derived from Cox regression testing. P values derived from log-rank tests. (A) Kaplan-Meier estimate of PFS of the full analysis set (primary end point) and (B) Kaplan-Meier estimate of OS of the full analysis set (secondary end point). FU/FA, fluorouracil and folinic acid; FU/FA/Pmab, panitumumab plus FU/FA; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; Pmab, panitumumab.
FIG 4.

Subgroup analysis of progression-free survival. Forest plot with indicated analyses. Hazard ratios for progression or death with 95% CI. ECOG, Eastern Cooperative Oncology Group; FU/FA, fluorouracil and folinic acid; left-sided primary tumor, splenic flexure to rectum; Pmab, panitumumab; right-sided primary tumor, caecum to transverse colon.
OS (event rate: 54.4%) numerically favored FU/FA and Pmab versus FU/FA alone (28.7 months; [95% CI, 25.4 to 39.1] v 25.7 months [95% CI, 22.2 to 28.2]), although this was not significant (HR, 0.84; 95% CI, 0.60 to 1.18; P = .32; Fig 3B).
Objective response according to RECIST 1.1 during maintenance therapy was observed in 51 patients (40.8%) receiving FU/FA plus Pmab and 32 patients (26.0%) receiving FU/FA alone (odds ratio, 1.96; 95% CI, 1.14 to 3.36; P = .02). In both arms of the trial, objective responses during maintenance therapy were primarily (90.2% v 93.8%, respectively) observed in patients who had achieved objective responses following prior induction therapy (Appendix Table A3, online only).
Survival Estimates From Start of Induction Therapy
PFS of the full analysis set from start of induction therapy until progression or death was 11.8 months (95% CI, 10.0 to 13.5) versus 8.6 months (95% CI, 8.1 to 9.1) in patients receiving FU/FA plus Pmab versus patients receiving FU/FA maintenance, respectively. OS from start of induction therapy until death from any cause was 31.5 months (95% CI, 26.7 to 36.4) in patients receiving FU/FA plus Pmab, 28.4 months (95% CI, 24.6 to 32.1) in patients receiving FU/FA maintenance, and 18.1 months (95% CI, 11.6 to 24.6) in patients without maintenance therapy (Appendix Fig A2, online only).
Efficacy of Reinduction Therapy
Objective response to reinduction therapy was 8.9% (4 of 45 patients) versus 34.7% (26 of 75 patients) in patients who had received FU/FA and Pmab versus FU/FA alone as maintenance therapy, respectively (odds ratio, 0.18; 95% CI, 0.06 to 0.57; P = .002). Correspondingly, PFS of reinduction therapy with FOLFOX plus Pmab was 3.8 months (95% CI, 2.5 to 4.8) versus 6.3 months (95% CI, 4.7 to 8.2) in patients who had received FU/FA and Pmab versus FU/FA alone as maintenance therapy (HR, 2.34; 95% CI, 1.54 to 3.56; P < .001).
Toxicity and Safety
Of 377 patients in the safety set of the trial, 138 patients (36.6%) experienced at least one serious adverse event related or unrelated to study medication; of those, 105 (27.9%) patients had a grade ≥ 3 event. Nine grade 5 serious adverse events (six during induction phase, two in the FU/FA plus Pmab arm, and one in the FU/FA arm, all of them not suspected as treatment-related) were reported (terms were as follows: aspiration, bleeding event, ischemic cardiac event, multiorgan failure [2×], renal failure, respiratory tract infection, road traffic accident, and sudden death).
The highest frequency of grade ≥ 3 events of the safety population was reported for acneiform rash with 73 of 377 (19.4%) patients (Appendix Table A4, online only).
Adverse events with first or most severe onset during maintenance therapy were observed with moderate frequency and hardly comprised further hematologic effects. The highest frequency of grade ≥ 3 adverse events with new onset during maintenance was acneiform rash (7.2%) in the Pmab arm (Table 2).
TABLE 2.
