Abstract
The subgranular zone of the dentate gyrus provides a local microenvironment (niche) for neural stem cells. In the adult brain, it has been established that the vascular compartment of such niches has a significant role in regulating adult hippocampal neurogenesis. More recently, evidence showed that neurovascular coupling, the relationship between blood flow and neuronal activity, also regulated hippocampal neurogenesis. Here, we review the most recent articles on addressing the intricate relationship between neurovasculature and adult hippocampal neurogenesis and a novel pathway where functional hyperemia enhances hippocampal neurogenesis. In the end, we have further reviewed recent research showing that impaired neurovascular coupling may cause declined neurogenesis and contribute to brain damage in neurodegenerative diseases.
Keywords: Hippocampus, Subgranular Zone, Adult Hippocampal Neurogenesis, Neurovasculature, Blood Brain Barrier, Neurovascular Coupling
Introduction and Goal of this Review
In the late 1800s and early 1900s, it was generally accepted that neurons in the brain do not have the capacity to regenerate in the adult brain. The birth of the “neuron doctrine” from Cajal’s seminal work and his claim that cells cannot regenerate allowed this idea to settle down as the central dogma of neurobiology [17]. However, in the 1960s, the first idea of adult brain neurogenesis was suggested in adult rats [3], followed by concrete evidence in songbirds [39, 103], changing the dogma that neurons are nonregenerative. Subsequent studies have solidified this idea and have provided many advancements in the field of adult neurogenesis, including evidence that it occurs in the human brain [29, 129].
Adult neurogenesis occurs in specialized microenvironments (niches), in which neural stem cells are regulated and maintained. Among the many components of these niches, the vasculature is becoming important as it has been shown to be crucial in regulating adult neural stem cells [68]. Neurotrophic and metabolic factors produced in the brain or delivered by blood flow have been found to regulate adult neurogenesis throughout its steps. This suggests that angiogenesis [99] and neurovascular coupling [122], a term used to explain the relationship between neuronal activity and local blood flow, are also correlated with adult neurogenesis. Moreover, decline or dysfunction of adult neurogenesis has been implicated in neurodegenerative diseases. Despite our extensive efforts to find cures for neurodegenerative diseases, we have continuously failed to make a breakthrough. Therefore, the focus has shifted to exploring the disruption of neurovasculature, blood flow and neurogenesis in the context of neurodegeneration and aging.
In this review, we first summarize the relationship between adult hippocampal neurogenesis, occurring in the subgranular zone of the dentate gyrus, and the surrounding vasculature. Next, we discuss the signaling pathways of neurovascular coupling and outline the role of functional hyperemia in regulating adult hippocampal neurogenesis. Finally, we review recent studies that have implicated neurovascular dysfunction from a clinical point of view, especially Alzheimer’s disease.
1. Neurogenesis Niche and Key Regulators
Neurogenesis, the process of generating newborn neurons, has mainly been understood and studied in the perspective of embryonic and perinatal developmental stages of mammals [83]. However, it was only recently that it was accepted that neurogenesis continues into adulthood in certain areas of the brain [29, 39, 82, 103, 129]. The subgranular zone (SGZ) of the hippocampal dentate gyrus (DG) is one of the most prominent areas where adult neurogenesis occurs. The SGZ is a thin layer located between the granule cell layer and hilus and provides a unique microenvironment for neural stem cells to proliferate into new dentate granule cells (Fig. 1A) [40]. Although we have become more knowledgeable of the signaling pathways of the SGZ niche (see Goncalves, 2016 [40] for detailed review on the different signaling pathways) and environmental conditions (e.g. exercise, enriched environment) involved in regulating the neural stem cell (NSC) niche, the mechanisms that regulate adult hippocampal neurogenesis (AHN) have not been fully elucidated. Nevertheless, extensive studies have provided insight on the different neurotrophic factors in the SGZ niche that affect neurogenesis.
Fig. 1. Neurogenesis in the SGZ.
(A) A general outline of the different layers of the dentate gyrus of the hippocampus. The most outer molecular layer is labeled in yellow, the granule cell layer is depicted by the gray cells, the subgranular zone is labeled with blue, and finally the innermost hilus. (B) A simplified version of the steps of adult hippocampal neurogenesis in association with different neurotrophic factors.
BDNF: brain-derived neurotrophic factor; IGF-1: insulin-like growth factor 1; FGF-2: fibroblast growth factor-2; VEGF: vascular endothelial growth factor.
A variety of extracellular proteins are known to take part in regulating and maintaining the development of newborn cells in adult neurogenesis. Neurotrophic factors and growth factors such as brain-derived neurotrophic factor (BDNF), insulin-like growth factor 1 (IGF-1), basic fibroblast growth factor (FGF-2) and vascular endothelial growth factor (VEGF) have been studied in the context of hippocampal neurogenesis (Fig. 1B). BDNF mediates the maturation of dendritic growth of adult-born cells through autocrine and angiocrine signaling and also functions as regenerative cues for NSCs [110, 141]. Therefore, these functions show that BDNF may influence proliferation [56, 65, 66] of neural progenitor cells and survival [65, 118] of newborn neurons in the DG. However, one study challenged BDNF’s role in proliferation as a decrease in BDNF actually caused an increase in proliferation [118]. Nevertheless, BDNF’s role in increasing survival has been consistent across studies. IGF-1 and FGF-2 also play a crucial role in AHN. IGF-1 supports neural progenitor cell proliferation and survival during embryonic development [44, 91], and continues to provide a wide range of action, including neuroprotection [74], in adulthood. Based on these functions, one can speculate that IGF-1 promotes the proliferation [1, 21, 85, 125] and survival [1, 21, 136] stages of adult neurogenesis. Interestingly, IGF-1 also facilitates the differentiation [1, 18, 21, 85] of progenitor cells, especially through the PI3K-Akt signaling pathway [85, 104, 155]. FGF-2 is identified as a factor that maintains the NSC population [54] and is highly expressed in areas of adult neurogenesis [109]. Therefore, similar to IGF-1, FGF-2 also influences the proliferation [125, 151, 156], differentiation [144, 151, 156] and survival [111, 156] stages of AHN. Finally, there is conflicting evidence for whether VEGF potentiates the proliferation stage [120, 142, 147, 150] or the survival stage [64, 119, 133]. However, this may be due to the fact that the release of VEGF is regulated by BDNF [27], which has been shown to have an effect on both proliferation and survival [75]. It is worth noting though that the variability in results may be due to differences in different animal species (e.g., mouse, rat) or the context it is being explored (e.g., type of injury or disease, age, pharmacological interventions). In the end, however, all of these factors come together to enhance AHN despite some discrepancies between studies.
More recent studies have shown that glial cell derived factors have an influence on hippocampal neurogenesis as well. BDNF, FGF-2, glial cell line-derived neurotrophic factor (GDNF), D-serine, ATP, and lactate are released from astrocytes, while BDNF and IGF-1 are released from microglia, increasing neurogenesis. On the other hand, interferon-γ (IFN-γ) from microglia was found to decrease neurogenesis [7].
2. Neurovasculature and Adult Hippocampal Neurogenesis
A common feature of the biomolecules discussed in the section above is that they are all vasculature-related factors. This brings up the question whether the vasculature of the brain can regulate adult neurogenesis and if so, what the key modulators are. Sure enough, recent evidence suggests that VEGF secreted from the SGZ niche and angiogenesis plays a crucial role in regulating adult neurogenesis [38, 68, 135]. In this section, we will review the vascular architecture of the brain and how it is intertwined with adult hippocampal neurogenesis.
2.1. General overview of Vasculature in the brain
The brain is considered to be one of the most highly perfused organs of the body. Therefore, it is not surprising that the formation and architecture of brain vessels are complex. The brain starts forming its vascular system by a process called extracerebral vascularization, which is when the perineural vascular plexus is formed. This is followed by capillaries branching out from the plexus through the process of intracerebral vascularization [107]. The general architecture of the brain arterial system can be summarized into four components. Pial arteries are located on the surface of the brain within the subarachnoid space [52, 152], followed by penetrating arterioles, intraparenchymal arterioles and finally, the smallest capillaries [47]. As with any blood vessel in the body, all four types of cerebral blood vessels have a single layer of endothelial cells, forming the endothelium [47]. In addition, the larger blood vessels may have an additional layer of smooth muscle cells (SMCs) positioned outside of the endothelium [115], and these SMCs are well known for mediating the dilation and constriction of blood vessels [12, 137]. Although there is much variation between species, the general rule is that the larger the blood vessel is, the more layers of SMCs it will have [80, 115]. On the other hand, the smaller capillaries are known to have pericytes, instead of SMCs, outside the endothelial cells. The role of pericytes in mediating capillary diameter has been controversial but recent evidence have suggested that its role is similar to that of SMCs in bigger blood vessels [33, 105, 145]. In addition, neural progenitor cells in the central nervous system secrete VEGF-A, which recruits endothelial cells and pericytes. Therefore, angiogenesis is highly likely to occur parallel to neurogenesis, and eventually the neurovasculature could spatially match the complicated neuronal network [49].
