Abstract
PURPOSE
BCL2 is overexpressed and confers prosurvival signaling in malignant lymphoplasmacytic cells in Waldenström macroglobulinemia (WM). Venetoclax is a potent BCL2 antagonist and triggers in vitro apoptosis of WM cells. The activity of venetoclax in WM remains to be clarified.
PATIENTS AND METHODS
We performed a multicenter, prospective phase II study of venetoclax in patients with previously treated WM (NCT02677324). Venetoclax was dose-escalated from 200 mg to a maximum dose of 800 mg daily for up to 2 years.
RESULTS
Thirty-two patients were evaluable, including 16 previously exposed to Bruton tyrosine kinase inhibitors (BTKis). All patients were MYD88 L265P–mutated, and 17 carried CXCR4 mutations. The median time to minor and major responses was 1.9 and 5.1 months, respectively. Previous exposure to BTKis was associated with a longer time to response (4.5 v 1.4 months; P < .001). The overall, major, and very good partial response rates were 84%, 81%, and 19%, respectively. The major response rate was lower in those with refractory versus relapsed disease (50% v 95%; P = .007). The median follow-up time was 33 months, and the median progression-free survival was 30 months. CXCR4 mutations did not affect treatment response or progression-free survival. The only recurring grade ≥ 3 treatment-related adverse event was neutropenia (n = 14; 45%), including one episode of febrile neutropenia. Laboratory tumor lysis without clinical sequelae occurred in one patient. No deaths have occurred.
CONCLUSION
Venetoclax is safe and highly active in patients with previously treated WM, including those who previously received BTKis. CXCR4 mutation status did not affect treatment response.
INTRODUCTION
Waldenström macroglobulinemia (WM) is characterized by the malignant accumulation of immunoglobulin M (IgM)-secreting lymphoplasmacytic cells in the bone marrow (BM) and other organs.1 Treatment options for WM include alkylating agents, anti-CD20 monoclonal antibodies, proteasome inhibitors, and Bruton tyrosine kinase inhibitors (BTKis).2 Highly recurring somatic mutations in MYD88 and CXCR4 are found in patients with WM, and can affect disease presentation, prognosis, and treatment response to BTKis.3-5 WM remains incurable, and new treatment options are needed.
CONTEXT
Key Objective
Is venetoclax monotherapy a safe and effective therapy in patients with previously treated Waldenström macroglobulinemia (WM)?
Knowledge Generated
Venetoclax monotherapy, up to a dose of 800 mg by mouth once daily for 2 years, induced a high response rate in patients with WM, including 50% of patients previously exposed to Bruton tyrosine kinase inhibitors (BTKis) and 38% refractory to the preceding line of therapy. Venetoclax was well tolerated with only one episode of laboratory (not clinical) tumor lysis syndrome and manageable neutropenia. There were no episodes of immunoglobulin M flare, neuropathy, secondary myeloid neoplasms, or arrhythmia associated with venetoclax therapy.
Relevance
The findings of our study establish venetoclax as an active and tolerable therapy in patients with previously treated WM, regardless of previous exposure to BTKis. Given the manageable safety profile, future studies evaluating venetoclax in combination with anti-CD20 monoclonal antibodies or BTKis are warranted.
BCL2 is an essential regulator of the intrinsic pathway of apoptosis in normal and malignant cells. BCL2 is overexpressed in WM lymphoplasmacytic cells, particularly in patients with WM with MYD88 mutations with similar levels of BCL2 expression regardless of CXCR4 mutational status.6 Venetoclax is an oral BCL2 antagonist approved for the treatment of chronic lymphocytic leukemia (CLL) and acute myeloid leukemia. In vitro treatment of MYD88-mutated WM cells engineered to express either wild-type or mutated CXCR4 with venetoclax triggered apoptosis and sensitized cells to various chemotherapeutics including BTKis.6 In a prospective phase I study, all four patients with WM attained a major response to venetoclax.7
Herein, we report the safety and efficacy of this first, prospective, multicenter study of venetoclax in relapsed or refractory WM.
PATIENTS AND METHODS
Study Design and Oversight
This study was an investigator-initiated, multicenter, prospective trial of single-agent venetoclax in patients with previously treated WM. The study enrolled patients at the Dana-Farber Cancer Institute, Weill Cornell Medical Center, City of Hope National Medical Center, and Stanford University Medical Center. Enrollment began in June 2016 and closed in February 2018. The date of data cutoff was December 31, 2020. The study was approved by the institutional review board at all study sites. All patients provided written informed consent for participation. AbbVie provided research funding and study drug. The trial was registered under ClinicalTrials.gov identifier NCT02677324.
Study Patients
Adults with a diagnosis of WM were eligible if they were previously treated, had an Eastern Cooperative Oncology Group performance of ≤ 2, adequate BM function (ie, neutrophil count ≥ 1.0 K/uL, platelet count ≥ 50 K/uL, and hemoglobin ≥ 8 g/dL), creatinine clearance ≥ 50 mL/min, adequate hepatic function, and met criteria to receive therapy.8 Relapsed disease was defined as attaining a response to previous therapy and meeting criteria for disease progression beyond 6 months after completing such therapy.9 Refractory disease was defined as not attaining a minor response (MR) or progressing while on or within 6 months of completing previous therapy. Exclusion criteria included CNS involvement, active HIV, hepatitis B or hepatitis C infection, concurrent therapy for other cancer, and current pregnancy or breastfeeding. A flow diagram is shown in Figure 1.
FIG 1.
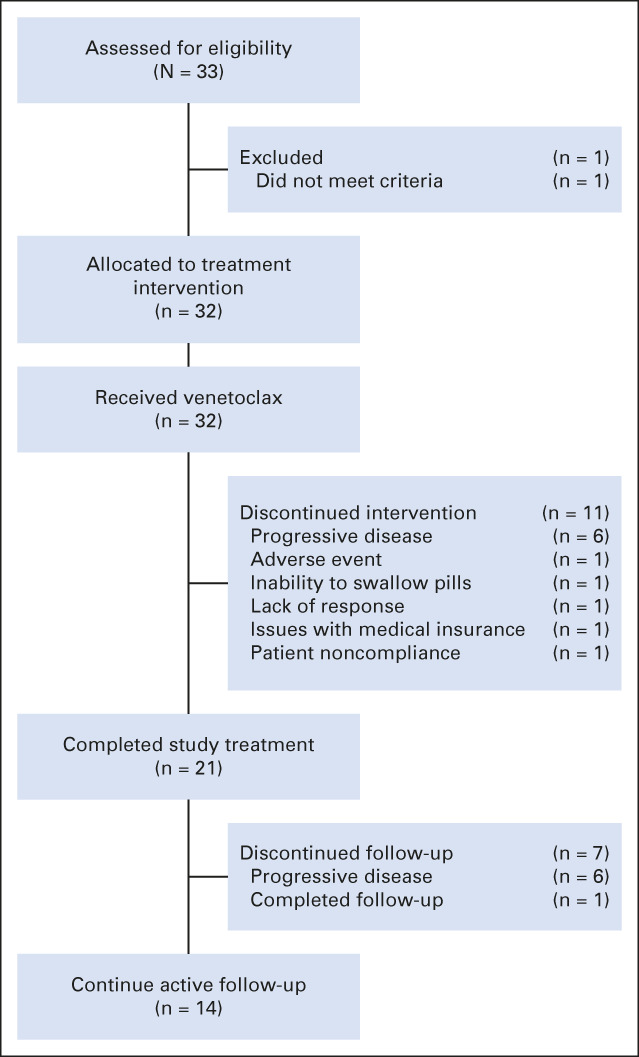
Flow diagram.
