Abstract
Purpose
As a subgroup of lung cancer, small cell lung cancer (SCLC) is characterized by a short tumor doubling time, high rates of early occurred distant cancer spread, and poor outcomes. Despite its exquisite sensitivity to chemotherapy and radiotherapy, acquired drug resistance and tumor progression are typical. This study aimed to develop a robust signature based on immune‐related genes to predict the outcome of patients with SCLC.
Methods
The expression data of 77 SCLC patients from George's cohort were divided into training set and testing set, and 1534 immune‐related genes from ImmPort database were used to generate and validate the signature. Cox proportional hazards and the Kaplan–Meier analysis were used for developing and testing the prognostic signature. Single‐sample gene set enrichment analysis was used to determine immune cell infiltration phenotypes.
Results
A 10‐gene model comprising NR3C1, NR1D2, TANK, ARAF, HDGF, INHBE, LRSAM1, PLXNA1, PML, and SP1 with the highest frequency after 1000 interactions, was chosen to construct immune‐related signature. This signature showed robust predictive value for SCLC patients’ survival in both training and testing sets. This signature was weakly associated with the clinic pathological values like TNM stage. Furthermore, patients with low risk presented with activation of immune signal pathways, and specific immune cell infiltration with high levels of CD56bright NK cells but low levels of CD8+ T cells, mast cells, and helper T cells.
Conclusion
The present study developed immune‐related signature that may help predict the prognosis of SCLC patients, which reflects an unappreciated level of heterogeneity of immunophenotype associated with diverse prognosis for specific subsets in this highly lethal cancer type.
Keywords: immune cell infiltration, immune‐related gene, prognosis, signature, small cell lung cancer, tumor mutational burden
Here, we constructed a prognostic immune‐related signature for predicting small cell lung cancer (SCLC) patients’ survival. The 10‐gene signature was enriched in growth factor activity and immune‐related TGF‐β signaling pathway. Furthermore, increased CD56dim NK cells and reduced plasmacytoid dendritic cells infiltration were significantly associated with survival prolongment. However, SCLC patients with low risk presents more CD56bright NK cells but less CD8+ T cells, mast cells, and helper T cells infiltration compared to those with high risk. Our findings indicate that this immune‐related signature may help predict the prognosis of SCLC patients, which reflects an unappreciated level of heterogeneity of immunophenotype associated with diverse prognosis for specific subsets in this highly lethal cancer type.

1. INTRODUCTION
Lung cancer, the most common cancer in male and female worldwide, accounts for approximately 19% of all cancer deaths. 1 In general, a majority of lung cancers are non‐small cell lung cancer (NSCLC), whereas 13%–15% is small cell lung cancer (SCLC). SCLC is an aggressive undifferentiated neuroendocrine tumor and clinically characterized by its high grade, rapid growth, and early spread of cancer cells. Thus, approximately 70% of SCLC patients are classified as having extensive disease, which leads to the extremely poor prognosis. 2 Although first‐line chemotherapy with etoposide plus either cisplatin or carboplatin produces a high response rate of up to 70%, SCLC patients fail to have an opportunity to receive molecular‐targeted therapy targeting specific driver genes. Furthermore, most patients relapse within 6 months of the completion of initial treatment due to acquired drug resistance, subsequent effective treatment options are still limited. 3 SCLC has been reported to have high tumor mutation burden and high neoantigens formation which are associated with increased sensitivity to immunotherapy with immune checkpoint inhibitors (ICIs). 4 Actually, ICIs targeting the programmed cell death 1 and programmed cell death‐ligand 1 (PD‐L1) pathway, such as nivolumab, pembrolizumab, atezolizumab, and durvalumab monotherapy or in combination with chemotherapy have been shown to prolong the survival of patients with SCLC with manageable toxicity profile. 3 However, the application of ICIs in SCLC appears to be less effective when compared to NSCLC, and only a minority of SCLC patients can benefit from immune checkpoint blockade. 4 In particular, low expression levels of major histocompatibility complex and PD‐L1 on tumor cells, less immune cells infiltration, and high ratio of suppressive immune cells all have compromised the efficacy of ICIs.
The importance of tumor immune microenvironment in SCLC has been demonstrated using antigen vaccines and dendritic cell vaccines treatment. 5 , 6 However, there is a lack of feasible cytogenetic signatures associated with immune microenvironment to predict SCLC patients' prognosis. 2 , 3 Therefore, it is essential to define immune‐related biomarkers as a predictor for SCLC patients' survival from the perspective of tumor immunity, which could help clinician identify a subgroup with a favorable outcome and might benefit from immunotherapy with ICIs. In this study, transcriptome data were utilized to create an immune‐related signature comprising 10 genes for SCLC prognostication.
2. MATERIALS AND METHODS
2.1. Construction of the immune‐related risk signature
Here we constructed a prognostic signature by focusing on immune‐related genes, which were downloaded from the ImmPort database (https://immport.niaid.nih.gov). ImmPort database is one of the largest open repositories of human immunological data. 7 We downloaded a list of 2,498 immune‐related genes from ImmPort database (Table S1). A variety of immune‐related genes were included, such as cytokine genes, cytokine receptor genes, and genes associated with the T‐cell receptor signaling pathway, B‐cell antigen receptor signaling pathway, natural killer cell cytotoxicity, antigen processing, and presentation pathways. All patients from George's cohort 7 were obtained, and 77 samples with OS information were randomly divided into a training set (n = 54) for identifying key immune‐related genes and a testing set (n = 23) for validating the immune‐related genes signature. The clinical and survival information of the 77 samples are summarized in Table 1. Univariate analysis was performed to identify prognostic immune‐related risk signature, and p < 0.05 indicates a significant correlation between immune‐related genes and prognosis. In order to identify the best gene model for predicting the outcome in SCLC patients, the Cox proportional hazards model with an elastic net penalty (iteration = 1000) was performed with R3.4.4 package “glmnet.” The penalty parameter was evaluated by 10‐fold cross‐validation with the training dataset. Based on a linear combination of Cox coefficient and gene expression, genes weighted value was yielded for further analysis.
TABLE 1.
