Abstract
Chronic viral hepatitis infection affects an estimated 325 million people globally. People who initiate treatment after significant disease progression face increased risk of severe liver complications and death. Data are scarce on the characteristics and risk factors of people who present late to care in Spain and globally. Data were collected from January 2018 to December 2019 to report late presentation (LP) to specialist care at 11 large university hospitals in Spain to assess related risk factors using a multivariable logistic regression model. 2290 (CHB = 505, CHC = 1785) patients were analysed, with 581 (25.2%) presenting late. Hepatitis C patients more frequently reported LP compared to hepatitis B patients (28.1% vs 15.0%; p < 0.001). Older age (p < 0.001), being male (p < 0.001), being Spanish-born (p < 0.001), and having an unknown origin of referral (p = 0.08) were associated with a higher likelihood of LP. Advanced liver disease was identified in 533 (23%) patients and late-stage liver disease in 124 (5.4%). LP, including with irreversible liver damage, to viral hepatitis specialist care is frequent in Spain, despite being a country with unrestricted treatment access. Initiatives to reduce LP should specifically target men, older individuals, foreign-born populations for CHB, and Spanish nationals for CHC.
Subject terms: Hepatology, Health services, Hepatitis B, Hepatitis C
Introduction
Viral hepatitis currently affects an estimated 325 million people globally1. In 2016, the World Health Organization (WHO) adopted the “Global Health Sector Strategy on Viral Hepatitis, 2016–2021”, which sets out to eliminate viral hepatitis as a public health threat by 2030 by reaching targets that principally aim to reduce new hepatitis infections by 90% and deaths by 65%2. Recent data in the WHO European region report that an estimated 15 million people live with chronic hepatitis B virus (HBV)3 and an estimated 14 million people with hepatitis C virus (HCV), equivalent to one in 50 people affected4. Diagnosis can be challenging as chronic HBV and HCV infections may remain clinically silent for decades with symptoms only occurring once the disease has significantly progressed.
HBV and HCV infected individuals are at an increased risk of developing liver cirrhosis, decompensated liver disease, hepatocellular carcinoma (HCC), and liver-related death5, 6. The risk of disease progression is related to several factors with viral replication being an important factor in fibrosis progression and thus, in the risk of developing HCC. Therefore, antiviral therapy that can suppress viral replication in the case of hepatitis B and direct-acting antivirals (DAAs) against HCV7, can modify the natural course of the disease and reduce or prevent the development of disease progression, particularly if therapy is initiated in early stages of the disease. In the case of chronic hepatitis C (CHC) infection, although antiviral therapy results in sustained virologic response (SVR) in ≥ 95% of cases, for patients that present late to care, there is still a risk of HCC for those that have developed advanced fibrosis or cirrhosis8, 9. In Spain, access to HCV DAA therapy has been unrestricted since mid-2017, yet only half of the patients with HCV-RNA know their status and are receiving therapy10. Viral elimination through completion of DAA treatment therapy can reduce all-cause mortality in HCV patients11. Similarly, timely diagnosis and treatment initiation of patients with HBV is required to prevent patients from progressing to severe adverse clinical outcomes, which is estimated to be as high as 15–40%12.
For virtually all transmissible infectious diseases, late presentation (LP) to care has both individual and population health implications. Screening to identify these individuals before significant liver damage occurs has reduced HBV and HCV incidence13 and prevalence and has been shown to maintain or improve quality of life14. Thus, to optimise the benefits of available treatment regimens and work towards the WHO elimination goal, patients need to be diagnosed earlier. In a 2017 consensus paper15, LP was defined as: (1) presentation with advanced liver disease (ALD) in untreated patients with chronic hepatitis B and C showing significant fibrosis (F ≥ 3); or (2) presentation with late-stage liver disease (LSLD) in untreated patients with chronic hepatitis B or C and including at least one symptom of decompensated cirrhosis (i.e., jaundice, hepatic encephalopathy, clinically detectable ascites, variceal bleeding) and/or HCC in patients with no previous antiviral treatment.
There are few studies evaluating late presentation in general, and population level studies are largely unexplored. The aim of this study is to report the prevalence of LP of chronic infection with HBV and HCV at first visits with specialists prescribing therapy and managing HBV and HCV in Spain, which has unrestricted treatment access, and describe the risk factors associated with HBV and HCV late presentation to care.
Methods
This is a retrospective (Jan 2018–Feb 2019) and prospective (Mar–Dec 2019) cohort study (data collection was first retrospective and subsequently prospective) of adult patients (18 years or older) with chronic HBV or HCV infection presenting for consultation with a liver specialist in 11 Spanish university hospitals in eight of Spain’s 17 autonomous communities from 1 January 2018 to 31 December 2019. Chronic HCV infection was defined by the presence of HCV-RNA for > 6 months and chronic HBV infection by HBsAg detection for > 6 months16, 17. Patients with acute hepatitis as well as those with acute liver failure were excluded. Those presenting for care with a liver specialist (hepatology department, infectious diseases department, internal medicine department, or “other”) were referred by a primary care office or other specialist. In Spain, only specialists are permitted to treat and manage chronic viral hepatitis patients and, therefore, referrals from other departments are needed before being seen by a specialist. Data from each patient’s visits with specialists were collected in a non-identifiable Excel collection template through review of medical records in each hospital’s medical record system by authorised personnel. Standard demographic variables (gender, nationality, date of birth), epidemiological (mode of transmission, year of diagnosis, vaccination status), laboratory parameters (alanine aminotransferase [ALT], aspartate aminotransferase [AST], platelet count), viral hepatitis B and C serologies and viral load (HBsAg, HBeAg, HBV-DNA, HCV-RNA, HCV genotype and subtype), fibrosis stage, and clinical outcome variables, such as decompensated cirrhosis and hepatocellular carcinoma (HCC), were collected. Variables on the type of centre and information on referral of patients were also collected. Late presentation was defined based on the consensus definition proposed by Mauss et al.15 (Box 1).
Box 1.
