Abstract
Tunnelling nanotubes (TNTs) are an emerging route of long-range intercellular communication that mediate cell-to-cell exchange of cargo and organelles and contribute to maintaining cellular homeostasis by balancing diverse cellular stresses. Besides their role in intercellular communication, TNTs are implicated in several ways in health and disease. Transfer of pathogenic molecules or structures via TNTs can promote the progression of neurodegenerative diseases, cancer malignancy, and the spread of viral infection. Additionally, TNTs contribute to acquiring resistance to cancer therapy, probably via their ability to rescue cells by ameliorating various pathological stresses, such as oxidative stress, reactive oxygen species (ROS), mitochondrial dysfunction, and apoptotic stress. Moreover, mesenchymal stem cells play a crucial role in the rejuvenation of targeted cells with mitochondrial heteroplasmy and oxidative stress by transferring healthy mitochondria through TNTs. Recent research has focussed on uncovering the key regulatory molecules involved in the biogenesis of TNTs. However further work will be required to provide detailed understanding of TNT regulation. In this review, we discuss possible associations with Rho GTPases linked to oxidative stress and apoptotic signals in biogenesis pathways of TNTs and summarize how intercellular trafficking of cargo and organelles, including mitochondria, via TNTs plays a crucial role in disease progression and also in rejuvenation/therapy.
Keywords: Intercellular transfer, Mitochondrial homeostasis, Reactive oxygen species (ROS), Apoptosis, Cellular stress, Chemotherapy resistance, Mesenchymal stem cells, Rejuvenation
Introduction
Cell-to-cell communication plays an important role in maintaining tissue homeostasis. Intercellular communication can be facilitated by many soluble factors such as growth factors, neurotransmitters, cytokines, and extracellular vesicles (EVs), such as exosomes. A study in 2004 [1], first described intercellular transfer of molecular information directly between distal cells forming f-actin containing membrane lipid bilayer encircled ‘tunnel’ structures. Since then, the term “tunnelling nanotube” (TNT) has referred to this membrane f-actin conduit. Originally, the diameter of TNTs was reported to be 50–200 nm [1]. Later studies reported a relatively thicker diameter of around 700–900 nm, using optical resolution limited methods [2]. Cancer cells form networks of TNT-like but relatively thicker membrane protrusions, termed as tumour microtubes (TMs), consisting of both f-actin and tubulin. They are closed-ended and connected via gap junctions at the ends to transfer electrical signals and small molecules [3, 4]. Several studies have also referred to thinner nano-scaled membrane actin closed-ended protrusions as TNTs. Conventionally, f-actin containing open ended nanostructures are termed as TNTs. Recently, correlative FIB-SEM, light- and cryo-electron microscopy of neuronal cells revealed that TNTs of diameter 550 nm are made of 2–11 bundles of thinner channels (iTNTs), where the average diameter of each iTNTs was 123 ± 66 nm [5]. TNTs allow for the intercellular transport of various cargos, including viruses, organelles, RNAs, proteins, and toxic materials such as neurodegenerative protein aggregates [6]. Transfer of mitochondria has been implicated in disease progression and also in regeneration. Several studies have shown that intracellular build-up of prions or prion-like proteins facilitate disease progression by transferring toxic aggregates of these proteins or stressed organelles such as lysosomes and mitochondria from pathological donor cells to healthier acceptor cells [7, 8]. On the other hand, healthy mitochondria from mesenchymal stem cells (MSCs) are transferred to targeted acceptor cells with non-functional mtDNA/mitochondria [6, 9, 10]
In addition to mediating intercellular communication, TNTs rescue cells by relieving diverse cellular stresses caused by pathological conditions, such as oxidative stress, reactive oxygen species (ROS), mitochondrial heteroplasmy and apoptotic stress [11, 12]. Although the molecular drivers for the formation of TNTs under various pathophysiological conditions are unclear, studies over the last two decades indicate that cells form direct long-range connections between neighbouring cells via TNTs to alleviate cellular stress. Cytoskeletal dynamics play a pivotal role in the formation of TNTs and several studies have implicated the localized control of Rho GTPases in TNT-linked actin polymerization pathways [3, 13]. It has become evident that classical Rho GTPases (Rac1, Cdc42, and RhoA) control the complex regulatory balance in cell cycle progression and apoptotic signalling pathways [13, 14]. The capacity of MSCs to donate healthy mitochondria to targeted acceptor cells via TNTs correlates with the activity and expression of the atypical mitochondrial Rho GTPases [15], Miro-1 [9] and Miro-2 [16]. In this review, we summarize the role of TNTs in counteracting oxidative stress, mitochondrial heteroplasmy and apoptosis-related diverse cellular stresses, and the possible association of Rho GTPase-linked apoptotic signalling pathways in cytoskeleton remodelling and plasma membrane surface dynamics in the biogenesis of TNTs.
TNTs in intercellular transport
The original report [1], showed the transfer of endocytic vesicles and organelles as intercellular mediators between pheochromocytoma (PC12) cells. Subsequently, several studies in various cellular systems have shown the presence of TNTs and a range of organelles and cargo transportation via TNTs. These cargos include cytosolic proteins [17], ions [18], and miRNAs [19] that propagate between cells.
Various cellular stresses and pathological conditions promote intercellular transfer of organelles including the endoplasmic reticulum, golgi [12], mitochondria [20], endosomes [21] and lysosomes [7] via TNTs. Transfer of lysosomes from healthy endothelial progenitor cells to stressed human umbilical vein endothelial cells (HUVEC) has been reported, and this transfer helps to maintain lysosomal pH [22]. Oxidative stress-induced transfer of aberrant mitochondria via TNTs helps to propagate pathology from stressed to healthy cells in several diseases [23]. On the other hand, the transfers of healthy mitochondria from MSCs to targeted stressed cells is emerging as a potential therapy in regeneration [9, 10, 24, 25]
TNTs in the spread of disease pathology
Studies in 2005–2010 reported the transfer of prion proteins [26], bacteria [27], and viruses [28] from cell to cell through nanotubes leading to the spread of pathology. Viruses such as human immunodeficiency virus (HIV), and herpesviruses use this intercellular mode of dissemination without exposing themselves to the extracellular environment, thereby escaping the humoral immunity of the host [29, 30]. The first report about the propagation of virus particles from infected to uninfected T cells via TNTs was described for HIV [28]. Later, the involvement of TNTs in the spread of viruses has also been demonstrated for the influenza A virus [31], DNA viruses including alpha herpesvirus [32], bovine herpesvirus 1 [33] and human T-cell leukemia virus type 1 [34].
Initial studies demonstrated in 2009 that prions can hijack TNTs to spread the prion pathology in a cell-to-cell manner [26]. Subsequently, the intercellular propagation of amyloidogenic proteins via TNTs has been widely studied. Several such studies have demonstrated the spread of neurodegenerative proteins such as α-synuclein [35, 36], tau [37, 38], amyloid β [12, 39] and huntingtin [40]. One of the major hallmarks of neurodegenerative diseases is insufficient degradative capacity of lysosomes due to the accumulation of proteotoxic aggregates [41, 42], and lysosomal accumulation generates mitochondrial toxicity and increased oxidative stress. Evidence from several studies indicates that lysosomes can mediate the spread of neurodegenerative protein aggregates via TNTs [7]. It has also been demonstrated that α-synuclein aggregates can be transferred from cell to cell bound to mitochondria travelling within TNTs between neuronal cells [8].
TNTs in cancer malignancy
TNTs that are formed between malignant cells or between malignant cells and other cells in the tumour matrix are known to initiate tumour formation and metastasis [43]. Cell-to-cell transfer of mitochondria via TNTs plays a crucial role in maintaining metabolic homeostasis in cancer cells [44]. Below we discuss several key reports regarding cancer malignancy and TNTs.
Tumour cells network via nano-sized actin membrane open-ended conduits (TNTs proper) or by relatively thicker closed-ended micro-sized tubes (TMs) containing tubulin to transport organelles. The initial study [45], first demonstrated TNT like structures in intact solid tumours dissected from patients with lung adenocarcinoma and pleural mesothelioma malignant tissues. More recently, tumour cell-derived networks of membrane-tubes were observed in animal models of astrocytic brain tumours, including glioblastomas (GBM tumours) [46]. The structures are longer and thicker in diameter, and referred to as TMs. Intercellular transfer of mitochondria from tumour-activated stromal cells (TASC) by means of TNTs, EVs or cannibalism promotes proliferation of patient derived primary cultures of GBM cells in a 3D environment [47]. GBM stem-like cells (GSLCs) used in 2D culture and 3D organoid culture showed mitochondrial transfer via TNTs. These studies proposed a role of TNTs and TMs in the context of malignancy spread in organoid tumour models [48].
Mitochondrial transfer by means of TNTs from non-malignant bone marrow stromal cells to multiple myeloma cells resulted in tumour progression [49]. The same study also showed that shRNA-mediated CD38 knockdown inhibited mitochondrial transfer in vivo. The same knockdown in the in vivo model resulted in attenuation of tumour growth and improved survival rate of animal. In addition, hypoxia elevated the formation of TNTs and malignancy in ovarian and colon cancer [50]. This state of oxygen insufficiency results in increased levels of ROS in tumour cells, which leads to increased metabolic rate, gene expression, mitochondrial peroxidation, cellular stress and apoptotic stress [51, 52]. Cancer cells can counteract ROS induced apoptosis by enzymatic and non-enzymatic antioxidant defences, and it is now well accepted that moderate levels of ROS contributes to tumour progression by promoting several signalling pathways and gene mutations [53]. Several recent studies have shown that ROS promotes formation of TNTs and TNTs contribute in developing malignancy and resistance to cancer therapy [54].
