Abstract
Background
Several concerns exist on the immunogenicity of SARS-CoV-2 vaccines in multiple sclerosis (MS) subjects due to their immunomodulating disease modifying therapies (DMTs). Here we report a comparison of the humoral response to BNT162b2-mRNA coronavirus (COVID)-19 vaccine and the immunological phenotype in a cohort of 125 MS subjects undergoing different DMTs, with no history of SARS-CoV-2 infection.
Methods
We collected serum and blood samples at the first day of vaccine (T0) and 21 days after the second vaccine dose (T1) from 125 MS subjects, undergoing eight different DMTs. Sera were tested using the Elecsys anti-SARS-CoV-2-IgG assay for the detection of IgG antibodies to SARS-CoV-2 spike protein. The anti-spike IgG titres from MS subjects were compared with 24 age- and sex-matched healthy controls (HC). Percentage and absolute number of B and T lymphocytes were evaluated by cytofluorimetric analysis in the same study cohort.
Results
When compared with SARS-CoV-2 IgG levels in HC (n = 24, median 1089 (IQR 652.5–1625) U/mL), we observed an increased secretion of SARS-CoV-2 IgG in interferon-beta 1a (IFN)-treated MS subjects (n = 22, median 1916 (IQR 1024–2879) U/mL) and an impaired humoral response in MS subjects undergoing cladribine (CLAD) (n = 10, median 396.9 (IQR 37.52–790.9) U/mL), fingolimod (FTY) (n = 19, median 7.9 (IQR 4.8–147.6) U/mL) and ocrelizumab (OCRE) (n = 15, median 0.67 (IQR 0.4–5.9) U/mL) treatment. Moreover, analysis of geometric mean titre ratio (GMTR) between different DMT's groups of MS subjects revealed that, when compared with IFN-treated MS subjects, intrinsic antibody production was impaired in teriflunomide (TERI)-, natalizumab (NAT)-, CLAD-, FTY- and OCRE-, while preserved in DMF- and GA-treated MS subjects.
Conclusion
Humoral response to BNT162b2-mRNA-vaccine was increased in IFN-treated MS subjects while clearly blunted in those under CLAD, FTY and OCRE treatment. This suggests that the DMTs could have a key role in the protection from SARS-CoV-2 related disease and complication in MS subjects, underlying a novel aspect that should be considered in the selection of the most appropriate therapy under COVID-19 pandemic.
Keywords: BNT162b2-mRNA coronavirus-19 vaccine, Disease modifying therapies, Multiple sclerosis, Humoral response, SARS-CoV-2 spike protein
Abbreviations: CLAD, Cladribine; COVID-19, Coronavirus disease 2019; DMF, Dimethyl fumatate; DMTs, Disease modifying therapies; EDSS, Expanded disability status scale; FTY, Fingolimod; GA, Glatiramer acetate; GMTR, Geometric mean titre ratio; HC, Healthy controls; IgG, Immunoglobulin G; IFN, Interferon β-1a; IQR, Interquartile range; MS, Multiple sclerosis; NAT, Natalizumab; OCRE, Ocrelizumab; TERI, Teriflunomide; WBC, White blood cells
1. Introduction
The current pandemic caused by the Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2), the virus causing the coronavirus disease 2019 (COVID-19), severely impacts national health systems around the world. The Pfizer-BioNtech (BNT162b2) has been the first vaccine approved; it is composed by a nucleoside-modified messenger (m)RNA encoding the full-length SARS-CoV-2 spike protein, encapsulated in lipid nanoparticles to deliver the genetic information inside target cells (Pardi et al., 2018; Walsh et al., 2020; Wrapp et al., 2020). It has been shown that BNT162b2 conferred strong protection against COVID-19 eliciting high titre of spike (S1)-binding neutralizing immunoglobulin G (IgG) together with CD8+ and CD4+ T cell-specific activation, thus combining both protective humoral and cellular immune response to constrain the virus (Polack et al., 2020; Sahin et al., 2020).
Multiple sclerosis (MS) subjects, especially those with older age, severe disabilities, progressive forms and comorbidities, have been shown to be at higher risk of serious illness or death secondarily to SARS-Co-V-2 infection; however, this point is still debated (Achiron et al., 2021; Naser Moghadasi, Shabany et al. 2021; Sormani et al., 2021). Preliminary data showed that COVID-19 vaccines are safe in MS subjects, with no evidence of increased risk of relapse (Achiron et al., 2021). It is well described that some disease-modifying-therapies (DMTs) induce immunomodulation through lymphocyte depletion, involving either B cells, T cells or both (Hauser and Cree 2020). However, there are still few data about the impact of the different DMTs on the induction of a protective humoral and cellular response to COVID-19 vaccine in MS subjects. Among them, rituximab, ocrelizumab (OCRE) and fingolimod (FTY) have been shown to affect SARS-CoV-2 IgG antibody production in MS-treated subjects (Achiron et al., 2021; Guerrieri et al., 2021; Sormani et al., 2021). However, studies evaluating whether distinct humoral and cellular immune response to COVID-19-vaccination could be observed in MS subjects undergoing several DMTs are still missing. Moreover, as currently available DMTs exhibit different mechanism of action, either impacting or not on lymphocyte count, it is extremely interesting to evaluate whether different vaccine responses could be observed in these groups of MS subjects.