Clinical characteristics of the total datasets
| Feature | Sample number | Ratio (%) |
|---|---|---|
| Age | ||
| ≤60 years | 20 | 26.0 |
| >60 years | 57 | 74.0 |
| Gender | ||
| Male | 54 | 70.1 |
| Female | 23 | 29.9 |
| AJCC stage | ||
| Stage I | 33 | 42.9 |
| Stage II | 14 | 18.2 |
| Stage III | 21 | 27.3 |
| Stage IV | 9 | 11.7 |
2.2. Performance assessment
The predictive efficiency of the immune‐related risk signature was assessed using Harrell's concordance index (C‐index) and time‐dependent receiver operating characteristic (ROC) analysis. The area under curve (AUC) was calculated using the “survival ROC” package in R3.4.4. In order to estimate survival differences of patients between high‐ and low‐risk groups, the Kaplan–Meier (K–M) survival curves were generated using the “survminer” package in R. Besides, principal component analysis (PCA) was performed to assess gene expression patterns.
2.3. Gene enrichment analysis
In order to explore the biological processes of differentially expressed genes (DEGs), Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses were performed with the Database for Annotation, Visualization, and Integrated Discovery (https://david.ncifcrf.gov/) with the cut‐off criterion of false discovery rate < 0.01. p < 0.05 was considered statistically significant. The 26 immune cell types enrichment score was calculated using single‐sample gene set enrichment analysis (ssGSEA) method implemented by R package Gene Set Variation Analysis (GSVA), to measure the level of immune cell infiltration. 8 , 9
2.4. Statistical analysis
Heatmaps were produced using R pheatmap package. Clustering of the heatmaps was performed by the standard R hclust (hierarchical clustering) method, using the “ward.D2” option. Multivariable cox analysis was performed with cox proportional hazard regression using R3.4.4 survival package for three datasets: (1) risk score, age, gender, and pathological stage; (2) proportion of eight immune cells infiltrated; and (3) 26 immune cells enrichment score. We obtained the gene set corresponding to the 26 immune cells mentioned in previous research and used the default parameters of the ssGSEA algorithm for immune cell infiltration analysis. 10 The boxplots were conducted using the R package called ggpubr. Differences among two and three groups were determined by the Wilcoxon rank‐sum test and the Kruskal–Wallis test, respectively. p < 0.05 was considered statistically significant.
3. RESULTS
3.1. Construction and validation of the immune‐related risk signature
All 77 samples were randomly divided into a training set (n = 54) (54/77, 70% for identifying key genes) and a testing set (n = 23) (23/77, 30% for validating). Using univariate Cox analysis, the correlation between gene expression and patient's overall survival (OS) was calculated and 77 genes with prognostic ability were obtained (p < 0.05). In order to develop the best gene model to predict the prognosis of SCLC patients, the Cox proportional hazards model with an elastic net penalty was performed. After 1000 iterations, 14 model feature gene sets were obtained, and one of which contained 10 feature genes was highly stable and reaches the frequency of 430 times, accounting for 43% in 1000 iterations (Figure 1). This 10‐gene model include ARAF, HDGF, INHBE, LRSAM1, NR1D2, NR3C1, PLXNA1, PML, SP1, and TANK and respective coefficients are listed in Table 2. Using the risk scoring formula as follows, the risk score for each SCLC patient was calculated based on expression level and coefficient of 10 characteristic genes.
FIGURE 1.
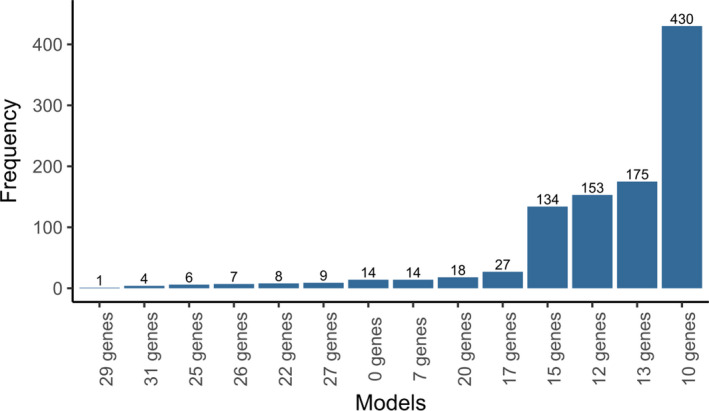
Frequency of each model in 1000 iterations. Generation of 14 model feature gene sets after 1000 iterations. One gene model contained 10 feature genes was highly stable and reaches the frequency of 430 times compared with other 13 gene models
TABLE 2.
The best gene set and coefficient related to prognosis
| Gene | Coef |
|---|---|
| ARAF | −0.0066322 |
| HDGF | −0.0015719 |
| INHBE | −0.0021426 |
| LRSAM1 | −0.0152107 |
| NR1D2 | 0.00920882 |
| NR3C1 | 0.0185948 |
| PLXNA1 | −0.0009105 |
| PML | −0.0081578 |
| SP1 | −0.0023929 |
| TANK | 0.00671622 |
Time‐dependent ROC and C‐index were applied to evaluate the prognostic values of the 10‐gene signature in terms of OS. The ROC curve analysis of 10‐gene signature in the training set has exhibited the favorable predictive value for survival of SCLC patients, and AUC was 0.83 at 1 year, 0.801 at 3 year, and 0.783 at 5 year (Figure 2A). Then, 10‐gene signature was validated in the testing set, and the 1‐, 3‐, and 5‐year AUC were 0.713, 0.701, and 0.719, respectively (Figure 2B). As for all cohorts, 10‐gene signature also achieved an accuracy to predict patient's OS, and the AUC value for 1‐, 3‐ and 5‐year was 0.806, 0.8, and 0.732, respectively (Figure 2C). Besides, the C‐index for the training, testing, and total data set was all above 0.75 (Figure 2D), indicating a superior prognostic value of constructed model.
FIGURE 2.
