Abstract
The epidemic of coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has increasingly attracted worldwide concern. Liver damage or dysfunction occurred in patients with COVID-19 (mainly characterized by moderately elevated serum aspartate aminotransferase levels). However, it is not yet clear whether the COVID-19-related liver injury is mainly caused by the virus infection, potentially hepatotoxic drugs, or other coexisting conditions. Progression of pre-existing chronic liver disease (CLD) may be the underlying mechanism of liver injury. Although COVID-19 patients with CLD, such as nonalcoholic fatty liver disease, liver cirrhosis, and liver cancer, have been deemed at increased risk for serious illness in many studies, little is known about the impact of CLD on the natural history and outcome of COVID-19 patients. Thereby, based on the latest evidence from case reports and case series, this paper discusses the clinical manifestations, treatment, prognosis, and management of the COVID-19 patients with different CLD. This article also reviews the effect of COVID-19 on liver transplantation patients (LT), hoping to work for future prevention, management, and control measures of COVID-19. However, due to the lack of relevant research, most of them are still limited to the theoretical stage, further study of COVID-19 and CLD needs to be improved in the future.
KeyWords: COVID-19, SARS-CoV-2, Chronic liver disease, Liver injury, Prognosis
Abbreviations: AASLD, American Association for the Study of Liver Diseases; ACE2, Angiotensin I converting enzyme 2; ACLF, Acute on chronic liver failure; AIFA, Italian Medical Agency; AIH, Autoimmune hepatitis; ALD, Alcohol-associated liver disease; APASL, Asian-Pacific Association for the Study of Liver Diseases; CLD, Chronic liver disease; COVID-19, Coronavirus disease 2019; EASL, European Association for the Study of the Liver; HCC, Hepatocellular carcinoma; LT, Liver transplantation; MAFLD, Metabolic dysfunction-associated fatty liver disease; NAFLD, Non-alcoholic fatty liver disease; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
1. Introduction
The COVID-19 pandemic, resulting from the virus SARS-CoV-2, continues to cause significant morbidity and mortality worldwide. By early April 2021, more than 120 million COVID-19 confirmed patients and 2 million and 600 thousand related deaths have been accumulated worldwide since the first reported patient was diagnosed [1].The main clinical characteristics of COVID-19 are fever and dry cough, while different degrees of liver damage have been observed in several patients with COVID-19 with gastrointestinal symptoms [2].
Liver injury has been reported as a common manifestation with unclear clinical significance. The potential mechanisms of liver injury in COVID-19 include direct damage by the virus (based on the presence of the ACE2 receptor in the liver), psychological stress, systemic inflammatory response, drug-induced liver injury, and the progression of pre-existing liver diseases, i.e., from simple fatty liver to steatohepatitis [3]. Although COVID-19 patients with chronic liver disease (CLD) have been deemed to be at an increased risk for serious illness in many studies, little is known about the impact of CLD on the natural history and outcome of COVID-19. Therefore, based on the existing evidence from case reports and case series, this article reviews the manifestations, treatment, prognosis, and management of COVID-19 patients with different CLDs in the clinic. This review also describes the influence of COVID-19 on liver transplantation patients to promote prevention, management, and control measures for COVID-19 in this patient population in the future.
2. Impact of COVID-19 on patients with pre-existing chronic liver disease
Whether CLD patients are more likely to develop COVID-19 is unclear, as there is a dearth of data on the CLD prevalence among patients with COVID-19. A meta-analysis showed that the risk of COVID-19 did not increase in CLD patients. However, it seems that patients with CLD were more likely to have severe or critical COVID-19 than those without CLD, and they were also more likely to have higher mortality [4], and acute on chronic liver failure (ACLF) can also occur in patients with compensated CLD who had severe COVID-19 [5] (Table 1 ). We summed all studies on COVID-19 with CLD patients in Table 2 .
Table 1.
Impact of COVID-19 in the management of chronic liver disease patients.
| Conditions | Impact of COVID-19 in the management |
|---|---|
| Chronic viral hepatitis6,9,13 |
It is unknown if patients with chronic HBV/HCV infection are more vulnerable to liver damage from SARS-CoV-2, or whether these patients have a greater risk of severe disease after acquiring COVID-19 or not. Treatment of SARS-CoV-2 in patients with chronic viral hepatitis requires close monitoring but does not necessarily preclude the use of antivirals such as remdesivir or immunosuppressors. |
| Non-alcoholic fatty liver disease (NAFLD)16,19-21 | NAFLD is indicated as an independent risk factor for disease progression to severe COVID-19, highlights the significance of monitoring patients with metabolic disorders during the COVID-19 crisis. |
| Alcohol associated liver disease (ALD)13,25 |
The disorder of the innate and adaptive immune system caused by alcohol may increase the risk of severe infection in COVID-19 patients and the follow-up work of related patients need to be more proactive. |
| Autoimmune hepatitis (AIH)27 |
No data suggests AIH patients, even if immunosuppressed, being at an increased risk of severe SARS-CoV-2 infection. Routine immunosuppressants and other therapeutic drugs should not be stopped or lower for AIH patients with COVID-19. |
| Cirrhosis34-36 | Evolving data indicate a poor prognosis and higher mortality in cirrhosis patients. Treatment and management of cirrhosis patients with COVID-19 should be more stringent in medication, monitoring, and follow-up. |
| Hepatocellular carcinoma (HCC)38,39 | There is no data on the prognosis of COVID-19 in HCC patients. Most of the patients present with two major diseases: cancer and concomitant CLD, they may have an increased risk of severe COVID-19. |
Table 2.
Studies on COVID-19 with chronic liver disease patients.
