Abstract
Background
Self-medication is one aspect of self-care that has been shown to benefit primary health care. When done correctly, it provides significant benefits to customers, such as self-reliance and cost savings. Inappropriate methods, on the other hand, such as incorrect self-diagnosis and therapy selection, can be disastrous. The COVID-19 pandemic context may benefit the community in easing the burden on the health system. There have been no studies conducted on this possibility in the context of COVID-19 in a selected area, hence the purpose of this study was to determine the extent of and factors associated with self-medication among clients visiting community pharmacies in west Harerghe, Ethiopia from June 1 to 30, 2020.
Methods
This institution-based cross-sectional study used a systemic random sample of 416 community-pharmacy clients. To collect data, face-to-face interviews were conducted using pretested semistructured questionnaires modified from established techniques. EpiData 3.1 was used to enter data and SPSS 24 for analysis. To determine factors associated with self-medications, bivariate and multivariate logistic regression analyses were performed. AORs with 95% CIs are used to report associations, and the level of significance was set at P<0.05.
Results
The proportion of people self-medicating was 73.6% (95% CI 69.2%–77.9%). Self-medications were significantly associated with age 18–24 years (AOR 9.28, 95% CI 3.56–24.21) and 25–34 years (AOR 3.54, 95% CI 1.35–9.27), Amhara ethnicity (AOR 1.72, 95% CI 1.01–2.94), current single status (AOR 0.28, 95% CI 0.15–0.51), government employment (AOR 0.31, 95% CI 0.12–0.82), and limited knowledge (AOR 2.31, 95% CI 1.40–3.79).
Conclusion
Three in four participants practiced self-medication in the era of COVID-19. Repetition was significantly associated with age, ethnicity, current marital status, type of occupation, and knowledge about self-medications. An alternative medical care–delivery system by all health-care providers and increasing community awareness should be promoted.
Keywords: self-medication, community pharmacy, COVID-19, Ethiopia
Plain-Language Summary
The rapid spread of COVID-19 has resulted in complicating already flimsy health-care systems in resource-limited countries. Due to the severity of the virus infection and the likelihood of nosocomial infections in health institutions, people identified with the disease have to be prioritized. Nationwide lockdowns have prohibited international movement, which can hamper drug supplies in health-care settings. Consequently, people can be discouraged from seeking medical attention, which might be used to promote self-medication.
In view of this, we intended to evaluate the extent of and factors associated with self-medication in Ethiopia, so conducted an institution-based cross-sectional study using systemic random selection on 416 community-pharmacy clients in June 2020. A total of 306 participants were using self-medication. The most common reasons for this were prior experience (n=177), past prescriptions (n=208), and preference for community drug-retail outlets to access drugs (n=286), mainly through disclosing signs and symptoms (n=212). Headache and fever (n=99) were prominent complaints. Painkillers (n=256) were the most common drug class. Age 18–34 years, ethnicity, being single, government employment, and poor knowledge were significantly associated with self-medication.
As such, integrated approaches for maximizing health-promotion activities among all health-care providers was indispensable in easing the burden. Moreover, increasing community awareness to avoid panic measures and establishing safe and effective knowledge might be imperative in a resource-scarce setting. This study made a context evaluation of self-medication that may give an experience in decreasing grave consequences secondary to risk-taking behaviors in situations like the COVID-19 pandemic.
Introduction
According to the World Health Organization, self-medication is “the selection and use of medicines by individuals to treat self-recognized illnesses or symptoms.” It entails obtaining pharmaceuticals without a prescription, taking medications on the advice of relatives or friends without consulting a physician, sharing drugs with family/friends, or using remaining medications stored at home.1 This may include not only the use of conventional remedies but also unconventional medicines. In 2013, it was estimated that 246 million people, or one in every 20 people aged 15–64 years, had used a self-medication drug, with each representing a different population segment.2
Self-medication in a responsible manner can be used to prevent and treat symptoms and disorders that do not require consistent medical supervision.2,3 As a result, the strain on medical services will be reduced, particularly in impoverished countries with limited health-care resources. However, many people lack a clear vision of themselves or their circumstances and unintentionally engage in self-medication.4 This can have serious consequences, particularly at extremes of age (children and the elderly) and in physiological situations, such as pregnancy and breastfeeding.5–7 Furthermore, it leads to antimicrobial-drug resistance,8,9 high drug dosage, long-term use, drug interactions,10 and adverse drug responses,11 all of which can lead to serious complications and even death.12,13
The extent of self-medication varies among locations, eg, Belagavi, India (35.1%),14 Togo (34.2%),15 east Nepal (44.04%),16 and Poland (45.6%).17 In Ethiopia, the extent of self-medication in Meket district, Gondar town, and Addis Ababa is 35.9%, 50.2%, and 75.5%, respectively.4,18,19 This demonstrates that usage may vary depending on the sociodemographic status of residents and such contexts as the COVID-19 pandemic.
This epidemic is said to have increased the power of social media in terms of drug disinformation, causing public misperception and anxiety, as well as augmented use of self-medication comprising home preparations lacking proven safety and efficacy information.20,21 The disease’s rapid spread has resulted in national lockdowns, prohibited international movement, and in some cases trade.22 This could have hampered drug supply in health-care facilities23 and discouraged people from seeking medical attention there.24 This may have led to people looking for medications from sources other than pharmacies, contributing to a rise in self-medication. Because the strength of the regulatory structure and understanding of the hazards associated with available pharmaceutical items varies by country, the ramifications of self-medication will be obvious contextually.3,25–28
The use of appropriate pharmaceutical care during the pandemic has aided in popularizing self-treatment and introducing a rational dimension.3 In some countries, pharmacists have been given the authority to manufacture hand and surface disinfectants, the ability to renew chronic treatment prescriptions, completion of COVID-19, influenza, and group A Streptococcus screening tests, and the delivery of vaccinations in the fight against the virus.29,30 As a result, it is critical to ensure the health and safety of frontline pharmacists and other health-care professionals, in order to ensure the continuity and functionality of their community roles31 in educating customers, particularly those at high risk of a pandemic,22 as evidenced by their competence and adequate knowledge, as well as positive attitudes and practice regarding COVID-19 management.31,32
A majority of COVID-19 symptomatology is also seen in common colds, flu, whooping cough, and malaria. As a result of these clinical similarities, as well as an increase in poor health-seeking behavior at usual health facilities,33 there have been fewer hospital visits due to fear of contracting the disease.34 Media promotion of medicines that are said to be active against COVID-19 and access to health information on the Internet35,36 have encouraged many people to engage in self-medicating behavior and look for alternative places to seek medical attention, such as community pharmacies.37
The responsibility for promoting proper drug use in the health-care setting cannot be left only to government authorities. Every country or individual should participate in existing laws on over-the-counter drug use and sales. Moreover, periodic and contextualized studies are imperative in order to quantify the extent of the problem. However, there is limited information on self-medication during the COVID-19 pandemic in Ethiopia. As such, the purpose of this study was to determine the extent of and factors associated with self-medication among clients visiting community pharmacies in west Harerghe, Ethiopia from June 1 to 30, 2020.
Methods
Study Area, Period, and Design
This institution-based cross-sectional study on community drug-outlet clients was carried out in Micheta, west Harerghe, Oromia, Ethiopia from June 1 to 30, 2020. Micheta is 494 km from Addis Ababa and has a total area of 280.51km2. According to the 2007 national census, there were 198,095 people in the district, 101,596 men and 96,499 women, and its urban population was 16,862. A majority of the population is Muslim — 94.21%.38 Currently, the town has ten retailcommunity drug outlets: Odabultum, Eman, Beza, Mariyam, Bergay, Aynage, Teka, Beha Biftu, Biftu Oda, and Ebsitu.
