Abstract
We report the first case of human infection by Phaeoisaria clematidis. This fungus caused a corneal ulcer in a Brazilian man who had previously suffered an eye injury. Diagnosis was established by positive direct examination and repeated cultures. The isolate was clearly resistant in vitro to the six antifungal agents tested.
Mycotic keratitis, a medical curiosity about 2 or 3 decades ago, is now a common manifestation, and its increasing frequency is of considerable concern in present-day medicine. Numerous fungi which were considered nonpathogenic to human beings have been isolated frequently as causal organisms from cases of keratitis. The genera that most frequently cause keratitis are Fusarium, Aspergillus, and Candida; Fenelon and Kennedy (4) listed 20 other genera that also have been involved in this type of infection. However, in recent years, a number of other genera have been reported, such as Phoma, Dichotomophthoropsis, Rhizoctonia, Cephaliophora, Lasiodiplodia, Colletotrichum, Cladorrhinum, and Metarhizium (1). Here we report the first case of human infection caused by Phaeoisaria clematidis. To our knowledge, this is the first report of keratitis or of any other infection caused by this organism in animals.
Case report.
A 47-year-old man was seen in the Ophthalmology Department of the Escuela Paulista de Medicina, São Paulo, Brazil. He complained of severe pain, redness, and the appearance of a white spot in the left eye. Approximately 2 months earlier he had suffered an injury with a broom. He was given local treatment in the form of gentamicin and dexamethasone, which initially produced a slight improvement. However, 1 month later, the above symptoms began. Clinical examination revealed visual acuities of 1 in the right eye and 0.6 in the left eye. The left eye showed conjunctival hyperemia, epithelial erosion, and a deep central corneal ulcer, measuring 1 by 1.5 mm, with irregular borders and a stromatic infiltrate. The right eye did not show any abnormality. Results of other local and systemic examinations were negative.
Deep corneal scrapings were collected with a sterile scalpel blade for direct mounts and cultures. Direct examination of lactophenol wet mounts of the scrapings revealed abundant septate and pigmented hyphal fragments. Corneal samples were directly inoculated onto Sabouraud glucose agar (Difco Laboratories, Detroit, Mich.) and potato dextrose agar (PDA; Difco) by making a series of C-shaped cuts on the medium. Cultures were incubated at 25, 30, and 35°C. After 3 days, numerous small colonies of a dematiaceous mold appeared on all cultures. Results of routine bacteriological cultures were negative.
Treatment was started with hourly topical 5% natamycin, ofloxacin every 6 h for 4 days, 1% atropine every 8 h for 8 days, and oral ketoconazole at 400 mg/day. After 3 weeks, the condition of the eye worsened, with an increase in the corneal edema and hypopyon. Antifungal therapy was discontinued, and more corneal scrapings were collected for new cultures. The patient was treated for presumptive bacterial infection with local antibiotics (cephalothin at 50 mg/ml and gentamicin at 14 mg/ml hourly for 9 days). The cultures were again negative for bacteria and positive for the same mold. Antibacterial treatment was therefore discontinued. A contact lens was prescribed, and treatment was started with antifungal drugs (0.5% amphotericin B topically and ketoconazole at 400 mg/day orally) supplemented with ofloxacin every 6 h. Amphotericin B was used for 2 weeks. The lesion improved slightly, and topical dexamethasone (0.05%) every 8 h for 8 days was added. The ulcer healed, a leukomatous opacity formed, and the corticoid was discontinued. At present, the patient is awaiting therapeutic keratoplasty.
Cultures obtained on the two occasions yielded molds with identical morphological characteristics. One isolate was sent to the Microbiology Unit of the Rovira i Virgili University, Reus, Tarragona, Spain, for identification and antifungal susceptibility testing.
Morphological study.