| Patient Number | Patient's Condition | Risk Factors of Severe COVID-19/Higher Mortality | Outcome | Treatment | |
|---|---|---|---|---|---|
| Chronic hepatitis B(CHB) | |||||
| Bin Zhang et al. [6] | N=23 | HBV carriers: 15 CHB: 7 Hepatitis B Cirrhosis: 1 |
/ | 3 (13.0%) patients progressed to severe, and 2 (8.7%) progressed to critically ill. All patients were discharged after treatment. | 23 patients were treated with antiviral drugs and 13 were treated with liver-protecting drugs. |
| Rui Liu et al. [8] | N=220 | COVID-19 patients with HBV: 50 COVID-19 alone: 6 HBeAg-negative CHB: 57 Healthy controls: 57 |
/ | COVID-19 with HCVpatients posed a higher extent of dysregulation of host functions at the onset of COVID-19. | 7 patients under continuous anti-HBV treatment (Entecavir or Lamivudine) in the HBV coinfected COVID-19 group. |
| Yang Li et al. [9] | N=28 | COVID-19 co-infected HBV: 7 COVID-19 without chronic HBV infection: 21 |
/ | No patient was admitted to the ICU or had any severe complicationsincluding liver failure during hospitalization. | All patients were treated with antiviral drugs (lopinavir/ritonavir; interferon α-2b; arbidol; oseltamivir) and empirical antibiotic therapy. 2 patients received anti-HBV treatment with entecavir. 2 patients were given corticosteroids and 1 patient was given gamma globulin. |
| Sergio Rodríguez-Tajes et al. [11] | N=61 | Patients with HBsAg negative/anti-HBc positive: 61 | / | Nocases of HBsAg seroreversion. | 38 (62%) patients received entecavir and 23 (38%) did not. The most frequently used immunosuppressant drug was tocilizumab (72%); 25 patients (41%) received high doses of steroids alone (n=5) or in combination with other immune modulators (n=20). 22 patients (36%) received prednisone for 1 month or longer; 15 of them had undergone high-dose steroid therapy. |
| HCVinfcction | |||||
| Bianca Cerbu et al. [12] (meta analysis) |
N=1,057 | / | Active HCV is associated with severe COVID-19. | Non-active HCV infection patients: 88 (92.6%) had mild or moderate COVID-19, while 7 patients (7.4%) presented a severe form. Active HCV infection patients: 21 (67.7%) had mild or moderate COVID-19, while 10 (32.3%) presented with severe COVID-19 infection. |
The treatment scheme for all patients with COVID-19included a standard association of remdesivir, dexamethasone, azithromycin antibiotic prophylaxis, and anticoagulation using enoxaparin. |
| Non-alcoholic/metabolic dysfunction-associatedfatty liver disease(NAFLD/MAFLD) | |||||
| Rui Huang et al. [17] | N=280 | COVID-19 with NAFLD diagnosed by HSI: 86 Abnormal liver function:100 |
Age over 50 yearsand concurrent NAFLD were independent risk factors of ALT elevation in COVID-19 patients. | No patient developed liver failure and death. 28 (10.0%) patients were transferred to the ICU during hospitalization. |
Atomized inhalation of IFNα-2b, lopinavir/ritonavir, and arbidol account for 57.1%, 72.5%, and 49.3% . 73.6% of patients received empirical antibiotic treatment. 73 (26.1%) patients were given corticosteroidsand 35 (12.5%) patients were treated with gamma globulin. |
| Giovanni Targher et al. [24] | N=310 | COVID-19 with MAFLD: 94 | MAFLD patients with increased FIB-4 or NFS are associated with severe COVID-19 illness. | / | / |
| Yujie Zhou et al. [25] | N=110 | COVID-19 with MAFLD: 45 | MAFLD is associated withsevere COVID-19. | / | / |
| Autoimmune hepatitis (AIH) | |||||
| Tatyana Kushner et al. [29] | N=110 | AIH: 110 Cirrhosis: 32 Comorbid conditions: 53 Malignancy: 5 (active in COVID-19:2) |
Cirrhosis with AIHwas the strongest predictor for severe COVID-19. | Antivirals was associated with liver injury, while continued immunosuppression during COVID-19 was associated with a lower rate of liver injury. The rates of severe COVID-19 and all-cause mortality were not different between AIH and non-AIH CLD. |
Most patients (92.7%) were on immunosuppressive therapy before the COVID-19 diagnosis, 65 (59.1%) patients were on prednisone therapy (alone or in combination with other immunosuppressants) with a median dose of 5 (range, 2.5-60) mg/day. |
| Thomas Marjot et al. [30] | N=932 | Non-alcoholic liver disease: 362, ALD: 233 Chronic HCV infection: 128 Chronic HBV infection: 121 AIH: 70 |
Age, Child-Pugh class B and C cirrhosis in the AIH cohort were associated with deathwithin the AIH cohort. | AIH and non-AIH CLD had no significant differences in the rates of all major outcomes. | 58 (83%) patients with AIH were taking immunosuppression: prednis (ol)one 41 (71%), thiopurines (azathioprine or 6-mercaptopurine) 32 (55%), mycophenolate mofetil (MMF) 9 (16%), tacrolimus 5 (9%), and budesonide 4 (7%). 30 (52%) patients with AIH were on combined immunosuppression with ≥2 agents. |
| VahinVuppalanchi et al. [31] | N=420 | AIH:420 Liver biopsy reported cirrhosis:78 |
Immune suppressants for the AIH treatment do not increase the risk of severe COVID-19. | / | / |
| Alessio Gerussi et al. [32] | N=10 | AIH patients:10 Cirrhosis: 4 Decompensated cirrhosis (Child-Pugh B): 6 |
/ | 9 patients are still alive and asymptomatic, and 1 (patient 6) died. Patient 9 has experienced a relapse of AIH and is now being treated with prednisone 50 mg/day. |
All subjects received a combination of an antiretroviral drug with an antimalarial medication; 2 cases were treated with azithromycin. 2 patients (patients 2 and 4) were under high-dose steroids at the time of COVID-19. |
| Chronic liver disease with cirrhosis | |||||
| Shiv Kumar Sarin et al. [36] | N=288 | CLD without cirrhosis: 185 Cirrhosis:43 MAFLD: 113 Viral: 26 |
Pugh score of 9 or more at presentation predicted high mortality. Rising bilirubin and AST/ALT ratio predicted mortality among cirrhosis patients. |
The COVID-19 produces acute liver injury in 43% of CLD patients without cirrhosis. 20% of compensated cirrhosis patients develop either ACLF or acute decompensation. Liver-related complications were seen in nearly half of the decompensated cirrhotics. |
Hydroxychloroquine with azithromycin and antiviral drugs at admission in mild and moderate cases. The moderate and severe cases received antibiotics, convalescent plasma, steroids in form of intravenous methylprednisolone or IVIG, on a case-to-case basis. |
| Xiaolong Qi et al. [37] | N=21 | Compensated cirrhosis: 81% Comorbidities other than cirrhosis: 66.7% |
Lower lymphocyte and platelet counts, and higher direct bilirubin level might represent poor prognostic indicators. | The cause of death in most patients was respiratory failure rather than progression of liver disease (ie, development of acute-on-chronic liver failure). | / |
| Furong Liu et al. [38] | N=17 | COVID-19 with cirrhosis:17 (6 CSPH and 11 non-CSPH) |
Cirrhosis, especially CSPH, may play an important role in the progression of COVID-19. | 66.7% (4/6) of CSPH patients were severe cases, compared with 18.2% (2/11) of non-CSPH patients. 3 patients (17.6%) were admitted to the ICU, and two of them died. 1 CSPH patient developed esophageal gastric variceal bleeding (EGVB). |
Antiviral (88.2%), antimicrobial (76.5%), hepatoprotective (64.7%) and oxygen (35.3%) therapy were the most commonly used, and CSPH patients had a higher proportion of hepatoprotective therapy (6/6 vs 5/11, P = 0.025) and oxygen therapy (4/6 vs 2/11, P = 0.046) than non-CSPH patients. |
| Jasmohan S Bajaj et al. [40] | N=272 | Cirrhosis+COVID-19: 37 COVID-19:108 Cirrhosis:127 |
Charlson Comorbidity Index (CCI) was the only independent mortality predictor in the entire matched cohort. | Patients with cirrhosis+COVID-19 had higher mortality compared with patients with COVID-19 (30% vs 13%, P=0.03) but not between patients with cirrhosis+COVID-19 and patients with cirrhosis (30% vs 20%, P=0.16). CCI was significantly higher in cirrhosis regardless of COVID-19 status compared with patients with COVID-19 only. |
Antibiotic in COVID-19 patients with and without cirrhosis are mostly beta-lactam antibiotics, macrolides, and vancomycin. patients with cirrhosis+COVID-19 and COVID-19 alone were similar on non-steroidal anti-inflammatory drugs, acetaminophen, chloroquine/hydroxychloroquine, and open-label remdesivir. |
