Abstract
Purpose
Marital status has emerged as an important influence on several cancer outcomes, but its role in medullary thyroid cancer (MTC) remains unclear. This study was to explore the effects of marital status on the prognosis of MTC patients and to determine whether its effects vary by age.
Patients and methods
We retrospectively extracted 1344 eligible patients diagnosed with MTC between 2004 and 2015 from the Surveillance, Epidemiology, and End Results (SEER) database. Based on the marital status, we divided those patients into married and unmarried groups. We compared the difference in overall survival (OS) and cancer‐specific survival (CSS) between married and unmarried via the Kaplan–Meier analysis. Univariate and multivariate Cox proportional models were performed to identify the prognostic factors of OS and CSS.
Results
There were 1344 MTC eligible patients in a total of which 883 (65.7%) were married and 461 (34.3%) were unmarried. The comparison observed between married and unmarried patients was as follows: male (45.2% vs. 28.0%), age (≥52 years) (55.9% vs. 44.6%), White (86.7% vs. 78.7%), and undergo surgery (97.7% vs. 93.3%). Multivariate analysis revealed unmarried status as a risk factor independently associated with worse OS (HR: 2.15, 95% CI: 1.59–2.92) rate and CSS (HR: 1.70, 95% CI: 1.17–2.47) rate. In a further analysis stratified by age, there was no significant difference in OS and CSS between married and unmarried patients younger than 52 years. For the remaining group with 52 years old and higher, unmarried patients showed significantly higher risk of OS and CSS than married patients at all stages of the pathology except M1 stage.
Conclusion
Married patients with MTC have a better prognosis than unmarried ones. Age can affect the association between marital status and the survival of MTC, and married elders may benefit more than youngers.
Keywords: cancer prognosis, marital status, MTC, SEER database, social support
Married patients with MTC have a better prognosis than unmarried ones. Age can affect the association between marital status and the survival of MTC, and married elders may benefit more than youngers.

1. INTRODUCTION
Medullary thyroid cancer (MTC) is a rare malignancy, which originates from the parafollicular C cells of the thyroid gland, accounting for nearly 3%–5% of all thyroid cancers. 1 , 2 Most MTC cases are sporadic, and 20%–30% of cases are multiple endocrine neoplasia type 2 (including MEN2A and MEN2B) or familial. 3 , 4 Despite low incidence, the 10‐year overall survival rate of regional MTC was estimated at approximately 75% and decreased to 40% in patients with metastasis. 5 Early diagnosis and surgery are effective methods to improve both cure and survival rates. Moreover, novel approaches include targeted agents that are proven means of antitumor therapeutics's for clarity. Vandetanib and cabozantinib are used as the evidence‐based treatment of advanced MTC. 6 , 7 However, a common limitation of targeted drug therapy is to develop drug resistance and this phenomenon is independent of the type of tumor. 8
Previous studies have intensively predicted the prognosis of MTC, mainly limiting to the clinicopathological characteristics and therapeutic strategies. 9 , 10 , 11 However, with the growing understanding of human health and disease, more attention is being paid to socio‐psychological factors. A previous clinical trial found that psychosocial factors were linked with low back pain and may affect the prognosis. 12 Marital status is recognized as one type of socio‐psychological factor that influences the cancer survival. 13 Extensive studies focused on marital status and cancers showed that married patients have significantly better survival compared to unmarried ones. 14 , 15 , 16 Nonetheless, in analyses to present, few studies on marital status among MTC patients have been conducted.
The Surveillance, Epidemiology, and End Results (SEER) program collects the data of 18 registries on cancer diagnosis, treatment, and survival for nearly 30% of the US population. 17 It creates a shared research field, thus we can easily analyze the effect of marital status among different cancers. In this study, we analyzed the data from MTC patients using the SEER database. Our work aims to explore the effects of marital status in MTC, especially for older patients.
2. MATERIALS AND METHODS
2.1. Study population
The data of MTC patients were downloaded from the SEER*Stat Database, version 8.3.6. Patients were chosen for this study if they met the following criteria: (1) Primary sites defined by the International Classification of Diseases for Oncology (ICD‐O‐3), code C73.9. (2) Patients diagnosed with primary cancer from 2004 to 2015. (3) Histological codes were limited to MTC (8345, 8510). Exclusion criteria included: (1) Patients aged less than 18. (2) Patients with incomplete clinical characteristics and treatment. (3) Patients with incomplete demographic and follow‐up information. Finally, 1344 eligible patients were selected for analysis according to inclusion and exclusion criteria.