Study Treatment
All treatments were provided in the outpatient setting. For the first six patients, venetoclax was administered orally once daily at 200 mg for 1 week, followed by 400 mg for 1 week, and then 800 mg for 2 years. Starting with the seventh patient, venetoclax was administered orally once daily at 400 mg for 1 week, followed by 800 mg for 2 years. Dose reductions were allowed for toxicity (Appendix Table A1, online only). Three days before venetoclax, all patients started on allopurinol 300 mg once daily and 2-liter-per-day oral hydration for tumor lysis prophylaxis. Allopurinol was continued for 4 weeks. If oral hydration was not optimal, 2 L of normal saline was administered intravenously before the first dose of venetoclax. Granulocyte colony-stimulating factor (G-CSF) support was permitted for neutrophil count < 0.5 K/uL.
Assessments
Complete blood counts, comprehensive metabolic panel, and serum immunoglobulin levels were measured at baseline, once monthly for the first 3 months, and quarterly thereafter for the study duration. Tumor lysis monitoring with comprehensive metabolic panel, lactate dehydrogenase, phosphorus, and uric acid levels took place at 0, 8, and 24 hours on day 1 of each dose increase. BM aspiration and biopsies were obtained at baseline and 6, 12, and 24 months, and were evaluated at each participating institution. Computerized tomography scans with intravenous contrast were obtained at baseline and repeated at 6, 12, and 24 months if there was evidence of extramedullary disease at screening.
The primary objective was to determine the overall response rate (ORR), which included MR, partial response (PR), very good partial response (VGPR), and complete response. Secondary objectives included determining major response rate (PR or better), time to response, duration of response (DOR), progression-free survival (PFS), time to next treatment (TTNT), and drug safety. The responses were defined according to modified criteria from the Sixth International Workshop on WM.9,10 Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
An allele-specific polymerase chain reaction assay was used to detect MYD88 L265P mutations in CD19-selected BM cells.11 CXCR4 mutational status was determined by Sanger sequencing and allele-specific polymerase chain reaction in CD19-selected BM cells.12
Statistical Analysis
A sample size of 32 participants was estimated using an alpha level of .05 and a power of 90%, assuming a null ORR ≤ 40% and an alternative ORR ≥ 70%, on the basis of response rates to other monotherapies in previously treated patients with WM. Data were categorized to facilitate analysis (Appendix Table A2, online only), and factors evaluated in response and survival analyses. Descriptive statistics, including proportions, medians, and ranges, were calculated. Differences between group categories were assessed using the Fisher exact test. The Kaplan-Meier method was used for time-to-event analyses with censoring, and the log-rank test to assess differences between group categories. P values < .05 indicate statistical significance. Statistical analyses and graphs were performed using STATA 17.0 (College Station, TX).
RESULTS
Patients and Disease Characteristics
A total of 33 patients were enrolled. One patient received venetoclax but was excluded from the analysis because further workup indicated this patient had IgM myeloma. The malignant cells expressed cyclin D1 and harbored the t(11;14) translocation. This patient attained an MR and progressed after 8 months of therapy.
The baseline characteristics of all participants are shown in Table 1. The median time from the previous therapy to venetoclax initiation was 9 months (range, 1-115 months). Seven patients were treated in first relapse, and previous treatment included bortezomib, dexamethasone and rituximab (n = 4), bendamustine and ofatumumab (n = 1), BTKi (n = 1), and ofatumumab (n = 1). Of the 11 refractory patients, four were refractory to proteasome inhibitor–based regimens, three to BTKi, two to single agent anti-CD20 monoclonal antibodies, one to bendamustine and rituximab, and one to a phosphatidylinositol-3 kinase inhibitor.
TABLE 1.
Baseline Characteristics of the Participants
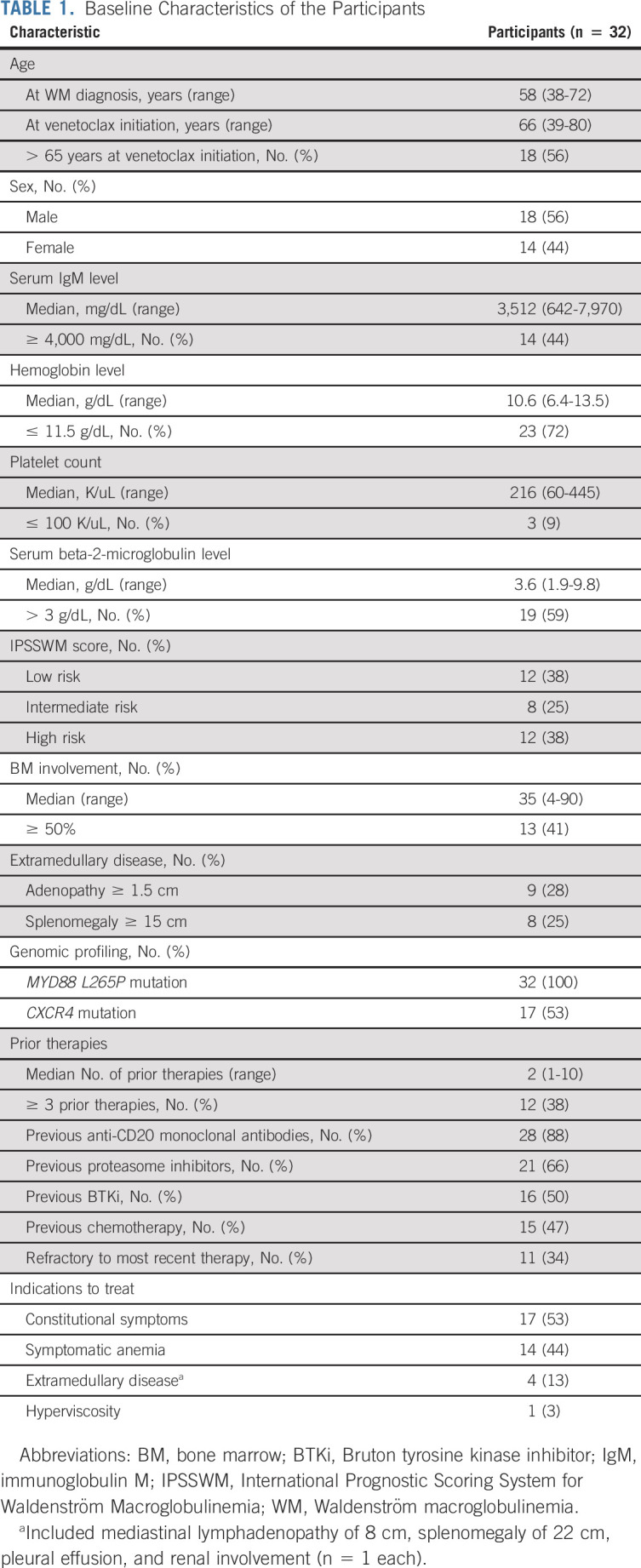
Sixteen patients (50%) were previously treated with a BTKi. BTKis were the last therapy before venetoclax in 11 patients with a median time from BTKi cessation to venetoclax initiation of 3 months (range, 1-15 months), of whom eight were intolerant and three had progressed on BTKis. In the remaining five patients, one was intolerant and four had progressed on BTKis. The median time of BTKi exposure was 10 months (range, 2-83 months).
