Summary
Terahertz (THz) waves are ranged between microwave and infrared region in the electromagnetic spectrum. THz technology has been demonstrated promising potential for biomedical applications. Exploration of biological effects of THz waves has emerged as a critical new area in life sciences. It is critical to uncover the effects of THz waves on complex biological systems in order to lay out the framework for THz technology development and future applications. Specifically, THz radiation has been shown to affect the nervous system, including the structure of nerve cell membranes, genes expressions, and cytokines level. In this review, we primarily discuss the biological impacts and mechanisms of THz waves on the nervous system at the organisms, cellular, and molecular levels. The future application perspectives of THz technologies in neuroscience are also highlighted and proposed.
Subject areas: Radiation physics, Neuroscience, Biomedical engineering
Graphical abstract
Radiation physics; Neuroscience; Biomedical engineering
Introduction
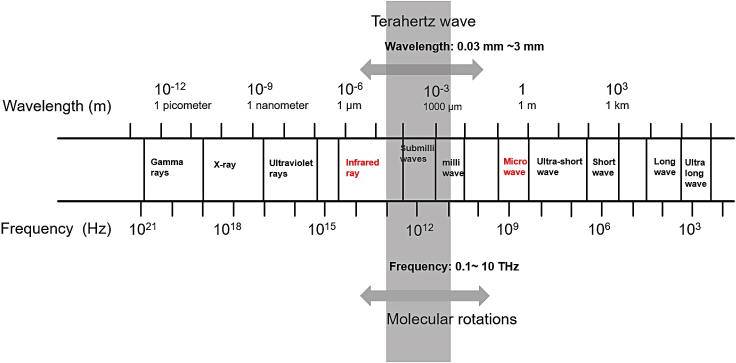
Terahertz (THz) wave is an electromagnetic wave with a frequency range of 0.1–10 THz (1 THz = 1012 Hz) and a wavelength of 0.03–3 mm (Figure 1). Due to the immaturity of THz wave generation, transmission, and detection systems, this band was once referred to as the "THz Gap" in the electromagnetic spectrum (Globus, 2016; Smye et al., 2001). With the promising advancement of scientific methodologies in materials and optics since the 1980s, this gap has been filled, resulting in the rapid development and progress of THz technology.
Figure 1.
Terahertz band in the electromagnetic spectrum and a molecular transition associated with it
THz waves are a novel type of radiation with several distinct advantages, including quantum and electronic properties, non-ionizing features, and easy absorbance by water (Kristensen et al., 2010). THz waves offer the following benefits in terms of application: first, THz waves, for example, have a high permeability to a wide range of materials, allowing them to be used for nondestructive detection (Mao et al., 2020). Second, THz photons have low energy, which means they will not cause tissue ionization or damage, making them ideal for live detection of the human body or biological samples (Sun et al., 2018). Third, THz imaging can be used to detect tumors because the water content of tumor tissues differs greatly from that of normal tissues (Chen et al., 2011; Yamaguchi et al., 2016). Forth, the THz wave's high coherence aids in determining the precise refractive index and absorption coefficient of samples (Han et al., 2018).
As a result, THz technology has shown a promising potential for application in a variety of fields in the future, including communication, security inspection, and biomedicine (Ghafoor et al., 2020; Son et al., 2019; Tzydynzhapov et al., 2020). More specifically, in neuroscience, THz technology has been used as a tool for the distinction between benign and malignant brain tumors (Ji et al., 2016; Oh et al., 2014; Wu et al., 2019). In the last decade, an increasing line of evidence has revealed the physical consequences of THz radiation on the structure and functions of the nervous system, and has predicted a promising perspective of THz for neuroscience research and clinical practice in neurology. In this review, we summarize the brief biological mechanisms of THz radiation and highlight the recent research progress on the biological impacts of THz waves in the nervous system. The applications of THz technology in neuroscience are further discussed.
Effects of Terahertz radiation on the nervous system
Effects of Terahertz waves on patients and animal models of neurological diseases
Nervous system is more vulnerable to exogenous stimuli of THz waves, due to its bioelectric basis of functional activity. THz therapy can be used under various neurological conditions to either ameliorate disease symptoms or rescue disease pathologies, in the same way, that physical therapy dose. For example, Reukov et al. treated patients with acute ischemia stroke using infrared radiation modulated by THz frequencies (Reukov et al., 2016). THz waves (0.02–8 THz, 2.4 mW/cm2, 22.5 min) were used to stimulate the Bai Hui acupuncture point on the top of the head, which is one of the most critical areas for regulating neurovascular activity (Wang et al., 2014). Patients in the THz group regained consciousness and resolved neurological symptoms faster than those in the control group. The tissue temperature did not rise more than 0.1°C during the entire irradiation process, suggesting a nonthermal effect of THz therapy. Improving oxygen delivery in the brain by increasing blood oxygen volume, promoting neuronal rejuvenation are all possible underlying mechanisms for the beneficial outcomes of THz radiation therapy.
In vivo, nitrogen oxides can be resonant with THz waves generated by an NO laser with a range of 150.176–150.664 GHz. Nitric oxide can regulate physiological, pathophysiological, and biochemical processes. THz radiation can affect the central nervous system and behavioral performance of animal. Under stress conditions, THz radiation (NO frequency range, 150.17–150.664 GHz, 0.2 mW/cm2, 30 min) could effectively prevent the changes in horizontal and vertical activity as well as exploratory behavior in male rats (Kirichuk et al., 2014). In contrast, THz (3.6 THz, 23.6 mW/cm2, 30 min) irradiation increased the anxiety level of animals (Bondar et al., 2008). In the experiment of THz radiation (the frequency range, 150.17–150.664 GHz, 3 mW/cm2, 60 min), it was found that THz radiation induced depression in rats (Kirichuk et al., 2009). Male rats exposed to low-intensity THz irradiation (167 GHz, 5 days) were able to maintain their natural exploratory abilities. When the frequency was simply decreased to 144 GHz, however, the experimental animals displayed anxious behavior, reduced appetite and sleep time, as well as aggressive behavior (Kirichuk and Ivanov, 2013). THz radiation has a variety of effects that are dependent on the frequency. On different frequencies of THz waves, experimental animals showed different behavioral changes. Effects of THz radiation on the nervous system in vivo are shown in Table 1.
Table 1.