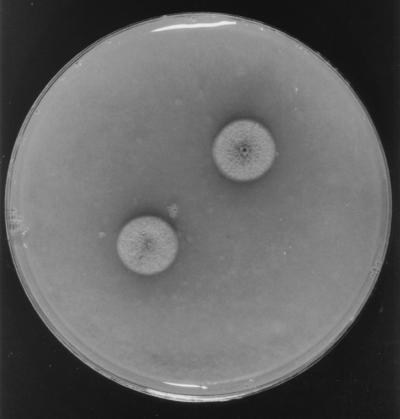
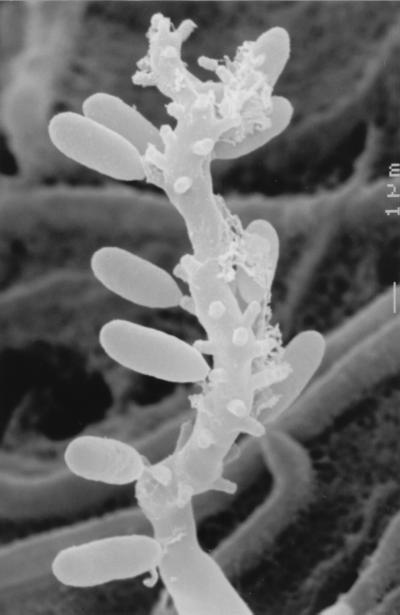
For identification purposes, this clinical isolate was subcultured on PDA, potato carrot agar (PCA; 20 g of potato, 20 g of carrot, 18 g of agar, 1,000 ml of tap water [homemade]), and oatmeal agar (OA; 30 g of oat flakes, 1 g of MgSO4 · 7H2O, 1.5 g of H2KPO4, 15 g of agar, 1,000 ml of tap water [homemade]) and incubated at ca. 25°C in the dark. After 10 days, the colonies on PDA were velvety, umbonate, mouse gray to brown, flat, and whitish at the edge, with a dark olivaceous reverse, and attained 9 to 11 mm in diameter. On PCA and OA, the colonies were very similar and developed more rapidly than on PDA, attaining a diameter of 12 to 14 mm after 10 days (Fig. 1). They were flat, brownish gray, and cottony at first, with thin fascicles at the center, and then became brown and powdery, with a dark brown reverse. The fungus sporulated profusely, and numerous synnemata (bundles of conidiophores sporulating in the apical part) (Fig. 2) appeared after 15 to 20 days of incubation on all the media.
FIG. 1.
P. clematidis FMR 6274 colonies on OA after 10 days of incubation at 25°C.
FIG. 2.
P. clematidis synnemata viewed by scanning electron microscopy. Magnification, ×280.
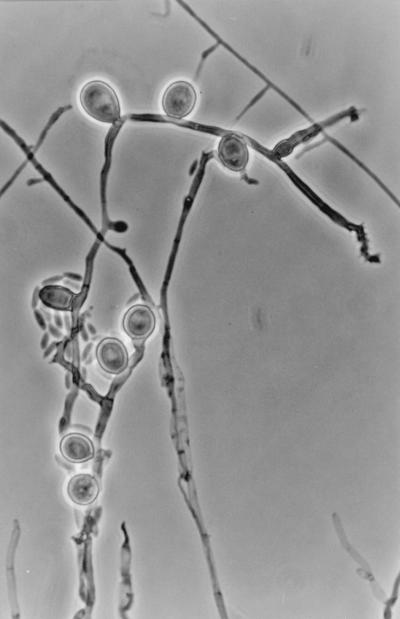
The microscopic characteristics of the isolate were determined by making wet mounts with acid lactic, which were then examined under a light microscope (Leitz Dialux 20). Synnemata were erect, cylindrical, or slightly clavate, were up to 1,500 μm long and 25 to 80 μm wide near the base, and had a dark brown stipe composed of parallel, smooth-walled, septate, branched hyphae 2 to 3 μm in width and conidiogenous cells that usually covered the upper two-thirds to one-third of the stipe. Conidiogenous cells (Fig. 3) that also arose from undifferentiated hyphae were denticulate, smooth walled, pale brown, subhyaline toward the apex, cylindrical or clavate, and 10 to 20 μm long by 2 to 3 μm wide when young but with age often considerably longer and geniculate. Denticles were terminal and lateral, scattered, or grouped, more or less cylindrical, and up to 1 μm long. Conidia were aseptate, smooth walled, subhyaline, ellipsoidal or narrowly clavate, often slightly curved, and 5 to 10 μm long by 2 to 3 μm wide. Chlamydospores (Fig. 4) were abundantly produced from submerged and superficial mycelium. They were usually lateral, sessile, thick and smooth walled, dark brown, subglobose, ovoid or ellipsoidal, 11 to 14 μm long by 8 to 10 μm wide, aseptate (rarely with one or two septa), and up to 17 μm long. Moreover, after approximately 2 weeks, fruiting body initials (up to 60 μm in diameter) of a probable ascomycete were detected on all the media, but they did not ripen under different culture conditions.