Consensus definition of late presentation with chronic viral hepatitis for medical care.
| Presentation with advanced liver disease (ALD) in untreated patients with chronic hepatitis B and C | A patient with chronic HBV or HCV and significant fibrosis assessed by one of the following: serologic fibrosis score ≥ 3 (assessed by APRI score > 1.5, FIB-4 > 3.25, Fibrotest > 0.59 or alternatively transient elastography (FibroScan) > 9.5 kPa or liver biopsy (≥ METAVIR stage F3) in patients with no previous antiviral treatment |
| Presentation with late-stage liver disease (LSLD) in untreated patients with chronic hepatitis B and C | A patient with a fibrosis score > F3 and presence of at least one symptom of decompensated cirrhosis (jaundice, hepatic encephalopathy, clinically detectable ascites, variceal bleeding) and/or hepatocellular carcinoma in patients with no previous antiviral treatment |
Source Mauss et al.15.
APRI aspartate aminotransferase to platelet ratio, F3 stage 3 fibrosis, FIB-4 Fibrosis 4, HBV hepatitis B virus, HCV hepatitis C virus, METAVIR Meta-analysis of Histological Data in Viral Hepatitis.
Variables
The date of birth of patients were collected and later birth year was extracted in order to calculate age at time of presentation to care. Country of origin was re-categorised into “Spanish-born” and “Foreign-born” and consisted of responses as “Spain” or “España” versus all other possible nationalities. HBV-DNA and HCV-RNA were recorded as UI/mL, ALT and AST values were recorded as UI/L, and platelet counts were standardised and recorded as × 103/µL. The upper limit of normality (ULN) for ALT and AST values was set at 40 UI/mL. HBeAg and HBsAg were collected dichotomously as “positive” or “negative” for HBV patients. In the case of HCV-related variables, genotype letter and number were collected. Fibrosis stage was reported as F0-F4 and later re-categorised to create the variable “advanced liver disease”, which included all patients with fibrosis ≥ F3 and an aminotransferase platelet ratio (APRI) score > 1.5 based on the initial (first or second) patient visits with the liver specialist. Patients who did not have a reported fibrosis stage had their APRI index score calculated using reported AST and platelet count values using the standard formula [(AST/upper limit of the normal AST range) × 100/platelet count]. The variable LSLD included any patient with a reported liver complication (jaundice, hepatic encephalopathy, ascites, variceal bleeding) defining decompensated cirrhosis and/or HCC in addition to a fibrosis score > F3. If fibrosis stage or HCC or liver complications were not available, the patient entry was excluded from analysis. Mode of transmission was collected categorically and included: sexual, same sex; sexual, hetero; IV drug use; blood transfusion; other; unknown. In the instance of HBV-related variables, being correctly vaccinated against HBV was collected as “yes,” “no,” and “unknown”. Year of diagnosis and was collected as a continuous variable in years. If an HCV patient had a reported year of diagnosis prior to 1989 (year of the discovery of the virus), this was considered a diagnosis of “non-A non-B” hepatitis. Origin of patient referral was also collected categorically (primary care; other specialty in the same centre; gastroenterology or hepatology specialist from another centre; other specialist from another centre; other; unknown). Blank answers were treated as missing values. Any anomalies in databases were verified with the co-author from each corresponding centre to be verified and modified if needed. Data that did not meet inclusion criteria were removed from the database and excluded from analysis; under the age of 18 (n = 12); HBsAg negative (n = 4); ALT > 600 UI/L (n = 31); no data on liver fibrosis, HCC, or liver complications to calculate LP (n = 11).
Statistical analyses
The prevalence of LP was calculated for the whole sample and stratified according to the following categories: chronic HBV or HCV infection, participant centre, sex, country of origin (Spanish-born vs foreign-born), mode of transmission, and origin of referral. Patients who presented late to care were compared to patients without late presentation. Binary logistic regression tested the odds ratios (OR) between late presentation and three variables of interest (sex, age, and country of origin). The level of significance was set to < 0.05, and data were analysed using Stata, version 16.
Ethics approval and consent to participate
This study received ethical clearance in 2019 from the Ethical Committee of the Hospital Clínic, Barcelona, Spain (n. HCB/2019/0111), and was performed in accordance with relevant guidelines and regulations. No personally identifiable information was collected from study participants.
Results
2290 patients were included in this study (HBV = 505, HCV = 1785). The mean age of the population was 53.8 years (SD: 14.4) and men represented 62.6% (n = 1434) of the population (Table 1). The majority (77.9%; 1783) of patients were Spanish-born and the majority of patients with viral hepatitis infection had an “unknown” (59.6%; 1366) mode of transmission, followed by injecting drug use (21.4%; 490). Most patients were referred to the liver specialist for the first time from primary care (44.4%; 1016), followed by an “other” referral (17.3%; 396). 8.8% (n = 203) of patients did not have a reported origin of referral. The mean year of diagnosis for 2199 patients with available data was 2011 (SD: 9.2) with a range of diagnosis year from 1966 to 2019. 54.4% (n = 1245) of the data were collected from a review of 2018 patient history and the remaining from 2019 (n = 1045). There were no changes in LP prevalence between the two years of data collection (25.9% vs 25.5%, respectively).
Table 1.
Baseline characteristics of all patients included in the 11 Spanish centres, 2018/19.
| OVERALL (N = 2290) N = 2290 |
HBV (N = 505) N (%) |
HCV (N = 1785) N (%) |
|
|---|---|---|---|
| Age, mean (SD) | 53.8 (14.4) | 46.2 (15.6) | 55.9 (13.3) |
| Sex | |||
| Male | 1434 (62.6) | 294 (58.2) | 1140 (63.9) |
| Female | 854 (37.3) | 211 (41.8) | 643 (36.0) |
| Country of origin | N = 2274 | ||
| Spanish-born | 1783 (77.9) | 224 (44.4) | 1559 (87.3) |
| Foreign-born | 491 (21.4) | 275 (54.4) | 2176 (12.1) |
| Missing values | 16 (0.7) | 6 (1.2) | 10 (0.6) |
| Mode of transmission | |||
| Sexual, same sex | 23 (1.0) | 9 (1.8) | 14 (0.8) |
| Sexual, hetero | 38 (1.7) | 11 (2.2) | 27 (1.5) |
| Injecting drug use | 490 (21.4) | 5 (0.9) | 485 (27.2) |
| Blood transfusion | 185 (8.1) | 15 (2.9) | 170 (9.5) |
| Other | 149 (6.5) | 78 (15.4) | 71 (4.0) |
| Unknown | 1366 (59.6) | 385 (76.2) | 981 (54.9) |
| Missing values | 39 (1.7) | 2 (0.4) | 37 (2.1) |
| Year of diagnosis (mean, SD) | 2011 (9.4) | 2013 (8.9) | 2010 (9.2) |
| Origin of referral | |||
| Primary care | 1016 (44.4) | 276 (54.6) | 740 (41.5) |
| Other specialty in the same centre | 319 (13.9) | 114 (22.6) | 205 (11.5) |
| Gastrohep specialist from another centre | 228 (9.9) | 30 (5.9) | 198 (11.1) |
| Other specialist from another centre | 79 (3.4) | 13 (2.6) | 66 (3.7) |
| Other | 396 (17.3) | 11 (2.2) | 385 (21.6) |
| Unknown | 203 (8.8) | 47 (9.3) | 156 (8.7) |
| Missing values | 49 (2.1) | 14 (2.8) | 35 (1.9) |
HBV hepatitis B virus, HCV hepatitis C virus.