One unique property of the brain’s vasculature is the Blood-Brain-Barrier (BBB). The BBB consists of specialized endothelial cells, which have continuous intercellular tight junction, low level of transcytosis and high level of specific transporters (e.g. Glucose transporter 1). This tightly sealed barrier ensures homeostasis of the nervous system, allowing intake of nutrition and preventing the entry of toxins (see Obermeier, 2013 [94] for detailed review on BBB). The BBB is one of the most important part of brain vasculature and abnormalities have been found to be associated with a variety of pathological conditions such as Alzheimer’s disease, stroke, epilepsy and neuroinflammation [16, 53, 88, 121, 138]. According to previous studies, induction, maturation, and maintenance of the BBB require signaling pathways similar to those utilized in neurogenesis. Wnt, Sonic Hedgehog (SHH), GDNF and FGF-2 are all the factors that are reported to support neurogenesis and BBB formation or maintenance [5, 14, 40, 48, 126, 132]. Moreover, BBB function has been found to be modulated by neural activity through numerous factors from neurons, astrocytes, and pericytes (see Kaplan, 2020 [55] for a comprehensive review on neuronal regulation of the BBB). More specifically, neuronal activity has been shown to have a role in barrier permeability [93]. This is important as trafficking of some factors such as IGF1 may be affected [93], which would have an effect on neurogenesis. Therefore, development and maintenance of the BBB, which usually accompany angiogenesis, is likely to be related to neurogenesis.
Despite our growing knowledge of the neurovascular system and hippocampal neurogenesis, there is a gap when trying to understand the vasculature of the hippocampus. Especially with the SGZ of the DG as an important niche for neurogenesis, it is highly likely that the vascular pattern differs in the hippocampus from other regions of the brain. As expected, recent efforts to map the vasculature of the brain have revealed that there is a unique vascular network in the DG. Starting in the molecular layer, capillaries show sudden change in their diameter and branching angle, which leads to the creation of a unique comb-like pattern [154]. Then, the capillaries perpendicularly enter the granule cell layer of the DG. Some continue down into the hilus, crossing the SGZ, while others change direction and start to run parallel to the SGZ [68]. This structural architecture provides ample opportunity for the neurovasculature to interact with NSCs in the SGZ. However, the hippocampus was seen to have a lower mean diameter, volume fraction and length density, meaning it is more vulnerable to decreased blood supply [154]. With further characterization of the vascular distribution and pattern in the DG, we hope to gain more insight into how the vasculature and neurogenesis is coupled.
2.2. Vasculature in a Stem Cell Niche
Among the many factors existing within the stem cell niche of adult NSCs, the microvasculature of the brain has become of increasing importance. The vasculature in the brain provides energy and helps maintain homeostasis. Blood flow will deliver oxygen and glucose to brain tissues, while removing CO2 and extra heat for homeostasis [11, 58, 59]. In addition to its metabolic function, blood flow in the brain also assists in the delivery of brain-derived molecules and passes peripheral signals to the brain [1, 22, 23, 113]. As seen above, many of the soluble factors regulating adult neurogenesis were vasculature-related factors. Then, does microvasculature in a stem cell niche have a role in regulating neurogenesis? This idea was first confirmed with the observation that cells labeled with Bromodeoxyuridine (BrdU), a compound used to identify proliferating cells, were clustered proximal to capillaries [99]. Thereafter, the coupling of angiogenesis and neurogenesis was examined in animal models and was first observed in songbirds [75] followed by confirmation in rodents and mammals [15, 62, 106].
Since then, multiple studies have put in the effort to demonstrate the functional relationship between the vasculature in the stem cell niche and neurogenesis. One study eloquently showed that soluble factors released from vascular endothelial cells were capable of stimulating self-renewal and neuron production [123]. Subsequent studies also suggested that NSCs were tightly associated with blood vessels, sometimes in close proximity or even through direct contact mediated by a specific integrin-laminin interaction [124, 134]. Through these efforts, it became clear that the NSC niche vasculature is integral to adult neurogenesis and serves as a conduit for signaling as well as enabling NSCs to maintain a close proximity to proliferative and survival signals.
2.3. Angiogenesis and Adult Hippocampal Neurogenesis
After the confirmation of coupling between angiogenesis and adult neurogenesis [75, 99], subsequent studies further elucidated that AHN induced by physical exercise is always accompanied by an increase of microvascular density or an increase of cerebral blood volume in the dentate gyrus [15, 106]. These findings indicated the possibility of signal pathways between angiogenesis and AHN.
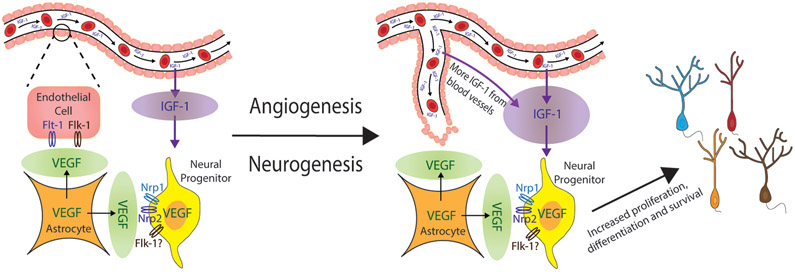
Over the past few years, VEGFs have been the main focus as the potential mediator between angiogenesis and AHN because VEGF is shown to contribute to both processes [31, 34, 51]. Specifically in the DG, in vivo studies showed that VEGF is mainly produced and expressed by astrocytes [67], while in vitro studies showed VEGF expression in cultured hippocampal neural progenitors [31], with confirmation from a recent in vivo study [108]. In addition, expression of the VEGF receptors VEGF-R1/Flt-1 and VEGF-R2/Flk-1 were localized to endothelial cells [67], while the coreceptors of VEGF, neuropilin-1 (Nrp1) and neuropilin-2 (Nrp2), were expressed on hippocampal granule cells and neural progenitors [31, 67]. Some studies also suggested expression of Flk-1 in neural progenitors [31, 51]. Therefore, one could expect to see enhanced angiogenesis and AHN with overexpression of VEGF in the DG [67]. However, a different group also revealed that peripheral VEGF in the DG is essential for physical exercise induced AHN but not for basal AHN [31]. Put together, these lines of evidence suggest that VEGF is sufficient for inducing adult hippocampal neurogenesis but is not necessary at all conditions. Moreover, it is likely that the increase in microvascular density will alter the microenvironment of the neurogenic niche by delivering more metabolic factors (e.g. insulin-like growth factor-1), ultimately leading to enhanced AHN [136] (Fig. 2).
Fig. 2. Coupling of Angiogenesis and Adult Hippocampal Neurogenesis.
A visual schematic of how angiogenesis and neurogenesis is coupled. VEGF is expressed in astrocytes and neural progenitors, while VEGF receptors (Flt-1, Flk-1) are expressed on endothelial cells and its coreceptors (Nrp1, Nrp2) are on neural progenitors. Proliferation and recruitment of endothelial cells allows for angiogenesis leading to more IGF-1 delivery through blood vessels. Both IGF-1 and VEGF ultimately increase adult hippocampal neurogenesis.
VEGF: vascular endothelial growth factor; Flt-1: VEGF-R1; Flk-1: VEGF-R2; Nrp1: neuropilin-1; Nrp2: neuropilin-2; IGF-1: insulin-like growth factor 1.