FIG. 3.
P. clematidis denticulate conidiogenous cells and conidia viewed by scanning electron microscopy. Magnification, ×11,000.
FIG. 4.
P. clematidis terminal and lateral chlamydospores, conidiogenous cells, and conidia viewed by phase-contrast microscopy. Magnification, ×640.
On the basis of the above characteristics, the isolate was tentatively identified as P. clematidis, although curved conidia had never before been described for this species, nor had its teleomorph. The only Phaeoisaria species with curved or at least in part slightly curved conidia are P. curvata (2), P. triseptata (6), and P. sparsa var. cubensis (7). However, the latter two species differ from our strain because they have septate conidia, and the conidia of the former are larger, are always curved, and never produce synnemata. In addition, the only known species with a teleomorph is the Phaeoisaria state of Peroneutypella echidna (Ascomycota, Diatripales) (3), but its conidia are smaller (5 to 5.5 μm long).
To achieve ripe fruiting body initials and verify the stability of the conidial morphology, we inoculated a conidial suspension of the isolate onto sterilized plant material by the technique described by Gené and Guarro (5). Under these conditions, the fruiting body initials did not ripen after 2 months of incubation; curved conidia were practically absent, and the other microscopic characteristics were very similar to those described above. This clinical strain was therefore identified as P. clematidis. Living cultures of the strain have been kept in the culture collection of the Faculty of Medicine, Rovira i Virgili University, Reus, Spain, as FMR 6274 and have also been deposited in the Commonwealth Agricultural Bureaux International Bioscience, Egham, United Kingdom (IMI 381458), and in the Centraalbureau voor Schimmelcultures, Baarn, The Netherlands (CBS 102276).
Antifungal susceptibility testing.
The fungal isolate was tested to determine its susceptibility to amphotericin B, miconazole, itraconazole, ketoconazole, fluconazole, and flucytosine (Table 1). Tests were carried out by a previously described microdilution method (10) mainly according to the guidelines recommended for molds by the National Committee for Clinical Laboratory Standards (8); we used RPMI 1640 medium buffered to pH 7.0 with 0.165 morpholinepropanesulfonic acid (MOPS), an inoculum of 4.7 × 104 CFU/ml, an incubation temperature of 30°C, a second-day reading (48 h), and an additive drug dilution procedure. MICs and minimum fungicidal concentrations were very high, demonstrating the inefficacy of all the drugs tested.
TABLE 1.