Late presentation was detected in 25.7% (n = 588) of all patients. ALD was reported in 552 (24.1%) patients and LSLD was reported in 5.3% (122) of all patients (Table 2). Overall, 15.7% (359) presented with stage F4 fibrosis and 7.4% (170) had stage F3 fibrosis at first consultation determined by Fb > 9.5 at most centres (1166; 50.9%) (Supplementary Table 1). An additional 23 (1%) who did not have fibrosis stage reported and had AST and platelet count values available had an APRI score greater than 1.5, indicating ALD. Mean ALT was 67.6 UI/L and AST was 57.6 UI/L. Seventy-five (3.3%) patients reported at least one liver complication defining decompensated cirrhosis. The most common reported liver complication was clinical ascites (17.9%; 35), followed by jaundice (12.3%; 24). An additional 113 “other” non-decompensated cirrhosis defining liver complications were reported.
Table 2.
Description of HBV and HCV LP patients (N = 2290), categorised by ALD/LSLD in 11 Spanish hospitals.
| Overall | HBV (N = 505) | HCV (N = 1785) | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Late presentation to carea | |||
| Yes | 588 (25.7) | 78 (15.4) | 510 (28.6) |
| Advanced liver disease | 552 (24.1) | 72 (14.3) | 480 (26.9) |
| Missing values | 10 (0.4) | 2 (0.4) | 8 (0.5) |
| Fibrosis stage | |||
| F3 | 170 (7.4) | 17 (3.4) | 153 (8.6) |
| F4 | 359 (15.7) | 48 (9.5) | 311 (17.4) |
| APRI > 1.5 | 23 (1.0) | 7 (1.4) | 16 (0.9) |
| Late-stage liver disease | 122 (5.3) | 28 (5.5) | 94 (5.2) |
| Hepatocellular carcinoma | 55 (2.4) | 10 (2.0) | 45 (2.5) |
| Missing values | 78 (3.4) | 0 (0) | 78 (4.3) |
| Decompensated cirrhosis defining liver complicationsb | |||
| Yes | 75 (3.3) | 19 (3.8) | 56 (3.1) |
| Jaundice | 21 (0.9) | 9 (1.8) | 12 (0.7) |
| Hepatic encephalopathy | 8 (0.3) | 0 (0) | 8 (0.4) |
| Ascites | 37 (1.6) | 11 (2.1) | 26 (1.4) |
| Variceal bleeding | 18 (0.8) | 3 (0) | 15 (0.8) |
| Missing values | 214 (9.3) | 133 (26.3) | 186 (10.4) |
ALD advanced liver disease, APRI aspartate aminotransferase to platelet ratio, F3 stage 3 fibrosis, F4 stage 4 fibrosis, HBV hepatitis B virus, HCV hepatitis C virus, LP late presentation, LSLD late-stage liver disease.
aLate presentation to care includes those with ALD and LSLD, of which, some patients may classify in both categories (i.e. a patient may have fibrosis F4 and a liver complication).
bNumber of patients for each type of liver complication sum to more than the “YES” total because some patients reported more than one liver complication.
Chronic HBV and HCV
Patients with chronic HCV infection were more likely to present late to care compared to those with chronic HBV (28.6% vs 15.4%; p < 0.001). ALD was reported more frequently in HCV patients compared to HBV patients (26.9% vs 14.3%; p < 0.001) and LSLD was described in 5.5% (n = 28) and 5.2% (n = 94) of HBV and HCV patients, respectively (p = 0.806). HCV patients had a higher mean ALT at baseline compared to HBV patients: 57 UI/L [95% CI 44.8–70.9] and 70 UI/L [95% CI 67.3–73.7]; p = 0.0069) and AST at baseline (59.9 UI/L [95% CI 57.2–62.6] and 49 UI/L [95% CI 40.2–59.5]; p = 0.0065). HBV patients presenting late to care had a significantly higher proportion of ALT and AST values above the upper limit of normality (≥ 40) in comparison to those not presenting late (ALT: 60.8% v. 26.9%; p < 0.001, AST: 60.8% v. 21.3%; p < 0.001). This same trend was similar among HCV patients (ALT: 78.2% v. 59.9%; p < 0.001, AST: 78.4% v. 50.1%; p < 0.001).
Hepatocellular carcinoma (HCC)
55 (2.4%) patients presented with HCC at first consultation (Table 3). The majority of HCC patients were Spanish born and male. Those with HCC were significantly older than those without HCC (p < 0.001) and 81.8% of those with HCC at first consultation (n = 55) were chronic HCV patients (n = 45) (p < 0.001). Those with HCV infection and HCC on average were older than those with HBV infection and HCC (64.8 years vs. 56 years).
Table 3.
Description of patients with and without HCC who presented late to care. Spain, 2018/2019.
| Patients with HCC (n = 55) | Patients without HCC (n = 2173) | |
|---|---|---|
| Age, mean (SD) | 63.2 (12.6) | 53.4 (14.2) |
| Sex | ||
| Male | 42 (2.9) | 1352 (94.3) |
| Female | 13 (1.5) | 803 (94.0) |
| Country of origin | ||
| Spanish-born | 50 (2.8) | 1683 (93.7) |
| Foreign-born | 5 (1.0) | 474 (96.1) |
Missing values not reported.
HCC hepatocellular carcinoma.