Bcl-2, a highly conserved anti-apoptotic protein plays a central role in acquiring resistance to cancer therapy. A recent study [55] has shown that TNTs contribute to the progression of colorectal cancer by upregulating ERK (extracellular signal regulated kinase) expression in recipient cells by transferring mutant KRAS to these cells. They tend to develop TNTs as a part of their invasion and migration processes, and to transfer miRNAs as regulators of signalling pathways [56–58]. All these recent reports and several other studies (summarized in the Table 1) document that TNT formation is directly related to tumour malignancy and plays a significant role in tumour adaptation.
Table 1.
Transfer of pathology spreading through TNTs in cancer malignancies and acquiring of cancer therapy resistance
| Type of cell | Type of study | Movement of mitochondria | Result |
|---|---|---|---|
| Rat PC12 derived from Pheochromocytoma | In vitro | A two-way motion of mitochondrial movement was observed in the healthy cells whereas in unhealthy cells the mitochondrial movement was unidirectional (healthy-unhealthy) | The UV-treated cells were retrieved [25] |
| MSCs, cisplatin treated NSCs | In vitro | Mitochondria transfer from MSCs to cisplatin induced NSCs | Survival of cisplatin induced NSCs [59] |
| Patient derived primary Glioblastoma stem cells (2D and 3D) | In vitro | Transfer of mitochondria from glioblastoma stem cells to tumour organoid | The transfer of mitochondria was observed after the irradiation treatment [56] |
| Human tumour activated stromal cells (TASCs) and glioblastoma cells | In vitro | The transfer occurred from TASCs to glioblastoma cells in 3D/organoid condition | The proliferation of GBM cells occurred along with chemoresistance [47] |
| U87 glioblastoma cells and chemo resistance U87RETO cells | In vitro | Cytotoxic stress by etoposide | Accumulation of mitochondria in chemo resistance cancer cells [60] |
| Multiple myeloma primary cells(human) and cell lines, bone marrow stromal cells (BMSC) | In vitro | The transmission occurred between BMSC and myeloma cells | The proliferation increased in myeloma cells and higher ATP production [49] |
| In vivo | Knockdown of CD38 inhibits transfer of mitochondria | CD38 knockdown in animal model improves their survival by inhibiting myeloma growth [49] | |
| Human AML (acute myeloid leukemia) blasts and BMSCs | In vitro | NOX2 induced superoxide promotes the transfer between BMSC to AML cells | Greater basal and highest mitochondrial respiration and ATP production was observed in AML cells [61] |
| In vivo | Inhibition of NOX2 prevents transfer in AML mouse | Apoptosis in AML and improved survival of AML mouse [61] | |
| Primary cells derived from human malignant mesothelioma, mesothelioma cell lines and healthy mesothelial cells | In vitro | The transfer occurred between malignant cells / among the healthy cells, but not between cancer cells and normal cells | Cancer cell etiology and conquest [45] |
| Human prostatic cancer cells (PCa), cancer-associated fibroblasts (CAFs) | In vitro | The transfer ensued from CAFs to PCa cancer cells | A higher migratory and metastatic capacities of PC3 cancer cells were observed [62] |
| In vivo | Tumour growth and transfer was observed in PCa tumour models | ||
| Mesenchymal stem cells, ECs, ovarian cancer cell line and breast cancer cell line | In vitro | A two-directional movement was seen | Chemoresistance was observed [63] |
| Human T24 urothelial carcinoma cells and non-malignant urinary papillary urothelial cell (RT4) | In vitro | The transfer happened between malignant to non-malignant cells | Increased non-malignant cell intrusiveness [64] |
| In vivo | Increased invasiveness of bladder cancer cells | ||
| MSCs and acute lymphoblastic leukemia (ALL) cells | In vitro | It occurred from chemotherapy activated MSCs to ALL cells | Chemoprotection occurred by the ROS-induced pathway [65] |
| In vivo | Chemotherapy activated MSCs disseminated mitochondria to ALL cells in murine NSG model | ||
| MSCs, Jurkat cells and T-ALL cells | In vitro | A mutual exchange occurred between the Human MSCs, Jurkat cells and T-ALL cells | Jurkat and T-ALL cells developed chemoresistance [66] |
| Wharton jelly mMSCs and osteosarcoma cells | In vitro | mtDNA deleted osteosarcoma (143 ρ0 cells) | OXYPHOS dependent cell proliferation and restoration of bioenergetics [67] |
| Senescent primary human fibroblast line HF043 | In vitro | The transfer happened between senescent cells | mTOR and Cdc-42 signalling pathways involve in TNT formation [68] |
| Chemoresistant and chemosensitive ovarian cancer cells | In vitro | Hypoxia | Cancer cells were synchronized against chemotherapy [69] |
TNTs in drug resistance
Intercellular communications were suggested as a potential target for anti-cancer therapies as early as 2004 [70]. Several recent studies have demonstrated that TNT and TM networks play crucial roles in making these tumours exceptionally resistant to therapy [48]. Mitochondrial transfer from tumour activated stromal cells (TASC) to glioblastoma (GBM) cells was observed via TNTs, and the process provided chemo- and radio-resistance of the GBM [47]. Another study around the same time showed, GBM cells import the DNA repair enzyme O6-methylguanine-DNA methyltransferase via TNTs, thus enhancing resistance to temozolomide [71]. A self-repair mechanism of laser irradiated brain tumour cells was observed, and it involved formation of a network of TNTs and TMs [46]. Furthermore, GBM cells irradiated with α- particles establish a network of TNTs more rapidly compared to control irradiated cells in vitro within 24 h [72].
TNT-mediated cancer drug resistance and rescue from apoptotic cell death is a great challenge in cancer treatment. Acquisition of mitochondria in cancer cells (MCF-7) from endothelial cells through TNTs resulted in doxorubicin resistance in MCF-7 cells [63]. Later, in 2015 [73], it was shown that disruption of TNTs decreased the resistance of B-cell precursor acute lymphoblastic leukemia (BCP-ALL) cell to antileukemic drug prednisolone. A study in pancreatic cancer cells showed, doxorubicin increased the formation of TNTs in vitro in a dose-dependent manner and the biogenesis of TNTs promotes resistance to chemotherapy. The observation of drug resistance was also demonstrated in vivo in tumour specimens from patients diagnosed with pancreatic adenocarcinoma and neuroendocrine carcinoma [74]. The study by Wang et al. [66], showed that mitochondrial exchange through TNTs from Jurkat cells to MSCs by ICAM-1 mediated cell adhesion led to chemoresistance (Ara C and Methotrexate) in Jurkat cells. They also showed inhibition of TNT formation led to reduced chemoresistance in primary T-ALL cells (T cell acute lymphoblastic leukemia). Chemotherapy drugs, cytarabine (Ara-C) and doxorubicin (DNR), activated MSCs to disseminate mitochondria to surrounding ALL cells, and as a result chemoresistance developed [65]. Moreover, transfer of myosin containing cellular vesicles from stromal cells to chronic myeloid leukemia cells resulted in increased resistance of leukemic cells to imatinib which is a tyrosine kinase inhibitor [75].
Mitochondrial transfer from mesenchymal stem cells via TNTs
From a therapeutic point of view, TNTs can play a significant role in stem cell therapy, while the same cellular processes can be detrimental in certain pathological conditions. Several studies have shown that the transfer of mitochondria primarily depends on the communication between MSCs and target cells, and this communication is governed by several mechanisms. They include EVs, gap junctions, cell fusions, and TNTs [76]. Mitochondria provide the capacity for aerobic respiration, play important roles in aging and dysfunction in various heritable and acquired diseases. The human mitochondrial genome has 16,568 bp and encodes for only a small set of mitochondria-specific proteins, rRNAs and tRNAs, while majority of proteins are encoded by the nucleus [77]. The mutation rate in the mtDNA genome is high because it is not protected by histones and has low-efficiency nucleotide repair mechanisms [78].The first report of mitochondrial transfer from MSCs was published in 2006, and showed rescue of aerobic respiration by transferring functioning mitochondria via TNTs to cancer cells devoid of mtDNA [10]. Following this, several studies reported a high propensity of mitochondrial propagation and dynamics through TNTs extended from MSCs to the targeted somatic cells [20, 79–81]. Researchers have documented transfer of mitochondria from MSCs to the HUVEC, which are initially subject to ischemia–reperfusion injury [82]. A study in a mouse model of lung injury showed transfer of mitochondria from bone marrow-derived stromal cells to pulmonary alveoli caused alleviation of respiratory damage [83].
Recent research has shown that MSCs from tissue of divers origins, such as bone marrow, Wharton's jelly, adipose, and dental pulp play a role in protecting damaged cells from oxidative stress by donating mitochondria [84]. Studies have also demonstrated that MSCs play a crucial role in reducing mitochondrial ROS levels during repair pathways [9, 85]. However, it is not clear why MSCs exclusively form TNTs to targeted cells and what signal stimulates healthy MSCs to induce TNTs and transfer functional mitochondria. Paracrine factors released from neighbouring stressed cells modulate MSCs to initiate its action of damage repair. One study has shown that phosphatidylserine externalized on the surface of damaged cells (apoptotic epithelial cell) prompted MSCs to form TNTs [80]. In another study, it has been shown that connexin 43 plays a vital role in the regulation of TNT formation [86]. The same study has shown that iPSC derived MSCs transfer mitochondria via TNTs to rescue injured lung epithelial cells in a mouse model as well as in an in vitro model. This “donation” of mitochondria helped in alleviating asthma-related inflammation levels due to hypoxic conditions, and also prevented apoptosis of epithelial cells. One study [87] has shown that transfer of mitochondria via TNTs from MSCs to ocular cells helped in increasing the aerobic capacity and upregulation of mitochondrial genes. The work [88] suggested that both paracrine factors and mitochondrial transfer protect cardiomyocytes against stress, independent of each other.