Inclusion and Exclusion Criteria
All those >18 years old visiting community drug outlets for illness within the past one month of the study period were included. Those who had to revisit, seriously ill ones, obtaining medicines for other persons, with communication problems, and refused to participate were left out of the study.
Sample-Size Determination
Sample size was determined using the single population–proportion formula (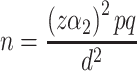 ) after referring to previous studies,2,10,39 with subsequent assumptions of confidence level 95% (
) after referring to previous studies,2,10,39 with subsequent assumptions of confidence level 95% (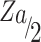 ) 1.96, margin of error of 5%, and self-medication extent among community pharmacies around Harar 50.2%.27 Then, the sample size was calculated as n = (1.96)2(0.502) (0.498)/(0.05)2 = 384. By assuming a 10% nonresponse rate and the objectives, the final largest sample size was determined to be 422.
) 1.96, margin of error of 5%, and self-medication extent among community pharmacies around Harar 50.2%.27 Then, the sample size was calculated as n = (1.96)2(0.502) (0.498)/(0.05)2 = 384. By assuming a 10% nonresponse rate and the objectives, the final largest sample size was determined to be 422.
Sampling Procedure/Technique
There were ten drug outlets in the selected study area. The number of individuals to be surveyed for each outlet was determined based on patient flow to the selected outlets. Each study participant was nominated using systematic random sampling considering inclusion criteria.
Variables
Dependent Variable
The extent of self-medication
Independent Variable
Sociodemographic factors (age, sex, religion, education, marital status, occupation, residence, income, knowledge)
Enabling factors (household income, health insurance, health service, similarity to medical illness, availability of over-the-counter drugs, lack of time, distance, advertisements, advice of others’ experiences)
Need factors (self-medication needs, severity of ill effects, urgency of an illness’s minor symptoms, type of illness, duration of illness)
Operational Definitions
Self-medication: an act in which a study participant suspects he or she has a medical condition and has taken drugs in the previous month without seeking medical advice, either with a prior prescription or not.4
Drug outlet: pharmacies (retail facilities overseen by licensed pharmacists that sell registered prescription-based medicines), drug shops (lower-tier retail outlets with no pharmacist on staff that sell over-the-counter drugs, chemical products, and household remedies).40,41
Nonpharmacological: nonpharmaceutical treatment (massage, exercise, steam inhalation).41
Over counter/nonprescribed drugs: are pharmaceuticals that can be legally obtained without the need for a prescription from a licensed medical professional from a drug outlet, eg, paracetamol, ibuprofen, Advil, cough syrup, antacids.8
Health-care professionals: licensed health-care workers providing necessary health-care services for patients/clients in a health institution.42
Self-medication: measures taken by a research participant while feeling disease and has used a drug drug outlet in the last month with a previous prescription or without prescription from a licensed medical professional.19
Physician: any person medically qualified to prescribe medications.19
Pharmacy professionals: regardless of academic level, pharmacy practitioners who worked in retail outlets.39
Knowledge of appropriate self-medication: if a respondent answered correctly 50% (eight items) or more of the questions on proper self-medication, he or she was considered knowledgeable.19
Data-Collection Tool
A semistructured questionnaire was used to collect data. Following a thorough assessment of the literature, a four-part questionnaire was created.1,19,39 To maintain uniformity, the survey was written in English and then translated into local languages before being returned to English by language experts and researchers. The first section included queries related to the demographic characteristics of participants. In the second section, there were questions about the characteristics of the community in health-seeking practices, and the third division addressed knowledge about self-medication. The last section assessed self-medication (Supplementary Material).
Data Collectors and Data-Collection Procedures
Five health workers able to speak local languages were recruited for data collection. Four were trained and certified druggists. The other one was a pharmacist and oversaw the data-collection process. Before data collection, the questionnaire was piloted using 5% of the total sample size in a nearby town where actual data collection had never been conducted, and slight modifications were made accordingly. The study was then carried out using face-to-face interviews and a questionnaire administered by an interviewer in local languages.
Data Quality Control
The data-collection tools in Afan Oromo were translated from the English version. The questionnaire was tested prior to data collection and instrument corrections made as needed. The supervisor and principal investigators directly and daily supervised the data to ensure its completeness and consistency.
Data Processing, Analysis, and Interpretation
Data were checked for completeness and labeled. The information was then entered into EpiData 3.1, and SPSS 23 was used for analysis. Descriptive analyses were performed, including frequency, percentage, means ± SD, and variance. Data are presented in the form of tables and figures. For each independent and dependent variable, bivariate logistic regression analysis was performed, and variables with P<0.25 were subjected to multivariate analysis to control for all potential confounders and identify variables significantly associated with the outcome variable. Model fitness was checked for multivariate logistic regression analysis using the Hosmer–Lemeshow test. ORs with 95% CIs were used to assess relationships between dependent and independent variables, and statistical significance was taken as P<0.05.
Results
Sociodemographic Characteristics
A total of 416 people took part in the study, for a response rate of 98.6%. A majority, (383, 92.1%) of participants were urban residents, 252 (60.6%) aged 18–24 years, with mean age 24.3±5.1 years. Nearly three-quarters (303, 72.8%) were male, more than half (249, 59.5%) married and 181 (43.5%) had primary education. In addition, 133 (32.0%) were merchants/traders (Table 1).
Table 1.
Sociodemographic characteristics of participants (n=416)
| n | % | ||
|---|---|---|---|
| Residence | Urban | 383 | 92.1 |
| Rural | 33 | 7.9 | |
| Age (years) | 18–24 | 252 | 60.6 |
| 25–34 | 100 | 24.0 | |
| 35–44 | 39 | 9.4 | |
| ≥45 | 25 | 6.0 | |
| Sex | Female | 113 | 27.2 |
| Male | 303 | 72.8 | |
| Religion | Orthodox | 188 | 45.2 |
| Protestant | 23 | 5.5 | |
| Muslim | 205 | 49.3 | |
| Ethnicity | Other | 16 | 3.8 |
| Amhara | 107 | 25.7 | |
| Oromo | 266 | 63.9 | |
| Marital status | Single | 158 | 38.0 |
| Divorce | 9 | 2.2 | |
| Married | 249 | 59.9 | |
| Education | None | 60 | 14.4 |
| Primary | 181 | 43.5 | |
| Secondary education | 120 | 28.8 | |
| Above sec | 55 | 13.2 | |
| Occupation | Housewife | 38 | 9.1 |
| Farmer | 24 | 5.8 | |
| Merchant/trader | 133 | 32.0 | |
| Laborer | 46 | 11.1 | |
| Private employee | 82 | 19.7 | |
| Government employee | 45 | 10.8 | |
| Other | 48 | 11.5 | |
| Monthly income (ETB) | ≤1,000 | 114 | 27.4 |
| 1,001–2,000 | 130 | 31.3 | |
| >2,000 | 172 | 41.3 | |
Abbreviation: ETB, Ethiopian birr.
Characteristics of the Community in Health-Seeking Practices
Nearly two-thirds (63.2%) of participants had experienced illness in the last 10 days before data collection. A majority (326, 78.4%) responded that self-medication was their immediate action whenever feeling sick, with the most frequent class, painkillers in 348 (83.6%). Most (83.6%) agreed that self-medication may have negative consequences, such as the development of drug dependence (88.9%, Table 2).
Table 2.