Country of origin
Of those that presented late to care, the majority (85.7%; 504) were Spanish-born individuals and foreign-born individuals made up 13.2% (n = 78) of those presenting late to care (p < 0.001). HCV patients were primarily Spanish-born individuals (87.3%; 1559) (Map 1) whereas about half of all HBV patients were foreign-born individuals (54.5%; 275) (Map 2). 34.6% (n = 27) of HBV patients who presented late to care were foreign-born (p = 0.001) compared to 10.0% (n = 51) of HCV patients (p = 0.079).
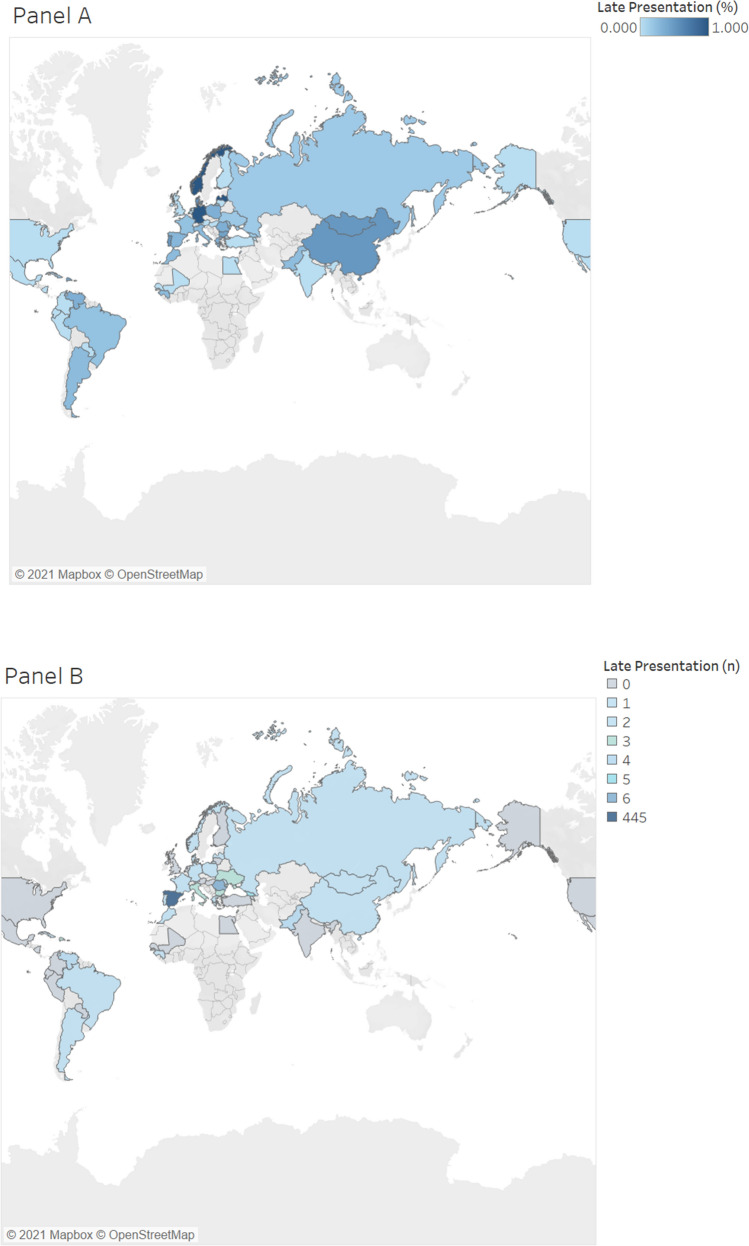
Map 1.
Percent of HCV LP patients by country of origin (A) and number of HCV LP patients (N = 510) from each corresponding country (B). HCV hepatitis C virus, LP late presentation. Map was created using Tableau version 2021.1.0 (www.tableau.com).
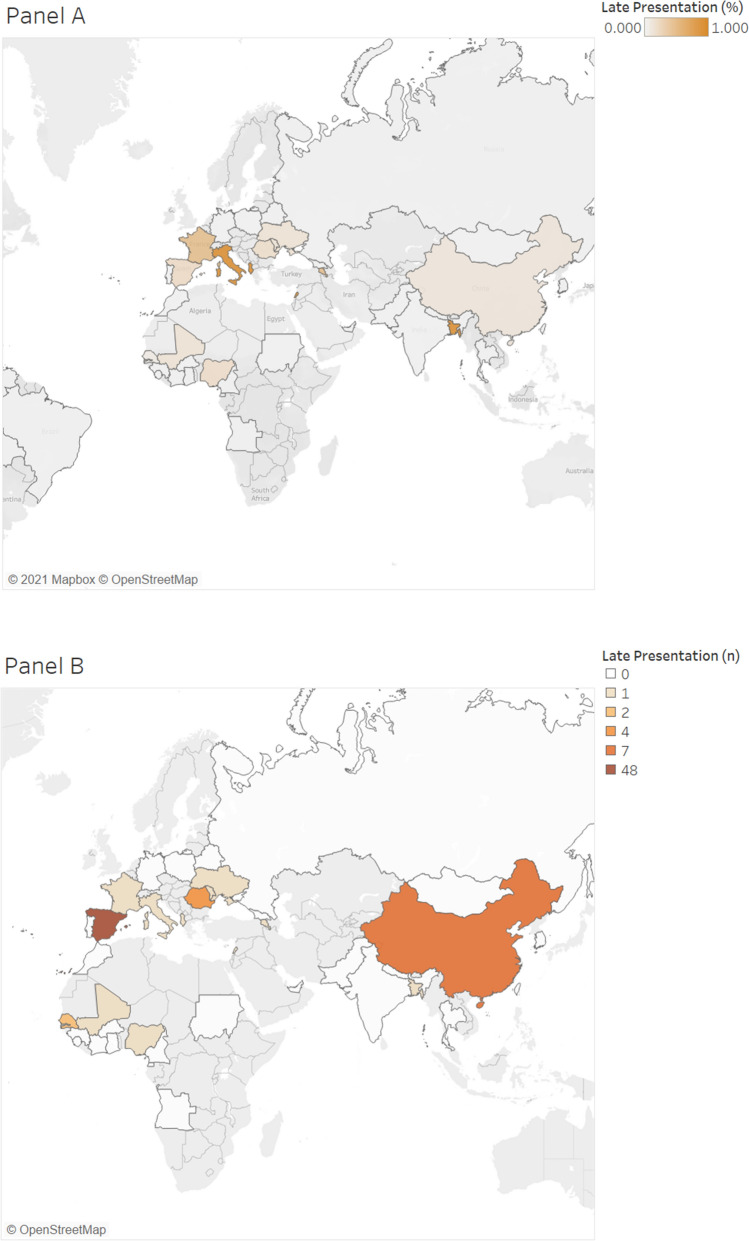
Map 2.
