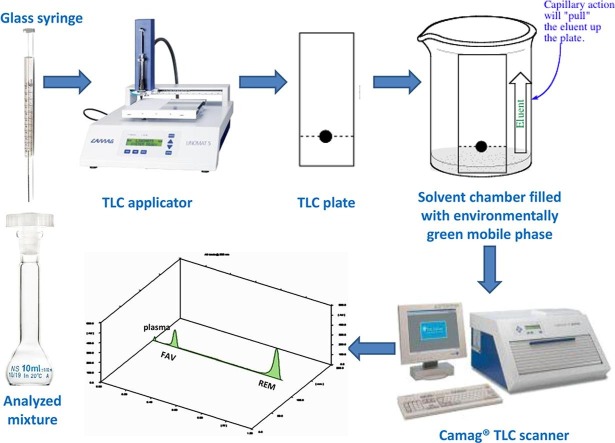
Graphical abstract
Keywords: Remdesivir, Favipiravir, COVID-19, TLC-densitometry, Human plasma
Abstract
A great demand for discovering new therapeutic solutions has been considered all over the world for managing the rapidly progressing COVID-19 pandemic. Remdesivir (REM) and Favipiravir (FAV) are introduced as promising newly developed antiviral agents against the corona virus as evidenced by the clinical findings. Hence, the optimization of an analytical method for their simultaneous determination acquires potential importance in quality control labs and further confirmatory investigations. Herein, a green, sensitive, and selective densitometric method has been proposed and validated for determination of REM and FAV in pharmaceutical formulations and spiked human plasma on normal phase TLC plates. A solvent mixture of ethyl acetate–methanol-ammonia (8:2:0.2 by volume) has been chosen as developing mobile phase system. Well resolved spots have been detected at 235 nm with retardation factors (Rf) of 0.18 and 0.98 for REM and FAV, respectively. A validation study has been carried out in the light of ICH guidelines. Remdesivir and FAV have shown excellent sensitivities with quantitation limits down to 0.12 and 0.07 μg/band, respectively. The developed method has been successfully applied to tablet formulations and spiked plasma with excellent recoveries ranged from 97.21 to 101.31%. The greenness of the method has been evaluated using the standards of greenness profile and Eco-Scale. It has passed the four greenness profile quadrants and achieved 80 score in Eco-Scale.
1. Introduction
Coronavirus disease (COVID-19) has been declared as a global pandemic by WHO on March 2020 with more than 227 million confirmed cases worldwide and up to 4.6 million deaths until September 2021 [1]. The riskiness is attributed to its severe acute respiratory syndrome affecting the lower respiratory tract inducing fatal pneumonia [2]. The recent approval of different vaccines failed to restrain the life threating pandemic. This could be due to the unavailability and inadequacy of vaccination, mutation in addition to the lack of alternative viable therapeutic options [3]. Consequently, repurposing the usage of the currently marketed antiviral drugs such as Remdesivir (REM) and Favipiravir (FAV) is considered a viable and instant option [4], [5].
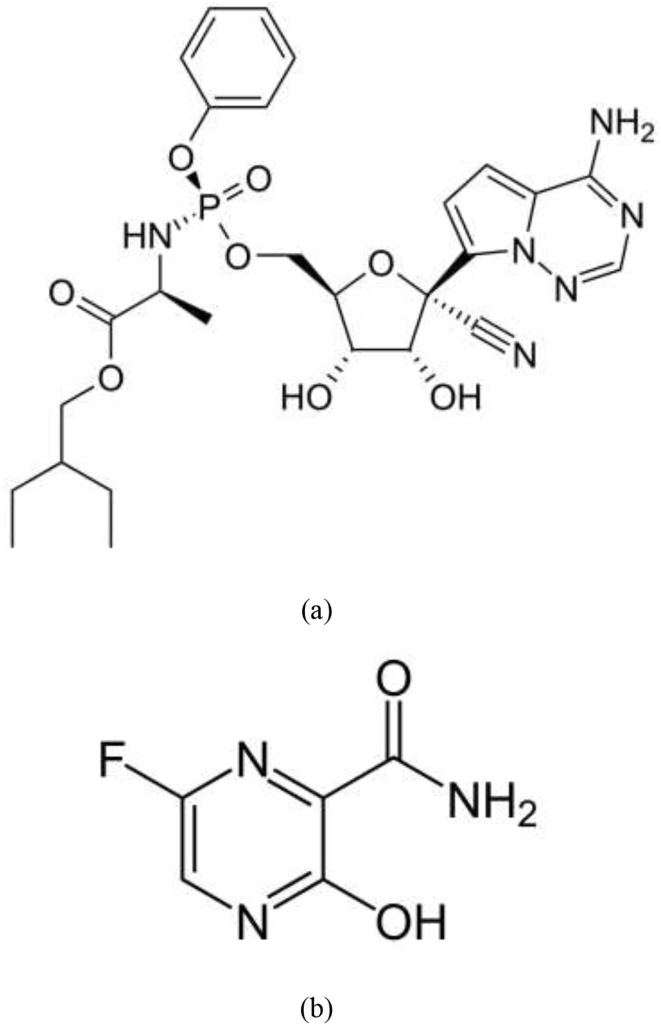
Chemically, REM is 2-ethylbutyl (2S)-2-[[[(2R,3S,4R,5R)-5-(4-aminopyrrolo [2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxyoxolan-2-yl] methoxy-phenoxyphosphoryl] amino] propanoate, Fig. 1 , which was firstly synthesized by Gilead Sciences for management of Ebola virus infections [6]. While, FAV, Fig. 1, is 6-fluoro-3-hydroxy-2-pyrazine carboxamide and it was developed by Toyama Chemical Company in Japan as anti-influenza therapy [7], [8]. Both antivirals are acting as RNA polymerase inhibitors and hence preventing replication of the coronavirus [9], [10].
