Abstract
A fraction of COVID-19 convalescent individuals mount a potent antibody response to SARS-CoV-2 with cross-reactivity to SARS-CoV-1. To uncover their humoral response in detail, we performed single B cell analysis from 10 SARS-CoV-2 elite neutralizers. We isolated and analyzed 126 monoclonal antibodies, many of which were sarbecovirus cross-reactive, with some displaying merbecovirus- and embecovirus-reactivity. Several isolated broadly neutralizing antibodies were effective against B.1.1.7, B.1.351, B.1.429, B.1.617, and B.1.617.2 variants and 19 prominent potential escape sites. Furthermore, assembly of 716,806 SARS-CoV-2 sequences predicted emerging escape variants, which were also effectively neutralized. One of these broadly neutralizing potent antibodies, R40-1G8, is a IGHV3-53 RBD-class-1 antibody. Remarkably, cryo-EM analysis revealed that R40-1G8 has a flexible binding mode, targeting both “up” and “down” conformations of the RBD. Given the threat of emerging SARS-CoV-2 variants, we demonstrate that elite neutralizers are a valuable source for isolating ultrapotent antibody candidates to prevent and treat SARS-CoV-2 infection.
Keywords: COVID-19, SARS-CoV-2, broadly neutralizing antibodies, cryo-EM, variants of concern, emerging variants, ultrapotent monoclonal antibodies
Graphical abstract
Vanshylla et al. deciphered the antibody response in SARS-CoV-2 convalescent elite neutralizers on a single B cell level. Isolated antibodies were highly potent and neutralized various mutants, including the predominant variants of concern and emerging variants. Structural analysis of one potent IGHV3-53/IGKV1-9 bNAb revealed a flexible binding mechanism to the RBD.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has led to loss of over 5 million lives worldwide in the last 2 years (Dong et al., 2020). Although a majority of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections are mild, hospitalizations and death can occur in all age groups, and older individuals with co-morbidities, in particular, are at risk (Williamson et al., 2020). The rapid and successful development of effective SARS-CoV-2 vaccines has been a critical breakthrough (Krammer, 2020). Vaccines can protect from severe disease and death as well as mitigate the spread of infection (Thompson et al., 2021). However, unvaccinated individuals and vulnerable groups such as immune-deficient patients who cannot mount adequate immune responses still remain susceptible to infection and severe complications (Choi et al., 2020). Moreover, the current rise in SARS-CoV-2 variants with antigenic escape mutations can reduce the efficacy of currently approved vaccines and result in increased incidents of breakthrough infections (Lopez Bernal et al., 2021). This warrants the need for interventions which can effectively treat SARS-CoV-2 infection by preventing severe disease and reducing morbidity.
Neutralizing antibodies (NAbs) are an important part of the humoral immune system for preventing viral infections and are crucial for protection (Khoury et al., 2021). They can block viral entry into cells and mediate clearance of viral particles through Fc-mediated effector functions (Zohar and Alter, 2020). Advanced single B cell analyses have helped decipher the B cell response to SARS-CoV-2 and resulted in the isolation of several highly potent monoclonal antibodies (mAbs) (Andreano et al., 2021; Ju et al., 2020; Kreer et al., 2020b; Liu et al., 2020; Robbiani et al., 2020; Rogers et al., 2020; Zost et al., 2020). These SARS-CoV-2 antibodies are directed against the spike (S) protein, which facilitates viral entry into human cells by binding to the human ACE-2 receptor (Hoffmann et al., 2020a; Walls et al., 2020) and is expressed on the virus surface (Piccoli et al., 2020). On the spike protein, a large fraction of the NAb response is directed at the receptor binding domain (RBD) (Piccoli et al., 2020) or the N-terminal domain (NTD) of the SARS-CoV-2 spike S1 domain (Liu et al., 2020). The S2 domain of the spike protein is more conserved than the S1 among β-coronaviruses (β-CoVs) (Cui et al., 2019); however, described NAbs targeting the S2 domain are rare and less potent (Pinto et al., 2021; Sauer et al., 2021). The highly potent neutralizing capacity of RBD-directed antibodies (Dejnirattisai et al., 2021) has led to the clinical development of mAbs for treating and preventing COVID-19 (Corti et al., 2021). Passive immunization with mAbs can prevent infection in exposed individuals (O’Brien et al., 2021) as well as treat COVID-19 and prevent progression to severe disease (Chen et al., 2021b; Dougan et al., 2021; Weinreich et al., 2021). Several of these mAbs have received emergency Food and Drug Administration and European Medicines Agency approval for treatment of COVID-19 or are currently being investigated in phase-III clinical trials (Corti et al., 2021).
Despite the success of antibody-mediated treatment, the recent emergence of SARS-CoV-2 variants with antigenic escape mutations in the spike has led to reduced effectiveness or rendered approved antibodies ineffective due to loss of neutralizing activity (Hoffmann et al., 2021; U.S. Department of Health & Human Services, 2021; Wang et al., 2021). Therefore, it is essential to develop next-generation mAbs that retain potency and effectiveness against circulating or emerging SARS-CoV-2 variants.
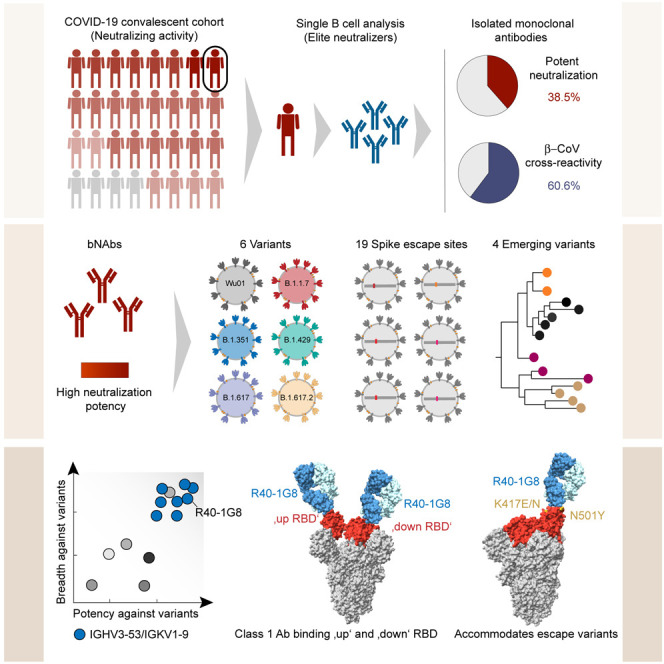
We recently studied the NAb response in a SARS-CoV-2 convalescent cohort of 963 individuals (Vanshylla et al., 2021). The NAb response was found to be highly diverse with some individuals lacking any detectable NAb response following natural infection, while others demonstrated a highly potent NAb response. The latter group of so-called “elite neutralizers” not only displayed high potency against SARS-CoV-2 but also had cross-reactive antibodies against SARS-CoV-1. In order to understand the Ab response in these individuals, we isolated 1,361 single B cells and produced mAbs from 10 donors. Based on a detailed B cell repertoire analysis, functional antibody characterization and cryoelectron microscopy (cryo-EM) structure determination, we identified diverse β-CoV cross-reactive antibodies as well as a large pool of SARS-CoV-2 broadly neutralizing antibodies (bNAbs). These bNAbs are ultrapotent against circulating SARS-CoV-2 variants of concern (VOCs) as well as emerging escape variants derived from phylogenetic tracking of global SARS-CoV-2 sequences. Therefore, these bNAbs isolated from elite neutralizers serve as an important resource to combat emerging SARS-CoV-2 variants as well as potential future CoV pandemics.
Results
Identification of SARS-CoV-2 elite neutralizers
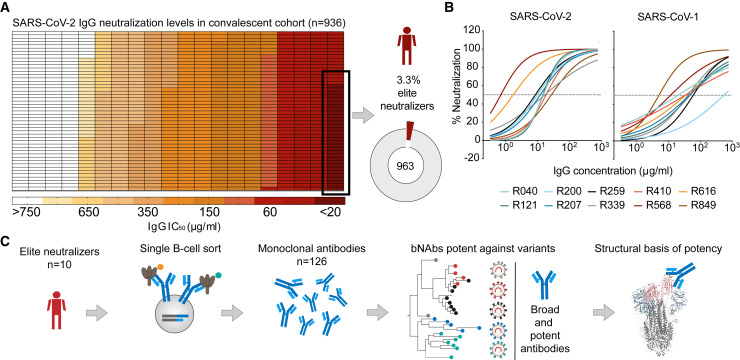
We recently analyzed the NAb response in a cohort of SARS-CoV-2 convalescent individuals (n = 963) (Figure 1 A) (Vanshylla et al., 2021). The neutralizing capacity of the cohort was measured by testing purified plasma immunoglobin G (IgG) in a Wu01-spike-based SARS-CoV-2 pseudovirus assay. The analysis revealed a small fraction of the cohort (3.3%) displaying a highly potent neutralizing response with IgG 50% inhibitory concentration (IC50) values of <20 μg/mL (Figure 1A) and were identified as elite neutralizers (Vanshylla et al., 2021). Ten of these individuals (R040, R121, R200, R207, R259, R339, R410, R568, R616, and R849), ranging in age from 32 to 60 years old (median age: 52), served as donors for single B cell evaluation (Table S1A).
Figure 1.
Identification of SARS-CoV-2 elite neutralizers
(A) Heatmap depicting the IgG neutralization IC50 values against the SARS-CoV-2 Wu01 pseudovirus in the COVID-19 convalescent cohort studied (Vanshylla et al., 2021). Pie chart shows the fraction of elite neutralizers in the cohort (3.3%).
(B) Neutralization curves depicting IgG neutralization from n = 10 donor elite neutralizers against SARS-CoV-2 and SARS-CoV-1 pseudovirus. Mean of two measurements plotted and dotted line represents 50% neutralization.
(C) Schematic of study design used to identify and isolate monoclonal antibodies from SARS-CoV-2 elite neutralizers.
The potency of the IgG response against SARS-CoV-2 in selected subjects ranged from 0.7 to 31 μg/mL (geometric mean IC50: 8.6 μg/mL) (Figure 1B; Table S1B). Moreover, all these individuals also had a highly potent IgG response against SARS-CoV-1 with IgG IC50 ranging between 5.1 and 391.7 μg/mL (geometric mean IC50: 36.3 μg/mL) (Figure 1B; Table S1B). Examination of the IgG binding to the SARS-CoV-2 RBD, NTD, and S1 and S2 domains, as well as soluble full-length trimer, revealed reactivity against all regions of the spike (Figure S1A; Table S1B). Moreover, elite-neutralizer-derived IgG demonstrated broad β-CoV reactivity against spike proteins of sarbecovirus (SARS-1) and merbecovirus (MERS) as well as the common cold embecoviruses (OC43 and HKU-1) (Figure S1A; Table S1B). The elite neutralizers also displayed neutralizing IgA antibodies with IC50s ranging from 3.0 to 110.5 μg/mL but with overall lower potency compared to IgG against the SARS-CoV-2 spike (Figures S1B and S1C; Table S1B). In addition to antibody responses, we also tested T cell reactivity against the SARS-CoV-2 spike protein using peptide pools spanning either the S1 or S2 domain (Figures S1D–S1F). PBMC samples from elite neutralizers were compared to high, average, or low neutralizers from the same convalescent cohort (Table S1C) (Vanshylla et al., 2021). We found higher levels of activation-induced marker positive (AIM+) CD4 T cells in elite neutralizers (geometric mean frequency: 0.06%) as compared to high (geometric mean frequency: 0.02%), average (geometric mean frequency: 0.008%), or low neutralizers (geometric mean frequency: 0.008%); however, these differences did not reach statistical significance (Figure S1F). This is in line with previous reports on partial correlation between B and T cell responses to SARS-CoV-2 spike in convalescent individuals (Figure S1F) (Rydyznski Moderbacher et al., 2020). Together, these results demonstrate that elite neutralizers generate a highly potent NAb response against SARS-2 and possess pan-β-CoV reactivity after natural SARS-CoV-2 infection. Thus, such individuals may serve as an ideal source for the identification of novel broad and ultrapotent SARS-CoV-2 mAbs (Figure 1C).
Convergent evolution of B cell response in elite neutralizers
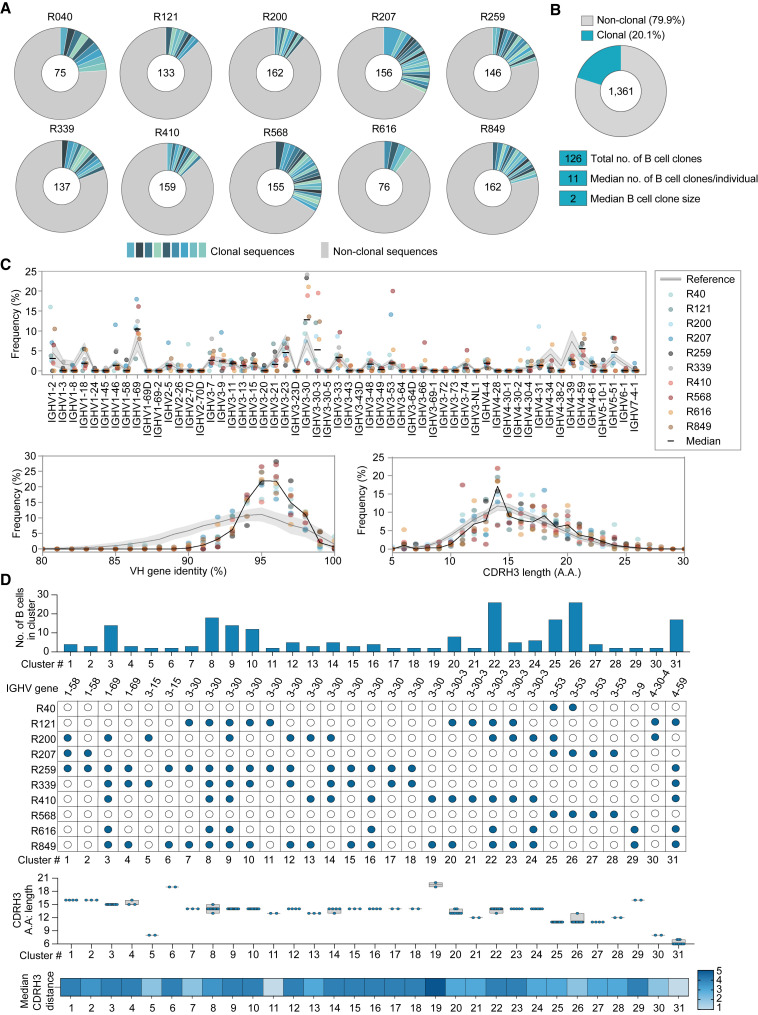
To decipher the B cell response on a single-cell level, we used a pre-fusion stabilized HexaPro SARS-CoV-2 spike protein (Hsieh et al., 2020) as bait to sort antigen-specific B cells from all 10 individuals. The frequency of spike-reactive B cells that bound to both Dylight488-labeled and Dylight650-labeled spike protein bait ranged from 0.4% to 2.1% of the enriched IgG+ B cell fraction (Figure S2A). Reverse transcription and PCR amplification from single-sorted B cells yielded a total of 1,361 productive IgG-heavy chain sequences with between 75–162 IgG-heavy chain sequences obtained from each donor (Figure 2 A). Within each individual, 10%–33% of the isolated sequences were clonal with a median B cell-clone size of two, indicating a low clonal expansion and a diverse polyclonal response (Figure 2B). Similar to other COVID-19 convalescent individuals (Cerutti et al., 2021; Chen et al., 2021a; Yuan et al., 2020), the SARS-CoV-2 spike-specific antibodies from elite neutralizers had a relative enrichment for IGHV3-30 (12.8%), IGHV1-69 (10.4%), IGHV4-59 (5.5%), IGHV3-30-3 (5.2%), IGHV1-2 (3.2%), and IGHV3-53 (1.9%) (Figure 2C). A median CDRH3 length of 14 amino acids (aa) (range 5–30 aa) (Figure 2C) and an inferred germline identity of 94% (range 87.4%–100%) was observed (Figure 2C).