Grades 1-4 Adverse Events of Interest With Onset During Maintenance Therapy (full analysis set population)
DISCUSSION
The development of maintenance therapies is based on the clinical necessity to discontinue or reduce oxaliplatin-based chemotherapies because of toxicity and intolerability (mostly accumulating neurotoxicity).1,18,19,27 Therefore, maintenance therapy concepts are usually free of oxaliplatin.19,27-29 In the context of antibody-based regimens, this leads to three potential strategies of maintenance therapy: monotherapy with a fluoropyrimidine as established by OPTIMOX,19 monotherapy with the monoclonal antibody used during induction therapy, or a combination of both. Accordingly, fluoropyrimidines plus bevacizumab became the preferred maintenance option following induction therapy with fluoropyrimidines, oxaliplatin, and bevacizumab.28,29 Interestingly, to the best of our knowledge, fluoropyrimidines as standard maintenance therapy of the preantibody era were not used as control arms in maintenance studies involving monoclonal antibodies. This is despite the fact that maintenance therapy with bevacizumab alone was not found to be sufficiently active28,30 and maintenance with an anti-EGFR antibody alone, despite activity signals,22-24,31 was inferior to a maintenance treatment combining FU/FA and Pmab (VALENTINO study).25 The latter trial emphasized the need for a randomized comparison of antibody-free maintenance with FU/FA versus the combination of FU/FA plus an anti-EGFR antibody. In the PANAMA study, maintenance treatment with Pmab and FU/FA significantly prolonged PFS compared with FU/FA with a relative improvement of 28%. In addition, OS was numerically prolonged in the FU/FA plus Pmab arm without reaching statistical significance. Immature event rates, sample size, and the higher frequency and superior efficacy of reinduction therapy in the FU/FA arm as compared with the FU/FA plus Pmab arm are likely explanations for this observation. The latter aspect may suggest that induction of a new treatment line rather than reinduction was a more favorable option after FU/FA plus Pmab maintenance therapy.
Maintenance therapy with FU/FA plus Pmab was generally well-tolerated with a slightly higher rate of adverse events as compared with FU/FA alone. Since patients were selected for favorable efficacy and tolerability before random assignment, these results should be interpreted with some caution. Nevertheless, the favorable toxicity profile during maintenance therapy supports FU/FA plus Pmab as an attractive maintenance option.
The PANAMA study has several limitations. First, without a standard of care, the control arm of the PANAMA study could have been considered experimental and vice versa. Second, the comparability of the trial results is limited by the duration of 3 months induction therapy, which is rather short compared with other maintenance trials.25,28,29 However, it could be argued that induction therapy involving an anti-EGFR antibody reaches the maximum depth of response after a median of 3-4 months and the induction interval might be considered adequate from a biologic standpoint.13,32 Furthermore, the evaluation of maintenance efficacy only in patients who actually underwent maintenance therapy was not consistently conducted in other maintenance therapy trials.22-25,29,31,33
Third, the PANAMA trial included subgroups of patients with right-sided primary tumor location, BRAF-mutated tumors, and/or microsatellite instability–high tumors who are not ideal candidates for anti-EGFR antibody–based first-line therapy.8-10,14,34 Interestingly, right-sided primary tumor location was slightly underrepresented within the PANAMA trial,9,10,14,15,25 suggesting a potential selection by the investigators. Of note, the Pmab-driven improvement of PFS appeared independent of primary tumor site. However, small sample size of patients with right-sided primary tumors and bias because of selection for anti-EGFR antibody–sensitive tumors before random assignment might explain this finding. Data concerning BRAF-mutant tumors and/or microsatellite instability–high tumors are not yet available for PANAMA. However, given the clearly positive primary efficacy end point, an exploratory analysis excluding subgroups unlikely to benefit from EGFR-targeted maintenance therapy will presumably not affect the interpretation of the trial.
Fourth, although unlikely to have driven the outcome of the trial, a little imbalance concerning negative prognostic characteristics (ie, peritoneal metastases and number of metastatic sites) may have favored the Pmab-based arm of the trial.
Fifth, with prolonged PFS as the primary end point and without a clear OS benefit in this trial, which compares favorably with various trials in this setting,35,36 the concept of maintenance therapy is questionable as a general standard of care. Therefore, complete treatment breaks as a quality of life–friendly alternative remain an option in selected patients.
Last, it is unclear to which extend our data on FU/FA plus Pmab as favorable maintenance therapy can be extrapolated to other anti-EGFR antibody–based induction therapy regimens.
In conclusion, in RAS WT mCRC, maintenance therapy with FU/FA plus Pmab induced a significantly superior PFS compared with FU/FA alone. If active maintenance therapy is aspired following induction therapy with FOLFOX plus Pmab, FU/FA plus Pmab appears to be the most favorable option. Longer follow-up and future studies may help to understand to which extent maintenance therapy including anti-EGFR antibodies affects OS.