Fig. 1.
Chemical structure of (a) Remdesivir and (b) Favipiravir.
To the best of our knowledge, few analytical methods have been proposed for determination of REM and FAV such as liquid chromatography [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], electrochemical techniques [23], [24], [25], and spectrofluorimetric methods [14], [26], [27]. It is worth noting that most of published methods suffer from using expensive analytical instruments and hazardous chemicals, time consuming, and need well-trained personnel.
Nowadays, the greenness of analytical methods [28], [29], [30], [31], [32] is considered prerequisite as it provides safety of both individuals and environment by reducing consumption of carcinogenic solvents or replacing them by more green ones [33]. Additionally, it provides several advantages over the traditional analytical methodologies such as reducing cost of analytical performance and amount of chemical waste through miniaturization, skipping pretreatment, improving precision through automation, and enhancing selectivity through involving of kinetic aspects. This manuscript has afforded a simple and environment friendly TLC-densitometric way for the simultaneous analysis of REM and FAV as raw, in pharmaceuticals, and in spiked plasma samples. The developed method provides greenness in addition to overcoming drawbacks of other techniques.
2. Experimental
2.1. Apparatus
Camag® TLC scanner with linomat 5 (Switzerland) equipped with WinCATS® programme (V 1.4.4) was utilized. The following specifications were properly considered; 4 mm and 0.45 mm as slit dimension, 20 mm/s as scanning speed, deuterium lamp as radiation source, absorbance mode as the scan mode, and chromatogram and integrated peak area as output. TLC tank (26.5 cm height × 27 cm width × 7 cm diameter; Sigma-Aldrich® Co., USA), 20 × 20 cm pre-coated silica gel aluminum plates (60 F254, 0.1 mm thickness), Allugram SIL G/UV 254 (Machenary-Nagel, Germany), and TLC-Hamilton® glass syringe. Digital analytical balance (AG 29, Meltter Toledo, Glattbrugg, Switzerland), tabletop low speed centrifuge model with maximum speed 4000 rpm (TD3, Taiwan), and sonicator (Sonix TV ss-series, New York, USA) were used throughout the investigation.
2.2. Materials and reagents
REM and FAV authentic samples were kindly supplied as gifts from EVA Pharma (Giza, Egypt) with claimed purity of 100.68 % ± 1.04 and 99.98 % ± 1.57, respectively, according to the reported HPLC methods [13], [18]. Methanol, ethanol, ethyl acetate, and ammonia of analytical grade were purchased from El-Nasr Pharmaceutical Chemicals Co. (Cairo, Egypt). The plasma samples were provided by the National Egyptian Blood Bank and stored at −20 °C until used.
2.3. Pharmaceutical formulation
Remdesivir-Rameda® for I.V injection lyophilized powder (batch no. 203242) was purchased from the local market and claimed to have 100.00 mg REM/vial (Rameda Pharmaceuticals, 6th of October city, Giza, Egypt). Avipiravir® tablets (EVA Pharma, Giza, Egypt; batch no. 2103008) were claimed to have 200.00 mg FAV/tablet and also obtained from the Egyptian market.
2.4. Standard solutions
Stock standard solutions of REM and FAV (1.00 mg/mL) were prepared by weighing 100.00 mg of each into two separate 100-mL volumetric flasks, and then the volumes were made up to the mark by ethanol and methanol for REM and FAV, respectively. The prepared stock solutions were stable in the refrigerator for 10 days.
2.5. Procedures
2.5.1. Chromatographic conditions
The TLC plates were saturated for 15 min in the chromatographic chamber by the eluent system; ethyl acetate–methanol-ammonia (8:2:0.2 by volume), and then samples were applied as bands 1 cm from the bottom edge. Afterwards, the chromatographic development took place and plates were air dried and scanned at 235 nm.
2.5.2. Construction of the calibration plots
Two concentration sets of REM and FAV were prepared in methanol and ranged; 20.00 – 450.00 and 8.00 – 500.00 μg/mL, respectively. About 10.00 μL aliquots of each flask were injected onto TLC plates in triplicates to get concentrations in the ranges of 0.20 – 4.50 and 0.08 – 5.00 μg/band of REM and FAV, respectively. Calibration plots were constructed as concentrations (μg/mL) against the corresponding areas under the peaks.