Figure 2.
Single B cell analysis of the antibody response against SARS-CoV-2 in elite neutralizers
(A) Pie charts depicting distribution of clonal (shades of blue) and non-clonal (gray) single B cell-derived heavy chain sequences from each elite neutralizer.
(B) Pie chart illustrating overall clonality of all productive (n = 1,361) SARS-CoV-2 reactive heavy chain B cell sequences. Total numbers of sequences analyzed shown in center of pie charts in (A) and (B).
(C) Frequencies of heavy chain V-gene distribution from SARS-CoV-2 elite neutralizers (upper panel) and analysis of heavy chain germline identity and CDRH3 length (lower panels) of IGHV sequences derived from elite neutralizers.
(D) Analysis of rates of sequence similarity in the heavy chain CDRH3 from the SARS-CoV-2 antibody repertoire of elite neutralizers. Top to bottom: analysis of size (number) of B cell clusters, V-gene information and individuals included in the cluster, length of the CDRH3s (bars show min. to max. with line at median), and the median CDRH3 distance. Reference in panels (C) and (D) (gray) refers to IGHV sequences derived from naive donors.
COVID-19 convalescent individuals have been shown to produce a shared public B cell clonotype response (Chen et al., 2021a; Nielsen et al., 2020). In order to study whether individuals who show elite neutralization generate similar antibodies, we performed a sequence similarity analysis of all heavy chain sequences. B cell sequences with the same immunoglobin heavy chain variable (IGHV) gene segments were allocated the same cluster if they shared ≥75% CDRH3 similarity (Figure 2D). IGHV3-30, IGHV3-30-3, IGHV3-53, and IGHV4-59 formed among the largest clusters encompassing up to 7 individuals within a cluster with 14 shared sequences (Figure 2D). One IGHV3-30-3 and one IGHV3-53 cluster comprised 26 shared B cell sequences derived from 5 and 3 individuals, respectively. Based on Levenshtein distance calculation, the median CDRH3 distance within clusters ranged from 1–4 amino acids and 6 clusters contained B cells with 2–6 identical CDRH3 sequences (Figure 2D). In summary, SARS-CoV-2 elite neutralizers mount a polyclonal antibody response with a high degree of IGHV sequence similarity, indicating convergent evolution of the antibody response.
SARS-CoV-2 elite neutralizers are a rich source of cross-reactive and ultrapotent antibodies
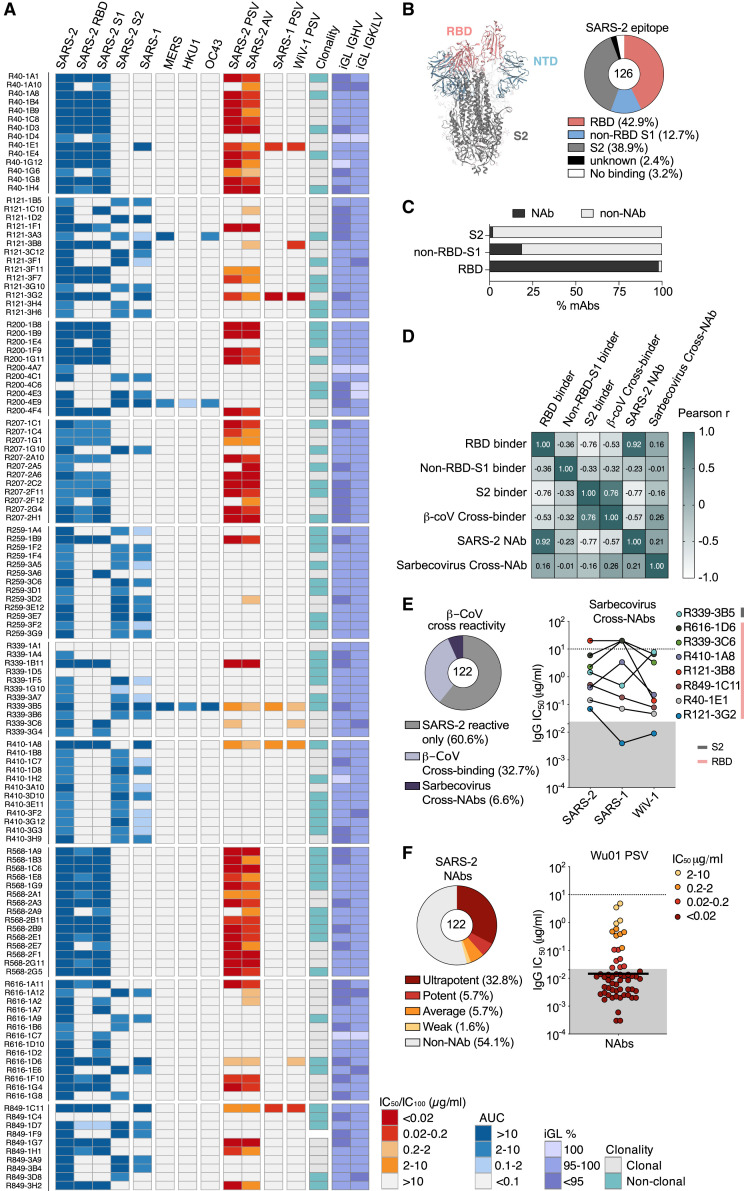
In order to functionally analyze the SARS-CoV-2 antibody response of elite neutralizers, we cloned and produced 126 mAbs, representing different heavy and light chain gene segment combinations including IgHV3-30 (16.6%), IgHV1-69 (9.5%), IgHV3-30-3 (7.9%), IgHV3-53 (11.1%), IgKV3-20 (19%), IgKV1-9 (11.9%), and IgLV1-40 (6.3%) (Figure S2B). All antibodies were tested in ELISA for reactivity against the SARS-CoV-2 trimer, RBD, and S1 and S2 domains, as well as SARS-1, MERS, HKU-1, and OC43 spike proteins (Figure 3 A). Neutralizing activity against SARS-CoV-2 was determined by using a spike-based pseudovirus assay (Figure 3A) as well as authentic virus assay (Figure S2C). In addition, neutralizing activity against SARS-CoV-1 and the bat CoV WiV1, which share the same receptor and have high sequence similarity with SARS-CoV-2 (Hoffmann et al., 2020a), was tested in pseudovirus assays (Figure 3A).
Figure 3.
CoV-cross-reactive and potent monoclonal antibodies derived from elite neutralizers
(A) Heatmap illustrating ELISA binding (AUC) against indicated CoV-spikes, pseudovirus neutralization (IC50) (SARS-2, SARS-1, and WIV-1 PSV column), authentic virus neutralization (IC100) (SARS-2 AV column), and clonality and germline identity of n = 126 elite neutralizer mAbs. iGL, inferred germline.
(B) Annotated P0DTC2 (6XKL) model of the SARS-CoV-2 spike (left) and pie chart showing epitope-binding distribution of the mAbs determined by ELISA (right).
(C) Bar graph presenting fraction of neutralizing mAbs (n = 122) binding to SARS-CoV-2 spike epitopes.
(D) Pearson correlation matrix of binding and neutralization data from (A) in order to study relationships between binding epitopes, SARS-2-specific neutralization, β-CoV cross-reactivity (SARS-1, MERS, HKU1, and OC43), and sarbecovirus cross-neutralization (SARS-1 and WiV-1).
(E) Pie chart depicting fraction of cross-reactive mAbs (left) and plot depicting IC50 values of sarbecovirus cross-neutralizing mAbs (right).
(F) Pie chart depicting fraction of NAbs, based on potency (left), and plot showing IC50s of NAbs against the SARS-CoV-2 Wu01 pseudovirus (right); black bar denotes geometic mean. Gray area in (E) and (F) highlights values below 0.02 μg/mL.
The majority of mAbs (42.8%) were directed against the RBD, while 38.9% bound to the S2 region and 12.7% of mAbs bound the S1 outside the RBD; only 4 out of 126 antibodies did not bind to the SARS-CoV-2 spike by ELISA (Figures 3A and 3B). Neutralizing activity was detected in 98.1% of RBD-targeting antibodies, 18.8% of non-RBD S1-directed mAbs and only in 2% of S2-binding antibodies (Figure 3C). In line with higher conservation of the S2 domain among β-CoVs (Cui et al., 2019), 41 of 47 cross-reactive mAbs (87.2%) bound to S2 and only 6 mAbs (12.8%) bound to the RBD. Therefore, most RBD-directed antibodies were SARS-CoV-2-specific neutralizers, whereas most non-neutralizing S2 antibodies displayed broader reactivity against other β-CoVs (Figure 3D). Derived from 9 of 10 donors, 39.3% of the mAbs displayed cross-reactivity to SARS-CoV-1 (Figure 3E). Three cross-reactive mAbs (R339-3B5, R121-3A3, and R200-4E9) were also reactive to MERS and OC43 spike proteins. The S2 antibody R339-3B5 displayed exceptional cross-reactivity and could also bind HKU-1, making it pan-β-CoV-reactive (Figure 3A). Finally, R339-3B5, along with seven RBD-directed antibodies, could cross-neutralize SARS-CoV-1 and WiV-1, with R121-3G2 showing IC50 values as low as 0.004 and 0.006 μg/mL, respectively (Figure 3E). The pan-β-CoV-reactive R339-3B5 had an IC50 of 0.921 μg/mL against SARS-CoV-2; hence, the β-CoV cross-reactive neutralizers were relatively less potent in their neutralizing activity.
Out of the isolated antibody pool, 57 antibodies (47.7%) could neutralize the pseudovirus expressing the Wu01 spike protein, and the vast majority of these—45 antibodies (32.8%)—were ultrapotent with IC50 values less than 0.02 μg/mL (20 ng/mL) (Figure 3F). To screen for any auto-reactive properties of selected mAbs, we performed HEp-2 cell assays that determine reactivity against nuclear and cytoplasmic antigens and found no or only minimal binding (Figure S3A). Taken together, elite neutralizers possess a large pool of highly potent RBD-directed NAbs. Additionally, elite neutralizers also possess a fraction of less potent NAbs targeting the RBD or S2 domains that are capable of cross-neutralizing β-Coronaviruses.
NAbs from SARS-CoV-2 elite neutralizers are highly effective against variants of concern
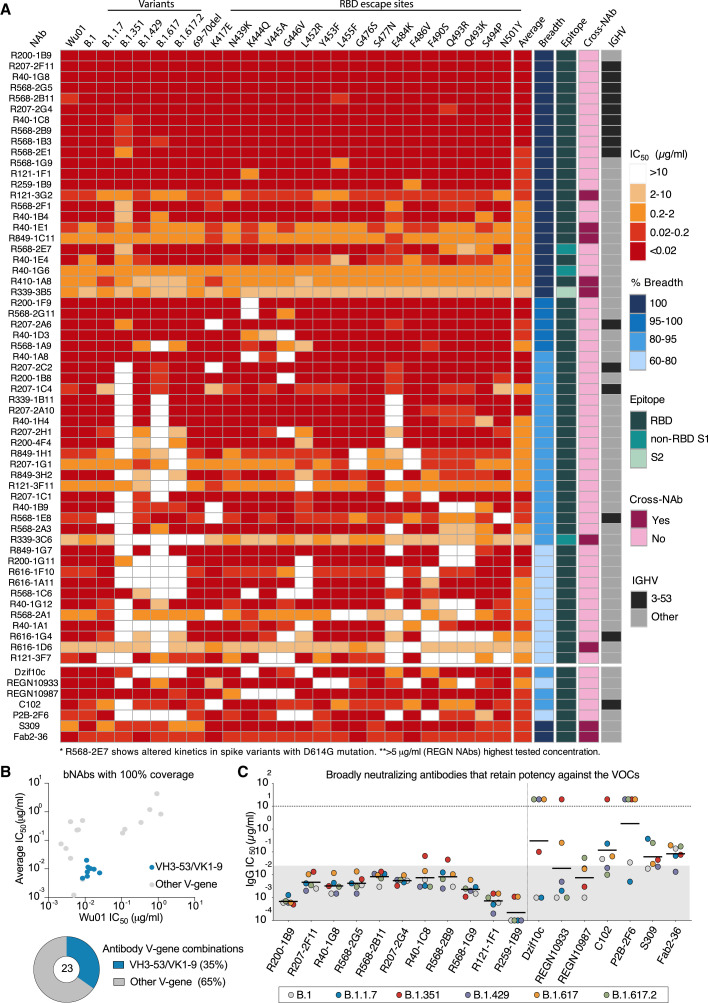
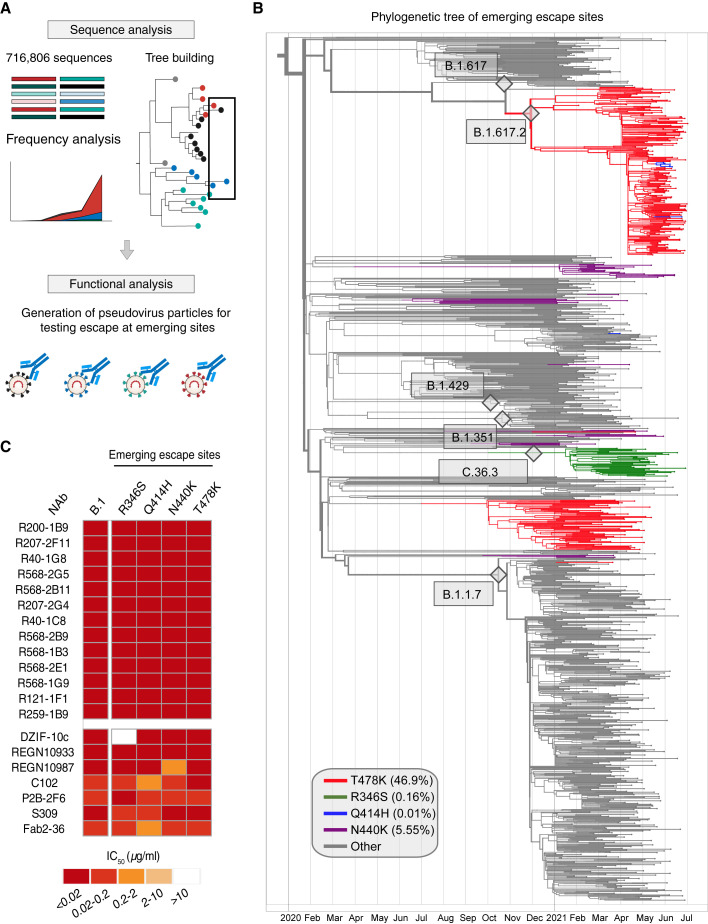
SARS-CoV-2 VoCs/variants of interest (VoIs) with higher transmission rates (Volz et al., 2021) and/or antigenic escape mutations (Harvey et al., 2021) have spread globally in recent months. These VoCs can not only reduce the neutralization capacity of serum from convalescent or vaccinated individuals but have also rendered some clinically tested or approved mAbs less effective (Hillus et al., 2021; Planas et al., 2021; Wang et al., 2021). With the aim to identify the next generation of SARS-CoV-2 bNAbs from elite neutralizers, we tested all 57 NAbs against 5 VoC/VoIs and their ancestor—namely, B.1, B.1.1.7, B.1.351, B.1.429, B.1.617, and B.1.617.2 (Figure S3B)—1 NTD escape mutant, and 17 known RBD escape mutants (Figure 4 A). Based on this neutralization map, 23 NAbs had 100% coverage by neutralizing all 24 variants with the most potent antibody, R200-1B9, having an average IC50 of 0.001 μg/mL (Figure 4A). In contrast, 3 out of 4 clinically tested mAbs, including DZIF-10c (Halwe et al., 2021; Kreer et al., 2020b), REGN10933 (casirivimab), and REGN10987 (imdevimab) (Hansen et al., 2020) covered only 83%, 78%, and 87% of tested variants, respectively. Only S309 (VIR7831) (Pinto et al., 2020) displayed 100% breadth across all variants tested but with lower potency (average IC50: 0.09 μg/mL) (Figure 4A). Evaluation of the somatic hypermutation rate and the CDR3 length of heavy and light chain sequences indicated slightly higher levels of somatic hypermutations and shorter CDRH3s for the bNAbs (Figure S3C). Of note, 20 out of 23 bNAbs bound to the RBD, two were non-RBD-S1, and one was an S2-binding antibody. Interestingly, we observed that among the 23 bNAbs with 100% breadth, 8 antibodies (35%) utilized the IGHV3-53/IGKV1-9 gene segment combination (Figure 4B; Table S2). When closely comparing bNAbs with 100% breadth against VoCs, 11 RBD-directed bNAbs retained very high potency in contrast to clinical antibodies like the REGN antibodies or DZIF-10c that showed loss of activity against B.1.351 or B.1.429, B.1.617, and a prominent variant, B.1.617.2 (Figure 4C).