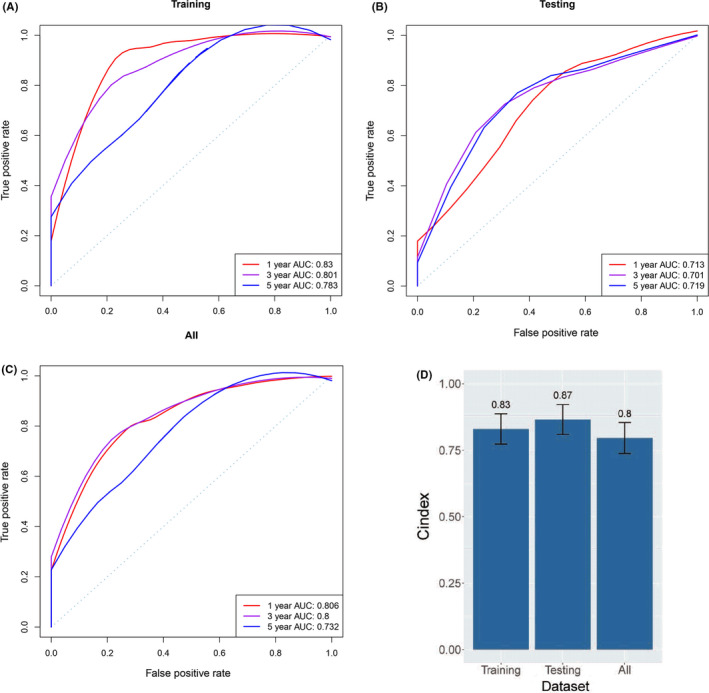
Model performance evaluation. Receiver operator characteristic analysis was performed to compare our 10‐gene signature in predicting 1‐, 3‐, and 5‐year overall survival in training (A), testing (B), and all data cohorts (C). Harrell's concordance index (C‐index) for the training, testing, and total data set was 0.83, 0.87, and 0.8, respectively (D). AUC, area under curve
3.2. Association between 10 immune‐related risk signature and SCLC patients' survival
The median value of the risk score is taken as the threshold to divide the high‐risk and low‐risk populations. PCA of the training, testing, and total SCLC cohort demonstrated a different distribution pattern of high risk and low risk based on 10 immune‐related gene expression, indicating their difference in immune phenotype (Figure 3), the training set was clustered and heatmap was created (Figure 4), and the NR3C1, NR1D2, and TANK gene expression levels were higher in high‐risk population, while ARAF, HDGF, INHBE, LRSAM1, PLXNA1, PML, and SP1 gene expression levels were higher in low‐risk population (Figure 4).
FIGURE 3.
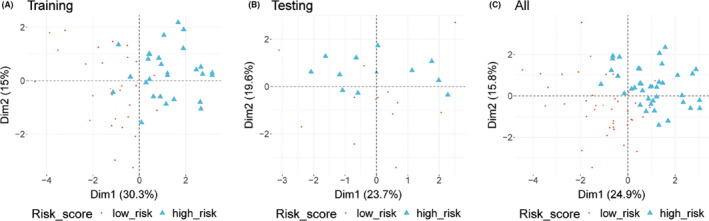
Principal component analysis analysis. Distribution patterns for high‐ and low‐risk population based on 10 genes. Most of the high‐risk population are well separated from the low‐risk population, displaying a different distribution pattern of high‐risk and low‐risk population. (A) Training data set. (B) Testing data set. (C) All data set
FIGURE 4.
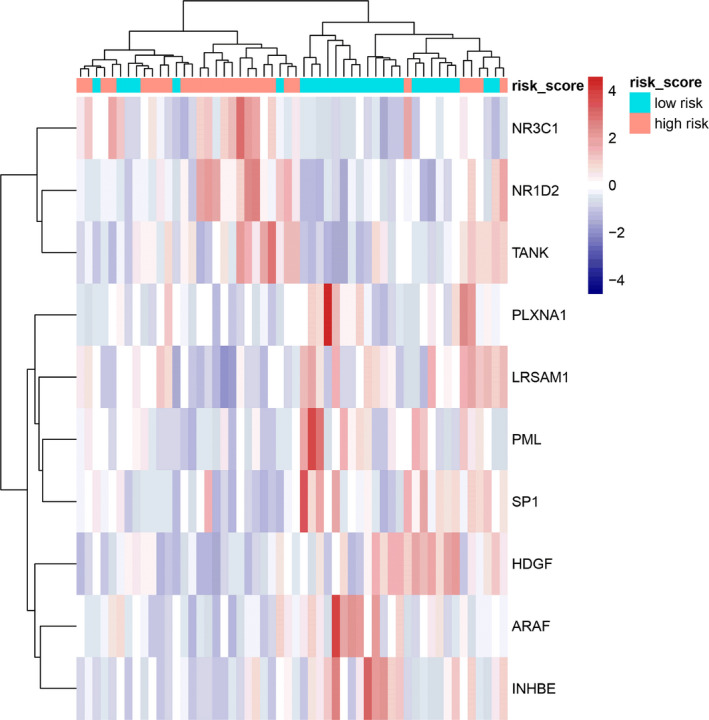
Cluster analysis of 10 characteristic genes expression in training data set. Ten genes with higher expression in the heatmap are shown in red color, and with lower expression are shown in blue. Tiffany blue represents cancer tissue from the low‐risk population, while the pink represents cancer tissue from the high‐risk population
In order to calculate the association between immune‐related risk signature and SCLC patients' survival outcome, the K–M survival analysis was performed in three data sets. In the training sets, SCLC patients from the low‐risk group had significantly better OS than patients from the high‐risk group (HR = 3.87, 95% CI: 1.79–8.36, p = 0.00027) (Figure 5A). The same trends were also observed in the validation sets (HR = 3.71, 95% CI: 1.25–11.05, p = 0.012) (Figure 5B) and total data sets (HR = 4.39, 95% CI: 2.33–8.24, p < 0.0001) (Figure 5C). Hazard ratio analysis showed risk score was a poor prognostic factor of the risk of survival in SCLC patients with a HR of 367.34 in training set (95% CI: 39–3460, p < 0.001), and 155.40 in testing set (95% CI: 1.91–13000, p = 0.025). (Figure 5D–F). In addition, in the validation and total data set, the gender of SCLC patients was a favorable prognostic factor of the risk of survival, and the risk of survival was significantly lower in female SCLC patients (HR = 0.078, 95% CI: 0.0085–0.71, p = 0.024; HR = 0.32, 95% CI: 0.14–0.70, p = 0.004) (Figure 5D–F). In the total data set, pathological stage is a poor prognostic factor of the risk of survival in SCLC patients (HR = 1.42, 95% CI: 1.07–1.9, p = 0.014). Of note, there was no significant association between the age and survival risk of SCLC patients in all three data sets (Figure 5D–F).
FIGURE 5.