In the last 15 years, several studies have documented transfer of mitochondria from different types of MSCs to aberrant cells via TNTs. In Table 2, we have summarized these studies, most of which have shown the involvement of oxidative stress, mitochondrial stress, ROS and/or apoptotic stress in the biogenesis of TNTs or cell-to-cell transfer via TNTs. Transfer of mtDNA and healthy mitochondria from MSCs via TNTs can be a potential remedy.
Table 2.
Transfer of mitochondria from different types of MSCs to aberrant disease models via TNTs
| Donor cell/source | Disease model | Experimental model used in the study | Mechanism/signals involve in TNT formation | Results |
|---|---|---|---|---|
| MSCs derived from human adipose tissues | Oxidative stress | In vitro: MSCs were subjected to hydrogen peroxide, N-acetyl-l-cysteine, and l-ascorbic acid 2-phosphate | Oxidative stress and mitochondrial dysfunction | Antioxidants increased the mitochondrial mass and respiratory capacity [89] |
| Rat bone marrow MSCs | Cardiovascular | In vitro model of ischemia–reperfusion injury | Hypoxia in the target cells | Decrease in the rate of apoptosis in H9c2s [90] |
| Wharton jelly mMSCs | MELAS patients (Mitochondrial Encephalopathy, Lactic Acidosis, and Stroke-like episodes) | In vitro: rotenone treated stressed Human MELAS fibroblasts | Eliminates mt.3243A>G mutation burden | Rescues bioenergetics of mitochondria in rotenone-stressed MELAS fibroblast [91] |
| Wharton jelly mMSCs | MERRF (myoclonus epilepsy associated with ragged-red fibers) | In vitro: increased ROS levels and oxidative stress | Eliminates mt.3243A>G mutation burden | Rescues bioenergetics of mitochondria and alleviates ROS levels in MERRF model [92] |
| Human iPSC-MSCs | Oxidative stress |
In vitro: rotenone was used in the corneal epithelial cells In vivo: sodium hydroxide induced a corneal alkaline burn in the rabbit model |
Oxidative stress and mitochondrial dysfunction |
In vitro: protection against rotenone oxidative stress In vivo: beneficial effects for corneal wound recovery [93] |
| Human bone marrow MSCs | Lung injury |
In vitro: secreted medium from the macrophages that were exposed to IL-13 was used to treat the mouse bronchial epithelial cells In vivo: epithelial injury and allergic airway inflammation was induced by rotenone treatment in a mouse model |
Epithelial mitochondrial dysfunction | A higher mitochondrial transfer was seen in the overexpressed Miro1 MSCs [9] |
| Mouse and human bone marrow MSCs | Lung injury | In vivo: mouse acute lung injury model | Acute lung injury caused dysfunction in the mitochondria | Shielding effects were observed by the mitochondrial transfer via TNTs [83] |
| Human iPSC-derived MSC | Mitochondria damage | In vitro: PC12 cells were exposed to the CoCl2 (a chemical inducer of hypoxia inducible factor-1) | ROS | Reduction in mitochondrial dysfunction was detected [94] |
| Mesenchymal Multipotent stromal cells (MMSCs) | Kidney transplantation | In vitro: rat renal tubular cells | Induction of differentiation | A two-directional exchange of cytoplasmic content was seen [95] |
| Human bone marrow MSCs | Inflammatory disease | In vitro: human vascular smooth muscle cells | Mitochondrial dysfunction in vascular smooth muscle cells | There was an enhanced MSC proliferation but not differentiation was detected [96] |
| Rat bone marrow MSCs | Inflammation | In vitro: rat nucleus pulposus cells subjected to IL-1β | Excessive apoptosis | There was a reduction in apoptosis in the direct co-culture method [97] |
| MMSCs | Post-ischemic stroke | In vitro: post-ischemic model in rat cortical neurons | Post-ischemic stress | Better rehabilitation after stroke [98] |
| MMSCs | Ischemic model | In vitro: ROS elevated ischemic model in neural cells, and astrocytes | Elevated ROS levels | Restored bioenergetics and stimulated proliferation [99] |
| BM-MSCs | Spinal cord injury | In vitro: oxygen-glucose deprived (OGD) injured VSC4.1 motor neurons or primary cortical neurons | Oxygen–glucose deprivation | Improved bioenergetics and recovery of OGD and spinal cord injury models [100] |
| BM-MSCs | Acute respiratory distress syndrome (ARDS) | In vitro: monocyte-derived macrophages | Stress due to E. coli infection | Enhanced phagocytosis [101] |
| BM-MSCs | Acute respiratory distress syndrome (ARDS) | In vitro: lipopolysaccharide (LPS) induced monocyte-derived macrophages | LPS-induced stress | Enhanced phagocytosis [102] |
| BM-MSCs, | Myocardial infarction | In vitro: ischemic H9c2 cardiomyoblasts | Oxygen–glucose deprivation | Increased survival rate of cardio-myoblasts [103] |
| MSCs, | Cardiomyopathy | In vitro: cardiomyocytes | LPS-induced stress | Enhancement of myocardioblast functions due to bioenergetics stimulus [104] |
| iPSCs-MSCs | Cardiomyopathy | In vitro: cardiomyocytes | Cardiomyopathy induced by anthracycline | Rescued of cardiomyopathy by transferring of Miro1 and TNFαip2 [105] |
| iPSCs-MSCs | Chronic obstructive pulmonary disease (COPD) | In vitro: bronchial epithelial cells | Cigarette smoking (CS) induced COPD | Rescued CS induced mitochondrial damage [106] |
However, a deeper understanding is needed to implement the transfer of mitochondria as a therapy, and focus should be given to unravelling various stress signals that could affect transcellular trafficking of mitochondria via TNTs, both in diseases and in rejuvenation [3, 107, 108].
Association of tunnelling nanotubes with oxidative stress, apoptosis, and mitochondrial homeostasis
Mitochondria play an important role in oxidative phosphorylation, aerobic metabolism, calcium signalling, and apoptosis [109]. Mitochondrial dysfunction-related oxidative stress is associated with diseases such as cardiomyopathy, ischemic heart diseases, lung disorders, brain injury, stroke, and neurodegenerative diseases like Alzheimer’s and Parkinson’s disease. Exchange of mtDNA between cells via transfer of mitochondria could modulate respiration and cell cycle arrest. Levels and homoplasmic polymorphism of mtDNA regulate mtDNA-processing enzymes, replication, and transcription of mtDNA and respiratory complexes. Dysfunction of these processes can result in aberrant mitochondria with formation of ROS and also cell cycle arrest due to impaired function of the respiration-linked enzyme dihydroorotate dehydrogenase [110]. Melanoma cancer cells devoid of mtDNA injected in to syngeneic C57BL/6Nsu9-DsRed2 mice expressed with red fluorescent mitochondrial protein can recover to form tumours after import of mtDNA by acquiring whole mitochondria from neighbouring healthy cells [81]. Oxidative stress and ROS promote the biogenesis of TNTs in several pathological conditions [54]. Hydrogen peroxide (H2O2) treatment in the primary hippocampal rat astrocytes and neurons promotes the biogenesis of TNTs, at the same time the induced cellular stress activates tumour suppressor protein p53 [12]. However, later studies were reported that p53 is not the key element for TNT formation, and the effect of H2O2 on TNTs is cell type-specific [111].
The crucial role of intercellular, horizontal transfer of mitochondria demonstrated recently under various pathophysiological conditions, primarily in rescuing tumourigenesis and bioenergetic deficiencies. Tan et al. [108] have shown that the mtDNA-deficient cells acquired functional mitochondrial genome from the surrounding tumour microenvironment or MSCs to regulate many factors related to mitochondrial respiration. In cancer cells, delaying apoptosis resulted in the restoration of cell survival and enhancement of tumourigenicity or metastasis. MSCs from different sources exert different rescue capacities against aerobic respiration ability and postpone apoptosis of the recipient cells [23, 107]. It is possible that paracrine factors related to oxidative stress and/or ROS sent from stressed cells trigger MSCs to make cellular bridges via TNT structures for transferring mitochondria.
The role of TNTs in rescue from apoptotic cell death has also been demonstrated in neuronal cells [25]. This study showed that PC12 cells that were treated with UV light were rescued by non-cancer cells by transfer of mitochondria via TNT-like structures when compared with untreated cells. The UV treated cells that had lost cytochrome C formed TNTs but did not enter the apoptotic cascade. The study suggests that transfer of mitochondria from healthy cells via TNTs reverses the cellular stress in early stage of apoptosis. A recent work [112] has shown that α-synuclein protofibril-induced defects in cellular degradation machineries in microglia enhance cell to cell networks via TNTs to transfer the burden of proteotoxic aggregates to neighbouring cells. The study has also shown that mitochondrial shuffling and sharing of proteotoxic burdens via TNTs alleviate ROS levels and rescue cells from ROS-induced apoptosis.
Rho GTPase related signals counteract apoptosis via tunnelling nanotubes
TNTs mediate direct intercellular transport between neighbouring cells and, structurally, they are open-ended membrane actin conduits. Thus, modulation of membrane and cytoskeleton dynamics may play a major role in the biogenesis of TNTs. Several studies have shown that actin-depolymerizing agents such as cytochalasin B and latrunculin B inhibit TNT formation [1, 113]. The master regulators of the cytoskeleton, Rho family of GTPases (Rac1, Cdc42, and RhoA), are implicated in TNT formation by many studies [13]. The localized control of Rho GTPase regulators, the GTPase activating proteins (GAPs) and guanine nucleotide exchange factors 42 (GEFs) have been proposed to play a role in TNT assembly. One study [114] in immune cells has reported that Cdc42 and Rac1, and their respective effector molecules WASP and WAVE2, are involved in the biogenesis of TNTs by modulating actin polymerization via the Arp2/3 complex. Using FRET-based biosensors, the study has demonstrated that Rac1 stays distributed throughout the TNT structures, while Cdc42 is involved in initiating the biogenesis of TNTs. Transfer of oncogenic KRAS promotes formation of TNTs by regulating the ERK pathway in colorectal cancer. It is thus important to note that Rho GTPase-regulated ERK signalling pathway controls the expression of pro-survival or anti-apoptotic Bcl-2 family of proteins [55].