| Shalimar et al. [41] | N=116 | COVID-19 with known chronic liver disease: 28 Cirrhotic without COVID-19: 78 |
Cirrhosis, mechanical ventilationare associated with poor outcomes. ACLF is associated with worst survival rates. | All COVID-19 patients with ACLF (9/9) died compared with 53.3% (16/30) in ACLF of historical controls. The mortality rate was higher in COVID-19 patients with compensated cirrhosis and AD as compared with historical controls 2/17 (11.8%) vs. 2/48 (4.2%). The requirement of mechanical ventilation independently predicted mortality (hazard ratio 13.68). | They followed the standard recommendation for the management of all complications. For patients who presented with UGIB, they delayed endoscopy and managed patients with vasoconstrictors like terlipressin, and patients were started on carvedilol for secondary prophylaxis. |
| Massimo Iavarone et al. [42] | N=50 | Cirrhosis with COVID-19:50 (38% virus-related and 52% previously compensated cirrhosis) |
The severity of lung and liver (according to CLIF-C, CLIF-OF, and MELD scores) diseases in cirrhosispatients independently predicted mortality. | 34% patients died from diagnosis of SARS-CoV-2 infection. COVID-19 with respiratory failure was considered the cause of death in 71% patients, while end stage-liver disease (ESLD) accounted for death in 29%. In the multivariate analysis, only CLIF-OF and moderate/ severe lung failure independently predicted mortality. |
26 (52%) patients received specific anti-SARS-CoV-2 treatment: 9 (18%) received hydroxychloroquine alone, 3 (6%) received antiviral therapy with lopinavir-ritonavir, and 14 (28%) received both antiviral treatment and hydroxychloroquine; none of the patients have been treated with tocilizumab or remdesivir. |
| Chronic liver disease | |||||
| Donghee Kim et al. [44] | N=867 | NAFLD:456 HCV infection:190 ALD: 94 HBV infection: 62 Cirrhosis: 227 |
ALD, decompensated cirrhosis, and HCC predict higher overall mortality among patients with CLD and COVID-19. Hispanic ethnicity and decompensated cirrhosis are associated with severe COVID-19. |
The overall all-cause mortality was 14.0%, and 61.7%had severe COVID-19. | One (or more) of Remdesivir, Chloroquine, Hydroxychloroquine, Azithromycin, Prednisone or Methylprednisolone, Lopinavir/ritonavir, Donor Plasma, Others or None. |
| Liver transplant | |||||
| Chiara Becchetti et al. [53] | N=57 | Liver transplant recipients:57 (with) cardiovascular disease: 21 (with)arterial hypertension: 32 (with) diabetes mellitus: 21 |
A history of cancer was more frequent in patients with poorer outcomes. | Among hospitalized recipients, 4 (10%, 95% CI 3% to 23%) were admitted to the ICU and required invasive mechanical ventilation, while 11 (19%, 95% CI 10% to 32%) developed ARDS. Death was registered in seven cases (12%, median time from transplant to death was 6 years, IQR 3–13), all of whom were hospitalized with ARDS. | Overall, the reduction was observed in 22 (39%) patients, and complete discontinuation was reported in 4 cases (7%). Concerning specific therapy for COVID-19,including steroids, hydroxychloroquine,lopinavir/ritonavir, darunavir/cobicistat, and remdesivir, tocilizumab. 35 (63%) and 16 (29%) subjects received at least one antibiotic drug. 15 (27%) were treated with azithromycin. |
| Jacqueline Fraser et al. [54] (meta analysis) |
N=223 | / | Dyspnea on presentation, diabetes mellitus, and age 60 years or older were significantly associated with increased mortality. | 77.7% required hospitalization, 24% had mild disease, 40% had moderate disease, and 36% had severe disease. Immunosuppression was modified in 32.8% of recipients. The case fatality rate was 19.3%. | 17.0% (38/223) of cases augmented the baseline immune suppression regimen, reduction or cessation of antimetabolite in 76.3% (29/38) and calcineurin inhibitor in 52% (20/38). A total of 93 of 141 (66%) patients were prescribed hydroxychloroquine. 6 patients received immune-modulatory therapy, with 9 patients prescribed IL-6 inhibitors and 4 patients receiving systemic interferon. |
| Ashraf Imam et al. [56] | N=120 | Liver transplant:120 | liver transplant recipientshad higher rates of severe disease, complications, and mortality. | ICU admission occurred in 28.6% of patients. 72.7% of patients had recovered and were discharged with a median illness duration of 17 days, 13.6% of patients were alive and hospitalized at the time of publication with a median illness duration of 16 days, and 13.6% of patients had died with a median illness duration of 24 days. | Liver transplant patients infected with COVID-19 were maintained on Tac (79%), mycophenolate (MMF) (48.4%), and Prednisone (29.6%) and were managed by reducing MMF in 14.3% of patients and reducing Tac in 14.3% of patients. |
| Jordi Colmenero et al. [57] | N=111 | Liver transplant:111 | Baseline immunosuppression containing mycophenolate was an independent predictor of severe COVID-19, particularly at doses higher than 1,000 mg/day. | 86.5% patients were admitted to the hospital and 19.8% patients required respiratory support. 10.8% patients were admitted to the ICU. The mortality rate was 18%, which was lower than in the matched general population. 31.5%patients met the criteria of severe COVID-19. | Patients would be treated with oral hydroxychloroquine and/or azithromycin for 5–7 days. In patients with moderate-severe COVID-19, antiviral therapy with lopinavir/ritonavir or remdesivir was allowed as compassionate use. Patients with acute distress respiratory syndrome could receive boluses of steroids and/or tocilizumab. |
Note: ARDS: acute respiratory distress syndrome, ACLF: acute on chronic liver failure, AD: acute decompensation, AIH: autoimmune hepatitis, ALD: alcohol associated liver disease, CCI :charlson comorbidity index, CHB:chronic hepatitis B, CI:confidence interval, CLD: chronic liver disease, CLIF:consortium organ failure score, COVID-19: coronavirus disease 2019, CSPH: clinically significant portal hypertension, EGVB: esophageal variceal bleeding, FIB-4:fibrosis-4 index, HBV/HCV:hepatitis B/C virus, HCC: hepatocellular carcinoma, HIS:hepatic steatosis index, ICU:intensive care unit, IVIG:intravenous immunoglobulin, LT: Lliver transplantation, MAFLD: metabolic dysfunction-associated fatty liver disease, NAFLD: non-alcoholic fatty liver disease, MELD: model for end-stage liver disease, NFS:nonalcoholic fatty liver fibrosis score, OR:odd ratio, UGBI:upper gastrointestinal bleeding.
2.1. COVID-19 in patients with chronic viral hepatitis
The relationship between patients with chronic hepatitis B or C infection and liver injury caused by SARS-CoV-2 infection is not clear. There is no evidence to show that HBV patients infected with SARS-CoV-2 have different clinical manifestations or prognoses. Most HBV carriers infected with SARS-CoV-2 will not become severely or critically ill [6, 7]. The monocyte and white blood cell counts in SARS-CoV-2-infected patients with HBV were reported to be significantly lower than those in patients with SARS-CoV-2 infection alone, while the level of CD8-T cells was significantly higher, and SARS-CoV-2-infected patients with HBV consistently had a higher risk of thrombocytopenia [8]. Higher prothrombin times within the normal range in chronic HBV patients indicated a risk of abnormal coagulation [9]. However, due to the small sample size of the above studies, we cannot draw general conclusions. An Arabian patient developed severe liver injury with high levels of AST, ALT, and TB, suggesting that COVID-19 may cause HBV reactivation [10]. However, the causal relationship between liver injury and HBV reactivation remains unclear. Other reports also indicated the possibility of HBV reactivation during SARS-CoV-2 infection, but the risk seems low [11]. Accordingly, it is best to assess the viral load promptly if possible, or HBV infection can be treated with antiviral therapy for a short time during SARS-CoV-2 infection.