2.5.3. In vitro calibration plots
Into 10-mL volumetric flasks, one milliliter plasma samples were spiked by different concentrations of the investigated analytes. About two milliliters acetonitrile as a protein denaturation agent were added and the volumes were made up with methanol. Flasks were vortexed for 2 min and centrifuged at 4000 rpm for 20 min. The clear supernatants were transferred into 10-mL volumetric flasks and made up to the mark by methanol to get final concentration ranges of 20.00 – 450.00 and 8.00–500.00 μg/mL for REM and FAV, respectively. Aliquots of 10.00 μL of each concentration flask were applied to the TLC plates in triplicate to get concentrations ranges of 0.20 – 4.50 and 0.08 – 5.00 μg/band of REM and FAV, respectively. Calibration plots were constructed and a blank experiment was carried out simultaneously.
2.5.4. Analysis of pharmaceutical formulations
Stock solutions (1.00 mg/mL) of both analytes were prepared. An amount of REM lyophilized powder equivalent to 25.00 mg was transferred into a 25-mL volumetric flask, diluted with 10.00 mL distilled water, shaked well, and made up to the mark with the same solvent. The contents of ten Avipiravir® tablets were precisely weighed and ground to fine powder. A quantity corresponding to 25.00 mg was transferred into a 25-mL volumetric flask. Ten milliliters methanol were added, and the flask was sonicated for about 20 min. The volume was made up to 25.00 mL with methanol and then filtered using a 0.45-mm membrane filter.
Definite volumes of each stock solution were diluted with methanol to get working ones. The percentage recoveries of REM and FAV were estimated using the relevant regression equations for each drug, and also standard addition was applied to determine the validity of the method.
3. Results and discussion
A validated, sensitive, selective, quick, and cost-effective TLC-densitometric method was developed for determination of REM and FAV with minimal environmental impact. The developed TLC method has the advantages of separating several analytes concurrently with low solvent consumption and with a relatively easy sample preparation procedure. To attain good resolution with acceptable Rf values and sharp symmetric peaks, different chromatographic conditions were optimized.
3.1. Method development and optimization
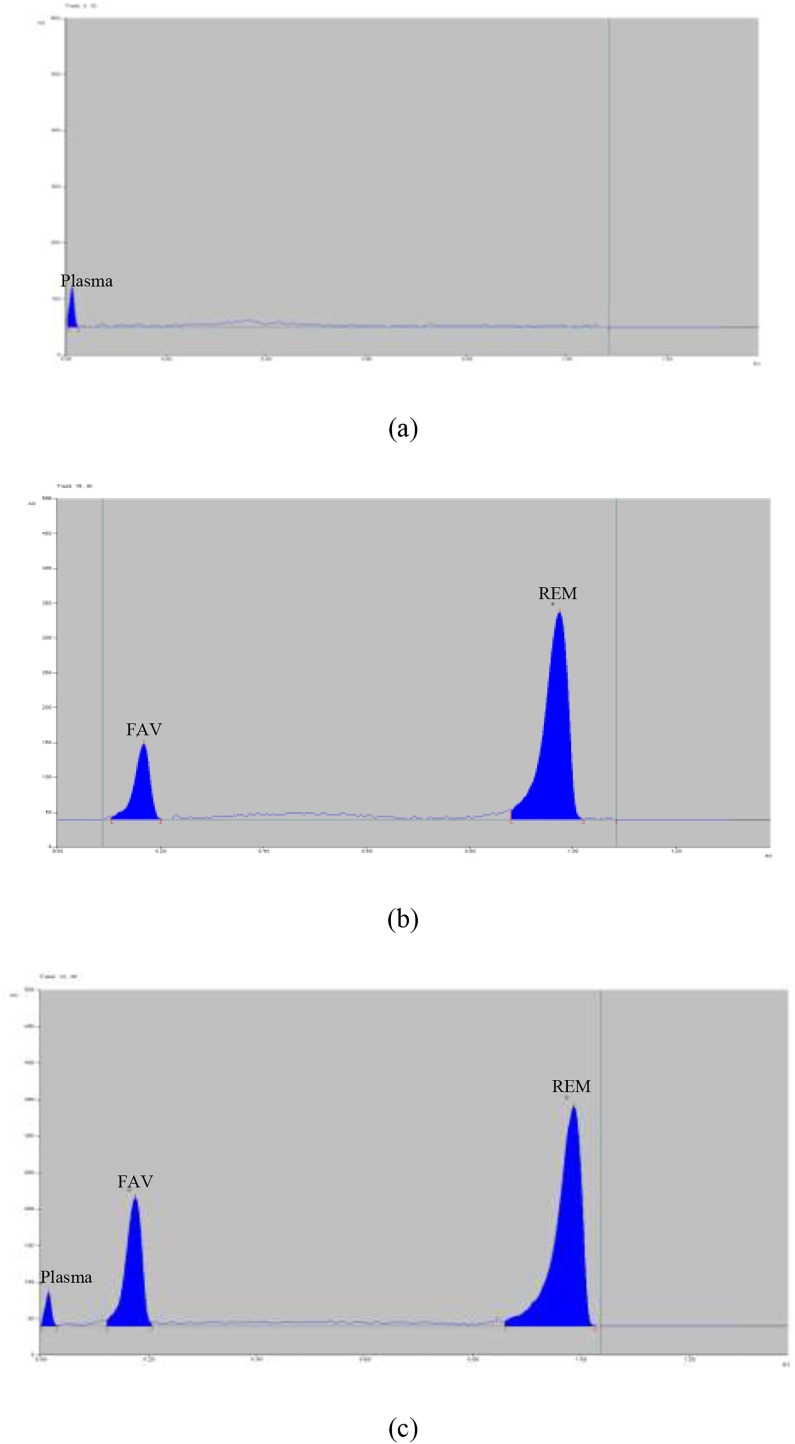
The factors affecting the proposed TLC-densitometric method were adjusted including; eluent composition, saturation time, and scanning wavelengths. Different mixtures of chloroform and methanol in different ratios were tested firstly but FAV had exhibited peak tailing. To improve greenness of the method as well analysis efficiency, different eco-friendly solvent mixtures were investigated as ethyl acetate-acetone (8:2, v/v), ethyl acetate-ethanol (8:2, v/v), and ethyl acetate–methanol (8:2, v/v). Upon testing the first solvent combination, only REM was eluted but FAV and plasma peaks were retained on the base line. The second one has afforded