The MYD88 L265P mutation was detected in all patients. CXCR4 mutations were detected in 17 patients, including 12 nonsense and five frameshift variants. Patients with CXCR4 mutations were less likely to have adenopathy ≥ 1.5 cm (12% v 53%; P = .01) and had a lower median serum beta-2-microglobulin level (2.6 v 4.9 mg/dL; P = .002) versus those without CXCR4 mutations.
Response to Therapy
Categorical responses included VGPR (n = 6; 19%), PR (n = 19; 61%), and MR (n = 1; 3%), for an ORR of 84% (95% CI, 66 to 95) and major response rate of 81% (95% CI, 63 to 93; Fig 2A). No patient attained a complete response. The ORR was lower in those with refractory versus relapsed disease (60% v 95%; P = .03; Fig 2B), and those who received ≥ 3 versus < 3 prior lines of therapy (63% v 95%; P = .04; Fig 2C). No other clinical or genomic factor, including prior BTKi exposure (Fig 2D), CXCR4 mutational status (Fig 2E), and International Prognostic Scoring System for Waldenström Macroglobulinemia (Fig 2F), was associated with a lower ORR. The major response rate was also significantly lower in those with refractory versus relapsed disease (50% v 95%; P = .007). No other clinical or genomic factor was associated with a lower major response rate. No baseline characteristic was associated with VGPR attainment. The kinetics of response to venetoclax are shown in Appendix Fig A1 (online only).
FIG 2.
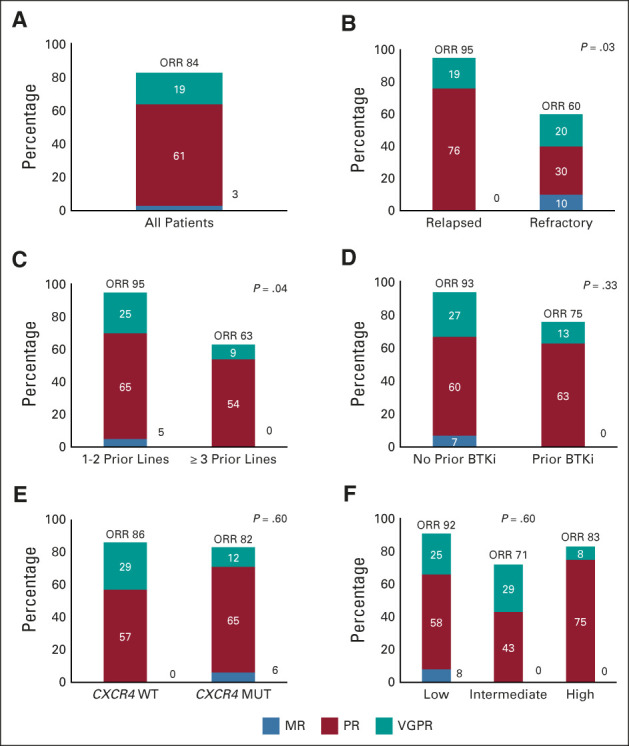
Categorical response rates to venetoclax for (A) all patients, and according to (B) relapsed or refractory disease, (C) number of previous lines of therapy, (D) prior BTKi exposure, (E) CXCR4 mutational status, and (F) IPSSWM. BTKi, Bruton tyrosine kinase inhibitor; IPSSWM, International Prognostic Scoring System for Waldenström Macroglobulinemia; MR, minor response; MUT, mutated; ORR, overall response rate; PR, partial response; VGFR, very good partial response; WT, wild-type.
The median times to minor and major response were 1.9 months (95% CI, 1.1 to 2.1) and 5.1 months (95% CI, 4.7 to 8.2), respectively. The median time to MR was longer in patients previously treated with BTKis (4.5 and 1.4 months; P < .001). The median time to MR for the 11 patients in whom BTKis were the last line before venetoclax was 4.5 months, and 4.7 months for the five who were treated with BTKis at a previous time point. A serum IgM rebound, defined by a ≥ 25% increase in serum IgM between baseline and cycle 2, was observed in only two of the 11 patients in whom BTKis were the last line of therapy before venetoclax. The median time to major response was longer in those with previous BTKi exposure (8.5 and 4.4 months; P < .001); those with ≥ 3 prior lines of therapy (10.8 v 4.8 months; P = .03); and those with refractory versus relapsed disease (8.2 v 4.7 months; P = .007).
Serum IgM levels correlated with decrease in BM disease burden. At 6, 12, and 24 months, the median decrease in serum IgM level was 55%, 69%, and 72% and in BM involvement was 65%, 70%, and 75%, respectively (correlation r = 0.94). Of the nine patients with baseline lymphadenopathy (≥ 1.5 cm), eight (89%) showed decreased adenopathy with follow-up scans, and one was unevaluable. Of the eight patients with baseline splenomegaly (≥ 15 cm), four (50%) showed reduction in spleen size and one (13%) showed stability. Three patients were unevaluable for extramedullary disease response, as venetoclax was discontinued before assessments were performed.