Two actin regulators downstream of Rho GTPases, βCamKII and cofilin, have recently been demonstrated to play a role in the biogenesis of TNTs. Cross-talk between the signalling cascades of Rho GTPases with the actin regulatory molecules βCamKII, cofilin and Arp2/3 is well documented in the early development of the dendritic spine [115]. Vargas et al. [116], showed that stability of TNTs depends on the activation of the Wnt/Ca2+ signal-dependent modulation of βCamKII in the CAD (mouse catecholaminergic neuronal cell line) cells and primary neurons. The actin-binding ability of the protein is modulated by phosphorylation of βCamKII [117]. Inactivation of cofilin by the RNA-binding protein nucleolin induces TNT biogenesis [118]. In addition, the alphaherpesvirus-induced biogenesis of TNTs depends on the US3 protein kinase-mediated activation of p21-activated kinases (PAKs) apparently by activation of Cdc42/Rac1 and Rho signalling axis, within a poorly understood complex mechanism [32]. PAK kinases are considered primarily the effector of the Rho family GTPases Cdc42 and Rac1. Additionally, studies have shown that PAK1 inhibitor IPA-3 attenuates alpha herpes virus-induced TNT-like membrane actin projections [32, 119]. PAK2 has also been reported in HIV-1 Nef protein-mediated TNT formation [120].
In our recent study, we have observed that Alzheimer’s pathogenesis, the amyloid-β oligomers internalize via PAK1 dependent actin mediated endocytic pathway, and the internalization process promotes formation of TNT-like structures and direct cell-to-cell transfer of oligomers in neuronal cells [39]. The study has also shown colocalization of activated PAK1 with f-actin throughout the TNT network.
Conversely, the Cdc42/IRSp53/VASP system plays a role in the filopodia-promoting network, being negatively correlated with formation of TNTs in neuronal cells [121]. Recently, another study has reported that Arp2/3 negatively regulates biogenesis of TNTs in CAD cells [5]. Another recent study [112] has shown that inhibition of ROCK (using chemical inhibitor Y-27632), a downstream signalling molecule of Rho/Rac/Cdc42, promotes biogenesis of TNTs. The study has indicated that ROCK inhibition promotes TNT formation via Myosin II regulated f-actin modulation. Altogether, these studies suggest a complex regulatory mechanism of Rho GTPases in TNT biogenesis. A further recent report [118], has also shown that M-sec regulated exocyst complex needs to function together with actin polymerization by inhibiting activity of cofilin in the biogenesis of TNTs in multiple mammalian cellular models. The study suggests that in addition to actin polymerization, M-Sec-dependent plasma membrane (PM) re-modelling is a necessary step in formation of TNTs.
The rescue capacity of MSCs mediated via TNTs correlates with the Miro-1 expression, as shown for the transfer capacity of mitochondria from MSCs to stressed alveolar epithelial cells via TNTs [9]. Miro-1 and -2 belong to a class of novel Rho-GTPase, amino acid sequence revealed GTPases domain homolog to the classical Rho-GTPases in the N-terminal part of the protein [16]. Interestingly, Miro proteins lack the membrane-binding motif CAAX in their C-terminal domains, unlike small GTPases but contain a second GTP-binding domain without homology to typical Rho-GTPases [15]. Studies have shown that overexpression of Miro-1 protein leads to an increase in the mitochondrial transfer capacity and, hence, there is a decrease in the apoptosis level and mitochondrial ROS production, and alleviation of respiratory dysfunction [122]. A recent study has shown that the monooxygenase domain of MICAL2PV, a spliced isoform product of the neuronal guidance gene MICAL2, interacts with Miro-2, inhibiting TNT formation by depolymerization of f-actin. MICAL2PV plays crucial role in cell survival and down-regulation of MICAL2PV, and protect lung cancer cells treated with chemotherapeutic drugs [123].
Rho GTPases in cell surface dynamics and TNT biogenesis
Several cytoskeleton remodelling signals are correlated with cell surface dynamics and PM remodelling [124]. Small GTPases Arf and Rab regulate exocytosis of specific vesicles to discrete sites of the PM. Rho GTPases and their regulatory factors contribute to the process by modulating the tethering and subsequent fusion of exocytic vesicles. One study [125], showed that formation of TNTs is regulated by the exocyst complex protein M-Sec in HeLa cells, which is involved in exosome fusion and membrane expansion. The exocyst complex contributes to PM recruitment of the actin remodelling proteins Ral-GTPase and filamin to promote TNTs. The regulatory molecules associated with the recycling of endocytic vesicles and vesicle trafficking, which regulates PM surface dynamics, have also been implicated by several studies in the biogenesis of TNTs. Rab class of small GTPases, Rab8a, Rab11a, and Rab35 are implicated in TNT formation by regulating membrane recycling in neuronal and cancer cells [126, 127]. Rab35-GTP, ACAP2, ARF6-GDP, and EHD1 promote TNT formation in a cascade-like manner in neuronal cells. It may therefore be that modulation of cytoskeleton remodelling via actin polymerization signalling cascades is linked to cell membrane surface dynamics to induce formation of membrane actin-derived TNTs.
Rho GTPases in cell cycle progression, apoptosis, and TNT biogenesis
RhoA, Rac1, and Cdc42 are the most studied typical Rho GTPases, not only involved in the regulation of distinct actin cytoskeleton and PM structures, they are also interlinked via complex molecular signalling events to regulate cell cycle progressions and apoptosis [128]. Rac1-regulated oxidase was reported to modulate acute cellular necrosis, apoptosis, and acute inflammatory response in hepatic ischemia. Rac1-induced production of ROS by an NADPH oxidase was also reported in both phagocytic and non-phagocytic cells [129]. Rac1 can also activate signalling downstream of NFκB, PAK, and ERK by ROS-mediated pathways in neuronal cells to counteract apoptosis. Neuronal cells have limited regenerative capability, and continuous ‘fitness’ of these cells is vital; these cells possess intrinsic competence to attenuate apoptosis [130]. Instead, apoptosis due to elevated stress/ROS levels in neuronal cells may induce formation of TNTs to ameliorate cellular stress [54]. In cancer cells, Rac1-mediated MAPK/ERK and Akt signalling involves the upregulation of the pro-survival or anti-apoptotic Bcl-2 family of proteins [131]. The pro-survival signalling of MAPK/ERK involving formation of TNTs occurs in various cancer cells [132], and TNTs promote cell proliferation and cancer malignancy levels [48]. In addition, TNTs are involved in transfer of apoptosis regulators from healthy cells to diseased cells. Several studies have also shown that the pro-apoptotic Fas ligand is transferred via TNTs to T lymphocytes to induce cell death [133, 134].
Conclusions
The discovery of TNTs in 2004, opened up a novel mechanism of long-range intercellular communication. TNTs are actin-membrane conduits, thereby, actin regulation together with dynamic PM modulatory cellular events play major roles in their biogenesis. The complex functions of Rho GTPase signalling cascades have been implicated by several studies in TNT biogenesis. However, some contradictions exist in the literature and there may be some variability in TNT regulation in different cell types. Moreover, discrepancies also exist in the definition of supercellularity of TNT structures in different studies. It is challenging to resolve TNTs and TMs in ex vivo organoid models or in vivo animal models. Detection methods using advanced imaging tools or exclusive markers need to be explored to make advancement in the field.
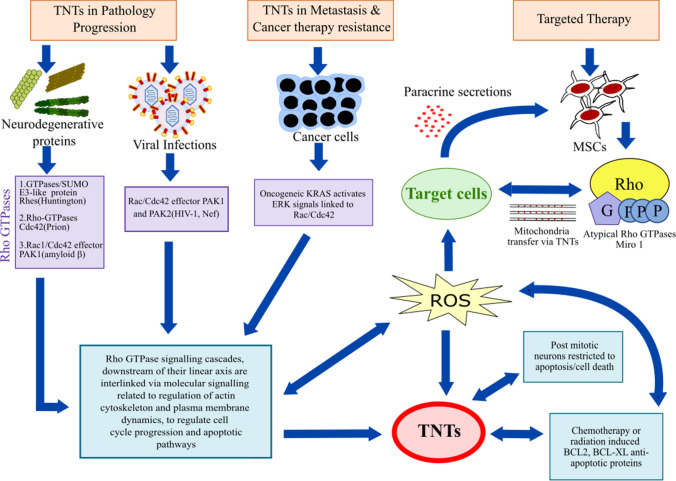
Rho GTPase signalling cascades, that are not only related to the regulation of distinct actin cytoskeleton and PM dynamics, downstream of their linear axis are interlinked via complex molecular signalling events to regulate cell cycle progression and apoptosis (Fig. 1) [128, 131]. Direct cell-to-cell transfer of organelles or cargo via TNTs has emerged as an important mechanism for maintaining cellular homeostasis, and this process has been implicated in disease spread and disease resistance [1]. The widespread association of oxidative stress, apoptosis, mitochondrial homeostasis, and mitochondrial heteroplasmy with the biogenesis of TNTs has been established by several studies [1, 113]. Cell types that possess an inherent mechanism to resist apoptosis, such as neuronal cells and cancer cells, promote the biogenesis of TNTs possibly to maintain cell survival under pathological stress. Some studies for example [10], have shown that ROS and apoptotic stress promotes the biogenesis of TNTs, however, the molecular events associated with apoptosis signalling or oxidative stresses are not the primary regulatory elements. Biogenesis of TNTs increases the survival of cancer cells treated with chemotherapy, radiotherapy, UV radiation, and laser-induced phototoxicity. MSCs rescue cells from apoptotic death triggered by oxidative stress or mitochondrial heteroplasmy. Therefore, MSC-mediated transfer of mitochondria could have therapeutic potential, for example, by promoting wound healing in response to mitochondrial import [135]. On the other hand, transfer of healthy mitochondria rescues ROS-induced apoptosis in cancer cells and promotes cancer malignancies. It is unclear to what extent damage to mitochondria triggers the formation of TNTs. Do damaged recipient cells actively form TNTs to healthy neighbouring cells? If not then, what signal triggers healthy cells to make direct connections via TNTs to transfer mitochondria. Several articles have shown that the atypical Rho GTPases, Miro 1 and Miro 2, play significant roles in cell to cell transfer of mitochondria from MSCs. Classical Rho GTPases are implicated in other cell types, such as neuronal cells, immune cells and in the transfer of virus spreading. Structurally and functionally these two types of Rho GTPases are distinct, although they do share several homologous domains and may have overlapping functions in TNT signalling pathways. Thus, future studies are required to investigate the emerging role of Rho GTPase signalling cascades in TNT biogenesis and in the formation of supercellular structures with potential importance in maintaining tissue homeostasis and pathophysiological conditions.