2.2. Study variables
Study variables included sex, age at diagnosis, race, marital status, tumor stage, nodal stage, metastasis, surgery, survival months, and vital status. Age was considered as a continuous variable, measured by means and standard deviations. X‐tile software (version 3.6.1) was used to analyze the best cut‐off point (52 year old) for the age. 18 After that, two age groups were defined as more than 52 years versus those aged 52 years and lower. The race was divided into three groups: White, Black, and Others (Asian or Pacific Islander, American Indian/Alaska Native). Marital status was categorized into married and unmarried (divorced, separated, widowed, never married, or domestic partner). Thyroid surgery was classed into three groups: none, lobectomy/isthmectomy, and total thyroidectomy. For tumor (T), nodal (N), and metastasis (M) status, the TNM status was assessed according to the sixth edition of TNM classification for medullary thyroid cancer. Overall survival (OS) and cancer‐specific survival (CSS) were also analyzed for all eligible patients. The former was calculated from the date of diagnosis to the date of any death, while the latter was estimated from diagnosis to cancer‐specific caused death.
2.3. Statistical analysis
Baseline data were expressed as frequency or mean ± standard deviation according to the data type. Categorical variables were assessed by the Pearson chi‐squared test, while the continuous variables were examined by the t‐test or the Mann–Whitney U test. The survival of marital status and age subgroups was analyzed by the Kaplan–Meier curves, and their differences were evaluated by the log‐rank test. Univariate and multivariate Cox proportional hazards models were used to distinguish the independent prognostic factors in MTC, and their effects were presented as hazard ratio (HR) with 95% confidence intervals (CIs). Similarly, we evaluated the effects of marital status stratified by age, using the multivariate Cox proportional hazards models to analyze the survival difference between married and unmarried in pathological subgroups. All statistical analyses in this study were performed using SPSS (version 26.0) and R software (version 4.1.0). A two‐tailed p value of less than 0.05 was considered statistically significant.
3. RESULTS
3.1. Patients baseline characteristics
We selected 1344 eligible MTC patients diagnosed between 2004 and 2015 in the SEER database. It included 532 (39.3%) male and 816 (60.7%) female patients, with a mean age of 52.9 ± 15.5 years at the diagnosis of MTC. Among them, 883 (65.8%) were married and 461 (34.2%) were unmarried. Between married and unmarried groups, we observed significant differences in sex, age, race, and surgery (all p < 0.001). Besides, married patients were male (45.2% vs. 28.0%), older (age ≥ 52) (55.9% vs. 44.6%), White (86.7% vs. 78.7%), more likely to undergo surgery (97.7% vs. 93.3%), and less to be in M1 status (7.2% vs. 10.2%) compared to unmarried patients. The summary of baseline patient characteristics grouped by marital status is described in detail in Table 1.
TABLE 1.
Baseline characteristics of patients with MTC in SEER database
| Characteristics | Total (%) | Married (%) | Unmarried (%) | p value b |
|---|---|---|---|---|
| Number | N = 1344 (100) | N = 883 (65.7) | N = 461 (34.3) | |
| Sex | ||||
| Female | 816 (60.7) | 484 (54.8) | 332 (72.0) | <0.001 |
| Male | 528 (39.3) | 399 (45.2) | 129 (28.0) | |
| Age a | ||||
| Mean ± SD | 52.9 ± 15.5 | 54.0 ± 14.1 | 50.7 ± 17.9 | <0.001 |
| <52 | 637 (47.4) | 389 (44.1) | 248 (53.4) | |
| ≥52 | 707 (52.6) | 494 (55.9) | 213 (46.6) | |
| Race | ||||
| White | 1129 (84.0) | 766 (86.7) | 363 (78.7) | <0.001 |
| Black | 127 (9.4) | 53 (6.0) | 74 (16.1) | |
| Others | 88 (6.5) | 64 (7.3) | 24 (5.2) | |
| Tumor stage | ||||
| T1 | 581 (43.2) | 365 (41.3) | 216 (46.9) | 0.281 |
| T2 | 347 (25.8) | 234 (26.5) | 113 (24.5) | |
| T3 | 282 (21.0) | 193 (21.9) | 89 (19.3) | |
| T4 | 134 (10.0) | 91 (10.3) | 43 (9.3) | |
| Nodal stage | ||||
| No | 783 (58.3) | 513 (58.1) | 270 (58.6) | 0.877 |
| N1a | 166 (12.4) | 107 (12.1) | 59 (12.8) | |
| N1b | 395 (29.4) | 263 (29.8) | 132 (28.6) | |
| Metastasis | ||||
| M0 | 1233 (91.7) | 819 (92.8) | 414 (89.8) | 0.062 |
| M1 | 111 (8.3) | 64 (7.2) | 47 (10.2) | |
| Surgery | ||||
| No | 51 (3.8) | 20 (2.3) | 31 (6.7) | <0.001 |
| IT/LT | 94 (7.0) | 63 (7.1) | 31 (6.7) | |
| TT | 1199 (89.2) | 800 (90.6) | 399 (86.6) | |
Variables with statistical significance were shown in bold. Abbreviations: IT/LT, isthmectomy/lobectomy; MTC, medullary thyroid cancer; SEER, The Surveillance, Epidemiology, and End Result; TT: total thyroidectomy or near total thyroidectomy.