Effects of THz radiation on nerve system in vivo
| In vivo models | Frequency (THz) | Intensity, (mW/cm2) | Exposure time | Behavioral effects | Reference |
|---|---|---|---|---|---|
| HUMAN human subjects | 0.02–8 THz | 2.4 mW/cm2 | 22.5 min | Improve the symptoms of neurological defects | (Reukov et al., 2016) |
| RAT male albino rats | 0.15 THz | 0.2 mW/cm2 | 30 min | Improve behavioral abnormalities | (Kirichuk et al., 2014) |
| Male albino rats | 0.15 THz | 3 mW/cm2 | 60 min | Induce signs of depression. | (Kirichuk et al., 2009) |
| Male rats | 0.167 THz | n.s | 5 days | Maintain normal ability to explore new things | (Kirichuk and Ivanov, 2013) |
| Male rats | 0.144 THz | n.s | 5 days | Increase anxious and aggressive behavior, reduce appetite and sleep time | (Kirichuk and Ivanov, 2013) |
| MOUSE male mice | 3.6 THz | 23.6 mW/cm2 | 30 min | Increase the anxiety level of animals | (Bondar et al., 2008) |
n.s, not specified.
Effects of Terahertz radiation on nerve cells
Olshevskaya et al. reported a change in cell membrane adhesion and axon structure in isolated lymnaea stagnalis neurons exposed to THz irradiation (0.72 THz, 10–20 mW/cm2, 60 min) (Olshevskaya et al., 2008). Structural changes in the soma membrane, axon, and growth cone were observed after THz irradiating the same neurons (3.68 THz, 10–20 mW/cm2, 60 min). The biological radiation effects described above mostly occurred during the early stages of neuronal development. Neurite growth cones are primarily destroyed at later stages of neurite outgrowth, disrupting neuronal contacts (Olshevskaya et al., 2008). When the same neurons were used, it was found that cell death varied depending on the frequency and power density of THz radiation (Cherkasova et al., 2020). At high-power THz radiation (30 mW/cm2), cell death occurred 2 h after irradiation with a constant frequency and exposure time (2.3 THz, 1 min). Low-power THz radiation (3 mW/cm2), on the other hand, significantly promoted cell survival. Moreover, the THz radiation with much lower power (0.3 mW/cm2) improved cell survival. Tsurkan et al. applied broadband pulsed THz radiation (frequency: 0.05–2 THz, power density: 0.5–50 μW/cm2) to the sensory ganglion of chicken embryos and found 0.5 but not 5 and 50 μW/cm2 power accelerated ganglion growth (Tsurkan et al., 2012). Interestingly, during the experiment, the sample's temperature remained constant, indicating a non-thermal effect of THz irradiation. Broadband THz wave irradiation on PC12 cells resulted in changes in the shape and length of synapses, implying stimulated nerve growth (Romanenko et al., 2020). These findings suggest that THz radiation has both stimulatory and inhibitory effects on nerve cells. THz radiation's effects on cells are proportional to the irradiation power, and these changes could be linked to molecular regulation or structural changes in individual neurons.
THz radiation (2.3 THz; Mean intensity, 0.5 to 20 mW/cm2, 0.6 min) was applied to neurons, increasing cell membrane permeability that allowed impermeable compound dyes to enter the cytoplasm (Cherkasova et al., 2020). When the radiant frequency was adjusted to 2.0 THz, most exposed cells did not alter much (Cherkasova et al., 2020). Only a few individual neurons were uniformly stained and had a low or no membrane potential, comparable to those seen in controls. As a result, the alteration of the cell membrane permeability is dependent on the THz radiation frequency. The biological effects described above occurred without a discernible increase in ambient temperature, implying a non-thermal effect caused by excessive THz radiation. It lays the groundwork for THz technology's future use in regulating the transport of specific compounds. In PC12 neuron-like pheochromocytoma cells, THz radiation (0.3–19.5 THz, 10 min) can increase membrane permeability (Perera et al., 2019). The passage of silica nanospheres (d = 23.5 nm) and their clusters (d ≈ 63.9 nm) through the cell membrane was observed using high-resolution transmission electron microscopy (Perera et al., 2019). Approximately 95% of PC12 cells could take up silica nanospheres after THz irradiation, compared to only 4.5% of PC12 cells without THz irradiation. These findings shed light on nanodrug delivery and gene therapy with the assistance of THz technology.
THz radiation (0.12–0.18 THz, 3.2 mW/cm2) was used to test the cytotoxicity of rat glial cells. The relative number of apoptotic cells was 1.5, 1.8, and 2.4 times at the first, third, and fifth minutes of irradiation (Borovkova et al., 2017). The increase of cell ambient temperature is less than 0.1°C (Borovkova et al., 2017). Considering the potential cytotoxicity on neurons, the biosafety of THz radiation deserves more attention. More research is required in the future to determine the biosafety threshold of THz radiation. Effects of THz radiation on nerve cells are shown in Table 2.
Table 2.
Effects of THz radiation on cells in vitro
| Cells in vitro study | Frequency (THz) | Intensity, (mW/cm2) | Exposure time | Effects | Reference |
|---|---|---|---|---|---|
| Neurons of L. stagnalis | 0.72 THz | 10–20 mW/cm2 | 60min | Changes in cell membrane adhesion | (Olshevskaya et al., 2008) |
| 3.68 THz | 10–20 mW/cm2 | 60min | Destruction of growth cones, breaking neural connections. | (Cherkasova et al., 2020) | |
| 2.3 THz | 30 mW/cm2 | 1 min | Cell death occurred 2 h after irradiation | (Cherkasova et al., 2020) | |
| 2.3 THz | 3 mW/cm2 | 1min | Cell death occurred 3 h after irradiation | (Cherkasova et al., 2020) | |
| 2.3 THz | 0.3mW/cm2 | 1 min | Membrane changes | (Cherkasova et al., 2020) | |
| 2.3 THz | 0.5 to 20 mW/cm2 | 0.6 min | Changes in the cell membrane permeability | (Cherkasova et al., 2020) | |
| Sensory ganglion of chicken embryos | 0.5 THz | 0.5–50 μW/cm2 | 3 min | Accelerating ganglion growth | (Tsurkan et al., 2012) |
| PC12 cells | n.s. | n.s. | n.s. | Changes in the shape and length of synapses | (Romanenko et al., 2020) |
| PC12 neuron-like pheochromocytoma cells | 0.3–19.5 THz | n.s. | 10 min | Changes in the cell membrane permeability | (Perera et al., 2019) |
| Glial cells | 0.12–0.18 THz, | 3.2 mW/cm2 | 5min | Cell death | (Borovkova et al., 2017) |
| Primary cultured neurons | 0.16–0.17 THz | n.s. | 6min,10min | Changes in neurotransmitter | (Tan et al., 2019) |
n.s, not specified.
Biological mechanisms involved in Terahertz waves
The energy of THz waves is as low as a few millivolts, and the mechanisms of their impacts on living beings are quite different from that of high-energy and irreversibly destructive electromagnetic radiation, such as X-rays and gamma rays. Thermal and non-thermal effects are two basic categories of the biological mechanisms of THz radiation.