Fig. 1.
Schematic summary of TNT studies indicating the involvement of Rho GTPase signalling cascades in the biogenesis of TNTs by modulating actin cytoskeleton proteins, PM dynamics and potentially alleviating cellular or apoptotic stress
Abbreviations
- ALL
Acute lymphoblastic leukemia
- AML
Acute myeloid leukemia
- BMSC
Bone marrow stromal cells
- CAD
Catecholaminergic neuronal cells
- EVs
Extracellular vesicles
- ERK
Extracellular signal-regulated kinase
- GBM
Glioblastoma Multiforme
- HIV
Human immunodeficiency virus
- HUVEC
Human umbilical vein endothelial cells
- iPSC
Induced pluripotent stem cells
- MIRO
Mitochondrial Rho GTPase
- MSCs
Mesenchymal stem cells
- mtDNA
Mitochondrial DNA
- PAK1
P21-activated kinase 1
- PC12
Pheochromocytoma cells
- Rho
Ras homologous protein
- ROS
Reactive oxygen species
- TASC
Tumour activated stromal cells
- TNTs
Tunnelling nanotubes
- TMs
Tumour microtubes
- UV
Ultraviolet
Author contributions
SN conceived the initial idea for the review; AR, PR and SN researched the literatures and wrote the manuscript; AR and PR drew the figure and collected the tables; JN and DLP edited, revised and added their ideas in the manuscript.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal. A.R thanks Manipal Academy of higher education for Dr. TMA pai fellowship. S.N thanks the Intramural fund of Manipal Academy of Higher Education, Manipal, India and the Indian Council of Medical Research of India (5/4-5/Ad-hoc/Neuro/216/2020-NCD-I) for financial support. D.L.P and J.N thank Griffith University for financial support.
Declarations
Conflict of interest
The authors declare that there are no competing interests associated with the manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303(5660):1007–1010. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- 2.Gerdes HH, Rustom A, Wang X. Tunneling nanotubes, an emerging intercellular communication route in development. Mech Dev. 2013;130(6–8):381–387. doi: 10.1016/j.mod.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Ljubojevic N, Henderson JM, Zurzolo C. The ways of actin: why tunneling nanotubes are unique cell protrusions. Trends Cell Biol. 2021;31(2):130–142. doi: 10.1016/j.tcb.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Roehlecke C, Schmidt MHH. Tunneling nanotubes and tumor microtubes in cancer. Cancers (Basel) 2020;12(4):857. doi: 10.3390/cancers12040857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sartori-Rupp A, Cordero Cervantes D, Pepe A, Gousset K, Delage E, Corroyer-Dulmont S, et al. Correlative cryo-electron microscopy reveals the structure of TNTs in neuronal cells. Nat Commun. 2019;10(1):342. doi: 10.1038/s41467-018-08178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mittal R, Karhu E, Wang JS, Delgado S, Zukerman R, Mittal J, et al. Cell communication by tunneling nanotubes: implications in disease and therapeutic applications. J Cell Physiol. 2019;234(2):1130–1146. doi: 10.1002/jcp.27072. [DOI] [PubMed] [Google Scholar]
- 7.Victoria GS, Zurzolo C. The spread of prion-like proteins by lysosomes and tunneling nanotubes: implications for neurodegenerative diseases. J Cell Biol. 2017;216(9):2633–2644. doi: 10.1083/jcb.201701047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valdinocci D, Kovarova J, Neuzil J, Pountney DL. Alpha-synuclein aggregates associated with mitochondria in tunnelling nanotubes. Neurotox Res. 2021;39(2):429–443. doi: 10.1007/s12640-020-00285-y. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad T, Mukherjee S, Pattnaik B, Kumar M, Singh S, Kumar M, et al. Miro1 regulates intercellular mitochondrial transport and enhances mesenchymal stem cell rescue efficacy. EMBO J. 2014;33(9):994–1010. doi: 10.1002/embj.201386030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci USA. 2006;103(5):1283–1288. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu D, Tan KS, Zhang X, Sun AY, Sun GY, Lee JC. Hydrogen peroxide alters membrane and cytoskeleton properties and increases intercellular connections in astrocytes. J Cell Sci. 2005;118(Pt 16):3695–3703. doi: 10.1242/jcs.02507. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Cui J, Sun X, Zhang Y. Tunneling-nanotube development in astrocytes depends on p53 activation. Cell Death Differ. 2011;18(4):732–742. doi: 10.1038/cdd.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S, Kazanietz MG, Cooke M. Rho GTPases and the emerging role of tunneling nanotubes in physiology and disease. Am J Physiol Cell Physiol. 2020;319(5):C877–C884. doi: 10.1152/ajpcell.00351.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olson MF, Ashworth A, Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science. 1995;269(5228):1270–1272. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- 15.Fransson A, Ruusala A, Aspenstrom P. Atypical Rho GTPases have roles in mitochondrial homeostasis and apoptosis. J Biol Chem. 2003;278(8):6495–6502. doi: 10.1074/jbc.M208609200. [DOI] [PubMed] [Google Scholar]
- 16.Nahacka Z, Zobalova R, Dubisova M, Rohlena J, Neuzil J. Miro proteins connect mitochondrial function and intercellular transport. Crit Rev Biochem Mol Biol. 2021;56(4):401–425. doi: 10.1080/10409238.2021.1925216. [DOI] [PubMed] [Google Scholar]
- 17.Biran A, Perelmutter M, Gal H, Burton DG, Ovadya Y, Vadai E, et al. Senescent cells communicate via intercellular protein transfer. Genes Dev. 2015;29(8):791–802. doi: 10.1101/gad.259341.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watkins SC, Salter RD. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity. 2005;23(3):309–318. doi: 10.1016/j.immuni.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Thayanithy V, Dickson EL, Steer C, Subramanian S, Lou E. Tumor-stromal cross talk: direct cell-to-cell transfer of oncogenic microRNAs via tunneling nanotubes. Transl Res. 2014;164(5):359–365. doi: 10.1016/j.trsl.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koyanagi M, Brandes RP, Haendeler J, Zeiher AM, Dimmeler S. Cell-to-cell connection of endothelial progenitor cells with cardiac myocytes by nanotubes: a novel mechanism for cell fate changes? Circ Res. 2005;96(10):1039–1041. doi: 10.1161/01.RES.0000168650.23479.0c. [DOI] [PubMed] [Google Scholar]
- 21.Onfelt B, Nedvetzki S, Benninger RK, Purbhoo MA, Sowinski S, Hume AN, et al. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J Immunol. 2006;177(12):8476–8483. doi: 10.4049/jimmunol.177.12.8476. [DOI] [PubMed] [Google Scholar]
- 22.Yasuda K, Khandare A, Burianovskyy L, Maruyama S, Zhang F, Nasjletti A, et al. Tunneling nanotubes mediate rescue of prematurely senescent endothelial cells by endothelial progenitors: exchange of lysosomal pool. Aging (Albany NY) 2011;3(6):597–608. doi: 10.18632/aging.100341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valdinocci D, Simoes RF, Kovarova J, Cunha-Oliveira T, Neuzil J, Pountney DL. Intracellular and intercellular mitochondrial dynamics in Parkinson's disease. Front Neurosci. 2019;13:930. doi: 10.3389/fnins.2019.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers RS, Bhattacharya J. When cells become organelle donors. Physiology (Bethesda) 2013;28(6):414–422. doi: 10.1152/physiol.00032.2013. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Gerdes HH. Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ. 2015;22(7):1181–1191. doi: 10.1038/cdd.2014.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gousset K, Schiff E, Langevin C, Marijanovic Z, Caputo A, Browman DT, et al. Prions hijack tunnelling nanotubes for intercellular spread. Nat Cell Biol. 2009;11(3):328–336. doi: 10.1038/ncb1841. [DOI] [PubMed] [Google Scholar]
- 27.Dubey GP, Ben-Yehuda S. Intercellular nanotubes mediate bacterial communication. Cell. 2011;144(4):590–600. doi: 10.1016/j.cell.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Kohler K, et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol. 2008;10(2):211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- 29.Okafo G, Prevedel L, Eugenin E. Tunneling nanotubes (TNT) mediate long-range gap junctional communication: Implications for HIV cell to cell spread. Sci Rep. 2017;7(1):16660. doi: 10.1038/s41598-017-16600-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jansens RJJ, Tishchenko A, Favoreel HW. Bridging the gap: virus long-distance spread via tunneling nanotubes. J Virol. 2020;94(8):e02120-19. doi: 10.1128/JVI.02120-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts KL, Manicassamy B, Lamb RA. Influenza A virus uses intercellular connections to spread to neighboring cells. J Virol. 2015;89(3):1537–1549. doi: 10.1128/JVI.03306-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van den Broeke C, Radu M, Deruelle M, Nauwynck H, Hofmann C, Jaffer ZM, et al. Alphaherpesvirus US3-mediated reorganization of the actin cytoskeleton is mediated by group A p21-activated kinases. Proc Natl Acad Sci USA. 2009;106(21):8707–8712. doi: 10.1073/pnas.0900436106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panasiuk M, Rychlowski M, Derewonko N, Bienkowska-Szewczyk K. Tunneling nanotubes as a novel route of cell-to-cell spread of herpesviruses. J Virol. 