On the other hand, overexpression of VEGF has been reported to disrupt the BBB, especially in pathological conditions such as epilepsy, traumatic brain injury and multiple sclerosis [8, 130, 146]. Specifically, epilepsy has been reported to be accompanied by an increasing expression of VEGF and its receptors in the temporal cortex [19], causing higher permeability and breakdown of the BBB. The mechanism of VEGF-mediated BBB breakdown is thought the be either through endothelial cells or matrix metalloproteinases [9, 130, 131]. VEGF overexpression in epilepsy has also been found in the CA3 region of the hippocampus, which led to BBB disruption [96, 148]. Although not fully elucidated, an epilepsy model showed damaged BBB in the dentate gyrus, which could potentially be a result of VEGF overexpression in the DG [78, 96]. Moreover, breakdown of BBB in this brain region might be related to cognitive dysfunction [87].
Interestingly, in post-ischemia models, increased expression of VEGF in the SGZ and SVZ enhanced the migration and proliferation of neural stem cells, thus enhancing neurogenesis [42, 50, 117]. Studies also showed that overexpression of VEGF attenuated the decline in hippocampal neurogenesis of aging animals and enhanced neurovascular coupling [70]. They also argued that their VEGF overexpression protocol did not induce BBB disruption [61, 70]. This discrepancy on the effect of VEGF overexpression, however, may be due to different experimental settings. Studies that reported BBB disruption were performed in either animal disease models or cell culture [9, 131, 146], while experiments from Licht’s lab mainly used transgenic VEGF overexpression onset in in vivo animal models. This difference in in vivo and in vitro models could have led different results. Moreover, in Litch’s earlier studies [61, 70], they treated animals with VEGF overexpression for no more than 1 month. This provides a definitive contrast to disease models, in which animals are exposed to VEGF overexpression for much longer periods of time. Thus, they themselves proved that if animals had longer VEGF overexpression, mature neurons were dramatically lost, although neurogenesis was still increased in the dentate gyrus [69]. This provides an explanation for why long-term VEGF overexpression in disease models is harmful to SGZ function.
3. Functional Neurovascular Coupling and Adult Hippocampal Neurogenesis
As the relationship between vasculature and AHN becomes defined, the role of metabolic factors is introduced. As these factors travel through blood, cerebral blood flow become more important. In other words, what are the key regulators of cerebral blood flow? The concept of the neurovascular unit (NVU) was first introduced in 2001, which called for interest in the relationship between the brain and its vasculature. One of the most intricate relationship within the NVU is the correlation between blood flow and neuronal activity, which is termed neurovascular coupling. Studies have shown that blood flow velocity is correlated with AHN during physical exercise [106] and more recent research has elucidated that neurovascular coupling is necessary for AHN in a novel environment [122]. Therefore, the signals flowing between neurons, blood vessels and neural stem cells could mediate the vascular-neurogenesis coupling discussed above. In this section we aim to briefly introduce the signal pathways in neurovascular coupling and introduce the pathway between neurovascular coupling and AHN.
3.1. Signaling Pathways of Neurovascular Coupling
The signaling pathways of neurovascular coupling are largely divided into feedback and feedforward schemes [11]. In the feedback pathways, neuronal activity leads to changes in environmental factors (e.g. oxygen concentration, glucose concentration, pH or other metabolic products). These environmental changes will activate the dilation or constriction of blood vessels and regulate blood flow velocity. This is a metabolic demand-driving process. However, the feedback mechanism was not sufficient to explain neurovascular coupling to its full extent [41, 71, 84, 140]. Fortunately, the discovery of neurotransmitter-mediated signaling was able to provide us with an alternative perspective. Neurotransmitter-mediated neurovascular coupling is the feedforward pathway, where neurons directly send signals to blood vessels or indirectly send signals for vascular regulation via astrocytes. The feedforward process largely depends on neurotransmitter systems, such as glutamate, acetylcholine or neuromodulatory signals (e.g. dopamine or serotonin) [24, 25, 47, 60, 63, 95, 114]. Among these neurotransmitters, the most well-established system is the glutamate-mediated pathway. Glutamate activates neuronal N-Methyl-D-aspartic acid (NMDA) receptors and leads to the activation of neuronal nitric oxide synthase (nNOS) [112, 149]. These activated neurons will then release nitric oxide (NO), which mediates the dilation of blood vessels (Fig. 3A) [20]. Glutamate can also regulate neurovascular coupling through astrocytes by binding to metabotropic glutamate receptors (mGluRs) [98]. Downstream of mGluRs will be the production of vasoactive substances such as arachidonic acid, prostaglandin and epoxyeicosatrienoic acid (Fig. 3A) [11]. Therefore, increasing neuronal activity will usually lead to increased local blood flow, which is called functional hyperemia.
Fig. 3. Neurovascular coupling is coupled to Adult Hippocampal Neurogenesis.
(A) A schematic of the feedforward signaling pathways of neurovascular coupling. (B) A schematic of how PV/nNOS interneurons mediate functional hyperemia through neurovascular coupling. This is followed by increased IGF-1, which increases the proliferation, differentiation and survival of neural precursors, enhancing adult hippocampal neurogenesis.
NMDAR: N-methyl-D-aspartate receptor); mGluR: metabotropic glutamate receptor; nNOS: Nitric oxide synthase 1 or neuronal Nitric oxide synthase; NO: Nitric oxide; AA: arachidonic acid; PGE2: Prostaglandin E2; EET: epoxyeicosatrienoic acid; PV: Parvalbumin; GABA: γ-aminobutyric acid; IGF-1: insulin-like growth factor 1.
Functional hyperemia can provide more oxygen and glucose to the brain tissue. In addition to serving as a source of energy, blood flow in the brain also delivers signal molecules. Important signal molecules such as IGF-I [73, 113] and VEGF can be perfused in the brain at a higher rate. Interestingly, these signal molecules match the factors mentioned in our neurogenesis niche and key regulators section. Therefore, this indicates the potential mechanism for neurovascular-neurogenesis coupling.
3.2. Coupling of Functional Hyperemia and Adult Hippocampal Neurogenesis
In AHN, it has been well established that there is a feedforward pathway of glutamatergic and GABAergic input from neuronal afferents, which provides signals to induce differentiation and maturation of neural stem cells and immature neurons [40, 127]. These feedforward pathways are driven by mature neuronal activity, induced by conditions that can stimulate neurogenesis (e.g. enriched environment, physical exercises, specific emotional or physiological condition). However, having seen a potential relationship between neurovascular coupling and AHN, we propose the possibility of a novel feedforward pathway where glutamatergic input from neuronal afferents induce neurovascular coupling in a neurovascular niche, which can change the microenvironment and enhance neurogenesis. Such a pathway has recently been outlined [122].
Previous studies have proved that vasculature-derived IGF-1 can mediate neurogenesis [1, 92]. A recent study [122] builds a more complete signaling pathway between mature neurons, blood vessels and newborn neurons in the dentate gyrus. In the setting of an enriched environment, mature granule cells of the DG will be stimulated causing release of glutamate. This activates a population of interneurons that co-express parvalbumin (PV) and neuronal nitric oxide synthase (nNOS), which were found to be located proximal to blood vessels. The PV/nNOS positive interneurons release nitric oxide (NO) which diffuses to blood vessels [76] and induces functional hyperemia. Ultimately, the increased local blood flow will deliver increased levels of IGF-1 to the neurogenesis niche and enhance neurogenesis.
In addition to mediating neurovascular coupling through the nNOS pathway, PV interneurons are also known to regulate AHN by directly forming GABAergic synapses on neural progenitors [4, 36, 127, 128]. Together, these findings define the feedforward pathways involving interneurons, neurovascular coupling and AHN. Figure 3B summarizes these pathways, illustrating how the glutamatergic signals from mature granule cells and GABAergic signals from interneurons regulate neural precursors in association with blood vessel-derived growth factors.
3.3. Neurovascular coupling regulating Neurogenesis in Alzheimer’s disease
As the relationship between functional neurovascular coupling and AHN is gradually explored in the adult brain, our interest also shifts toward how this relationship is altered with aging or pathological conditions. As our population continues to age, the prevalence of dementia is increasing, and the burden of healthcare costs is becoming a significant problem to the current society [10]. Alzheimer disease (AD) is the leading cause of dementia and is one of the most devastating neurodegenerative diseases [10]. However, despite extensive amounts of effort, we have continuously failed to find a definitive cure for AD. With increasing evidence that neurovascular dysfunction is observed in AD, disruption of blood flow [28, 32, 46, 77, 116], damage to the blood-brain-barrier [13, 72, 81, 90, 153] and the potential impairment of adult neurogenesis [89, 97, 143, 157] are being highlighted.