Antifungal susceptibilities of the clinical isolate of P. clematidis
| Antifungal agent | MIC (μg/ml) | Minimum fungicidal concn (μg/ml) |
|---|---|---|
| Amphotericin B | 32 | 32 |
| Miconazole | 32 | 32 |
| Itraconazole | 32 | 32 |
| Ketoconazole | 32 | 32 |
| Fluconazole | 128 | 128 |
| Flucytosine | 256 | 256 |
Species of Phaeoisaria are easily recognized by the erect synnemata bearing numerous denticulate conidiogenous cells in the upper region (2). However, when they are grown in cultures, these typical structures are sometimes not produced. P. clematidis is a soilborne fungus, ubiquitous, cosmopolitan, and a well-known plant pathogen. It has been isolated on dead plant material and is very common in the tropics (3). Using a murine model, Okeke et al. (9) demonstrated that this species was able to survive passage in mice and proliferate in tissue, although infections of the cornea were not studied. The fungus produced mainly localized, subcutaneous nodular lesions and visceral effects, depending on the means of inoculation. The tissue form comprised short septate hyphae and brown, thick-walled chlamydoconidium-like cells.
In the present case, the fungus may have been introduced at the time of injury. It is more likely, however, that it was introduced later to the wound by airborne spores and that topical corticoids and antibiotics facilitated its penetration. In the treatment of the fungal infection, both antifungal and antibacterial drugs were used with corticoids, but there is no clear evidence that either of them had any real effect. On the other hand, the antifungal agents used last (amphotericin B and ketoconazole), which seemed to be the ones that produced the corneal healing, were clearly inefficient in vitro. It is unfortunate that the patient received a topical steroid prior to proper diagnosis and treatment. Topical steroids exacerbate infections and should never be considered in keratomycosis or bacterial keratitis unless there is secure knowledge that the correct antimicrobial agent is already being given. Although some authors have used corticosteroids as adjunctive therapy, these probably have no place in the treatment of fungal keratitis and are more likely to result in deeper penetration of the infections (4).
In conclusion, P. clematidis is a saprophytic fungus which has been demonstrated, both experimentally and in this study, to be pathogenic for animals, including humans. It should be added to the list of opportunistic fungi that can infect an immunocompetent host.
REFERENCES
- 1.de Hoog G S, Guarro J, editors. Atlas of clinical fungi. Baarn, The Netherlands: Centraalbureau voor Schimmelcultures; 1995. [Google Scholar]
- 2.de Hoog G S, Papendorf M C. The genus Phaeoisaria. Persoonia. 1976;8:407–414. [Google Scholar]
- 3.Deighton F C. Four synnematous hyphomycetes. Trans Br Mycol Soc. 1974;62:243–252. [Google Scholar]
- 4.Fenelon L E, Kennedy S M. Fungal diseases in ophthalmology. In: Kibbler C C, Mackenzie D W R, Odds F C, editors. Principles and practice of clinical mycology. Chichester, England: John Wiley & Sons Ltd.; 1996. pp. 221–223. [Google Scholar]
- 5.Gené J, Guarro J. A new Chaetomium from Thailand. Mycol Res. 1996;100:1005–1009. [Google Scholar]
- 6.Holubová-Jechová V. Studies on Hyphomycetes from Cuba. VII. Seven new taxa of dematiaceous Hyphomycetes. Ceska Mykol. 1988;42:23–30. [Google Scholar]
- 7.Mercado-Sierra A, Figueras M J, Gené J. New or rare hyphomycetes from Cuba. VIII. Species of Lylea, Phaeoisaria, Arxiella, Graphium, Periconia, and Ramichloridium. Mycotaxon. 1997;63:369–375. [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi; proposed standard M38-P. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 9.Okeke C N, Gugnani H C, Onuigbo W I B. Potential pathogenicity of Cladosporium tenuissimum, Phaeoisaria clematidis and Ramichloridium subulatum in a mouse model. Mycopathologia. 1991;114:65–70. doi: 10.1007/BF00436423. [DOI] [PubMed] [Google Scholar]
- 10.Pujol I, Guarro J, Llop C, Soler L, Fernández J. Comparison study of broth macrodilution and microdilution antifungal susceptibility tests for the filamentous fungi. Antimicrob Agents Chemother. 1996;40:2106–2110. doi: 10.1128/aac.40.9.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]