Health-related characteristics of participants (n=416)
| n | % | ||
|---|---|---|---|
| Time since most recent illness (days) | <10 | 263 | 63.2 |
| 10–20 | 129 | 31.0 | |
| >20 | 24 | 5.8 | |
| Enrollment in CBHI scheme | No | 290 | 69.7 |
| Yes | 126 | 30.3 | |
| Immediate action taken when they got sick | Self-medicated | 326 | 78.4 |
| Consulted a doctor | 67 | 16.1 | |
| Ignored it | 15 | 3.6 | |
| Discussed with friends | 8 | 1.9 | |
| Do you think SM has negative consequences? | Yes | 348 | 83.6 |
| No | 68 | 16.4 | |
| Type of negative consequence | Drug dependence | 370 | 88.9 |
| Drug resistance | 27 | 6.6 | |
| Adverse drug reaction | 17 | 4.0 | |
| Worsening of disease condition | 2 | 0.5 | |
Abbreviation: CBHI, community-based health insurance.
Self-Medication
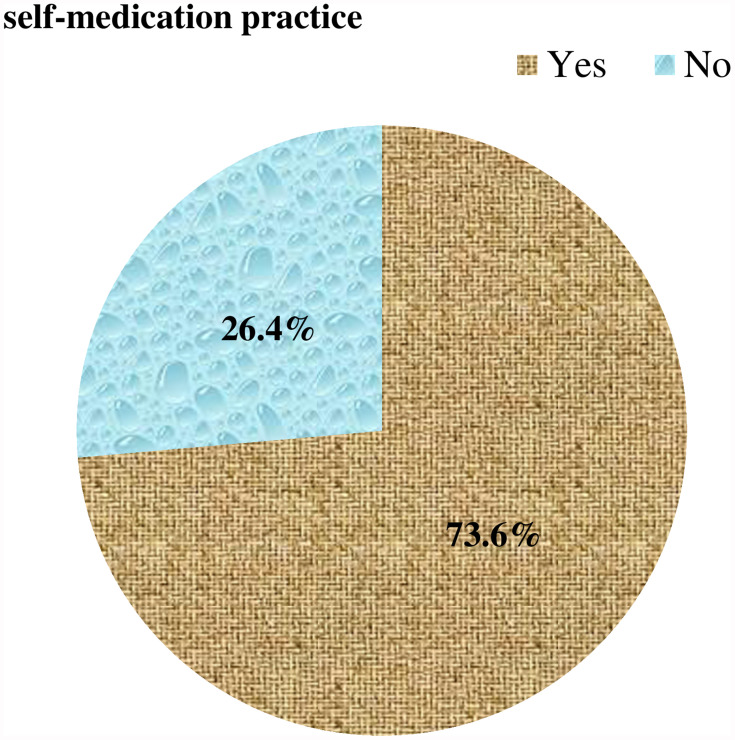
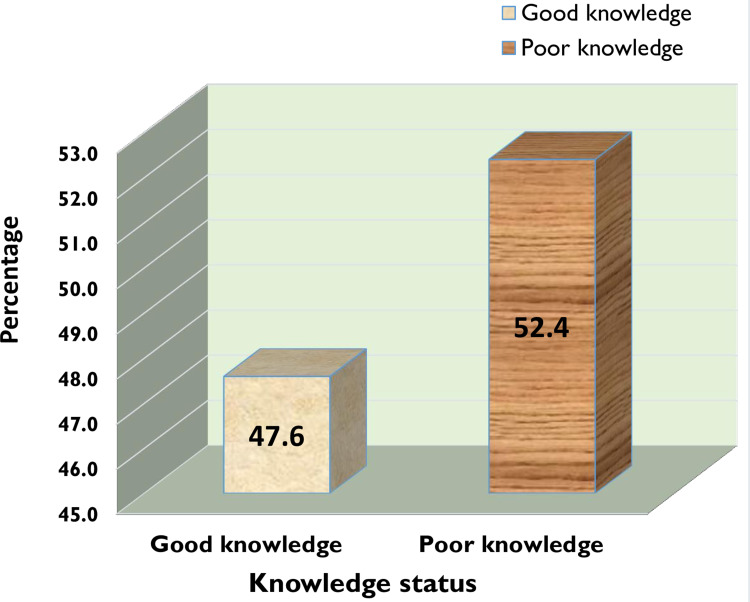
Participants self-medicating numbered 306 (73.6%, 95% CI 69.2%–77.9%) (Figure 1). Fifteen items were used to measure knowledge of self-medication, and showed less than half (47.6%) the participants had good awareness (Figure 2). Complaints frequently treated in 99 (23.5%) were fever and headache. The most frequent reason for self-medication was prior illness 177 (57.8%). A total of 208 (68%) used drug information from prior prescriptions, while 286 (93.5%) said their source for self-medication was pharmacies or drug shops, mostly via request by disclosing signs and symptoms of their disease (212, 69.3%). Fortunately, 283 (92.5%) had not faced any adverse reactions, so 277 (90.5%) would recommend others to practice self-medication (Table 3).
Figure 1.
Extent of self-medication (n=416).
Figure 2.
Knowledge about self-medication (n=416).
Table 3.
SM by participants (n=306)
| n | % | ||
|---|---|---|---|
| Complaints driving SM | Headache and fever | 99 | 32.4 |
| Common cold or cough | 75 | 24.5 | |
| Pain and chills | 25 | 8.1 | |
| Sore throat | 14 | 4.6 | |
| Diarrhea | 28 | 9.2 | |
| Vomiting | 19 | 6.2 | |
| Gastritis | 46 | 15 | |
| Type of medication used for SM | Painkillers | 256 | 83.7 |
| Antibiotics | 32 | 10.5 | |
| Cough syrup | 5 | 1.6 | |
| Antacid | 5 | 1.6 | |
| Oral contraceptive | 4 | 1.3 | |
| Other | 4 | 1.3 | |
| Reason for SM | Previous experience | 177 | 57.8 |
| Mildness of illness | 47 | 15.4 | |
| Sufficient knowledge about drugs | 43 | 14.1 | |
| Ready availability of drugs | 20 | 6.5 | |
| To save time | 5 | 1.6 | |
| To save money | 5 | 1.6 | |
| Too far from the health institution | 9 | 2.9 | |
| Source of drug information | Previous prescription | 208 | 68 |
| Academic knowledge | 50 | 16.3 | |
| Family or neighbors | 17 | 5.6 | |
| Reading materials | 13 | 4.2 | |
| Friends | 10 | 3.3 | |
| Advertising | 8 | 2.6 | |
| Source of drugs for SM | Pharmacy or drug shop | 286 | 93.5 |
| Family or relatives | 7 | 2.3 | |
| Leftover prescription | 4 | 1.3 | |
| Friends | 3 | 1.0 | |
| Other (magazines, Internet) | 6 | 2.0 | |
| Way of requesting drugs for SM | Disclosing signs and symptoms | 212 | 69.3 |
| Giving drug name | 52 | 17 | |
| Providing a piece of paper | 21 | 6.9 | |
| Taking drug container | 21 | 6.9 | |
| Adverse reactions to SM | No | 283 | 92.5 |
| Yes | 23 | 7.5 | |
| Would recommend or advise on SM to others | Yes | 277 | 90.5 |
| No | 28 | 9.2 | |
| Not certain | 1 | 0.3 | |
Abbreviation: SM, self-medication.
