Abstract
We report a case of disseminated histoplasmosis and COVID-19 infection in a renal transplant recipient in Argentina. The patient exhibited respiratory symptoms, and a chest computed tomography scan (CT) showed multiple bilateral centrilobular opacities with a tree-in-bud pattern in both lobes. The patient was initially treated as having bacterial community-acquired pneumonia, and then tuberculosis. A month later, histoplasmosis was diagnosed, and Histoplasma capsulatum LAmB clade was isolated from sputum, skin and oral lesions. The patient was hospitalized and treatment was started with intravenous liposomal amphotericin B. During the course of the antifungal therapy the respiratory symptoms worsened, a new chest CT showed a unilateral lesion with a ground glass appearance and SARS-CoV-2 was detected in a new nasopharyngeal sample. In addition, plasma therapy was administered, and the immunosuppressive regimen was adjusted (everolimus was interrupted, mycophenolate mofetil reduced, and meprednisone increased). Finally, the patient's progress was favorable and was discharged after five days on oral itraconazole treatment for histoplasmosis.
Keywords: COVID-19, Fungal infection, Histoplasma capsulatum, Renal transplant, SARS-CoV-2, Argentina
Abstract
Se presenta un caso de histoplasmosis diseminada e infección por COVID-19 en un paciente trasplantado renal en Argentina. El paciente presentó un cuadro clínico respiratorio, y la tomografía computarizada (TC) de tórax mostró múltiples opacidades centrolobulillares bilaterales con patrón de árbol en brote. El paciente fue tratado inicialmente con antibióticos para agentes causantes de neumonía bacteriana adquirida en la comunidad y luego como tuberculosis. Un mes después se le diagnosticó una histoplasmosis diseminada y el hongo fue aislado del esputo, la piel y la mucosa oral. El hongo fue tipificado molecularmente como Histoplasma capsulatum clado LAmB. El paciente fue hospitalizado y se inició tratamiento con anfotericina B liposomal vía intravenosa. Durante el transcurso de la terapia antifúngica los síntomas respiratorios del paciente empeoraron, una nueva TC de tórax mostró una lesión unilateral con apariencia de vidrio esmerilado y se detectó SARS-CoV-2 en el hisopado nasofaríngeo. El paciente fue tratado con plasmoterapia y se modificó el régimen de inmunosupresión (se interrumpió everolimus, se redujo micofenolato de mofetilo y se incrementó la meprednisona). La evolución del paciente fue favorable y fue dado de alta con tratamiento oral con itraconazol.
Palabras clave: COVID-19, Infección fúngica, Histoplasma capsulatum, Trasplante renal, SARS-CoV-2, Argentina
Histoplasmosis is an endemic systemic mycosis caused by the Histoplasma capsulatum species complex divided into eight geographic clades (phylogenetic species): Nam1, Nam 2, LamA, LamB, Australian, The Netherlands (Indonesian?); Eurasian and African7. In Argentina, the median estimated prevalence of previous exposure to H. capsulatum in the general population ranged from 30 to 40%1. Histoplasmosis is the fourth systemic mycosis diagnosed in Argentina, and its estimated incidence based on laboratory data is 0.45/100 00014. The risk of developing diverse mycoses is increased in kidney transplant recipients, although the incidence rate of histoplasmosis is low (0.16 per 100 person years) in the post-transplant period, even in endemic areas2, 13. Currently, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been associated with individuals with chronic comorbidities, such as solid organ transplant recipients, and various opportunistic mycoses have been described as a coinfection15. In this study, we report a case of disseminated histoplasmosis with cutaneous involvement, and SARS-CoV-2 coinfection in a renal transplant recipient in Argentina. The Hospital Aleman of Buenos Aires city approved this retrospective case report, and all procedures performed were in accordance with the ethical standards of the institution.