Figure 4.
Broadly neutralizing next-generation SARS-CoV-2 bNAbs
(A) Neutralization escape map profile of n = 57 elite neutralizer NAbs against 25 SARS-CoV-2 spike pseudovirus variants. Average IC50 values, relative neutralization breadth across the variants, the spike epitope determined by ELISA, and cross-neutralizing capacity, as well as IGHV3-53 usage, are depicted in columns to the right.
(B) Dot plot depicting average IC50s and IC50s against Wu01 for all isolated bNAbs (n = 23) with the IGHV3-53/IGKV1-9 bNAbs highlighted in blue. Pie chart shows fraction of IGHV3-53/IGKV1-9 bNAbs among nBAns obtained from elite neutralizers.
(C) Plot evaluating the IC50 values of the broadest (100%) and most potent NAbs against SARS-CoV-2 pseudovirus variants with spike sequence of B.1, B.1.1.7, B.1.351, B.1.429, B.1.617, and B.1.617.2 are compared to published monoclonal antibodies. REGN antibodies tested up to 5 μg/mL. Gray area in (C) highlights values below 0.02 μg/mL, and black bars denote geometric means.
In summary, our evaluation of the isolated NAbs from elite neutralizers revealed a large pool of highly potent SARS-CoV-2 bNAbs that exhibit full coverage against the most prevalent VoCs as well as RBD escape sites with retention of potent neutralizing activity.
Next-generation ultrapotent bNAbs against emerging SARS-CoV-2 variants
With constant evolution of the SARS-CoV-2 spike, new sites of possible antigenic escape are continually emerging (Harvey et al., 2021; Hodcroft et al., 2021). From GISAID sequencing data from July 21, 2021, over 716,806 unique quality-controlled sequences were used to construct a full, timed phylogenetic tree of circulating SARS-CoV-2 variants with a computational pipeline utilizing software as described in the methods section (Figures 5A and 5B). This was used to obtain frequencies for the VoCs and RBD mutants (Figures 5A and 5B) as well as frequency trajectories from October 2020 onward (Figure S4A). Global frequencies were also compared to frequencies in regions with high (>60%) or low (<30%) vaccination rates (Figure S4A). Here, no substantial differences in trajectories were observed except for the B.1.1.7 VoC and its corresponding mutations, showing higher distributions in countries with high vaccination, which likely reflects the high prevalence of this VoC in the UK (Figure S4A). Using the phylogenetic tree and trajectory profiles, we could track which spike mutations were circulating in the past, which ones are currently circulating, and which new spike mutations show an increase in frequency (Figure S4A). Using global data, four RBD mutants were identified as potential candidates as emerging spike escape sites: R346S, Q414H, N440K, and T478K (Figures 5B and S4A). Among these sites, the T478K mutation is a defining mutation in the B.1.617.2 (delta) VoC spike, R346S in the VoI C.36.3, or R346K in the VoI B.1.621 (mu); Q414H appeared multiple times in the B.1.617.2 clade; and N440K was spread across the phylogenetic tree on multiple backgrounds. We generated pseudovirus variants expressing these mutations in the B.1 (D614G) background and tested all NAbs against them (Figures 5B and S4B). A large fraction of NAbs isolated from elite neutralizers had exceptionally high potency against all 24 variants tested (Figures 4 and S4B). Notably, all 11 RBD ultrapotent bNAbs with exceptional breadth and potency (Figure 4) remained effective against all predicted emerging mutations (Figure 5C). In contrast, the clinical NAbs like DZIF-10c and REGN10987 were affected by mutations at R346 and N440, respectively (Figure 5C).
Figure 5.
Maintenance of bNAb potency amidst emerging escape variants
(A) Schematic of study design used to analyze emerging escape variants.
(B) A full phylogenetic tree with leaves corresponding to isolated sequences collected after January 01, 2020, highlighting the 4 RBD mutations: T478K (red), R346S (green), Q414H (blue), and N440K (purple). The number of leaves for each clade reflects the frequency of that clade in 2021, where the 4 RBD mutations are upweighted.
(C) Neutralization analysis of the 11 most potent and broad bNAbs along with published antibodies tested against B.1 pseudoviruses carrying the 4 emerging spike mutations, R346S, Q414H, N440K, and T478K.
In summary, bNAbs from elite neutralizers are not only highly potent against the prevalent circulating VoCs but are also effective against emerging escape sites in the spike RBD, thereby constituting next-generation SARS-CoV-2 NAbs that may retain activity despite further virus evolution in the future.
Cryo-EM complex of R40-1G8 Fab with SARS-CoV-2 spike reveals binding to both “up” and “down” RBD
Notably, antibodies utilizing the IGHV3-53 and IGKV1-9 V gene segments are reported to exhibit exceptional SARS-CoV-2 neutralizing potency (Dejnirattisai et al., 2021). We wanted to obtain insight into the binding pattern of the RBD bNAbs to better understand their ability to their high coverage of variants. RBD antibodies are structurally characterized into 4 epitope classes based on their binding epitope, as well as their ability to bind an “up” or “down” RBD conformation and to block ACE2 binding. IGHV3-53 antibodies are typically class 1 or class 2 antibodies, and mutations at K417 or E484, respectively, can knock out the function of these antibodies (Barnes et al., 2020; Harvey et al., 2021). We tested the RBD bNAbs by competition ELISA against C102 (Robbiani et al., 2020), P2B-2F6 (Ju et al., 2020), S309 (Pinto et al., 2020), and Fab2-36 (Liu et al., 2020) as class 1, 2, 3, and 4 reference antibodies, respectively (Figure S5A). Based on these competition data, potent bNAbs showed a high degree of overlap (>90%) among each other and are closely related to class 1 or class 2, which bind to the RBD in an “up” (class 1) or “up and down” (class 2) orientation.
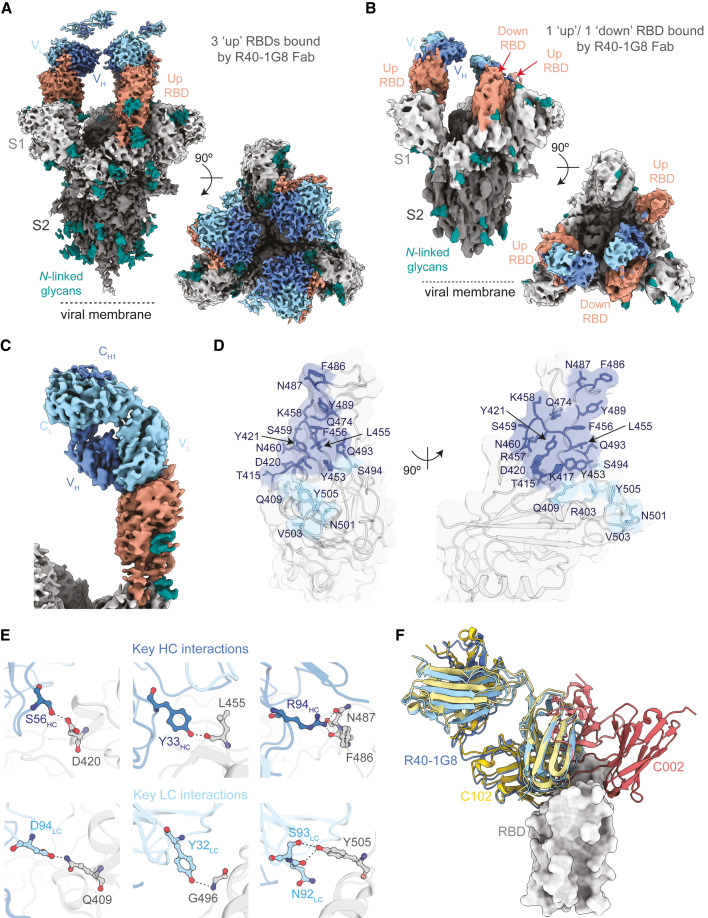
To pin down the binding mode, we selected one of the most potent and broad bNAbs from the pool with overlapping RBD binding for cryo-EM analysis (Figures 6 and S5). The selected bNAb, R40-1G8, is an IGHV3-53/IGKV1-9 antibody with an IC50 of 0.001 μg/mL against the Wu01 spike and an average IC50 of 0.004 μg/mL across the variant panel (Figure 4). To define the binding epitope, we determined a single-particle cryo-EM structure of SARS-CoV-2 S HexaPro trimer in complex with R40-1G8 Fab in two conformational states of the RBD (Figures 6A and 6B). R40-1G8 Fab was found to bind to both up (state 1) and down (state 2) RBDs, but due to the relative low resolution for the RBD and Fab in state 2 (Figure S5), we only built the model for state 1 with R40-1G8 Fab bound to all up RBDs. Focused refinement of the region of RBD of SARS-CoV-2 S HexaPro trimer bound R40-1G8 resulted in a 3.5 Å local resolution (Table S3; Figure S5). The interaction between R40-1G8 and the RBD was mainly mediated through the heavy chain contacting 16 residues, while the light chain had 6 sites of contact (Figures 6D and 6E). Structural alignments with complex structures of class 1 (C102 and C105) and class 2 (C002) revealed that R40-1G8 binds to a similar epitope as C102 or C105, suggesting it is a class 1 antibody (Figures 6F and S6).
Figure 6.
Structural basis of SARS-CoV-2 neutralization breadth of R40-1G8
(A and B) Cryo-EM density maps for R40-1G8 Fab-SARS-CoV-2 S protein complexes at 3.2 Å (state 1) (A) and 3.7 Å (state 2) (B), revealing binding of R40-1G8 Fab to both up and down RBDs as indicated by the orange arrows.
(C) Locally refined cryo-EM map of the R40-1G8 Fab-RBD complex from which the R40-1G8 Fab was built.
(D) Surface representation of the R40-1G8 Fab epitope on the surface of RBD. Epitope residues are shown as sticks in blue (for interactions with the R40-1G8 heavy chain) and light blue (interactions with the R40-1G8 light chain).
(E) Close-up showing interactions between the heavy and light chains of R40-1G8 and RBD with the contact residues involved in key interactions shown in sticks.
(F) Structural alignment of C102 (PDB 7K8M), C002 (PDB 7K8T), and R40-1G8 on the RBD, revealing that R40-1G8 binds at a similar location as the class 1 anti-RBD antibody C102.
C102 is a class 1 antibody encoded by IGHV3-53 and binds the RBD only in an “up” conformation (Barnes et al., 2020). R40-1G8 seem to be an exception in the class-1 RBD antibody class as it binds both an “up” and “down” RBD conformation (Figures 5, S5, and S6). In addition, many IGHV3-53 antibodies like C102 with a short CDRH3 are affected by the K417 mutation (Wu et al., 2020; Yuan et al., 2021), but despite the similar binding mode (Figures 6F and S6) and CDRH3 length (Table S2), R40-1G8 is not affected by the K417E/N/T mutation, which is a prominent escape site found in VoCs like B.1.351 (Figures 4 and S6). The K417 site in the C102 structure is almost forming a cation-pi interaction in addition to a hydrogen bonding interaction. However, K417 in the R40-1G8 structure does not have this interaction, which explains lack of escape at this residue (Figure S6). R40-1G8 is also not affected by another key mutation at N501Y, which is present in the VoC B.1.1.7, as the antibody can accommodate Y at RBD position 501 (Figure S6). In summary, by examining structural binding properties of R40-1G8, we found a class 1 RBD antibody binding mechanism which encompasses binding to both “up” and “down” RBD, thereby providing structural insights into the high potency and breadth of this bNAb.
Discussion
SARS-CoV-2 elite neutralizers mount a highly potent NAb response following natural infection, which is accompanied by the presence of cross-reactive antibodies against other closely related β-CoVs (Vanshylla et al., 2021). The Ab response in such individuals has not been studied in detail and could prove to be a critical source of potent NAbs as therapeutics to treat or prevent SARS-CoV-2 infection. In this study, we isolated mAbs from 10 elite neutralizers that were identified from screening 963 COVID-19 convalescent individuals. We discovered several ultrapotent RBD-directed antibodies that potently neutralize prevalent VoCs and a panel of prominent RBD escape mutants, as well as an S2-directed NAb with cross-reactivity spanning the SARS-1, MERS, and embecovirus family. Importantly, the isolated bNAbs are able to neutralize all VoCs as well as emerging variants.