slightly resolved peaks with bad resolution. On the other hand, the third one has yielded more resolved peaks but with tailed FAV peak. Different ratios of acetic acid and ammonia solution were investigated to the third solvent mixture. Bad resolution was obtained with acetic acid, while ammonia solution had improved the resolution with sharp peaks. Saturation times of (10–30 min) were examined as it had a significant impact on chromatographic separation [34]. Satisfactory results were obtained with saturation time of 15 min. Different scanning wavelengths were investigated (215, 220, 230, 235 nm) and 235 nm has shown best sensitivity with optimum signal-to-noise ratios. Finally, ethyl acetate–methanol-ammonia (8:2:0.2, by volume) mixture was selected as the developing system at 235 nm. The Rf values were found to be 0.18 and 0.98 for plasma, FAV and REM, respectively Fig. 2 .
Fig. 2.
TLC-densitograms of (a) blank plasma, (b) mixture of pure favipiravir and remdesivir and (c) mixture of favipiravir and remdesivir in spiked human plasma.
3.2. Method validation
Validation was carried out according to the ICH guidelines regarding linearity range, accuracy, precision, detection and quantitation limits, robustness, and system suitability parameters [35].
3.2.1. Range of linearity
The calibration plots were conducted under the specified chromatographic conditions. Good correlation coefficients were obtained over the concentration range of 0.20 – 4.50 and 0.08–5.00 μg/band, for REM, and FAV, respectively. Regression equations were found to be:
y1 = 4.1936 xREM + 0.7534 r = 0.9999
y2 = 1.7891 xFAV + 0.3069 r = 0.9999where, y is the peak area at 235 nm, x is the concentration in μg/band, and r is the correlation coefficient. This has proven the good linearity of the developed method. Regression and analytical parameters were summarized in Table 1 .
Table 1.
Analytical parameters for determination of REM and FAV by the proposed TLC-densitometric method in pure form and spiked human plasma.
| Parameters | Pure samples |
Spiked human plasma samples |
||
|---|---|---|---|---|
| REM | FAV | REM | FAV | |
| Linearity range (μg/band) | 0.20 – 4.50 | 0.08 – 5.00 | 0.20 – 4.50 | 0.08 – 5.00 |
| Correlation coefficient (r) | 0.9999 | 0.9999 | 0.9998 | 0.9999 |
| Determination coefficient (r2) | 0.9999 | 0.9999 | 0.9998 | 0.9999 |
| Slope (b) | 4.20 | 1.7891 | 3.7877 | 1.61 |
| Intercept (a) | 0.7534 | 0.3069 | 0.9321 | 0.6428 |
| SD of slope | 0.01863 | 0.0009014 | 0.02436 | 0.004249 |
| SD of intercept | 0.05317 | 0.002443 | 0.06953 | 0.01193 |
| LOD (μg/band) | 0.04 | 0.02 | 0.06 | 0.02 |
| LOQ (μg/band) | 0.12 | 0.07 | 0.18 | 0.07 |
LOD, limit of detection; LOQ, limit of quantitation.
3.2.2. Accuracy
The accuracy of the developed method was assessed by analyzing five concentrations within linearity range of analytes in triplicates and expressed as percentage recoveries. The results obtained have demonstrated the high reliability and accuracy of the developed method, Table 2 .
Table 2.