In the current work, we presented a prospective monocentric study assessing the immunogenicity and the immune phenotype in a cohort of 125 MS patients treated with eight different DMTs, without previous SARS-CoV-2 infection, all receiving BNT162b2 vaccine.
2. Materials and methods
2.1. Subjects and study design
This is a prospective monocentric study to evaluate the efficacy of SARS-CoV-2 mRNA BNT162b2 vaccine in MS subjects undergoing vaccination at the Multiple Sclerosis centre of the Cardarelli Hospital (Naples, Italy) from March to June 2021. All human subjects were enrolled after obtaining informed consent. The study was approved by the Institutional Review Board of the Cardarelli Hospital. We enrolled 125 MS and 24 healthy controls receiving the two doses of BNT162b2 vaccine according to the recommendations of Italian Authority of Health, all without previous SARS-CoV-2 infection. MS subjects were vaccinated according to specific timing (Centonze et al., 2021); more in detail, MS subjects treated with IFN, GA, TERI, DMF, FTY and NAT were vaccinated without any interruption of immunomodulatory treatment, while CLAD- and OCRE-treated MS subjects were vaccinated at least 1 or 3 months respectively after the last therapeutic administration, according to the recommendations of Italian Authority of Health. Blood samples were collected at 9:00AM into heparinized Vacutainers (BD Biosciences) and processed within the following 4 h. Demographic and clinical characteristics of the study cohort are shown in Table 1 . Inclusion criteria were patients aged between 18 and 65 years, diagnosed with Multiple Sclerosis treated with DMTs for at least 6 months. Exclusion criteria were previous SARS-CoV-2 infection (antibodies screening at baseline), any relapse and/or steroid use in the last 30 days before enrolment. Healthy subjects were matched for age and sex and had no history of inflammation, endocrine or autoimmune disease. The ethnic distribution among the groups was comparable, with all participants being Caucasian.
Table 1.
Clinical and demographic characteristics of the study cohort.
| MS subjects (N = 125) | Healthy controls(N = 24) | |
|---|---|---|
| Gender, n (%) | ||
| Female | 69 (55.2) | 13 (54.2) |
| Male | 56 (44.8) | 11 (45.8) |
| Age, years | ||
| Mean age (±SD) | 41 (±10.7) | 47.3 (±11) |
| 25–75 IQR | 16.5 | 20.5 |
| MS type, n (%) | ||
| RRMS | 117 (93.6) | |
| PPMS | 4 (3.2) | |
| SPMR | 4 (3.2) | |
| EDSS, n (%) | ||
| ≤3.0 | 104 (83.2) | |
| 3.5–5.5 | 17 (13.6) | |
| ≥6.0 | 4 (3.2) | |
| DMT type, n (%) | ||
| Interferon β 1a | 22 (17.6) | |
| Dimethyl fumarate | 18 (14.4) | |
| Teriflunomide | 10 (8.0) | |
| Natalizumab | 22 (17.6) | |
| Glatiramer acetate | 9 (7.2) | |
| Cladribine | 10 (8.0) | |
| Fingolimod | 19 (15.2) | |
| Ocrelizumab | 15 (12.0) | |
| DMT duration, mean (months) | ||
| Interferon β 1a | 82.1 | |
| Dimethyl fumarate | 45.4 | |
| Teriflunomide | 29.7 | |
| Natalizumab | 52 | |
| Glatiramer acetate | 61.4 | |
| Cladribine | 14.8 | |
| Fingolimod | 55.5 | |
| Ocrelizumab | 24.2 | |
| Last 12-months relapse, n (%) | 23 (18.4) |
DMTs: disease-modifying therapies; EDSS: Expanded Disability Status Scale; MS: Multiple Sclerosis; PPMS: Primary Progressive Multiple Sclerosis; RRMS: Relapsing Remitting Multiple Sclerosis; SPSM: Secondary Progressive Multiple Sclerosis.
2.2. SARS- COV 2 antibody detection
Quantitative determination of antibodies to the SARS-CoV-2 spike protein was carried out by Roche Elecsys® Anti‑SARS‑CoV‑2 S assay (Roche Diagnostics International Ltd, Rotkreuz, Switzerland). The assay was performed using a recombinant protein representing the RBD of the S antigen leading to a double-antigen sandwich assay complex which favors detection of high affinity antibodies against SARS-CoV-2 (range between 0.4 to 250 U/mL), resulting in a sensitivity of 98.8% (95% CI: 98.1 – 99.3%).