Brain changes that are well known in AD include accumulation of amyloid β (Aβ) plaques and intracellular neurofibrillary tangles made of hyperphosphorylated tau-protein, atrophy of the brain, especially the hippocampus, and chronic inflammation [10]. Not surprisingly, it has also been found that cerebral blood flow is significantly decreased in AD [32, 46, 116] and neurovascular coupling is impaired [47]. Cerebral blood flow is essential for brain energy homeostasis and decreased cerebral blood flow is thought to reflect neuronal dysfunction and synaptic failure [57]. However, the factors mediating this change in cerebral blood flow in AD are not fully understood.
As the pathogenesis of AD has been attributed to Aβ and hyperphosphorylated tau accumulation, one could speculate that neurovascular alterations may occur with increasing amounts of these pathogenic factors. Sure enough, recent research has shown that both tau [101] and Aβ [102] attenuates blood flow and disrupts neurovascular coupling in mice. Moreover, it has been suggested that Aβ plaques can cause loss of hippocampal interneurons [37] and tau can accumulate in GABAergic interneurons [157], causing deficits in adult hippocampal neurogenesis. Interestingly, PVIs have been identified as one of the interneurons to be impaired in both human AD studies [6] and AD mouse models [43, 45, 79]. Neuronal loss [2, 37], intrinsic electrical properties [79, 139], hypersynchrony [100], and altered neural network activity [100, 139] have been reported for PVIs in various regions of the brain. In addition, astrocytes, which are known to have a role in neurovascular coupling, is known to be activated in AD [86]. Cytokines from astrocytes, including interleukin (IL) −1β, IL-6, and IL-17, seem to cause neuronal death and impair neurogenesis [35] with the help of apolipoprotein E4 (ApoE4) from astrocytes, which prevents synaptogenesis and inhibits dendritic formation of newborn neurons [7]. These deficits allow us to continuously question what role neurovascular coupling dysfunction has in AD.
4. Conclusion and perspectives
In this review, we first summarized the different neurotrophic factors that mediate adult hippocampal neurogenesis. We then examined the structure and architecture of the neurovasculature in the brain and the dentate gyrus, followed by the relationship between angiogenesis and AHN. These studies supported the idea that neurovasculature plays an important role in regulating AHN. Finally, we introduced the concept of neurovascular coupling and illustrated how it relates to AHN. However, it has not been elucidated whether there are additional modulators and regulators along the pathway, nor has it been explored whether different blood-born factors are involved. Nonetheless, this provides the first step in understanding AHN from a new perspective and opens the door to investigating neurodegenerative diseases from a different point of view.
One point to note is the limitation of imaging blood flow at the capillary level. There have been many efforts to image the vasculature of the brain. However, it has always been a challenge to image microvasculature in deeper structures such as the DG. A novel method to record microvasculature in the dentate gyrus was illustrated in Shen’s 2019 paper [122]. Nevertheless, the invasive nature of surgical procedures may produce drawbacks. The development of new imaging systems is promising, especially as some of them are non-invasive [26, 30]. We hope that further advancements in technology will allow us to image blood flow in a more sophisticated manner.
Highlights.
Neurotrophic factors and growth factors enhance adult hippocampal neurogenesis.
VEGF couples angiogenesis and adult hippocampal neurogenesis.
PV/nNOS interneurons induces functional hyperemia through nitric oxide signaling.
Functional hyperemia enhances adult hippocampal neurogenesis by increased delivery of IGF-1 to the neurogenesis niche.
Neurovascular dysfunction is indicated in Alzheimer’s disease.
Funding
This work was supported by the National Institute of Health [R21 AG046875].
Footnotes
Declaration of competing interests
None
References
- [1].Åberg MAI, Åberg ND, Hedbäcker H, Oscarsson J, Eriksson PS, Peripheral Infusion of IGF-I Selectively Induces Neurogenesis in the Adult Rat Hippocampus, The Journal of Neuroscience 20 (2000) 2896–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ali F, Baringer SL, Neal A, Choi EY, Kwan AC, Parvalbumin-Positive Neuron Loss and Amyloid-β Deposits in the Frontal Cortex of Alzheimer’s Disease-Related Mice, J Alzheimers Dis 72 (2019) 1323–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Altman J, Das GD, Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats, Journal of Comparative Neurology 124 (1965) 319–335. [DOI] [PubMed] [Google Scholar]
- [4].Alvarez DD, Giacomini D, Yang SM, Trinchero MF, Temprana SG, Büttner KA, Beltramone N, Schinder AF, A disynaptic feedback network activated by experience promotes the integration of new granule cells, Science 354 (2016) 459–465. [DOI] [PubMed] [Google Scholar]
- [5].Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bourbonniere L, Bernard M, van Horssen J, de Vries HE, Charron F, Prat A, The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence, Science 334 (2011) 1727–1731. [DOI] [PubMed] [Google Scholar]
- [6].Arai H, Emson PC, Mountjoy CQ, Carassco LH, Heizmann CW, Loss of parvalbumin-immunoreactive neurones from cortex in Alzheimer-type dementia, Brain Res 418 (1987) 164–169. [DOI] [PubMed] [Google Scholar]
- [7].Araki T, Ikegaya Y, Koyama R, The effects of microglia- and astrocyte-derived factors on neurogenesis in health and disease, Eur J Neurosci (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Argaw AT, Asp L, Zhang J, Navrazhina K, Pham T, Mariani JN, Mahase S, Dutta DJ, Seto J, Kramer EG, Ferrara N, Sofroniew MV, John GR, Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease, J Clin Invest 122 (2012) 2454–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR, VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown, Proc Natl Acad Sci U S A 106 (2009) 1977–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].A.s. Association, 2019 Alzheimer's disease facts and figures, Alzheimer's & Dementia 15 (2019) 321–387. [Google Scholar]
- [11].Attwell D, Buchan AM, Charpak S, Lauritzen M, MacVicar BA, Newman EA, Glial and neuronal control of brain blood flow, Nature. 468 (2010) 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bayliss WM, On the local reactions of the arterial wall to changes of internal pressure, J Physiol. 28 (1902) 220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bell RD, Zlokovic BV, Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer's disease, Acta Neuropathol 118 (2009) 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bonafina A, Trinchero MF, Rios AS, Bekinschtein P, Schinder AF, Paratcha G, Ledda F, GDNF and GFRalpha1 Are Required for Proper Integration of Adult-Born Hippocampal Neurons, Cell Rep 29 (2019) 4308–4319 e4304. [DOI] [PubMed] [Google Scholar]
- [15].Borght K.V.d., Kóbor-Nyakas DE, Klauke K, Eggen BJL, Nyakas C, Zee E.A.V.d., Meerlo P, Physical exercise leads to rapid adaptations in hippocampal vasculature: temporal dynamics and relationship to cell proliferation and neurogenesis, Hippocampus. 19 (2009) 928–936. [DOI] [PubMed] [Google Scholar]
- [16].Cai Z, Qiao PF, Wan CQ, Cai M, Zhou NK, Li Q, Role of Blood-Brain Barrier in Alzheimer's Disease, J Alzheimers Dis 63 (2018) 1223–1234. [DOI] [PubMed] [Google Scholar]
- [17].Cajal SR, Degeneration & Regeneration of the Nervous System, Hafner Publishing Company, 1959. [Google Scholar]
- [18].Carlson SW, Madathil SK, Sama DM, Gao X, Chen J, Saatman KE, Conditional Overexpression of Insulin-Like Growth Factor-1 Enhances Hippocampal Neurogenesis and Restores Immature Neuron Dendritic Processes After Traumatic Brain Injury, Journal of Neuropathology & Experimental Neurology 73 (2014) 734–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Castaneda-Cabral JL, Beas-Zarate C, Rocha-Arrieta LL, Orozco-Suarez SA, Alonso-Vanegas M, Guevara-Guzman R, Urena-Guerrero ME, Increased protein expression of VEGF-A, VEGF-B, VEGF-C and their receptors in the temporal neocortex of pharmacoresistant temporal lobe epilepsy patients, J Neuroimmunol 328 (2019) 68–72. [DOI] [PubMed] [Google Scholar]