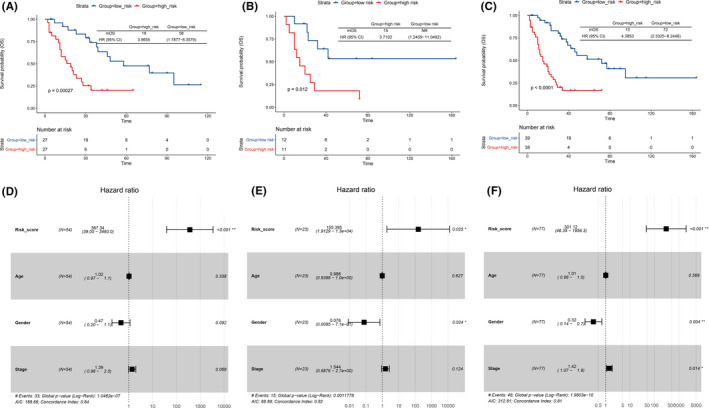
K–M survival and hazard ratio analysis. The Kaplan–Meier curves of overall survival (OS) for SCLC patients with high risk and low risk in training set (A), testing set (B), and all data set (C). Hazard ratios (HRs) and 95% CIs are for high‐risk group versus low‐risk population. p values were calculated with the log‐rank test. Gender (p = 0.024) in testing set (E) and gender (p = 0.004) and stage (p = 0.014) in all data set (p = 0.004) (F) were significantly related to the prognosis by Cox regression analysis
3.3. Enrichment of GO and KEGG pathway by immune‐related risk signature
To elucidate the molecular mechanism of the 10 immune‐related risk gene signature, GO and KEGG pathway enrichment analyses were applied to explore the functions of the 10 genes. Fifteen Go terms were significantly enriched in GO enrichment and four pathways were enriched in KEGG pathway enrichment analyses. KEGG pathway enrichment analyses revealed that the DEGs participated in acute myeloid leukemia (p = 0.04), TGF‐β signaling pathway (p = 0.04), endocrine resistance (p = 0.04), and parathyroid hormone synthesis, secretion, and action (p = 0.04) (Table 3). After GO enrichment analyses, the 10 genes were significantly enriched in biological processes including small ubiquitin‐like modifier binding, core promoter binding, transcription factor activity, RNA polymerase II transcription factor binding, steroid hormone receptor activity, ubiquitin‐like protein binding, core promoter sequence‐specific DNA binding, growth factor activity, and ubiquitin protein ligase binding (Table 4).
TABLE 3.
Pathways with significant enrichment of characteristic genes
| ID | Description | q value |
|---|---|---|
| hsa05221 | Acute myeloid leukemia | 0.04 |
| hsa04350 | TGF‐beta signaling pathway | 0.04 |
| hsa01522 | Endocrine resistance | 0.04 |
| hsa04928 | Parathyroid hormone synthesis, secretion, and action | 0.04 |
TABLE 4.
Go term with significantly enriched characteristic genes
| ID | Description | q value | Gene ID |
|---|---|---|---|
| GO:0032183 | SUMO binding | 8.65E–04 | NR3C1/PML |
| GO:0001047 | Core promoter binding | 9.74E–04 | NR1D2/NR3C1/SP1 |
| GO:0001076 | Transcription factor activity, RNA polymerase II transcription factor binding | 1.57E–03 | HDGF/NR1D2/NR3C1 |
| GO:0003707 | Steroid hormone receptor activity | 4.01E–03 | NR1D2/NR3C1 |
| GO:0032182 | Ubiquitin‐like protein binding | 5.80E–03 | NR3C1/PML |
| GO:0001046 | Core promoter sequence‐specific DNA binding | 5.80E–03 | NR1D2/SP1 |
| GO:0000982 | Transcription factor activity, RNA polymerase II proximal promoter sequence‐specific DNA binding | 6.49E–03 | NR1D2/NR3C1/SP1 |
| GO:0008083 | Growth factor activity | 1.33E–02 | HDGF/INHBE |
| GO:0001077 | Transcriptional activator activity, RNA polymerase II proximal promoter sequence‐specific DNA binding | 2.31E–02 | NR3C1/SP1 |
| GO:0031625 | Ubiquitin protein ligase binding | 2.43E–02 | PML/TANK |
| GO:0044389 | Ubiquitin‐like protein ligase binding | 2.50E–02 | PML/TANK |
| GO:0001228 | Transcriptional activator activity, RNA polymerase II transcription regulatory region sequence‐specific DNA binding | 2.70E–02 | NR3C1/SP1 |
| GO:0000978 | RNA polymerase II proximal promoter sequence‐specific DNA binding | 2.70E–02 | NR3C1/SP1 |
| GO:0000987 | Proximal promoter sequence‐specific DNA binding | 2.70E–02 | NR3C1/SP1 |
| GO:0048018 | Receptor ligand activity | 2.70E–02 | HDGF/INHBE |
3.4. Correlation of the immune‐related risk signature with clinicopathologic features
The relationship between 10‐gene signature and tumor staging, age, and gender was analyzed. We have observed that SCLC patients who were above 60 years old (Figure 6A) and male SCLC patients (Figure 6B) tend to have higher risk. In our case, the numbers of stages I, II, III, and IV patients in dataset were 33, 14, 21, and 9, respectively. We have found that the mean of risk score in advanced SCLC was higher than early stage SCLC, but the difference was not significant (Figure 6C).
FIGURE 6.