2.3. Immunophenotypic analysis
Blood samples from HC and MS subjects were collected in EDTA-coated tubes and were used for immune cell profiling and analysed within 24 h from blood draw. Human whole blood was incubated with FcR Blocking Reagent (Becton Dickinson) and subsequently stained for 30 min at 4 °C with the following specific fluorescent-labelled Abs combinations: APC—H7 anti-CD3 (clone SK7), PE anti-CD4 (clone SK3), APC anti-CD4 (clone SK3), PE-Cy7 anti-CD8 (clone SK1), FITC anti-CD45 (clone 2D1), APC anti-CD45 (clone 2D1), BV605 anti-CD45RO (clone UCHL1), PE anti-CD19 (clone SJ25C1) and PE-Cy7 anti-CD56 (clone NCAM 16.2) from Becton Dickson (BD); APC anti-CD45RA (clone 2H4LDH11LDB9 (2H4)) form Beckman Coulter; FITC anti-HLA-DR. (clone G46–6), PE anti-CD25 (clone M-A251) and FITC anti-CD20 (clone 2H7) from Pharmingen. After incubation, cells were washed and resuspended in phosphate-buffered saline (PBS). Immunophenotypic analysis was performed using FACS Canto and FACS Lyric flow cytometers (Becton Dickinson).
2.4. Ethics (Standard protocol approvals, registrations and patient consents)
The study was conducted according the Good Clinical Practice guidelines and the ethical principles of the Declaration of Helsinki. Investigators obtained ethic committee approval for the study protocol and amendments by the local Ethic Committee of A.O.R.N. A. Cardarelli/Santobono-Pausilipon (protocol number 2821). All subjects give written informed consent to participate to the study.
2.5. Statistical analysis
Descriptive analyses were presented as means (± standard error of the mean), median and interquartile range (IQR). Categorial variables were described as frequency and percentage. A Shapiro-wilk test was performed to assess the normal distribution of data. In case of not-normal distribution appropriate non-parametric tests were performed. Mann-Whitney U test and Wilcoxon test were used when appropriate. Geometric mean titrers (GMTs) at baseline (T0) and 21 days after the second vaccine dose (T1) and the respective ratios (GMTRs) were summarized by treatment group. P-value less than 0.05 indicated significance. A multilinear regression model was used to compare the antibody levels across subjects treated with different DMTs after adjusting for age, sex, EDSS levels, disease duration, DMT duration and antibody levels in the pre-vaccination samples. Data analyses were performed using Graphpad Prism (version 8).
3. Results
3.1. Study cohort
Data were collected from March to June 2021. At the time of enrolment in this prospective monocentric study, 149 MS and 26 HC have been invited to participate. We excluded subjects previously infected with SARS-CoV-2, through the measurement of nucleocapsid-specific antibodies, the most sensitive target for serologic diagnosis of SARS-CoV-2 infection. After assessment, 125 MS and 24 healthy subjects without previous SARS-CoV-2 infection were enrolled. The demographic and clinical characteristics are reported in Table 1. In the MS group, 69 were female (55.2%) and 56 male (44.8%) and the mean age was 41 ± 10.7 (mean ± SD) years. In the control group, 13 subjects were females (54.2%) and 11 males (45.8%), with a mean age of 47.3 ± 11 (mean ± SD) years. In the MS cohort, 117 had relapsing-remitting (RR) (93.6%), 4 primary-progressive (PP) (3.2%) and 4 secondary-progressive (SP) (3.2%) MS. Different types of disease-modifying therapies (DMTs) were: interferon β 1a (IFN) (n = 22; 17.6%), dimethyl fumarate (DMF) (n = 18; 14.4%), teriflunomide (TERI) (n = 10; 8%), natalizumab (NAT) (n = 22; 17.6%), glatiramer acetate (GA) (n = 9; 7.2%), cladribine (CLAD) (n = 10; 8%), fingolimod (FTY) (n = 19; 15.2%) and ocrelizumab (OCRE) (n = 15; 12%).