2018;92(10):e00090-18. doi: 10.1128/JVI.00090-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Omsland M, Pise-Masison C, Fujikawa D, Galli V, Fenizia C, Parks RW, et al. Inhibition of tunneling nanotube (TNT) formation and human T-cell leukemia virus type 1 (HTLV-1) transmission by cytarabine. Sci Rep. 2018;8(1):11118. doi: 10.1038/s41598-018-29391-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abounit S, Bousset L, Loria F, Zhu S, de Chaumont F, Pieri L, et al. Tunneling nanotubes spread fibrillar alpha-synuclein by intercellular trafficking of lysosomes. EMBO J. 2016;35(19):2120–2138. doi: 10.15252/embj.201593411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dieriks BV, Park TI, Fourie C, Faull RL, Dragunow M, Curtis MA. Alpha-synuclein transfer through tunneling nanotubes occurs in SH-SY5Y cells and primary brain pericytes from Parkinson's disease patients. Sci Rep. 2017;7:42984. doi: 10.1038/srep42984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11(7):909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tardivel M, Begard S, Bousset L, Dujardin S, Coens A, Melki R, et al. Tunneling nanotube (TNT)-mediated neuron-to neuron transfer of pathological Tau protein assemblies. Acta Neuropathol Commun. 2016;4(1):117. doi: 10.1186/s40478-016-0386-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dilna A, Deepak KV, Damodaran N, Kielkopf CS, Kagedal K, Ollinger K, et al. Amyloid-beta induced membrane damage instigates tunneling nanotube-like conduits by p21-activated kinase dependent actin remodulation. Biochim Biophys Acta Mol Basis Dis. 2021;1867(12):166246. doi: 10.1016/j.bbadis.2021.166246. [DOI] [PubMed] [Google Scholar]
- 40.Costanzo M, Abounit S, Marzo L, Danckaert A, Chamoun Z, Roux P, et al. Transfer of polyglutamine aggregates in neuronal cells occurs in tunneling nanotubes. J Cell Sci. 2013;126(Pt 16):3678–3685. doi: 10.1242/jcs.126086. [DOI] [PubMed] [Google Scholar]
- 41.Domert J, Rao SB, Agholme L, Brorsson AC, Marcusson J, Hallbeck M, et al. Spreading of amyloid-beta peptides via neuritic cell-to-cell transfer is dependent on insufficient cellular clearance. Neurobiol Dis. 2014;65:82–92. doi: 10.1016/j.nbd.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 42.Nath S, Agholme L, Kurudenkandy FR, Granseth B, Marcusson J, Hallbeck M. Spreading of neurodegenerative pathology via neuron-to-neuron transmission of beta-amyloid. J Neurosci. 2012;32(26):8767–8777. doi: 10.1523/JNEUROSCI.0615-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sahu P, Jena SR, Samanta L. Tunneling nanotubes: a versatile target for cancer therapy. Curr Cancer Drug Targets. 2018;18(6):514–521. doi: 10.2174/1568009618666171129222637. [DOI] [PubMed] [Google Scholar]
- 44.Hekmatshoar Y, Nakhle J, Galloni M, Vignais ML. The role of metabolism and tunneling nanotube-mediated intercellular mitochondria exchange in cancer drug resistance. Biochem J. 2018;475(14):2305–2328. doi: 10.1042/BCJ20170712. [DOI] [PubMed] [Google Scholar]
- 45.Lou E, Fujisawa S, Morozov A, Barlas A, Romin Y, Dogan Y, et al. Tunneling nanotubes provide a unique conduit for intercellular transfer of cellular contents in human malignant pleural mesothelioma. PLoS One. 2012;7(3):e33093. doi: 10.1371/journal.pone.0033093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Osswald M, Jung E, Sahm F, Solecki G, Venkataramani V, Blaes J, et al. Brain tumour cells interconnect to a functional and resistant network. Nature. 2015;528(7580):93–98. doi: 10.1038/nature16071. [DOI] [PubMed] [Google Scholar]
- 47.Salaud C, Alvarez-Arenas A, Geraldo F, Belmonte-Beitia J, Calvo GF, Gratas C, et al. Mitochondria transfer from tumor-activated stromal cells (TASC) to primary glioblastoma cells. Biochem Biophys Res Commun. 2020;533(1):139–147. doi: 10.1016/j.bbrc.2020.08.101. [DOI] [PubMed] [Google Scholar]
- 48.Pinto G, Brou C, Zurzolo C. Tunneling nanotubes: the fuel of tumor progression? Trends Cancer. 2020;6(10):874–888. doi: 10.1016/j.trecan.2020.04.012. [DOI] [PubMed] [Google Scholar]
- 49.Marlein CR, Piddock RE, Mistry JJ, Zaitseva L, Hellmich C, Horton RH, et al. CD38-driven mitochondrial trafficking promotes bioenergetic plasticity in multiple myeloma. Cancer Res. 2019;79(9):2285–2297. doi: 10.1158/0008-5472.CAN-18-0773. [DOI] [PubMed] [Google Scholar]
- 50.Lou E, Zhai E, Sarkari A, Desir S, Wong P, Iizuka Y, et al. Cellular and molecular networking within the ecosystem of cancer cell communication via tunneling nanotubes. Front Cell Dev Biol. 2018;6:95. doi: 10.3389/fcell.2018.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mattson MP, Culmsee C, Yu ZF. Apoptotic and antiapoptotic mechanisms in stroke. Cell Tissue Res. 2000;301(1):173–187. doi: 10.1007/s004419900154. [DOI] [PubMed] [Google Scholar]
- 52.Ham PB, 3rd, Raju R. Mitochondrial function in hypoxic ischemic injury and influence of aging. Prog Neurobiol. 2017;157:92–116. doi: 10.1016/j.pneurobio.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perillo B, Di Donato M, Pezone A, Di Zazzo E, Giovannelli P, Galasso G, et al. ROS in cancer therapy: the bright side of the moon. Exp Mol Med. 2020;52(2):192–203. doi: 10.1038/s12276-020-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rustom A. The missing link: does tunnelling nanotube-based supercellularity provide a new understanding of chronic and lifestyle diseases? Open Biol. 2016;6(6):160057. doi: 10.1098/rsob.160057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Desir S, Wong P, Turbyville T, Chen D, Shetty M, Clark C, et al. Intercellular transfer of oncogenic KRAS via tunneling nanotubes introduces intracellular mutational heterogeneity in colon cancer cells. Cancers (Basel) 2019;11(7):892. doi: 10.3390/cancers11070892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pinto G, Saenz-de-Santa-Maria I, Chastagner P, Perthame E, Delmas C, Toulas C, et al. Patient-derived glioblastoma stem cells transfer mitochondria through tunneling nanotubes in tumor organoids. Biochem J. 2021;478(1):21–39. doi: 10.1042/BCJ20200710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma L, Weinberg RA. Micromanagers of malignancy: role of microRNAs in regulating metastasis. Trends Genet. 2008;24(9):448–456. doi: 10.1016/j.tig.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 58.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449(7163):682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 59.Boukelmoune N, Chiu GS, Kavelaars A, Heijnen CJ. Mitochondrial transfer from mesenchymal stem cells to neural stem cells protects against the neurotoxic effects of cisplatin. Acta Neuropathol Commun. 2018;6(1):139. doi: 10.1186/s40478-018-0644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diaz-Carballo D, Klein J, Acikelli AH, Wilk C, Saka S, Jastrow H, et al. Cytotoxic stress induces transfer of mitochondria-associated human endogenous retroviral RNA and proteins between cancer cells. Oncotarget. 2017;8(56):95945–95964. doi: 10.18632/oncotarget.21606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marlein CR, Zaitseva L, Piddock RE, Robinson SD, Edwards DR, Shafat MS, et al. NADPH oxidase-2 derived superoxide drives mitochondrial transfer from bone marrow stromal cells to leukemic blasts. Blood. 2017;130(14):1649–1660. doi: 10.1182/blood-2017-03-772939. [DOI] [PubMed] [Google Scholar]
- 62.Ippolito L, Morandi A, Taddei ML, Parri M, Comito G, Iscaro A, et al. Cancer-associated fibroblasts promote prostate cancer malignancy via metabolic rewiring and mitochondrial transfer. Oncogene. 2019;38(27):5339–5355. doi: 10.1038/s41388-019-0805-7. [DOI] [PubMed] [Google Scholar]
- 63.Pasquier J, Guerrouahen BS, Al Thawadi H, Ghiabi P, Maleki M, Abu-Kaoud N, et al. Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance. J Transl Med. 2013;11:94. doi: 10.1186/1479-5876-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lu J, Zheng X, Li F, Yu Y, Chen Z, Liu Z, et al. Tunneling nanotubes promote intercellular mitochondria transfer followed by increased invasiveness in bladder cancer cells. Oncotarget. 2017;8(9):15539–15552. doi: 10.18632/oncotarget.14695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burt R, Dey A, Aref S, Aguiar M, Akarca A, Bailey K, et al. Activated stromal cells transfer mitochondria to rescue acute lymphoblastic leukemia cells from oxidative stress. Blood. 2019;134(17):1415–1429. doi: 10.1182/blood.2019001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang J, Liu X, Qiu Y, Shi Y, Cai J, Wang B, et al. Cell adhesion-mediated mitochondria transfer contributes to mesenchymal stem cell-induced chemoresistance on T cell acute lymphoblastic leukemia cells. J Hematol Oncol. 2018;11(1):11. doi: 10.1186/s13045-018-0554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin HY, Liou CW, Chen SD, Hsu TY, Chuang JH, Wang PW, et al. Mitochondrial transfer from Wharton's jelly-derived mesenchymal stem cells to mitochondria-defective cells recaptures impaired mitochondrial function. Mitochondrion. 2015;22:31–44. doi: 10.1016/j.mito.