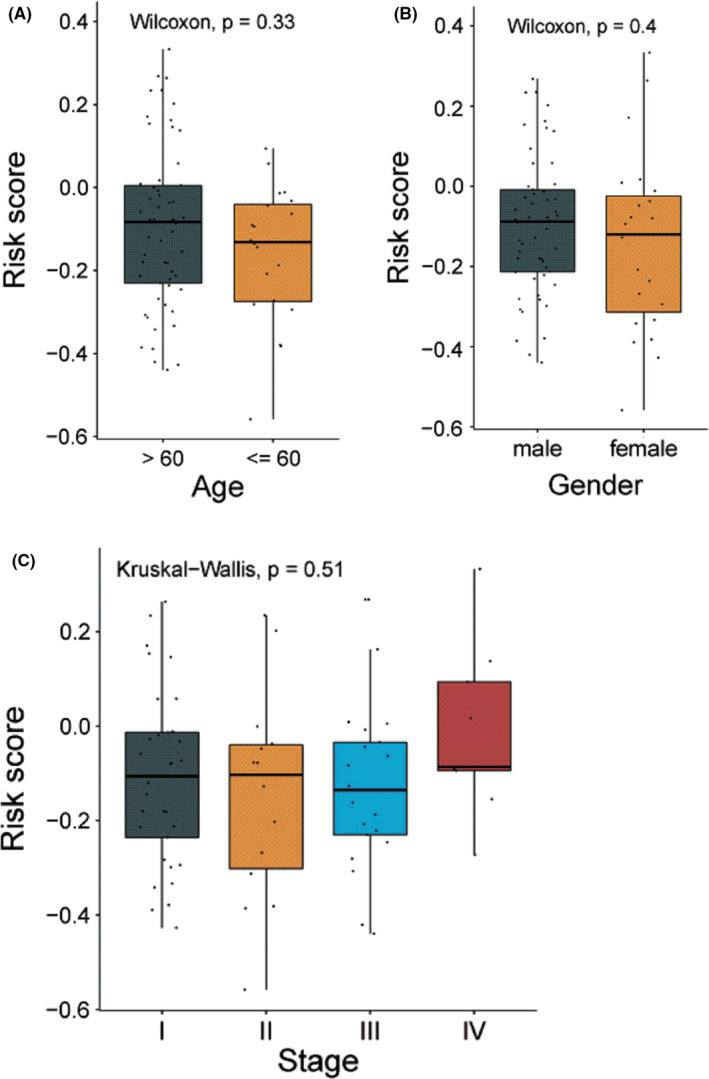
Difference test of risk score between different pathological stages. (A) Comparison of risk score between small cell lung cancer (SCLC) patients who were above 60 years old and those at or below 60 years old. (B) Comparison of risk score between male and female SCLC patients. (C) Comparison of risk score between SCLC patients at different pathological stages
3.5. Tumor immunity relevance of immune‐related risk signature
The abundance of 26 immune cells in the total data set was calculated by ssGSEA method. The relationship between the abundance of immune cells in tumor immune microenvironment and overall survival (OS) was analyzed by multivariate Cox analysis. We have observed that abundance of specific immune cells was associated with OS of SCLC patients. The abundance of CD56dim NK cells is a favorable prognostic factor for survival of SCLC patients (p = 0.035), while the abundance of the plasmacytoid dendritic cells (pDC) is a poor prognostic factor for survival of SCLC patients (p = 0.044) (Figure 7). There was no significant association between other immune cell subsets including CD8+ T cells, macrophages, or T cells and increased patients' survival.
FIGURE 7.
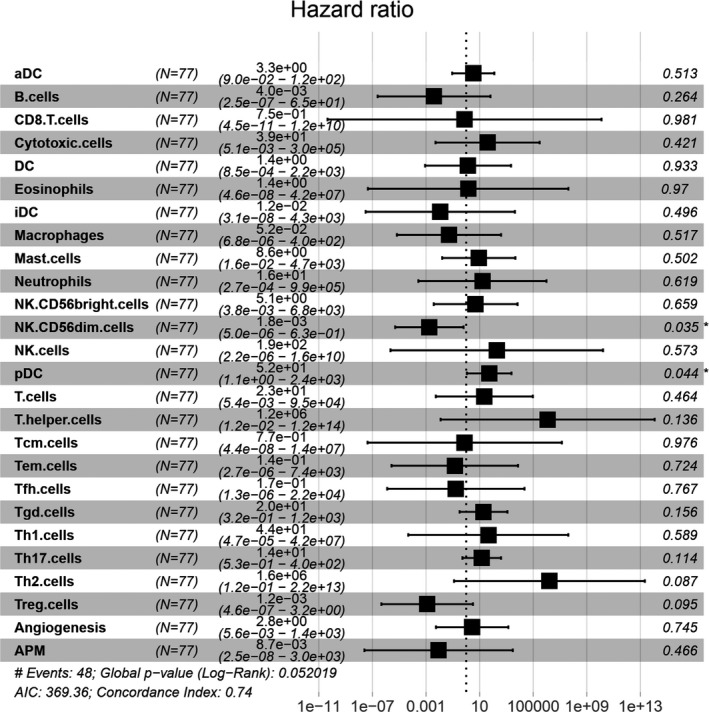
Hazard ratio analysis of score values of immune cells. The relationship between abundance of immune cells and overall survival was investigated. NK56dim cells (p = 0.035) and pDC (p = 0.044) were significantly related to the prognosis in multivariate Cox regression model. APM, antigen‐presenting machinery; DC, dendritic cell; HR, hazard ratios; NK, natural killing cell; Tcm, central memory T cells; Tem, effector memory T cells; Tfh, follicular helper T cells; Treg, regulatory T cells
In order to interpret survival difference between high‐ and low‐risk population from the perspective of tumor immunity, the immune cell infiltration profile in patients with high and low risk was analyzed. We failed to observe a significant difference regarding CD56dim NK cells and pDC infiltration between low‐risk and high‐risk groups, indicating that immune‐related risk signature and immune microenvironment have independent effects on prognosis (Figure 8A,B). In addition, patients with high risk had more CD8+ T cells, helper T cells, mast cells, and follicular helper T (Tfh) cells but less Treg cells compared to those with low risk. Interestingly, patients with low risk had more CD56bright cell infiltration than patients with high risk (Figure 8C–H).
FIGURE 8.
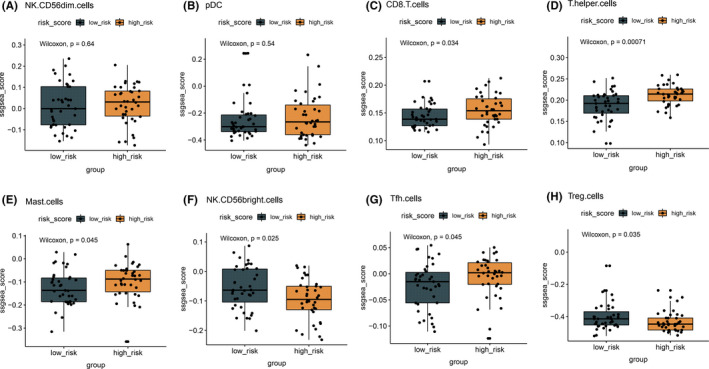
Difference in immune cell score in patients with high‐ and low‐risk score. The abundance of different immune cell infiltration status between high‐ and low‐risk populations is analyzed, and results are shown in box plots. p < 0.05 was considered statistically significant
4. DISCUSSION
Lung cancer is the leading cause of cancer‐related death worldwide. Based on the histological differences, lung cancer is broadly classified into two subtypes: SCLC and NSCLC. SCLC comprises about 15% of all lung cancer cases. 11 Given that SCLC is an incurable cancer type, it is essential to develop immune‐related biomarkers to identify patients who have a good prognosis and might benefit more from immunotherapy. 12 Here, we constructed a prognostic immune‐related signature for predicting SCLC patients' survival. The 10‐gene prognostic immune‐related signature was enriched in growth factor activity and immune‐related TGF‐β signaling pathway. Furthermore, increased CD56dim NK cells and reduced pDC infiltration were significantly associated with survival prolongment. However, according to the present prognostic immune‐related signature, SCLC patients with low risk presents more CD56bright NK cells but less CD8+ T cells, mast cells, and helper T cells infiltration compared to those with high risk. Our findings indicate that the present study developed immune‐related signature that may help predict the prognosis of SCLC patients, and SCLC has an unappreciated level of heterogeneity of SCLC immunophenotype that determines the diverse prognosis for specific subsets.