3.2. Distinctive SARS-COV-2 humoral response in ms subjects undergoing different types of disease modifying therapies (DMTs)
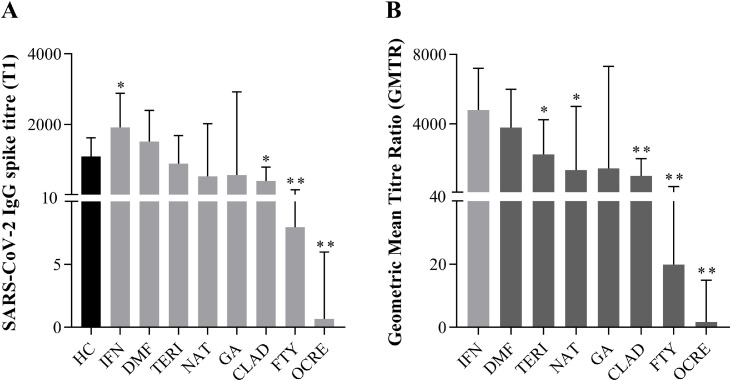
We measured SARS-CoV-2 IgG titre 21 days after the second BNT162b2 mRNA vaccine dose (T1) in MS subjects undergoing different DMTs, compared to age- and sex-matched HC. We found that IFN-treated MS subjects showed a significant increase of anti-spike IgG levels compared to HC (median 1916 (IQR: 1024–2879) vs 1089 (IQR: 652.5–1625) U/mL; p = 0.029); on the contrary, IgG levels were significantly reduced in MS subjects undergoing CLAD (median 396.9 (IQR: 37.52–790.9) vs 1089 (IQR: 652.5–1625) U/mL; p = 0.002), FTY (median 7.9 (IQR: 4.8–147.6) vs 1089 (IQR: 652.5–1625) U/mL; p < 0.0001) and OCRE (median 0.67 (IQR: 0.4–5.9) vs 1089 (IQR: 652.5–1625) U/mL; p < 0.0001) treatment (Fig. 1 A). The percentage of MS subjects on CLAD, FTY and OCRE with antibody levels above the cut-off of positivity was 100% (10/10), 89% (17/19) and 60% (9/15), respectively. An augmented antibody production that did not reach any statistical significance was also found in DMF-treated MS subjects (median 1514 (IQR: 767.7–2398) U/ml vs 1089 (IQR: 652.5–1625) U/mL; p = 0.23). Then, we measured anti-spike IgG titre in all DMT-groups before the first (T0) and after the second (T1) vaccine dose to calculate the geometric mean titre ratio (GMTR). Compared to the highest value found in IFN-treated MS subjects (median 4789 (IQR: 2560–7196) U/ml), we observed a reduced GMTR in TERI- (median 2223 (IQR: 552.3–4226) U/ml), NAT- (median 1331 (IQR: 362.8–5004) U/ml), CLAD- (median 992.1 (IQR: 93.7–1977) U/ml), FTY- (median 19.8 (IQR: 11.9–369) U/ml) and OCRE- (median 1.7 (IQR: 1–14.9) U/ml) treated MS subjects (Fig. 1 B and Supplementary Table 1). We performed linear regression analyses to rule out that individual factors or clinical variables (age, sex, EDSS, DMT duration, time to last therapeutic administration) could affect the SARS-CoV-2 vaccine humoral response, but we did not find any significant correlation between these parameters and IgG-spike titre in the different DMTs groups (data not shown). Our data highlight how DMTs could differentially affect or promote the protective SARS-CoV-2 humoral response in MS subjects.
Fig. 1.
(A) SARS CoV-2 IgG Spike titre – median difference between healthy controls (HC) and different DMT-treated multiple sclerosis (MS) subjects 3 weeks post second vaccine dose (T1). Data are reported as median with interquartile range (IQR) of MS or HC. Mann U-Whitney two tailed test vs HC was performed and a p-value less than 0.05 was considered statistically significant. *p < 0.05, **p < 0.0001. (B) Geometric mean titre ratio (GMTR) between IFN and the different DMTs. GMTR was obtained as the ratio between post-vaccination (T1) and pre-vaccination (T0) SARS CoV-2 IgG titre. Data are reported as median with interquartile range (IQR) of MS or HC. Mann U-Whitney two tailed test vs IFN was performed and a p-value less than 0.05 was considered statistically significant. *p < 0.05, **p < 0.0001.
3.3. Immunological phenotype in different DMT-treated MS subjects
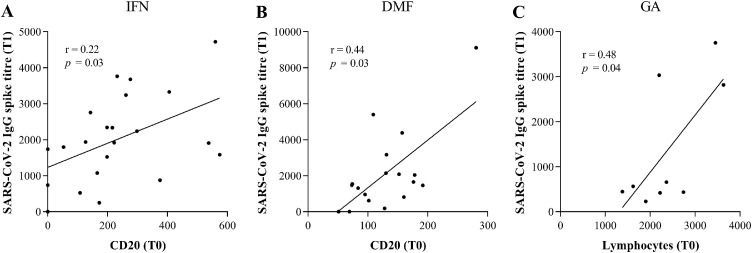
We performed a basic immunological phenotype to evaluate B and T cell frequency in our study cohort. As reported in Table 2 , we did not find any significant difference in the absolute number of peripheral leukocytes (WBC), B (CD20), T (CD3), CD4 and CD8 cells, before (T0) and 3 weeks after the second (T1) SARS-CoV-2 vaccine in all the DMT's groups of MS subjects. At T1, we observed an increased frequency of B cells in OCRE- and of CD4 T lymphocytes in TERI-treated MS subjects, and a reduction of leukocytes in DMF-treated MS subjects, despite these changes do not reach any statistical significance. Then, we performed linear regression analysis to evaluate the association between humoral response and the absolute number of leukocytes, B, T, CD4 and CD8 cell counts in the different groups of DMTs. In IFN- and DMF-treated MS subjects we observed a direct correlation between anti-spike IgG levels at T1 and B cell count at baseline (T0) (IFN: r = 0.22, p = 0.03; DMF: r = 0.44, p = 0.03) (Fig. 2 A and 2 B); moreover, anti-spike IgG levels directly correlated with lymphocyte number at baseline (T0) in GA-treated MS subjects (r = 0.48, p = 0.04) (Fig. 2 C). These findings underline that the impact of DMTs on B and T cell count could affect the humoral response to SARS-CoV-2 vaccine in MS subjects.