Thermal effect
The thermal effect of THz radiation is usually observed using a high-energy THz radiation approach since THz radiation has a low photon energy of about 0.3 meV and does not induce a considerable temperature rise in the biological matter (Dalzell et al., 2010; Wilmink et al., 2011). Biological mediums including water have a solid ability to absorb THz radiation and transform the absorbed radiation energy into heat energy, leading to the thermal effect (Kristensen et al., 2010). The thermal effect of THz wave radiation is influenced by various factors, including the frequency, spot size, exposure duration, radiation capacity density, and beam profile of THz exposure, as well as the refractive index, absorption rate, and scattering rate of biological tissues (Wilmink and Grundt, 2011). The frequency domain of THz wave contains the stretching and bending vibration modes of hydrogen bonding between water molecules. The substantial absorption of THz waves by water is promoted by these vibration models (Suzuki et al., 2009). As a result, without the above energy conversion mechanism, the temperature of the object irradiated by THz waves will rise directly. Thermal effects in biological tissues frequently result in structural protein degeneration, cell death, tissue coagulation, activation of the intracellular stress response, and organelle dysfunction (Dalzell et al., 2010; Wilmink and Grundt, 2011; Wilmink et al., 2011). As a result, thermal effects are critical for defining THz waveband safety guidelines. The thermal effects of THz radiation on organism vary greatly, according to the types of organs, tissues, cells, and the exposure period (Wilmink and Grundt, 2011). The temperature change is normally on a macroscopic level, which is correlated with the organism's microenvironment, including water content, acid-base balance, energy metabolism, etc (Wilmink and Grundt, 2011). Different tissues respond to THz radiation in different ways due to the complexity and variation of these factors. Despite of this, all biological tissues have a comparable response to THz wave radiation.
Nonthermal effect
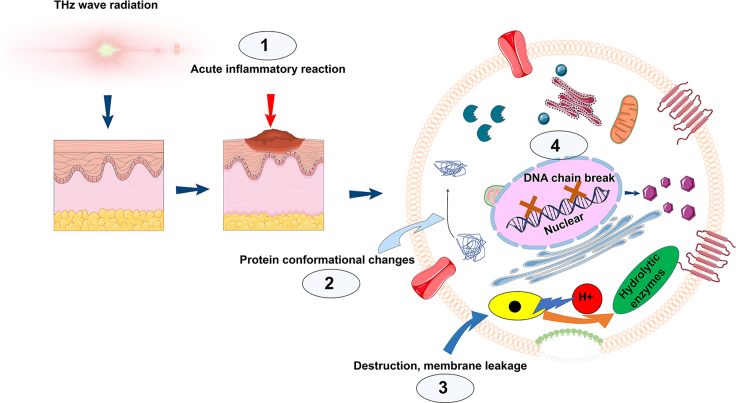
Compare to the thermal effect, nonthermal effect of THz wave is much more complicated and worth of thorough investigation. Non-thermal effect is revealed when the radiation temperature is controlled. Frohlich was the first to propose the concept of "coherent states" (Fedorov et al., 2003; Fröhlich, 1980), which was a watershed moment for the theory of non-thermal effects of electromagnetic radiation. THz radiation primarily interacts with hydrogen bonds in biomolecules (Fischer et al., 2002), creating low-frequency intramolecular vibrations that cause protein structural changes (Cherkasova et al., 2009). THz radiation has an energy scale that is close to that of hydrogen bonds, so it is thought that such nonionizing electromagnetic fields can have a significant impact on cells and biomolecules (Goodman and Blank, 2002). These motions typically occur in the THz range (0.1–2 THz) (Scaramozzino et al., 2020). A mathematical model of DNA respiration predicted that THz radiation can affect gene expression and DNA replication by forming local openings in the DNA helix via nonlinear resonance (Alexandrov et al., 2010). Nonthermal effects can change protein conformation (Borovkova et al., 2017), influence DNA double chain stability (Alexandrov et al., 2010), change membrane permeability (Cherkasova et al., 2020), and generate acute inflammatory reactions (Figure 2) (Hwang et al., 2014), therefore impacting the morphology and structure of neurons and inducing apoptosis (Borovkova et al., 2017; Zhao et al., 2014). Further experiments in animals revealed that THz radiation can induce animal behavior changes (Bondar et al., 2008; Kirichuk et al., 2014).
Figure 2.
Non-thermal biological effects of Thz radiation
THz waves generated by THz sources have been proven to trigger acute inflammatory reactions in skin tissues (1), protein conformational changes (2), disruption or leakage of plasma membranes (3), and disruption of DNA double-strand replication or repair (4).
Molecular basis of the impacts of Terahertz radiation on the nervous system
Proteins and nucleic acids are critical components of the nervous system. Low-frequency resonances of biomacromolecules are generated by THz wave radiation, which affects protein spatial conformation and DNA double-strand stability (Alexandrov et al., 2010). An in-depth investigation of the biomolecular action mechanism of THz wave radiation on the nervous system will provide a theoretical foundation for its future use in neuroscience.
In primary hippocampal neurons, the expression of synaptic-related proteins (SYN) was significantly reduced in the group that received THz radiation (0.16 THz, 60 min). Synaptic-related proteins (PSD-95) expression decreased significantly in primary cortical neurons, and the extent of PSD-95 expression was closely related to exposure time and wavelength. After THz wave irradiation, there was no significant difference in the expression of SYN and PSD95 in MN9D cells (Tan et al., 2019). THz radiation can thus regulate different protein expressions in different neuronal cells.
Glial cells are characterized by a high density of proteins associated with ion channels at the cell membrane surface. THz radiation was used to irradiate rat glial cells (0.12–0.18 THz, 3.2 mW/cm2, 1 min). Depolarization of the plasma membrane, lysosomal, and mitochondrial membranes causes conformational changes in membrane ion channel proteins, affecting intracellular environmental homeostasis and resulting in the release of apoptotic proteins from mitochondria and proteolytic enzymes from lysosomes. Glial cells also contain a variety of aquaporins, which can disrupt cell morphology and metabolic function if their conformation changes (Borovkova et al., 2017). THz radiation at specific frequencies can change the permeability of nerve cell membranes (Cherkasova et al., 2020; Perera et al., 2019). It could be linked to membrane protein conformational changes caused by THz radiation and the formation of hydrophilic pores (Borovkova et al., 2017). THz radiation (2.31 THz, 0.18 W/cm2, 35 s) caused E. coli/pKatG-gfp biosensor cells to produce green fluorescent protein (Serdyukov et al., 2021).