Evaluation of accuracy for determination of REM and FAV using the proposed method.
| Sample number | REM |
FAV |
||||
|---|---|---|---|---|---|---|
| Taken (μg/band) | Founda (μg/band) | % Recovery | Taken (μg/band) | Founda (μg/band) | % Recovery | |
| 1 | 0.20 | 0.202 | 101.10 | 0.08 | 0.078 | 98.65 |
| 2 | 0.80 | 0.804 | 100.61 | 0.40 | 0.39 | 98.59 |
| 3 | 1.50 | 1.49 | 99.95 | 2.50 | 2.51 | 100.58 |
| 4 | 2.50 | 2.49 | 99.76 | 4.00 | 4.005 | 100.14 |
| 5 | 4.50 | 4.54 | 100.90 | 5.00 | 4.95 | 99.11 |
| Mean | 100.46 | 99.41 | ||||
| SD | 0.58 | 0.89 | ||||
| %RSD | 0.58 | 0.90 | ||||
SD, standard deviation; RSD, relative standard deviation.
Average of three determinations.
3.2.3. Precision
The precision was evaluated through intra-day (repeatability) and inter-day fluctuations by analyzing three concentrations of each drug three times on the same and on three successive days, respectively. The relative standard deviation (%RSD) values obtained were less than 2.00 %, demonstrating that the proposed method is precise, Table 3 .
Table 3.
Precision study for the developed TLC-densitometric method.
| Parameters | REM |
FAV |
|||||
|---|---|---|---|---|---|---|---|
| 0.20 μg/band | 2.60 μg/band | 4.50 μg/band | 0.40 μg/band | 2.50 μg/band | 5.00 μg/band | ||
| Intraday | 1 | 100.51 | 100.88 | 100.42 | 100.58 | 100.58 | 100.10 |
| 2 | 101.10 | 101.62 | 100.90 | 99.59 | 100.96 | 99.11 | |
| 3 | 100.96 | 100.35 | 101.85 | 99.68 | 99.97 | 100.26 | |
| Mean | 100.86 | 100.95 | 101.06 | 99.95 | 100.50 | 99.82 | |
| SD | 0.31 | 0.63 | 0.72 | 0.54 | 0.49 | 0.62 | |
| %RSD | 0.30 | 0.63 | 0.72 | 0.54 | 0.49 | 0.62 | |
| Interday | 1 | 100.51 | 100.88 | 100.42 | 100.58 | 100.58 | 100.10 |
| 2 | 101.96 | 99.42 | 99.57 | 98.59 | 99.68 | 99.11 | |
| 3 | 101.58 | 99.77 | 98.56 | 99.68 | 98.76 | 100.86 | |
| Mean | 101.35 | 100.02 | 99.52 | 99.61 | 99.68 | 100.02 | |
| SD | 0.75 | 0.76 | 0.93 | 0.99 | 0.90 | 0.87 | |
| %RSD | 0.74 | 0.76 | 0.93 | 1.00 | 0.90 | 0.87 | |
SD, standard deviation; RSD, relative standard deviation.
3.2.4. Limits of detection and quantitation
Sensitivity of the developed method was evaluated through determining limits of detection (LOD) and quantitation (LOQ); LOD; (3.3 * σ)/ S and LOQ; (10* σ)/ S, where (σ) is the standard deviation of intercept and (S) is the average slope. The limits of detection and quantitation were 0.04 and 0.12 ng/band for REM, and 0.02 and 0.07 μg/band for FAV, demonstrating excellent sensitivity of the method, Table 1.
3.2.5. Robustness
In order to evaluate the robustness, minor but deliberate variations in chromatographic method parameters were made and expressed as the percentage relative standard deviation (% RSD). Small variations were performed in the mobile phase system composition; ethyl acetate, methanol volumes (±0.10 mL), formic acid volume (±0.05 mL), and saturation time (±5 min). The results have shown that the investigated parameters had no significant influence, and that the procedures were both robust and reliable, Supporting information Table 1.
3.2.6. System suitability parameters
The system suitability parameters were investigated namely; symmetry factors, selectivity, and resolution parameters. The obtained values were within the acceptable limits as summarized in Table 4 .
Table 4.
System suitability testing parameters of the developed TLC-densitometric method.
3.3. Application to pharmaceutical formulations
The proposed method was applied to REM and FAV in their pharmaceutical formulations. Satisfactory percentage recoveries were obtained. The results were compared to previously reported ones [13], [18] using variance f-test and student's t-test. No significant differences were observed, Table 5 , and this proves that there is no excipients interference. The standard addition technique was examined for more assessment of accuracy of the TLC-densitometric method. The results were acceptable, demonstrating the high accuracy of the proposed method, Table 5.
Table 5.
Determination of REM and FAV in their pharmaceutical formulations by the proposed TLC-densitometric method with application of standard addition technique, and statistical comparison of the obtained results using the reported HPLC methods [13], [18].
| Pharmaceutical formulation | Founda (% ± S.D) | Standard addition technique |
Reported methoda | t-testc | F- testc | |
|---|---|---|---|---|---|---|
| Added (µg/band) | % Recoveryb | |||||
| Remdesivir-Rameda® lyophilized powder for I.V injection (1.00 µg/band) | 99.86 ± 0.82 | 0.50 | 101.31 | 100.68 ± 0.61 | 1.77 | 1.76 |
| 1.00 | 100.10 | |||||
| 1.50 | 101.2 | |||||
| (Mean ± S.D) | 100.90 ± 0.69 | |||||
| Avipiravir® tablets (1.00 µg/band) | 104.24 ± 0.70 | 0.50 | 98.57 | 103.18 ± 0.99 | 1.93 | 1.98 |
| 1.00 | 100.25 | |||||
| 1.50 | 100.66 | |||||
| (Mean ± S.D) | 99.82 ± 1.10 | |||||
The values are the mean of five determinations.