Active HCV infection and HCV viral load seem to be related to poor prognosis [12]. However, HCV antibodies may be suggestive of “protection” against COVID-19 because recently cured HCV patients were less likely to be infected with SARS-CoV-2 [13].
The case of a 47-year-old male with HCV and past exposure to hepatitis B virus who developed severe COVID-19 pneumonia has also been reported. The administration of remdesivir resulted in a temporary lowering of the HCV viral load, followed by a significant rebound after its discontinuation with a hepatitis flare. A decrease in the anti-HBV titer was also noted, probably related to the administration of tocilizumab. Thus, the treatment of SARS-CoV-2 infection in patients with chronic viral hepatitis requires close monitoring but does not necessarily preclude the use of antivirals such as remdesivir or immunosuppressors [14].
Some HBV and HCV antiviral medications, such as sofosbuvir and tenofovir, may have therapeutic activity against SARS-CoV-2, but there is no current evidence of benefit, and the relevant clinical trials are still in progress. Medications could not be stopped for patients receiving anti-HBV therapy; therefore, the changes may be due to the immunological response to COVID-19 or an outbreak of HBV infection [15].
2.2. COVID-19 in patients with nonalcoholic fatty liver disease
Nonalcoholic fatty liver disease (NAFLD), recently renamed metabolic dysfunction-associated fatty liver disease (MAFLD) [16], represents an increasing cause of liver disease [14] and is predicted to be present in the majority of COVID-19 patients with pre-existing CLD worldwide.
NAFLD patients are generally considered to have a longer viral shedding time, a higher possibility of liver dysfunction, and an increased risk of disease progression to severe COVID-19 than non-NAFLD patients [17], [18], [19], [20]. In a retrospective study of 202 consecutive patients with COVID-19 and MAFLD, liver injury occurred in 50% and 75.2% of patients on admission and during hospitalization, respectively. These findings may be attributed to the chronic inflammatory state of the liver in NAFLD patients. Additionally, the high expression of the ACE2 receptor was one of the possible reasons for liver injury based on an animal model of hepatic steatosis [21]. However, the relevant evidence is still not sufficient, and further research is needed. NAFLD was indicated as an independent risk factor for COVID-19 progression [18, [21], [22], [23]]. In addition, the liver fibrosis degree in NAFLD might influence the outcome of COVID-19; for example, the severity of COVID-19 illness was associated with intermediate or high FIB4 scores among patients with MAFLD [24]. MAFLD was associated with an approximately four times higher probability for severe disease [25]. In NAFLD patients, the polarization status of hepatic macrophages, switching from inflammation-promoting M1 to inflammation-suppressing M2, was presumed to favor COVID-19 progression [18, 26]. The cytokine storm hypothesis suggests that fibrosis in the background of MAFLD may aggravate the severity of infection through the release of inflammatory cytokines in the liver [18].
At present, except for several monoclonal antibody drugs that are still in clinical trials [27], there is no specific drug for the treatment of COVID-19, and antiviral drugs and corresponding symptomatic therapy are the main treatments. However, the drugs seem to be more hepatotoxic and thus aggravate pre-existing steatosis through different proinflammatory factors in NAFLD patients. Proinflammatory cytokines, such as TNF-a, IL1-b, and IL-6, have been shown to significantly enhance the hepatotoxicity potential of various drugs, including antibiotics. Other pharmaceuticals, such as corticoids, antiretroviral agents, and methotrexate, might trigger the transition of simple fatty liver to NASH or worsen pre-existing steatosis, necroinflammation, and fibrosis [19]. There seems to be a vicious cycle between COVID-19 and NAFLD steatosis. The prevalence of NAFLD and the risk of patients with NAFLD progressing to severe COVID-19 highlight the significance of identifying and monitoring patients with liver diseases, especially those with metabolic disorders, during and after the COVID-19 crisis.
2.3. COVID-19 in patients with alcohol-related liver disease
Alcohol intake may have become an uncontrollable condition due to the influence of relevant social and economic factors during the epidemic period, which may lead to adverse effects in alcohol-associated liver disease (ALD) patients. Few studies have mentioned the effect of COVID-19 on alcohol-associated liver disease patients. Disorders of the innate and adaptive immune systems caused by alcohol may increase the risk of severe infection in patients with COVID-19. Common underlying medical conditions, including obesity with metabolic syndrome and chronic kidney disease, in ALD patients can also affect prognosis, acting as risk factors. The use of glucocorticoids in the treatment of alcoholic hepatitis patients should be more cautious during the COVID-19 pandemic, especially in high incidence areas. The follow-up of these patients also needs to be more proactive [15, 28].
2.4. COVID-19 in autoimmune hepatitis patients
Despite some reports, the effect of immunosuppression on COVID-19 remains inconclusive. The immune function of autoimmune hepatitis (AIH) patients is passively suppressed, and their liver may be decompensated at the same time, which may have adverse effects on the recovery of COVID-19. Several large multicenter studies have found that COVID-19 patients seemed to have a similar disease process as the general patients among the patients who received immunosuppressive therapy, and immunosuppression may also reduce the risk of new-onset liver injury [29], [30], [31]. Reducing immunosuppression in AIH (applicable to other autoimmune diseases) patients during the pandemic may be detrimental, as it may expose individuals to a higher risk of disease recurrence. In addition, immunosuppression may counteract the excessive inflammation driven by COVID-19. It was reported that if AIH patients suddenly reduce/stop immunosuppressive drugs during the stable period of disease, for most immunosuppressive drugs, these patients will have a high risk of recurrence [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48]. According to APASL expert panel consensus recommendations, routine immunosuppressants and other therapeutic drugs should not be stopped for patients with autoimmune liver disease even if they are infected with SARS-CoV-2 [33].
2.5. COVID-19 in compensated cirrhosis patients
Since liver cirrhosis patients are often in a state of impaired immune function, these patients are susceptible to SARS-CoV-2 infection [34]. The overexpression of the ACE-2 enzyme in COVID-19 patients with decompensated cirrhosis may lead to an increase in SARS-CoV-2 viral titers [35]. In patients with cirrhosis, the ACE-2 reduction by SARS-CoV-2-induced internalization may aggravate liver fibrosis and portal hypertension and exacerbate disease severity.
Interestingly, COVID-19 patients with cirrhosis seem to have a different liver injury pattern. These patients usually had rapid and early worsening of jaundice, higher AST than ALT, and a low R-value, all of which were more pronounced in those who did not survive [36]. However, these findings still need to be confirmed. Lower platelet and lymphocyte counts, hypoproteinemia, and higher direct bilirubin levels, which are relatively more common manifestations in COVID-19 patients with liver cirrhosis than in COVID-19 patients without liver disease, might be indicators of a poor prognosis [37], [38], [39]. Evolving data from the global registry indicate a poor prognosis and higher mortality in cirrhosis patients [40], [41], [42].
Considering the adverse effect of cirrhosis on COVID-19, management and treatment should be more stringent in terms of medication, monitoring, and follow-up. Remote management is necessary. Otherwise, the subtle differences between COVID-19 with cirrhosis caused by different etiologies and COVID-19 alone and the long-term effects of SARS-CoV-2 infection remain undefined. Furthermore, it is unclear whether COVID-19 patients with cirrhosis are affected by other complications associated with the novel coronavirus. Therefore, there are still many problems that need to be solved.