Percent of HBV LP patients by country of origin (A) and number of HBV LP patients (N = 78) from each corresponding country (B). HBV hepatitis B virus, LP late presentation. Map was created using Tableau version 2021.1.0 (www.tableau.com).
After Spain (1783), patients were primarily from China (66), Romania (39), Georgia (37), Pakistan (39), and Senegal (29). Patients from these mentioned countries all presented late to care (Maps 1 and 2). Chinese and Senegalese patients primarily had chronic HBV, representing 97% (64) and 96.5% (28) of their infections, respectively. In comparison, patients from Georgia primarily had chronic HCV (83.8%; 31). Romanian patients represented both chronic HBV (53.8%; 21) and HCV (46.2%; 18) infections. Similarly, patients from Pakistan also represented both chronic HBV (41.4%; 12) and HCV (58.6%; 17) infections.
Age
Overall, those who presented late to care were older (59 years vs 51 years; p < 0.001) than those who did not present late. This trend did not change for patients with ALD (59 years vs 52 years; p < 0.001) or LSLD (59 years vs 53 years; p < 0.001). HCV patients were significantly older compared to HBV patients (56 years vs 46 years; p < 0.001).
Sex
Men were more likely to present late to care compared to women (30.3% vs17.8%; p < 0.001). This trend did not change for patients with ALD where 28.7% (n = 412) of men and 16.3% (139) of women had ALD (p < 0.001). The proportion of patients who presented with LSLD was double in men (6.4%; 92) compared to women (3.5%; 30) (p = 0.011).
Origin of referral
Patients who were referred from primary care made up 39.3% (n = 231) of patients who presented late to care (Table 4).
Table 4.
Referral origin of LP patients, compared to those who did not present late (Spain, 2018/2019).
| Origin of referral | Late presentation, n (%) | No late presentation, n (%) |
|---|---|---|
| Primary care | 231 (22.7) | 785 (77.3) |
| Other specialty in the same centre | 90 (28.2) | 229 (71.8) |
| GI specialist from another centre | 67 (29.4) | 161 (70.6) |
| Other specialist from another centre | 24 (30.4) | 55 (69.6) |
| Other | 101 (25.5) | 295 (75.5) |
| Unknown | 64 (31.5) | 139 (68.5) |
| Missing values | 11 (22.4) | 38 (77.6) |
| Total | 588 (100) | 1702 (100) |
LP late presentation.
Participating centres
Eleven tertiary hospitals, representing six out of the 17 (35%) autonomous communities in Spain, provided data. Late presentation to care ranged from 4.6% to 34% across the centres and autonomous communities (Table 5). HCV patients presented late to care more frequently than HBV patients across all centres.
Table 5.
Proportion of HBV and HCV LP patients categorised by 11 participating Spanish centres, 2018/2019.
| Hospital (city) | HBV patients n (%)* |
% HBV patients with LP n (%) |
HCV patients n (%)* |
% HCV patients with LP n (%) |
Total patients (n) | Overall LP n (%) |
|---|---|---|---|---|---|---|
| Hospital 1 (Tenerife) | 36 (12.2) | 6 (16.7) | 260 (87.9) | 68 (26.1) | 296 | 74 (12.6) |
| Hospital 2 (Santander) | 25 (18.4) | 4 (16.0) | 111 (81.6) | 40 (36.0) | 136 | 44 (7.6) |
| Hospital 3 (Madrid) | N/A | N/A | 143 (100) | 45 (31.5) | 143 | 45 (7.6) |
| Hospital 4 (San Sebastián) | 27 (10.6) | 8 (29.6) | 226 (89.3) | 69 (30.5) | 253 | 77 (13.1) |
| Hospital 5 (Barcelona) | 125 (31.0) | 16 (12.8) | 278 (69.0) | 39 (14.0) | 403 | 55 (9.3) |
| Hospital 6 (Madrid) | 21 (17.8) | 4 (19.0) | 97 (82.2) | 29 (29.9) | 118 | 33 (5.6) |
| Hospital 7 (Pontevedra) | N/A | N/A | 136 (100) | 26 (19.1) | 136 | 26 (4.4) |
| Hospital 8 (Sevilla) | 48 (35.8) | 13 (27.1) | 86 (64.2) | 33 (38.4) | 137 | 46 (7.8) |
| Hospital 9 (Barcelona) | 163 (38.6) | 10 (6.2) | 258 (61.4) | 90 (34.8) | 420 | 100 (17.0) |
| Hospital 10 (Málaga) | 47 (30.7) | 16 (34.0) | 106 (69.3) | 45 (42.4) | 153 | 61 (10.4) |
| Hospital 11 (Zaragoza) | 14 (14.3) | 1 (7.1) | 84 (54.7) | 26 (30.9) | 98 | 27 (4.6) |
N/A not available—did not provide HBV data.
HBV hepatitis B virus, HCV hepatitis C virus, LP late presentation.
*(%) reported in relation to the total of all patients in the hospital.
Bivariate logistic regression analyses with late presentation as the outcome (Table 6) were conducted for sex, age, origin of referral, and country of origin (Spanish-born vs. foreign-born). Being female was associated with a 50% decreased odds of LP and being Spanish born was associated with double the odds of presenting late. Each additional year of age corresponded to 0.3% greater odds of LP. Compared to primary care as the origin of referral, each of the other origins of referral showed greater odds of late presentation and in particular, having an unknown origin of referral had the greatest odds of LP.
Table 6.