PFS, TTNT, and Overall Survival
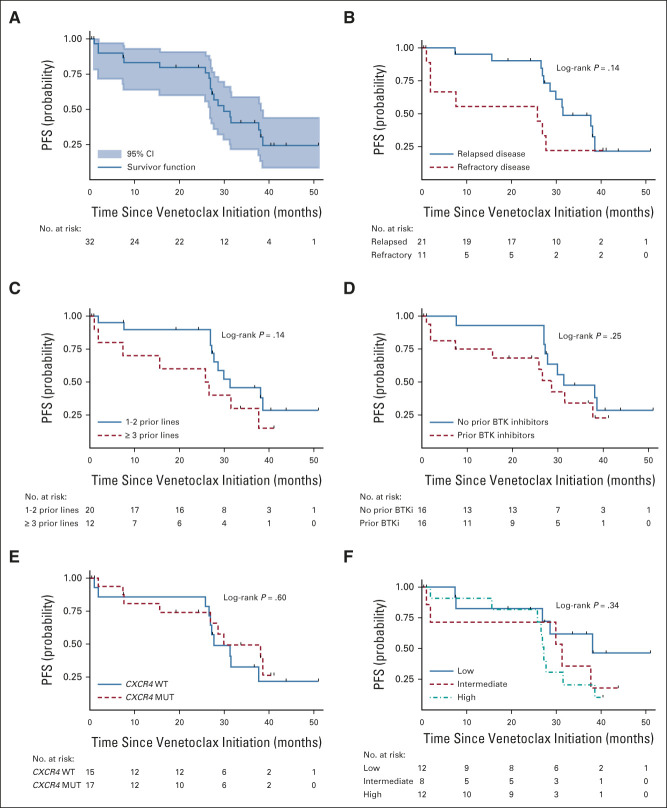
With a median follow-up of 33 months (range, 24-38 months), 19 patients have progressed. The median PFS was 30 months (95% CI, 27 to 38; Fig 3A). The 12- and 24-month PFS rates were 83% (95% CI, 64 to 93) and 80% (95% CI, 60 to 90), respectively. A trend for shorter PFS was observed for those with refractory disease (Fig 3B) and ≥ 3 lines of prior therapy (Fig 3C), but there was no difference in PFS on the basis of prior BTK inhibition (Fig 3D), CXCR4 mutational status (Fig 3E), or International Prognostic Scoring System for Waldenström Macroglobulinemia (Fig 3F). No other factor was associated with a worse PFS.
FIG 3.
Kaplan-Meier curves for PFS for (A) the entire cohort, and according to (B) relapsed or refractory disease, (C) number of previous lines of therapy, (D) prior BTKi exposure, (E) CXCR4 mutational status, and (F) IPSSWM. BTKi, Bruton tyrosine kinase inhibitor; IPSSWM, International Prognostic Scoring System for Waldenström Macroglobulinemia; MUT, mutated; PFS, progression-free survival; WT, wild-type.
Six patients progressed within the first 24 months, and 13 after completion of venetoclax therapy, including 10 between 24 and 36 months, and three after 36 months. Among patients who progressed after completion of 24 months of venetoclax therapy, 10 were in PR and three in VGPR. Attainment of VGPR was not associated with progression after 24 months of treatment completion (P = .65). The median DOR for the 19 patients who attained PR was 34 months (95% CI, 29 to 37) and for the six patients who attained VGPR was 37 months (95% CI, 10 to not evaluable). The DOR for the one patient who attained MR was 9 months.
Twelve patients have started a new therapy after venetoclax. The median TTNT has not been reached. The 30-month rate without subsequent therapy was 76% (57%-88%). The 30-month rate without subsequent therapy was lower for refractory patients than those with relapsed disease at 60% (95% CI, 25 to 83) and 85% (95% CI, 60 to 95), respectively (P = .006). All 32 patients were alive at time of data cutoff, for a 30-month overall survival rate of 100% (95% CI, 89 to 100).
Patient Disposition
Twenty-one patients (66%) completed all scheduled therapy cycles and received a median number of 25.5 cycles (range, 1-26 cycles) of treatment. Eleven patients (34%) did not complete study therapy because of disease progression and initiation of new treatment (n = 6); inability to swallow pills (n = 1); Protocol (online only) noncompliance (n = 1); nonresponse (n = 1); related to medical insurance coverage (n = 1); and persistent pancytopenia (n = 1). Of the 21 patients who completed therapy, 15 continue in active follow-up; five had disease progression during follow-up and new treatment initiated; and one patient completed all follow-ups. Of the 12 patients who started a new therapy during the study course, successor therapies included BTKi (n = 4); alkylators (n = 3), proteasome inhibitor (n = 1), and investigational agent (n = 4). Of the 13 patients who relapsed after 24 months, six required treatment at a median time from progression to next therapy of 6 months (range, 1-15 months). Seven patients have not yet required therapy. All the patients exposed to BTKis have responded.
Drug Administration and Safety
Seven patients (22%) had venetoclax dose reduction related to neutropenia (n = 2), pancytopenia (n = 1), fatigue not otherwise attributable (n = 1), diarrhea (n = 1), multiple grade 2 events (n = 1), and dosing noncompliance, that is, self-reduced (n = 1). Among these patients, four were reduced to 600; two to 400; and one to 300 mg daily. The only factor associated with dose reduction was female sex (70% v 14% in males). Dose reductions were not associated with worse ORR, major response rate, VGPR rate, or PFS (P > .05 in all instances). Venetoclax was discontinued early in one patient because of pancytopenia.
Thirty-one patients (94%) experienced a grade ≥ 2 adverse event (Table 2). The only grade 4 adverse events were neutropenia (n = 6) and febrile neutropenia (n = 1). All seven participants received G-CSF, and all events resolved with a median of 4 days (range, 0-17 days). One participant was dose-reduced twice for neutropenia. One patient, who had generalized lymphadenopathy (3.7 cm), splenomegaly (27 cm), and leukocytosis (59 K/uL), experienced a laboratory tumor lysis syndrome (TLS) that was managed as an inpatient and responded to intravenous fluids and one dose of rasburicase. No paradoxical IgM flare, secondary myeloid neoplasms, aggressive transformation, peripheral neuropathy, cardiac arrhythmia, or clinical TLS were observed on venetoclax therapy. No serum IgM rebound was documented after stopping venetoclax therapy.
TABLE 2.