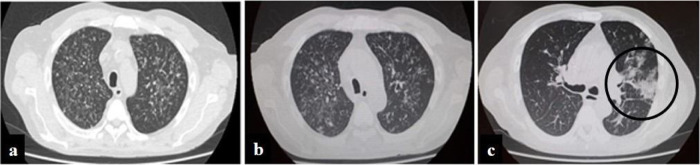
A 57-year-old man, living in San Miguel County, Buenos Aires Province, who had a kidney transplant with cadaveric donor in 2014, was admitted to the emergency department on April 24, 2020, with odynophagia and a history of cough and expectoration for 15 days (Fig. 1 ). He had been receiving immunosuppressive maintenance therapy including everolimus, mycophenolate mofetil, and meprednisone. The chest computed tomography (CT) image showed diffusely distributed multiple bilateral centrilobular opacities, and a tree-in-bud pattern in both lobes (Fig. 2 a).
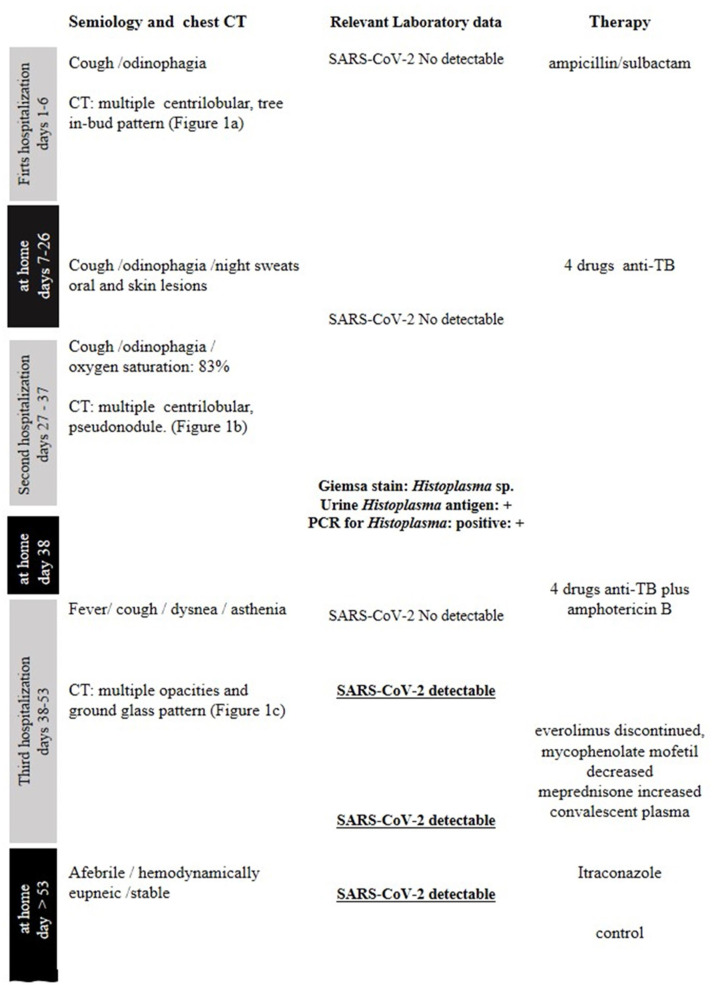
Figure 1.
Timeline of symptom onset, chest CT, laboratory data, and treatment.
Figure 2.
Evolution of chest computed tomography images. Multiple bilateral centrilobular opacities, with tree-in-bud pattern during first hospitalization. Slight increase of multiple bilateral centrilobular opacities during second hospitalization. Follow-up CT image obtained 10 days later of the third hospitalization shows multifocal peripheral abnormalities (circle) with ground glass pattern involve right lung.
Nasopharyngeal swab samples were analyzed by real-time RT-PCR based SARS-CoV-2 detection, and by Multiplex Real-Time PCR using FilmArray® Respiratory Panel 2.0 (BioFire Diagnostics, LLC). The results of RT-PCR and FilmArray were negative.