ACKNOWLEDGMENT
The authors thank all patients and families as well as all participating study centers. Marlen Susan Winkler, Melanie Schemberg, and Juliane Stintzing provided expert help in coordination of the trial. The list of recruiting study centers can be found in Appendix 1.
APPENDIX 1. List of Recruiting Study Centers
TABLE.
List of Recruiting Study Centers

FIG A1.
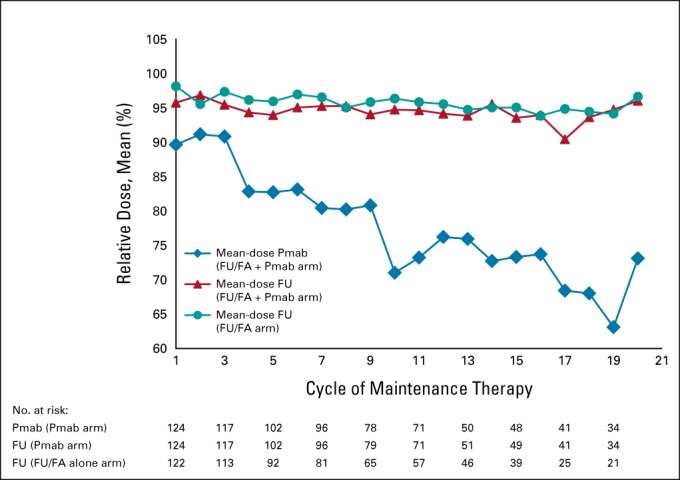
Dose intensity per treatment cycle and substance during maintenance therapy (full analysis set). Relative doses are expressed as means. Patients evaluated = patients evaluated in respective cycle; reference doses were fluorouracil 2,400 mg/m2 and Pmab 6 mg/kg. FU/FA, fluorouracil and folinic acid; Pmab, panitumumab.
FIG A2.

Kaplan-Meier estimates of the safety set for OS since from start of induction therapy. P values derived from log-rank tests. No maintenance = patients with induction therapy who did not receive maintenance therapy. The no maintenance therapy group includes 112 patients who were not randomly assigned and 17 patients who were randomly assigned but did not receive maintenance therapy (refer to CONSORT diagram). FU/FA, fluorouracil and folinic acid; FU/FA/Pmab, panitumumab plus FU/FA; OS, overall survival; Pmab, panitumumab.
TABLE A1.
Reason for End of Study-Therapy
TABLE A2.
Reported Second-Line Therapies After Study Therapy
TABLE A3.
Response to Maintenance Therapy—By Response to Induction Therapy
TABLE A4.
Adverse Events by Maximum National Cancer Institute Common Terminology Criteria for Adverse Event Grade of the Safety Population (n = 377 patients)
Dominik Paul Modest
Honoraria: Merck Serono, Amgen, Roche, Servier, Bristol Myers Squibb, Taiho Pharmaceutical, Merck Sharp & Dohme, Pierre Fabre, Onkowissen, Sanofi, Lilly
Consulting or Advisory Role: Merck Serono, Amgen, Merck Sharp & Dohme, Roche, Servier, Incyte, Bristol Myers Squibb, Pierre Fabre, Lilly, Cor2Ed, IQvia, Onkowissen
Research Funding: Amgen, Servier
Travel, Accommodations, Expenses: Amgen, Merck Serono, Servier
Meinolf Karthaus
Consulting or Advisory Role: Amgen
Travel, Accommodations, Expenses: Amgen
Stefan Fruehauf
Stock and Other Ownership Interests: AbbVie, Bristol Myers Squibb/Pfizer, Johnson & Johnson/Janssen, Merck
Ullrich Graeven
Honoraria: Daiichi Sankyo, Boehringer Ingelheim, Amgen, Servier, AstraZeneca, Bristol Myers Squibb, MSD Oncology
Consulting or Advisory Role: Merck KGaA, Bristol Myers Squibb, Hexal, Amgen, Celgene, Johnson & Johnson, MSD Oncology
Travel, Accommodations, Expenses: Merck KGaA, Amgen, Boehringer Ingelheim, GlaxoSmithKline
Lothar Müller
Honoraria: Roche
Alexander Otto König
Honoraria: Ipsen, Pierre Fabre
Consulting or Advisory Role: Roche Pharma AG
Ludwig Fischer von Weikersthal
Honoraria: Novartis, Roche Pharma AG, AstraZeneca, Pierre Fabre, Lilly GmbH
Albrecht Kretzschmar