Odds ratios and their 95% confidence intervals univariate logistic regressions for late presentation.
| Variable | Odds ratio (OR) | 95% Confidence interval | p-value |
|---|---|---|---|
| Age | 1.03 | [1.03–1.04] | < 0.001 |
| Sex | |||
| Male (reference group) | – | – | < 0.001 |
| Female | 0.49 | [0.40–0.61] | |
| Country of origin | |||
| Foreign-born (reference group) | – | – | – |
| Spanish born | 2.08 | [1.60–2.71] | < 0.001 |
| Origin of referral | |||
| Primary care (reference group) | – | – | – |
| Other specialty in the same centre | 1.33 | [1.00–1.77] | 0.046 |
| GI specialist from another centre | 1.41 | [1.02–1.94] | 0.034 |
| Other specialist from another centre | 1.48 | [0.89–2.44] | 0.124 |
| Other | 1.16 | [0.88–1.52] | 0.271 |
| Unknown | 1.56 | [1.12–2.17] | 0.008 |
GI gastrointestinal.
Discussion
Late presentation to hepatitis specialist care (liver units or gastroenterologist specialists) continues to be a challenge in Spain, with nearly one-fourth of the patients in this study of 11 large university hospitals presenting with advanced liver disease or late-stage liver disease at first consultation with a liver specialist. Patients with chronic hepatitis C infection presented late to care more frequently than those with hepatitis B and were predominantly Spanish-born individuals.
Late presentation to care results in more advanced disease progression at baseline, making treatment (for both HBV and HCV) and subsequent cure (in the case of HCV) more challenging. In Spain, cirrhosis and other chronic liver diseases due to HCV and HBV accounted for 1598 deaths (0.38% of total) and 618 (0.15%) deaths in 2017, respectively. Liver cancer accounted for 2860 (0.69%) deaths due to HCV and 409 (0.1%) deaths due to HBV in the same year17. Health systems must ensure that those who are in need of treatment are screened, diagnosed, and linked to specialist care before their liver disease progresses to cause substantial liver damage18. In the instance of human immunodeficiency virus (HIV), a definition of late presentation at the time of diagnosis has aided in surveillance and the identification of risk factors for presenting late with HIV19 in addition to monitoring trends over time20.
Delayed linkage to care or loss to follow-up after referral to specialised care poses a significant barrier to timely treatment initiation21 and poses additional risk for ALD and LSLD. A delayed referral for specialised treatment from primary care, where viral hepatitis diagnoses are often made, extends the amount of time individuals live undertreated or untreated with either or both diseases, highlighting deficiencies within the health system. Not only should screening for viral hepatitis be strengthened at the primary care level, but the referral pathway needs to be improved. In our study, we identified patients being referred from other specialists in the same hospital or even other gastroenterology and hepatology services from other hospitals. The former suggests that referral processes in the hospitals need to be strengthened, which is a healthcare failure. The latter possibly highlights that some patients may have difficult-to-treat hepatic conditions, rather than them presenting late to viral hepatitis care. This nuance should be considered by healthcare providers at the point of care engagement, when, despite presenting with ALD or LSLD, patients may not have initially presented late to care. Strong care pathways (e.g. referral processes) are a particular challenge during the ongoing COVID-19 pandemic as primary care centres in Spain are overburdened with COVID-19 cases, and may have less capacity and resources to address other morbidities, including viral hepatitis. In our study, the mean year of patients’ diagnosis was 2011; 7–8 years prior to reaching specialist care with a gastroenterologist or hepatologist despite possibly having been in hospital care for comorbidities. Patients who present with hepatocellular carcinoma at first consultation with a specialist initiate treatment once the impact of prolonged and untreated viral hepatitis infection has already resulted in irreversible liver damage. Deaths due to cirrhosis constituted 2.4% of total deaths globally in 201722. In a single-centre study conducted in Denmark, 5.3% (28/527) of patients presenting late (n = 169) had LSLD at first consultation23. Our study reported a similar prevalence of LSLD (5.8%), of which 55 patients presented with HCC at first consultation between the years 2018 and 2019. Similarly, in a study using newly-diagnosed hepatitis B related HCC patients (n = 1276) in Korea, Sinn et al. reported that in 2013, 23.1% of patients with newly diagnosed HCC cases had no prior clinic visits before their HCC diagnosis24. In contrast, in 2003, this prevalence was 50.9%, highlighting the impact of effective targeted screening in Korea. However, any proportion of LP, and specifically of HCC at first consultation, highlights that timely diagnosis and referral needs to be optimized and targeted as a public health challenge.
In the study by Sinn et al.24, authors reported that a lower income was associated with a higher risk of LP. Our study did not collect income level variables; however, it would be interesting to further explore potential barriers to accessing specialised care in Spain, which could include socioeconomic barriers. Healthcare is free and universal for all legal residents of Spain in all autonomous communities of the country so cost of care would not be anticipated to be a main factor contributing to LP to care. However, HBV patients were predominantly foreign-born individuals, particularly from sub-Saharan African and Asian countries. Migrants who have irregular legal status in the country would not be entitled to access the public healthcare system, which could be a risk factor contributing to the 15.0% of LP HBV cases in our study. Furthermore, 35% of all HBV patients who presented late to care were not Spanish natives, highlighting the importance of early detection of HBV among migrant populations residing in Spain. In addition, HBV vaccination coverage of foreign-born individuals was not well reported across all centres, with nearly 50% reporting incorrect vaccination among patients and an additional 40.8% in missing values (data not reported in results). Incomplete or incorrect vaccination of HBV among patients arriving to Spain from endemic countries should suggest an urgency for timely HBV screening. Proper vaccination coverage reporting will consolidate current monitoring and surveillance methods and may detect gaps in testing and referral services.
In contrast, HCV patients were predominantly Spanish natives (87.3%) and had a history of past or current injecting drug use (27.1%). ALD was reported in 26.9% of HCV patients, similar to those described in another cohort study (GECCO cohort, Germany) which reported an ALD prevalence of 32.5%25. However, 210/863 of the patients included in the GECCO cohort were HIV/HCV co-infected. HIV patients are increasingly aware of their status and are regularly in care, which would suggest that screening for HCV would be done in a timely manner, reducing risk for LP. Integrated care is a reported best practice for increasing screening, management, and treatment monitoring for HCV care26 and in Spain, elimination of HCV among those who are HIV/HCV is feasible27, as is the case in other settings28. Of those who had a documented mode of transmission in our study, injecting drug use was the most common route of HCV infection. Targeted interventions for this key population should be implemented if lacking in particular regions and scaled up widely to reach the WHO 2030 elimination targets.