Table 2.
Absolute number of white blood cells (WBC), lymphocytes, CD20, CD3, CD4 and CD8 before the first (T0) and 21 days after the second (T1) vaccine dose in multiple sclerosis subjects under different disease modifying therapies (DMTs). Mann-Whitney two-tailed was performed and a p<0.05 was considered statistically significant.
| DMTs | T0 | T1 | p-value | |
|---|---|---|---|---|
| WBC (cells/μL) |
Interferon β 1a | 6162 | 6169 | 0.94 |
| Dimethyl fumarate | 6487 | 5543 | 0.07 | |
| Teriflunomide | 7268 | 8267 | 0.35 | |
| Natalizumab | 8981 | 8557 | 0.79 | |
| Glatiramer acetate | 8250 | 7872 | 0.99 | |
| Cladribine | 5794 | 5992 | 0.92 | |
| Fingolimod | 5565 | 5390 | 0.56 | |
| Ocrelizumab | 6808 | 6142 | 0.17 | |
| Lymphocytes (cells/mm3) |
Interferon β 1a | 1979 | 2002 | 0.84 |
| Dimethyl fumarate | 1284 | 1322 | 0.75 | |
| Teriflunomide | 1809 | 2359 | 0.20 | |
| Natalizumab | 3590 | 3418 | 0.66 | |
| Glatiramer acetate | 2389 | 2214 | 0.54 | |
| Cladribine | 1135 | 1203 | 0.72 | |
| Fingolimod | 809 | 828 | 0.82 | |
| Ocrelizumab | 1594 | 1648 | 0.94 | |
| CD20 (cells/mL) |
Interferon β 1a | 233 | 273 | 0.74 |
| Dimethyl fumarate | 130 | 129 | 0.77 | |
| Teriflunomide | 188 | 225 | 0.63 | |
| Natalizumab | 638 | 623 | 0.88 | |
| Glatiramer acetate | 154 | 167 | 0.65 | |
| Cladribine | 105 | 133 | 0.58 | |
| Fingolimod | 30 | 51 | 0.26 | |
| Ocrelizumab | 10 | 32 | 0.07 | |
| CD3 (cells/mL) |
Interferon β 1a | 1441 | 1488 | 0.97 |
| Dimethyl fumarate | 871 | 853 | 0.93 | |
| Teriflunomide | 1331 | 1814 | 0.22 | |
| Natalizumab | 2333 | 2319 | 0.79 | |
| Glatiramer acetate | 1795 | 1741 | 0.60 | |
| Cladribine | 800 | 828 | 0.91 | |
| Fingolimod | 506 | 498 | 0.84 | |
| Ocrelizumab | 1320 | 1382 | 0.77 | |
| CD4 (cells/mL) |
Interferon β 1a | 957 | 1009 | 0.80 |
| Dimethyl fumarate | 628 | 650 | 0.85 | |
| Teriflunomide | 816 | 1079 | 0.06 | |
| Natalizumab | 1424 | 1317 | 0.45 | |
| Glatiramer acetate | 1134 | 1075 | 0.99 | |
| Cladribine | 451 | 477 | 0.85 | |
| Fingolimod | 289 | 277 | 0.84 | |
| Ocrelizumab | 839 | 891 | 0.71 | |
| CD8 (cells/mL) |
Interferon β 1a | 202 | 223 | 0.81 |
| Dimethyl fumarate | 114 | 115 | 0.88 | |
| Teriflunomide | 189 | 272 | 0.19 | |
| Natalizumab | 373 | 341 | 0.54 | |
| Glatiramer acetate | 273 | 286 | 0.49 | |
| Cladribine | 124 | 125 | 0.85 | |
| Fingolimod | 88 | 77 | 0.91 | |
| Ocrelizumab | 234 | 254 | 0.46 |
Fig. 2.
(A) Linear regression analysis between the absolute number of CD20 pre-vaccination (T0) and SARS CoV-2 IgG Spike titre 3 weeks post-vaccination (T1) in IFN-treated MS subjects. Analysis was assessed by Spearman rank correlation and reported as coefficient of correlation (r). p-value less than 0.05 was considered statistically significant. (B) Linear regression analysis between the absolute number of CD20 pre-vaccination (T0) and SARS CoV-2 IgG Spike titre 3 weeks post-vaccination (T1) in DMF-treated MS subjects. Analysis was assessed by Spearman rank correlation and reported as coefficient of correlation (r). p-value less than 0.05 was considered statistically significant. (C) Linear regression analysis between the absolute number of lymphocytes pre-vaccination (T0) and SARS CoV-2 IgG Spike titre 3 weeks post-vaccination (T1) in GA-treated MS subjects. Analysis was assessed by Spearman rank correlation and reported as coefficient of correlation (r). p-value less than 0.05 was considered statistically significant.