Factors Associated with Self-Medication
On the binary logistic regression analysis, age, knowledge, and education were significantly related to self-medication (Table 4). After controlling for potential confounders and taking variables with P<0.25 into account (religion, ethnicity, marital status, occupation, and income), age, marital status, knowledge, ethnicity, and occupation were found to be significantly associated with self-medication (P<0.05) on the final multivariate logistic regression analysis.
Table 4.
Bivariate logistic regression of factors associated with SM among participants (n=416)
| SM | COR (95% CI) | P | ||||
|---|---|---|---|---|---|---|
| Yes, n (%) | No, n (%) | |||||
| Age (years) | 18–24 | 201(79.8) | 51(20.2) | 5.02(2.15–11.71)*** | 0 | |
| 25–34 | 69(69.0) | 31(31.0) | 2.83(1.16, 6.94)* | 0.023 | ||
| 35–44 | 25(64.1) | 14(35.9) | 2.278(0.82, 6.34) | 0.117 | ||
| ≥45 | 11(44.0) | 14(56.0) | 1 | |||
| Sex | Female | 91(79.8) | 23(20.2) | 1.60(0.95, 2.70) | 0.361 | |
| Male | 215(71.2) | 87(28.8) | 1 | |||
| Religion | Orthodox | 144(76.6) | 44(23.4) | 1.37(0.88, 2.17) | 0.156 | |
| Protestant | 18(78.3) | 5(21.7) | 1.53(0.54, 4.29) | 0.424 | ||
| Muslim | 144(70.2) | 61(29.8) | 1 | |||
| Ethnicity | Other | 14(87.5) | 2(13.5) | 2.90(0.65,13.08) | 0.165 | |
| Amhara | 104(77.6) | 3(22.4) | 1.44(0.89, 2.34) | 0.141 | ||
| Oromo | 188(70.7) | 78(29.3) | 1 | |||
| Marital status | Single | 108(68.4) | 50(31.6) | 0.60(0.42,11.02) | 0.064 | |
| Divorce | 7(77.8) | 2(22.2) | 1.06(0.22, 5.26) | 0.940 | ||
| Married | 191(76.7) | 58(23.3) | 1 | |||
| Education | None | 49(81.7) | 11(18.3) | 2.55(1.08,5.98)* | 0.032 | |
| Primary | 132(72.9) | 49(27.1) | 1.54(0.81, 2.92) | 0.186 | ||
| Secondary | 90(75.0) | 30(25.0) | 0.71(0.86,3.41) | 0.124 | ||
| Above secondary | 35(63.6) | 20(36.4) | 1 | |||
| Residence | Urban | 283(73.9) | 100(26.1) | 1.23(0.57, 2.68) | 0.600 | |
| Rural | 23(69.7) | 10(30.3) | 1 | |||
| Occupation | Housewife | 30(78.9) | 8(21.1) | 1.39(0.51, 3.81) | 0.519 | |
| Farmer | 18(75.0) | 6(25.0) | 1.11(0.36, 3.42) | 0.850 | ||
| Merchant/trader | 104(78.2) | 29(21.8) | 1.33(0.62, 2.84) | 0.459 | ||
| Laborer | 35(76.1) | 11(23.9) | 1.18(0.47, 2.99) | 0.725 | ||
| Private employee | 59(72.0) | 23(28.0) | 0.95(0.43, 2.12) | 0.906 | ||
| Government employee | 25(55.6) | 20(44.4) | 0.46(0.20, 1.10) | 0.083 | ||
| Other | 35(72.9) | 13(27.1) | 1 | |||
| Monthly income (ETB) | ≤1,000 | 82(71.9) | 32(28.1) | 1.02(0.603, 1.73) | 0.939 | |
| 1,001–2,000 | 101(77.7) | 29(22.3) | 1.39(0.82, 2.34) | 0.225 | ||
| >2,000 | 123(71.5) | 49(22.5) | 1 | |||
| Time since most recent illness (days) | <10 | 196(74.5) | 67(25.5) | 1.46(0.60, 3.57) | 0.404 | |
| 10–20 | 94(72.9) | 35(27.1) | 1.34(0.53, 3.41) | 0.536 | ||
| >20 | 16(66.7) | 8(33.3) | 1 | |||
| Enrollment in CBHI scheme | No | 217(74.8) | 73(25.2) | 1.24(0.78, 1.97) | 0.373 | |
| Yes | 89(70.6) | 37(29.4) | 1 | |||
| Knowledge about SM | Poor | 159(80.3) | 39(19.7) | 1.97(1.26, 3.09)** | 0.003 | |
| Good | 147(67.4) | 71(32.6) | 1 | |||
Notes: ***P<0.001; **P<0.01; *P<0.05.
Abbreviations: ETB, Ethiopian birr; SM, self-medication; CBHI, community-based health insurance.
On multivariate analysis, the odds of self-medication in clients aged 18–24 years and 25–34 years, were 9.28 times (95% CI 3.56–24.21) and 3.54 times (95% CI 1.35–9.27) those of participants aged ≥45 years, while odds were nearly double among ethnicity (AOR 1.72, 95% CI 1.01–2.94). Self-medication among clients with poor knowledge was 2.31-fold (95% CI 1.40–3.79) that of those with good knowledge. Singles had lower odds of self-medication (AOR 0.28, 95% CI0.15–0.51) than married participants, and government employee also (AOR 0.31, 95% CI 0.12–0.82) than others (Table 5).
Table 5.
Multivariate logistic regression of factors associated with SM among participants (n=416)
| SM | AOR (95% CI) | P | |||
|---|---|---|---|---|---|
| Yes, n (%) | No, (%) | ||||
| Age (in years) | 18–24 | 201(79.8) | 51(20.2) | 9.28(3.56, 24.21)*** | 0 |
| 25–34 | 69(69.0) | 31(31.0) | 3.54(1.35, 9.27)* | 0.010 | |
| 35–44 | 25(64.1) | 14(35.9) | 2.27(0.76, 6.81) | 0.141 | |
| ≥45 | 11(44.0) | 14(56.0) | 1 | ||
| Religion | Orthodox | 144(76.6) | 44(23.4) | 1.24(0.64, 2.39) | 0.528 |
| Protestant | 18(78.3) | 5(21.7) | 2.07(0.63, 15.30) | 0.231 | |
| Muslim | 144(70.2) | 61(29.8) | 1 | ||
| Ethnicity | Other | 14(87.5) | 2(12.5) | 3.07(0.63,115.10) | 0.167 |
| Amhara | 104(77.6) | 3(22.4) | 1.72(1.01, 2.94)* | 0.045 | |
| Oromo | 188(70.7) | 78(29.3) | 1 | ||
| Marital status | Single | 108(68.4) | 50(31.6) | 0.28(0.15, 0.51)*** | 0 |
| Divorce | 7(77.8) | 2(22.2) | 1.41(0.23, 8.62) | 0.707 | |
| Married | 191(76.7) | 58(23.3) | 1 | ||
| Education | None | 49(81.7) | 11(18.3) | 1.81(0.59, 5.58) | 0.304 |
| Primary | 132(72.9) | 49(27.1) | 1.01(0.41, 2.46) | 0.994 | |
| Secondary | 90(75.0) | 30(25.0) | 1.21(0.51, 2.89) | 0.662 | |
| Above secondary | 35(63.6) | 20(36.4) | 1 | ||
| Occupation | Housewife | 30(78.9) | 8(21.1) | 0.62(0.19, 1.97) | 0.412 |
| Farmer | 18(75.0) | 6(25.0) | 0.72(0.21, 2.52) | 0.606 | |
| Merchant/trader | 104(78.2) | 29(21.8) | 1.23(0.53, 2.86) | 0.629 | |
| Laborer | 35(76.1) | 11(23.9) | 1.06(0.40, 2.82) | 0.900 | |
| Private employee | 59(72.0) | 23(28.0) | 0.79(0.33, 1.88) | 0.594 | |
| Government employee | 25(55.6) | 20(44.4) | 0.31(0.12, 0.82)* | 0.018 | |
| Other | 35(72.9) | 13(27.1) | 1 | ||
| Monthly income (ETB) | ≤1,000 | 82(71.9) | 32(28.1) | 0.95(0.49, 1.88) | 0.892 |
| 1,001–2,000 | 101(77.7) | 29(22.3) | 1.24(0.67, 2.27) | 0.494 | |
| >2,000 | 123(71.5) | 49(28.5) | 1 | ||
| Knowledge about SM | Poor | 159(80.3) | 39(19.7) | 2.31(1.40, 3.79)** | 0.001 |
| Good | 147(67.4) | 71(32.6) | 1 | ||
Notes: ***P<0.001; **P<0.01; *P<0.05.