The immune‐related signature consisted of 10 immune‐related genes with prognostic ability. Three (NR3C1, Sp1, and PML) of the genes in the 10‐gene signature were previously reported to be associated with SCLC. NR3C1 (nuclear receptor subfamily three group C member 1) gene encodes glucocorticoid receptor (GR). GR displays anti‐inflammatory effects through transcriptional activation of glucocorticoid‐induced leucine zipper genes 13 or transrepression via interferences with the activity of many other immune‐related transcription factors, including nuclear factor‐κB, nuclear factor of activated T cells, activator protein 1, interferon regulatory factor 3, cyclic‐AMP response binding protein, T‐box transcription factor 21, GATA binding protein 3, 14 and higher NR3C1 expression in high‐risk group patients who have worse OS might compromise pro‐inflammatory and antitumor immune response in vivo. Sp1 gene encoded Sp1 protein which is a well‐known zinc finger transcription factor. 15 Zhu et al. have reported Sp1 directly regulate decoy receptor 3 (DcR3) expression in hepatocellular carcinoma which promotes Th2 and Treg cell differentiation but inhibits Th1 cell differentiation. 16 DcR3 expression was also significantly higher in SCLC cancer tissues compared to normal lung tissue, 17 thus inhibition of DcR3 expression by interfering with upstream Sp1 pathway may provide a novel immunotherapeutic target to restore antitumor immune response in low‐risk group SCLC patients. PML (promyelocytic leukemia) gene was originally identified in acute PML. 18 PML and the PML nuclear domain have been regarded as a tumor‐suppressive role in several different types of cancer. 19 Zhang et al. have found decreased PML protein expression in SCLC. Furthermore, there is evidence that PML was involved in regulation of innate immune response through affecting interferon and targeting cytokines secretion, such as pro‐inflammatory cytokines IL‐1β and IL‐6, 20 , 21 thus OS difference between high‐ and low‐risk patients might be partly ascribed to the regulatory role of PML on innate immune signaling in these groups. Besides, the roles of seven genes (NR1D2, TANK, LRSAM1, PLXNA1, INHBE, HDGF, and ARAF) in SCLC have not been reported, however those genes have been reported to play a vital role in other type cancer. 22
Furthermore, we attempted to investigate the potential molecular background of the prognostic immune‐related signature. Go and KEGG pathways were further analyzed and proved the robust connection of the signature with growth factor activity and immune‐regulatory TGF‐β signaling pathway. Unlike NSCLC, SCLC had different expression levels of TGF‐β and its receptors. Autocrine and paracrine growth inhibition by TGF‐β has been found in SCLC because of the inhibitory synthesis of TGF‐β isoforms and TGF‐β II. 23 In addition, SCLC cell lines suppressed IL‐2‐dependent T cell growth via secreting active TGF‐β1. 24 A specific anti‐TGF‐β1 antibody or a recently developed novel bifunctional anti‐PD‐L1/TGF‐β checkpoint inhibitor, the fusion protein M7824, decreased tumor burden and increased survival in mice through promoting CD8+ T cell and NK cell activation and blocking the immunosuppressive activity induced by the SCLC cells. 24 , 25 Therefore, blockade of TGF‐β pathway represents a novel therapeutic strategy for SCLC in terms of combination immunotherapy.
CD56dim NK cells possess a strong cytolytic capacity, but with low levels of cytokines production. 26 , 27 Picard et al. have found that lower rate of the cytotoxic CD56dim CD16+ NK cells was observed in NSCLC patients compared with healthy control, indicating CD56dim NK cells play an important role in cancer immunosurveillance. 28 NanoString transcriptomic analysis of melanomas revealed that there was a trend of increased CD56dim NK cell gene signature expression associated with better clinical outcome. 29 In the sophisticated, genetically engineered mouse models, Best et al. found that the lack of NK cells, but not CD8+ T cells, substantially promote metastatic dissemination of SCLC tumor cells in vivo, 30 indicating that NK cells play a vital role in the prognosis of SCLC patients. In our study, in total population we observed that the abundance of CD56dim but not CD56bright NK cells is positively associated with the increased survival of SCLC patients, and the abundance of pDC is inversely associated with the increased survival. Therefore, CD56 dim and CD56 bright NK cells might differentially affect the prognosis of SCLC patients. It has been reported that CD56bright NK cells inversely correlate with the survival of melanoma patients, also IFN‐γ production from CD56bright NK cells correlated inversely with the OS of patients, 31 however, the comprehensive role of the subpopulation of NK cells in SCLC has not yet been clarified.
It has been widely observed that tumor‐associated pDCs are associated with an increase in Tregs and the decrease in OS in gliomas, 32 ovarian, 33 and breast cancer, 34 and lung cancer. Sorrentino et al. have found that depletion of pDCs with a specific antibody (m927) in a mouse model of Lewis lung carcinoma cell‐induced lung cancer reversed the immune‐suppressive microenvironment, including decreased tumor burden, activation of mDC and CD8+ T cells, and Th1‐ and Th17‐like cytokine production. 35 Additionally, Munn et al. have found that a subset of pDCs in mouse tumor‐draining lymph nodes that constitutively expressed immunosuppressive indoleamine 2,3‐dioxygenase suppressed T‐cell responses and induced T anergy. 36 Thus, these results indicate an unappreciated level of heterogeneity of SCLC immunophenotype associated with diverse clinical outcome.