Grade 2 or Higher Adverse Events Associated With Venetoclax Therapy
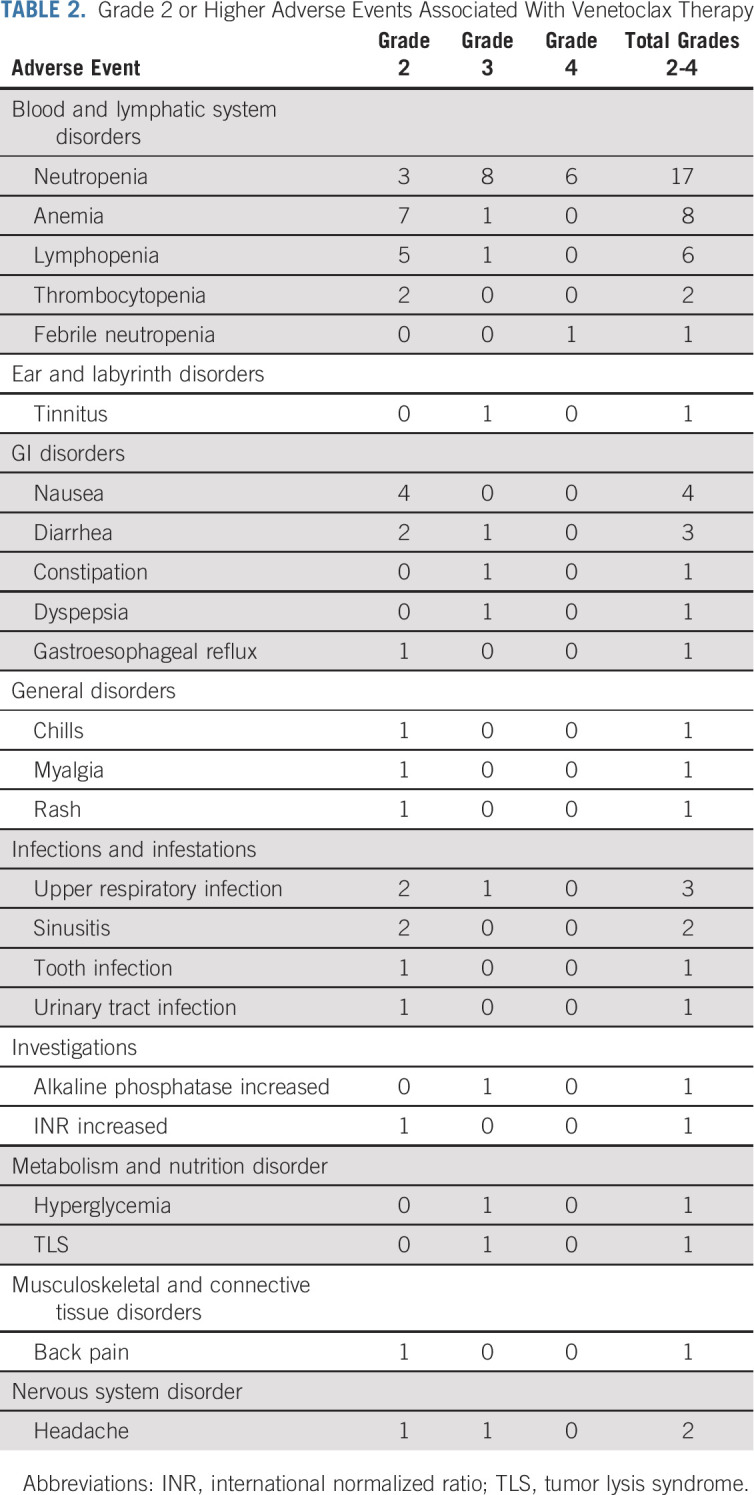
There were 21 temporary venetoclax holds in 14 participants. Reasons for drug holds included neutropenia (n = 6), infections (n = 4), diarrhea (n = 3), and mouth sores, appendicitis, hip fracture, fatigue, stem-cell collection, laboratory TLS, jaw injury, and nausea (n = 1 each). The median duration of the drug hold was 6 days (range, 2-35 days). There was no difference in median serum IgM levels at the start and the end of the drug hold (P = .25).
There was no difference in median serum IgG and immunoglobulin A levels between baseline and end of treatment (P > .05 in both instances).
DISCUSSION
Herein, we present the results of, to our knowledge, this first, prospective phase II study evaluating the oral BCL2 antagonist venetoclax in previously treated WM. We found that venetoclax monotherapy was highly effective in patients with previously treated WM with an ORR of 84%, on the basis of which our study met its end point. Responders included those with refractory disease and those who received ≥ 3 prior therapies, although lower response rates were observed in these patients. Prior BTK exposure affected time to minor and major response attainment but did not affect depth of response or PFS. The delay in response for this patient cohort is unlikely to be fully explained by an IgM rebound following cessation of BTKis,13 as this was observed in only two patients. Conversely, BTKi-promoted selection of malignant clones overexpressing BCL2 family members (eg, BCLXL) has been postulated to contribute to relative resistance associated with venetoclax in patients with CLL and could be etiologic for this observation in patients with WM.14
Important secondary findings were a major response of 81% and the relatively long PFS of 30 months in this population of patients whose median prior therapies was two, and of whom 34% were refractory to previous therapy, including 50% previously exposed to BTKis. These efficacy outcomes compare favorably with non-BTKi regimens in previously treated WM, wherein PFS durations of 8-20 months have been observed.2 Importantly, a rapid decline in PFS between months 24 and 36 was observed in 10 patients who completed the 2-year course of Protocol therapy, suggesting that indefinite continuation of venetoclax may be necessary in patients with WM. Importantly, CXCR4 mutation status did not affect response or PFS to venetoclax, in contrast to experiences with BTKis.10,15 Since MYD88 mutations were detected in all the participants, we were unable to assess the impact of venetoclax on MYD88 wild-type WM patients. Other markers of clinical benefit included improvement in hemoglobin levels and decrease in lymphadenopathy and splenomegaly. Notably, venetoclax led to impressive decreases in BM disease involvement. In comparison to BTKis, venetoclax offers a more robust clearance of BM disease burden.13,16,17
The precise sequencing of BTKis and BCL2 inhibitors in WM remains to be clarified, and further study will be required to address this point. In CLL, resistance to venetoclax post-BTKi exposure has been observed, whereas BTKis appeared effective after venetoclax.18,19 Our results suggested similar levels of efficacy for venetoclax for either BTKi-naive or previously exposed patients, thereby offering a meaningful, active treatment option for those previously exposed to a BTKi.