Honoraria: Roche Pharma AG, Merck Serono, Shire, Amgen, Medac, Servier, Sanofi, MSD, Bristol Myers Squibb, Bayer Schering Pharma, Aspen Pharma, Roche Pharma
Consulting or Advisory Role: Roche Pharma AG, Shire, Amgen
Travel, Accommodations, Expenses: PharmaMar, Merck Serono, Ipsen, Medac
Eray Goekkurt
Consulting or Advisory Role: MSD, Bristol Myers Squibb, Roche, Sanofi
Annika Kurreck
Honoraria: Servier
Travel, Accommodations, Expenses: Roche, Medac
Arndt Stahler
Honoraria: Roche, Servier, Taiho Pharmaceutical
Travel, Accommodations, Expenses: Amgen, Roche, Lilly, Pfizer
Anke Reinacher-Schick
Honoraria: Amgen, Roche, Pfizer, Sanofi/Aventis, Merck Serono, Celgene, Lilly, Bristol Myers Squibb, Servier, MSD, Aurikamed, IOMEDICO, Promedicis, MCI Group, AstraZeneca
Consulting or Advisory Role: Amgen, Roche, Pfizer, Merck Serono, Celgene, Bristol Myers Squibb, Servier, Baxalta, MSD, AstraZeneca, Pierre Fabre
Research Funding: Roche, Celgene, Ipsen, Amgen, Alexion Pharmaceuticals, AstraZeneca, Lilly, Servier, AIO-Studien, Georgius Agricola Stiftung Ruhr, Rafael Pharmaceuticals, ERYTECH Pharma, BioNTech
Travel, Accommodations, Expenses: Ipsen, Amgen, Roche, Servier, MCI Group, Pierre Fabre, AstraZeneca, Merck Serono, MSD
Stefan Kasper
Honoraria: Bristol Myers Squibb, MSD Oncology, AstraZeneca, Merck Serono, Amgen, Roche, Servier, Lilly, Sanofi/Aventis
Consulting or Advisory Role: Roche, Merck Serono, Amgen, MSD Oncology, Sanofi, Bristol Myers Squibb, Lilly, AstraZeneca, Servier, Janssen-Cilag
Research Funding: Merck Serono, Bristol Myers Squibb, Celgene, Lilly, Servier, Roche/Genentech
Travel, Accommodations, Expenses: Merck Serono, Lilly, Amgen, Sanofi, Roche
Other Relationship: Sanofi, Amgen, Merck Serono, Bristol Myers Squibb, Roche, Lilly
Volker Heinemann
Honoraria: Roche, Celgene, Amgen, Sanofi, Merck, Sirtex Medical, Baxalta, Lilly, Boehringer Ingelheim, Taiho Pharmaceutical, Servier
Consulting or Advisory Role: Merck, Amgen, Roche, Sanofi, Boehringer Ingelheim, Celgene, Sirtex Medical, Baxalta, Servier, Halozyme, MSD, Bristol-Myers Squibb, MSD Oncology
Research Funding: Merck, Amgen, Roche, Celgene, Boehringer Ingelheim, Sirtex Medical, Shire, Servier
Travel, Accommodations, Expenses: Merck, Roche, Sirtex Medical, Amgen, Servier, Shire, MSD, Bristol Myers Squibb
Sebastian Stintzing
Honoraria: Merck KGaA, Roche, Amgen, Bayer, Sanofi, Lilly, Pierre Fabre, Takeda, Taiho Pharmaceutical, Servier, MSD
Consulting or Advisory Role: Merck KGaA, Roche, Sanofi, Bayer, Amgen, Boehringer Ingelheim, Lilly, Takeda, MSD, Servier, Pierre Fabre
Research Funding: Pierre Fabre, Roche Molecular Diagnostics, Merck Serono
Travel, Accommodations, Expenses: Merck KGaA, Roche, Sanofi, Bayer, Sirtex Medical, Amgen, Lilly, Takeda, Pierre Fabre
Tanja Trarbach
Research Funding: Amgen
Travel, Accommodations, Expenses: Ipsen, Takeda, OMT, AbbVie, Novartis, MSD, Sanofi/Aventis, Amgen, Johnson & Johnson/Janssen
No other potential conflicts of interest were reported.