Notably, however, our study also had a large proportion of unknown modes of transmission, both for HBV and HCV patients. This poses a particular challenge when designing targeted interventions for key populations, as described in the Spanish Ministry of Health screening guidelines29. Age was identified as a risk factor for LP and the presence of hepatocellular carcinoma in our study, which can serve as a basis for increased screening among older age groups, including multiple generations, similar to what is recommended in the United States30.
Current patient-centred models of care exist across Spain to increase testing and linkage to care for people who inject drugs. These models of care have high cure rates and should be expanded, especially given that normal care pathways with referral systems in the Spanish hospital system show large proportions of LP and un-tracked origins of referral. These models of care often use point-of-care testing31 or reflex testing32, helping reduce potential lost to follow-up.
Our findings may be used to identify vulnerable groups (e.g. people who inject drugs and migrants); risk factors, and characteristics (e.g. age) that contribute to LP; track the effectiveness of current interventions in the region; and potentially initiate additional studies and improve timely access to treatment. Given that hospitals serving various and diverse Spanish cities were included in this study, further studies investigating potential barriers to specialist care are warranted.
The main advantages of our study include that it is a multicentre study with a large number of patients included during a two-year period. However, the study has several limitations such as the inconsistency in data quality, including missing data for some variables across centres. Additionally, conflation between late presentation to care and late presentation to treatment should be highlighted. While our study focused on describing late presentation to specialist care because liver specialists are designated to oversee the treatment and care of viral hepatitis patients in Spain, data also show that some patients may have been engaged in care in other specialities before reaching the appropriate specialty for their viral hepatitis care. Additionally, fibrosis stage was reported by each individual participating hospital using different liver staging methods by each. Our data cannot reflect the accuracy of each reported fibrosis stage; hospitals classified their patients’ fibrosis stage according to one of the methods described in “Supplementary material”.
Conclusions
In conclusion, one in four patients in Spain with chronic viral hepatitis B or C present late to specialist care, of which more than 5% already have irreversible liver damage. Despite universal access to DAA therapy in Spain, late presentation did not decrease from the start of 2018 to the end of 2019. Initiatives to reduce late presentation should specifically target men, foreign-born populations for HBV, Spanish nationals for HCV, and people with past or current drug use, while following the local epidemiology of each autonomous community.
Supplementary Information
Acknowledgements
CAP, TMW, JVL acknowledge support to ISGlobal from the Spanish Ministry of Science, Innovation and Universities through the “Centro de Excelencia Severo Ochoa 2019–2023” Programme (CEX2018-000806-S), and support from the Government of Catalonia through the CERCA Programme. CAP acknowledges support from the Secretaria d’Universitats i Recerca de la Generalitat de Catalunya and the European Social Fund as an AGAUR-funded PhD fellow. The authors would like to thank those who contributed to data collection: From the Basque Country: Leire Samaniego, Maria Vaamonde, and Edurne Almandoz. From Madrid; Dr Yza Frias, and Ana Robles. From Málaga; Enrique del Campo Herrera. From Sevilla; Bianca Sánchez-Barbero, Javier Ampuero.
Abbreviations
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- CHB
Chronic hepatitis B
- CHC
Chronic hepatitis C
- WHO
World Health Organization
- HCC
Hepatocellular carcinoma
- DAAs
Direct-acting antivirals
- SVR
Sustained virologic response
- LP
Late presentation
- ALD
Advanced liver disease
- LSLD
Late-stage liver disease
- AST
Aspartate aminotransferase
- ALT
Alanine transaminase
- APRI
Aminotransferase platelet ratio
- OR
Odd’s ratio
- HIV
Human immunodeficiency virus
Author contributions
J.V.L. and M.B. conceived of the study and developed the data collection tool. C.A.P. led the data management and S.L., M.H.G., J.A., R.J.A., J.C., J.G.S., M.R., J.T., J.L.C., M.A.S., M.R.B., A.P., D.M.A., and A.G. collected and provided the data. Data analysis was undertaken by C.A.P., J.V.L., M.B. and T.M.W. C.A.P. drafted the first iteration of manuscript with assistance from J.V.L. and M.B. All authors reviewed the full draft of the article, subsequent revisions and approved the final version for submission.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Maria Buti and Jeffrey V. Lazarus.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-01885-0.
References
- 1.World Health Organization. Hepatitis C. Fact sheet 9 July 2019 [Internet]. https://www.who.int/news-room/fact-sheets/detail/hepatitis-c. Accessed 15 Nov 2021.
- 2.World Health Organization . Global Health Sector Strategy on Viral Hepatitis, 2016–2021. World Health Organization; 2016. [Google Scholar]
- 3.World Health Organization Regional Office for Europe. Hepatitis B in the WHO European region. Fact sheet July 2019 [Internet]. World Health Organization; 2019. http://www.euro.who.int/__data/assets/pdf_file/0007/377251/Fact-Sheet-Hepatitis-B_2019-ENG.pdf?ua=1. Accessed 15 Nov 2021.
- 4.World Health Organization Regional Office for Europe. Hepatitis C in the WHO European region. Fact sheet July 2019 [Internet]. World Health Organization; 2019. http://www.euro.who.int/__data/assets/pdf_file/0009/377253/Fact-Sheet-Hepatitis-C_2019_ENG.PDF?ua=1. Accessed 15 Nov 2021.