These data confirm that antibody responses of SARS-CoV-2 elite neutralizers are both potent and diverse. Moreover, our analysis resulted in the isolation of NAbs that are ultrapotent against SARS-CoV-2 with 82% of the NAb fraction having IC50 values below 0.02 μg/mL. While we do not know why these individuals generate this potent NAb response, the higher anti-spike response in elite neutralizers may help enhance development of cross-reactive antibodies. This is confirmed by β-CoV cross-reactivity in 39% of isolated mAbs, some of which could neutralize SARS-1 with IC50s as low as 0.004 μg/mL. Most cross-reactive mAbs were directed against the S2 domain of the spike protein, which is more conserved among β-CoVs (Pinto et al., 2021; Sauer et al., 2021). However, unlike the RBD, the S2 domain does not induce a high neutralizing response as supported by 89% (41 of 46) of our isolated S2-directed mAbs being non-neutralizing mAbs. The mAb R339-3B5 had neutralizing activity against S2 domain of SARS-CoV-2 with an IC50 of 0.9 μg/mL. Although relatively less potent than RBD NAbs, this antibody displayed exceptional cross-reactivity against SARS-1, WiV-1, MERS, HKU1, and OC43, making it one of the few pan-β-CoV reactive antibodies described so far (Pinto et al., 2021). Less potent RBD NAbs were cross-reactive with SARS-CoV-1 and Wiv1 CoVs, and several of these NAbs maintained reactivity against the SARS-CoV-2 variants, suggesting that they target highly conserved sites on the RBD. Cross-neutralizing antibodies targeting the RBD are quite rare (Starr et al., 2021), and S309 (VIR7831), isolated from a SARS-1 survivor, is currently the only cross-reactive RBD antibody in advanced clinical use (Pinto et al., 2020). Targeted introduction of mutations to improve potency of pan-β-CoV-reactive mAbs could make them good candidates for further clinical development.
Although highly efficacious vaccines have been rapidly developed, unvaccinated or immunocompromised patients are in need for effective therapeutics (Choi et al., 2020). SARS-CoV-2 mAbs can reduce the risk of hospitalization (Dougan et al., 2021); however, several clinical SARS-CoV-2 mAb programs have suffered setbacks due to the emergence of SARS-CoV-2 escape variants (Harvey et al., 2021). Thus, the next generation of mAbs need to block circulating and emerging escape variants of SARS-CoV-2. From the elite neutralizers, we isolated NAbs with an average IC50 of 0.001 μg/mL against B.1.1.7, B.1.429, B.1.617, B.1.617.2, B.1.351, and 19 single escape sites. Out of the isolated fraction, 23 bNAbs (19%) were identified with 100% coverage across all tested variants. Importantly, potent bNAbs could also cover emerging RBD escape sites at R346S, Q414H, N440K, and T478K. R346 and N440 mutants were previously only reported in cell culture-based escape assays (Liu et al., 2021; Weisblum et al., 2020). The prediction of escape sites by using frequency trajectories and building a phylogenetic tree using 700,000 sequences helps studying variants that are circulating at low levels but might expand in the near future. Therefore, by testing such sites, we predict that the isolated bNAbs are likely to cover variants that may arise in the near future.
Potent antibodies of the IGHV1-24 clonotype targeting the NTD supersite have been described before (Cerutti et al., 2021), but these antibodies are also prone to escape observed within the NTD of VoCs (McCallum et al., 2021). We did not find SARS-CoV-2-specific IGHV1-24 antibodies in the investigated elite neutralizers, but interestingly, many of the highly potent bNAbs targeted the RBD and utilized the IGHV3-53/IGKV1-9 gene segment combination. It was previously shown that antibodies with short CDRH3s are generally class 1 RBD binders and those with long CDRH3s are class 2 RBD binders and are typically knocked out by K417 or E484 mutants respectively (Barnes et al., 2020; Wu et al., 2020; Yuan et al., 2021). Using our set of 5 VoCs and 19 RBD mutants, we identified several IGHV3-53 antibodies that defy this paradigm. Despite being IGHV3-53 antibodies with 93.5%–97.3% germline identity, minor differences in the antibody sequence seem to render them immune from the typical escape seen for this VH class. Cryo-EM structure of one of the IGHV3-53/IGKV1-9 bNAbs, R40-1G8, revealed a unique class 1 antibody mechanism that allows binding of both “up” and “down” conformations. Class 1 antibodies were previously shown to bind an “up” RBD because binding to “down” RBD will have clashes with the nearby RBD. However, in the R40-1G8-spike map, there is one “up” RBD with R40-1G8-Fab, one “up” RBD without R40-1G8-Fab, and a “down” RBD with R40-1G8-Fab. This current state is possible because Fab-free “up” RBD moves out of the way and the “down” RBD can be bound by R40-1G8. This alternative approach to binding the RBD could potentially explain the breadth of R40-1G8 against SARS-CoV-2 variants, as it may provide higher flexibility to this bNAb in approaching the RBD. Such insights will help to guide structure-based vaccine design strategies to effectively counter current and emerging VoCs.
In summary, we demonstrate that SARS-CoV-2 elite neutralizers generate a highly diverse and potent Ab response that can yield β-CoV cross-reactive and SARS-CoV-2 bNAbs with up to 100% coverage against RBD escape variants and VoCs. Together, these findings illustrate that elite neutralizers are excellent candidates for the isolation of next-generation SARS-CoV-2 bNAbs and pan β-CoV antibodies.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| APC Mouse Anti-Human IgG; Clone G18-145 | BD Biosciences | Catalog# 550931; RRID: AB_398478 |
| Alexa Fluor® 700 Mouse Anti-Human CD20; Clone 2H7 | BD Biosciences | Catalog# 560631; RRID: AB_1727447 |
| FITC Mouse Anti-Human IgM; Clone G20-127 | BD Biosciences | Catalog# 555782; RRID: AB_396117 |
| PE Mouse Anti-Human CD27; Clone M-T271 | BD Biosciences | Catalog# 560985; RRID: AB_10563213 |
| HRP-conjugated polyclonal goat anti-human IgG | Southern Biotech | Catalog# 2040-05; RRID: AB_2795644 |
| Ultra-LEAF™ Purified anti-human CD28 Antibody | Biolegend | Catalog# 302934; RRID: AB_11148949 |
| Brilliant Violet 605™ anti-human CD3 Antibody; Clone UCTH1 | Biolegend | Catalog# 300460; RRID: AB_2564380 |
| Alexa Fluor® 700 anti-human CD8a Antibody; Clone RPA-T8 | Biolegend | Catalog# 301028; RRID: AB_493745 |
| APC/Fire™ 750 anti-human CD4 Antibody; Clone RPA-T4 | Biolegend | Catalog# 300560; RRID: AB_2629693 |
| Brilliant Violet 785™ anti-human CD45RA Antibody; Clone HI-100 | Biolegend | Catalog# 304140; RRID: AB_2563816 |
| Brilliant Violet 650™ anti-human CD27 Antibody; Clone O323 | Biolegend | Catalog# 302828; RRID: AB_2562096 |
| Brilliant Violet 421™ anti-human CD137 (4-1BB) Antibody; Clone 4B4-1 | Biolegend | Catalog# 309820; RRID: AB_2563830 |
| FITC anti-human CD197 (CCR7) Antibody; Clone G043H7 | Biolegend | Catalog# 353216; RRID: AB_10916386 |
| APC anti-human CD69 Antibody; Clone FN50 | Biolegend | Catalog# 310910; RRID: AB_314845 |
| PE/Dazzle™ 594 anti-human CD154 Antibody; Clone 24-31 | Biolegend | Catalog# 310840; RRID: AB_2566245 |
| PE/Cyanine7 anti-human IFN-γ Antibody; Clone 4S.B3 | Biolegend | Catalog# 502528; RRID: AB_2123323 |
| PE anti-human CD185 (CXCR5) Antibody; Clone J252D4 | Biolegend | Catalog# 356904; RRID: AB_2561813 |
| S309 monoclonal antibody | Pinto et al., 2020 | N/A |
| C102 monoclonal antibody | Robbiani et al., 2020 | N/A |
| Fab2-36 monoclonal antibody | Liu et al., 2020 | N/A |
| P2B-2F6 monoclonal antibody | Ju et al., 2020 | N/A |
| REGN-10987 monoclonal antibody | Hansen et al., 2020 | N/A |
| REGN-10989 monoclonal antibody | Hansen et al., 2020 | N/A |
| DZIF-10c monoclonal antibody | (Halwe et al., 2021) (Kreer et al., 2020b) | N/A |
| IgG from donors R40, R121, R200, R259, R39, R410, R568, R616, R849 | Vanshylla et al., 2021 and this study | N/A |
| IgA from donors R40, R121, R200, R259, R39, R410, R568, R616, R849 | This study | N/A |
| Monoclonal antibodies from donors R40, R121, R200, R259, R39, R410, R568, R616, R849 | This study | N/A |
| Bacterial and virus strains | ||
| SARS-CoV-2 CoV2-P3 authentic virus | Vanshylla et al., 2021 | N/A |
| SARS-2-S Wu01 pseudovirus | Vanshylla et al., 2021 | N/A |
| SARS-2-S SARS-1 pseudovirus | This study | N/A |
| SARS-2-S WiV-1 pseudovirus | This study | N/A |
| SARS-2-S B.1 variant pseudovirus | Vanshylla et al., 2021 | N/A |
| SARS-2-S B.1.1.7, B.1.351, B.1.429, B.1.617 and B.1.617.2 variants pseudovirus | This study | N/A |
| SARS-2-S B.1 variants with RBD mutations: R346S; Q414H; K417E; N439K; N440K; K444Q; V445A; G446V; Y453F; G476S; S477N; T478K; E484K; F486V; F490S; Q493R; Q493K; S494P and N501Y pseudovirus | This study | N/A |
| Biological samples | ||
| Plasma/serum from convalescent donors | Vanshylla et al., 2021 and this study | N/A |
| PBMCs from convalescent donors | Vanshylla et al., 2021 and this study | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| FuGENE® 6 Transfection Reagent | Promega | Catalog# E2691 |
| Adenosine 5′-triphosphate disodium salt hydrate ATP | Sigma-Aldrich | Catalog# A2383-10G |
| Coenzyme A sodium salt hydrate,cofactor for acyl transfer | Sigma-Aldrich | Catalog# C3144-500MG |
| Igepal® CA-630 for molecular biology | Sigma-Aldrich | Catalog# I8896-100ML |
| D-Luciferin, Sodium Salt | ZellBio | Catalog# LUCNA-1G |
| Protein G Sepharose® 4 Fast Flow | Sigma-Aldrich | Catalog# GE17-0618-05 |
| Peptide M Agarose | Invivogen | Catalog# gel-pdm-2 |
| DAPI (4’,6-Diamidino-2-Phenylindole, Dihydrochloride) | Thermo Fisher | Catalog# D1306 |
| FreeStyle™ 293 Expression Medium | Thermo Fisher | Catalog# 12338018 |
| RNaseOUT | Thermo Fisher | Catalog# 10777-019 |
| RNasin | Promega | Catalog# N2515 |
| DTT (Superscript IV Kit) | Thermo Fisher | Catalog# 18090050 |
| Random Hexamer Primer | Thermo Fisher | Catalog# S0142 |
| NP-40 | Thermo Fisher | Catalog# 85124 |
| 5x RT Buffer (Superscript IV Kit) | Thermo Fisher | Catalog# 18090050 |
| dNTPs | Thermo Fisher | Catalog# R1122 |
| Superscript IV | Thermo Fisher | Catalog# 18090050 |
| Platinum™ Taq polymerase | Thermo Fisher | Catalog# 10-966-026 |
| Platinum™ Taq Green Hot Start DNA Polymerase | Thermo Fisher | Catalog# 11966034 |
| Q5 Hot Start High Fidelity DNA Polymerase | NEB | Catalog# M0493L |
| Branched Polyethylenimine, 25 kDa | Sigma-Aldrich | Catalog# 408727 |
| Puromycin | Sigma | Catalog# P4512 |
| Doxycyclin | Sigma | Catalog# D3447 |
| Strep-Tactin®XT 4Flow® high capacity resin | IBA Lifesciences | Catalog# 2-5030-500 |
| Biotin Blocking Buffer | IBA Lifesciences | Catalog# 2-0501-002 |
| ABTS Substrate Solution | Thermo Fisher | Catalog# 002024 |
| Tween-20 | Carl Roth | Catalog# 9127.20 |
| EDTA 0.5 M, pH 8.0, RNase-free | Thermo Fisher | Catalog# AM9260G |
| HRP-conjugated Streptavidin | Thermo Fisher | Catalog# 21130 |
| Crystallized Papain | Sigma Aldrich | Catalog# P3125 |
| Octyl Maltoside, Fluorinated, Anagrade | Anatrace | Catalog# O310F |
| Pierce™ Universal Nuclease for Cell Lysis | Thermo Fisher | Catalog# 88702 |
| SEB (Staphylococcal enterotoxin B from Staphylococcus aureus) | Merck/ Sigma-Aldrich | Catalog# 11100-45-1 |
| eBioscience™ Brefeldin A-Lösung (1000x) | Thermo Fisher | Catalog# 00-4506-51 |
| Dimethylsulfoxid | Sigma | Catalog# D4540 |
| Zombie UV™ Fixable Viability Kit | Biolegend | Catalog# 423108 |
| Human TruStain FcX Fc Receptor Blocking Solution | Biolegend | Catalog# 422302 |
| eBioscience™ Foxp3/Transcription Factor Staining Buffer Set | Invitrogen | Catalog# 00-5523-00 |
| PepMix™ SARS-CoV-2 (Spike SUB1), Mix of 166 peptides (15mers, overlap 11) | JPT | Catalog# PM-WCPV-S-SU1-1 |
| PepMix™ SARS-CoV-2 (Spike SUB2) Mix of 145 peptides (15mers, overlap 11) | JPT | Catalog# PM-WCPV-S-SU2-1 |
| SARS-CoV-2 HexaPro bait protein | Jason McLellan lab; Hsieh et al., 2020 | N/A |
| SARS-CoV-2 S1 domain protein for ELISA | This study | N/A |
| SARS-CoV-2 RBD domain protein for ELISA | This study | N/A |
| SARS-CoV-2 NTD domain protein for ELISA | This study | N/A |