Age was a continuous variable and grouped by cut‐off point using x‐tile software.
Categorical variables were assessed by the chi‐squared test and continuous variables were examined by the Mann–Whitney U test.
3.2. Effects of marital status on OS and CSS
The effects of marital status on OS were examined by K‐M curves, which showed married patients had a significantly superior OS compared to unmarried ones (Figure 1A). Similarly, the significant differences of CSS were also observed among different marital groups (Figure 1B) with a better CSS in married populations than in unmarried ones. To evaluate the prognosis‐related factors of MTC, we first performed the univariate cox analysis. The results showed that marital status, sex, age, tumor stage, nodal stage, metastasis, and surgery were regarded as significant prognostic factors for both OS (all p < 0.05) (Table 2) and CSS (all p < 0.05) (Table 3). Race was a prognostic factor for CSS (p < 0.05), but not OS (p > 0.05) in univariate analysis. Subsequently, those above‐mentioned significant factors were analyzed in a multivariate cox model. After multivariate adjustment, marital status remained a significant prognostic factor in OS (p < 0.001) and CSS (p = 0.006), with worse OS (HR: 2.15, 95% CI: 1.59–2.92) rate and CSS (HR: 1.70, 95% CI: 1.17–2.47) rate in unmarried patients compared to married ones. However, there was no significant survival difference observed in sex (OS: p = 0.858, CSS: p = 0.826).
FIGURE 1.
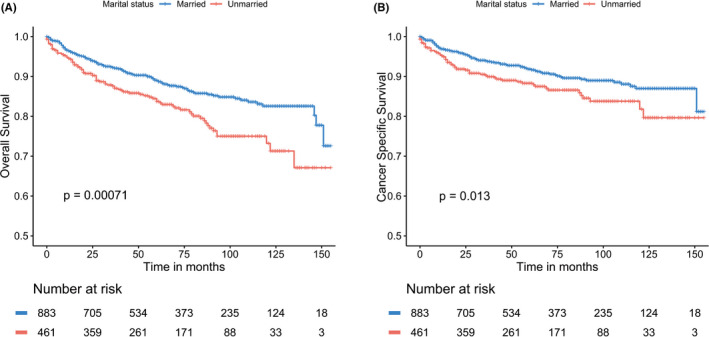
The Kaplan–Meier survival curves: (A) The overall survival and (B) the cancer‐specific survival according to marital status
TABLE 2.