Abbreviations: ETB, Ethiopian birr; SM, self-medication .
Discussion
Self-medication is one aspect of self-care that has been shown to benefit primary health care. When done correctly, it provides significant benefits to consumers, such as self-reliance and cost savings. On the other hand, erroneous self-diagnosis, harmful drug–drug interactions, improper administration, incorrect dosage, and incorrect therapy selection can pose such risks as disguising serious disease, dependence, and misuse.
In this study, the extent of self-medication was found to be 73.6%, relatively similar to studies in Pakistan (64%),43 Bangladesh (71.4%),44 and Ethiopia (71.4%)8 that were conducted in the analogous context of the COVID-19 pandemic, though greater than findings in Brazil (14.9%),45 Tamil Nadu (17.8%),46 Saudi Arabia (35.4%),47 and India (35.1%).14 Other results from Ethiopia in different towns further strengthen this difference: Welaita Soddo (33.7%),48 Jijiga (37.5%),2 Dessie (42.4%),49 and Gonder (50.2%).4
This substantial increase might be due to the study period coinciding with the timing of the country’s state-of-emergency announcement for implementing pandemic-prevention measures.50 Due to the severity of diseases and the risk of nosocomial infections in health facilities, this has burdened the already shaky health-care system, forcing it to prioritize people identified with COVID-19 who may require intensive care. As a result, the pandemic has had a negative influence on the care-seeking behavior of people with chronic medical illnesses, with the impact being greater among those with severe disease and in dread of COVID-19,51 as further augmented by a drop in emergency admissions and follow-up clinic appointments at referral hospitals.52 Therefore, self-medication may be of particular interest to a significant segment of the population, because it allows people with minor ailments to make independent decisions about how to manage their health and well-being without having to go to the hospital.35 This might also explain the finding that 78.4% of the participants responded that self-medication would be their immediate action if they got sick. This might be further attributed to the wide practice of uncontrolled and nonselective dispensing of drugs without prescription in developing countries.53–57
The most common reasons for self-medication were headache and fever (32.4%). Comparable results have been reported elsewhere (32–38%)44,48,49,58,59 and for common complaints.60,61 Very high proportions (>90%) have been reported in other studies.8,14,62 This study also revealed that prior experience was the major (57.8%) reason for the practice by extrapolating drug information from previous prescriptions (68%), and other studies have found repeated prescriptions as primary sources — 63%63 and 51.4%14 — and prior experience (57.8%) increase confidence in repetition.39 Most of them (93.5%) accessed drugs largely from community drug outlets through asking pharmacy professionals for drugs by disclosing symptoms of illness (69.3%). Similar trends have been reported in other studies, with 79.3%,8 73%,62 and 40.3%39 preferring drug outlets to access drugs. In this study, good knowledge was reported in 47.6%, comparable to a study (45.4%) conducted in Gondar, Ethiopia.4 Lack of awareness about the medicine used in self-medication was 82%64 and incorrect understanding 81%65 in studies conducted in Malaysia and Malawi, respectively. Sufficient knowledge of self-medication was reported in almost all respondents (96.7%) to a population-based survey in Nigeria.66
Our participants were trying to get information from community pharmacists, unlike prior research in which participants were counseled by neighbors, colleagues, or relations to take drugs,2,4 which might tell us that the pandemic has contributed to increasing conscious health-seeking behavior which were taken as alternative and safe by the community. In this regard, we could learn a lesson that community-pharmacy professionals have been dedicated to ensuring that the public has access to pharmaceutical care services and minimizing the pandemic’s negative impact.67–69 With the growth in COVID-19 cases, there has been unprecedented strain on the health-care system, which is wreaking havoc on hospitals and clinics. To avoid unnecessary scares, pharmacists were raising awareness of the disease at the public level during this critical time. Symptom-related counseling will assist a patient in determining when medical assistance is required. At this juncture, community pharmacists might educate the public on the differential signs, because the bulk of clients visit the emergency department for coughs or flu, putting an extra burden on hospitals.70–73 Similarly, self-medication is growing more common during the current epidemic17,74 as a result of increased drug-related material on social media and news outlets, as well as an increase in information-seeking behavior.35,75
The most common drugs used were analgesics (83.7%), followed by antimicrobials (10.5%). Pain-relieving agents are the most frequent self-medicating agents,8,14,39,58,59,61,62,76 though high antibiotic use has been reported in other studies: 74.4%8 and 23.5%.59 One of the challenges identified in the COVID-19 era has been the use of antimicrobials as self-medication agents, such as ivermectin, azithromycin, doxycycline, and hydroxychloroquine, which might predispose to toxicity and resistance development.44,66,77–80 Medications for the prevention of COVID-19 are mainly vitamins15,66,79,81 and zinc.81 Integrated public health education at the community level has an indispensable role in increasing awareness and decreasing the burden on facility-based health-care delivery, and efforts should be made to help mitigate risks of self-medication by the active involvement of pharmacists and other members of the health-care team to correct false claims about drugs.
On multivariate logistic regression analysis, age, marital status, knowledge, ethnicity, and occupation were significantly associated with self-medication. Odds of self-medication were higher among those aged 18–34 years than those aged ≥45 years, similar to other studies.45,47,62 The Amhara ethnic group showed nearly double the odds of others.
self-medication practices were 2.3 times higher among clients with poor knowledge than those with good knowledge. Parallel4 and converse66 findings have been reported in previous research. Unmarried clients had lower odds of self-medication than married clients, unlike other findings where the reverse was true.4,18,82 This might be due to fear and feeling great responsibility to take prompt action in keeping the family safe from disease transmission related to the pandemic. Working for the government was associated with lower odds of self-medication than others. This occupation might be linked with low incomes, as other research has reported that high incomes increase the affordability of medication.2
The threat of coronavirus made the practice a fittest will survive action with the assumption of something is better than nothing. Self-medication may have a lot of cons in terms of severe consequences, but the advantages might be life-saving in an emergency. This pandemic could be taken as a lesson to think about integrative approaches to maximizing health-promotion activities among all health-care providers and utilizing indigenous practices with known safety and efficacy, especially in resource-limited settings.
Conclusion
This study showed that three quarters of participants were engaged in self-medication. Driving this practice was prior experience using old prescriptions as the main source of drug information and a preference for community drug outlets and pharmacy professionals to access drugs and consultation. Headache and fever were prominent complaints. Painkillers were the major drug class used. self-medication practices were significantly associated with age <34 years, ethnic differences, marital status, government employment, and poor knowledge of self-medication. Fear of COVID-19 might make the practice or malpractice a survival of the fittest choice leaving a lesson on how to handle health emergencies. An alternative medical care–delivery system with integrated approaches for maximizing health-promotion activities among all health-care providers is indispensable. Moreover, increasing community awareness in this state of emergency and utilizing safe and effective indigenous knowledge might be imperative, especially in resource-limited settings.