The values are the mean of three determinations.
The tabulated values of t-test and F test at 0.05% are 2.306 and 6.388, respectively.
3.4. Application to spiked human plasma
The developed method has exhibited high sensitivity in human plasma. The results obtained prove the ability of the developed method for estimation of the studied drugs in human plasma without any interference from plasma components, Fig. 2, Supporting information Table 2.
3.5. Developed method greenness assessment
Green analysis is defined by the absence or minimum use of hazardous chemicals, the absence of waste, and the reduction of energy usage. The greenness profile and Eco-Scale methodology were investigated in order to assess the greenness of the proposed TLC-densitometric method [36], [37].
3.5.1. Proposed method greenness profile
Greenness profile of the developed method was assessed according to National Environmental Method Index (NEMI) [36]. It depends on using of non-persistent, bio accumulative and toxic solvents (PBT). The solvents utilized in the developed method were ethyl acetate and methanol, which are not PBT. Furthermore, the developing system pH was around 8 and therefore it was not deemed corrosive. Also, the waste generated per sample was 5 g/run (volume of developing system used per run/ number of samples). According to the aforementioned findings, the recommended TLC-densitometric method saved solvents with producing minimal quantities of waste. For these reasons, it passed the four quadrants of the greenness profile and considered an ecofriendly green method, Table 6 .
Table 6.
Greenness assessment of the developed TLC method by Analytical Eco-scale and NEMI.
| Eco-scale | NEMI | ||
|---|---|---|---|
| Parameters | Penalty points |  |
|
| Reagents | Ethyl acetate | 4 | |
| Methanol | 6 | ||
| Ammonia solution | 6 | ||
| Instrument | 1 | ||
| Occupational hazard | 0 | ||
| Waste | 3 | ||
| Total penalty points | 20 | ||
| Analytical Eco-Scale score | 80 | ||
3.5.2. Eco-Scale of the developed method
Eco-scale is a simple approach that can easily apply in quality control laboratory practice. It is determined by calculating penalty points to all of the established method's parameters that include reagents amount, occupational hazards, waste, and energy using the following equation (analytical Eco − Scale score = 100 − total penalty) [37]. If the score is greater than 75, the analytical method is regarded to be outstanding green analysis. The developed TLC-densitometric method had an Eco-Scale score of 80, Table 6; for that it is considered to be a green one.
4. Conclusion
New, simple, green and highly sensitive TLC-densitometeric method has been developed for the first time for monitoring REM and FAV simultaneously. The proposed method is deemed green and ecologically friendly that could be utilized in quality control laboratories with elimination of carcinogenic and environmentally hazardous solvents that are commonly employed in chromatographic procedures. It was successfully validated and applied for estimation of the studied components in pure form, pharmaceutical dosage forms, and plasma samples. It has the advantages of being rapid, low cost, and small amount of solvents consumption.
CRediT authorship contribution statement
Deena A.M. Noureldeen: Methodology, Resources, Data curation, Writing – review & editing. John M. Boushra: Formal analysis, Investigation, Validation, Writing – original draft, Visualization, Data curation. Adel S. Lashien: Resources, Supervision, Project administration. Ahmed F. Abdel Hakiem: Methodology, Software, Writing – review & editing. Tamer Z. Attia: Conceptualization, Methodology, Software, Validation, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgment
The authors would like to express their gratitude to EVA Pharma (Giza, Egypt) for providing the supplies needed to complete this study.
Formatting of funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.microc.2021.107101.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.World Health Organization, 2021. COVID-19 Weekly Epidemiological Update, 21 September 2021. https://www.who.int/docs/default-source/coronaviruse/situation reports/20210921_weekly_epi_update_58.pdf?sfvrsn=2ec52077_3&download=true.