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 68.Walters HE, Cox LS. Intercellular transfer of mitochondria between senescent cells through cytoskeleton-supported intercellular bridges requires mTOR and CDC42 signalling. Oxid Med Cell Longev. 2021;2021:6697861. doi: 10.1155/2021/6697861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Desir S, Dickson EL, Vogel RI, Thayanithy V, Wong P, Teoh D, et al. Tunneling nanotube formation is stimulated by hypoxia in ovarian cancer cells. Oncotarget. 2016;7(28):43150–43161. doi: 10.18632/oncotarget.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vidulescu C, Clejan S, O'Connor KC. Vesicle traffic through intercellular bridges in DU 145 human prostate cancer cells. J Cell Mol Med. 2004;8(3):388–396. doi: 10.1111/j.1582-4934.2004.tb00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Valdebenito S, Audia A, Bhat KPL, Okafo G, Eugenin EA. Tunneling nanotubes mediate adaptation of glioblastoma cells to temozolomide and ionizing radiation treatment. iScience. 2020;23(9):101450. doi: 10.1016/j.isci.2020.101450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matejka N, Reindl J. Influence of alpha-particle radiation on intercellular communication networks of tunneling nanotubes in U87 glioblastoma cells. Front Oncol. 2020;10:1691. doi: 10.3389/fonc.2020.01691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Polak R, de Rooij B, Pieters R, den Boer ML. B-cell precursor acute lymphoblastic leukemia cells use tunneling nanotubes to orchestrate their microenvironment. Blood. 2015;126(21):2404–2414. doi: 10.1182/blood-2015-03-634238. [DOI] [PubMed] [Google Scholar]
- 74.Desir S, O'Hare P, Vogel RI, Sperduto W, Sarkari A, Dickson EL, et al. Chemotherapy-induced tunneling nanotubes mediate intercellular drug efflux in pancreatic cancer. Sci Rep. 2018;8(1):9484. doi: 10.1038/s41598-018-27649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kolba MD, Dudka W, Zaręba-Kozioł M, Kominek A, Ronchi P, Turos L, et al. Tunneling nanotube-mediated intercellular vesicle and protein transfer in the stroma-provided imatinib resistance in chronic myeloid leukemia cells. Cell Death Dis. 2019;10(11):817. doi: 10.1038/s41419-019-2045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Torralba D, Baixauli F, Sanchez-Madrid F. Mitochondria know no boundaries: mechanisms and functions of intercellular mitochondrial transfer. Front Cell Dev Biol. 2016;4:107. doi: 10.3389/fcell.2016.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brandon MC, Lott MT, Nguyen KC, Spolim S, Navathe SB, Baldi P, et al. MITOMAP: a human mitochondrial genome database—2004 update. Nucleic Acids Res. 2005;33(Database issue):D611–D613. doi: 10.1093/nar/gki079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mason PA, Matheson EC, Hall AG, Lightowlers RN. Mismatch repair activity in mammalian mitochondria. Nucleic Acids Res. 2003;31(3):1052–1058. doi: 10.1093/nar/gkg167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shanmughapriya S, Langford D, Natarajaseenivasan K. Inter and intracellular mitochondrial trafficking in health and disease. Ageing Res Rev. 2020;62:101128. doi: 10.1016/j.arr.2020.101128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu K, Ji K, Guo L, Wu W, Lu H, Shan P, et al. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia-reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc Res. 2014;92:10–18. doi: 10.1016/j.mvr.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 81.Dong LF, Kovarova J, Bajzikova M, Bezawork-Geleta A, Svec D, Endaya B, et al. Horizontal transfer of whole mitochondria restores tumorigenic potential in mitochondrial DNA-deficient cancer cells. Elife. 2017;6:e22187. doi: 10.7554/eLife.22187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sarmah D, Kaur H, Saraf J, Pravalika K, Goswami A, Kalia K, et al. Getting closer to an effective intervention of ischemic stroke: the big promise of stem cell. Transl Stroke Res. 2018;9(4):356–374. doi: 10.1007/s12975-017-0580-0. [DOI] [PubMed] [Google Scholar]
- 83.Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Paliwal S, Chaudhuri R, Agrawal A, Mohanty S. Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J Biomed Sci. 2018;25(1):31. doi: 10.1186/s12929-018-0429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 2016;535(7613):551–555. doi: 10.1038/nature18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yao Y, Fan XL, Jiang D, Zhang Y, Li X, Xu ZB, et al. Connexin 43-mediated mitochondrial transfer of iPSC-MSCs alleviates asthma inflammation. Stem Cell Rep. 2018;11(5):1120–1135. doi: 10.1016/j.stemcr.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jiang D, Chen FX, Zhou H, Lu YY, Tan H, Yu SJ, et al. Bioenergetic crosstalk between mesenchymal stem cells and various ocular cells through the intercellular trafficking of mitochondria. Theranostics. 2020;10(16):7260–7272. doi: 10.7150/thno.46332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mahrouf-Yorgov M, Augeul L, Da Silva CC, Jourdan M, Rigolet M, Manin S, et al. Mesenchymal stem cells sense mitochondria released from damaged cells as danger signals to activate their rescue properties. Cell Death Differ. 2017;24(7):1224–1238. doi: 10.1038/cdd.2017.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li CJ, Chen PK, Sun LY, Pang CY. Enhancement of mitochondrial transfer by antioxidants in human mesenchymal stem cells. Oxid Med Cell Longev. 2017;2017:8510805. doi: 10.1155/2017/8510805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Han H, Hu J, Yan Q, Zhu J, Zhu Z, Chen Y, et al. Bone marrow-derived mesenchymal stem cells rescue injured H9c2 cells via transferring intact mitochondria through tunneling nanotubes in an in vitro simulated ischemia/reperfusion model. Mol Med Rep. 2016;13(2):1517–1524. doi: 10.3892/mmr.2015.4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin TK, Chen SD, Chuang YC, Lan MY, Chuang JH, Wang PW, et al. Mitochondrial transfer of Wharton's jelly mesenchymal stem cells eliminates mutation burden and rescues mitochondrial bioenergetics in rotenone-stressed MELAS fibroblasts. Oxid Med Cell Longev. 2019;2019:9537504. doi: 10.1155/2019/9537504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chuang YC, Liou CW, Chen SD, Wang PW, Chuang JH, Tiao MM, et al. Mitochondrial transfer from Wharton's jelly mesenchymal stem cell to MERRF cybrid reduces oxidative stress and improves mitochondrial bioenergetics. Oxid Med Cell Longev. 2017;2017:5691215. doi: 10.1155/2017/5691215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jiang D, Gao F, Zhang Y, Wong DS, Li Q, Tse HF, et al. Mitochondrial transfer of mesenchymal stem cells effectively protects corneal epithelial cells from mitochondrial damage. Cell Death Dis. 2016;7(11):e2467. doi: 10.1038/cddis.2016.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang Y, Ye G, Zhang YL, He HW, Yu BQ, Hong YM, et al. Transfer of mitochondria from mesenchymal stem cells derived from induced pluripotent stem cells attenuates hypoxia-ischemia-induced mitochondrial dysfunction in PC12 cells. Neural Regen Res. 2020;15(3):464–472. doi: 10.4103/1673-5374.266058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Plotnikov EY, Khryapenkova TG, Galkina SI, Sukhikh GT, Zorov DB. Cytoplasm and organelle transfer between mesenchymal multipotent stromal cells and renal tubular cells in co-culture. Exp Cell Res. 2010;316(15):2447–2455. doi: 10.1016/j.yexcr.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 96.Vallabhaneni KC, Haller H, Dumler I. Vascular smooth muscle cells initiate proliferation of mesenchymal stem cells by mitochondrial transfer via tunneling nanotubes. Stem Cells Dev. 2012;21(17):3104–3113. doi: 10.1089/scd.2011.0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hu J, Deng G, Tian Y, Pu Y, Cao P, Yuan W. An in vitro investigation into the role of bone marrowderived mesenchymal stem cells in the control of disc degeneration. Mol Med Rep. 2015;12(4):5701–5708. doi: 10.3892/mmr.2015.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Babenko VA, Silachev DN, Zorova LD, Pevzner IB, Khutornenko AA, Plotnikov EY, et al. Improving the post-stroke therapeutic potency of mesenchymal multipotent stromal cells by cocultivation with cortical neurons: the role of crosstalk between cells. Stem Cells Transl Med. 2015;4(9):1011–1020. doi: 10.5966/sctm.2015-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Babenko VA, Silachev DN, Popkov VA, Zorova LD, Pevzner IB, Plotnikov EY, et al. Miro1 enhances mitochondria transfer from multipotent mesenchymal stem cells (MMSC) to neural cells and improves the efficacy of cell recovery. Molecules. 2018;23(3):687. doi: 10.3390/molecules23030687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li H, Wang C, He T, Zhao T, Chen YY, Shen YL, et al. Mitochondrial transfer from bone marrow mesenchymal stem cells to motor neurons in spinal cord injury rats via Gap Junction. Theranostics. 