| SARS-CoV-2 S2 domain protein for ELISA | This study | N/A |
| SARS-CoV-2 HexaPro trimer for ELISA | This study | N/A |
| SARS-CoV-1 spike trimer for ELISA | This study | N/A |
| MERS-CoV trimer for ELISA | This study | N/A |
| HCoV-HKU1 spike trimer for ELISA | This study | N/A |
| HCoV-OC43 spike trimer for ELISA | This study | N/A |
| Critical commercial assays | ||
| NOVA Lite HEp-2 ANA Kit | Inova Diagnostics | Catalog# 708100 |
| Q5® Site-Directed Mutagenesis Kit | NEB | Catalog# E0554 |
| NEBuilder® HiFi DNA Assembly | NEB | Catalog# E2621S |
| DyLight 488 Antibody Labeling Kit | Thermo Fisher | Catalog# 53024 |
| DyLight 650 Antibody Labeling Kit | Thermo Fisher | Catalog# 84535 |
| Deposited data | ||
| R40-1G8 spike structure coordinates | Protein Data Bank | PDB ID 7SC1 |
| R40-1G8 spike structure EM map | Microscopy Data Bank | EMDB 25008 |
| Tested monoclonal antibodies antibody V-gene sequences from donors R40, R121, R200, R259, R39, R410, R568, R616, R849 | This paper | GenBank accession # OL741060 - OL741311 |
| Experimental models: Cell lines | ||
| HEK293T-ACE2 cells | Jesse Bloom lab; Crawford et al., 2020 | BEI Resources Catalog# NR-52511 |
| VeroE6 cells | ATCC | Catalog# CRL-1586 |
| HEK293T | ATCC | Catalog# CRL-11268 |
| 2936E | National research Council Canada | NRC file 11565 |
| HEK293 EBNA | Invitrogen | Catalog# R620907 |
| Expi293F cells | GIBCO | Catalog# A14527 |
| Oligonucleotides | ||
| 5′ oPR-IGHV primer mix | Kreer et al., 2020a | N/A |
| 5′ oPR-IGKV primer mix | Kreer et al., 2020a | N/A |
| 5′ oPR-IGLV primer mix | Kreer et al., 2020a | N/A |
| 3′ Cg-RT primer | Ozawa et al., 2006 | N/A |
| 3′ Cκ-543 primer | Tiller et al., 2008 | N/A |
| 3′ Cκ-494 primer | Tiller et al., 2008 | N/A |
| 3′ IgG internal | Tiller et al., 2008 | N/A |
| 3′ XhoI Cλ | Tiller et al., 2008 | N/A |
| 5′ SLIC-oPR-IGHV primer | Kreer et al., 2020a | N/A |
| 5′ SLIC-oPR-IGKV primer | Kreer et al., 2020a | N/A |
| 5′ SLIC-oPR-IGLV primer | Kreer et al., 2020a | N/A |
| 3′ SLIC_IgG_HC_rev | (Kreer et al., 2020b) | N/A |
| 3′ SLIC_KC_rev | (Kreer et al., 2020b) | N/A |
| 3′ SLIC_LC_rev | (Kreer et al., 2020b) | N/A |
| Random Hexamer Primer | Thermo Fisher | Catalog #SO142 |
| Recombinant DNA | ||
| Human antibody expression vectors (IgG1, Igκ, Igλ) | Tiller et al., 2008 | N/A |
| Human antibody expression vectors (IgG1, Igκ, Igλ) with V-gene antibody sequences from donors R40, R121, R200, R259, R39, R410, R568, R616, R849 | This study | N/A |
| pHDM-tat1b | Jesse Bloom lab; Crawford et al., 2020 | N/A |
| pHDM-Hgpm2 | Jesse Bloom lab; Crawford et al., 2020 | N/A |
| pRC-CMV-Rev1b | Jesse Bloom lab; Crawford et al., 2020 | N/A |
| pHAGE-CMV-Luc2-IRES-ZsGreen-W | Jesse Bloom lab; Crawford et al., 2020 | N/A |
| SARS-CoV-2 Wu01 codon optimized spike | (Hoffmann et al., 2020b) | N/A |
| pcDNA™3.1/V5-His TOPO™ SARS-2-S Wu01 spike | Vanshylla et al., 2021 | N/A |
| pcDNA™3.1/V5-His TOPO™ SARS-2-S B.1 spike variant | Vanshylla et al., 2021 | N/A |
| pcDNA™3.1/V5-His TOPO™ SARS-2-S B.1.1.7, B.1.351, B.1.429, B.1.617 and B.1.617.2 spike variants | This study | N/A |
| pcDNA™3.1/V5-His TOPO™ SARS-2-S B.1 variants with RBD mutations: R346S; Q414H; K417E; N439K; N440K; K444Q; V445A; G446V; Y453F; G476S; S477N; T478K; E484K; F486V; F490S; Q493R; Q493K; S494P and N501Y | This study | N/A |
| pCAGGS-SARS-CoV2-S-HexaPro spike | Jason McLellan lab; Hsieh et al., 2020 | N/A |
| pCDNA3.1-SARS-CoV-1 spike | Jason McLellan lab | N/A |
| pCG1-WiV-1 spike | This study | N/A |
| pVRC-MERS-CoV spike | Jason McLellan lab; (Pallesen et al., 2017) | N/A |
| phCMV3-HCoV-HKU1 spike | Raiees Andrabi lab; (Song et al., 2021) | N/A |
| phCMV3- HCoV-OC43 spike | Raiees Andrabi lab; (Song et al., 2021) | N/A |
| Software and algorithms | ||
| GraphPad PRISM, Version 7 and 9 | GraphPad Software, Inc | https://www.graphpad.com |
| Geneious R10v10.0.9 | Biomatters | https://www.geneious.com |
| Illustrator® CC 2018 | Adobe | https://www.adobe.com |
| BertholdTech TriStar2S ICE, Version 1.0.9.5 | Berthold Technologies | https://www.berthold.com/en |
| IgBLAST | Ye et al., 2013 | N/A |
| SerialEM automated data collection software | Mastronarde, 2005 | N/A |
| cryoSPARC v3.2 | Punjani et al., 2017 | N/A |
| Chimera visualization software | Pettersen et al., 2004 | N/A |
| Coot molecular-graphics application | Emsley et al., 2010 | N/A |
| Phenix | Liebschner et al., 2020 | N/A |
| GISAID EpiCov database | Elbe and Buckland-Merrett, 2017 | N/A |
| MAFFTv7.467 | Katoh and Standley, 2013 | N/A |
| IQTree | Minh et al., 2020 | N/A |
| TreeTime | Sagulenko et al., 2018 | N/A |
| Other | ||
| C102 spike structure | Protein Data Bank; Barnes et al., 2020 | PDB ID 7K8M |
| C105 spike structure | Protein Data Bank; Barnes et al., 2020 | PDB ID 6XCM |
| C002 spike structure | Protein Data Bank; Barnes et al., 2020 | PDB ID 7K8T |
| CD19 MicroBeads, human | Miltenyi Biotec | Catalog# 130-050-301 |
| Amicon Ultra-0.5 Centrifugal Filter Unit 10 kDa | Merck | Catalog# UFC501096 |
| Amicon Ultra-0.5 Centrifugal Filter Unit Ultracel-30, 0.5 mL sample | Millipore Sigma | Catalog# UFC503096 |
| Amicon® Ultra-4 | Sigma-Aldrich | Catalog# UFC803096 |
| Corning® 96-well EIA/RIA Easy Wash™ Clear Flat Bottom Polystyrene High Bind Microplate | Corning | Catalog# 3369 |
| 96-well Black Flat Bottom Polystyrene Not Treated Microplate | Corning | Catalog# 3628 |
| 96-well Clear Flat Bottom TC-treated Microplate | Corning | Catalog# 3915 |
| NucleoSpin® 96 PCR Clean-up | Macherey-Nagel | Catalog# 740658.4 |
| Q5® High-Fidelity 2X Master Mix, | NEB | Catalog# M0492S |
| EZ-Link Sulfo-NHS-Biotin | Thermo Fisher | Catalog# A39256 |
| HiTrapTM MabSelect SuReTM | GE Healthcare Life Sciences | Catalog# 11-0034-94 |
| Superdex 200 Increase 10/300 column | GE Healthcare Life Sciences | Catalog# 28-9909-44 |
| QuantaFoil 300 mesh 1.2/1.3 grids | Electron Microscopy Sciences | Catalog# Q310CR1.3 |
| PELCO easiGlow™ Glow system | Ted Pella | Catalog# 91000 |
| Vitrobot Mark IV | Thermo Fisher | N/A |
| Leica DMI3000 B microscope | Leica | N/A |
| Sunrise™ microplate reader | Tecan | N/A |
| BertholdTech TriStar2S luminometer | Berthold Technologies | N/A |
| BD FACSAria Fusion™ | Becton Dickinson | N/A |
| Krios G4 Cryo-Transmission Electron Microscope with Gatan K3 camera | Thermo Fisher | N/A |
Resource availability
Lead contact
Requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Florian Klein (florian.klein@uk-koeln.de).
Materials availability
Request for reagents will be made available by the lead contact with a Material Transfer Agreement.
Experimental model and subject details
Enrollment of human subjects and study design
Blood samples were collected from donors (for details please refer to Table S1) who gave their written consent under the protocols 20-1187 and 16-054, approved by the Institutional Review Board (IRB) of the University Hospital Cologne. The COVID-19 convalescent cohort of 963 individuals was previously described in detail (Vanshylla et al., 2021). Samples for single B cell analysis were collected from 5 male and 5 female participants (median age 43 years) at a median of 19 weeks post disease onset. Additional samples for IgG/IgA neutralization and T cell analyses were obtained from 18 male and 13 female participants (median age 47 years) at a median of 7.4 weeks post disease onset. The COVID-19 convalescent donors tested SARS-CoV-2 positive between March and April 2020, and were not treated with SARS-CoV-2 monoclonal antibodies or received a COVID-19 vaccine prior to sample collection.
Cell lines
VeroE6 cells, HEK293T cells and 293T-ACE2 cells were maintained in DMEM (GIBCO) containing 10% FBS, 1% PS, 1mM L-Glutamine and 1mM Sodium pyruvate. Cells were grown on tissue culture treated T75 flask (Sarstedt) at 37°C and 5% CO2. 293-6E cells were maintained at 37°C and 6% CO2 in FreeStyle Expression Medium (Thermo Fisher) and kept under constant shaking at 110-120 rpm.
Method details
Processing of serum, plasma and whole blood samples
Blood samples were collected in Heparin syringes or EDTA monovette tubes (Sarstedt) and fractionated into plasma and peripheral blood mononuclear cell (PBMC) by density gradient centrifugation using Histopaque-1077 (Sigma). PBMCs were stored at −150°C. Plasma aliquots were stored at −80°C till use. Serum was collected from Serum-gel tubes (Sarstedt) by centrifugation and stored at −80°C till use.
Isolation of IgGs and IgAs from serum and plasma samples
For the isolation of total IgG, 0.5-1 mL plasma was heat inactivated for 45 min at 56°C and incubated with Protein G Sepharose 4 Fast Flow beads (GE Healthcare) at 4°C overnight. For isolation of IgA, 0.5 mL heat inactivated plasma was incubated with Peptide M Agarose beads (Invivogen) at 4°C overnight. On the next day, the beads were washed on chromatography columns (BioRad) and Protein G bound IgG or Peptide M bound IgA was eluted using 0.1M Glycine pH = 3 and immediately buffered with 1M Tris pH = 8. Buffer exchange to PBS (GIBCO) was performed using 30 kDa cut-off Amicon Ultra-15 columns (Millipore) and the purified IgG or IgA was stored at 4°C.
Single B cell sorting
Single B cell analyses were performed as previously described (Gieselmann et al., 2021). The SARS-CoV-2 variant called HexaPro, which has a pre-fusion spike confirmation (Hsieh et al., 2020) was used as bait protein for sorting spike-specific B cells from elite neutralizers using a 2-color sorting strategy. The HexaPro protein was labeled using the DyLight 488 or Dylight 650 antibody labeling kits (Thermo Fisher) as per manufacturer’s protocol. B cells were enriched from the peripheral blood mononuclear cell (PBMC) fraction using the CD19 Microbeads kit (Miltenyi Biotec). Enriched B cells were stained with antibodies against human CD20, human IgG, DAPI, HexaPro-Dylight-488 and HexaPro-Dylight-650. B cells that were DAPI-negative, CD20-positive, IgG-positive, HexaPro-Dylight-488-positive and HexaPro-Dylight-650-positive were sorted onto 96 well plates containing sorting buffer comprised of 0.2 μL RNAsin (40U/μl Promega), 0.1 μL RNaseOut (40 U/μl Thermo Fisher), 0.2 μL 10X PBS, 0.4 μL DTT (100mM Promega) and 3.1 μL Nuclease free H2O (Thermo Fisher). Sorts were done on a BD FACSAria Fusion cell sorter (Becton Dickinson) and sorted cells were frozen at −80°C until further processing.
Single cell cDNA production and PCR
Sorted single B cells were lysed at 65°C for 1 min with 0.75 μL Random Hexamer Primer (200 ng/μl Thermo Fisher), 0.5 μL NP-40 (10% Thermo Fisher), 0.15 μL RNaseOUT (100mM Thermo Fisher) and 5.6 μL Nuclease-free H2O (Thermo Fisher). Thereafter, 2 μL Nuclease-free H2O (Thermo Fisher), 3 μL 5X RT Buffer (Invitrogen), 0.5 μL dNTPs (25mM Thermo Fisher), 1 μL DTT (100mM Invitrogen), 0.1 μL RNAsin (40 U/μl Promega), 0.1 μL RNaseOUT (40 U/μl Thermo Fisher) and 0.25 μL Superscript IV (200 U/μl Invitrogen) were added and reverse transcription performed by incubating at RT for 10 min, 42°C for 10 min, 25°C for 10 min, 50°C for 10 min and 94°C for 5 min. Individual antibody sequences were amplified using semi-nested PCR. Heavy, kappa and lambda chains were simultaneously amplified in the 1st PCR using Platinum Taq DNA polymerase (Thermo Fisher) using the 5′ oPR-IGHV, oPR-IGKV and oPR-IGLV primer mix (Kreer et al., 2020a) along with the 3′ Cg-RT (Ozawa et al., 2006), 3′ Cκ 543 (Tiller et al., 2008) and 3′ Cκ 494 (Tiller et al., 2008). 1st PCR was run at 94°C for 1 min, 50 cycles of 94°C for 30 s, 57°C for 30 s, 72°C for 55 s and final extension at 72°C for 5 min. In the 2nd PCR, the antibody chains were amplified in separate reaction using primer pairs of 5′ oPR-IGHV + 3′ IgG internal (Tiller et al., 2008) (heavy chain), 5′ oPR-IGKV + 3′ Cκ 494 (kappa chain) and 5′ oPR-IGLV + 3′ XhoI Cλ (Tiller et al., 2008) (lambda chain). 2nd PCR was run at 94°C for 1 min, 50 cycles of 94°C for 30 s, 57°C for 30 s, 72°C for 45 s and final extension at 72°C for 5 min. 2nd PCR products were sequenced by Sanger sequencing for subsequent sequence analysis.