Neutropenia was the only grade 4 adverse event and was effectively managed with G-CSF. TLS, a major concern in patients with CLL receiving venetoclax,20 was only observed as a laboratory anomaly without clinical manifestations in one patient in this study. A previous study also reported low rates of tumor lysis events in patients with indolent lymphomas treated with venetoclax, comparable with our findings.7 The favorable safety profile of venetoclax provides a platform to study combination regimens in patients with WM. It would be of interest to investigate venetoclax in combination with anti-CD20 monoclonal antibodies in WM, given encouraging experience with this combination in CLL.21
Another promising combination is concurrent use of venetoclax with BTKis as suggested by in vitro WM studies, and ongoing findings from clinical trials examining the combination of ibrutinib and venetoclax in CLL and mantle cell lymphoma.6,22-25 As suggested by these combination studies, concurrent inhibition of BTK and BCL2 could potentially lead to more robust tumor killing and deeper responses including minimal residual disease achievement, a finding that remains elusive for most patients with WM with current pharmacotherapy. Given these observations, a prospective study evaluating ibrutinib and venetoclax in treatment-naive patients with WM has been initiated (NCT04273139).
In conclusion, venetoclax is safe and highly active in patients with previously treated WM, including those previously treated with BTKis. CXCR4 mutation status did not affect treatment response. The optimal duration of venetoclax therapy in WM is yet to be determined.
ACKNOWLEDGMENT
The authors would like to acknowledge the help and effort of their respective clinical research teams and the willingness of the study participants and their families. J.J.C. would like to acknowledge Kathleen Kacena for her help with the statistical analysis.
APPENDIX
FIG A1.
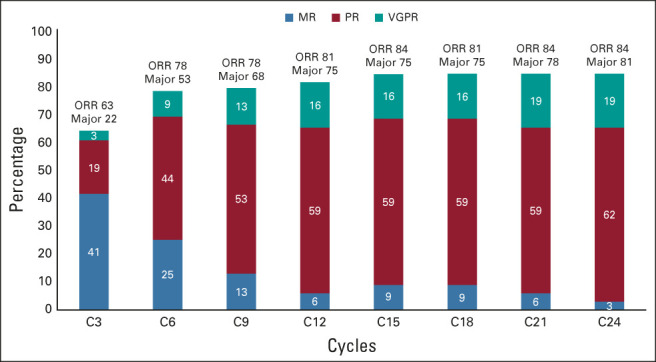
Response kinetics to venetoclax in patients with previously treated WM. MR, minor response; ORR, overall response rate; PR, partial response; VGFR, very good partial response; WM, Waldenström macroglobulinemia.
TABLE A1.
Venetoclax Dose Modification Guidelines
TABLE A2.
Data Categorization for Analysis
Jorge J. Castillo
Consulting or Advisory Role: Janssen, Roche/Genentech, Beigene, AbbVie/Pharmacyclics
Research Funding: Pharmacyclics (Inst), AbbVie (Inst), Janssen (Inst), BeiGene (Inst), TG Therapeutics (Inst)
John N. Allan
Honoraria: AstraZeneca, AbbVie, Pharmacyclics/Janssen, BeiGene
Consulting or Advisory Role: AbbVie/Genentech, Pharmacyclics, Ascentage Pharma, BeiGene, Janssen Oncology, Epizyme, AstraZeneca, TG Therapeutics, ADC Therapeutics
Research Funding: Genentech, Janssen, Celgene, TG Therapeutics
Tanya Siddiqi
Consulting or Advisory Role: Juno Therapeutics, AstraZeneca, BeiGene, Celgene, Pharmacyclics, Bristol Myers Squibb/Celgene
Speakers' Bureau: Pharmacyclics/Janssen, AstraZeneca, BeiGene, Bristol Myers Squibb/Celgene
Research Funding: Juno Therapeutics (Inst), Kite, a Gilead company (Inst), Acerta Pharma (Inst), TG Therapeutics (Inst), BeiGene (Inst), Pharmacyclics (Inst), Celgene (Inst), Oncternal Therapeutics (Inst), Bristol-Myers Squibb/Sanofi (Inst)
Ranjana H. Advani
Consulting or Advisory Role: Genentech/Roche, Seattle Genetics, Takeda, Portola Pharmaceuticals, Celgene, Sanofi, Kura Oncology, Merck, Karyopharm Therapeutics, ADC Therapeutics, Daiichi Sankyo, Bristol Myers Squibb/Celgene, Epizyme, Incyte
Research Funding: Millennium (Inst), Seattle Genetics (Inst), Genentech/Roche (Inst), Pharmacyclics (Inst), Janssen (Inst), Merck (Inst), Kura Oncology (Inst), Forty Seven (Inst), Cyteir (Inst)
Catherine A. Flynn
Consulting or Advisory Role: Pharmacyclics, Karyopharm Therapeutics
Shayna Sarosiek
Research Funding: Acrotech Biopharma (Inst)
Andrew R. Branagan
Consulting or Advisory Role: Pharmacyclics/Janssen, Genzyme, Adaptive Biotechnologies, BeiGene, Karyopharm Therapeutics, CSL Behring
Xia Liu
Employment: Merck (I)
Guang Yang
Employment: AbbVie (I), Blueprint Medicines
Stock and Other Ownership Interests: Blueprint Medicines
Honoraria: Janssen
Matthew S. Davids
Honoraria: Research to Practice
Consulting or Advisory Role: Genentech, Janssen, Pharmacyclics, TG Therapeutics, AbbVie, AstraZeneca, Celgene, MEI Pharma, Adaptive Biotechnologies, Verastem, Ascentage Pharma, BeiGene, Lilly, Novartis, Takeda, Bristol Myers Squibb
Research Funding: Genentech (Inst), TG Therapeutics (Inst), Pharmacyclics (Inst), Surface Oncology (Inst), MEI Pharma (Inst), Verastem (Inst), Ascentage Pharma (Inst), AstraZeneca (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: TG Therapeutics, Janssen, AbbVie, BeiGene, Genentech
Richard R. Furman
Honoraria: Janssen, AstraZeneca, Pharmacyclics, Janssen Biotech, Genentech/Roche, Loxo, TG Therapeutics, Verastem, Acerta Pharma, AstraZeneca, Beigene, Incyte, OncoTracker, AbbVie, MorphoSys, Sanofi
Research Funding: Acerta Pharma, TG Therapeutics
Expert Testimony: AbbVie, Janssen Oncology
Travel, Accommodations, Expenses: TG Therapeutics, Janssen Oncology
Other Relationship: Incyte, Janssen Biotech
Steven P. Treon
Honoraria: Abbvie/Pharmacyclics, Janssen Oncology, BeiGene
Consulting or Advisory Role: Janssen, Pharmacyclics, Beigene, X4 Pharmaceuticals, Bristol Myers Squibb
Research Funding: Pharmacyclics, Bristol Myers Squibb (Inst), X4 Pharmaceuticals (Inst), Lilly (Inst), Beigene (Inst), AbbVie (Inst)
Patents, Royalties, Other Intellectual Property: My institution holds patents related to use of MYD88 and CXCR4 testing for which a predetermined financial distribution to the laboratory and individuals is provided. I have not received any income to this date related to these patents (Inst)
Travel, Accommodations, Expenses: Janssen Oncology
Other Relationship: Janssen, Pharmacyclics, Beigene
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the 23rd Congress of the European Hematology Association, Stockholm, Sweden, June 14-17, 2018; at the 10th International Workshop for Waldenström Macroglobulinemia, New York, NY, October 11-13, 2018; at the 60th Annual Meeting of the American Society of Hematology, San Diego, CA, December 1-4, 2018; and at the 17th International Myeloma Workshop, Boston, MA, September 12-15, 2019.