- [20].Cauli B, Tong XK, Rancillac A, Serluca N, Lambolez B, Rossier J, Hamel E, Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways, J Neurosci 24 (2004) 8940–8949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chen BH, Ahn JH, Park JH, Song M, Kim H, Lee TK, Lee JC, Kim YM, Hwang IK, Kim DW, Lee CH, Yan BC, Kang IJ, Won MH, Rufinamide, an antiepileptic drug, improves cognition and increases neurogenesis in the aged gerbil hippocampal dentate gyrus via increasing expressions of IGF-1, IGF-1R and p-CREB, Chem Biol Interact 286 (2018) 71–77. [DOI] [PubMed] [Google Scholar]
- [22].Chen G, Castro WL, Chow HH, Reichlin S, Clearance of 125I-labeled interleukin-6 from brain into blood following intracerebroventricular injection in rats, Endocrinology. 138 (1997) 4830–4836. [DOI] [PubMed] [Google Scholar]
- [23].Chen G, Reichlin S, Clearance of [125I]-tumor necrosis factor-alpha from the brain into the blood after intracerebroventricular injection in rats, Neuroimmunomodulation. 5 (1998) 261–269. [DOI] [PubMed] [Google Scholar]
- [24].Choi JK, Chen YI, Hamel E, Jenkins BG, Brain hemodynamic changes mediated by dopamine receptors: Role of the cerebral microvasculature in dopamine-mediated neurovascular coupling, Neuroimage 30 (2006) 700–712. [DOI] [PubMed] [Google Scholar]
- [25].Cohen Z, Bonvento G, Lacombe P, Hamel E, Serotonin in the regulation of brain microcirculation, Prog Neurobiol 50 (1996) 335–362. [DOI] [PubMed] [Google Scholar]
- [26].Demené C, Tiran E, Sieu LA, Bergel A, Gennisson JL, Pernot M, Deffieux T, Cohen I, Tanter M, 4D microvascular imaging based on ultrafast Doppler tomography, Neuroimage 127 (2016) 472–483. [DOI] [PubMed] [Google Scholar]
- [27].Deyama S, Bang E, Kato T, Li X-Y, Duman RS, Neurotrophic and Antidepressant Actions of Brain-Derived Neurotrophic Factor Require Vascular Endothelial Growth Factor, Biological Psychiatry 86 (2019) 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Duan W, Sehrawat P, Balachandrasekaran A, Bhumkar AB, Boraste PB, Becker JT, Kuller LH, Lopez OL, Gach HM, Dai W, Cerebral Blood Flow Is Associated with Diagnostic Class and Cognitive Decline in Alzheimer's Disease, J Alzheimers Dis 76 (2020) 1103–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn A-M, Nordborg C, Peterson DA, Gage FH, Neurogenesis in the adult human hippocampus, Nature Medicine 4 (1998) 1313–1317. [DOI] [PubMed] [Google Scholar]
- [30].Errico C, Pierre J, Pezet S, Desailly Y, Lenkei Z, Couture O, Tanter M, Ultrafast ultrasound localization microscopy for deep super-resolution vascular imaging, Nature 527 (2015) 499–502. [DOI] [PubMed] [Google Scholar]
- [31].Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD, VEGF is necessary for exercise-induced adult hippocampal neurogenesis, European Journal of Neuroscience 18 (2003) 2803–2812. [DOI] [PubMed] [Google Scholar]
- [32].Farkas E, Luiten PG, Cerebral microvascular pathology in aging and Alzheimer’s disease, Prog Neurobiol 64 (2001) 575–611. [DOI] [PubMed] [Google Scholar]
- [33].Fernández-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U, Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain, Proceedings of the National Academy of Sciences 107 (2010) 22290–22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ferrara N, Gerber H-P, LeCouter J, The biology of VEGF and its receptors, Nat Med. 9 (2003) 669–676. [DOI] [PubMed] [Google Scholar]
- [35].Garwood CJ, Pooler AM, Atherton J, Hanger DP, Noble W, Astrocytes are important mediators of Aβ-induced neurotoxicity and tau phosphorylation in primary culture, Cell Death Dis 2 (2011) e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ge S, Goh ELK, Sailor KA, Kitabatake Y, Ming G.-l., Song H, GABA regulates synaptic integration of newly generated neurons in the adult brain, Nature 439 (2006) 589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Giesers NK, Wirths O, Loss of Hippocampal Calretinin and Parvalbumin Interneurons in the 5XFAD Mouse Model of Alzheimer’s Disease, ASN Neuro 12 (2020) 1759091420925356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Goldberg JS, Hirschi KK, Diverse roles of the vasculature within the neural stem cell niche, Regenerative Medicine 4 (2009) 879–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Goldman SA, Nottebohm F, Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain, Proceedings of the National Academy of Sciences 80 (1983) 2390–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gonçalves JT, Schafer ST, Gage FH, Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior, Cell 167 (2016) 897–914. [DOI] [PubMed] [Google Scholar]
- [41].Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA, Brain metabolism dictates the polarity of astrocyte control over arterioles, Nature 456 (2008) 745–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hatakeyama M, Ninomiya I, Kanazawa M, Angiogenesis and neuronal remodeling after ischemic stroke, Neural Regen Res 15 (2020) 16–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hijazi S, Heistek TS, Scheltens P, Neumann U, Shimshek DR, Mansvelder HD, Smit AB, van Kesteren RE, Early restoration of parvalbumin interneuron activity prevents memory loss and network hyperexcitability in a mouse model of Alzheimer’s disease, Molecular Psychiatry 25 (2020) 3380–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hodge RD, D'Ercole AJ, O'Kusky JR, Insulin-like growth factor-I (IGF-I) inhibits neuronal apoptosis in the developing cerebral cortex in vivo, Int J Dev Neurosci 25 (2007) 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, Mathys H, Seo J, Kritskiy O, Abdurrob F, Adaikkan C, Canter RG, Rueda R, Brown EN, Boyden ES, Tsai LH, Gamma frequency entrainment attenuates amyloid load and modifies microglia, Nature 540 (2016) 230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Iadecola C, Neurovascular regulation in the normal brain and in Alzheimer's disease, Nat Rev Neurosci 5 (2004) 347–360. [DOI] [PubMed] [Google Scholar]
- [47].Iadecola C, The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease, Neuron 96 (2017) 17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Igarashi Y, Utsumi H, Chiba H, Yamada-Sasamori Y, Tobioka H, Kamimura Y, Furuuchi K, Kokai Y, Nakagawa T, Mori M, Sawada N, Glial cell line-derived neurotrophic factor induces barrier function of endothelial cells forming the blood-brain barrier, Biochem Biophys Res Commun 261 (1999) 108–112. [DOI] [PubMed] [Google Scholar]
- [49].James JM, Mukouyama YS, Neuronal action on the developing blood vessel pattern, Semin Cell Dev Biol 22 (2011) 1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA, Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat, Proc Natl Acad Sci U S A 98 (2001) 4710–4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA, Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo, Proc Natl Acad Sci U S A. 99 (2002) 11946–11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Jones EG, On the mode of entry of blood vessels into the cerebral cortex, J Anat 106 (1970) 507–520. [PMC free article] [PubMed] [Google Scholar]
- [53].Kahles T, Luedike P, Endres M, Galla HJ, Steinmetz H, Busse R, Neumann-Haefelin T, Brandes RP, NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke, Stroke 38 (2007) 3000–3006. [DOI] [PubMed] [Google Scholar]
- [54].Kang W, Hébert JM, FGF Signaling Is Necessary for Neurogenesis in Young Mice and Sufficient to Reverse Its Decline in Old Mice, J Neurosci. 35 (2015) 10217–10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kaplan L, Chow BW, Gu C, Neuronal regulation of the blood-brain barrier and neurovascular coupling, Nat Rev Neurosci 21 (2020) 416–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Katoh R-S, Asano T, Ueda H, Morishita R, Takeuchi IK, Inaguma Y, Kato K, Riluzole enhances expression of brain-derived neurotrophic factor with consequent proliferation of granule precursor cells in the rat hippocampus, The FASEB Journal 16 (2002) 1328–1330. [DOI] [PubMed] [Google Scholar]