Sputum and bronchoalveolar lavage (BAL) samples were processed for microbiological studies. Ziehl–Neelsen stained sputum and BAL smears did not show acid-fast bacilli. Direct microscopic examination and Giemsa-staining to detect fungi were also negative. Common bacteria, fungi and mycobacterium cultures were negative.
The clinical diagnostic criteria, CT features and microbiological results suggested bacterial community-acquired pneumonia (CAP), and the patient received empiric antimicrobial (ampicillin/sulbactam combination) plus anti-viral (oseltamivir) treatments. After five days the patient was discharged from hospital and outpatient follow-up was indicated.
Two weeks later, on May 12, the patient returned to the outpatient unit with persistent odynophagia, cough with expectoration, night sweats and subfebrile state. The clinical examination revealed inflammation to the right peri-tonsillar pillar with soft tissue injury, and a painless, heatless non-scaly circular rash in the forearm. Because of the CT images and a suspicion of disseminated tuberculosis, empiric anti-TB therapy was indicated while waiting for the culture results. After 15 days, both oral and skin biopsies were performed on an outpatient basis.
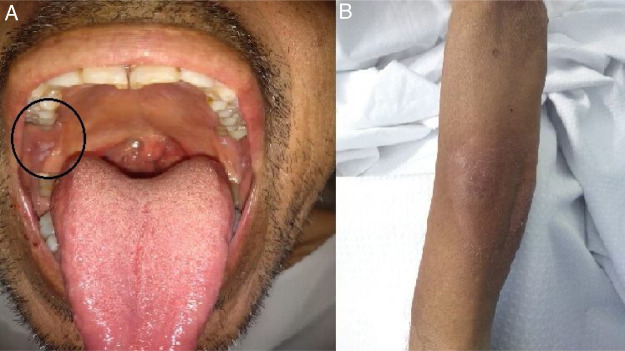
On May 26, the patient presented to the emergency department with dyspnea on mild exertion (class 3), low blood-oxygen saturation (83%), odynophagia and severe dysphagia. He was re-admitted for close monitoring in the transplantation unit. A new chest CT scan showed slight enlargement of the bilateral centrilobular opacities and small consolidations with a pseudonodular appearance on the upper lung lobes (Fig. 2b). Clinical examination showed worsening of the oral and left forearm skin lesions (Fig. 3 a and b). Routine laboratory tests showed a white blood cell count of 3280 cells/mm3, with elevated fibrinogen (609 mg/dl) and D-dimer (650 ng/ml) in plasma. In serum, the results showed low albumin (2.50 g/dl), elevated lactate dehydrogenase (266 UI/l), as well as C-reactive protein (66.8 mg/l), and ferritin (1728 ng/ml).
Figure 3.
Mucocutaneous lesions. a. Right peri-tonsillar pillar injury (circle). b. Erythematous, indurated plaques on the left forearm.
Considering the pandemic situation, new samples of nasopharyngeal swab were collected for the SARS-CoV-2 test and the results of the RT-PCR test were negative for the virus. Biopsies of both lesions were performed for histological and microbiological studies and a new sputum sample was processed for fungus and mycobacterial culture.
The microbiological direct microscopic examination of the oral mucosa smear showed intracellular yeasts measuring 2–5 μm in diameter compatible with H. capsulatum on the Giemsa stain. Under direct microscopic examination the skin and sputum samples were negative for fungi and acid-fast bacilli. Coinciding with the previous findings, histopathology showed yeasts compatible with H. caspulatum in the oral mucosa. Urinary antigen detection was positive with a value of 2.1 ng/ml using the Histoplasma GM enzyme immunoassay kit (IMMY, Norman, Oklahoma).
The cultures for fungi on SDA at 28 °C showed mold in both oral, skin and sputum samples, after 10 and 18 days, respectively. All isolates were identified by their microscopic characteristics and MALDI-TOF MS (matrix-assisted laser desorption/ionization time-of-flight mass spectrometry) as H. capsulatum.