2019;9(7):2017–2035. doi: 10.7150/thno.29400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jackson MV, Krasnodembskaya AD. Analysis of mitochondrial transfer in direct co-cultures of human monocyte-derived macrophages (MDM) and mesenchymal stem cells (MSC) Bio Protoc. 2017;7(9):e2255. doi: 10.21769/BioProtoc.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jackson MV, Morrison TJ, Doherty DF, McAuley DF, Matthay MA, Kissenpfennig A, et al. Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem Cells. 2016;34(8):2210–2223. doi: 10.1002/stem.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cselenyak A, Pankotai E, Horvath EM, Kiss L, Lacza Z. Mesenchymal stem cells rescue cardiomyoblasts from cell death in an in vitro ischemia model via direct cell-to-cell connections. BMC Cell Biol. 2010;11:29. doi: 10.1186/1471-2121-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang H, Borg TK, Ma Z, Xu M, Wetzel G, Saraf LV, et al. Biochip-based study of unidirectional mitochondrial transfer from stem cells to myocytes via tunneling nanotubes. Biofabrication. 2016;8(1):015012. doi: 10.1088/1758-5090/8/1/015012. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Y, Yu Z, Jiang D, Liang X, Liao S, Zhang Z, et al. iPSC-MSCs with High intrinsic MIRO1 and sensitivity to TNF-alpha yield efficacious mitochondrial transfer to rescue anthracycline-induced cardiomyopathy. Stem Cell Rep. 2016;7(4):749–763. doi: 10.1016/j.stemcr.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li X, Zhang Y, Yeung SC, Liang Y, Liang X, Ding Y, et al. Mitochondrial transfer of induced pluripotent stem cell-derived mesenchymal stem cells to airway epithelial cells attenuates cigarette smoke-induced damage. Am J Respir Cell Mol Biol. 2014;51(3):455–465. doi: 10.1165/rcmb.2013-0529OC. [DOI] [PubMed] [Google Scholar]
- 107.Paliwal S, Chaudhuri R, Agrawal A, Mohanty S. Correction to: Human tissue-specific MSCs demonstrate differential mitochondria transfer abilities that may determine their regenerative abilities. Stem Cell Res Ther. 2019;10(1):215. doi: 10.1186/s13287-019-1343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tan AS, Baty JW, Dong LF, Bezawork-Geleta A, Endaya B, Goodwin J, et al. Mitochondrial genome acquisition restores respiratory function and tumorigenic potential of cancer cells without mitochondrial DNA. Cell Metab. 2015;21(1):81–94. doi: 10.1016/j.cmet.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 109.Wallace DC. Mitochondrial DNA sequence variation in human evolution and disease. Proc Natl Acad Sci USA. 1994;91(19):8739–8746. doi: 10.1073/pnas.91.19.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bajzikova M, Kovarova J, Coelho AR, Boukalova S, Oh S, Rohlenova K, et al. Reactivation of dihydroorotate dehydrogenase-driven pyrimidine biosynthesis restores tumor growth of respiration-deficient cancer cells. Cell Metab. 2019;29(2):399–416 e10. doi: 10.1016/j.cmet.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Andresen V, Wang X, Ghimire S, Omsland M, Gjertsen BT, Gerdes HH. Tunneling nanotube (TNT) formation is independent of p53 expression. Cell Death Differ. 2013;20(8):1124. doi: 10.1038/cdd.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Scheiblich H, Dansokho C, Mercan D, Schmidt SV, Bousset L, Wischhof L, et al. Microglia jointly degrade fibrillar alpha-synuclein cargo by distribution through tunneling nanotubes. Cell. 2021;184(20):5089–5106 e21. doi: 10.1016/j.cell.2021.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Austefjord MW, Gerdes HH, Wang X. Tunneling nanotubes: diversity in morphology and structure. Commun Integr Biol. 2014;7(1):e27934. doi: 10.4161/cib.27934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hanna SJ, McCoy-Simandle K, Miskolci V, Guo P, Cammer M, Hodgson L, et al. The role of Rho-GTPases and actin polymerization during macrophage tunneling nanotube biogenesis. Sci Rep. 2017;7(1):8547. doi: 10.1038/s41598-017-08950-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rangamani P, Levy MG, Khan S, Oster G. Paradoxical signaling regulates structural plasticity in dendritic spines. Proc Natl Acad Sci USA. 2016;113(36):E5298–E5307. doi: 10.1073/pnas.1610391113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vargas JY, Loria F, Wu YJ, Cordova G, Nonaka T, Bellow S, et al. The Wnt/Ca(2+) pathway is involved in interneuronal communication mediated by tunneling nanotubes. EMBO J. 2019;38(23):e101230. doi: 10.15252/embj.2018101230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shen K, Meyer T. Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science. 1999;284(5411):162–166. doi: 10.1126/science.284.5411.162. [DOI] [PubMed] [Google Scholar]
- 118.Dagar S, Pushpa K, Pathak D, Samaddar S, Saxena A, Banerjee S, et al. Nucleolin regulates 14-3-3zeta mRNA and promotes cofilin phosphorylation to induce tunneling nanotube formation. FASEB J. 2021;35(1):e21199. doi: 10.1096/fj.202001152R. [DOI] [PubMed] [Google Scholar]
- 119.Jacob T, Broeke CVD, Waesberghe CV, Troys LV, Favoreel HW. Pseudorabies virus US3 triggers RhoA phosphorylation to reorganize the actin cytoskeleton. J Gen Virol. 2015;96(8):2328–2335. doi: 10.1099/vir.0.000152. [DOI] [PubMed] [Google Scholar]
- 120.Mukerji J, Olivieri KC, Misra V, Agopian KA, Gabuzda D. Proteomic analysis of HIV-1 Nef cellular binding partners reveals a role for exocyst complex proteins in mediating enhancement of intercellular nanotube formation. Retrovirology. 2012;9:33. doi: 10.1186/1742-4690-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Delage E, Cervantes DC, Penard E, Schmitt C, Syan S, Disanza A, et al. Differential identity of filopodia and tunneling nanotubes revealed by the opposite functions of actin regulatory complexes. Sci Rep. 2016;6:39632. doi: 10.1038/srep39632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Las G, Shirihai OS. Miro1: new wheels for transferring mitochondria. EMBO J. 2014;33(9):939–941. doi: 10.1002/embj.201488441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang F, Chen X, Cheng H, Song L, Liu J, Caplan S, et al. MICAL2PV suppresses the formation of tunneling nanotubes and modulates mitochondrial trafficking. EMBO Rep. 2021;22(7):e52006. doi: 10.15252/embr.202052006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16(10):522–529. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 125.Hase K, Kimura S, Takatsu H, Ohmae M, Kawano S, Kitamura H, et al. M-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex. Nat Cell Biol. 2009;11(12):1427–1432. doi: 10.1038/ncb1990. [DOI] [PubMed] [Google Scholar]
- 126.Bhat S, Ljubojevic N, Zhu S, Fukuda M, Echard A, Zurzolo C. Rab35 and its effectors promote formation of tunneling nanotubes in neuronal cells. Sci Rep. 2020;10(1):16803. doi: 10.1038/s41598-020-74013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Burtey A, Wagner M, Hodneland E, Skaftnesmo KO, Schoelermann J, Mondragon IR, et al. Intercellular transfer of transferrin receptor by a contact-, Rab8-dependent mechanism involving tunneling nanotubes. FASEB J. 2015;29(11):4695–4712. doi: 10.1096/fj.14-268615. [DOI] [PubMed] [Google Scholar]
- 128.Aznar S, Lacal JC. Rho signals to cell growth and apoptosis. Cancer Lett. 2001;165(1):1–10. doi: 10.1016/S0304-3835(01)00412-8. [DOI] [PubMed] [Google Scholar]
- 129.Ozaki M, Deshpande SS, Angkeow P, Bellan J, Lowenstein CJ, Dinauer MC, et al. Inhibition of the Rac1 GTPase protects against nonlethal ischemia/reperfusion-induced necrosis and apoptosis in vivo. FASEB J. 2000;14(2):418–429. doi: 10.1096/fasebj.14.2.418. [DOI] [PubMed] [Google Scholar]
- 130.Lin L, Zhang M, Stoilov P, Chen L, Zheng S. Developmental attenuation of neuronal apoptosis by neural-specific splicing of Bak1 microexon. Neuron. 2020;107(6):1180–1196 e8. doi: 10.1016/j.neuron.2020.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stankiewicz TR, Linseman DA. Rho family GTPases: key players in neuronal development, neuronal survival, and neurodegeneration. Front Cell Neurosci. 2014;8:314. doi: 10.3389/fncel.2014.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cole JM, Dahl R, Cowden Dahl KD. MAPK signaling is required for generation of tunneling nanotube-like structures in ovarian cancer cells. Cancers (Basel) 2021;13(2):274. doi: 10.3390/cancers13020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Arkwright PD, Luchetti F, Tour J, Roberts C, Ayub R, Morales AP, et al. Fas stimulation of T lymphocytes promotes rapid intercellular exchange of death signals via membrane nanotubes. Cell Res. 2010;20(1):72–88. doi: 10.1038/cr.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Luchetti F, Canonico B, Arcangeletti M, Guescini M, Cesarini E, Stocchi V, et al. Fas signalling promotes intercellular communication in T cells. PLoS One. 2012;7(4):e35766. doi: 10.1371/journal.pone.0035766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Levoux J, Prola A, Lafuste P, Gervais M, Chevallier N, Koumaiha Z, et al. Platelets facilitate the wound-healing capability of mesenchymal stem cells by mitochondrial transfer and metabolic reprogramming. Cell Metab. 2021;33(3):688–690. doi: 10.1016/j.cmet.2021.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.