SUPPORT
AbbVie provided drug and funds for this study.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Deidentified data, study protocol, and informed consent form will be available immediately and up to 3 years from publication. Requests should be made via e-mail to jorgej_castillo@dfci.harvard.edu.
AUTHOR CONTRIBUTIONS
Conception and design: Jorge J. Castillo, Matthew S. Davids, Steven P. Treon
Financial support: Christopher J. Patterson
Administrative support: Kirsten Meid, Carly Leventoff, Timothy P. White, Christopher J. Patterson
Provision of study materials or patients: John N. Allan, Tanya Siddiqi, Ranjana H. Advani, Shayna Sarosiek, Richard R. Furman
Collection and assembly of data: Jorge J. Castillo, John N. Allan, Tanya Siddiqi, Ranjana H. Advani, Kirsten Meid, Carly Leventoff, Timothy P. White, Catherine A. Flynn, Andrew R. Branagan, Maria G. Demos, Maria L. Guerrera, Xia Liu, Manit Munshi, Nicholas Tsakmaklis, Lian Xu, Guang Yang, Christopher J. Patterson, Richard R. Furman, Steven P. Treon
Data analysis and interpretation: Jorge J. Castillo, John N. Allan, Tanya Siddiqi, Kirsten Meid, Shayna Sarosiek, Andrew R. Branagan, Zachary R. Hunter, Matthew S. Davids, Richard R. Furman, Steven P. Treon
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Venetoclax in Previously Treated Waldenström Macroglobulinemia
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jorge J. Castillo
Consulting or Advisory Role: Janssen, Roche/Genentech, Beigene, AbbVie/Pharmacyclics
Research Funding: Pharmacyclics (Inst), AbbVie (Inst), Janssen (Inst), BeiGene (Inst), TG Therapeutics (Inst)
John N. Allan
Honoraria: AstraZeneca, AbbVie, Pharmacyclics/Janssen, BeiGene
Consulting or Advisory Role: AbbVie/Genentech, Pharmacyclics, Ascentage Pharma, BeiGene, Janssen Oncology, Epizyme, AstraZeneca, TG Therapeutics, ADC Therapeutics
Research Funding: Genentech, Janssen, Celgene, TG Therapeutics
Tanya Siddiqi
Consulting or Advisory Role: Juno Therapeutics, AstraZeneca, BeiGene, Celgene, Pharmacyclics, Bristol Myers Squibb/Celgene
Speakers' Bureau: Pharmacyclics/Janssen, AstraZeneca, BeiGene, Bristol Myers Squibb/Celgene
Research Funding: Juno Therapeutics (Inst), Kite, a Gilead company (Inst), Acerta Pharma (Inst), TG Therapeutics (Inst), BeiGene (Inst), Pharmacyclics (Inst), Celgene (Inst), Oncternal Therapeutics (Inst), Bristol-Myers Squibb/Sanofi (Inst)
Ranjana H. Advani
Consulting or Advisory Role: Genentech/Roche, Seattle Genetics, Takeda, Portola Pharmaceuticals, Celgene, Sanofi, Kura Oncology, Merck, Karyopharm Therapeutics, ADC Therapeutics, Daiichi Sankyo, Bristol Myers Squibb/Celgene, Epizyme, Incyte
Research Funding: Millennium (Inst), Seattle Genetics (Inst), Genentech/Roche (Inst), Pharmacyclics (Inst), Janssen (Inst), Merck (Inst), Kura Oncology (Inst), Forty Seven (Inst), Cyteir (Inst)
Catherine A. Flynn
Consulting or Advisory Role: Pharmacyclics, Karyopharm Therapeutics
Shayna Sarosiek
Research Funding: Acrotech Biopharma (Inst)
Andrew R. Branagan
Consulting or Advisory Role: Pharmacyclics/Janssen, Genzyme, Adaptive Biotechnologies, BeiGene, Karyopharm Therapeutics, CSL Behring
Xia Liu
Employment: Merck (I)
Guang Yang
Employment: AbbVie (I), Blueprint Medicines
Stock and Other Ownership Interests: Blueprint Medicines
Honoraria: Janssen
Matthew S. Davids
Honoraria: Research to Practice
Consulting or Advisory Role: Genentech, Janssen, Pharmacyclics, TG Therapeutics, AbbVie, AstraZeneca, Celgene, MEI Pharma, Adaptive Biotechnologies, Verastem, Ascentage Pharma, BeiGene, Lilly, Novartis, Takeda, Bristol Myers Squibb
Research Funding: Genentech (Inst), TG Therapeutics (Inst), Pharmacyclics (Inst), Surface Oncology (Inst), MEI Pharma (Inst), Verastem (Inst), Ascentage Pharma (Inst), AstraZeneca (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: TG Therapeutics, Janssen, AbbVie, BeiGene, Genentech
Richard R. Furman
Honoraria: Janssen, AstraZeneca, Pharmacyclics, Janssen Biotech, Genentech/Roche, Loxo, TG Therapeutics, Verastem, Acerta Pharma, AstraZeneca, Beigene, Incyte, OncoTracker, AbbVie, MorphoSys, Sanofi
Research Funding: Acerta Pharma, TG Therapeutics
Expert Testimony: AbbVie, Janssen Oncology
Travel, Accommodations, Expenses: TG Therapeutics, Janssen Oncology
Other Relationship: Incyte, Janssen Biotech
Steven P. Treon
Honoraria: Abbvie/Pharmacyclics, Janssen Oncology, BeiGene
Consulting or Advisory Role: Janssen, Pharmacyclics, Beigene, X4 Pharmaceuticals, Bristol Myers Squibb
Research Funding: Pharmacyclics, Bristol Myers Squibb (Inst), X4 Pharmaceuticals (Inst), Lilly (Inst), Beigene (Inst), AbbVie (Inst)
Patents, Royalties, Other Intellectual Property: My institution holds patents related to use of MYD88 and CXCR4 testing for which a predetermined financial distribution to the laboratory and individuals is provided. I have not received any income to this date related to these patents (Inst)
Travel, Accommodations, Expenses: Janssen Oncology
Other Relationship: Janssen, Pharmacyclics, Beigene
No other potential conflicts of interest were reported.