The serum sample, isolates, and Formalin-Fixed Paraffin-Embedded (FFPE) tissue slices of the oral mucosa and skin samples were sent to the National Reference Laboratory for Mycology of Argentina-INEI “Carlos G. Malbrán” – ANLIS for additional tests. Anti-H. capsulatum antibodies were not detected in the serum sample using counterimmunoelectrophoresis and immunodiffusion methods.
In addition, FFPE tissue specimens were analyzed using both PCRs, a nested conventional PCR that amplified a fragment of the HP100 gene and a real time quantitative PCR that amplified the ITS1 fragment4. The nested PCR amplified a 220 bp fragment specific for H. capsulatum while the quantitative PCR detected 2.2 × 102 fg DNA/μl in the oral mucosa sample. Neither of the two PCRs detected Histoplasma DNA in the skin samples.
The isolate was analyzed using phylogenetic multi-locus sequence analysis according to Kasuga et al.7, and the fungus was identified as H. capsulatum clade LamB. Gene sequences of H. capsulatum strain (Culture collection DMIc206235) were deposited in GenBank under accession numbers MW027017 (arf) MW027018 (ole), MW027019 (tub1) and MW002769 (H-anti).
After ten days in the hospital, the patient developed fever (38 °C), night sweats, malaise and asthenia. Based on the recent diagnosis of disseminated histoplasmosis, treatment with intravenous liposomal amphotericin B (3 mg/kg/day) was initiated with daily renal function measurements, while maintaining the TB treatment. A new nasopharyngeal swab was collected for SARS-CoV-2 detection, which was not detectable by RT-PCR.
During his hospital stay, on June 16, due to a worsening of the symptoms, and chest CT findings (Fig. 2c), a new nasopharyngeal swab sample for SARS-CoV-2 detection was performed in the context of the COVID-19 pandemic, and viral RNA was detected.
The patient's immunosuppressive regimen was changed, everolimus was discontinued, mycophenolate mofetil was reduced and meprednisone was increased. The patient received convalescent plasma therapy (one unit, title 1:800 IgG), with good clinical evolution, afebrile, hemodynamically stable, eupneic, and without supplemental oxygen requirements.
Two weeks later, liposomal amphotericin B therapy was switched to oral therapy with itraconazole 200 mg t.i.d. for three days, and 200 mg b.i.d. for one year2.
On July 4, the patient was discharged with favorable evolution, and SARS-CoV-2 RNA was detected in a nasopharyngeal swab sample until July 24.
Figure 1 summarizes the timeline of symptom onset, chest CT, laboratory data, and treatment.
Cases of COVID-19-associated histoplasmosis have already been reported in a patient living with HIV and advanced immunosuppression3, 10. Here, we are describing a new case of disseminated histoplasmosis with mucocutaneous and lung involvement in a renal transplant patient who acquired SARS-CoV-2 infection probably during hospitalization for his treatment with amphotericin B.
Histoplasmosis is often mistaken for tuberculosis because this condition often shows similar chest CT images in patients with clinical epidemiological criteria. Furthermore, kidney transplant recipients seem to have greater predisposition to acquire tuberculosis9. In this case, the patient showed mucocutaneous lesions that led to the diagnosis of histoplasmosis. This mycosis has a low incidence rate in transplant recipients, and respiratory manifestations and cutaneous lesions are the most common13.
Finally, COVID-19 was suspected because, when the patient had already been diagnosed with histoplasmosis, on day 10 of the antifungal therapy, his respiratory symptoms had worsened. A new CT showed progression of abnormalities with unifocal and peripheral lesions in the right lung with a characteristic ground glass pattern compatible with coronavirus disease5.