- [57].Kisler K, Nelson AR, Montagne A, Zlokovic BV, Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease, Nature Reviews Neuroscience 18 (2017) 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kiyatkin EA, Brain temperature fluctuations during physiological and pathological conditions, Eur J Appl Physiol. 101 (2007) 3–17. [DOI] [PubMed] [Google Scholar]
- [59].Kiyatkin EA, Brown PL, Wise RA, Brain temperature fluctuation: a reflection of functional neural activation, Eur J Neurosci 16 (2002) 164–168. [DOI] [PubMed] [Google Scholar]
- [60].Kocharyan A, Fernandes P, Tong XK, Vaucher E, Hamel E, Specific subtypes of cortical GABA interneurons contribute to the neurovascular coupling response to basal forebrain stimulation, J Cereb Blood Flow Metab 28 (2008) 221–231. [DOI] [PubMed] [Google Scholar]
- [61].Kreisel T, Wolf B, Keshet E, Licht T, Unique role for dentate gyrus microglia in neuroblast survival and in VEGF-induced activation, Glia 67 (2019) 594–618. [DOI] [PubMed] [Google Scholar]
- [62].Kuhn H, Dickinson-Anson H, Gage F, Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation, The Journal of Neuroscience 16 (1996) 2027–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lecrux C, Hamel E, Neuronal networks and mediators of cortical neurovascular coupling responses in normal and altered brain states, Philos Trans R Soc Lond B Biol Sci 371 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Lee C, Agoston DV, Vascular endothelial growth factor is involved in mediating increased de novo hippocampal neurogenesis in response to traumatic brain injury, J Neurotrauma 27 (2010) 541–553. [DOI] [PubMed] [Google Scholar]
- [65].Lee J, Duan W, Mattson MP, Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice, Journal of Neurochemistry 82 (2002) 1367–1375. [DOI] [PubMed] [Google Scholar]
- [66].Li Y, Luikart BW, Birnbaum S, Chen J, Kwon C-H, Kernie SG, Bassel-Duby R, Parada LF, TrkB Regulates Hippocampal Neurogenesis and Governs Sensitivity to Antidepressive Treatment, Neuron 59 (2008) 399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Licht T, Goshen I, Avital A, Kreisel T, Zubedat S, Eavri R, Segal M, Yirmiya R, Keshet E, Reversible modulations of neuronal plasticity by VEGF, Proc Natl Acad Sci U S A. 108 (2011) 5081–5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Licht T, Keshet E, The vascular niche in adult neurogenesis, Mechanisms of Development 138 (2015) 56–62. [DOI] [PubMed] [Google Scholar]
- [69].Licht T, Kreisel T, Biala Y, Mohan S, Yaari Y, Anisimov A, Alitalo K, Keshet E, Age-Dependent Remarkable Regenerative Potential of the Dentate Gyrus Provided by Intrinsic Stem Cells, J Neurosci 40 (2020) 974–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Licht T, Rothe G, Kreisel T, Wolf B, Benny O, Rooney AG, Ffrench-Constant C, Enikolopov G, Keshet E, VEGF preconditioning leads to stem cell remodeling and attenuates age-related decay of adult hippocampal neurogenesis, Proc Natl Acad Sci U S A 113 (2016) E7828–E7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lindauer U, Leithner C, Kaasch H, Rohrer B, Foddis M, Fuchtemeier M, Offenhauser N, Steinbrink J, Royl G, Kohl-Bareis M, Dirnagl U, Neurovascular coupling in rat brain operates independent of hemoglobin deoxygenation, J Cereb Blood Flow Metab 30 (2010) 757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Liu Y, Huber CC, Wang H, Disrupted blood-brain barrier in 5×FAD mouse model of Alzheimer's disease can be mimicked and repaired in vitro with neural stem cell-derived exosomes, Biochem Biophys Res Commun (2020). [DOI] [PubMed] [Google Scholar]
- [73].Llorens-Martín M, Torres-Alemán I, Trejo JL, Exercise modulates insulin-like growth factor 1-dependent and -independent effects on adult hippocampal neurogenesis and behaviour, Mol Cell Neurosci. 44 (2010) 109–117. [DOI] [PubMed] [Google Scholar]
- [74].LLorens-Martín M, Torres-Alemán I, Trejo JL, Reviews: Mechanisms Mediating Brain Plasticity: IGF1 and Adult Hippocampal Neurogenesis, The Neuroscientist 15 (2009) 134–148. [DOI] [PubMed] [Google Scholar]
- [75].Louissaint A Jr., Rao S, Leventhal C, Goldman SA, Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain, Neuron 34 (2002) 945–960. [DOI] [PubMed] [Google Scholar]
- [76].Lourenço CF, Santos RM, Barbosa RM, Cadenas E, Radi R, Laranjinha J, Neurovascular coupling in hippocampus is mediated via diffusion by neuronal-derived nitric oxide, Free Radical Biology and Medicine 73 (2014) 421–429. [DOI] [PubMed] [Google Scholar]
- [77].Luo YP, Zhang L, Wu XY, Hou WS, Chen L, Tian XL, Wen HZ, Cerebral blood microcirculation measurement in APP/PS1 double transgenic mice at the preclinical stage of Alzheimer's disease: preliminary data on the early intervention of anodal transcranial direct current stimulation(), Annu Int Conf IEEE Eng Med Biol Soc 2020 (2020) 3557–3560. [DOI] [PubMed] [Google Scholar]
- [78].Marchi N, Oby E, Batra A, Uva L, De Curtis M, Hernandez N, Van Boxel-Dezaire A, Najm I, Janigro D, In vivo and in vitro effects of pilocarpine: relevance to ictogenesis, Epilepsia 48 (2007) 1934–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Martinez-Losa M, Tracy TE, Ma K, Verret L, Clemente-Perez A, Khan AS, Cobos I, Ho K, Gan L, Mucke L, Alvarez-Dolado M, Palop JJ, Nav1.1-Overexpressing Interneuron Transplants Restore Brain Rhythms and Cognition in a Mouse Model of Alzheimer’s Disease, Neuron 98 (2018) 75–89.e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].McHedlishvili G, Kuridze N, The Modular Organization of the Pial Arterial System in Phylogeny, Journal of Cerebral Blood Flow & Metabolism 4 (1984) 391–396. [DOI] [PubMed] [Google Scholar]
- [81].Miners JS, Kehoe PG, Love S, Zetterberg H, Blennow K, CSF evidence of pericyte damage in Alzheimer’s disease is associated with markers of blood-brain barrier dysfunction and disease pathology, Alzheimer's Research & Therapy 11 (2019) 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Ming G.-l., Song H, Adult Neurogenesis in the Mammalian Brain: Significant Answers and Significant Questions, Neuron 70 (2011) 687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ming GL, Song H, Adult neurogenesis in the mammalian central nervous system, Annu Rev Neurosci 28 (2005) 223–250. [DOI] [PubMed] [Google Scholar]
- [84].Mintun MA, Lundstrom BN, Snyder AZ, Vlassenko AG, Shulman GL, Raichle ME, Blood flow and oxygen delivery to human brain during functional activity: Theoretical modeling and experimental data, Proceedings of the National Academy of Sciences 98 (2001) 6859–6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Mir S, Cai W, Carlson SW, Saatman KE, Andres DA, IGF-1 mediated Neurogenesis Involves a Novel RIT1/Akt/Sox2 Cascade, Scientific Reports 7 (2017) 3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Mohamed A, Posse de Chaves E, Aβ internalization by neurons and glia, Int J Alzheimers Dis 2011 (2011) 127984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV, Blood-brain barrier breakdown in the aging human hippocampus, Neuron 85 (2015) 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Montagne A, Nation DA, Sagare AP, Barisano G, Sweeney MD, Chakhoyan A, Pachicano M, Joe E, Nelson AR, D'Orazio LM, Buennagel DP, Harrington MG, Benzinger TLS, Fagan AM, Ringman JM, Schneider LS, Morris JC, Reiman EM, Caselli RJ, Chui HC, Tcw J, Chen Y, Pa J, Conti PS, Law M, Toga AW, Zlokovic BV, APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline, Nature 581 (2020) 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Moreno-Jiménez EP, Flor-García M, Terreros-Roncal J, Rábano A, Cafini F, Pallas-Bazarra N, Ávila J, Llorens-Martín M, Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer's disease, Nat Med 25 (2019) 554–560. [DOI] [PubMed] [Google Scholar]