Antibody sequence analysis
Antibody sequence analysis was performed using a python-based in-house pipeline as previously described (Kreer et al., 2020b). B cell sequences with a minimum length of 240 nucleotides and a mean Phred score of >= 28 amino acids were annotated with IgBLAST (Ye et al., 2013) and trimmed from Framework region (FWR) 1 to the end of the J gene of the variable region. Base calls with Phred score of < 16 were masked and sequences with > 15 masked nucleotides, frameshifts or stop codons were excluded from further analyses. From these productive sequences, clonality was analyzed by grouping identical V genes, and the pairwise Levenshtein distance of their CDRH3 was determined. Individual sequences were grouped into a clonal culster when they shared the same VH gene and the minimal CDRH3 identity (defined by Levenshtein distance in relation to the length of the shorter CDRH3) was >=75%. Sequences that remained unassigned after 20 rounds of randomized input were designated as being non-clonal. CDRH3 length was calculated based on IMGT numbering and % of sequences with a particular V-gene out of all sequences within an individual. Healthy reference shown in gray in Figure 2C. For the similarity network analysis in Figure 2D, B cells with the same VH gene from one individual was linked to a sequence from another individual when CDRH3 amino acid similarity (defined by Levenshtein distance in relation to the length of the shorter CDRH3) is >= 75%. Reference B cell heavy chain sequences in Figure 2 were derived from naive donors (Kreer et al., 2020b).
Antibody cloning for protein synthesis
For cloning of single B cell-derived antibodies, the 1st PCR product was used to amplify the variable regions for each antibody chain in separate reactions using the 5′ primers SLIC-oPR-IGHV, SLIC-oPR-IGKV and SLIC-oPR-IGLV (Kreer et al., 2020a) and 3′ primers SLIC_IgG_HC_rev, SLIC_KC_rev and SLIC_LC_rev (Kreer et al., 2020b) for heavy, kappa and lambda chain respectively. PCR was performed using the Q5 Hot Start High Fidelity DNA Polymerase (NEB) and run at 98°C for 30 s, 35 cycles of 98°C for 10 s, 72°C for 45 s and final extension at 72°C for 2 min. The PCR product was purified using the NucleoSpin® 96 PCR Clean-up kit (Macherey-Nagel) and cloned into the respective pIg expression vectors (pIgG1, pIgK, or pIgL) by restriction digest and SLIC assembly (von Boehmer et al., 2016).
Antibody synthesis
Antibodies were produced by transfection of 293-6E cells (National Research Council Canada) using branched polyethylenimine (PEI) 25kDa (Sigma-Aldrich) with 0.5 μg heavy chain plasmid and 0.5 μg light chain plasmid per 1 mL 293-6E culture. Cells were maintained at 37°C and 6% CO2 FreeStyle 293 Expression Medium (Thermo Fisher) and 0.2% Penicillin/Streptomycin 7 days post transfection, the cell culture supernatant was harvested, filtered with a 0.45 μM Nalgene Rapid Flow filter (Thermo Fisher) and incubated overnight at 4°C with Protein G Sepharose 4 Fast Flow (GE Healthcare) overnight. Antibody bound Sepharose beads were washed on chromatography columns (BioRad) and antibodies were eluted using 0.1 M Glycine pH = 3 and immediately buffered in 1 M Tris pH = 8. Thereafter, buffer exchange to PBS was performed using 50 kDa Amicon Ultra-15 spin columns (Millipore) and the antibodies were stored at 4°C.
Cloning of SARS-CoV-2 spike variants
The codon optimized SARS-CoV-2 Wu01 spike (Hoffmann et al., 2020b) (EPI_ISL_406716) was cloned into pCDNA™3.1/V5-HisTOPO vector (Invitrogen). The D614G, 69-70 deletion and RBD mutants were generated by introducing the corresponding amino acid mutations using the Q5® Site-Directed Mutagenesis Kit (NEB) and per manufacturer’s protocol. SARS-2-S RBD variants were generated in the B.1 background and included R346S; Q414H; K417E; N439K; N440K; K444Q; V445A; G446V; Y453F; G476S; S477N; T478K; E484K; F486V; F490S; Q493R; Q493K; S494P and N501Y. SARS-2-S variants B.1.1.7, B.1.351, B.1.429, B.1.617 and B.1.617.2 were generated by introducing the corresponding amino acid mutations (Figure S3) using PCR and HiFi assembly (NEBuilder® HiFi DNA Assembly Kit, New England Biolabs) of overlapping segments as per manufacturer’s protocol.
Production of SARS-CoV pseudovirus particles
Pseudovirus particles were generated by co-transfection of individual plasmids encoding HIV-1 Tat, HIV-1 Gag/Pol, HIV-1 Rev, luciferase followed by an IRES and ZsGreen, and the SARS-CoV-2, (Crawford et al., 2020), SARS-CoV-1 or WiV-1 spike protein. In brief, HEK293T cells were transfected with the pseudovirus encoding plasmids using FuGENE® 6 Transfection Reagent (Promega). The virus culture supernatant was harvested at 48 h and 72 h post transfection and stored at −80°C until use. Each virus batch was titrated by infecting 293T-ACE2 and after a 48 h incubation period at 37°C and 5% CO2, luciferase activity was determined after addition of luciferin/lysis buffer (10 mM MgCl2, 0.3 mM ATP, 0.5 mM Coenzyme A, 17 mM IGEPAL (all Sigma-Aldrich), and 1 mM D-Luciferin (GoldBio) in Tris-HCL) using a microplate reader (Berthold). Pseudovirus dilution resulting in a RLU of approximately 1000-fold in infected cells versus non-infected cells was used for neutralization assays.
Pseudovirus assay to determine IgG/plasma/serum neutralizing activity
For testing neutralizing activity of IgG or serum/plasma samples, serial dilutions of IgG or serum/plasma (heat inactivated at 56°C for 45 min) were co-incubated with pseudovirus supernatants for 1 h at 37°C prior to addition of 293T cells engineered to express ACE2 (Crawford et al., 2020). Following a 48 h incubation at 37°C and 5% CO2, luciferase activity was determined using the reagents described above. After subtracting background relative luminescence units (RLUs) of non-infected cells, 50% inhibitory concentrations (IC50s) were determined as the IgG concentrations resulting in a 50% RLU reduction compared to untreated virus control wells. 50% Inhibitory dose (ID50) was determined as the serum dilution resulting in a 50% reduction in RLU compared to the untreated virus control wells. Each IgG and serum sample were measured in two independent experiments on different days and the average IC50 or ID50 values have been reported. For each run, a SARS-CoV-2 neutralizing monoclonal antibody was used as control to ensure consistent reproducibility in experiments carried out on different days. Assay specificity calculated using pre-COVID-19 samples was found to be 100%. IgG IC50 or serum/plasma ID50 values were calculated in GraphPad Prism 7.0 by plotting a dose response curve.
SARS-CoV-2 authentic virus neutralization assay
Authentic SARS-CoV-2 was previously grown out from a swab from Cologne using VeroE6 cells (Vanshylla et al., 2021). For the neutralization assay, dilutions of monoclonal IgG were co-incubated with the virus (1000-2000 TCID50) for 1 h at 37°C prior to addition of VeroE6 cells in DMEM (GIBCO) containing 2% FBS, 1% PS, 1mM L-Glutamine and 1mM Sodium pyruvate. After 4 days of incubation at 37°C, 5% CO2, neutralization was analyzed by observing cytopathic effects (CPE) using a brightfield microscope and the highest dilution well with no CPE was noted to be the IC100 for the antibody. All samples were measured in two independent experiments on separate days and the average IC100 from all measurements is reported.
SARS-CoV-2 T cell reactivity assay
To detect SARS-CoV-2 spike-specific T cells, an activation induced marker (AIM) assay was used. Frozen PBMCs were thawed in pre-warmed RPMI 1640 supplemented with 10% FCS (Sigma-Aldrich), 1% Glutamax (GIBCO) and Pierce Universal Nuclease for Cell Lysis (ThermoFisher) and washed before resuspention in cRPMI (RPMI containing 10% heat-inactivated FCS (Sigma-Aldrich), 1% Penicillin-Streptomycin (Sigma-Aldrich), 1% Glutamax (GIBCO), 1% NEAA (Thermo Fisher), 1% HEPES (Thermo Fisher), 1% Sodium Pyruvate (Thermo Fisher) and rested for 5 h at 37°C and 5% CO2 in a humidified incubator. Live cells were counted on a MACSQuant Analyzer 16 (Miltenyi Biotec) with DAPI (Miltenyi Biotec) and seeded in 96 well U-bottom plates at 5 × 105 live cells per well in 100 μL in cRPMI. To stimulate cells, 1 μg/mL PepMix SARS-CoV-2 spike glycoprotein (JPT) peptide pool 1 (S1 domain) or 2 (S2 domain) in the presence of 1 μg/mL anti-CD28 antibody was added to up to six technical replicates per condition. Staphyoccocal enterotoxin B (SEB, Sigma-Aldrich) stimulated positive controls and negative controls using equivalent amount of DMSO and 1 μg/mL anti-CD28 antibody were included in parallel. Stimulated cells were incubated for 2 h before cytokine secretion inhibition with Brefeldin A (eBioscience). 19 h post stimulation, anti-human CXCR5 antibody was added to the culture for 30 min. Cells were then washed in PBS, technical replicates pooled and incubated with Zombie UV Live/Dead and Human TruStain FcX for 30 min at 4°C. Cells were then washed twice and incubated with the surface stain cocktail for 30 min at 4°C (all anti-human; CD45RA BV785, CD27 BV650, CCR7 FITC, CD69 APC and PD-1 eFluor506). Cells were permeabilized and fixed (eBioscience Foxp3/Transcription Factor Staining Buffer set) for 30 min at RT, washed and stained with the intracellular antibody cocktail for 30 min at 4°C (all anti-human; CD3 BV605, CD8 AF700, CD4 APC-Fire750, CD137 BV421, CD154 PE-Dazzle594, IFNg PE-Cy7). Cells were washed twice and acquired on a Cytoflex LX (Beckman Coulter) within 24 h of staining. Data were analyzed using FlowJo v10 (BD Bioscience). AIM+ CD4 T cells were defined by co-expression of CD137 and CD154 and frequencies calculated by background-subtraction of paired unstimulated controls. Researchers were blinded to grouping of samples during the experiment and data analysis. Antibody details can be found in the key resources table.
Production of coronavirus spike proteins
The following coronavirus regions were amplified from synthetic gene plasmids and cloned into modified Sleeping Beauty transposon expression vectors (Kowarz et al., 2015). Proteins used in ELISA included SARS-2 S1 domain: MN908947, A.A. 14-609, RRAR to GGGG, N-terminal BM40 signal peptide followed by a Twin strep tag, 83 kDa; SARS-2 S2 domain: MN908947, A.A. 686-1208, K986P, V987P, BM40 signal peptide; C-terminal Twin strep tag, 61 kDa; SARS-2 RBD domain: MN908947, A.A. 331-524, N-terminal Twin strep tag, 26 kDa; SARS-2 NTD domain: MN908947, A.A. 1-330, C-terminal Twin strep tag, 40 kDa; HexaPro spike trimer: MN908947, A.A. 1-1208, RRAR to GSAS, F817P, A892P, A899P, A942P, K986P, V987P, C-terminal T4 foldon followed by a Twin strep tag, 139 kDa; SARS-1 spike trimer: AAP13567, A.A. 18-1190, BM40 signal peptide, C-terminal T4 foldon – Twin strep tag, codon Optimized, 137 kDa; MERS spike trimer: AHE78097, A.A. 18-1291, RSVR to ASVG, V1060P, L1061P, BM40 signal peptide, C-terminal T4 foldon – Twin strep tag, 147 kDa ; HKU1 spike trimer: YP_173238, A.A. 1-1295, RRKRR to GSAG, A1071P, L1072P, C-terminal T4 foldon – Twin strep tag, 149 kDa; OC43 spike trimer: AAX84792, A.A. 1-1300, RRSRR to GSAS, A1078P, L1079P, C-terminal T4 foldon – Twin strep tag, 150 kDa. For recombinant protein production, stable HEK293 EBNA cell lines were generated employing the sleeping beauty transposon system (Kowarz et al., 2015). Briefly, expression constructs were co-transfected with a transposase plasmid (10:1) into the HEK293 EBNA cells, and after puromycin selection (3 μg/mL; Sigma), cells were expanded in triple flasks and protein production induced with doxycycline (0.5 μg/mL, Sigma). Supernatants of confluent cells were harvested every 3 days, filtered and recombinant proteins purified via Strep-Tactin®XT (IBA Lifescience, Göttingen, Germany) resin. Proteins were eluted with biotin containing TBS-buffer (IBA Lifescience, Göttingen, Germany), dialyzed against TBS-buffer, checked on an SDS-gel and aliquots stored at −80°C.
Detection of spike-specific reactivity by ELISA
For assessing binding reactivity, 2 μg/mL protein was coated overnight at 4°C on high binding 96-well assay plates (Corning). Proteins used were SARS-CoV-2 spike trimer (aa 1-1204), SARS-CoV-2 S1 monomer (aa 16-609), SARS-CoV-2 RBD monomer (aa 331-524), SARS-CoV-2 NTD monomer (aa 1-330), SARS-CoV-2 S2 monomer (aa 610-1208), SARS-1 spike trimer (aa 18-1190), MERS spike trimer (aa 18-1291), OC43 spike trimer (aa 1-1300), HKU1 spike trimer (aa 1-1295). Wells were washed at each step with washing buffer containing 0.05% Tween-20 (Carl Roth) in PBS. Uncoated sites were blocked with blocking buffer containing 3% milk powder (Carl Roth), 1 μM EDTA (Thermo Fisher) and 0.1% Tween-20 by incubating for 120 min at RT. After this, a dilution series of the samples, starting with a highest concentration of 1 mg/mL for total plasma IgG and 10 μg/mL for mAbs was added for 90 min at RT. For detection of binding signal from the primary antibody, goat anti-human IgG-HRP (Southern Biotech) was added for 60 min at RT. ABTS solution (Thermo Fisher) was added as substrate and the absorbance at 415 nm with reference at 695 nm was measured on the Sunrise microplate reader (Tecan). The optical density (OD) was used to plot binding curves and calculate area under curve (AUC) in GraphPad Prism 9.0.
Competition ELISA
For competition ELISA, 0.5 mg antibody was biotinylated with a 50X molar excess of biotin using the EZ-Link Sulfo-NHS-Biotin kit (Thermo Fisher) as per manufacturer’s protocol. 2 μg/mL SARS-CoV-2 spike trimer (aa 1-1204) was coated on overnight at 4°C on high binding 96-well assay plates (Corning). Wells were washed at each step with washing buffer containing 0.05% Tween-20 (Carl Roth) in PBS. Uncoated sites were blocked with blocking buffer containing 3% milk powder (Carl Roth), 1 μM EDTA (Thermo Fisher) and 0.1% Tween-20 at RT for 120 min. After this, a dilution series of the samples to be tested, starting with a highest concentration of 40 μg/mL for mAbs was added for 60 min at RT. Following this, 0.4 μg/mL of the biotinylated antibody was added on top of the primary antibody for an additional 60 min at RT. For detection of binding signal from the biotinylated antibody, HRP-conjugated Streptavidin (Thermo Fisher) was added for 60 min at RT. ABTS solution (Thermo Fisher) was added as substrate and the absorbance at 415 nm with reference at 695 nm was measured on the Sunrise microplate reader (Tecan). The OD values were normalized to a negative control showing no competition in order to calculate the % competition and the non-biotinylated version of the same antibody was tested in parallel to confirm maximum competition (over 95%).