DISCLAIMER
Amgen had no role in the design and conduct of the trial; collection, management, analysis, and interpretation of the data; or the decision to submit the manuscript for publication. Amgen Inc reviewed the manuscript before journal submission. D.P.M. and T.T. had full access to all study data. D.P.M. had the final responsibility for the decision to submit for publication.
PRIOR PRESENTATION
Presented in part as oral presentation at the 2021 ASCO Virtual Annual Meeting, June 4-8, 2021.
SUPPORT
The legal funder (sponsor) of the trial was the AIO Studien gGmbH, Berlin, Germany. Amgen Inc supported the trial with study medication and a research grant to the AIO Studien gGmbH, Thousand Oaks, CA.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
With respect to the clinical trial, Randomized phase II study for evaluation of efficacy and safety of maintenance treatment with 5-FU/FA plus panitumumab versus 5-FU/FA alone after prior induction treatment with mFOLFOX6 plus panitumumab and re-induction with mFOLFOX6 plus panitumumab in case of progression for first-line treatment of patients with metastatic colorectal cancer (PanaMa), sponsor code AIO-KRK-0212, EudraCT-No. 2012-005422-30, AIO Studien gGmbH acting as the legal sponsor is committed to provide information about its results to researchers with the goal of facilitating scientific progress. Information that will be considered for disclosure includes individual participant data that underlie the results reported in this article (text, tables, figures, and appendices). Additionally, study Protocol and statistical analysis plan can be made available. All data shared must be anonymized to protect the privacy of the patients who participated in the trial, in accordance with applicable laws and regulations and in compliance with the International Council for Harmonisation and Good Clinical Practice (ICH/GCP). Researchers should provide a scientifically sound proposal directed to info@aio-studien-ggmbh.de for approval to gain access to the requested data. Shared data are only to be used to achieve aims of the approved proposal.
AUTHOR CONTRIBUTIONS
Conception and design: Dominik Paul Modest, Stefan Fruehauf, Ullrich Graeven, Karel Caca, Albrecht Kretzschmar, Stefan Kasper, Volker Heinemann, Sebastian Stintzing, Tanja Trarbach
Administrative support: Dominik Paul Modest, Anke Reinacher-Schick, Sebastian Stintzing
Provision of study materials or patients: Dominik Paul Modest, Stefan Fruehauf, Albrecht Kretzschmar, Eray Goekkurt, Anke Reinacher-Schick, Sebastian Stintzing, Tanja Trarbach
Collection and assembly of data: Dominik Paul Modest, Meinolf Karthaus, Stefan Fruehauf, Ullrich Graeven, Lothar Müller, Alexander Otto König, Ludwig Fischer von Weikersthal, Karel Caca, Albrecht Kretzschmar, Eray Goekkurt, Siegfried Haas, Arndt Stahler, Armin Jarosch, David Horst, Anke Reinacher-Schick, Stefan Kasper, Volker Heinemann, Sebastian Stintzing
Data analysis and interpretation: Dominik Paul Modest, Meinolf Karthaus, Stefan Fruehauf, Ullrich Graeven, Lothar Müller, Alexander Otto König, Ludwig Fischer von Weikersthal, Karel Caca, Albrecht Kretzschmar, Annika Kurreck, Swantje Held, David Horst, Volker Heinemann, Sebastian Stintzing, Tanja Trarbach
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Panitumumab Plus Fluorouracil and Folinic Acid Versus Fluorouracil and Folinic Acid Alone as Maintenance Therapy in RAS Wild-Type Metastatic Colorectal Cancer: The Randomized PANAMA Trial (AIO KRK 0212)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Dominik Paul Modest
Honoraria: Merck Serono, Amgen, Roche, Servier, Bristol Myers Squibb, Taiho Pharmaceutical, Merck Sharp & Dohme, Pierre Fabre, Onkowissen, Sanofi, Lilly
Consulting or Advisory Role: Merck Serono, Amgen, Merck Sharp & Dohme, Roche, Servier, Incyte, Bristol Myers Squibb, Pierre Fabre, Lilly, Cor2Ed, IQvia, Onkowissen