Worth noting, THz waves, on the other hand, showed no or minimal effect on neuronal differentiation and survival. The differentiation ability of neurons and the expressions of glutathione were not affected when dorsal root ganglion neuroblasts (ND7/23 cell line) were exposed to THz radiation (0.14 THz, power density: 24 mW/cm2–62 mW/cm2, 24 h) (Bourne et al., 2008). The influence of THz radiation on cells is directly related to the irradiation parameters, and cells themselves also make certain adaptive adjustments to external stimuli, which may be related to the regulation of macromolecules in neurons or the changes in structural morphology.
Despite proteins, THz radiation may also have biological effects on nucleic acids. THz waves (0.1 THz, average power density: 33 mW/cm2, 20 min) were used to irradiate primary hippocampal neurons that their gene expressions were analyzed using the RNA-SEQ technique (Shang et al., 2021). A total of 111 genes were up-regulated while 54 were downregulated, implying that THz radiation impacts gene expression. The transcription products involved in regulating neuronal growth, axonal genesis, and synaptic protein expression are enriched in DEGs and DETs analysis and GO/KEGG enrichment analysis (Shang et al., 2021). THz radiation may affect the interaction between transcription factor (TF) and DNA, and further regulate gene expression. Studies showed that THz radiation reduced the binding efficiency of transcription factor (TF) AP-1 and its transcription factor binding site (TFBS) in DNA (Shang et al., 2021). THz irradiation is thought to cause biological macromolecular interactions that regulate neuronal function. Irradiation with THz waves (0.22 THz, 25 mW/cm2, 5 min) significantly and dose-dependently reduced the length and number of protrusions in Neuro-2A cells, significantly decreased the expression of TUBB3 and SYP genes involved in protrusion growth and function, but had no effect on the expression of BAX and BCL2 genes (Ma et al., 2020). Highly differentiated neuroepithelial cells make up the photoreceptor cells in the retina's outer layer. Lu et al. irradiated retinal tissue of C57BL/6J mice with a THz wave (1–3.5 THz, 80 mW/cm2, 2 min), and RNA-SEQ technique analysis revealed 625 up-regulated and 9 down-regulated genes (Lu et al., 2020). KEGG signaling pathway analysis showed that the differential genes mainly affected the PPAR signaling pathway, cancer-related signaling pathway, and calcium ion signaling pathway. The expression of these highly abnormal genes can damage retinal tissue and interfere with photosensitive neurons' signal transduction. Skin is the most direct target of THz irradiation. Mice were exposed to femtosecond-THz pulses (2.5 THz, 0.32 W/cm2, 1 h) and 149 genes were found to be differentially expressed (Kim et al., 2013). Wound healing and tissue growth involved 82 genes that were upregulated and 67 genes that had their activity downregulated. Genomics has revealed that TGF plays a major role in trauma response (Kim et al., 2013). THz radiation activates the TGF-signaling pathway in wound skin tissue, which disrupts the wound healing process in vivo. These findings may shed light for future application of THz in neuroscience to modulate various growth factors or neurotrophic factors.
Neurotransmitters are important mediators of information transmission that control basic physiological functions in humans. As a result, further research into the generation and transmission mechanisms of neurotransmitters by THz radiation is required. In terms of neurotransmitter metabolisms, four rat brain regions (hippocampus, cerebral cortex, cerebellum, and brainstem) and three types of neurons-like cells (MN9D, PC12, and HT22 cells) showed significant changes in neurotransmitter content after radiations of two different THz parameters (0.16 THz, output power 50 mW; or 0.17 THz, output power 10 mW) (Tan et al., 2019). The changes in neurotransmitters after THz radiation are described below. Glutamate (Glu) decreased significantly in hippocampal neurons for 6 and 60 min of radiation at the same output of 50 mW, while alanine (Ala) and glycine (Gly) increased significantly at the same exposure time of 60 min for 50 mW and 10 mW output. Only Ala increased significantly in cerebellar neurons under all irradiation conditions. There was a significant reduction in Glu in brainstem neurons for 6 and 60 min of radiation at the same output of 50 mW. Glu increased and Ala decreased significantly in cortical neurons at the exposure time of 60 min for 10 mW. Compared with the controls, there was a subtle significant difference in neurotransmitters in PC12 and HT22 cells. Only ALa was significantly increased in MN9D cells at the exposure time of 60 min for 10 mW. Effects of THz radiation on neurotransmitters are shown in Table 3.
Table 3.
Effects of THz radiation on neurotransmitters
| Cells | Different treatments | Neurotransmitter changes |
Reference | ||
|---|---|---|---|---|---|
| Glu | Gly | Ala | |||
| Primary hippocampal neurons | 10 mW-6 min | NS | NS | NS | All data from (Tan et al., 2019) |
| 10 mW-60 min | NS | ↑ | ↑ | ||
| 50 mW-6 min | ↓↓ | NS | NS | ||
| 50 mW-60 min | ↓↓ | ↑ | NS | ||
| Primary cortical neurons | 10 mW-6 min | NS | NS | NS | |
| 10 mW-60 min | ↑↑ | NS | ↓↓ | ||
| 50 mW-6 min | NS | NS | NS | ||
| 50 mW-60 min | NS | NS | NS | ||
| Primary cerebellar neurons | 10 mW-6 min | ↓↓ | NS | ↑↑ | |
| 10 mW-60 min | ↓↓ | NS | ↑↑ | ||
| 50 mW-6 min | ↓↓ | NS | ↑↑ | ||
| 50 mW-60 min | ↓↓ | NS | ↑↑ | ||
| Primary brainstem neurons | 10 mW-6 min | NS | ↑↑ | NS | |
| 10 mW-60 min | NS | ↑↑ | NS | ||
| 50 mW-6 min | ↓↓ | ↑↑ | ↑ | ||
| 50 mW-60 min | ↓↓ | ↑↑ | ↑↑ | ||
| MN9D cells | 10 mW-6 min | NS | NS | NS | |
| 10 mW-60 min | NS | NS | ↑↑ | ||
| 50 mW-6 min | NS | NS | NS | ||
| 50 mW-60 min | ↓ | NS | ↑ | ||
| PC12 cells | 10 mW-6 min | NS | NS | NS | |
| 10 mW-60 min | NS | NS | NS | ||
| 50 mW-6 min | NS | NS | NS | ||
| 50 mW-60 min | NS | ↑ | NS | ||
| HT22 cells | 10 mW-6 min | NS | NS | NS | |
| 10 mW-60 min | NS | NS | NS | ||
| 50 mW-6 min | NS | NS | NS | ||
| 50 mW-60 min | NS | NS | NS | ||
(↑ or ↓), p < 0.05 vs control group; (↑↑ or ↓↓), p < 0.01 vs control group; NS: no significance.
These findings show that neuronal cells are sensitive to THz radiation and that THz radiation has a significant impact on neuronal functions such as protein expression, gene regulation, neurotransmitter production. As a result, elucidating the properties and safety of the THz effect requires a thorough examination of the function, dose-effect characteristics, and possible mechanisms on sensitive cells and tissues in the nervous system.