We also analyzed the immune cell infiltration profile for both low‐risk and high‐risk patients. We failed to observe a significant difference in CD56dim NK cells and pDC subset between low‐risk and high‐risk groups. However, patients with high risk had more CD8+ T cells, helper T cells, mast cells, and Tfh cells but less Treg cells compared to those with low risk. Although some studies showed that tumor‐associated CD45‐positive cells, 37 , 38 tumor‐infiltrating lymphocytes, 37 and CD8+ T cells 39 in SCLC specimens were a good clinical marker to identify patients with favorable prognosis, but there was no significant association between CD45‐positive cell counts and advanced disease stage. 40 In addition, high‐risk patients had a high level of mast cells that have been found to relate to unfavorable survival. 41 Interestingly, our results demonstrated that patients with low risk had more CD56bright cell subset that were responsible for large amounts of pro‐inflammatory cytokines production but not cytotoxic ability than patients with high risk. Thus, at least the present cohort reflected a different immunological microenvironment in SCLC patients with diverse prognosis.
Taken together, unlike NSCLC and other solid tumors, the immune microenvironment of SCLC is characterized as few tumor‐infiltrating lymphocytes and low PD‐L1 expression. Nevertheless, immunotherapy with immune‐checkpoint inhibitors still holds promise for SCLC patients independent of PD‐L1 expression status. 42 Therefore, it is essential to characterize SCLC patients who have a poor prognosis or benefits from immune checkpoint blockade, and the future research focusing on the identification of predictive biomarkers of prognosis and efficacy of immunotherapy and the characteristics of the SCLC immune microenvironment is urgently needed. 42 In this study, we constructed the 10‐gene signature which successfully predict patients’ prognosis and validated its accuracy in SCLC.
Our research has certain limitations. First, this study was based on bioinformatics analyses from one public database with a limited number of patients, which indeed weaken the strength of our findings. Second, of 10 immune‐related genes in our study, the role of three genes in SCLC has been investigated; however, the roles of other seven genes in SCLC have not been identified. Third, it is hard to validate the predictive value of our model in immunotherapy for SCLC patients as a lack of treatment‐related information. In future, the expression and function of immune‐related genes in SCLC tumor cells or infiltrated immune cells within tumor should be elucidated. The predictive value of this immune‐related signature should be further validated using different or real‐word SCLC cohorts with larger patient size, especially with detailed information on immunotherapy for SCLC patients. Flow cytometry and real‐time quantitative PCR as alternative tools should be used to verify our findings.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONFLICT OF INTEREST
Author Jian Yi, Hui Yu, and Tiantian Gu were employed by the company Yuce Biotechnology Co., Ltd. (Shenzhen, China). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (grant no. 81572875), the CSCO‐MSD Cancer Research Foundation (grant no. Y‐MSD2020‐0350), the CSCO‐PILOT Cancer Research Foundation (grant no. Y‐2019AZMS‐0440), the Wu Jieping Medical Foundation for Clinical Scientific Research (grant no. 320.6750.2020‐12‐16), the Major Science and Technology Innovation Project of Shandong Province (grant no. 2018CXGC1220), the Natural Science Foundation of Shandong Province (grant no. ZR202102190539), the Traditional Chinese Medicine Technology Development Plan of Shandong Province (grant no. 2019‐0377), and the Shandong Provincial Qianfoshan Hospital Grant (grant no. QYPY2020NSFC1015).
Xie Q, Chu H, Yi J, et al. Identification of a prognostic immune‐related signature for small cell lung cancer. Cancer Med. 2021;10:9115–9128. doi: 10.1002/cam4.4402
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87‐108. [DOI] [PubMed] [Google Scholar]
- 2. Taniguchi H, Sen T, Rudin CM. Targeted therapies and biomarkers in small cell lung cancer. Front Oncol. 2020;10:741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Calles A, Aguado G, Sandoval C, Alvarez R. The role of immunotherapy in small cell lung cancer. Clin Transl Oncol. 2019;21(8):961‐976. [DOI] [PubMed] [Google Scholar]
- 4. Hamilton G, Rath B. Immunotherapy for small cell lung cancer: mechanisms of resistance. Expert Opin Biol Ther. 2019;19(5):423‐432. [DOI] [PubMed] [Google Scholar]
- 5. Krug LM, Ragupathi G, Hood C, et al. Immunization with N‐propionyl polysialic acid‐KLH conjugate in patients with small cell lung cancer is safe and induces IgM antibodies reactive with SCLC cells and bactericidal against group B meningococci. Cancer Immunol Immunother. 2012;61(1):9‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Antonia SJ, Mirza N, Fricke I, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res. 2006;12(3 Pt 1):878‐887. [DOI] [PubMed] [Google Scholar]
- 7. George J, Lim JS, Jang SJ, et al. Comprehensive genomic profiles of small cell lung cancer. Nature. 2015;524(7563):47‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hanzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA‐seq data. BMC Bioinform. 2013;14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer‐cell subsets. Trends Immunol. 2001;22(11):633‐640. [DOI] [PubMed] [Google Scholar]
- 10. Zhang X‐C, Wang J, Shao G‐G, et al. Comprehensive genomic and immunological characterization of Chinese non‐small cell lung cancer patients. Nat Commun. 2019;10(1):1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oser MG, Niederst MJ, Sequist LV, Engelman JA. Transformation from non‐small‐cell lung cancer to small‐cell lung cancer: molecular drivers and cells of origin. Lancet Oncol. 2015;16(4):e165‐e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gadgeel SM. Targeted therapy and immune therapy for small cell lung cancer. Curr Treat Options Oncol. 2018;19(11):53. [DOI] [PubMed] [Google Scholar]
- 13. Hoppstädter J, Hachenthal N, Valbuena‐Perez JV, et al. Induction of Glucocorticoid‐induced Leucine Zipper (GILZ) contributes to anti‐inflammatory effects of the natural product curcumin in macrophages. J Biol Chem. 2016;291(44):22949‐22960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Bosscher K, Haegeman G, Elewaut D. Targeting inflammation using selective glucocorticoid receptor modulators. Curr Opin Pharmacol. 2010;10(4):497‐504. [DOI] [PubMed] [Google Scholar]