Research Funding: Amgen, Servier
Travel, Accommodations, Expenses: Amgen, Merck Serono, Servier
Meinolf Karthaus
Consulting or Advisory Role: Amgen
Travel, Accommodations, Expenses: Amgen
Stefan Fruehauf
Stock and Other Ownership Interests: AbbVie, Bristol Myers Squibb/Pfizer, Johnson & Johnson/Janssen, Merck
Ullrich Graeven
Honoraria: Daiichi Sankyo, Boehringer Ingelheim, Amgen, Servier, AstraZeneca, Bristol Myers Squibb, MSD Oncology
Consulting or Advisory Role: Merck KGaA, Bristol Myers Squibb, Hexal, Amgen, Celgene, Johnson & Johnson, MSD Oncology
Travel, Accommodations, Expenses: Merck KGaA, Amgen, Boehringer Ingelheim, GlaxoSmithKline
Lothar Müller
Honoraria: Roche
Alexander Otto König
Honoraria: Ipsen, Pierre Fabre
Consulting or Advisory Role: Roche Pharma AG
Ludwig Fischer von Weikersthal
Honoraria: Novartis, Roche Pharma AG, AstraZeneca, Pierre Fabre, Lilly GmbH
Albrecht Kretzschmar
Honoraria: Roche Pharma AG, Merck Serono, Shire, Amgen, Medac, Servier, Sanofi, MSD, Bristol Myers Squibb, Bayer Schering Pharma, Aspen Pharma, Roche Pharma
Consulting or Advisory Role: Roche Pharma AG, Shire, Amgen
Travel, Accommodations, Expenses: PharmaMar, Merck Serono, Ipsen, Medac
Eray Goekkurt
Consulting or Advisory Role: MSD, Bristol Myers Squibb, Roche, Sanofi
Annika Kurreck
Honoraria: Servier
Travel, Accommodations, Expenses: Roche, Medac
Arndt Stahler
Honoraria: Roche, Servier, Taiho Pharmaceutical
Travel, Accommodations, Expenses: Amgen, Roche, Lilly, Pfizer
Anke Reinacher-Schick
Honoraria: Amgen, Roche, Pfizer, Sanofi/Aventis, Merck Serono, Celgene, Lilly, Bristol Myers Squibb, Servier, MSD, Aurikamed, IOMEDICO, Promedicis, MCI Group, AstraZeneca
Consulting or Advisory Role: Amgen, Roche, Pfizer, Merck Serono, Celgene, Bristol Myers Squibb, Servier, Baxalta, MSD, AstraZeneca, Pierre Fabre
Research Funding: Roche, Celgene, Ipsen, Amgen, Alexion Pharmaceuticals, AstraZeneca, Lilly, Servier, AIO-Studien, Georgius Agricola Stiftung Ruhr, Rafael Pharmaceuticals, ERYTECH Pharma, BioNTech
Travel, Accommodations, Expenses: Ipsen, Amgen, Roche, Servier, MCI Group, Pierre Fabre, AstraZeneca, Merck Serono, MSD
Stefan Kasper
Honoraria: Bristol Myers Squibb, MSD Oncology, AstraZeneca, Merck Serono, Amgen, Roche, Servier, Lilly, Sanofi/Aventis
Consulting or Advisory Role: Roche, Merck Serono, Amgen, MSD Oncology, Sanofi, Bristol Myers Squibb, Lilly, AstraZeneca, Servier, Janssen-Cilag
Research Funding: Merck Serono, Bristol Myers Squibb, Celgene, Lilly, Servier, Roche/Genentech
Travel, Accommodations, Expenses: Merck Serono, Lilly, Amgen, Sanofi, Roche
Other Relationship: Sanofi, Amgen, Merck Serono, Bristol Myers Squibb, Roche, Lilly
Volker Heinemann
Honoraria: Roche, Celgene, Amgen, Sanofi, Merck, Sirtex Medical, Baxalta, Lilly, Boehringer Ingelheim, Taiho Pharmaceutical, Servier
Consulting or Advisory Role: Merck, Amgen, Roche, Sanofi, Boehringer Ingelheim, Celgene, Sirtex Medical, Baxalta, Servier, Halozyme, MSD, Bristol-Myers Squibb, MSD Oncology
Research Funding: Merck, Amgen, Roche, Celgene, Boehringer Ingelheim, Sirtex Medical, Shire, Servier
Travel, Accommodations, Expenses: Merck, Roche, Sirtex Medical, Amgen, Servier, Shire, MSD, Bristol Myers Squibb
Sebastian Stintzing
Honoraria: Merck KGaA, Roche, Amgen, Bayer, Sanofi, Lilly, Pierre Fabre, Takeda, Taiho Pharmaceutical, Servier, MSD
Consulting or Advisory Role: Merck KGaA, Roche, Sanofi, Bayer, Amgen, Boehringer Ingelheim, Lilly, Takeda, MSD, Servier, Pierre Fabre
Research Funding: Pierre Fabre, Roche Molecular Diagnostics, Merck Serono
Travel, Accommodations, Expenses: Merck KGaA, Roche, Sanofi, Bayer, Sirtex Medical, Amgen, Lilly, Takeda, Pierre Fabre
Tanja Trarbach
Research Funding: Amgen
Travel, Accommodations, Expenses: Ipsen, Takeda, OMT, AbbVie, Novartis, MSD, Sanofi/Aventis, Amgen, Johnson & Johnson/Janssen
No other potential conflicts of interest were reported.