Applications of Terahertz technology in neuroscience
The physicochemical properties of THz wave make it a promising candidate for biomedical applications. THz wave can be used to distinguish different biomolecules and analyze the conformational changes in molecules because many vital molecules vibrate at a low frequency and the energy level of intermolecular forces is in the frequency range of the THz wave (Mehrotra et al., 2019). THz technology can currently be used in two major biomedical fields: THz spectrum and THz imaging.
Applications of Terahertz spectroscopy in neuroscience
THz spectroscopy is widely used to investigate protein conformational changes and quantify intermolecular interactions (Xie et al., 2014). The THz time-domain system (THz-TDS) is the most commonly used technology in current research to classify tissues in various disease models. Amyloid aggregation and neurofibrillary tangles play a key role in Alzheimer's disease in terms of neurodegeneration. THz spectrum in mouse brain tissues with Alzheimer's disease was compared with normal mice in an experimental animal study (Shi et al., 2016). The absorption coefficient of mice with Alzheimer's disease was higher than that of normal mice at 1.44 THz, 1.8 THz, and 2.14 THz, while the refraction coefficient was significantly higher in mice with Alzheimer's disease than in normal mice. Using THZ-TDS detection, Png et al. discovered that the refractive index of brain tissue from patients with Alzheimer's disease was significantly higher than that of healthy patients (Png et al., 2009), which was linked to amyloid aggregation and fibrosis in Alzheimer's disease brain tissue samples. THz-TDS was found up to 95.5% accurate in the early diagnosis of explosive brain injuries in another study (Wang et al., 2020). The findings revealed that in the early stages of the disease, there were differences in the serum and cerebrospinal fluid of blast-induced traumatic brain injury (bTBI) rats. Lipid is a non–water-soluble organic compound whose primary functions include the storage of energy transduction signals and the construction of cell membranes. Myelin deficiency can lead to a variety of central nervous system diseases, but there are no reliable methods to detect it. In 2017, Zou et al. attempted to use THz spectroscopy to detect myelin deficiency in rhesus monkey models (Zou et al., 2017). The standard amplitude of myelin deficient monkey was significantly lower than that of control monkey at 0.5 THz, 1.0 THz, respectively. It demonstrates that this technique can quickly and effectively detect myelin deficiency in brain tissue. Water has a significant impact on THz detection, which can be classified as dehydrated or nondehydrated status. THz spectroscopy can be used to distinguish between normal and tumor tissue that is dehydrated. The refractive index, absorption coefficient, and dielectric constant of paraffin-embedded gliomas and normal brain tissue were measured in the range of 0.2–2.0 THz using the THz-TDS system (Meng et al., 2014). Paraffin-embedded gliomas had a higher refractive index, absorption coefficient, and dielectric constant than normal brain tissue. Because of these differences, the THz spectrum may be used to identify tumor regions. The dielectric constants of cancer cells (DLD-1, HEK293, and HeLa) were studied using THz time-domain attenuated total reflection spectroscopy (THZ-ATR). Water molecules in cancer cells exhibit a different dielectric response than extracellular fluid below 1.0 THz (Shiraga et al., 2014).
Application of Terahertz imaging in neuroscience
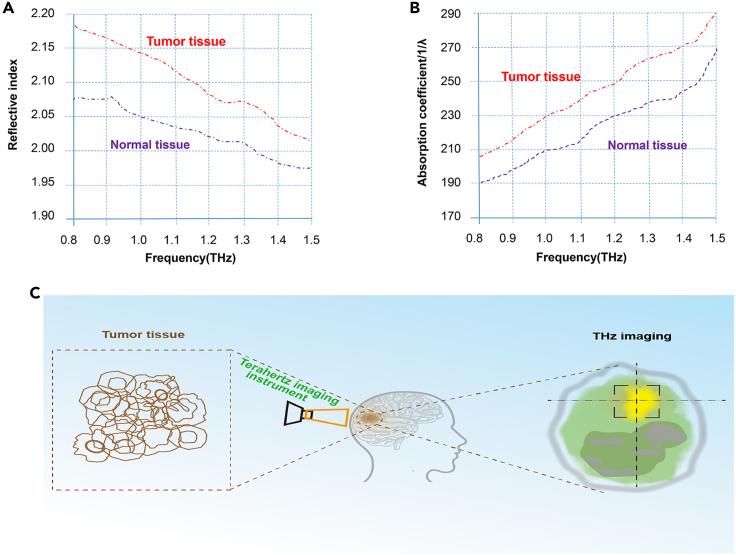
THz imaging is a new high technology with promising application perspectives in the field of neuroscience. In vivo imaging experiments are relatively rare, mostly focusing on the in vitro identification of benign and malignant tumors. THz imaging is a image spectrum synthesis that uses amplitude and phase information to extract tissue morphological characteristics in real time (Oh et al., 2014). THz imaging relies on changes in tissue's water content. Based on their water content, different tissues exert different THz spectrum characteristics, which help distinguish tumor from nontumor tissue, as schematically illustrated in Figures 3A and 3B (Yamaguchi et al., 2016). Using a reflect-type THz imaging system, Oh et al. compared viewable images in brain tissue section samples, as schematically illustrated in Figure 3C. Depending on the water content of tumor or non-tumor tissue, different THz spectrum performances were observed (Oh et al., 2014). The diagnostic value of THz imaging has been demonstrated in other glioma models (Ji et al., 2016; Wu et al., 2019; Yamaguchi et al., 2016).
Figure 3.
Application of THz imaging in tumor diagnosis
(A) The refractive index of THz wave at different frequencies of tumor tissue was significantly different from those of normal tissue (Figure 3A). Figure 3A was adapted from Figure 1 in Yamaguchi et al. (2016), used under CC BY 4.0.
(B) The absorption spectra of THz wave at different frequencies of tumor tissue were significantly different from those of normal tissue (Figure 3B). Figure 3B was adapted from Figure 1 in Yamaguchi et al. (2016), used under CC BY 4.0.
(C) The THz imaging pattern is shown in (Figure 3C).
Compare to THz spectroscopy, THz imaging allows for more intuitive and convenient tissue analysis, and future THz spectroscopy and THz imaging technique sets may be combined to allow for the collection of more comprehensive tissue data for clinical applications.
The current challenges and future perspectives
Electronic and photonic properties of the THz wave have made it a frontier field of study. Due to the advancement of THz wave emission source and detection technology, the THz technology has rapidly developed in medical diagnosis and therapy. THz wave has a complex interaction with biomacromolecules, and exploring the biological effects of THz radiation and its mechanism of action has become a major hot topic in life science. Though current research in this area is still limited, there is a lot to be explored about its potential medical applications.