Univariate and multivariate analyses for OS in MTC patients
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Sex | ||||
| Female | Reference | Reference | ||
| Male | 1.61 (1.21–2.14) | 0.001 | 0.97 (0.71–1.33) | 0.858 |
| Age a | ||||
| <52 | Reference | Reference | ||
| ≥52 | 3.58 (2.55–5.02) | <0.001 | 4.33 (3.06–6.13) | <0.001 |
| Race | ||||
| White | Reference | |||
| Black | 1.22 (0.78–1.91) | 0.373 | ||
| Others | 0.50 (0.22–1.12) | 0.093 | ||
| Marital status | ||||
| Married | Reference | Reference | ||
| Unmarried | 1.63 (1.23–2.17) | 0.001 | 2.15 (1.59–2.92) | <0.001 |
| Tumor stage | ||||
| T1 | Reference | Reference | ||
| T2 | 1.86 (1.18–2.93) | 0.007 | 1.86 (1.18–2.94) | 0.008 |
| T3 | 3.54 (2.33–5.38) | <0.001 | 2.26 (1.42–3.59) | 0.001 |
| T4 | 9.65 (6.39–14.58) | <0.001 | 3.96 (2.43–6.44) | <0.001 |
| Nodal stage | ||||
| N0 | Reference | Reference | ||
| N1a | 2.26 (1.44–3.54) | <0.001 | 1.77 (1.10–2.86) | 0.019 |
| N1b | 4.11 (2.99–5.65) | <0.001 | 1.84 (1.22–2.76) | 0.004 |
| Metastasis | ||||
| M0 | Reference | Reference | ||
| M1 | 11.50 (8.51–15.54) | <0.001 | 3.86 (2.65–5.61) | <0.001 |
| Surgery | ||||
| No | Reference | Reference | ||
| IT/LT | 0.06 (0.03–0.13) | <0.001 | 0.31 (0.14–0.67) | 0.003 |
| TT | 0.08 (0.06–0.12) | <0.001 | 0.30 (0.20–0.46) | <0.001 |
Variables with statistical significance were shown in bold. Abbreviations: IT/LT, isthmectomy/lobectomy; MTC, medullary thyroid cancer; OS, overall survival; TT: total thyroidectomy or near total thyroidectomy.
Age was a continuous variable and grouped by cut‐off point using x‐tile software.
TABLE 3.
Univariate and multivariate analyses for CSS in MTC patients
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Sex | ||||
| Female | Reference | Reference | ||
| Male | 2.05 (1.46–2.88) | <0.001 | 0.96 (0.66–1.39) | 0.826 |
| Age a | ||||
| <52 | Reference | Reference | ||
| ≥52 | 2.48 (1.71–3.59) | <0.001 | 2.96 (2.01–4.38) | <0.001 |
| Race | ||||
| White | Reference | Reference | ||
| Black | 1.32 (0.79–2.19) | 0.291 | 1.90 (1.11–3.23) | 0.019 |
| Others | 0.11 (0.02–0.81) | 0.030 | 0.10 (0.01–0.70) | 0.021 |
| Marital status | ||||
| Married | Reference | Reference | ||
| Unmarried | 1.53 (1.09–2.16) | 0.014 | 1.70 (1.17–2.47) | 0.006 |
| Tumor stage | ||||
| T1 | Reference | Reference | ||
| T2 | 2.36 (1.24–4.49) | 0.009 | 2.23 (1.16–4.28) | 0.016 |
| T3 | 6.03 (3.40–10.72) | <0.001 | 2.86 (1.53–5.33) | 0.001 |
| T4 | 19.17 (10.98–33.48) | <0.001 | 5.24 (2.78–9.86) | <0.001 |
| Nodal stage | ||||
| N0 | Reference | Reference | ||
| N1a | 3.93 (2.21–6.99) | <0.001 | 2.81 (1.52–5.20) | 0.001 |
| N1b | 7.98 (5.15–12.36) | <0.001 | 2.77 (1.62–4.73) | <0.001 |
| Metastasis | ||||
| M0 | Reference | Reference | ||
| M1 | 19.56 (13.89–27.54) | <0.001 | 6.05 (3.95–9.25) | <0.001 |
| Surgery | ||||
| No | Reference | Reference | ||
| IT/LT | 0.04 (0.02–0.10) | <0.001 | 0.34 (0.13–0.93) | 0.036 |
| TT | 0.06 (0.04–0.10) | <0.001 | 0.32 (0.20–0.50) | <0.001 |
Variables with statistical significance were shown in bold. Abbreviations: CSS, cancer‐specific survival; IT/LT, isthmectomy/lobectomy; MTC, medullary thyroid cancer; TT: total thyroidectomy or near total thyroidectomy.
Age was a continuous variable and grouped by cut‐off point using x‐tile software.
3.3. Effects of marital status on OS and CSS according to age stratification
We first assessed the association between age and survival by the Kaplan–Meier curves, which indicated that older patients were more likely to present worse survival compared to youngers (OS: p < 0.001; CSS: p < 0.001) (Figure 2A,B). Furthermore, to determine the effects of marital status on the survival of different pathological stages varies with age. We compared the OS and CSS of unmarried versus married based on age stratification by multivariate cox models, which were adjusted for the sex, race, marital status, TNM stage, and surgery. As shown in Table 4, marital status had no effect on survival in MTC patients younger than 52 years of age. In older patients, however, marital status had a significant effect on survival at all pathological subgroups except M1 stage, with unmarried groups presented a higher risk of OS and CSS (all p < 0.05) compared to married ones. These results showed that marriage had a significant protective effect in older patients among different pathological stages and its effect declined when the tumor progressed.