Hep-2 autoreactivity assay
HEp-2 cell autoreactivity assay was performed as per manufacturer’s protocol using the NOVA Lite Hep-2 ANA kit (Inova Diagnostics) using 100 μg/mL of monoclonal antibody in DPBS. As a positive control, the Hep-2 cell-reactive HIV-1 neutralizing antibody 4E10 was included. Images were acquired using a Leica DMI3000 B microscope with 1500 ms exposure and a gain of 10. Images were processed in Adobe Photoshop and assembled in Adobe Illustrator CC 2018®.
Production of Fab fragments and spike protein and sample preparation for cryo-EM
The expression and purification of SARS-CoV-2 HexaPro (6P) stabilized S trimers (Hsieh et al., 2020) and antibody Fabs was carried out based on a previously published protocol (Muecksch et al., 2021). Briefly, Expi293F cells (GIBCO) were transiently transfected and supernatants used to purify protein using Ni2+-NTA affinity size exclusion chromatography (SEC). SEC peak fractions were identified with SDS-PAGE and pooled and stored at 4°C. Fabs were generated with papain digestion from purified IgG at a 1:100 enzyme: IgG ratio by using crystallized papain (Sigma-Aldrich) in 50 mM sodium phosphate, 2mM EDTA, 10 mM L-cysteine and pH 7.4 for 30-60 min at 37°C. Fc fragments and undigested IgG were removed by applying digested products to a 1 mL HiTrap MabSelect SuRe column (GE Healthcare Life Sciences), and flow-through containing the cleaved Fabs was collected. SEC was done using a Superdex 200 Increase 10/300 column (GE Healthcare Life Sciences) in TBS to further purify the Fabs which were finally concentrated and stored at 4°C. SARS-CoV-2 S 6P trimer was mixed with purified R40-1G8 Fabs to a final concentration of 2 mg/mL with a 1.1:1 molar ratio Fab per SARS-CoV-2 S 6P protomer. The complex was incubated at room temperature for 30 min. QuantaFoil 300 mesh 1.2/1.3 grids (Electron Microscopy Sciences) were glow-discharged for 1 min at 20 mA using a PELCO easiGLOW (Ted Pella). Immediately before depositing 3 μL of the protein complex on to the glow-discharged grid, fluorinated octyl-maltoside (Anatrace) was added to the protein complex solution to a final concentration of 0.02% (w/v). The grids were blotted for 3 s with 0 blot force using Whatman No.1 filter paper at room temperature and 100% humidity and vitrified in 100% liquid ethane using Mark IV Vitrobot (Thermo Fisher).
Cryo-EM data collection, processing and refinement
Single-particle cryo-EM dataset for SARS-CoV-2 S 6P trimer in complex with R40-1G8 was collected using SerialEM automated data collection software (Mastronarde, 2005) on a 300 keV Titan Krios (Thermo Fisher) equipped with a K3 camera (Gatan). Detailed data processing workflow is outlined in Figure S5. 4,496 movies were recorded using a 3x3 beam image shift pattern with 3 exposures each hole with a pixel size of 0.416 Å in the superresolution mode. These cryo-EM movies were patch motion corrected with a binning factor of 2 and the CTF parameters were estimated using Patch CTF in cryoSPARC v3.2 (Punjani et al., 2017). Particles were picked using blob picker in cryoSPARC using a particle diameter of 100 to 200 Å, and movies and picked particles were inspected before extraction. A total of 840,417 particles were extracted and used to 2D classification. After discarding ice particles, the remaining 776,803 particles were used to generate four ab initio models. The particles that contributed to the reconstruction of a SARS-CoV-2 trimer looking model was used for the subsequent heterogeneous refinement of four models with the same ab initio volume. These four reconstructions revealed three different states, and the heterogeneous refinement was then repeated with three models. The resulting three particle stacks and three volumes were separately refined using homogeneous and non-uniform refinements in cryoSPARC. After rounds of refinement, the first particle stack with 178,597 particles yielded a reconstruction of 3.2 Å resolution with C3 symmetry. The second particle stack with 62,090 particles yielded a reconstruction of 3.7 Å resolution with C1 symmetry. And the last particle stack with 75,973 particles were discarded since the reconstruction had preferred orientation problem and it was similar to the first reconstruction. To resolve the residues at the interface of SARS-CoV-2 S 6P RBD and R40-1G8, a mask was generated using Chimera (Pettersen et al., 2004), and used for local refinement in cryoSPARC, which resulted in a final map of 3.5 Å local resolution using symmetry expanded (C3) particles. As the density for the RBD-Fab of the second reconstruction was bad, model buildings and structural refinements were only done for the first reconstruction. The initial model of SARS-CoV-2 S trimer in complex with R40-1G8 was obtained using the SARS-CoV-2 S 6P trimer (PDB 7K8T) and the C002 Fab structure (PDB 7K8O) as starting models. Initial model fitting was done in Chimera and Coot (Emsley et al., 2010). Rounds of refinements and manual model buildings were separately carried out in Phenix (Liebschner et al., 2020) and Coot, with final structure validation in Phenix.
Quantification and statistical analysis
Statistical modeling of SARS-CoV-2 sequences for identification of variants
The phylogeny was reconstructed using isolates of human SARS-CoV-2 retrieved from the GISAID EpiCov database (Elbe and Buckland-Merrett, 2017) as of 21-07-2021. Sequences that contained more than 1% ambiguous sites, with incomplete collection date or that diverged with more than 0.1 mutations per day from the root date, were removed, leaving more than 700.000 unique sequences. The sequences were aligned with MAFFTv7.467 (Katoh and Standley, 2013) to a reference isolate from GenBank (Wuhan-Hu-1, collected December 19th 2019 in Wuhan, China). This alignment of the selected isolates was used to infer the maximum likelihood phylogeny under the nucleotide substitution model GTR+G in IQTree (Minh et al., 2020). The tree topology was assessed using the ultrafast bootstrap function with 1000 replicates (Hoang et al., 2018). To root the tree, we specified the reference isolate hCoV-19/Wuhan/Hu-1/2019 (GISAID-Accession: EPI ISL 402125), which is identical in sequence to the GenBank isolate used in the alignment step. The sequences, as well as timing, of internal nodes were inferred using TreeTime. A fixed clock rate of (mutations / (bp date) was used under a skyline coalescent tree prior. The tree was rooted using the same reference isolate as with the IQTree step of topology reconstruction. The clock rate was computed as the total number of mutations on the tree, divided by the total length of branches of the timed tree. This rate was optimized by iterative runs of TreeTime (Sagulenko et al., 2018) until convergence. The maximum-likelihood time of the root of the tree is December 23, 2019.
Frequencies are computed as follows: (1) individual isolates, index with i, are assigned a smoothened multiplicity factor, , where is the collection date of the isolate, and the squared Gaussian kernel is σ = 11 days. The regional epidemic factor is the number of COVID-19 cases that are reported for each collected sequence on the tree in a region R: the multiplicity factor of an isolate reflects the likely number of SARS-CoV-2 cases in a given region R. Sample frequencies of the isolates are computed as , where Global frequencies of particular clades or RBD mutants are then where the regional epidemic factor up- or downweighs regions that are under- or over-represented in the sequencing data. By construction of frequency trajectories , the growth/decline of mutants can be tracked over time indicating mutations that might increase viral fitness.
Statistical analysis
Neutralizing levels of IgG (IC50) or serum (ID50) were calculated in GraphPad Prism 7.0 by using a non-linear fit model to plot an agonist versus normalized dose response curve with variable slope using the least-squares fitting method. A Y-value of 50% was used to calculate the corresponding X-value or IC50 based on this dose response curve. The statistical tests reported throughout the study were performed using GraphPad Prism 9.0. Data was checked for normality and the appropriate statistical test was applied. Significance was defined by considering P values < 0.05 as significant. The test applied for the individual analyses and P values are reported in the corresponding figure or figure legend. No additional methods were used to determine whether the data met assumptions of the statistical approach. Central tendencies are indicated in the corresponding figure or figure legend as median or geometric mean and denoted with bars. n values are defined in the corresponding figure legend to represent number of individuals, number of B cells or number of antibodies analyzed.
Acknowledgments
We would like to thank all members of the Klein lab for helpful discussions; Clara Lehmann for critical review of this manuscript and facilitating our work with the COVID-19 outpatient unit; Petra Meyer, Nicole Riet, and Ricarda Stumpf for technical assistance; Daniela Weiland, Nadine Henn, and Susanne Salomon for project and laboratory management; Knut Elbers for support with SARS-CoV-2 spike escape variants; Ching-Lin Hsieh and Jason S. McLellan for the SARS-CoV-2 HexaPro spike construct; Michael Korenkov and the team at Boehringer Ingelheim for antibody plasmids and antibodies; Pauline Hoffman, Dr. Jost Vielmetter, and the Caltech Beckman Institute Protein Expression Center for protein expression and purification; and Dr. Songye Chen for maintaining the electron microscope. Cryo-EM was performed in the Beckman Institute Resource Center for Transmission Electron Microscope at Caltech. This work was funded by grants to M.K. (DFG FOR2722); to P.J.B. by National Institutes of Health (1P01AI138938-S1); to M. Laessig by German Research Foundation (DFG) (CRC1310); to L.E.S. from COVIM “NaFoUniMedCovid19” (FKZ: 01KX2021); to F. Klein from the German Center for Infection Research (DZIF), the DFG (CRC1279 and CRC1310), European Research Council (ERC) (ERC-StG639961), and COVIM “NaFoUniMedCovid19” (FKZ: 01KX2021).
Author contributions
Conceptualization, F. Klein and K.V.; methodology, F. Klein, K.V., M.M., C.K., D.R., and C.F.; formal analysis, K.V., C.F., M.M., C.K., M.W., N.P., F.M., K.P., and H.G.; investigation, K.V., C.F., M.W., N.P., C.K., F. Kleipass, H.J., M.S.E., D.R., H.G., T.N., S.B., M.K., F.M., and K.P.; phylogenetic analysis, M.M.; sequence analysis, C.K.; resources, S.C.S., M.A., F.D., L.G., P.S., and M. Luksza; writing – original draft, K.V. and F. Klein; writing – reviewing and editing, C.F., M.M., H.G., N.P., C.K., and M.K.; supervision; F. Klein, P.J.B., M. Laessig, T.F.S., and L.E.S.; funding acquisition, F. Klein, L.E.S., M.K., M. Laessig, and P.J.B.
Declaration of interests
F. Klein, K.V., and H.G. are listed as inventors on a patent application that covers aspects of this work. F.K., C.K., and H.G. are listed as inventors on a patent application regarding neutralizing antibodies against SARS-related coronaviruses. All other authors declare no competing interests.
Published: December 18, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.chom.2021.12.010.
Supplemental information
Data and code availability
-
•
All data has been included in main figures or supplementary information.