Strengths and Limitations
This study made a context evaluation of self-medication that may give a lesson in decreasing grave consequences secondary to risk-taking behaviors in panic situations like the COVID-19 pandemic. However, we focused on contemporary medicines only and thus not incorporate traditional medicinal methods, which may have limited our findings in this research domain. This study may also be prone to recall bias as a result of participants’ self-reporting earlier experiences.
Funding Statement
There was no funding provided for this research.
Data Sharing
The data used to support the findings of this study are included in the article.
Ethics
This study was conducted following the principles of the Declaration of Helsinki. Before starting any data collection, the Institutional Health Research Ethics Review Committee (IHRERC) of Haramaya University’s College of Health and Medical Sciences gave their approval (IHRERC/159/2020). The research’s purposes and objectives, as well as risks and advantages, were all explained to participants to ensure that they had all the information they needed. During the study, data were kept completely anonymous and the information gathered was used only for this study. Participants’ provided informed, voluntary, written, and signed consent. COVID-19 prevention was maximized through social distancing and supplying protective items like facemasks and sanitizing inputs.
Author Contributions
All authors made a significant contribution to the work reported, whether in conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas, took part in drafting, revising, or critically reviewing the article, gave final approval to the version to be published, have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there are no competing interests in this work.
References
- 1.Ateshim Y, Bereket B, Major F, et al. Prevalence of self-medication with antibiotics and associated factors in the community of Asmara, Eritrea: a descriptive cross-sectional survey. BMC Public Health. 2019;19(1):1–7. doi: 10.1186/s12889-019-7020-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amaha MH, Alemu BM, Atomsa GE. Self-medication practice and associated factors among adult community members of Jigjiga town, Eastern Ethiopia. PLoS One. 2019;14(6):e0218772. doi: 10.1371/journal.pone.0218772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oleszkiewicz P, Krysinski J, Religioni U, Merks P. Access to Medicines via Non-Pharmacy Outlets in European Countries—A Review of Regulations and the Influence on the Self-Medication Phenomenon. Paper presented at: Healthcare; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jember E, Feleke A, Debie A, Asrade G. Self-medication practices and associated factors among households at Gondar town, Northwest Ethiopia: a cross-sectional study. BMC Res Notes. 2019;12(1):1–7. doi: 10.1186/s13104-019-4195-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jambo A, Mengistu G, Sisay M, Amare F, Edessa D. Self-medication and contributing factors among pregnant women attending antenatal care at public hospitals of Harar town, Ethiopia. Front Pharmacol. 2018;9:1063. doi: 10.3389/fphar.2018.01063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jamhour A, El-Kheir A, Salameh P, Abi Hanna P, Mansour H. Antibiotic knowledge and self-medication practices in a developing country: a cross-sectional study. Am J Infect Control. 2017;45(4):384–388. doi: 10.1016/j.ajic.2016.11.026 [DOI] [PubMed] [Google Scholar]
- 7.Ararsa A, Bekele A. Assessment of self-medication practice and drug storage on private pharmacy clients in Jimma town, Oromia, South West Ethiopia. AJPS. 2015;1(1):20–32. [Google Scholar]
- 8.Mohammed SA, Tsega G, Hailu AD. Self-medication practice and associated factors among health care professionals at Debre Markos comprehensive specialized hospital, Northwest Ethiopia. Drug Healthc Patient Saf. 2021;13:19. doi: 10.2147/DHPS.S290662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esan DT, Fasoro AA, Odesanya OE, Esan TO, Ojo EF, Faeji CO. Assessment of self-medication practices and its associated factors among undergraduates of a private university in Nigeria. J Environ Public Health. 2018;2018:1–7. doi: 10.1155/2018/5439079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abeje G, Admasie C, Wasie B. Factors associated with self-medication practice among pregnant mothers attending antenatal care at governmental health centers in Bahir Dar city administration, Northwest Ethiopia, a cross-sectional study. Pan Afr Med J. 2015;20. doi: 10.11604/pamj.2015.20.276.4243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wakjira G, Boru B, Labata B. Prevalence of self-medication and its associated factors among pregnant women attending antenatal care at Nekemte Referral Hospital, Oromia Regional State, West Ethiopia. J Bioanal Biomed. 2019;11:160–165. [Google Scholar]
- 12.Almasdy D, Sharrif A. Self-medication practice with nonprescription medication among university students: a review of the literature. Arch Pharm Pract. 2011;2(3):95. [Google Scholar]
- 13.Yadav S, Rawal G. Self-medication practice in low-income countries. Int J Pharmaceut Chem Anal. 2015;2(3):139–142. [Google Scholar]
- 14.Chari HS, Kadeangadi DM, Mallapur M. Practice of self medication among urban households–a community based cross-sectional study. Natl J Community Med. 2015;6(2):93–96. [Google Scholar]
- 15.Sadio AJ, Gbeasor-Komlanvi FA, Konu RY, et al. Assessment of self-medication practices in the context of the COVID-19 outbreak in Togo. BMC Public Health. 2021;21(1):1–9. doi: 10.1186/s12889-020-10145-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parajuli SB, Mishra A, Heera K, et al. Self-medication practices in surrounding communities of Birat Medical College and Teaching Hospital of Eastern Nepal. J Coll Med Sci Nepal. 2019;15(1):45–52. doi: 10.3126/jcmsn.v15i1.23021 [DOI] [Google Scholar]
- 17.Makowska M, Boguszewski R, Nowakowski M, Podkowińska M. Self-medication-related behaviors and Poland’s COVID-19 lockdown. Int J Environ Res Public Health. 2020;17(22):8344. doi: 10.3390/ijerph17228344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kassie AD, Bifftu BB, Mekonnen HS. Self-medication practice and associated factors among adult household members in Meket district, Northeast Ethiopia, 2017. BMC Pharmacol Toxicol. 2018;19(1):1–8. doi: 10.1186/s40360-018-0205-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shafie M, Eyasu M, Muzeyin K, Worku Y, Martín-Aragón S. Prevalence and determinants of self-medication practice among selected households in Addis Ababa community. PLoS One. 2018;13(3):e0194122. doi: 10.1371/journal.pone.0194122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Y. Use of herbal drugs to treat COVID-19 should be with caution. Lancet. 2020;395(10238):1689–1690. doi: 10.1016/S0140-6736(20)31143-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erku DA, Belachew SA, Abrha S, et al. When fear and misinformation go viral: pharmacists’ role in deterring medication misinformation during the’infodemic’surrounding COVID-19. Res Social Adm Pharm. 2021;17(1):1954–1963. doi: 10.1016/j.sapharm.2020.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bahlol M, Dewey RS. Pandemic preparedness of community pharmacies for COVID-19. Res Social Adm Pharm. 2021;17(1):1888–1896. doi: 10.1016/j.sapharm.2020.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tirivangani T, Alpo B, Kibuule D, Gaeseb J, Adenuga BA. Impact of COVID-19 pandemic on pharmaceutical systems and supply chain–a phenomenological study. Explorat Res Clin Social Pharm. 2021;2:100037. doi: 10.1016/j.rcsop.2021.100037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mudenda S, Witika BA, Sadiq MJ, et al. Self-medication and its consequences during & after the Coronavirus Disease 2019 (COVID-19) pandemic: a global health problem. Eur J Env Public Health. 2020;5(1):em0066. doi: 10.29333/ejeph/9308 [DOI] [Google Scholar]