- 5.Lok A, McMahon B. Chronic hepatitis B. Hepatology. 2007;45:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 6.Nelson K. The impact of chronic hepatitis C virus infection on mortality. J. Infect. Dis. 2012;206:461–463. doi: 10.1093/infdis/jis394. [DOI] [PubMed] [Google Scholar]
- 7.European Association for the Study of the Liver EASL recommendations on treatment of hepatitis C 2018. J. Hepatol. 2018;69(2):P461–511. doi: 10.1016/j.jhep.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 8.He S, Lockart I, Alavi M, Danta M, Hajarizadeh B, Dore GJ. Systematic review with meta-analysis: Effectiveness of direct-acting antiviral treatment for hepatitis C in patients with hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2020;51:34–52. doi: 10.1111/apt.15598. [DOI] [PubMed] [Google Scholar]
- 9.European Association for the Study of the Liver (EASL) Clinical Practice Guidelines. EASL recommendations on treatment of hepatitis C: Final update of the series. J. Hepatol. (2020). https://easl.eu/wp-content/uploads/2020/09/EASL-recommendations-on-treatment-of-hepatitis-C.pdf. (Accessed 22 September 2020). [DOI] [PubMed]
- 10.Crespo J, et al. El diagnóstico de la infección por el virus de la hepatitis C en España: una oportunidad para mejorar. Enferm. Infecc. Microbiol. Clín. 2019;37:231–238. doi: 10.1016/j.eimc.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Negro F, Forton D, Craxi A, Sulkowski MS, Feld JJ, Manns MP. Extrahepatic morbidity and mortality of chronic hepatitis C. Gastroenterology. 2015;149:1345–1360. doi: 10.1053/j.gastro.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 12.Yang CH, Shih YF, Liu CJ. Viral factors affecting the clinical outcomes of chronic hepatitis B. J. Infect. Dis. 2017;216:S757–S764. doi: 10.1093/infdis/jix461. [DOI] [PubMed] [Google Scholar]
- 13.Hajarizadeh B, Grebely J, Martinello M, Matthews GV, Lloyd AR, Dore GJ. Hepatitis C treatment as prevention: Evidence, feasibility, and challenges. Lancet Gastroenterol. Hepatol. 2016;1:317–327. doi: 10.1016/S2468-1253(16)30075-9. [DOI] [PubMed] [Google Scholar]
- 14.Smith-Palmer J, Cerri K, Valentine W. Achieving sustained virologic response in hepatitis C: A systematic review of the clinical, economic and quality of life benefits. BMC Infect. Dis. 2015;15:19. doi: 10.1186/s12879-015-0748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mauss S, et al. Late presentation of chronic viral hepatitis for medical care: A consensus definition. BMC. Med. 2017;15:1–5. doi: 10.1186/s12916-017-0856-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.European Association for the Study of the Liver EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 17.Institute for Health Metrics and Evaluation. GBD Compare [Internet]. 2017. https://vizhub.healthdata.org/gbd-compare/. Accessed 15 Nov 2021.
- 18.Lazarus JV, Picchio C, Dillon JF, Rockstroh JK, Weis N, Buti M. Too many people with viral hepatitis are diagnosed late with dire consequences. Nat. Rev. Gastroenterol. Hepatol. 2019;16:451–452. doi: 10.1038/s41575-019-0177-z. [DOI] [PubMed] [Google Scholar]
- 19.Montlahuc C, et al. Impact of late presentation on the risk of death among HIV-infected people in France (2003–2009) J. Acquir. Immune. Defic. Syndr. 2013;64:197–203. doi: 10.1097/QAI.0b013e31829cfbfa. [DOI] [PubMed] [Google Scholar]
- 20.Mocroft A, et al. Risk factors and outcomes for late presentation for HIV-positive persons in Europe: Results from the Collaboration of Observational HIV Epidemiological Research Europe Study (COHERE) PLoS Med. 2013;10:e1001510. doi: 10.1371/journal.pmed.1001510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuckerman A, Douglas A, Nwosu S, Choi L, Chastain C. Increasing success and evolving barriers in the hepatitis C cascade of care during the direct acting antiviral era. PLoS ONE. 2018;13:e0199174. doi: 10.1371/journal.pone.0199174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.GBD 2017 Cirrhosis Collaborators The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020;5:245–266. doi: 10.1016/S2468-1253(19)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen FJ, Hallager S, Øvrehus A, Weis N, Christensen PB, Pedersen C. Late presentation for Care among patients with chronic hepatitis C: Prevalence and risk factors. Open Forum Infect. Dis. 2018;5:ofx257. doi: 10.1093/ofid/ofx257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinn DY, et al. Late presentation of hepatitis B among patients with newly diagnoses hepatocellular carcinoma: A national cohort study. BMC Cancer. 2019;19:286. doi: 10.1186/s12885-019-5508-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rockstroh, J.K. et al. Has increased rollout of direct acting antiviral therapy decreased the burden of late presentation and advanced liver disease in patients starting hepatitis C virus therapy in Germany? in Presented at the International Liver Congress 2018. Abstract n. THU-432. [DOI] [PubMed]
- 26.Lazarus JV, et al. Hepatitis C standards of care: A review of good practices since the advent of direct acting antiviral therapy. Clin. Res. Hepatol. Gastroenterol. 2021;45:101564. doi: 10.1016/j.clinre.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Berenguer J, et al. Human immunodeficiency virus/hepatitis C virus coinfection in Spain: Elimination is feasible, but the burden of residual cirrhosis will be significant. Open Forum Infect. Dis. 2018;5:ofx258. doi: 10.1093/ofid/ofx258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garvey LJ, et al. Decline in hepatitis C virus (HCV) incidence in men who have sex with men living with human immunodeficiency virus: Progress to HCV microelimination in the United Kingdom? Clin. Infect. Dis. 2021;72:233–238. doi: 10.1093/cid/ciaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guía de cribado de la infección por el VHC. Ministerio de Sanidad de España. July 2020. https://mscbs.gob.es/ciudadanos/enfLesiones/enfTransmisibles/sida/docs/GUIA_DE_CRIBADO_DE_LA_INFECCION_POR_EL_VHC_2020.pdf. (Accessed 3 November 2020).
- 30.Centers for Disease Control and Prevention (CDC). Beyond baby boomers: hepatitis C now heavily impacting multiple generations. 9 April 2020. https://www.cdc.gov/nchhstp/newsroom/2020/hepatitis-c-impacting-multiple-generations-press-release.html. (Accessed 3 November 2020).
- 31.Saludes V, et al. Evaluation of the Xpert HCV VL Fingerstick point-of-care assay and dried blood spot HCV-RNA testing as simplified diagnostic strategies among people who inject drugs in Catalonia, Spain. Int. J. Drug. Pol. 2020;80:102734. doi: 10.1016/j.drugpo.2020.102734. [DOI] [PubMed] [Google Scholar]
- 32.Crespo J, et al. Hepatitis C reflex testing in Spain in 2019: A story of success. Enferm. Infecc. Microbiol. Clin. 2021;39:119–126. doi: 10.1016/j.eimc.2020.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.