Biosafety concern of Terahertz radiation
Today, electromagnetic radiation is nearly everywhere, and low-intensity radiation has little effect on people's health. On the other hand, a high quantity of electromagnetic radiation will be damaging to the human body in the long run (Miller et al., 2019). Biosafety concern is thus a prerequisite for THz radiation's future usage. To provide evidence for a safe THz radiation threshold, further validation at the organism, biological tissue level is required. The consequences of long-term chronic exposure on humans must be taken into consideration. A thorough and systematic investigation into the mechanisms by which THz waves affect every structural level of the organism is required.
The standardization of experimental Terahertz system
THz radiation experimental parameters such as radiation frequency, power density, pulse parameters, and others directly influence biological outcomes. Different biological species genera and experimental environmental temperatures indirectly affect biological effects. In the future, THz radiation parameters must be scientifically standardized to ensure reproducibility and comparability of experiments.
Exploration insight into the effects and mechanisms of Terahertz radiation on neurological diseases
To explore the systemic impacts of THz radiation, biomacromolecules, gene networks, and signaling pathways must be involved (Kim et al., 2013; Weightman and Peter, 2012). Autophagy, oxidative stress, cell inflammation, and cell apoptosis may be influenced during THz radiation. It is expected that an in-depth look at biological events such as membrane permeability and cell death will provide theoretical support for the development of THz wave biomedical applications in a variety of fields. On the other hand, THz radiation's effect on biological tissues is closely related to the species of biological tissues as well as their dielectric properties as well as their biomacromolecule composition, water content, and origin. THz radiation's biological effects are complex and diverse because of the factors listed above. THz energy absorption and distribution studies should be conducted in the future using dielectric characteristics of biological tissues, water content, transmittance, refractive index, and macromolecular composition to reveal the biological effect characteristics and corresponding mechanisms of different organisms and clarify the critical biological mechanism.
Conclusions
THz science is a multidisciplinary field that combines elements of electronics and photonics. THz technology and related equipment are useful in fields such as medicine, security inspection, and the military. THz radiation's neurobiological effects have emerged as a hot topic in biomedical research, and they will need to be investigated further in the future. Its mechanism of action, as well as its biosafety, must be clarified. These research results will help in the exploration of THz wave applications in the nervous system and have far-reaching implications. Therapeutic systems using THz wave generators will open up countless possibilities for the medical field if THz science can be better integrated with other related disciplines such as physics, medicine, materials science, and others.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC81771521). All the information prepared for this review paper came from PubMed, Springer, Web of Science, and Google Scholar with the relevant publications until June 2021, including original clinical and basic research articles, and prospective/retrospective reviews.
Author contributions
Jun Zhang: Collect published data; write original draft and revise the manuscript according to reviewer's suggestion.
Song Li: Help collect supplementary materials; help edit the revised manuscript.
Weidong Le: Project administration; proposing the original concept of this review paper, and help edit the revised manuscript.
Declaration of interests
The authors declare no competing interests.
References
- Alexandrov B.S., Gelev V., Bishop A.R., Usheva A., Rasmussen K.Ø. DNA breathing dynamics in the presence of a terahertz field. Phys. Lett. A. 2010;374:1214–1217. doi: 10.1016/j.physleta.2009.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondar N.P., Kovalenko I.L., Avgustinovich D.F., Khamoyan A.G., Kudryavtseva N.N. Behavioral effect of terahertz waves in male mice. Bull. Exp. Biol. Med. 2008;145:401–405. doi: 10.1007/s10517-008-0102-x. [DOI] [PubMed] [Google Scholar]
- Borovkova M., Serebriakova M., Fedorov V., Sedykh E., Vaks V., Lichutin A., Salnikova A., Khodzitsky M. Investigation of terahertz radiation influence on rat glial cells. Biomed. Opt. Express. 2017;8:273–280. doi: 10.1364/BOE.8.000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne N., Clothier R.H., D'Arienzo M., Harrison P. The effects of terahertz radiation on human keratinocyte primary cultures and neural cell cultures. Altern. Lab. Anim. 2008;36:667–684. doi: 10.1177/026119290803600610. [DOI] [PubMed] [Google Scholar]
- Chen H., Lee W.J., Huang H.Y., Chiu C.M., Tsai Y.F., Tseng T.F., Lu J.T., Lai W.L., Sun C.K. Performance of THz fiber-scanning near-field microscopy to diagnose breast tumors. Opt. Express. 2011;19:19523–19531. doi: 10.1364/Oe.19.019523. [DOI] [PubMed] [Google Scholar]
- Cherkasova O.P., Fedorov V.I., Nemova E.F., Pogodin A.S. Influence of terahertz laser radiation on the spectral characteristics and functional properties of albumin. Opt. Spectrosc. 2009;107:534–537. doi: 10.1134/s0030400x09100063. [DOI] [Google Scholar]
- Cherkasova O.P., Serdyukov D.S., Ratushnyak A.S., Nemova E.F., Kozlov E.N., Shidlovskii Y.V., Zaytsev K.I., Tuchin V.V. Effects of terahertz radiation on living cells: a review. Opt. Spectrosc. 2020;128:855–866. doi: 10.1134/s0030400x20060041. [DOI] [Google Scholar]
- Dalzell D.R., McQuade J., Vincelette R., Ibey B., Payne J., Thomas R., Roach W.P., Roth C.L., Wilmink G.J. 4. Optical Interactions with Tissues and Cells XXI Held in San Francisco, California, United States, 25 February 2010. SPIE; 2010. Damage thresholds for terahertz radiation; p. 75620M. [DOI] [Google Scholar]