- 25.Dulęba J, Religioni U, Słodka E, Fal A, Krysiński J, Merks P. The Awareness of risks associated with OTC drugs available in non-pharmacy outlets among polish patients—a cross-sectional study. Paper presented at: Healthcare; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wafula FN, Miriti EM, Goodman CA. Examining characteristics, knowledge and regulatory practices of specialized drug shops in Sub-Saharan Africa: a systematic review of the literature. BMC Health Serv Res. 2012;12(1):1–18. doi: 10.1186/1472-6963-12-223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glass BD. Counterfeit drugs and medical devices in developing countries. Res Rep Trop Med. 2014;5:11. doi: 10.2147/RRTM.S39354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suleman S, Woliyi A, Woldemichael K, et al. Pharmaceutical regulatory framework in Ethiopia: a critical evaluation of its legal basis and implementation. Ethiop J Health Sci. 2016;26(3):259–276. doi: 10.4314/ejhs.v26i3.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanzi MG. Pharmacists’ role during the COVID-19 pandemic. Pharm Today. 2021;27(3):54–63. doi: 10.1016/j.ptdy.2021.05.021 [DOI] [Google Scholar]
- 30.Hess K, Bach A, Won K, Seed SM. Community pharmacists roles during the COVID-19 pandemic. J Pharm Pract. 2020:0897190020980626. doi: 10.1177/0897190020980626 [DOI] [PubMed] [Google Scholar]
- 31.Hedima EW, Adeyemi MS, Ikunaiye NY. Community pharmacists: on the frontline of health service against COVID-19 in LMICs. Res Social Adm Pharm. 2021;17(1):1964–1966. doi: 10.1016/j.sapharm.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.AlRasheed MM, AlShahrani AH, AlMuhaini SA, et al. Knowledge, attitude, and practice towards COVID-19 among pharmacists: a cross-sectional study. Risk Manag Healthc Policy. 2021;14:3079. doi: 10.2147/RMHP.S317779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hussain R, Rashidian A, Hafeez A, Mirzaee N. Factors influencing healthcare seeking behaviour at primary healthcare level, in Pakistan. Ayub Med Coll Abbottabad. 2019;31(2):201–206. [PubMed] [Google Scholar]
- 34.Jeffery MM, D’Onofrio G, Paek H, et al. Trends in emergency department visits and hospital admissions in health care systems in 5 states in the first months of the COVID-19 pandemic in the US. JAMA Intern Med. 2020;180(10):1328–1333. doi: 10.1001/jamainternmed.2020.3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Onchonga D. A Google Trends study on the interest in self-medication during the 2019 novel coronavirus (COVID-19) disease pandemic. Saudi Pharm J. 2020;28(7):903. doi: 10.1016/j.jsps.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malik M, Tahir MJ, Jabbar R, Ahmed A, Hussain R. Self-medication during Covid-19 pandemic: challenges and opportunities. Drugs Ther Perspect. 2020;36(12):565–567. doi: 10.1007/s40267-020-00785-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merks P, Świeczkowski D, Bilmin K, et al. Community pharmacy during the COVID-19 pandemic: a cross-sectional study. Przegl Epidemiol. 2020;74(4):620–633. doi: 10.32394/pe.74.53 [DOI] [PubMed] [Google Scholar]
- 38.Central Statistical Agency. The 2007 population and housing census of Ethiopia. Addis Ababa: CSA; 2007. [Google Scholar]
- 39.Mamo S, Ayele Y, Dechasa M. Self-medication practices among community of Harar city and its surroundings, Eastern Ethiopia. J Pharm. 2018;2018:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bradley S, Shiras T. Regulation of drug shops and pharmacies relevant to family planning: a scan of 32 developing countries. Bethesda, MD: Sustaining Health Outcomes through the Private Sector Plu; 2017. [Google Scholar]
- 41.Solomon W, Abede G. Practice of self-medication in Jimma Town. Ethiop J Health Dev. 2003;17(2):111–116. [Google Scholar]
- 42.Tesfaye ZT, Yismaw MB, Negash Z, Ayele AG. COVID-19-related knowledge, attitude and practice among hospital and community pharmacists in Addis Ababa, Ethiopia. Integr Pharm Res Pract. 2020;9:105. doi: 10.2147/IPRP.S261275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rafiq K, Nesar S, Anser H, et al. Self-medication in COVID-19 pandemic: the survival of the fittest. Disaster Med Public Health Prep;2021:1–19. doi: 10.1017/dmp.2021.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nasir M, Chowdhury A, Zahan T. Self-medication during COVID-19 outbreak: a cross sectional online survey in Dhaka city. Int J Basic Clin Pharmacol. 2020;9(9):1325–1330. doi: 10.18203/2319-2003.ijbcp20203522 [DOI] [Google Scholar]
- 45.Domingues PHF, Galvão TF, KRCd A, Araújo PC, Silva MT, Pereira MG. Prevalence and associated factors of self-medication in adults living in the Federal District, Brazil: a cross-sectional, population-based study. Epidemiol Serv Saude. 2017;26:319–330. doi: 10.5123/S1679-49742017000200009 [DOI] [PubMed] [Google Scholar]
- 46.Dutta R, Raja D, Anuradha R, Dcruze L, Jain T, Sivaprakasam P. Self-medication practices versus health of the community. Int J Community Med Public Health. 2017;4:2757–2761. doi: 10.18203/2394-6040.ijcmph20173169 [DOI] [Google Scholar]
- 47.Alghanim S. Self-medication practice among patients in a public health care system. East Mediterr Health J. 2011;17(5):409–416. doi: 10.26719/2011.17.5.409 [DOI] [PubMed] [Google Scholar]
- 48.Mathewos T, Daka K, Bitew S, Daka D. Self-medication practice and associated factors among adults in Wolaita Soddo town, Southern Ethiopia. Int J Infect Control. 2021;17(1). doi: 10.3396/ijic.v17.20322 [DOI] [Google Scholar]
- 49.Baye A, Sada O. Self-medication practice in community pharmacies: the case of Dessie town, Northeast Ethiopia. Adv Pharmacoepidemiol Drug Saf. 2018;7(1):1–3. [Google Scholar]
- 50.Zikargae MH. COVID-19 in Ethiopia: assessment of how the Ethiopian government has executed administrative actions and managed risk communications and community engagement. Risk Manag Healthc Policy. 2020;13:2803. doi: 10.2147/RMHP.S278234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aklilu TM, Abebe W, Worku A, et al. The impact of COVID-19 on care seeking behavior of patients at tertiary care follow-up clinics: a cross-sectional telephone survey. Addis Ababa, Ethiopia. medRxiv. 2020. doi: 10.1101/2020.11.25.20236224 [DOI] [Google Scholar]
- 52.Abebe W, Worku A, Moges T, et al. Trends of follow-up clinic visits and admissions three-months before and during COVID-19 pandemic at Tikur Anbessa specialized hospital, Ethiopia: an interrupted time series analysis; 2021. [DOI] [PMC free article] [PubMed]