Histoplasmosis has often resulted in allograft loss and overall mortality6. Moreover, COVID-19 co-infection may be severe, requiring intensive care admission of kidney transplant recipients due to long-term immunosuppression12. In the case reported here, the patient had a favorable outcome probably because histoplasmosis was rapidly diagnosed and treated. In addition, he was treated early, after the diagnosis of SARS-CoV-2, with convalescent plasma therapy in line with the management of COVID-19 in kidney transplant recipients published by some authors11.
The molecular analysis of four genes using MLST identified the isolate as belonging to the LAmB clade, which did not surprise us since this clade is the predominant one in South America and the major clade circulating in Argentina7.
Twenty days after the last discharge, SARS-CoV-2 RNA was detected in a nasopharyngeal swab sample. This coincides with some authors who noted that in immunosuppressed renal transplant recipients SARS-CoV-2 viral shedding could be prolonged15.
The Centers for Disease Control and Prevention (CDC) listed patients requiring immunosuppressive therapy following organ transplantation, as being at high risk for severe SARS-CoV-disease. However, it is known that a low proportion of COVID-19 patients have post fungal co-infections8; in this case, histoplasmosis was prior to the viral infection, and subsequent hospitalizations and discharges could have been the factors influencing the acquisition of COVID-19 infection, in addition to the immunosuppressive status.
In the context of the COVID-19 pandemic it is important to pay attention to endemic mycoses such as histoplasmosis, since they exhibit respiratory symptoms that can be mistaken with viral or bacterial community-acquired pneumonia, and in pulmonary and disseminated histoplasmosis they can resemble other infections such as tuberculosis. To the best of our knowledge, this is the first report in the medical literature of COVID-19 associated with disseminated histoplasmosis in a renal transplant recipient. COVID-19 disease should be considered in patients with histoplasmosis, as well as other endemic mycoses and prolonged immunosuppression, particularly during the pandemic.
Conflict of interest
The authors declare that they have no conflicts of interest.
Acknowledgments
We thank Liliana Guelfand and Mariana Andreani Hospital Fernández, CABA, Argentina for the Histoplasma urinary antigen detection.
References
- 1.Adenis A.A., Valdes A., Cropet C., McCotter O.Z., Derado G., Couppie P., Chiller T., Nacher M. Burden of HIV-associated histoplasmosis compared with tuberculosis in Latin America: a modelling study. Lancet Infect Dis. 2018;18:1150–1159. doi: 10.1016/S1473-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assi M., Martin S., Wheat L.J., Hage C., Freifeld A., Avery R., Baddley J.W., Vergidis P., Miller R., Andes D., Young J.A.H., Hammoud K., Huprikar S., McKinsey D., Myint T., Garcia-Diaz J., Esguerra E., Kwak E.J., Morris M., Mullane K.M., Prakash V., Burdette S.D., Sandid M., Dickter J., Ostrander D., Abou Antoun S., Kaul D.R. Histoplasmosis after solid organ transplant. Histoplasmosis after solid organ transplant. Clin Infect Dis. 2013;57:1542–1549. doi: 10.1093/cid/cit593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basso R.P., Poester V.R., Benelli J.L., Stevens D.A., Zogbi H.E., Vasconcellos I.C.D.S., Pasqualotto A.C., Xavier M.O. COVID-19-associated histoplasmosis in an AIDS patient. Mycopathologia. 2020;6:1–4. doi: 10.1007/s11046-020-00505-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buitrago M.J., Canteros C.E., Frías De León G., González Á., Marques-Evangelista De Oliveira M., Muñoz C.O., Ramirez J.A., Toranzo A.I., Zancope-Oliveira R., Cuenca-Estrella M. Comparison of PCR protocols for detecting Histoplasma capsulatum DNA through a multicenter study. Rev Iberoam Micol. 