FIGURE 2.
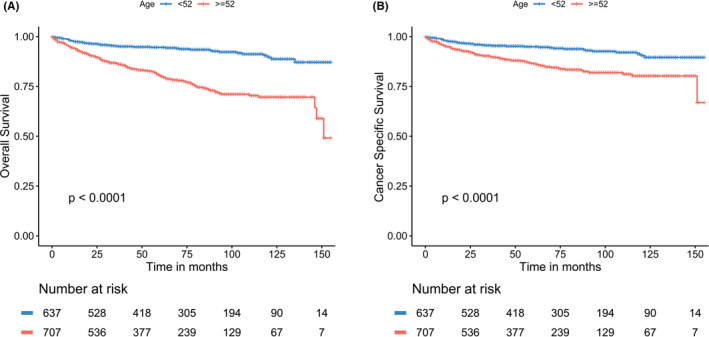
The Kaplan–Meier survival curves: (A) The overall survival and (B) the cancer‐specific survival according to age
TABLE 4.
The OS and CSS associated with being unmarried (vs. married) among MTC patients stratified by age, sex, and pathological stages
| Age a | OS | CSS | ||
|---|---|---|---|---|
| HR (95% CI) b | p value | HR (95% CI) b | p value | |
| <52 | ||||
| Sex | ||||
| Female | 0.90 (0.35–2.31) | 0.834 | 1.07 (0.42–2.68) | 0.892 |
| Male | 1.29 (0.19–3.39) | 0.613 | 1.29 (0.19–3.39) | 0.613 |
| Tumor stage | ||||
| T1/T2 | 1.86 (0.55–6.28) | 0.317 | 3.27 (1.03–10.42) | 0.045 |
| T3/T4 | 1.03 (0.49–2.18) | 0.932 | 0.95 (0.44–2.06) | 0.895 |
| Nodal stage | ||||
| N0 | 0.78 (0.08–7.17) | 0.829 | 1.52 (0.15–15.38) | 0.720 |
| N1 | 1.13 (0.55–2.33) | 0.732 | 1.14 (0.54–2.41) | 0.740 |
| Metastasis | ||||
| M0 | 0.94 (0.41–2.16) | 0.883 | 1.03 (0.42–2.54) | 0.944 |
| M1 | 2.67 (0.60–11.78) | 0.196 | 2.67 (0.60–11.78) | 0.196 |
| ≥52 | ||||
| Sex | ||||
| Female | 1.96 (1.21–3.18) | 0.007 | 1.17 (0.60–2.28) | 0.649 |
| Male | 2.99 (1.75–5.10) | <0.001 | 3.39 (1.83–6.30) | <0.001 |
| Tumor stage | ||||
| T1/T2 | 2.80 (1.62–4.85) | <0.001 | 2.90 (1.33–6.34) | 0.007 |
| T3/T4 | 2.32 (1.40–3.82) | 0.001 | 2.16 (1.23–3.79) | 0.007 |
| Nodal stage | ||||
| N0 | 3.32 (1.84–5.99) | <0.001 | 3.36 (1.38–8.21) | 0.008 |
| N1 | 1.76 (1.10–2.81) | 0.018 | 1.62 (0.95–2.77) | 0.074 |
| Metastasis | ||||
| M0 | 2.44 (1.57–3.78) | <0.001 | 1.97 (1.01–3.84) | 0.046 |
| M1 | 1.74 (0.90–3.38) | 0.101 | 1.78 (0.92–3.49) | 0.087 |
Variables with statistical significance were shown in bold. Abbreviations: CSS, cancer‐specific survival; MTC, medullary thyroid cancer; OS, overall survival.
Age was a continuous variable and grouped by cut‐off point using x‐tile software.
Models were adjusted for sex, marital status, race, tumor stage (T1/T2, T3/T4), nodal stage (N0, N1), metastasis (M0, M1), and surgery.
4. DISCUSSION
In this study, we investigated the association between marital status and survival based on a large cohort of MTC patients. Since age is an important factor affecting thyroid cancer outcomes, we also evaluated the effects of marital status at different age groups on MTC outcomes. 19 To our knowledge, this is the first study to explore the interaction of age and marital status in MTC survival.