-
•
SARS-CoV-2 antibody sequences are available at GenBank with accession numbers OL741060 - OL741311. The atomic coordinate and 3D EM reconstruction for SARS-CoV-2 S 6P in complex with R40-1G8 Fab has been deposited in the Protein Data Bank (PDB) with PDB ID 7SC1 and the Electron Microscopy Data Bank (EMDB) with EMDB 25008, respectively.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Andreano E., Nicastri E., Paciello I., Pileri P., Manganaro N., Piccini G., Manenti A., Pantano E., Kabanova A., Troisi M., et al. Extremely potent human monoclonal antibodies from COVID-19 convalescent patients. Cell. 2021;184:1821–1835.e16. doi: 10.1016/j.cell.2021.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes C.O., Jette C.A., Abernathy M.E., Dam K.A., Esswein S.R., Gristick H.B., Malyutin A.G., Sharaf N.G., Huey-Tubman K.E., Lee Y.E., et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature. 2020;588:682–687. doi: 10.1038/s41586-020-2852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti G., Guo Y., Zhou T., Gorman J., Lee M., Rapp M., Reddem E.R., Yu J., Bahna F., Bimela J., et al. Potent SARS-CoV-2 neutralizing antibodies directed against spike N-terminal domain target a single supersite. Cell Host Microbe. 2021;29:819–833.e817. doi: 10.1016/j.chom.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E.C., Gilchuk P., Zost S.J., Suryadevara N., Winkler E.S., Cabel C.R., Binshtein E., Chen R.E., Sutton R.E., Rodriguez J., et al. Convergent antibody responses to the SARS-CoV-2 spike protein in convalescent and vaccinated individuals. Cell Rep. 2021;36:109604. doi: 10.1016/j.celrep.2021.109604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Nirula A., Heller B., Gottlieb R.L., Boscia J., Morris J., Huhn G., Cardona J., Mocherla B., Stosor V., et al. BLAZE-1 Investigators SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19. N. Engl. J. Med. 2021;384:229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi B., Choudhary M.C., Regan J., Sparks J.A., Padera R.F., Qiu X., Solomon I.H., Kuo H.H., Boucau J., Bowman K., et al. Persistence and Evolution of SARS-CoV-2 in an Immunocompromised Host. N. Engl. J. Med. 2020;383:2291–2293. doi: 10.1056/NEJMc2031364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti D., Purcell L.A., Snell G., Veesler D. Tackling COVID-19 with neutralizing monoclonal antibodies. Cell. 2021;184:3086–3108. doi: 10.1016/j.cell.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford K.H.D., Eguia R., Dingens A.S., Loes A.N., Malone K.D., Wolf C.R., Chu H.Y., Tortorici M.A., Veesler D., Murphy M., et al. Protocol and Reagents for Pseudotyping Lentiviral Particles with SARS-CoV-2 Spike Protein for Neutralization Assays. Viruses. 2020;12:513. doi: 10.3390/v12050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejnirattisai W., Zhou D., Ginn H.M., Duyvesteyn H.M.E., Supasa P., Case J.B., Zhao Y., Walter T.S., Mentzer A.J., Liu C., et al. The antigenic anatomy of SARS-CoV-2 receptor binding domain. Cell. 2021;184:2183–2200.e2122. doi: 10.1016/j.cell.2021.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan M., Nirula A., Azizad M., Mocherla B., Gottlieb R.L., Chen P., Hebert C., Perry R., Boscia J., Heller B., et al. BLAZE-1 Investigators Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19. N. Engl. J. Med. 2021;385:1382–1392. doi: 10.1056/NEJMoa2102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbe S., Buckland-Merrett G. Data, disease and diplomacy: GISAID’s innovative contribution to global health. Glob Chall. 2017;1:33–46. doi: 10.1002/gch2.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P., Lohkamp B., Scott W.G., Cowtan K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieselmann L., Kreer C., Ercanoglu M.S., Lehnen N., Zehner M., Schommers P., Potthoff J., Gruell H., Klein F. Effective high-throughput isolation of fully human antibodies targeting infectious pathogens. Nat. Protoc. 2021;16:3639–3671. doi: 10.1038/s41596-021-00554-w. [DOI] [PubMed] [Google Scholar]
- Halwe S., Kupke A., Vanshylla K., Liberta F., Gruell H., Zehner M., Rohde C., Krähling V., Gellhorn Serra M., Kreer C., et al. Intranasal Administration of a Monoclonal Neutralizing Antibody Protects Mice against SARS-CoV-2 Infection. Viruses. 2021;13:1498. doi: 10.3390/v13081498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J., Baum A., Pascal K.E., Russo V., Giordano S., Wloga E., Fulton B.O., Yan Y., Koon K., Patel K., et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science. 2020;369:1010–1014. doi: 10.1126/science.abd0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey W.T., Carabelli A.M., Jackson B., Gupta R.K., Thomson E.C., Harrison E.M., Ludden C., Reeve R., Rambaut A., Peacock S.J., Robertson D.L., COVID-19 Genomics UK (COG-UK) Consortium SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021;19:409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillus D., Schwarz T., Tober-Lau P., Vanshylla K., Hastor H., Thibeault C., Jentzsch S., Helbig E.T., Lippert L.J., Tscheak P., et al. EICOV/COVIM Study Group Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet Respir. Med. 2021;9:1255–1265. doi: 10.1016/S2213-2600(21)00357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang D.T., Chernomor O., von Haeseler A., Minh B.Q., Vinh L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018;35:518–522. doi: 10.1093/molbev/msx281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodcroft E.B., Zuber M., Nadeau S., Vaughan T.G., Crawford K.H.D., Althaus C.L., Reichmuth M.L., Bowen J.E., Walls A.C., Corti D., et al. SeqCOVID-SPAIN consortium Spread of a SARS-CoV-2 variant through Europe in the summer of 2020. Nature. 2021;595:707–712. doi: 10.1038/s41586-021-03677-y. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Hofmann-Winkler H., Krüger N., Kempf A., Nehlmeier I., Graichen L., Arora P., Sidarovich A., Moldenhauer A.S., Winkler M.S., et al. SARS-CoV-2 variant B.1.617 is resistant to bamlanivimab and evades antibodies induced by infection and vaccination. Cell Rep. 2021;36:109415. doi: 10.1016/j.celrep.2021.109415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C.L., Goldsmith J.A., Schaub J.M., DiVenere A.M., Kuo H.C., Javanmardi K., Le K.C., Wrapp D., Lee A.G., Liu Y., et al. Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science. 2020;369:1501–1505. doi: 10.1126/science.abd0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju B., Zhang Q., Ge J., Wang R., Sun J., Ge X., Yu J., Shan S., Zhou B., Song S., et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584:115–119. doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- Kowarz E., Löscher D., Marschalek R. Optimized Sleeping Beauty transposons rapidly generate stable transgenic cell lines. Biotechnol. J. 2015;10:647–653. doi: 10.1002/biot.201400821. [DOI] [PubMed] [Google Scholar]
- Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- Kreer C., Döring M., Lehnen N., Ercanoglu M.S., Gieselmann L., Luca D., Jain K., Schommers P., Pfeifer N., Klein F. openPrimeR for multiplex amplification of highly diverse templates. J. Immunol. Methods. 2020;480:112752. doi: 10.1016/j.jim.2020.112752. [DOI] [PubMed] [Google Scholar]
- Kreer C., Zehner M., Weber T., Ercanoglu M.S., Gieselmann L., Rohde C., Halwe S., Korenkov M., Schommers P., Vanshylla K., et al. Longitudinal Isolation of Potent Near-Germline SARS-CoV-2-Neutralizing Antibodies from COVID-19 Patients. Cell. 2020;182:843–854.e812. doi: 10.1016/j.cell.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebschner D., Afonine P.V., Urzhumtsev A.G., Adams P.D. Implementation of the riding hydrogen model in CCTBX to support the next generation of X-ray and neutron joint refinement in Phenix. Methods Enzymol. 2020;634:177–199. doi: 10.1016/bs.mie.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Wang P., Nair M.S., Yu J., Rapp M., Wang Q., Luo Y., Chan J.F., Sahi V., Figueroa A., et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584:450–456. doi: 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- Liu Z., VanBlargan L.A., Bloyet L.M., Rothlauf P.W., Chen R.E., Stumpf S., Zhao H., Errico J.M., Theel E.S., Liebeskind M.J., et al. Identification of SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization. Cell Host Microbe. 2021;29:477–488.e474. doi: 10.1016/j.chom.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., Stowe J., Tessier E., Groves N., Dabrera G., et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronarde D.N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 2005;152:36–51. doi: 10.1016/j.jsb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- McCallum M., De Marco A., Lempp F.A., Tortorici M.A., Pinto D., Walls A.C., Beltramello M., Chen A., Liu Z., Zatta F., et al. N-terminal domain antigenic mapping reveals a site of vulnerability for SARS-CoV-2. Cell. 2021;184:2332–2347.e2316. doi: 10.1016/j.cell.2021.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minh B.Q., Schmidt H.A., Chernomor O., Schrempf D., Woodhams M.D., von Haeseler A., Lanfear R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020;37:1530–1534. doi: 10.1093/molbev/msaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muecksch F., Weisblum Y., Barnes C.O., Schmidt F., Schaefer-Babajew D., Wang Z., Lorenzi J.C.C., Flyak A.I., DeLaitsch A.T., Huey-Tubman K.E., et al. Affinity maturation of SARS-CoV-2 neutralizing antibodies confers potency, breadth, and resilience to viral escape mutations. Immunity. 2021;54:1853–1868.e7. doi: 10.1016/j.immuni.2021.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S.C.A., Yang F., Jackson K.J.L., Hoh R.A., Roltgen K., Jean G.H., Stevens B.A., Lee J.Y., Rustagi A., Rogers A.J., et al. Human B Cell Clonal Expansion and Convergent Antibody Responses to SARS-CoV-2. Cell Host Microbe. 2020;28:516–525.e515. doi: 10.1016/j.chom.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien M.P., Forleo-Neto E., Musser B.J., Isa F., Chan K.C., Sarkar N., Bar K.J., Barnabas R.V., Barouch D.H., Cohen M.S., et al. Covid-19 Phase 3 Prevention Trial Team Subcutaneous REGEN-COV Antibody Combination to Prevent Covid-19. N. Engl. J. Med. 2021;385:1184–1195. doi: 10.1056/NEJMoa2109682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa T., Kishi H., Muraguchi A. Amplification and analysis of cDNA generated from a single cell by 5′-RACE: application to isolation of antibody heavy and light chain variable gene sequences from single B cells. Biotechniques. 2006;40:469–470, 472, 474 passim. doi: 10.2144/000112123. [DOI] [PubMed] [Google Scholar]
- Pallesen J., Wang N., Corbett K.S., Wrapp D., Kirchdoerfer R.N., Turner H.L., Cottrell C.A., Becker M.M., Wang L., Shi W., et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc. Natl. Acad. Sci. USA. 2017;114:E7348–E7357. doi: 10.1073/pnas.1707304114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Piccoli L., Park Y.J., Tortorici M.A., Czudnochowski N., Walls A.C., Beltramello M., Silacci-Fregni C., Pinto D., Rosen L.E., Bowen J.E., et al. Mapping Neutralizing and Immunodominant Sites on the SARS-CoV-2 Spike Receptor-Binding Domain by Structure-Guided High-Resolution Serology. Cell. 2020;183:1024–1042.e21. doi: 10.1016/j.cell.2020.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D., Park Y.J., Beltramello M., Walls A.C., Tortorici M.A., Bianchi S., Jaconi S., Culap K., Zatta F., De Marco A., et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583:290–295. doi: 10.1038/s41586-020-2349-y. [DOI] [PubMed] [Google Scholar]
- Pinto D., Sauer M.M., Czudnochowski N., Low J.S., Tortorici M.A., Housley M.P., Noack J., Walls A.C., Bowen J.E., Guarino B., et al. Broad betacoronavirus neutralization by a stem helix-specific human antibody. Science. 2021;373:1109–1116. doi: 10.1126/science.abj3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planas D., Veyer D., Baidaliuk A., Staropoli I., Guivel-Benhassine F., Rajah M.M., Planchais C., Porrot F., Robillard N., Puech J., et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- Punjani A., Rubinstein J.L., Fleet D.J., Brubaker M.A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods. 2017;14:290–296. doi: 10.1038/nmeth.4169. [DOI] [PubMed] [Google Scholar]
- Robbiani D.F., Gaebler C., Muecksch F., Lorenzi J.C.C., Wang Z., Cho A., Agudelo M., Barnes C.O., Gazumyan A., Finkin S., et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584:437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T.F., Zhao F., Huang D., Beutler N., Burns A., He W.T., Limbo O., Smith C., Song G., Woehl J., et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science. 2020;369:956–963. doi: 10.1126/science.abc7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydyznski Moderbacher C., Ramirez S.I., Dan J.M., Grifoni A., Hastie K.M., Weiskopf D., Belanger S., Abbott R.K., Kim C., Choi J., et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell. 2020;183:996–1012.e19. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagulenko P., Puller V., Neher R.A. TreeTime: Maximum-likelihood phylodynamic analysis. Virus Evol. 2018;4:vex042. doi: 10.1093/ve/vex042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer M.M., Tortorici M.A., Park Y.J., Walls A.C., Homad L., Acton O.J., Bowen J.E., Wang C., Xiong X., de van der Schueren W., et al. Structural basis for broad coronavirus neutralization. Nat. Struct. Mol. Biol. 2021;28:478–486. doi: 10.1038/s41594-021-00596-4. [DOI] [PubMed] [Google Scholar]
- Song G., He W., Callaghan S., Anzanello F., Huang D., Ricketts J., Torres J.L., Beutler N., Peng L., Vargas S., et al. Cross-reactive serum and memory B-cell responses to spike protein in SARS-CoV-2 and endemic coronavirus infection. Nat. Comm. 2021;12:2938. doi: 10.1038/s41467-021-23074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr T.N., Czudnochowski N., Liu Z., Zatta F., Park Y.J., Addetia A., Pinto D., Beltramello M., Hernandez P., Greaney A.J., et al. SARS-CoV-2 RBD antibodies that maximize breadth and resistance to escape. Nature. 2021;597:97–102. doi: 10.1038/s41586-021-03807-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M.G., Burgess J.L., Naleway A.L., Tyner H., Yoon S.K., Meece J., Olsho L.E.W., Caban-Martinez A.J., Fowlkes A.L., Lutrick K., et al. Prevention and Attenuation of Covid-19 with the BNT162b2 and mRNA-1273 Vaccines. N. Engl. J. Med. 2021;385:320–329. doi: 10.1056/NEJMoa2107058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiller T., Meffre E., Yurasov S., Tsuiji M., Nussenzweig M.C., Wardemann H. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J. Immunol. Methods. 2008;329:112–124. doi: 10.1016/j.jim.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health & Human Services Pause in the Distribution of bamlanivimab/etesevimab. 2021. https://www.phe.gov/emergency/events/COVID19/investigation-MCM/Bamlanivimab-etesevimab/Pages/bamlanivimab-etesevimab-distribution-pause.aspx
- Vanshylla K., Di Cristanziano V., Kleipass F., Dewald F., Schommers P., Gieselmann L., Gruell H., Schlotz M., Ercanoglu M.S., Stumpf R., et al. Kinetics and correlates of the neutralizing antibody response to SARS-CoV-2 infection in humans. Cell Host Microbe. 2021;29:917–929.e4. doi: 10.1016/j.chom.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz E., Hill V., McCrone J.T., Price A., Jorgensen D., O’Toole A., Southgate J., Johnson R., Jackson B., Nascimento F.F., et al. Evaluating the Effects of SARS-CoV-2 Spike Mutation D614G on Transmissibility and Pathogenicity. Cell. 2021;184:64–75.e11. doi: 10.1016/j.cell.2020.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer L., Liu C., Ackerman S., Gitlin A.D., Wang Q., Gazumyan A., Nussenzweig M.C. Sequencing and cloning of antigen-specific antibodies from mouse memory B cells. Nat. Protoc. 2016;11:1908–1923. doi: 10.1038/nprot.2016.102. [DOI] [PubMed] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181:281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Nair M.S., Liu L., Iketani S., Luo Y., Guo Y., Wang M., Yu J., Zhang B., Kwong P.D., et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593:130–135. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- Weinreich D.M., Sivapalasingam S., Norton T., Ali S., Gao H., Bhore R., Musser B.J., Soo Y., Rofail D., Im J., et al. Trial Investigators REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N. Engl. J. Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum Y., Schmidt F., Zhang F., DaSilva J., Poston D., Lorenzi J.C., Muecksch F., Rutkowska M., Hoffmann H.H., Michailidis E., et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. eLife. 2020;9:e61312. doi: 10.7554/eLife.61312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., Curtis H.J., Mehrkar A., Evans D., Inglesby P., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N.C., Yuan M., Liu H., Lee C.D., Zhu X., Bangaru S., Torres J.L., Caniels T.G., Brouwer P.J.M., van Gils M.J., et al. An Alternative Binding Mode of IGHV3-53 Antibodies to the SARS-CoV-2 Receptor Binding Domain. Cell Rep. 2020;33:108274. doi: 10.1016/j.celrep.2020.108274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J., Ma N., Madden T.L., Ostell J.M. IgBLAST: an immunoglobulin variable domain sequence analysis tool. Nucleic Acids Res. 2013;41:W34-40. doi: 10.1093/nar/gkt382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M., Liu H., Wu N.C., Lee C.D., Zhu X., Zhao F., Huang D., Yu W., Hua Y., Tien H., et al. Structural basis of a shared antibody response to SARS-CoV-2. Science. 2020;369:1119–1123. doi: 10.1126/science.abd2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M., Huang D., Lee C.D., Wu N.C., Jackson A.M., Zhu X., Liu H., Peng L., van Gils M.J., Sanders R.W., et al. Structural and functional ramifications of antigenic drift in recent SARS-CoV-2 variants. Science. 2021;373:818–823. doi: 10.1126/science.abh1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohar T., Alter G. Dissecting antibody-mediated protection against SARS-CoV-2. Nat. Rev. Immunol. 2020;20:392–394. doi: 10.1038/s41577-020-0359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zost S.J., Gilchuk P., Chen R.E., Case J.B., Reidy J.X., Trivette A., Nargi R.S., Sutton R.E., Suryadevara N., Chen E.C., et al. Rapid isolation and profiling of a diverse panel of human monoclonal antibodies targeting the SARS-CoV-2 spike protein. Nat. Med. 2020;26:1422–1427. doi: 10.1038/s41591-020-0998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data has been included in main figures or supplementary information.
-
•
SARS-CoV-2 antibody sequences are available at GenBank with accession numbers OL741060 - OL741311. The atomic coordinate and 3D EM reconstruction for SARS-CoV-2 S 6P in complex with R40-1G8 Fab has been deposited in the Protein Data Bank (PDB) with PDB ID 7SC1 and the Electron Microscopy Data Bank (EMDB) with EMDB 25008, respectively.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.