- 2.Sohrabi C., Alsafi Z., O'Neill N., Khan M., Kerwan A., Al-Jabir A., Iosifidis C., Agha R. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int. J. Surgery. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wouters O.J., Shadlen K.C., Salcher-Konrad M., Pollard A.J., Larson H.J., Teerawattananon Y., Jit M. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet. 2021;397(10278):1023–1034. doi: 10.1016/S0140-6736(21)00306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sreekanth Reddy O., Lai W.-F. Tackling COVID-19 using Remdesivir and Favipiravir as therapeutic options. ChemBioChem. 2021;22(6):939–948. doi: 10.1002/cbic.202000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eroglu E., Toprak C. Overview of favipiravir and remdesivir treatment for COVID-19. Int. J. Pharm. Sci. Res. 2021:1950–1957. doi: 10.13040/IJPSR.0975-8232.12(4).1950-57. [DOI] [Google Scholar]
- 6.Eastman R.T., Roth J.S., Brimacombe K.R., Simeonov A., Shen M., Patnaik S., Hall M.D. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent. Sci. 2020;6(5):672–683. doi: 10.1021/acscentsci.0c00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furuta Y., Takahashi K., Kuno-Maekawa M., Sangawa H., Uehara S., Kozaki K., Nomura N., Egawa H., Shiraki K. Mechanism of action of T-705 against influenza virus. Antimicrob. Agents Chemother. 2005;49(3):981–986. doi: 10.1128/AAC.49.3.981-986.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiraki K., Daikoku T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol. Ther. 2020;209:107512. doi: 10.1016/j.pharmthera.2020.107512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon C.J., Tchesnokov E.P., Woolner E., Perry J.K., Feng J.Y., Porter D.P., Götte M. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. 2020;295(20):6785–6797. doi: 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du Y.-X., Chen X.-P. Favipiravir: pharmacokinetics and concerns about clinical trials for 2019-nCoV infection. Clin. Pharmacol. Ther. 2020;108(2):242–247. doi: 10.1002/cpt.1844. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen R., Goodell J.C., Shankarappa P.S., Zimmerman S., Yin T., Peer C.J., Figg W.D. Development and validation of a simple, selective, and sensitive LC-MS/MS assay for the quantification of remdesivir in human plasma. J. Chromatogr. B. 2021;1171:122641. doi: 10.1016/j.jchromb.2021.122641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avataneo V., de Nicolò A., Cusato J., Antonucci M., Manca A., Palermiti A., Waitt C., Walimbwa S., Lamorde M., di Perri G., D’Avolio A. Development and validation of a UHPLC-MS/MS method for quantification of the prodrug remdesivir and its metabolite GS-441524: a tool for clinical pharmacokinetics of SARS-CoV-2/COVID-19 and Ebola virus disease. J. Antimicrob. Chemother. 2020;75(7):1772–1777. doi: 10.1093/jac/dkaa152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bulduk İ. HPLC-UV method for quantification of favipiravir in pharmaceutical formulations. Acta Chromatogr. 2021;33(3):209–215. doi: 10.1556/1326.2020.00828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mikhail I.E., Elmansi H., Belal F., Ehab Ibrahim A. Green micellar solvent-free HPLC and spectrofluorimetric determination of favipiravir as one of COVID-19 antiviral regimens. Microchem. J. 2021;165:106189. doi: 10.1016/j.microc.2021.106189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.R. Nadendla, P. Abhinandana, A Validated high Performance Liquid Chromatographic Method for the Quantification of Favipiravir by PDA Detector.(2021). Int. J. Life Sci. Pharma Res, 2021. 11(2): 181–188.
- 16.Elmansi H., Ibrahim A.E., Mikhail I.E., Belal F. Green and sensitive spectrofluorimetric determination of Remdesivir, an FDA approved SARS-CoV-2 candidate antiviral; application in pharmaceutical dosage forms and spiked human plasma. Anal. Methods. 2021;13(23):2596–2602. doi: 10.1039/D1AY00469G. [DOI] [PubMed] [Google Scholar]
- 17.Alvarez J.-C., Moine P., Etting I., Annane D., Larabi I.A. Quantification of plasma remdesivir and its metabolite GS-441524 using liquid chromatography coupled to tandem mass spectrometry. Application to a Covid-19 treated patient. Clin. Chem. Lab. Med. (CCLM) 2020;58(9):1461–1468. doi: 10.1515/cclm-2020-0612. [DOI] [PubMed] [Google Scholar]
- 18.Pasupuleti R.R., Tsai P.-C., Ponnusamy V.K., Pugazhendhi A. Rapid determination of remdesivir (SARS-CoV-2 drug) in human plasma for therapeutic drug monitoring in COVID-19-Patients. Process Biochem. 2021;102:150–156. doi: 10.1016/j.procbio.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raasi K.M. Analytical method development and validation of Remdesivir in bulk and pharmaceutical dosage forms using reverse-phase-high performance liquid chromatography. BR Nahata Smriti Sansthan Int. J. Phram. Sci. Clin. Res. 2021;1(2) [Google Scholar]
- 20.Morsy M.I., Nouman E.G., Abdallah Y.M., Zainelabdeen M.A., Darwish M.M., Hassan A.Y., Gouda A.S., Rezk M.R., Abdel-Megied A.M., Marzouk H.M. A novel LC-MS/MS method for determination of the potential antiviral candidate favipiravir for the emergency treatment of SARS-CoV-2 virus in human plasma: Application to a bioequivalence study in Egyptian human volunteers. J. Pharm. Biomed. Anal. 2021;199:114057. doi: 10.1016/j.jpba.2021.114057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rezk M.R., Badr K.A., Abdel‐Naby N.S., Ayyad M.M. A novel, rapid and simple UPLC–MS/MS method for quantification of favipiravir in human plasma: application to a bioequivalence study. Biomed. Chromatogr. 2021;35(7) doi: 10.1002/bmc.5098. [DOI] [PubMed] [Google Scholar]