In our study, we found that married patients showed a lower M1 stage of tumor compared to unmarried ones, but opposite results were observed in the T stage. Moreover, only 20 (2.3%) married patients were not treated with surgery, which is significantly lower than 31 (6.7%) of unmarried patients. In univariate analysis, we identified marital status as an independent prognostic factor, with unmarried patients showed a higher risk of death. After adjustment for demographic and pathological characteristics, increased death risk was also found in unmarried patients compared to married ones. In addition, we investigated the effects of marital status in different pathological stages and sex groups based on age. Our results demonstrated that marriage has a significant protective effect on older (age ≥ 52) patients, while this effect decreased with the progression of the tumor. However, we found no significant difference in OS and CSS between married and unmarried patients younger than 52 years. In the previous studies, married younger patients were found to have significant better outcomes than unmarried ones in some other cancers, including breast cancer, multiple myeloma, and oral cavity cancer. 20 , 21 , 22 , 23 But, a latest study focused on differentiated thyroid cancer (DTC) showed the impact of marital status on survival varied with age, which was similar to our results. 24 It is well known that the prognosis of thyroid cancer is usually better in younger patients than older ones, thus we suggest that there is an interaction between age and marital status. In younger patients, the benefits of age may far beyond the benefits of marital status, which masked the survival difference between married and unmarried, and the opposite was in old patients.
The association of marital status and survival was explored in many tumors, including breast cancer, rectal cancer, and non‐small cell lung cancer. 14 , 15 , 16 Consistent with the findings of the above researches, our results presented that marriage was a factor associated with superior survival. In a previous study focusing on differentiated thyroid cancer patients, Shi et al. found unmarried patients increased the risk of tumor mortality. 24 A study of breast cancer showed greater protection of marriage among patients aged 70 years or older. 20 By collating and analyzing more than a million patients diagnosed with different cancers, unmarried patients presented a higher risk of metastatic cancer and death resulting from cancer. 13
Two possibilities could explain the superior survival of married patients than unmarried ones. On the one hand, these married patients were supervised by their spouses for regular physical examinations before being diagnosed, which contributes to the early detection of MTC. Spouses may also provide more economic support for subsequent treatments. In MTC, early stage surgical intervention has a benefit for survival, because the surgical cure is possible for patients without metastasis or with regional lymph nodes confined to the neck. 25 However, the therapeutic efficacy of surgery is limited when the tumor metastasizes outside the neck. Thus, married patients could obtain a better prognosis according to the early surgery.
On the other hand, the psychological disorder is common among cancer patients with more than four times higher than the ordinary individuals. 26 Recent studies showed that patients obtaining a cancer diagnosis have a greater susceptibility to develop psychiatric disorders, such as stress, depression, anxiety, etc. 27 , 28 However, married people showed less depression and psychological distress after being diagnosed with cancer, which may be attributed to the encouragement and support from their spouses. 29 The impact of psychological distress on tumor progression has been implicated by epidemiological studies. 30 For example, stress exposure promotes tumor cell proliferation, angiogenesis, and invasion by stimulating the adrenergic pathway. 31 Additionally, the cortisol circadian rhythm of patients is changed due to cancer‐related psychological stress, which will cause poor survival. 32 Some cytokines that are involved in inflammation response, including IL‐1β, IL‐6, TNF, and C‐reactive protein (CRP), are increased in depressed patients and promote tumor progression. 33 , 34 Therefore, the lack of social support and suffering from psychological problems perhaps partly explain the higher mortality in unmarried patients.
Despite these findings between marital status and survival, there remain several limitations in our study. First, an inherent limitation is related to our retrospective study, limiting our ability to define a cause‐and‐effect relationship between marital status and survival. Second, marital status in the SEER database was recorded at diagnosis. However, marital status is dynamic and may have changed throughout the follow‐up period, thus affecting the final results. In addition, the quality of marriage was not recorded, which may lead to different survival outcomes. Third, some information regarding socioeconomic status and education is not available in the SEER database. Finally, data on the type of chemotherapy or targeted agents are unavailable and thus are not included in our study, which may bias our present results.
5. CONCLUSION
In summary, our present results showed marital status was an independent prognostic factor in MTC. Unmarried patients showed a higher risk of OS and CSS, which is particularly evident in older patients. Further studies could investigate the mechanism for age affecting the benefits of marriage. Returning to our research, unmarried patients (especially the old unmarried patients) need to be noticed with more social care and psychological support to improve their survival.