- [90].Nation DA, Sweeney MD, Montagne A, Sagare AP, D’Orazio LM, Pachicano M, Sepehrband F, Nelson AR, Buennagel DP, Harrington MG, Benzinger TLS, Fagan AM, Ringman JM, Schneider LS, Morris JC, Chui HC, Law M, Toga AW, Zlokovic BV, Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction, Nature Medicine 25 (2019) 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Nieto-Estévez V, Defterali Ç, Vicario-Abejón C, IGF-I: A Key Growth Factor that Regulates Neurogenesis and Synaptogenesis from Embryonic to Adult Stages of the Brain, Front Neurosci 10 (2016) 52–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Nieto-Estévez V, Oueslati-Morales CO, Li L, Pickel J, Morales AV, Vicario-Abejón C, Brain Insulin-Like Growth Factor-I Directs the Transition from Stem Cells to Mature Neurons During Postnatal/Adult Hippocampal Neurogenesis, Stem Cells. 34 (2016) 2194–2209. [DOI] [PubMed] [Google Scholar]
- [93].Nishijima T, Piriz J, Duflot S, Fernandez AM, Gaitan G, Gomez-Pinedo U, Verdugo JM, Leroy F, Soya H, Nuñez A, Torres-Aleman I, Neuronal activity drives localized blood-brain-barrier transport of serum insulin-like growth factor-I into the CNS, Neuron 67 (2010) 834–846. [DOI] [PubMed] [Google Scholar]
- [94].Obermeier B, Daneman R, Ransohoff RM, Development, maintenance and disruption of the blood-brain barrier, Nat Med 19 (2013) 1584–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Offenhauser N, Thomsen K, Caesar K, Lauritzen M, Activity-induced tissue oxygenation changes in rat cerebellar cortex: interplay of postsynaptic activation and blood flow, J Physiol. 565 (2005) 279–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Ogaki A, Ikegaya Y, Koyama R, Vascular Abnormalities and the Role of Vascular Endothelial Growth Factor in the Epileptic Brain, Front Pharmacol 11 (2020) 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Ortega-Martinez S, Palla N, Zhang X, Lipman E, Sisodia SS, Deficits in Enrichment-Dependent Neurogenesis and Enhanced Anxiety Behaviors Mediated by Expression of Alzheimer's Disease-Linked Ps1 Variants Are Rescued by Microglial Depletion, J Neurosci 39 (2019) 6766–6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Otsu Y, Couchman K, Lyons DG, Collot M, Agarwal A, Mallet JM, Pfrieger FW, Bergles DE, Charpak S, Calcium dynamics in astrocyte processes during neurovascular coupling, Nat Neurosci 18 (2015) 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Palmer TD, Willhoite AR, Gage FH, Vascular niche for adult hippocampal neurogenesis, Journal of Comparative Neurology 425 (2000) 479–494. [DOI] [PubMed] [Google Scholar]
- [100].Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L, Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease, Neuron 55 (2007) 697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Park L, Hochrainer K, Hattori Y, Ahn SJ, Anfray A, Wang G, Uekawa K, Seo J, Palfini V, Blanco I, Acosta D, Eliezer D, Zhou P, Anrather J, Iadecola C, Tau induces PSD95-neuronal NOS uncoupling and neurovascular dysfunction independent of neurodegeneration, Nat Neurosci 23 (2020) 1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Park L, Zhou J, Koizumi K, Wang G, Anfray A, Ahn SJ, Seo J, Zhou P, Zhao L, Paul S, Anrather J, Iadecola C, tPA Deficiency Underlies Neurovascular Coupling Dysfunction by Amyloid-β, J Neurosci 40 (2020) 8160–8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Paton JA, Nottebohm FN, Neurons generated in the adult brain are recruited into functional circuits, Science 225 (1984) 1046–1048. [DOI] [PubMed] [Google Scholar]
- [104].Peltier J, O'Neill A, Schaffer DV, PI3K/Akt and CREB regulate adult neural hippocampal progenitor proliferation and differentiation, Dev Neurobiol 67 (2007) 1348–1361. [DOI] [PubMed] [Google Scholar]
- [105].Peppiatt CM, Howarth C, Mobbs P, Attwell D, Bidirectional control of CNS capillary diameter by pericytes, Nature 443 (2006) 700–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA, An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus, Proc Natl Acad Sci U S A. 104 (2007) 5638–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Plate KH, Mechanisms of Angiogenesis in the Brain, Journal of Neuropathology & Experimental Neurology 58 (1999) 313–320. [DOI] [PubMed] [Google Scholar]
- [108].Pombero A, Garcia-Lopez R, Estirado A, Martinez S, Vascular pattern of the dentate gyrus is regulated by neural progenitors, Brain Struct Funct. 223 (2018) 1971–1987. [DOI] [PubMed] [Google Scholar]
- [109].Powell PP, Finklestein SP, Dionne CA, Jaye M, Klagsbrun M, Temporal, differential and regional expression of mRNA for basic fibroblast growth factor in the developing and adult rat brain, Brain Res Mol Brain Res 11 (1991) 71–77. [DOI] [PubMed] [Google Scholar]
- [110].Rafii S, Butler JM, Ding B-S, Angiocrine functions of organ-specific endothelial cells, Nature 529 (2016) 316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Rai KS, Hattiangady B, Shetty AK, Enhanced production and dendritic growth of new dentate granule cells in the middle-aged hippocampus following intracerebroventricular FGF-2 infusions, European Journal of Neuroscience 26 (2007) 1765–1779. [DOI] [PubMed] [Google Scholar]
- [112].Rancillac A, Rossier J, Guille M, Tong X-K, Geoffroy H, Amatore C, Arbault S, Hamel E, Cauli B, Glutamatergic Control of Microvascular Tone by Distinct GABA Neurons in the Cerebellum, J Neurosci. 26 (2006) 6997–7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Reinhardt RR, Bondy CA, Insulin-like growth factors cross the blood-brain barrier, Endocrinology. 135 (1994) 1753–1761. [DOI] [PubMed] [Google Scholar]
- [114].Rivadulla C, de Labra C, Grieve KL, Cudeiro J, Vasomotion and neurovascular coupling in the visual thalamus in vivo, PLoS One 6 (2011) e28746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Roggendorf W, Cervós-Navarro J, Ultrastructure of arterioles in the cat brain, Cell Tissue Res 178 (1977) 495–515. [DOI] [PubMed] [Google Scholar]
- [116].Roher AE, Debbins JP, Malek-Ahmadi M, Chen K, Pipe JG, Maze S, Belden C, Maarouf CL, Thiyyagura P, Mo H, Hunter JM, Kokjohn TA, Walker DG, Kruchowsky JC, Belohlavek M, Sabbagh MN, Beach TG, Cerebral blood flow in Alzheimer's disease, Vasc Health Risk Manag 8 (2012) 599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Ruan L, Wang B, ZhuGe Q, Jin K, Coupling of neurogenesis and angiogenesis after ischemic stroke, Brain Res 1623 (2015) 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Sairanen M, Lucas G, Ernfors P, Castrén M, Castrén E, Brain-Derived Neurotrophic Factor and Antidepressant Drugs Have Different But Coordinated Effects on Neuronal Turnover, Proliferation, and Survival in the Adult Dentate Gyrus, The Journal of Neuroscience 25 (2005) 1089–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Schänzer A, Wachs F-P, Wilhelm D, Acker T, Cooper-Kuhn C, Beck H, Winkler J, Aigner L, Plate KH, Kuhn HG, Direct Stimulation of Adult Neural Stem Cells In Vitro and Neurogenesis In Vivo by Vascular Endothelial Growth Factor, Brain Pathology 14 (2004) 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Segi-Nishida E, Warner-Schmidt JL, Duman RS, Electroconvulsive seizure and VEGF increase the proliferation of neural stem-like cells in rat hippocampus, Proc Natl Acad Sci U S A 105 (2008) 11352–11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Seiffert E, Dreier JP, Ivens S, Bechmann I, Tomkins O, Heinemann U, Friedman A, Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex, J Neurosci 24 (2004) 7829–7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Shen J, Wang D, Wang X, Gupta S, Ayloo B, Wu S, Prasad P, Xiong Q, Xia J, Ge S, Neurovascular Coupling in the Dentate Gyrus Regulates Adult Hippocampal Neurogenesis, Neuron 103 (2019) 878–890.e873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S, Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells, Science 304 (2004) 1338–1340. [DOI] [PubMed] [Google Scholar]
- [124].Shen Q, Wang Y, Kokovay E, Lin G, Chuang S-M, Goderie SK, Roysam B, Temple S, Adult SVZ Stem Cells Lie in a Vascular Niche: A Quantitative Analysis of Niche Cell-Cell Interactions, Cell Stem Cell 3 (2008) 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Shetty AK, Hattiangady B, Shetty GA, Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: Role of astrocytes, Glia 51 (2005) 173–186. [DOI] [PubMed] [Google Scholar]