2.6. COVID-19 in hepatocellular carcinoma patients
Available evidence suggests that patients with cancer seem to be more vulnerable to SARS-CoV-2 infection. In a systematic review, it was reported that the prevalence of COVID-19 in patients with previously diagnosed cancer was 2% [43]. There are few reports about hepatocellular carcinoma (HCC) in COVID-19 patients, and only a multicenter, observational cohort found that the all-cause mortality was 52.4% (n = 11) in their HCC subgroup, almost seven times that of patients without HCC [44]. Even so, the data are insufficient to draw a definite conclusion. However, due to the close correlation between liver dysfunction and COVID-19, the low immune capability of these HCC patients, and the necessity of regular monitoring for tumor progression, liver cancer patients have become a vulnerable group that needs close attention.
In terms of treatment for this special group, a management guideline recommended that risk assessment and grading is a feasible method for HCC patients, and it is also recommended to lower the risk of SARS-CoV-2 infection during the convalescence of hospitalization by less invasive or noninvasive treatment [45]. Most HCC patients have cirrhosis and are thus afflicted with two comorbidities, which means that their liver immune function may not be favorable for the prognosis of COVID-19 [46]. Studies concerning the risk of progression and the prognosis of HCC patients are limited. COVID-19 patients with HCC may have exacerbated progression, and further SARS-CoV-2 infection may aggravate the status of existing liver disease [47], based on the physical condition of patients and other data on disease progression in cancer patients, as a higher mortality rate has been demonstrated in cancer patients (28%) than in those without (14%) [48].
The particularity and complexity of HCC patients indicate that the management of HCC patients will face many obstacles during the pandemic. However, due to the necessity of follow-up and timely treatment, relevant medical workers can choose remote follow-up to replace in-person management. The use of a clear indicator to determine the operation priority is difficult. Some patients can delay the operation after evaluation, but for high-risk patients, delayed treatment is not recommended. Clinicians can refer to some published guidelines [45, 46], and the choice can be based on the local situation to balance the risk of liver cancer and SARS-CoV-2 infection. APASL recommended no delayed treatment for patients with highly malignant HCC. However, it is necessary to triage patients when resources are insufficient. Radical treatment should be carried out regularly, while nonradical treatment can be postponed appropriately. For patients with confirmed or suspected COVID-19, diagnosis or treatment should be delayed until the virus is eliminated. Medical staff should take measures to reduce virus exposure in the process of diagnosis and treatment [49].
3. Effect of COVID-19 on liver transplantation patients
Organ donation and transplantation are facing new challenges due to the global spread of COVID-19. Liver transplantation is the second most common solid-organ transplantation in the world, second only to kidney transplantation [50]. LT patients are often in a state of chronic immunosuppression, which can increase opportunistic infection. Therefore, the effect of COVID-19 on LT patients has attracted wide attention in the context of the pandemic.
In terms of clinical symptoms, the existing evidence shows that the clinical symptoms and signs of LT patients with COVID-19 are relatively atypical in contrast to the general population. Some cases reported that LT patients infected with SARS-CoV-2 had low-grade or no fever. However, LT patients seem to be affected by diarrhea complications more easily [51, 52]. Interestingly, it seems that patients with short-term transplantation have fewer symptoms of fever and dyspnea than long-term transplantation patients [53]. Therefore, the neglect of early infection in this group should be vigilant. Gastrointestinal symptoms were more common in the LT population (28.6%) but were similar to those in other solid-organ transplant cohorts [54]. More extensive damage manifestations on chest CT and lymphopenia in LT patients with COVID-19 were common, as well as elevated C-reactive protein and erythrocyte sedimentation rates [55, 56]. In addition, a review showed that LT patients with COVID-19 may have some unreported symptoms, including chest distress, pain, and jaundice [56].
A systematic review, including available databases up to June 2020, showed that the COVID-19 case-fatality rate in LT patients was 19.3% (mean age=59.6 years), higher than the 8% mortality in the general population of a similar age, from 70 to 79 years [54]. However, another study in Spain found that the number of deaths observed in LT patients (mortality rate 18%) was slightly lower than expected after adjusting for age and sex, with a standardized mortality rate of 95.55 (95% CI: 94.25-96.85). In this study, the standardized incidence ratio was 206.48 (95% CI 205.45–207.52) in the subset of patients older than 60 years [57]. A positive correlation between death and the length of time on immunosuppressants was reported from an Italian LT center. All deaths associated with COVID-19 were related to patients receiving minimal long-term immunosuppression after LT but not to recently transplanted, fully immunosuppressed patients [58]. Therefore, LT patients seem to be more vulnerable to SARS-CoV-2 due to the use of immunosuppressants, but there is no significant difference in case mortality between LT patients and the general population. More caution should be exercised in COVID-19 patients with long-term immunosuppression.
Although immunosuppressive drugs may attenuate the inflammatory response in the early stages of the disease, the increased risk of viral toxicity arrives concomitantly. Previous data related to coronavirus infection (SARS-2003 and MERS) did not show a greater threat to mortality with the use of immunosuppressive drugs in LT patients [59]. Patients taking mycophenolate or discontinuing immunosuppression were more prone to develop severe COVID-19, while baseline therapy with a calcineurin inhibitor or everolimus had no statistical significance. The calcineurin inhibitor seemed to contribute to inhibiting virus replication. Therefore, it may be feasible to adjust mycophenolate drugs to calcineurin inhibitors or everolimus [57]. The Beijing liver transplantation working conference pointed out that patients with severe or rapidly progressive COVID-19 can decrease the dose of calcineurin inhibitors and consider discontinuing antimetabolite drugs while taking antiviral therapy; dose reductions in patients with mild to moderate COVID-19 are not recommended, but relevant indicators should be well monitored. Glucocorticoids can be maintained at a low dose in cases of adrenal insufficiency [55].
The presence of the virus may be hidden in immunosuppressed recipients, thus allowing them to carry the virus for a longer time, increasing the risk of contact transmission. A case report described a patient who turned positive after a negative viral test as an LT patient, which provides evidence of the presence of SARS-CoV-2 being hidden in these patients [60]. Relevant personnel, including health care workers, should be alert to this possibility for similar patients and take measures for self-protection. However, a case report from Italy showed that LT between SARS-CoV-2-infected (with antibody-positive) donors and recipients seemed to be feasible, which could potentially provide a life-saving opportunity for SARS-CoV-2-positive wait-listed patients [61]. According to the LTSI guidelines in India, as well as the AASLD Expert Panel Consensus Statement, the relevant indications for LT should be strictly observed during the COVID-19 pandemic, in addition to basic epidemic prevention measures. It is necessary to limit the patient's condition and consider telemedicine alternatives. Both donors and recipients are supposed to be tested for the virus before transplantation. There is no obvious evidence that the immunosuppressive regimen needs to be modified. Symptomatic patients with COVID-19 can appropriately reduce immunosuppressive drugs, and high-dose immunosuppression can be initiated only when acute rejection occurs. Postoperative follow-up should be carried out as much as possible [62, 63].
4. Treatment and vaccination suggestions for CLD (including LT) patients
At present, most COVID-19 patients can recover without special treatment, and clinical data on these drugs are limited, especially for CLD patients. Drugs for COVID-19 include antimalarials (chloroquine and hydroxychloroquine), antivirals (remdesivir, favipiravir, and lopinavir/ritonavir), convalescents, and some monoclonal antibody products. Both antimalarial and antiviral drugs have been reported to be associated with a risk of liver injury [19, 64]. The monoclonal antibody drugs discovered recently provide a new possibility for COVID-19 treatment [27]. In addition, COVID-19 patients usually have a fever, and many use acetaminophen, which is known to cause liver injury. Therefore, it seems that there is no optimal treatment plan for CLD patients infected with SARS-CoV-2. Antibody drugs may be appropriate, but there is also a lack of evidence. Regardless, it is necessary to monitor liver function during treatment and avoid a combination of multiple drugs as much as possible.