CONFLICT OF INTEREST
The authors declare no conflict of interest in this work.
ETHICAL APPROVAL STATEMENT
No ethical approval is required in this study.
ACKNOWLEDGMENTS
The authors acknowledge the data provided by the Surveillance, Epidemiology, and End Results program.
Ai L, Li N, Tan H‐L, et al. Effects of marital status on survival of medullary thyroid cancer stratified by age. Cancer Med. 2021;10:8829–8837. doi: 10.1002/cam4.4388
Funding information
This work was supported by the National Natural Science Foundation of China (grant numbers 81974423, 81902729), the Key Research and Development Program of Hunan Province (grant number 2019SK2031), the China Postdoctoral Science Foundation (grant numbers 2020M672517, 2021T140749), the Natural Science Foundation of Hunan Province (grant number 2020JJ5904), and the Xiangya Hospital Foundation for Young Scholars (grant number 2018Q01).
Contributor Information
Peng Huang, Email: xiangyahp@csu.edu.cn, Email: changshi@csu.edu.cn.
Shi Chang, Email: changshi@csu.edu.cn.
DATA AVAILABILITY STATEMENT
The dataset of this study is available in the Surveillance, Epidemiology, and End Results program (www.seer.cancer.gov) SEER*Stat Database: Incidence–SEER 18 Regs Custom Data (with additional treatment fields), Nov 2018 Sub (1975–2016 varying).
REFERENCES
- 1. Hoff AO, Hoff PM. Medullary thyroid carcinoma. Hematol Oncol Clin North Am. 2007;21(3):475‐488. [DOI] [PubMed] [Google Scholar]
- 2. Spitzweg C, Morris JC, Bible KC. New drugs for medullary thyroid cancer: new promises? Endocr Relat Cancer. 2016;23(6):R287‐R297. [DOI] [PubMed] [Google Scholar]
- 3. Lam ET, Ringel MD, Kloos RT, et al. Phase II clinical trial of sorafenib in metastatic medullary thyroid cancer. J Clin Oncol. 2010;28(14):2323‐2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim BH, Kim IJ. Recent updates on the management of medullary thyroid carcinoma. Endocrinol Metab (Seoul). 2016;31(3):392‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roman S, Lin R, Sosa JA. Prognosis of medullary thyroid carcinoma: demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer. 2006;107(9):2134‐2142. [DOI] [PubMed] [Google Scholar]
- 6. Wells SA, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double‐blind phase III trial. J Clin Oncol. 2012;30(2):134‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elisei R, Schlumberger MJ, Müller SP, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31(29):3639‐3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ma J, Zhang YI, Tang KE, et al. Reversing drug resistance of soft tumor‐repopulating cells by tumor cell‐derived chemotherapeutic microparticles. Cell Res. 2016;26(6):713‐727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Al‐Qurayshi Z, Khadra H, Chang K, Pagedar N, Randolph GW, Kandil E. Risk and survival of patients with medullary thyroid cancer: national perspective. Oral Oncol. 2018;83:59‐63. [DOI] [PubMed] [Google Scholar]
- 10. Schlumberger M, Elisei R, Müller S, et al. Overall survival analysis of EXAM, a phase III trial of cabozantinib in patients with radiographically progressive medullary thyroid carcinoma. Ann Oncol. 2017;28(11):2813‐2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Byun J‐K, Choi Y‐K, Kang YN, et al. Retinoic acid‐related orphan receptor alpha reprograms glucose metabolism in glutamine‐deficient hepatoma cells. Hepatol. 2015;61(3):953‐964. [DOI] [PubMed] [Google Scholar]
- 12. Thomas E, Silman AJ, Croft PR, Papageorgiou AC, Jayson MIV, Macfarlane GJ. Predicting who develops chronic low back pain in primary care: a prospective study. BMJ. 1999;318(7199):1662‐1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aizer AA, Chen M‐H, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31(31):3869‐3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen Z, Yin K, Zheng D, et al. Marital status independently predicts non‐small cell lung cancer survival: a propensity‐adjusted SEER database analysis. J Cancer Res Clin Oncol. 2020;146(1):67‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jin J‐J, Wang W, Dai F‐X, et al. Marital status and survival in patients with gastric cancer. Cancer Med. 2016;5(8):1821‐1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang X, Cao W, Zheng C, Hu W, Liu C. Marital status and survival in patients with rectal cancer: an analysis of the Surveillance, Epidemiology and End Results (SEER) database. Cancer Epidemiol. 2018;54:119‐124. [DOI] [PubMed] [Google Scholar]