- 15. Vizcaino C, Mansilla S, Portugal J. Sp1 transcription factor: a long‐standing target in cancer chemotherapy. Pharmacol Ther. 2015;152:111‐124. [DOI] [PubMed] [Google Scholar]
- 16. Zhu HF, Liu YP, Liu DL, et al. Role of TGFbeta3‐Smads‐Sp1 axis in DcR3‐mediated immune escape of hepatocellular carcinoma. Oncogenesis. 2019;8(8):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang Y, Luo J, He R, et al. Expression and clinicopathological implication of DcR3 in lung cancer tissues: a tissue microarray study with 365 cases. OncoTargets Ther. 2016;9:4959‐4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salomoni P, Bellodi C. New insights into the cytoplasmic function of PML. Histol Histopathol. 2007;22(8):937‐946. [DOI] [PubMed] [Google Scholar]
- 19. Salomoni P, Ferguson BJ, Wyllie AH, Rich T. New insights into the role of PML in tumour suppression. Cell Res. 2008;18(6):622‐640. [DOI] [PubMed] [Google Scholar]
- 20. Scherer M, Stamminger T. Emerging role of PML nuclear bodies in innate immune signaling. J Virol. 2016;90(13):5850‐5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lunardi A, Gaboli M, Giorgio M, et al. A role for PML in innate immunity. Genes Cancer. 2011;2(1):10‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yu M, Li W, Wang Q, Wang Y, Lu F. Circadian regulator NR1D2 regulates glioblastoma cell proliferation and motility. Oncogene. 2018;37(35):4838‐4853. [DOI] [PubMed] [Google Scholar]
- 23. Lopez‐Gonzalez JS, Aguilar‐Cazares D, Prado‐Garcia H, et al. Lack of correlation between growth inhibition by TGF‐beta and the percentage of cells expressing type II TGF‐beta receptor in human non‐small cell lung carcinoma cell lines. Lung Cancer. 2002;38(2):149‐158. [DOI] [PubMed] [Google Scholar]
- 24. Fischer JR, Darjes H, Lahm H, Schindel M, Drings P, Krammer PH. Constitutive secretion of bioactive transforming growth factor beta 1 by small cell lung cancer cell lines. Eur J Cancer. 1994;30A(14):2125‐2129. [DOI] [PubMed] [Google Scholar]
- 25. Knudson KM, Hicks KC, Luo X, Chen JQ, Schlom J, Gameiro SR. M7824, a novel bifunctional anti‐PD‐L1/TGFbeta Trap fusion protein, promotes anti‐tumor efficacy as monotherapy and in combination with vaccine. Oncoimmunology. 2018;7(5):e1426519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Forconi CS, Oduor CI, Oluoch PO, et al. A new hope for CD56(neg)CD16(pos) NK cells as unconventional cytotoxic mediators: an adaptation to chronic diseases. Front Cell Infect Microbiol. 2020;10:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aktas ON, Ozturk AB, Erman B, Erus S, Tanju S, Dilege S. Role of natural killer cells in lung cancer. J Cancer Res Clin Oncol. 2018;144(6):997‐1003. [DOI] [PubMed] [Google Scholar]
- 28. Picard E, Godet Y, Laheurte C, et al. Circulating NKp46(+) Natural Killer cells have a potential regulatory property and predict distinct survival in non‐small cell lung cancer. Oncoimmunology. 2019;8(2):e1527498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vujanovic L, Chuckran C, Lin Y, et al. CD56(dim) CD16(−) natural killer cell profiling in melanoma patients receiving a cancer vaccine and interferon‐alpha. Front Immunol. 2019;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Best SA, Hess JB, Souza‐Fonseca‐Guimaraes F, et al. Harnessing natural killer immunity in metastatic SCLC. J Thorac Oncol. 2020;15(9):1507‐1521. [DOI] [PubMed] [Google Scholar]
- 31. de Jonge K, Ebering A, Nassiri S, et al. Circulating CD56(bright) NK cells inversely correlate with survival of melanoma patients. Sci Rep. 2019;9(1):4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gousias K, von Ruecker A, Voulgari P, Simon M. Phenotypical analysis, relation to malignancy and prognostic relevance of ICOS+T regulatory and dendritic cells in patients with gliomas. J Neuroimmunol. 2013;264(1–2):84‐90. [DOI] [PubMed] [Google Scholar]
- 33. Labidi‐Galy SI, Treilleux I, Goddard‐Leon S, et al. Plasmacytoid dendritic cells infiltrating ovarian cancer are associated with poor prognosis. Oncoimmunology. 2012;1(3):380‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sisirak V, Vey N, Goutagny N, et al. Breast cancer‐derived transforming growth factor‐beta and tumor necrosis factor‐alpha compromise interferon‐alpha production by tumor‐associated plasmacytoid dendritic cells. Int J Cancer. 2013;133(3):771‐778. [DOI] [PubMed] [Google Scholar]
- 35. Faith A, Peek E, McDonald J, et al. Plasmacytoid dendritic cells from human lung cancer draining lymph nodes induce Tc1 responses. Am J Respir Cell Mol Biol. 2007;36(3):360‐367. [DOI] [PubMed] [Google Scholar]
- 36. Munn DH, Sharma MD, Hou D, et al. Expression of indoleamine 2,3‐dioxygenase by plasmacytoid dendritic cells in tumor‐draining lymph nodes. J Clin Investig. 2004;114(2):280‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhao X, Kallakury B, Chahine JJ, et al. Surgical resection of SCLC: prognostic factors and the tumor microenvironment. J Thorac Oncol. 2019;14(5):914‐923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang W, Hodkinson P, McLaren F, et al. Histologic assessment of tumor‐associated CD45(+) cell numbers is an independent predictor of prognosis in small cell lung cancer. Chest. 2013;143(1):146‐151. [DOI] [PubMed] [Google Scholar]
- 39. Zhou N, Luo P, Wen Y, Meng H, Zhang J. Immune cell infiltration is a strong prognostic indicator in surgical resection of SCLC. J Thorac Oncol. 2019;14(10):e242‐e243. [DOI] [PubMed] [Google Scholar]
- 40. Eerola AK, Soini Y, Paakko P. A high number of tumor‐infiltrating lymphocytes are associated with a small tumor size, low tumor stage, and a favorable prognosis in operated small cell lung carcinoma. Clin Cancer Res. 2000;6(5):1875‐1881. [PubMed] [Google Scholar]
- 41. Soo RA, Chen Z, Yan Teng RS, et al. Prognostic significance of immune cells in non‐small cell lung cancer: meta‐analysis. Oncotarget. 2018;9(37):24801‐24820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sabari JK, Lok BH, Laird JH, Poirier JT, Rudin CM. Unravelling the biology of SCLC: implications for therapy. Nat Rev Clin Oncol. 2017;14(9):549‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.