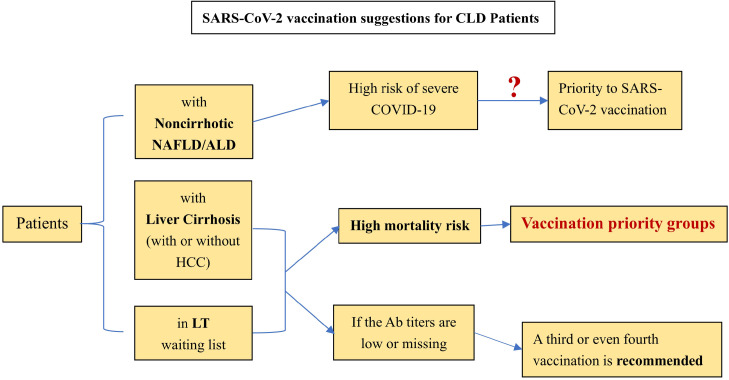
For preventative treatment, the effect of SARS-CoV-2 vaccination in special groups (CLD patients) is still unknown. However, early vaccination is beneficial for antibody production and T lymphocyte proliferative responses, thus reducing the risk of COVID-19 severity. Liane Rabinovich et al. [65] pointed out that the immunogenicity of liver transplant recipients to the vaccination was low, and neutralizing antibodies could be detected in only 47.5% of the included LT patients (n=80). Another study similarly confirmed this finding. The seroconversion and the T cell response rates of the second SARS-CoV-2 vaccination in LT patients were only 63% and 36.6%, respectively. Double-negative patients even accounted for 28%. For patients with liver cirrhosis in this cohort, the serum conversion rate after the second vaccination can reach 100% [66]. Based on the above research and position statements issued by the EASL and AIFA [67, 68], the following suggestions are listed for reference: [1] Patients with noncirrhotic NAFLD and ALD seem to be at high risk of severe COVID-19. Nonetheless, further studies are needed to clarify whether patients with NAFLD/ALD should be given priority for SARS-CoV-2 vaccination. [2] Patients with liver cirrhosis (with or without HCC) have priority to be vaccinated owing to their mortality risk. This priority also applies to patients waiting for LT. [3] A third or even fourth vaccination is recommended for LT patients and patients with cirrhosis with low or no antibody titers. [4] Research on the scope and duration of vaccination protection for CLD patients still needs to be improved (Figure 1 ).
Fig. 1.
Current Suggestions for SARS-CoV-2 Vaccianation on CLD patient.
(Ab: antibody, NAFLD: non-alcoholic fatty liver disease, ALD: alcohol associated liver disease, HCC: hepatocellular carcinoma, LT: liver transplantation, SARS-CoV-2: severe acute respiratory syndrome coronavirus 2, COVID-19: coronavirus disease 2019).
5. Conclusions
In general, limited information is available on the impact of SARS-CoV-2 infection in CLD patients. COVID-19 with different pre-existing CLDs has slightly different clinical characteristics. Most patients with CLD have problems of low immune function and abnormal metabolic function. Their capacity for SARS-CoV-2 viral clearance is inadequate compared with the general population, which leads to a higher risk of disease progression and death. Although the available clinical data are limited, it seems that immunosuppressed patients have a greater likelihood of atypical symptoms after SARS-CoV-2 infection, which suggests the greater potential for liver injury from inflammation. NAFLD patients often have many other metabolic diseases, such as hypertension and diabetes, which have been reported as independent risk factors for a poor prognosis of COVID-19, and patients with viral hepatitis need to be aware of the possibility of the reactivation of HBV.
Currently, the uncertainties in the order of occurrence make it difficult to identify whether liver injury is a risk factor for or an outcome of COVID-19 progression from a bystander perspective. There is also the problem of missing data related to the underlying liver disease.
Authorship
Project administration: Jie Li, Xin Yu
Conceptualization and Supervision: Jie Li, Xin Yu, Zhaoyang Guo
Investigation and Formal analysis: Xinyu Hu
Writing - original draft: Xinyu Hu, Longyan Sun, Jie Li
Writing - review & editing: All authors
Conflicts of interest
The authors declare that they have no conflict of interest.
Acknowledgments
Funding
None.
Acknowledge
Dr. Jie Li wishes to acknowledge the support from the National Natural Science Fund (No.82170609, 81970545), Natural Science Foundation of Shandong Province (Major Project) (No.ZR2020KH006) and Ji'nan Science and Technology Development Project (No.202019079).
References
- 1.Li J., Zou B., Yeo Y.H., Feng Y., Xie X., Lee D.H., et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4:389–398. doi: 10.1016/S2468-1253(19)30039-1. [DOI] [PubMed] [Google Scholar]
- 2.Li J., Huang D.Q., Zou B., Yang H., Hui W.Z., Rui F., et al. Epidemiology of COVID-19: A systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J Med Virol. 2021;93:1449–1458. doi: 10.1002/jmv.26424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J., Fan J.G. Characteristics and mechanism of liver injury in 2019 coronavirus disease. J Clin Transl Hepatol. 2020;8:13–17. doi: 10.14218/JCTH.2020.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kovalic A.J., Satapathy S.K., Thuluvath P.J. Prevalence of chronic liver disease in patients with COVID-19 and their clinical outcomes: a systematic review and meta-analysis. Hepatol Int. 2020;14:612–620. doi: 10.1007/s12072-020-10078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji D., Zhang D., Yang T., Mu J., Zhao P., Xu J., et al. Effect of COVID-19 on patients with compensated chronic liver diseases. Hepatol Int. 2020;14:701–710. doi: 10.1007/s12072-020-10058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang B., Huang W., Zhang S. Clinical features and outcomes of coronavirus disease 2019 (COVID-19) patients with chronic Hepatitis B virus infection. Clin Gastroenterol Hepatol. 2020;18:2633–2637. doi: 10.1016/j.cgh.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarkar S., Khanna P., Singh A.K. Impact of COVID-19 in patients with concurrent co-infections: a systematic review and meta-analyses. J Med Virol. 2021;93:2385–2395. doi: 10.1002/jmv.26740. [DOI] [PubMed] [Google Scholar]
- 8.Liu R., Zhao L., Cheng X., Han H., Li C., Li D., et al. Clinical characteristics of COVID-19 patients with hepatitis B virus infection - a retrospective study. Liver Int. 2020 doi: 10.1111/liv.14774. [DOI] [PubMed] [Google Scholar]
- 9.Li Y., Li C., Wang J., Zhu C., Zhu L., Ji F., et al. A case series of COVID-19 patients with chronic hepatitis B virus infection. J Med Virol. 2020;92:2785–2791. doi: 10.1002/jmv.26201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdulla S., Hussain A., Azim D., Abduallah E.H., Elawamy H., Nasim S., et al. COVID-19-induced hepatic injury: a systematic review and meta-analysis. Cureus. 2020;12:e10923. doi: 10.7759/cureus.10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez-Tajes S., Miralpeix A., Costa J., Lopez-Sune E., Laguno M., Pocurull A., et al. Low risk of hepatitis B reactivation in patients with severe COVID-19 who receive immunosuppressive therapy. J Viral Hepat. 2021;28:89–94. doi: 10.1111/jvh.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cerbu B., Pantea S., Bratosin F., Vidican I., Turaiche M., Frent S., et al. Liver impairment and hematological changes in patients with chronic Hepatitis C and COVID-19: a retrospective study after one year of pandemic. 2021:57. doi: 10.3390/medicina57060597. [DOI] [PMC free article] [PubMed]