- 17. Hankey BF, Ries LA, Edwards BK. The surveillance, epidemiology, and end results program: a national resource. Cancer Epidemiol Biomarkers Prev. 1999;8(12):1117‐1121. [PubMed] [Google Scholar]
- 18. Camp RL, Dolled‐Filhart M, Rimm DL. X‐tile: a new bio‐informatics tool for biomarker assessment and outcome‐based cut‐point optimization. Clin Cancer Res. 2004;10(21):7252‐7259. [DOI] [PubMed] [Google Scholar]
- 19. Verburg FA, Mader U, Tanase K, et al. Life expectancy is reduced in differentiated thyroid cancer patients ≥45 years old with extensive local tumor invasion, lateral lymph node, or distant metastases at diagnosis and normal in all other DTC patients. J Clin Endocrinol Metab. 2013;98(1):172‐180. [DOI] [PubMed] [Google Scholar]
- 20. Zhai Z, Zhang F, Zheng YI, et al. Effects of marital status on breast cancer survival by age, race, and hormone receptor status: a population‐based study. Cancer Med. 2019;8(10):4906‐4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gomez SL, Hurley S, Canchola AJ, et al. Effects of marital status and economic resources on survival after cancer: a population‐based study. Cancer. 2016;122(10):1618‐1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Costa LJ, Brill IK, Brown EE. Impact of marital status, insurance status, income, and race/ethnicity on the survival of younger patients diagnosed with multiple myeloma in the United States. Cancer. 2016;122(20):3183‐3190. [DOI] [PubMed] [Google Scholar]
- 23. Liao PH, Lee CC. The influence of marital status on survival for patients aged 65 years and younger with oral cavity cancer. Auris Nasus Larynx. 2018;45(6):1227‐1232. [DOI] [PubMed] [Google Scholar]
- 24. Shi R‐L, Qu N, Lu Z‐W, Liao T, Gao YI, Ji Q‐H. The impact of marital status at diagnosis on cancer survival in patients with differentiated thyroid cancer. Cancer Med. 2016;5(8):2145‐2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ernani V, Kumar M, Chen AY, Owonikoko TK. Systemic treatment and management approaches for medullary thyroid cancer. Cancer Treat Rev. 2016;50:89‐98. [DOI] [PubMed] [Google Scholar]
- 26. Bortolato B, Hyphantis TN, Valpione S, et al. Depression in cancer: the many biobehavioral pathways driving tumor progression. Cancer Treat Rev. 2017;52:58‐70. [DOI] [PubMed] [Google Scholar]
- 27. Zhu J, Fang F, Sjolander A, Fall K, Adami HO, Valdimarsdottir U. First‐onset mental disorders after cancer diagnosis and cancer‐specific mortality: a nationwide cohort study. Ann Oncol. 2017;28(8):1964‐1969. [DOI] [PubMed] [Google Scholar]
- 28. Lu D, Andersson TML, Fall K, et al. Clinical diagnosis of mental disorders immediately before and after cancer diagnosis. JAMA Oncology. 2016;2(9):1188. [DOI] [PubMed] [Google Scholar]
- 29. Goldzweig G, Andritsch E, Hubert A, et al. Psychological distress among male patients and male spouses: what do oncologists need to know? Ann Oncol. 2010;21(4):877‐883. [DOI] [PubMed] [Google Scholar]
- 30. Lutgendorf SK, Andersen BL. Biobehavioral approaches to cancer progression and survival: mechanisms and interventions. Am Psychol. 2015;70(2):186‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim‐Fuchs C, Le CP, Pimentel MA, et al. Chronic stress accelerates pancreatic cancer growth and invasion: a critical role for beta‐adrenergic signaling in the pancreatic microenvironment. Brain Behav Immun. 2014;40:40‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weinrib AZ, Sephton SE, DeGeest K, et al. Diurnal cortisol dysregulation, functional disability, and depression in women with ovarian cancer. Cancer. 2010;116(18):4410‐4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16(1):22‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860‐867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset of this study is available in the Surveillance, Epidemiology, and End Results program (www.seer.cancer.gov) SEER*Stat Database: Incidence–SEER 18 Regs Custom Data (with additional treatment fields), Nov 2018 Sub (1975–2016 varying).