REFERENCES
- 1.Owen RG, Treon SP, Al-Katib A, et al. : Clinicopathological definition of Waldenstrom's macroglobulinemia: Consensus panel recommendations from the Second International Workshop on Waldenstrom's Macroglobulinemia. Semin Oncol 30:110-115, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Castillo JJ, Advani RH, Branagan AR, et al. : Consensus treatment recommendations from the tenth International Workshop for Waldenstrom Macroglobulinaemia. Lancet Haematol 7:e827-e837, 2020 [DOI] [PubMed] [Google Scholar]
- 3.Hunter ZR, Xu L, Yang G, et al. : The genomic landscape of Waldenstrom macroglobulinemia is characterized by highly recurring MYD88 and WHIM-like CXCR4 mutations, and small somatic deletions associated with B-cell lymphomagenesis. Blood 123:1637-1646, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Treon SP, Xu L, Yang G, et al. : MYD88 L265P somatic mutation in Waldenstrom's macroglobulinemia. N Engl J Med 367:826-833, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Treon SP, Xu L, Guerrera ML, et al. : Genomic landscape of Waldenstrom macroglobulinemia and its impact on treatment strategies. J Clin Oncol 38:1198-1208, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao Y, Yang G, Hunter ZR, et al. : The BCL2 antagonist ABT-199 triggers apoptosis, and augments ibrutinib and idelalisib mediated cytotoxicity in CXCR4 Wild-type and CXCR4 WHIM mutated Waldenstrom macroglobulinaemia cells. Br J Haematol 170:134-138, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Davids MS, Roberts AW, Seymour JF, et al. : Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol 35:826-833, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyle RA, Treon SP, Alexanian R, et al. : Prognostic markers and criteria to initiate therapy in Waldenstrom's macroglobulinemia: Consensus panel recommendations from the Second international Workshop on Waldenstrom's Macroglobulinemia. Semin Oncol 30:116-120, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Owen RG, Kyle RA, Stone MJ, et al. : Response assessment in Waldenstrom macroglobulinaemia: Update from the VIth International Workshop. Br J Haematol 160:171-176, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Treon SP, Meid K, Gustine J, et al. : Long-term follow-up of ibrutinib monotherapy in symptomatic, previously treated patients with Waldenstrom macroglobulinemia. J Clin Oncol 39:565-575, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu L, Hunter ZR, Yang G, et al. : MYD88 L265P in Waldenstrom macroglobulinemia, immunoglobulin M monoclonal gammopathy, and other B-cell lymphoproliferative disorders using conventional and quantitative allele-specific polymerase chain reaction. Blood 121:2051-2058, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu L, Hunter ZR, Tsakmaklis N, et al. : Clonal architecture of CXCR4 WHIM-like mutations in Waldenstrom macroglobulinaemia. Br J Haematol 172:735-744, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Treon SP, Tripsas CK, Meid K, et al. : Ibrutinib in previously treated Waldenstrom's macroglobulinemia. N Engl J Med 372:1430-1440, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Haselager MV, Kielbassa K, Ter Burg J, et al. : Changes in Bcl-2 members after ibrutinib or venetoclax uncover functional hierarchy in determining resistance to venetoclax in CLL. Blood 136:2918-2926, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Castillo JJ, Xu L, Gustine JN, et al. : CXCR4 mutation subtypes impact response and survival outcomes in patients with Waldenstrom macroglobulinaemia treated with ibrutinib. Br J Haematol 187:356-363, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Dimopoulos MA, Trotman J, Tedeschi A, et al. : Ibrutinib for patients with rituximab-refractory Waldenstrom's macroglobulinaemia (iNNOVATE): An open-label substudy of an international, multicentre, phase 3 trial. Lancet Oncol 18:241-250, 2017 [DOI] [PubMed] [Google Scholar]
- 17.Treon SP, Gustine J, Meid K, et al. : Ibrutinib monotherapy in symptomatic, treatment-naive patients with Waldenstrom macroglobulinemia. J Clin Oncol 36:2755-2761, 2018 [DOI] [PubMed] [Google Scholar]
- 18.Roberts AW, Ma S, Kipps TJ, et al. : Efficacy of venetoclax in relapsed chronic lymphocytic leukemia is influenced by disease and response variables. Blood 134:111-122, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mato AR, Roeker LE, Jacobs R, et al. : Assessment of the efficacy of therapies following venetoclax discontinuation in CLL reveals BTK inhibition as an effective strategy. Clin Cancer Res 26:3589-3596, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davids MS, Hallek M, Wierda W, et al. : Comprehensive safety analysis of venetoclax monotherapy for patients with relapsed/refractory chronic lymphocytic leukemia. Clin Cancer Res 24:4371-4379, 2018 [DOI] [PubMed] [Google Scholar]
- 21.Seymour JF, Kipps TJ, Eichhorst B, et al. : Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med 378:1107-1120, 2018 [DOI] [PubMed] [Google Scholar]
- 22.Kuo HP, Ezell SA, Schweighofer KJ, et al. : Combination of ibrutinib and ABT-199 in diffuse large B-cell lymphoma and follicular lymphoma. Mol Cancer Ther 16:1246-1256, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Zhao X, Bodo J, Sun D, et al. : Combination of ibrutinib with ABT-199: Synergistic effects on proliferation inhibition and apoptosis in mantle cell lymphoma cells through perturbation of BTK, AKT and BCL2 pathways. Br J Haematol 168:765-768, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Jain N, Keating M, Thompson P, et al. : Ibrutinib and venetoclax for first-line treatment of CLL. N Engl J Med 380:2095-2103, 2019 [DOI] [PubMed] [Google Scholar]
- 25.Tam CS, Anderson MA, Pott C, et al. : Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N Engl J Med 378:1211-1223, 2018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified data, study protocol, and informed consent form will be available immediately and up to 3 years from publication. Requests should be made via e-mail to jorgej_castillo@dfci.harvard.edu.