- [126].Sobue K, Yamamoto N, Yoneda K, Hodgson ME, Yamashiro K, Tsuruoka N, Tsuda T, Katsuya H, Miura Y, Asai K, Kato T, Induction of blood-brain barrier properties in immortalized bovine brain endothelial cells by astrocytic factors, Neurosci Res 35 (1999) 155–164. [DOI] [PubMed] [Google Scholar]
- [127].Song J, Sun J, Moss J, Wen Z, Sun GJ, Hsu D, Zhong C, Davoudi H, Christian KM, Toni N, Ming GL, Song H, Parvalbumin interneurons mediate neuronal circuitry-neurogenesis coupling in the adult hippocampus, Nat Neurosci 16 (2013) 1728–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Song J, Zhong C, Bonaguidi MA, Sun GJ, Hsu D, Gu Y, Meletis K, Huang ZJ, Ge S, Enikolopov G, Deisseroth K, Luscher B, Christian KM, Ming G.-l., Song H, Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision, Nature 489 (2012) 150–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Spalding Kirsty L., Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner Hagen B., Boström E, Westerlund I, Vial C, Buchholz Bruce A., Possnert G, Mash Deborah C., Druid H, Frisén J, Dynamics of Hippocampal Neurogenesis in Adult Humans, Cell 153 (2013) 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Spampinato SF, Bortolotto V, Canonico PL, Sortino MA, Grilli M, Astrocyte-Derived Paracrine Signals: Relevance for Neurogenic Niche Regulation and Blood-Brain Barrier Integrity, Front Pharmacol 10 (2019) 1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Spampinato SF, Merlo S, Sano Y, Kanda T, Sortino MA, Astrocytes contribute to Abeta-induced blood-brain barrier damage through activation of endothelial MMP9, J Neurochem 142 (2017) 464–477. [DOI] [PubMed] [Google Scholar]
- [132].Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP, Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature, Science 322 (2008) 1247–1250. [DOI] [PubMed] [Google Scholar]
- [133].Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA, VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia, The Journal of Clinical Investigation 111 (2003) 1843–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F, A Specialized Vascular Niche for Adult Neural Stem Cells, Cell Stem Cell 3 (2008) 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Thau-Zuchman O, Shohami E, Alexandrovich AG, Leker RR, Combination of Vascular Endothelial and Fibroblast Growth Factor 2 for Induction of Neurogenesis and Angiogenesis after Traumatic Brain Injury, Journal of Molecular Neuroscience 47 (2012) 166–172. [DOI] [PubMed] [Google Scholar]
- [136].Trejo JL, Carro E, Torres-Alemán I, Circulating Insulin-Like Growth Factor I Mediates Exercise-Induced Increases in the Number of New Neurons in the Adult Hippocampus, The Journal of Neuroscience 21 (2001) 1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Tykocki NR, Boerman EM, Jackson WF, Smooth Muscle Ion Channels and Regulation of Vascular Tone in Resistance Arteries and Arterioles, Compr Physiol. 7 (2017) 485–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Varatharaj A, Galea I, The blood-brain barrier in systemic inflammation, Brain Behav Immun 60 (2017) 1–12. [DOI] [PubMed] [Google Scholar]
- [139].Verret L, Mann EO, Hang GB, Barth AM, Cobos I, Ho K, Devidze N, Masliah E, Kreitzer AC, Mody I, Mucke L, Palop JJ, Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model, Cell 149 (2012) 708–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Powers WJ, Hirsch IB, Cryer PE, Effect of stepped hypoglycemia on regional cerebral blood flow response to physiological brain activation., Am J Physiol. 270 (1996) 554–559. [DOI] [PubMed] [Google Scholar]
- [141].Wang L, Chang X, She L, Xu D, Huang W, Poo M.-m., Autocrine Action of BDNF on Dendrite Development of Adult-Born Hippocampal Neurons, The Journal of Neuroscience 35 (2015) 8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Warner-Schmidt JL, Duman RS, VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants, Proceedings of the National Academy of Sciences 104 (2007) 4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Wen PH, Hof PR, Chen X, Gluck K, Austin G, Younkin SG, Younkin LH, DeGasperi R, Gama Sosa MA, Robakis NK, Haroutunian V, Elder GA, The presenilin-1 familial Alzheimer disease mutant P117L impairs neurogenesis in the hippocampus of adult mice, Exp Neurol 188 (2004) 224–237. [DOI] [PubMed] [Google Scholar]
- [144].Werner S, Unsicker K, von Bohlen und Halbach O, Fibroblast growth factor-2 deficiency causes defects in adult hippocampal neurogenesis, which are not rescued by exogenous fibroblast growth factor-2, Journal of Neuroscience Research 89 (2011) 1605–1617. [DOI] [PubMed] [Google Scholar]
- [145].Winkler EA, Bell RD, Zlokovic BV, Central nervous system pericytes in health and disease, Nature Neuroscience 14 (2011) 1398–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Wu L, Ye Z, Pan Y, Li X, Fu X, Zhang B, Li Y, Lin W, Li X, Gao Q, Vascular endothelial growth factor aggravates cerebral ischemia and reperfusion-induced blood-brain-barrier disruption through regulating LOC102640519/HOXC13/ZO-1 signaling, Exp Cell Res 369 (2018) 275–283. [DOI] [PubMed] [Google Scholar]
- [147].Xiao Z, Kong Y, Yang S, Li M, Wen J, Li L, Upregulation of Flk-1 by bFGF via the ERK pathway is essential for VEGF-mediated promotion of neural stem cell proliferation, Cell Research 17 (2007) 73–79. [DOI] [PubMed] [Google Scholar]
- [148].Yan BC, Xu P, Gao M, Wang J, Jiang D, Zhu X, Won MH, Su PQ, Changes in the Blood-Brain Barrier Function Are Associated With Hippocampal Neuron Death in a Kainic Acid Mouse Model of Epilepsy, Front Neurol 9 (2018) 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Yang G, Zhang Y, Ross ME, Iadecola C, Attenuation of activity-induced increases in cerebellar blood flow in mice lacking neuronal nitric oxide synthase, Am J Physiol Heart Circ Physiol. 285 (2003) H298–304. [DOI] [PubMed] [Google Scholar]
- [150].Yang Y, Wei H, Zhou X, Zhang F, Wang C, Hyperbaric oxygen promotes neural stem cell proliferation by activating vascular endothelial growth factor/extracellular signal-regulated kinase signaling after traumatic brain injury, NeuroReport 28 (2017) 1232–1238. [DOI] [PubMed] [Google Scholar]
- [151].Yoshimura S, Takagi Y, Harada J, Teramoto T, Thomas SS, Waeber C, Bakowska JC, Breakefield XO, Moskowitz MA, FGF-2 regulation of neurogenesis in adult hippocampus after brain injury, Proceedings of the National Academy of Sciences 98 (2001) 5874–5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Zhang ET, Inman CB, Weller RO, Interrelationships of the pia mater and the perivascular (Virchow-Robin) spaces in the human cerebrum, J Anat 170 (1990) 111–123. [PMC free article] [PubMed] [Google Scholar]
- [153].Zhang Q, Xie C, Apolipoprotein E Drives Early Blood-Brain Barrier Damage in Alzheimer's Disease, Neurosci Bull (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Zhang X, Yin X, Zhang J, Li A, Gong H, Luo Q, Zhang H, Gao Z, Jiang H, High-resolution mapping of brain vasculature and its impairment in the hippocampus of Alzheimer's disease mice, National Science Review 6 (2019) 1223–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Zhang X, Zhang L, Cheng X, Guo Y, Sun X, Chen G, Li H, Li P, Lu X, Tian M, Qin J, Zhou H, Jin G, IGF-1 promotes Brn-4 expression and neuronal differentiation of neural stem cells via the PI3K/Akt pathway, PLoS One 9 (2014) e113801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Zhao M, Li D, Shimazu K, Zhou Y-X, Lu B, Deng C-X, Fibroblast Growth Factor Receptor-1 is Required for Long-Term Potentiation, Memory Consolidation, and Neurogenesis, Biological Psychiatry 62 (2007) 381–390. [DOI] [PubMed] [Google Scholar]
- [157].Zheng J, Li H-L, Tian N, Liu F, Wang L, Yin Y, Yue L, Ma L, Wan Y, Wang J-Z, Interneuron Accumulation of Phosphorylated tau Impairs Adult Hippocampal Neurogenesis by Suppressing GABAergic Transmission, Cell Stem Cell 26 (2020) 331–345.e336. [DOI] [PubMed] [Google Scholar]