REFERENCES
- 1.Van Cutsem E, Cervantes A, Adam R, et al. : ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 27:1386-1422, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Douillard JY, Oliner KS, Siena S, et al. : Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 369:1023-1034, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Heinemann V, von Weikersthal LF, Decker T, et al. : FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol 15:1065-1075, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Loupakis F, Cremolini C, Masi G, et al. : Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 371:1609-1618, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Saltz LB, Clarke S, Diaz-Rubio E, et al. : Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol 26:2013-2019, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Venook AP, Niedzwiecki D, Lenz HJ, et al. : Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: A randomized clinical trial. JAMA 317:2392-2401, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Modest DP, Martens UM, Riera-Knorrenschild J, et al. : FOLFOXIRI plus panitumumab as first-line treatment of RAS wild-type metastatic colorectal cancer: The randomized, open-label, phase II VOLFI study (AIO KRK0109). J Clin Oncol 37:3401-3411, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Andre T, Shiu KK, Kim TW, et al. : Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med 383:2207-2218, 2020 [DOI] [PubMed] [Google Scholar]
- 9.Arnold D, Lueza B, Douillard JY, et al. : Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol 28:1713-1729, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holch JW, Ricard I, Stintzing S, et al. : The relevance of primary tumour location in patients with metastatic colorectal cancer: A meta-analysis of first-line clinical trials. Eur J Cancer 70:87-98, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Khattak MA, Martin H, Davidson A, et al. : Role of first-line anti-epidermal growth factor receptor therapy compared with anti-vascular endothelial growth factor therapy in advanced colorectal cancer: A meta-analysis of randomized clinical trials. Clin Colorectal Cancer 14:81-90, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Schwartzberg LS, Rivera F, Karthaus M, et al. : PEAK: A randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol 32:2240-2247, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Stintzing S, Modest DP, Rossius L, et al. : FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): A post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol 17:1426-1434, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Tejpar S, Stintzing S, Ciardiello F, et al. : Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: Retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol 3:194-201, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venook A: Impact of primary (1º) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): Analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol 34, 2016. (suppl; abstr 3504) [Google Scholar]
- 16.Van Cutsem E, Lenz HJ, Kohne CH, et al. : Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol 33:692-700, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Douillard JY, Siena S, Cassidy J, et al. : Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: The PRIME study. J Clin Oncol 28:4697-4705, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Grothey A, Sobrero AF, Shields AF, et al. : Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med 378:1177-1188, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tournigand C, Cervantes A, Figer A, et al. : OPTIMOX1: A randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer—A GERCOR study. J Clin Oncol 24:394-400, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Van Cutsem E, Kohne CH, Hitre E, et al. : Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360:1408-1417, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Modest DP, Rivera F, Bachet JB, et al. : Panitumumab-based maintenance after oxaliplatin discontinuation in metastatic colorectal cancer: A retrospective analysis of two randomised trials. Int J Cancer 145:576-585, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wasan H, Meade AM, Adams R, et al. : Intermittent chemotherapy plus either intermittent or continuous cetuximab for first-line treatment of patients with KRAS wild-type advanced colorectal cancer (COIN-B): A randomised phase 2 trial. Lancet Oncol 15:631-639, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aranda E, Garcia-Alfonso P, Benavides M, et al. : First-line mFOLFOX plus cetuximab followed by mFOLFOX plus cetuximab or single-agent cetuximab as maintenance therapy in patients with metastatic colorectal cancer: Phase II randomised MACRO2 TTD study. Eur J Cancer 101:263-272, 2018 [DOI] [PubMed] [Google Scholar]