2013;30:256–260. doi: 10.1016/j.riam.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Caruso D., Polidori T., Guido G., Nicolai M., Bracci B., Cremona A., Zerunian M., Polici M., Pucciarelli F., Rucci C., Dominicis C., Girolamo M.D., Argento G., Sergi D., Laghi A. Typical and atypical COVID-19 computed tomography findings. World J Clin Cases. 2020;6:3177–3187. doi: 10.12998/wjcc.v8.i15.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grim S.A., Proia L., Miller R., Alhyraba M., Costas-Chavarri A., Oberholzer J., Clark N.M. A multicenter study of histoplasmosis and blastomycosis after solid organ transplantation. Transpl Infect Dis. 2012;14:17–23. doi: 10.1111/j.1399-3062.2011.00658.x. [DOI] [PubMed] [Google Scholar]
- 7.Kasuga T., White T.J., Koenig G., McEwen J., Restrepo A., Castañeda E., Da Silva Lacaz C., Heins-Vaccari E.M., De Freitas R.S., Zancopé-Oliveira R.M., Qin Z., Negroni R., Carter D.A., Mikami Y., Tamura M., Taylor M.L., Miller G.F., Poonwan N., Taylor J.W. Phylogeography of the fungal pathogen Histoplasma capsulatum. Mol Ecol. 2003;12:3383–3401. doi: 10.1046/j.1365-294x.2003.01995.x. [DOI] [PubMed] [Google Scholar]
- 8.Lansbury L., Lim B., Baskaran V., Lim W.S. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81:266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mamishi S., Pourakbari B., Moradzadeh M., van Leeuwen W.B., Mahmoudi S. Prevalence of active tuberculosis infection in transplant recipients: a systematic review and meta-analysis. Microb Pathog. 2020;139:103894. doi: 10.1016/j.micpath.2019.103894. [DOI] [PubMed] [Google Scholar]
- 10.Messina F.A., Marin E., Caceres D.H., Romero M., Depardo R., Priarone M.M., Rey L., Vázquez M., Verweij P.E., Chiller T.M., Santiso G. Coronavirus Disease 2019 (COVID-19) in a patient with disseminated histoplasmosis and HIV – a case report from argentina and literature review. J Fungi (Basel) 2020;10:E275. doi: 10.3390/jof6040275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naeem S., Gohh R., Bayliss G., Cosgrove C., Farmakiotis D., Merhi B., Morrissey P., Osband A., Bailey J.A., Sweeney J., Rogers R. Successful recovery from COVID-19 in three kidney transplant recipients who received convalescent plasma therapy. Transpl Infect Dis. 2020;19:e13451. doi: 10.1111/tid.13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nair V., Jandovitz N., Hirsch J.S., Nair G., Abate M., Bhaskaran M., Grodstein E., Berlinrut I., Hirschwerk D., Cohen S.L., Davidson K.W., Dominello A.J., Osorio G.A., Richardson S., Teperman L.W., Molmenti E.P. COVID-19 in kidney transplant recipients. Am J Transplant. 2020;20:1819–1825. doi: 10.1111/ajt.15967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parajuli S., Wick A., Pandeya S., Astor B.C., Smith J., Djamali A., Mandelbrot D.A. The feared five fungal infections in kidney transplant recipients: a single-center 20-year experience. Clin Transplant. 2018;32:e13289. doi: 10.1111/ctr.13289. [DOI] [PubMed] [Google Scholar]
- 14.Riera F.O., Caeiro J.P., Denning D.W. Burden of serious fungal infections in Argentina. J Fungi (Basel) 2018;24:51. doi: 10.3390/jof4020051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu L., Gong N., Liu B., Lu X., Chen D., Chen S., Shu H., Ma K., Xu X., Guo Z., Lu E., Chen D., Ge Q., Cai J., Jiang J., Wei L., Zhang W., Chen G., Chen Z. Coronavirus disease 2019 pneumonia in immunosuppressed renal transplant recipients: a summary of 10 confirmed cases in Wuhan, China. Eur Urol. 2020;77:748–754. doi: 10.1016/j.eururo.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]