- 13.Mangia A., Cenderello G., Verucchi G., Ciancio A., Fontana A., Piazzolla V., et al. Is positivity for hepatitis C virus antibody predictive of lower risk of death in COVID-19 patients with cirrhosis? World J Clin Cases. 2020;8:5831–5834. doi: 10.12998/wjcc.v8.i22.5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.da Cunha M.R., Rodrigues I.C., Trigueiros F., Freitas L.C., Braz S., Cota Medeiros F. Vol. 8. 2021. A case of severe acute respiratory syndrome coronavirus 2 treatment with remdesivir in a Hepatitis C-coinfected patient resulting in temporary viral control and posttreatment flare. (Open Forum Infect Dis). ofab100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamid S., Alvares da Silva M.R., Burak K.W., Chen T., Drenth J.P.H., Esmat G., et al. WGO guidance for the care of patients with COVID-19 and liver disease. J Clin Gastroenterol. 2021;55:1–11. doi: 10.1097/mcg.0000000000001459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eslam M., Sanyal A.J., George J. International Consensus P. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158 doi: 10.1053/j.gastro.2019.11.312. 1999-2014 e1. [DOI] [PubMed] [Google Scholar]
- 17.Huang R., Zhu L., Wang J., Xue L., Liu L., Yan X., et al. Clinical features of COVID-19 patients with non-alcoholic fatty liver disease. Hepatol Commun. 2020 doi: 10.1002/hep4.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma P., Kumar A. Metabolic dysfunction associated fatty liver disease increases risk of severe Covid-19. Diabetes Metab Syndr. 2020;14:825–827. doi: 10.1016/j.dsx.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferron P.J., Gicquel T., Megarbane B., Clement B., Fromenty B. Treatments in Covid-19 patients with pre-existing metabolic dysfunction-associated fatty liver disease: A potential threat for drug-induced liver injury? Biochimie. 2020;179:266–274. doi: 10.1016/j.biochi.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dongiovanni P., Meroni M., Longo M., Fracanzani A.L. MAFLD in COVID-19 patients: an insidious enemy. Expert Rev Gastroenterol Hepatol. 2020;14:867–872. doi: 10.1080/17474124.2020.1801417. [DOI] [PubMed] [Google Scholar]
- 21.Pan L., Huang P., Xie X., Xu J., Guo D., Jiang Y. Metabolic associated fatty liver disease increases the severity of COVID-19: a meta-analysis. Dig Liver Dis2020. doi: 10.1016/j.dld.2020.09.007. [DOI] [PMC free article] [PubMed]
- 22.Sachdeva S., Khandait H., Kopel J., Aloysius M.M., Desai R., Goyal H. NAFLD and COVID-19: a pooled analysis. SN Compr Clin Med. 2020:1–4. doi: 10.1007/s42399-020-00631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Portincasa P., Krawczyk M., Smyk W., Lammert F., Di Ciaula A. COVID-19 and non-alcoholic fatty liver disease: two intersecting pandemics. Eur J Clin Invest. 2020;50:e13338. doi: 10.1111/eci.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Targher G., Mantovani A., Byrne C.D., Wang X.B., Yan H.D., Sun Q.F., et al. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. 2020;69:1545–1547. doi: 10.1136/gutjnl-2020-321611. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y.J., Zheng K.I., Wang X.B., Sun Q.F., Pan K.H., Wang T.Y., et al. Metabolic-associated fatty liver disease is associated with severity of COVID-19. Liver Int. 2020;40:2160–2163. doi: 10.1111/liv.14575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lefere S., T.a.c.k.e. FJJriih. Macrophages in obesity and non-alcoholic fatty liver disease: crosstalk with metabolism. 2019:1:30-43. doi: 10.1016/j.jhepr.2019.02.004. [DOI] [PMC free article] [PubMed]
- 27.Weinreich D.M., Sivapalasingam S., Norton T., Ali S., Gao H., Bhore R., et al. REGEN-COV antibody combination and outcomes in outpatients with Covid-19. N Engl J Med. 2021 doi: 10.1056/NEJMoa2108163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kushner T., Cafardi J. Chronic liver disease and COVID-19: alcohol use disorder/alcohol-associated liver disease, nonalcoholic fatty liver disease/nonalcoholic steatohepatitis, autoimmune liver disease, and compensated cirrhosis. Clin Liver Dis (Hoboken) 2020;15:195–199. doi: 10.1002/cld.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Efe C., Dhanasekaran R., Lammert C., Ebik B., Higuera-de la Tijera F., Aloman C., et al. Outcome of COVID-19 in patients with autoimmune hepatitis: an international multicenter study. Hepatology. 2021;73:2099–2109. doi: 10.1002/hep.31797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marjot T., Buescher G., Sebode M., Barnes E., Barritt A.S.t., Armstrong M.J., et al. SARS-CoV-2 infection in patients with autoimmune hepatitis. J Hepatol. 2021;74:1335–1343. doi: 10.1016/j.jhep.2021.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vuppalanchi V., Gelow K., Green K., Vuppalanchi R., Lammert C. Behaviors, symptoms, and outcomes of North American patients with autoimmune hepatitis during the COVID-19 pandemic. J Investig Med. 2021 doi: 10.1136/jim-2021-001871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerussi A., Rigamonti C., Elia C., Cazzagon N., Floreani A., Pozzi R., et al. Coronavirus Disease 2019 (COVID-19) in autoimmune hepatitis: a lesson from immunosuppressed patients. Hepatol Commun. 2020 doi: 10.1002/hep4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lau G., Sharma M. Clinical practice guidance for hepatology and liver transplant providers during the COVID-19 pandemic: APASL expert panel consensus recommendations. Hepatol Int. 2020;14:415–428. doi: 10.1007/s12072-020-10054-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernardi M., Moreau R., Angeli P., Schnabl B., Arroyo V. Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63:1272–1284. doi: 10.1016/j.jhep.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Gao F., Zheng K.I., Fan Y.C., Targher G., Byrne C.D., Zheng M.H. ACE2: a linkage for the interplay between COVID-19 and decompensated cirrhosis. Am J Gastroenterol. 2020:115:1544. doi: 10.14309/ajg.0000000000000780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarin S.K., Choudhury A., Lau G.K., Zheng M.H., Ji D., Abd-Elsalam S., et al. Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study) Hepatol Int. 2020;14:690–700. doi: 10.1007/s12072-020-10072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qi X., Liu Y., Wang J., Fallowfield J.A., Wang J., Li X., et al. Clinical course and risk factors for mortality of COVID-19 patients with pre-existing cirrhosis: a multicentre cohort study. Gut. 2020 doi: 10.1136/gutjnl-2020-321666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu F., Long X., Ji G., Zhang B., Zhang W., Zhang Z., et al. Clinically significant portal hypertension in cirrhosis patients with COVID-19: Clinical characteristics and outcomes. J Infect. 2020;81:e178–e180. doi: 10.1016/j.jinf.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qi X., Wang J., Li X., Wang Z., Liu Y., Yang H., et al. Clinical course of COVID-19 in patients with pre-existing decompensated cirrhosis: initial report from China. Hepatol Int. 2020;14:478–482. doi: 10.1007/s12072-020-10051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bajaj J.S., Garcia-Tsao G., Biggins S.W., Kamath P.S., Wong F., McGeorge S., et al. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort. Gut. 2020 doi: 10.1136/gutjnl-2020-322118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shalimar E., lhence A., Vaishnav M., Kumar R., Pathak P., Soni K.D., et al. Poor outcomes in patients with cirrhosis and Corona Virus Disease-19. Indian J Gastroenterol. 2020;39:285–291. doi: 10.1007/s12664-020-01074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iavarone M., D'Ambrosio R., Soria A., Triolo M., Pugliese N., Del Poggio P., et al. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73:1063–1071. doi: 10.1016/j.jhep.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desai A., Sachdeva S., Parekh T., Desai R.J. Jgo. COVID-19 and Cancer: Lessons From a Pooled Meta-Analysis. 2020:6:557-559. doi: 10.1200/go.20.00097. [DOI] [PMC free article] [PubMed]