- Fedorov V.I., Popova S.S., Pisarchik A.N. Dynamic effects of submillimeter wave radiation on biological objects of various levels of organization. Int. J. Infrared Millim. Waves. 2003;24:1235–1254. doi: 10.1023/a:1024801304083. [DOI] [Google Scholar]
- Fischer B.M., Walther M., Uhd Jepsen P. Far-infrared vibrational modes of DNA components studied by terahertz time-domain spectroscopy. Phys. Med. Biol. 2002;47:3807–3814. doi: 10.1088/0031-9155/47/21/319. [DOI] [PubMed] [Google Scholar]
- Fröhlich H. In: Advances in Electronics and Electron Physics. Marton L., Marton C., editors. Academic Press; 1980. The biological effects of microwaves and related questions; pp. 85–152. [DOI] [Google Scholar]
- Ghafoor S., Boujnah N., Rehmani M.H., Davy A. MAC protocols for terahertz communication: a comprehensive survey. IEEE Commun. Surv. Tutor. 2020;22:2236–2282. doi: 10.1109/comst.2020.3017393. [DOI] [Google Scholar]
- Globus T. Sub-terahertz vibrational spectroscopy with high resolution for biological molecules and cells identification. J. Biomol. Res. Ther. 2016;5:e150. doi: 10.4172/2167-7956.1000e150. [DOI] [Google Scholar]
- Goodman R., Blank M. Insights into electromagnetic interaction mechanisms. J. Cell. Physiol. 2002;192:16–22. doi: 10.1002/jcp.10098. [DOI] [PubMed] [Google Scholar]
- Han X., Yan S., Zang Z., Wei D., Cui H.L., Du C. Label-free protein detection using terahertz time-domain spectroscopy. Biomed. Opt. Express. 2018;9:994–1005. doi: 10.1364/BOE.9.000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang Y., Ahn J., Mun J., Bae S., Jeong Y.U., Vinokurov N.A., Kim P. In vivo analysis of THz wave irradiation induced acute inflammatory response in skin by laser-scanning confocal microscopy. Opt. Express. 2014;22:11465–11475. doi: 10.1364/OE.22.011465. [DOI] [PubMed] [Google Scholar]
- Ji Y.B., Oh S.J., Kang S.-G., Heo J., Kim S.-H., Choi Y., Song S., Son H.Y., Kim S.H., Lee J.H. Terahertz reflectometry imaging for low and high grade gliomas. Sci. Rep. 2016;6:1–9. doi: 10.1038/srep36040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.T., Park J., Jo S.J., Jung S., Kwon O.S., Gallerano G.P., Park W.Y., Park G.S. High-power femtosecond-terahertz pulse induces a wound response in mouse skin. Sci. Rep. 2013;3:1–7. doi: 10.1038/srep02296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirichuk V.F., Antipova O.N., Krylova Y.A. Effect of continuous irradiation with terahertz electromagnetic waves of the NO frequency range on behavioral reactions of male albino rats under stress conditions. Bull. Exp. Biol. Med. 2014;157:184–189. doi: 10.1007/s10517-014-2521-1. [DOI] [PubMed] [Google Scholar]
- Kirichuk V.F., Efimova N.V., Andronov E.V. Effect of high power terahertz irradiation on platelet aggregation and behavioral reactions of albino rats. Bull. Exp. Biol. Med. 2009;148:746–749. doi: 10.1007/s10517-010-0807-5. [DOI] [PubMed] [Google Scholar]
- Kirichuk V.F., Ivanov A.N. Regulatory effects of terahertz waves. Russ. Open Med. J. 2013;2 doi: 10.15275/rusomj.2013.0402. [DOI] [Google Scholar]
- Kristensen T., Withayachumnankul W., Jepsen P.U., Abbott D. Modeling terahertz heating effects on water. Opt. Express. 2010;18:4727–4739. doi: 10.1364/OE.18.004727. [DOI] [PubMed] [Google Scholar]
- Lu Y.H., Cheng C.H., Gao P., Ma Q.L., He M.D., Zhang L., Yu Z.P. Terahertz radiation exposure results in altered gene expression profile in mouse retina. J. Third Mil. Med. Univ. 2020;42:2273–2281. doi: 10.16016/j.1000-5404.202008140. (in Chinese with English abstract) [DOI] [Google Scholar]
- Ma Q., Chen C., Lin M., Tao J., Deng P., Gao P. Non-thermal effects of 0.22 terahertz electromagnetic radiation exposure-induced injury in Neuro-2a cells. J. Third Mil. Med. Univ. 2020;42:2267–2273. doi: 10.16016/j.1000-5404.202008145. (in Chinese with English abstract) [DOI] [Google Scholar]
- Mao Q., Zhu Y., Lv C., Lu Y., Yan X., Yan S., Liu J. Convolutional neural network model based on terahertz imaging for integrated circuit defect detections. Opt. Express. 2020;28:5000–5012. doi: 10.1364/OE.384146. [DOI] [PubMed] [Google Scholar]
- Mehrotra P., Chatterjee B., Sen S. EM-wave biosensors: a review of RF, microwave, mm-wave and optical sensing. Sensors. 2019;19:1013. doi: 10.3390/s19051013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng K., Chen T.N., Chen T., Zhu L.G., Liu Q., Li Z., Li F., Zhong S.C., Li Z.R., Feng H., Zhao J.H. Terahertz pulsed spectroscopy of paraffin-embedded brain glioma. J. Biomed. Opt. 2014;19 doi: 10.1117/1.JBO.19.7.077001. [DOI] [PubMed] [Google Scholar]
- Miller A.B., Sears M.E., Morgan L.L., Davis D.L., Hardell L., Oremus M., Soskolne C.L. Risks to health and well-being from radio-frequency radiation emitted by cell phones and other wireless devices. Front. Public Health. 2019;7:223. doi: 10.3389/fpubh.2019.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S.J., Kim S.H., Ji Y.B., Jeong K., Suh J.S. Study of freshly excised brain tissues using terahertz imaging. Biomed. Opt. Express. 2014;5:2837–2842. doi: 10.1364/BOE.5.002837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshevskaya J., Ratushnyak A., Petrov A., Kozlov A., Zapara T. Region 8 International Conference on Computational Technologies in Electrical and Electronics Engineering Held in Novosibirsk, Russia, 21-25 July 2008. IEEE; 2008. Effect of terahertz electromagnetic waves on neurons systems; pp. 210–211. [DOI] [Google Scholar]
- Perera P.G.T., Appadoo D.R.T., Cheeseman S., Wandiyanto J.V., Linklater D., Dekiwadia C., Truong V.K., Tobin M.J., Vongsvivut J., Bazaka O., et al. PC 12 pheochromocytoma cell response to super high frequency terahertz radiation from synchrotron source. Cancers. 2019;11:162. doi: 10.3390/cancers11020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Png G.M., Flook R., Ng B.W.H., Abbott D. Terahertz spectroscopy of snap-frozen human brain tissue: an initial study. Electron. Lett. 2009;45:343–345. doi: 10.1049/el.2009.3413. [DOI] [Google Scholar]
- Reukov A.S., Naymushin A.V., Simakov K.V., Moroshkin V.S., Kozlenok A.V., Presnukhina A.P. Use of the infrared radiation modulated by terahertz frequencies in complex therapy of patients with acute ischemic stroke. Arter. Gipertenz. 2016;22:94–102. doi: 10.18705/1607-419x-2016-22-1-94-102. [DOI] [Google Scholar]