4. Discussion
This study reports SARS-CoV-2-specific vaccine response in MS subjects treated with different DMTs, all receiving BNT162b2 mRNA vaccine, with no history of previous COVID-19 infection. We evaluated vaccine-specific anti-spike immunoglobulin levels together with lymphocyte (B, T, CD4, CD8) cell counts before the first (T0) and 3 weeks after the second (T1) vaccination. Firstly, we demonstrated that BNT162b2 vaccine elicited comparable humoral response in healthy individuals and in MS subjects treated with GA, TERI, DMF and NAT. This evidence substantially confirms what previously reported on the immune competence of MS subjects undergoing these therapies (Sormani et al., 2021). Interestingly, we were first in showing that, when compared to HC, IFN-treated MS subjects showed higher IgG titre at T1 and this parameter strongly correlated with their B cell count at T0. Our findings expand the well-known preservation of B and T lymphocyte count, the good humoral response to seasonal influenza vaccination (Olberg et al., 2018; Metze et al., 2019) and, more importantly, the reduced susceptibility to develop SARS-CoV-2 (or other) infections in IFN-treated MS subjects (Sormani et al., 2021a, Sormani et al., 2021c; Sormani et al., 2021). In addition, we also unveiled that IFN treatment preserved a good B cell reservoir that correlated with SARS-CoV-2 specific humoral response in MS subjects. Moreover, we found a direct correlation between IgG titre at T1 and B cell count at T0 in DMF-treated MS subjects or between IgG titre at T1 and lymphocyte count at T0 in GA-treated MS subjects. This is extremely interestingly, as these two treatments are those with anti-spike IgG levels comparable to HC.
On the contrary, post-vaccination humoral response resulted impaired in MS subjects treated with CLAD, FTY and OCRE, despite with different serological positivity. Indeed, although commonly reduced in terms of levels, detectable anti-spike IgG were observed 21 days after the second vaccination in 100% of CLAD-, 89% FTY- while only in 60% of OCRE-treated MS subjects. It is important to mention that the reduction observed in OCRE- and CLAD-groups of MS subjects was independent to time from last infusion, in contrast with recently published data, by Sormani et al. (Sormani et al. (2021); however, this difference could be due either to the smaller subject number or to the exclusion of COVID-19 previously-infected MS subjects enrolled in our monocentric study. In line with recent data, we found that MS subjects treated with FTY and OCRE failed to mount a good anti-SARS-CoV-2 humoral response (Achiron et al., 2021; Bigaut et al., 2021; Sormani et al., 2021); conversely, we are first in showing increased anti-spike IgG titre in IFN- and a reduction in CLAD-treated MS subjects. While the well-known effect of the B-cell depleting therapies (Bar-Or et al., 2020) could anticipate the OCRE result, the effects of FTY and CLAD were less obvious. Our findings are supported by recent data showing the failure to mount an effective response to influenza vaccine in FTY-treated MS subjects (Kappos et al., 2015; Olberg et al., 2018). Moreover, as FTY interferes with lymphocyte migration in the periphery, a reduced antibody response could reflect T and B cell retention in the lymph nodes (Cross and Naismith, 2014). The same holds true also for CLAD, which have been described to inhibit both proliferating T and memory B cells (Moser et al., 2020). According to these data, we also observed reduced B, T, CD4 and CD8 cell count in both FTY- and CLAD-treated MS subjects enrolled in our study.
Analysis of the GMTR in the different DMT groups revealed that, when compared to IFN, treatment with TERI, NAT, CLAD, FTY and OCRE significantly reduced the intrinsic ability to produce anti-spike specific antibodies in MS subjects. It is also important to note that, among all the DMTs, IFN increased while DMF and GA preserved an efficient humoral response. Since the antibody production in these DMTs-treated MS subjects directly correlated with the B- or lymphocyte-count before vaccination, we hypothesized that these therapies preserve the antibody production because they did not affect peripheral T lymphocyte function in MS treated subjects.
Interestingly, despite its well-known activity as CD20-depletor, immunophenotypic analysis of OCRE-treated MS subjects revealed a surprising rise in B cell count at T1, thus testifying that a small, albeit insufficient, B cell response was still preserved in this group of subjects. However, as these data did not reach a statistical significance, they should be expanded in a larger cohort of OCRE-treated MS subjects to be confirmed.
5. Conclusion
In conclusion, this study is first in showing that IFN treatment boosts the SARS-CoV-2 specific humoral response, which is profoundly impaired in CLAD-, FTY- and OCRE-treated MS subjects. Also, we highlight that the preservation of the peripheral lymphocyte frequency and/or function before vaccination should represent a novel aspect to consider at the time of SARS-CoV-2 vaccination in MS subjects. Finally, our findings on the humoral response should benefit of future studies assessing the effect of the different DMTs on the SARS-CoV-2-specific T cell response in MS subjects.
Authors disclosures
G.T. Maniscalco received personal compensations from Serono, Biogen, Novartis, Roche and TEVA for public speaking and advisory boards. The other authors have nothing to disclose.