- 22.Eryavuz Onmaz D., Abusoglu S., Onmaz M., Yerlikaya F.H., Unlu A. Development and validation of a sensitive, fast and simple LC-MS/MS method for the quantitation of favipiravir in human serum. J. Chromatogr. B. 2021;1176:122768. doi: 10.1016/j.jchromb.2021.122768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tkach V.V., et al. Theoretical description for anti-COVID-19 drug Remdesivir electrochemical determination, assisted by squaraine Dye–Ag2O2 composite. Biointerface Res. Appl. Chem. 2021;11(2):9201–9208. [Google Scholar]
- 24.Mohamed M.A., Eldin G.M.G., Ismail S.M., Zine N., Elaissari A., Jaffrezic-Renault N., Errachid A. Innovative electrochemical sensor for the precise determination of the new antiviral COVID-19 treatment Favipiravir in the presence of coadministered drugs. J. Electroanal. Chem. 2021;895:115422. doi: 10.1016/j.jelechem.2021.115422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allahverdiyeva S., Yunusoğlu O., Yardım Y., Şentürk Z. First electrochemical evaluation of favipiravir used as an antiviral option in the treatment of COVID-19: A study of its enhanced voltammetric determination in cationic surfactant media using a boron-doped diamond electrode. Anal. Chim. Acta. 2021;1159:338418. doi: 10.1016/j.aca.2021.338418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao D., John Ling K.H., Tarnowski T., Humeniuk R., German P., Mathias A., Chu J., Chen Y.-S., van Ingen E. Validation of LC-MS/MS methods for determination of remdesivir and its metabolites GS-441524 and GS-704277 in acidified human plasma and their application in COVID-19 related clinical studies. Anal. Biochem. 2021;617:114118. doi: 10.1016/j.ab.2021.114118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Megahed S.M., Habib A.A., Hammad S.F., Kamal A.H. Experimental design approach for development of spectrofluorimetric method for determination of favipiravir; a potential therapeutic agent against COVID-19 virus: Application to spiked human plasma. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021;249:119241. doi: 10.1016/j.saa.2020.119241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arabi M., Ostovan A., Li J., Wang X., Zhang Z., Choo J., Chen L. Molecular imprinting: green perspectives and strategies. Adv. Mater. 2021;33(30):2100543. doi: 10.1002/adma.202100543. [DOI] [PubMed] [Google Scholar]
- 29.Ostovan A., Ghaedi M., Arabi M., Yang Q., Li J., Chen L. Hydrophilic multitemplate molecularly imprinted biopolymers based on a green synthesis strategy for determination of B-family vitamins. ACS Appl. Mater. Interfaces. 2018;10(4):4140–4150. doi: 10.1021/acsami.7b17500. [DOI] [PubMed] [Google Scholar]
- 30.Arabi M., Ostovan A., Zhang Z., Wang Y., Mei R., Fu L., Wang X., Ma J., Chen L. Label-free SERS detection of Raman-inactive protein biomarkers by Raman reporter indicator: toward ultrasensitivity and universality. Biosens. Bioelectron. 2021;174:112825. doi: 10.1016/j.bios.2020.112825. [DOI] [PubMed] [Google Scholar]
- 31.Arabi M., Ostovan A., Bagheri A.R., Guo X., Li J., Ma J., Chen L. Hydrophilic molecularly imprinted nanospheres for the extraction of rhodamine B followed by HPLC analysis: a green approach and hazardous waste elimination. Talanta. 2020;215:120933. doi: 10.1016/j.talanta.2020.120933. [DOI] [PubMed] [Google Scholar]
- 32.Bagheri A.R., Arabi M., Ghaedi M., Ostovan A., Wang X., Li J., Chen L. Dummy molecularly imprinted polymers based on a green synthesis strategy for magnetic solid-phase extraction of acrylamide in food samples. Talanta. 2019;195:390–400. doi: 10.1016/j.talanta.2018.11.065. [DOI] [PubMed] [Google Scholar]
- 33.Capello C., Fischer U., Hungerbühler K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007;9(9):927–934. doi: 10.1039/B617536H. [DOI] [Google Scholar]
- 34.B. Fried, Thin-layer chromatography: techniques and applications, 1982.
- 35.I. ICH Q2 (R1): Validation of analytical procedures: text and methodology, in: International Conference on Harmonization, Geneva, 2005.
- 36.Keith L.H., Gron L.U., Young J.L. Green analytical methodologies. Chem. Rev. 2007;107(6):2695–2708. doi: 10.1021/cr068359e. [DOI] [PubMed] [Google Scholar]
- 37.Van Aken K., Strekowski L., Patiny L. EcoScale, a semi-quantitative tool to select an organic preparation based on economical and ecological parameters. Beilstein J. Org. Chem. 2006;2(1):3. doi: 10.1186/1860-5397-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.F. FDA, Guidance for industry: bioanalytical method validation. http://www.fda.gov/cder/guidance/4252fnl.pdf, 2001.
- 39.Srivastava M.M. High-Performance Thin-Layer Chromatography (HPTLC) Springer Berlin Heidelberg; Berlin, Heidelberg: 2011. An overview of HPTLC: A modern analytical technique with excellent potential for automation, optimization, hyphenation, and multidimensional applications; pp. 3–24. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.