- Romanenko S., Appadoo D., Lawler N., Hodgetts S.I., Harvey A.R., Wallace V.P. 45th. International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW-THz) Held in Buffalo, NY, USA, 8-13 Nov 2020. IEEE; 2020. Terahertz radiation stimulates neurite growth in PC12 derived neurons during development phase: preliminary study; pp. 1–2. [DOI] [Google Scholar]
- Scaramozzino D., Lacidogna G., Carpinteri A. In: Grady M.E., editor. vol. 4. Springer; 2020. Protein conformational changes and low-frequency vibrational modes: a similarity analysis; pp. 7–10. (Mechanics of Biological Systems and Materials & Micro-and Nanomechanics). [DOI] [Google Scholar]
- Serdyukov D.S., Goryachkovskaya T.N., Mescheryakova I.A., Kuznetsov S.A., Popik V.M., Peltek S.E. Fluorescent bacterial biosensor E. coli/pTdcR-TurboYFP sensitive to terahertz radiation. Biomed. Opt. Express. 2021;12:705–721. doi: 10.1364/BOE.412074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang S., Wu X., Zhang Q., Zhao J., Hu E., Wang L., Lu X. 0.1 THz exposure affects primary hippocampus neuron gene expression via alternating transcription factor binding. Biomed. Opt. Express. 2021;12:3729–3742. doi: 10.1364/BOE.426928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L., Shumyatsky P., Rodriguez-Contreras A., Alfano R. Terahertz spectroscopy of brain tissue from a mouse model of Alzheimer's disease. J. Biomed. Opt. 2016;21 doi: 10.1117/1.JBO.21.1.015014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraga K., Ogawa Y., Suzuki T., Kondo N., Irisawa A., Imamura M. Characterization of dielectric responses of human cancer cells in the terahertz region. J. Infrared Millim. Te. 2014;35:493–502. doi: 10.1007/s10762-014-0067-y. [DOI] [Google Scholar]
- Smye S.W., Chamberlain J.M., Fitzgerald A.J., Berry E. The interaction between terahertz radiation and biological tissue. Phys. Med. Biol. 2001;46:101–112. doi: 10.1088/0031-9155/46/9/201. [DOI] [PubMed] [Google Scholar]
- Son J.-H., Oh S.J., Cheon H. Potential clinical applications of terahertz radiation. J. Appl. Phys. 2019;125:190901. doi: 10.1063/1.5080205. [DOI] [Google Scholar]
- Sun C.K., Chen H.Y., Tseng T.F., You B., Wei M.L., Lu J.Y., Chang Y.L., Tseng W.L., Wang T.D. High sensitivity of T-ray for thrombus sensing. Sci. Rep. 2018;8:1–10. doi: 10.1038/s41598-018-22060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Takayama K., Yamauchi S., Imai Y., Tonouchi M. 34th. International Conference on Infrared, Millimeter, and Terahertz Waves Held in Busan, Korea (South), 10 November 2009. IEEE; 2009. Measurement of water absorption coefficient using terahertz time-domain spectroscopy; pp. 1–2. [DOI] [Google Scholar]
- Tan S.Z., Tan P.C., Luo L.Q., Chi Y.L., Yang Z.L., Zhao X.L., Zhao L., Dong J., Zhang J., Yao B.W., et al. Exposure effects of terahertz waves on primary neurons and neuron-like cells under nonthermal conditions. Biomed. Environ. Sci. 2019;32:739–754. doi: 10.3967/bes2019.094. [DOI] [PubMed] [Google Scholar]
- Tsurkan M., Smolyanskaya O., Bespalov V., Penniyainen V., Kipenko A., Lopatina E., Krylov B. SPIE - The International Society for Optical Engineering Held in San Francisco, California, United States, 1 March 2012. International Society for Optics and Photonics; 2012. Changing growth of neurites of sensory ganglion by terahertz radiation; p. 82610S. [DOI] [Google Scholar]
- Tzydynzhapov G., Gusikhin P., Muravev V., Dremin A., Nefyodov Y., Kukushkin I. New real-time sub-terahertz security body scanner. J. Infrared Millim. Te. 2020;41:632–641. doi: 10.1007/s10762-020-00683-5. [DOI] [Google Scholar]
- Wang W.-w., Xie C.-l., Lu L., Zheng G.-q. A systematic review and meta-analysis of Baihui (GV20)-based scalp acupuncture in experimental ischemic stroke. Sci. Rep. 2014;4:1–16. doi: 10.1038/srep03981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang G., Xu D., Jiang B., Ge M., Wu L., Yang C., Mu N., Wang S., Chang C., et al. Terahertz spectroscopic diagnosis of early blast-induced traumatic brain injury in rats. Biomed. Opt. Express. 2020;11:4085–4098. doi: 10.1364/BOE.395432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weightman, Peter Prospects for the study of biological systems with high power sources of terahertz radiation. Phys. Biol. 2012;9 doi: 10.1088/1478-3975/9/5/053001. [DOI] [PubMed] [Google Scholar]
- Wilmink G.J., Grundt J.E. Invited review article: current state of research on biological effects of terahertz radiation. J. Infrared Millim. Te. 2011;32:1074–1122. doi: 10.1007/s10762-011-9794-5. [DOI] [Google Scholar]
- Wilmink G.J., Rivest B.D., Roth C.C., Ibey B.L., Payne J.A., Cundin L.X., Grundt J.E., Peralta X., Mixon D.G., Roach W.P. In vitro investigation of the biological effects associated with human dermal fibroblasts exposed to 2.52 THz radiation. Lasers Surg. Med. 2011;43:152–163. doi: 10.1002/lsm.20960. [DOI] [PubMed] [Google Scholar]
- Wu L., Xu D., Wang Y., Liao B., Jiang Z., Zhao L., Sun Z., Wu N., Chen T., Feng H., Yao J. Study of in vivo brain glioma in a mouse model using continuous-wave terahertz reflection imaging. Biomed. Opt. Express. 2019;10:3953–3962. doi: 10.1364/BOE.10.003953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L., Yao Y., Ying Y. The application of terahertz spectroscopy to protein detection: a review. Appl. Spectrosc. Rev. 2014;49:448–461. doi: 10.1080/05704928.2013.847845. [DOI] [Google Scholar]
- Yamaguchi S., Fukushi Y., Kubota O., Itsuji T., Ouchi T., Yamamoto S. Brain tumor imaging of rat fresh tissue using terahertz spectroscopy. Sci. Rep. 2016;6:1–6. doi: 10.1038/srep30124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Hao Y.H., Peng R.Y. Advances in the biological effects of terahertz wave radiation. Mil. Med. Res. 2014;1:1–4. doi: 10.1186/s40779-014-0026-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y., Li J., Cui Y., Tang P., Du L., Chen T., Meng K., Liu Q., Feng H., Zhao J., et al. Terahertz spectroscopic diagnosis of myelin deficit brain in mice and rhesus monkey with chemometric techniques. Sci. Rep. 2017;7:1–9. doi: 10.1038/s41598-017-05554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]