CRediT authorship contribution statement
Giorgia Teresa Maniscalco: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft. Valentino Manzo: Conceptualization, Project administration, Validation, Visualization, Writing – review & editing. Anne Lise Ferrara: Data curation, Formal analysis, Software. Alessandro Perrella: Project administration, Validation, Visualization, Writing – review & editing. Mariaelena Di Battista: Project administration, Validation, Visualization, Writing – review & editing. Simona Salvatore: Project administration, Validation, Visualization, Writing – review & editing. Daniela Graziano: Resources. Assunta Viola: Resources. Gerardino Amato: Resources. Ornella Moreggia: Data curation, Software. Daniele Di Giulio Cesare: Data curation, Formal analysis, Software. Stefano Barbato: Project administration, Validation, Visualization, Writing – review & editing. Giovanna Servillo: Project administration, Validation, Visualization, Writing – review & editing. Katia Longo: Project administration, Validation, Visualization, Writing – review & editing. Mario Di Giovanni: Project administration, Validation, Visualization, Writing – review & editing. Barbara Scarpati: Resources. Simona Maria Muggianu: Resources. Giuseppe Longo: Project administration, Validation, Visualization, Writing – review & editing. Giuseppe Russo: Project administration, Validation, Visualization, Writing – review & editing. Vincenzo Andreone: Project administration, Supervision, Validation, Visualization, Writing – review & editing. Veronica De Rosa: Investigation, Methodology, Supervision, Writing – original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
This work was supported by FISM 2018/R/4 from Fondazione Italiana Sclerosi Multipla, STAR Program Linea 1-2018 by UniNA and by Compagnia di San Paolo from the Università degli Studi di Napoli “Federico II”, Bando PRIN 2017 Prot. 2017K7FSYB from Ministry of Education, University and Research (MIUR).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.msard.2021.103455.
Appendix. Supplementary materials
References
- Achiron A., Dolev M., Menascu S., Zohar D.N., Dreyer-Alster S., Miron S., Shirbint E., Magalashvili D., Flechter S., Givon U., Guber D., Stern Y., Polliack M., Falb R., Gurevich M. COVID-19 vaccination in patients with multiple sclerosis: what we have learnt by February 2021. Mult. Scler. 2021;27(6):864–870. doi: 10.1177/13524585211003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achiron A., Mandel M., Dreyer-Alster S., Harari G., Magalashvili D., Sonis P., Dolev M., Menascu S., Flechter S., Falb R., Gurevich M. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther. Adv. Neurol. Disord. 2021;14 doi: 10.1177/17562864211012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or A., Calkwood J.C., Chognot C., Evershed J., Fox E.J., Herman A., Manfrini M., McNamara J., Robertson D.S., Stokmaier D., Wendt J.K., Winthrop K.L., Traboulsee A. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology. 2020;95(14):e1999–e2008. doi: 10.1212/WNL.0000000000010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigaut K., Kremer L., Fleury M., Lanotte L., Collongues N., de Seze J. Impact of disease-modifying treatments on humoral response after COVID-19 vaccination: a mirror of the response after SARS-CoV-2 infection. Rev. Neurol. (Paris) 2021 doi: 10.1016/j.neurol.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D., Rocca M.A., Gasperini C., Kappos L., Hartung H.P., Magyari M., Oreja-Guevara C., Trojano M., Wiendl H., Filippi M. Disease-modifying therapies and SARS-CoV-2 vaccination in multiple sclerosis: an expert consensus. J. Neurol. 2021 doi: 10.1007/s00415-021-10545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A.H., Naismith R.T. Established and novel disease-modifying treatments in multiple sclerosis. J. Intern. Med. 2014;275(4):350–363. doi: 10.1111/joim.12203. [DOI] [PubMed] [Google Scholar]
- Guerrieri S., Lazzarin S., Zanetta C., Nozzolillo A., Filippi M., Moiola L. Serological response to SARS-CoV-2 vaccination in multiple sclerosis patients treated with fingolimod or ocrelizumab: an initial real-life experience. J. Neurol. 2021 doi: 10.1007/s00415-021-10663-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser S.L., Cree B.A.C. Treatment of multiple sclerosis: a review. Am. J. Med. 2020;133(12):1380–1390e1382. doi: 10.1016/j.amjmed.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappos L., Mehling M., Arroyo R., Izquierdo G., Selmaj K., Curovic-Perisic V., Keil A., Bijarnia M., Singh A., von Rosenstiel P. Randomized trial of vaccination in fingolimod-treated patients with multiple sclerosis. Neurology. 2015;84(9):872–879. doi: 10.1212/WNL.0000000000001302. [DOI] [PubMed] [Google Scholar]