- 24.Boige V: Maintenance treatment with cetuximab versus observation in RAS wild-type metastatic colorectal cancer: Results of the randomized phase II PRODIGE 28-time UNICANCER study. J Clin Oncol 39, 2021. (suppl 3; abstr 15) [Google Scholar]
- 25.Pietrantonio F, Morano F, Corallo S, et al. : Maintenance therapy with panitumumab alone vs panitumumab plus fluorouracil-leucovorin in patients with RAS wild-type metastatic colorectal cancer: A phase 2 randomized clinical trial. JAMA Oncol 5:1268-1275, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peeters M, Price TJ, Cervantes A, et al. : Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 28:4706-4713, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Adams RA, Meade AM, Seymour MT, et al. : Intermittent versus continuous oxaliplatin and fluoropyrimidine combination chemotherapy for first-line treatment of advanced colorectal cancer: Results of the randomised phase 3 MRC COIN trial. Lancet Oncol 12:642-653, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hegewisch-Becker S, Graeven U, Lerchenmuller CA, et al. : Maintenance strategies after first-line oxaliplatin plus fluoropyrimidine plus bevacizumab for patients with metastatic colorectal cancer (AIO 0207): A randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol 16:1355-1369, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Simkens LH, van Tinteren H, May A, et al. : Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): A phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet 385:1843-1852, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Koeberle D, Betticher DC, von Moos R, et al. : Bevacizumab continuation versus no continuation after first-line chemotherapy plus bevacizumab in patients with metastatic colorectal cancer: A randomized phase III non-inferiority trial (SAKK 41/06). Ann Oncol 26:709-714, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Munemoto Y, Nakamura M, Takahashi M, et al. : SAPPHIRE: A randomised phase II study of planned discontinuation or continuous treatment of oxaliplatin after six cycles of modified FOLFOX6 plus panitumumab in patients with colorectal cancer. Eur J Cancer 119:158-167, 2019 [DOI] [PubMed] [Google Scholar]
- 32.Kurreck A, Geissler M, Martens UM, et al. : Dynamics in treatment response and disease progression of metastatic colorectal cancer (mCRC) patients with focus on BRAF status and primary tumor location: Analysis of untreated RAS-wild-type mCRC patients receiving FOLFOXIRI either with or without panitumumab in the VOLFI trial (AIO KRK0109). J Cancer Res Clin Oncol 146:2681-2691, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heinemann V, Stintzing S, Modest DP, et al. : Early tumour shrinkage (ETS) and depth of response (DpR) in the treatment of patients with metastatic colorectal cancer (mCRC). Eur J Cancer 51:1927-1936, 2015 [DOI] [PubMed] [Google Scholar]
- 34.Pietrantonio F, Petrelli F, Coinu A, et al. : Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: A meta-analysis. Eur J Cancer 51:587-594, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Adams R, Goey K, Chibaudel B, et al. : Treatment breaks in first line treatment of advanced colorectal cancer: An individual patient data meta-analysis. Cancer Treat Rev 99:102226, 2021 [DOI] [PubMed] [Google Scholar]
- 36.Sonbol MB, Mountjoy LJ, Firwana B, et al. : The role of maintenance strategies in metastatic colorectal cancer: A systematic review and network meta-analysis of randomized clinical trials. JAMA Oncol 6:e194489, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
With respect to the clinical trial, Randomized phase II study for evaluation of efficacy and safety of maintenance treatment with 5-FU/FA plus panitumumab versus 5-FU/FA alone after prior induction treatment with mFOLFOX6 plus panitumumab and re-induction with mFOLFOX6 plus panitumumab in case of progression for first-line treatment of patients with metastatic colorectal cancer (PanaMa), sponsor code AIO-KRK-0212, EudraCT-No. 2012-005422-30, AIO Studien gGmbH acting as the legal sponsor is committed to provide information about its results to researchers with the goal of facilitating scientific progress. Information that will be considered for disclosure includes individual participant data that underlie the results reported in this article (text, tables, figures, and appendices). Additionally, study Protocol and statistical analysis plan can be made available. All data shared must be anonymized to protect the privacy of the patients who participated in the trial, in accordance with applicable laws and regulations and in compliance with the International Council for Harmonisation and Good Clinical Practice (ICH/GCP). Researchers should provide a scientifically sound proposal directed to info@aio-studien-ggmbh.de for approval to gain access to the requested data. Shared data are only to be used to achieve aims of the approved proposal.