- 44.Kim D., Adeniji N., Latt N., Kumar S., Bloom P., Aby E., et al. Predictors of outcomes of COVID-19 in patients with chronic liver disease: US Multi-center Study. 2021:19:1469-1479.e19. doi: 10.1016/j.cgh.2020.09.027. [DOI] [PMC free article] [PubMed]
- 45.Santambrogio R., Farina G., D'Alessandro V., Iacob G., Gemma M., Zappa M.A. Guidelines adaptation to the COVID-19 outbreak for the management of hepatocellular carcinoma. J Laparoendosc Adv Surg Tech A. 2020 doi: 10.1089/lap.2020.0559. [DOI] [PubMed] [Google Scholar]
- 46.Chagas A.L., Fonseca L.G.D., Coelho F.F., Saud L., Abadala E., Andraus W., et al. Management of hepatocellular carcinoma during the COVID-19 pandemic - Sao Paulo clinicas liver cancer group multidisciplinary consensus statement. Clinics. 2020;75:e2192. doi: 10.6061/clinics/2020/e2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan Stephen L., Kudo M. Impacts of COVID-19 on liver cancers: during and after the pandemic. Liver Cancer. 2020;9:491–502. doi: 10.1159/000510765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Gerven N.M., Verwer B.J., Witte B.I., van Hoek B., Coenraad M.J., van Erpecum K.J., et al. Relapse is almost universal after withdrawal of immunosuppressive medication in patients with autoimmune hepatitis in remission. J Hepatol. 2013;58:141–147. doi: 10.1016/j.jhep.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 49.Shiina S., Gani R.A., Yokosuka O., Maruyama H., Nagamatsu H., Payawal D.A., et al. APASL practical recommendations for the management of hepatocellular carcinoma in the era of COVID-19. Hepatol Int. 2020 doi: 10.1007/s12072-020-10103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grinyo J.M. Why is organ transplantation clinically important? Cold Spring Harb Perspect Med. 2013;3 doi: 10.1101/cshperspect.a014985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao F., Zheng K.I., Gu J.Y., George J., Zheng M.H. COVID-19 and liver transplantation: Lessons learned from three reported cases. Transpl Infect Dis. 2020;22:e13335. doi: 10.1111/tid.13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sessa A., Mazzola A., Lim C., Atif M., Pappatella J., Pourcher V., et al. COVID-19 in a liver transplant recipient: Could iatrogenic immunosuppression have prevented severe pneumonia? A case report. World J Gastroenterol. 2020;26:7076–7084. doi: 10.3748/wjg.v26.i44.7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Becchetti C., Zambelli M.F., Pasulo L., Donato M.F., Invernizzi F., Detry O., et al. COVID-19 in an international European liver transplant recipient cohort. Gut. 2020;69:1832–1840. doi: 10.1136/gutjnl-2020-321923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fraser J., Mousley J., Testro A., Smibert O.C., Koshy A.N. Clinical presentation, treatment, and mortality rate in liver transplant recipients with coronavirus disease 2019: a systematic review and quantitative analysis. Transplant Proc. 2020;52:2676–2683. doi: 10.1016/j.transproceed.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu H., He X., Wang Y., Zhou S., Zhang D., Zhu J., et al. Management of COVID-19 in patients after liver transplantation: Beijing working party for liver transplantation. Hepatol Int. 2020;14:432–436. doi: 10.1007/s12072-020-10043-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Imam A., Abukhalaf S.A., Merhav H., Abu-Gazala S., Cohen-Arazi O., Pikarsky A.J., et al. Prognosis and treatment of liver transplant recipients in the COVID-19 era: a literature review. Ann Transplant. 2020;25 doi: 10.12659/AOT.926196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Colmenero J., Rodríguez-Perálvarez M., Salcedo M., Arias-Milla A., Muñoz-Serrano A., Graus J., et al. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021;74:148–155. doi: 10.1016/j.jhep.2020.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taher M., Miroliaee A., Ebrahimi Daryani N., Alborzi Avanaki F., Aletaha N., Nasiri-Toosi M., et al. Management of patients with liver transplant and chronic liver diseases during COVID-19 pandemic: a brief review. Arch Iran Med. 2020;23:713–717. doi: 10.34172/aim.2020.92. [DOI] [PubMed] [Google Scholar]
- 59.D'Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transpl. 2020;26:832–834. doi: 10.1002/lt.25756. [DOI] [PubMed] [Google Scholar]
- 60.Qin J., Wang H., Qin X., Zhang P., Zhu L., Cai J., et al. Perioperative presentation of COVID-19 disease in a liver transplant recipient. Hepatology. 2020;72:1491–1493. doi: 10.1002/hep.31257. [DOI] [PubMed] [Google Scholar]
- 61.Manzia T.M., Gazia C., Lenci I., Angelico R., Toti L., Monaco A., et al. Liver transplantation performed in a SARS-CoV-2 positive hospitalized recipient using a SARS-CoV-2 infected donor. Am J Transplant. 2021;21:2600–2604. doi: 10.1111/ajt.16548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saigal S., Gupta S., Sudhindran S., Goyal N., Rastogi A., Jacob M., et al. Liver transplantation and COVID-19 (Coronavirus) infection: guidelines of the liver transplant Society of India (LTSI) Hepatol Int. 2020;14:429–431. doi: 10.1007/s12072-020-10041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fix O.K., Hameed B., Fontana R.J., Kwok R.M., McGuire B.M., Mulligan D.C., et al. Clinical best practice advice for hepatology and liver transplant providers during the COVID-19 pandemic: AASLD expert panel consensus statement. Hepatology. 2020;72:287–304. doi: 10.1002/hep.31281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sultan S., Altayar O., Siddique S.M., Davitkov P., Feuerstein J.D., Lim J.K., et al. AGA institute rapid review of the gastrointestinal and liver manifestations of COVID-19, meta-analysis of international data, and recommendations for the consultative management of patients with COVID-19. Gastroenterology. 2020;159 doi: 10.1053/j.gastro.2020.05.001. 320-334 e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rabinowich L., Grupper A., Baruch R., Ben-Yehoyada M., Halperin T., Turner D., et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75:435–438. doi: 10.1016/j.jhep.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ruether D.F., Schaub G.M., Duengelhoef P.M., Haag F., Brehm T.T., Fathi A., et al. SARS-CoV2-specific humoral and T-cell immune response after second vaccination in liver cirrhosis and transplant patients. Clin Gastroenterol Hepatol. 2021 doi: 10.1016/j.cgh.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Russo F.P., Piano S., Bruno R., Burra P., Puoti M., Masarone M., et al. Italian association for the study of the liver position statement on SARS-CoV2 vaccination. Dig Liver Dis. 2021;53:677–681. doi: 10.1016/j.dld.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cornberg M., Buti M., Eberhardt C.S., Grossi P.A., Shouval D. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol. 2021;74:944–951. doi: 10.1016/j.jhep.2021.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]