- 53.Belachew SA, Hall L, Selvey LA. Non-prescription dispensing of antibiotic agents among community drug retail outlets in Sub-Saharan African countries: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2021;10(1):1–15. doi: 10.1186/s13756-020-00880-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bahta M, Tesfamariam S, Weldemariam DG, et al. Dispensing of antibiotics without prescription and associated factors in drug retail outlets of Eritrea: a simulated client method. PLoS One. 2020;15(1):e0228013. doi: 10.1371/journal.pone.0228013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Damisie G, Hambisa S, Yimam M. Over-the-counter sale of antibiotics at drug stores found in Mizan-Aman Town, Southwest Ethiopia: a cross-sectional simulated client visit study. J Pharm. 2019;2019:1–6. doi: 10.1155/2019/3510659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ocan M, Obuku EA, Bwanga F, et al. Household antimicrobial self-medication: a systematic review and meta-analysis of the burden, risk factors and outcomes in developing countries. BMC Public Health. 2015;15(1):1–11. doi: 10.1186/s12889-015-2109-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacobs TG, Robertson J, van den Ham HA, Iwamoto K, Pedersen HB, Mantel-Teeuwisse AK. Assessing the impact of law enforcement to reduce over-the-counter (OTC) sales of antibiotics in low-and middle-income countries; a systematic literature review. BMC Health Serv Res. 2019;19(1):1–15. doi: 10.1186/s12913-019-4359-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sridevi K, Subbaiah MV, Surekha M, Harini J, Sujana D, Sankar AR. Assessment of self-medication practices among community people. IOSR J Dent Med Sci. 2017;16:75–82. doi: 10.9790/0853-1605017582 [DOI] [Google Scholar]
- 59.Zeid W, Hamed M, Mansour N, Diab R. Prevalence and associated risk factors of self-medication among patients attending El-Mahsama family practice center, Ismailia, Egypt. Bull Natl Res Cent. 2020;44(1):1–5. doi: 10.1186/s42269-020-00351-7 [DOI] [Google Scholar]
- 60.Sivasakthi K, Koshila K, Viswa S. Assessment of knowledge, attitude and practice about self medication among rural areas in Erode district. Indian J Pharm Pract. 2020;13(3):228–231. doi: 10.5530/ijopp.13.3.37 [DOI] [Google Scholar]
- 61.Oche OM, Godwin GJ, Yahaya M, Gambo AA, Abdulkarim A, Emoh M. Prevalence and factors associated with self-medication among people living in urban slums of Sokoto Metropolis, Sokoto State, Nigeria. Cent African J Public Heal. 2019;5(6):302–309. [Google Scholar]
- 62.Khan H, Maheen S, Alamgeer GA, et al. Determinants of increasing trend of self-medication in a Pakistani community. Trop J Pharm Res. 2014;13(3):437–443. doi: 10.4314/tjpr.v13i3.19 [DOI] [Google Scholar]
- 63.Isameldin E, Saeed AA, Mousnad MA Self-medication practice among patients living in Soba Sudan. 2020.
- 64.Azhar MIM, Gunasekaran K, Kadirvelu A, Gurtu S, Sadasivan S, Kshatriya BM. Self-medication: awareness and attitude among Malaysian urban population. Int J Collab Res Intern Med Public Health. 2013;5(6):436–443. [Google Scholar]
- 65.Sambakusi CS. Knowledge, attitudes and practices related to self-medication with antimicrobials in Lilongwe, Malawi. Malawi Med J. 2019;31(4):225–232. doi: 10.4314/mmj.v31i4.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wegbom AI, Edet CK, Raimi O, Fagbamigbe AF, Kiri VA. Self-medication practices and associated factors in the prevention and/or treatment of COVID-19 virus: a population-based survey in Nigeria. Front Public Health. 2021;9. doi: 10.3389/fpubh.2021.606801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ali E, Thulasika P, Sarah A, Cindy T. Pharmacists and COVID-19. J Pharm Policy Pract. 2020;13:36. doi: 10.1186/s40545-020-00241-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kretchy IA, Asiedu-Danso M, Kretchy J-P. Medication management and adherence during the COVID-19 pandemic: perspectives and experiences from LMICs. Res Social Adm Pharm. 2020;17:2023–2026. doi: 10.1016/j.sapharm.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hussain K, Ambreen G, Muzammil M, Raza SS, Ali U. Pharmacy services during COVID-19 pandemic: experience from a tertiary care teaching hospital in Pakistan. J Pharm Policy Pract. 2020;13(1):1–4. doi: 10.1186/s40545-020-00277-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cadogan CA, Hughes CM. On the frontline against COVID-19: community pharmacists’ contribution during a public health crisis. Res Social Adm Pharm. 2021;17(1):2032–2035. doi: 10.1016/j.sapharm.2020.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mallhi TH, Liaqat A, Abid A, et al. Multilevel engagements of pharmacists during the COVID-19 pandemic: the way forward. Front Public Health. 2020;8:726. doi: 10.3389/fpubh.2020.561924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mukattash TL, Jarab AS, Mukattash I, et al. Pharmacists’ perception of their role during COVID-19: a qualitative content analysis of posts on Facebook pharmacy groups in Jordan. Pharm Pract. 2020;18(3):1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Visacri MB, Figueiredo IV, de Mendonça Lima T. Role of pharmacist during the COVID-19 pandemic: a scoping review. Res Social Adm Pharm. 2021;17(1):1799–1806. doi: 10.1016/j.sapharm.2020.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oliveira JVL, da Costa FB, Do Nascimento Porfirio V, et al. A automedicação no período de pandemia de COVID-19: revisão integrativa. Res Soc Dev. 2021;10(3):e58610313762. doi: 10.33448/rsd-v10i3.13762 [DOI] [Google Scholar]
- 75.Kalayou MH, Tilahun B, Endehabtu BF, Nurhussien F, Melese T, Guadie HA. Information seeking on Covid-19 pandemic: care providers’ experience at the University of Gondar Teaching Hospital, Northwest of Ethiopia. J Multidiscip Healthc. 2020;13:1957. doi: 10.2147/JMDH.S283563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arrais PSD, Fernandes MEP, TdSD P, et al. Prevalence of self-medication in Brazil and associated factors. Rev Saude Publica. 2016;50. doi: 10.1590/s1518-8787.2016050006117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Molento MB. COVID-19 and the rush for self-medication and self-dosing with ivermectin: a word of caution. One Health. 2020;10:100148. doi: 10.1016/j.onehlt.2020.100148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Quispe-Cañari JF, Fidel-Rosales E, Manrique D, et al. Self-medication practices during the COVID-19 pandemic among the adult population in Peru: a cross-sectional survey. Saudi Pharm J. 2021;29(1):1–11. doi: 10.1016/j.jsps.2020.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Quincho-Lopez A, Benites-Ibarra CA, Hilario-Gomez MM, Quijano-Escate R, Taype-Rondan A. Self-medication practices to prevent or manage COVID-19: a systematic review. PLoS One. 2021;16(11):e0259317. doi: 10.1371/journal.pone.0259317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Romano S, Galante H, Figueira D, Mendes Z, Rodrigues AT. Time-trend analysis of medicine sales and shortages during COVID-19 outbreak: data from community pharmacies. Res Social Adm Pharm. 2021;17(1):1876–1881. doi: 10.1016/j.sapharm.2020.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Elayeh E, Akour A, Haddadin RN. Prevalence and predictors of self-medication drugs to prevent or treat COVID-19: experience from a Middle Eastern country. Int J Clin Pract. 2021;75:e14860. doi: 10.1111/ijcp.14860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mishra D, Shetty B, Guddattu V, Chandrasekaran V. Self-medication among adults in urban Udupi taluk, southern India. Int J Med Sci Public Health. 2016;6(3):126–129. doi: 10.5530/ijmedph.2016.3.6 [DOI] [Google Scholar]