- Metze C., Winkelmann A., Loebermann M., Hecker M., Schweiger B., Reisinger E.C., Zettl U.K. Immunogenicity and predictors of response to a single dose trivalent seasonal influenza vaccine in multiple sclerosis patients receiving disease-modifying therapies. CNS Neurosci. Ther. 2019;25(2):245–254. doi: 10.1111/cns.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser T., Schwenker K., Seiberl M., Feige J., Akgun K., Haschke-Becher E., Ziemssen T., Sellner J. Long-term peripheral immune cell profiling reveals further targets of oral cladribine in MS. Ann. Clin. Transl. Neurol. 2020;7(11):2199–2212. doi: 10.1002/acn3.51206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naser Moghadasi A., Shabany M., Heidari H., Eskandarieh S. Can pulse steroid therapy increase the risk of infection by COVID-19 in patients with multiple sclerosis? Clin. Neurol. Neurosurg. 2021;203 doi: 10.1016/j.clineuro.2021.106563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olberg H.K., Eide G.E., Cox R.J., Jul-Larsen A., Lartey S.L., Vedeler C.A., Myhr K.M. Antibody response to seasonal influenza vaccination in patients with multiple sclerosis receiving immunomodulatory therapy. Eur. J. Neurol. 2018;25(3):527–534. doi: 10.1111/ene.13537. [DOI] [PubMed] [Google Scholar]
- Pardi N., Hogan M.J., Naradikian M.S., Parkhouse K., Cain D.W., Jones L., Moody M.A., Verkerke H.P., Myles A., Willis E., LaBranche C.C., Montefiori D.C., Lobby J.L., Saunders K.O., Liao H.X., Korber B.T., Sutherland L.L., Scearce R.M., Hraber P.T., Tombacz I., Muramatsu H., Ni H., Balikov D.A., Li C., Mui B.L., Tam Y.K., Krammer F., Kariko K., Polacino P., Eisenlohr L.C., Madden T.D., Hope M.J., Lewis M.G., Lee K.K., Hu S.L., Hensley S.E., Cancro M.P., Haynes B.F., Weissman D. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J. Exp. Med. 2018;215(6):1571–1588. doi: 10.1084/jem.20171450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Perez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Jr., Hammitt L.L., Tureci O., Nell H., Schaefer A., Unal S., Tresnan D.B., Mather S., Dormitzer P.R., Sahin U., Jansen K.U., Gruber W.C., Group C.C.T. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin U., Muik A., Derhovanessian E., Vogler I., Kranz L.M., Vormehr M., Baum A., Pascal K., Quandt J., Maurus D., Brachtendorf S., Lorks V., Sikorski J., Hilker R., Becker D., Eller A.K., Grutzner J., Boesler C., Rosenbaum C., Kuhnle M.C., Luxemburger U., Kemmer-Bruck A., Langer D., Bexon M., Bolte S., Kariko K., Palanche T., Fischer B., Schultz A., Shi P.Y., Fontes-Garfias C., Perez J.L., Swanson K.A., Loschko J., Scully I.L., Cutler M., Kalina W., Kyratsous C.A., Cooper D., Dormitzer P.R., Jansen K.U., Tureci O. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- Sormani M.P., De Rossi N., Schiavetti I., Carmisciano L., Cordioli C., Moiola L., Radaelli M., Immovilli P., Capobianco M., Trojano M., Zaratin P., Tedeschi G., Comi G., Battaglia M.A., Patti F., Salvetti M., Musc-19 Study G. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann. Neurol. 2021;89(4):780–789. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., Inglese M., Schiavetti I., Carmisciano L., Laroni A., Lapucci C., Da Rin G., Serrati C., Gandoglia I., Tassinari T., Perego G., Brichetto G., Gazzola P., Mannironi A., Stromillo M.L., Cordioli C., Landi D., Clerico M., Signoriello E., Frau J., Ferro M.T., Sapio A.D., Pasquali L., Ulivelli M., Marinelli F., Callari G., Iodice R., Liberatore G., Caleri F., Repice A.M., Cordera S., Battaglia M.A., Salvetti M., Franciotta D., Uccelli A., s. g. o. b. o. t. I. C.-A. i. M. S. CovaXi M.S. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine. 2021 doi: 10.1016/j.ebiom.2021.103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., Salvetti M., Labauge P., Schiavetti I., Zephir H., Carmisciano L., Bensa C., De Rossi N., Pelletier J., Cordioli C., Vukusic S., Moiola L., Kerschen P., Radaelli M., Theaudin M., Immovilli P., Casez O., Capobianco M., Ciron J., Trojano M., Stankoff B., Creange A., Tedeschi G., Clavelou P., Comi G., Thouvenot E., Battaglia M.A., Moreau T., Patti F., De Seze J., Louapre C., Musc and g. Covisep study DMTs and Covid-19 severity in MS: a pooled analysis from Italy and France. Ann. Clin. Transl. Neurol. 2021;8(8):1738–1744. doi: 10.1002/acn3.51408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E.E., Frenck R.W., Jr., Falsey A.R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M.J., Bailey R., Swanson K.A., Li P., Koury K., Kalina W., Cooper D., Fontes-Garfias C., Shi P.Y., Tureci O., Tompkins K.R., Lyke K.E., Raabe V., Dormitzer P.R., Jansen K.U., Sahin U., Gruber W.C. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N. Engl. J. Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.