Abstract
In this study, we prepared a kind of novel microecologics, namely Chinese medicine–probiotic compound microecological preparation (CPCMP), which is composed of 5 traditional Chinese medicine herbs (Galla Chinensis, Andrographis paniculata, Arctii Fructus, Glycyrrhizae Radix, and Schizonepeta tenuifolia) fermented by Aspergillus niger and a kind of compound probiotics (Lactobacillus plantarum A37 and L. plantarum MIII). The effects of the CPCMP in broilers on growth performance, serum parameters, immune function, and intestinal health were investigated. A total of 450 one-day-old male Arbor Acres broilers were randomly divided into 6 treatment groups with 5 replicates, 15 birds per replicate. Treatments consisted of: blank control, CPCMP, positive control, commercial CPCMP, traditional Chinese medicine, and probiotics groups, which were birds fed with basal diet supplemented with no extra additives, 0.2% CPCMP, 0.0035% chlortetracycline, 0.2% commercially available CPCMP, 0.2% fermented traditional Chinese medicines, and 0.2% compound probiotics, respectively. CPCMP obviously increased the average body weight and average daily gain (P < 0.05, compared with any other group) and decreased the feed:gain ratio of broilers (P < 0.05, compared with the blank control, commercial CPCMP, traditional Chinese medicine, or probiotics group). Moreover, it significantly increased glutathione peroxidase and secretory immunoglobulin A levels and spleen/bursa indices (P < 0.05 for all, compared with the blank control, commercial CPCMP, traditional Chinese medicine, or probiotics group). Villus heights in duodenum, jejunum, and ileum were also elevated by CPCMP treatment (P < 0.05, compared with any other group). Furthermore, CPCMP substantially increased jejunal mRNA levels of occludin and zonula occludens-1 (P < 0.05, compared with the blank control, positive control, or probiotics group) and facilitated the growth and colonization of beneficial cecal bacteria, such as Olsenella, Barnesiella, and Lactobacillus. Overall results show that the CPCMP prepared in our work contributes to improving growth performance, serum parameters, immune function, and intestinal health of broilers and exerts synergistic effects of traditional Chinese medicines and probiotics to some extent. Our findings suggest that CPCMP is a promising antibiotic substitute in the livestock and poultry industry in the future.
Key words: microecologics, traditional Chinese medicine, probiotics, intestinal health, broiler
INTRODUCTION
Since the discovery of penicillin in the late 1920s, the application of antibiotics has brought great changes to human life (Zaffiri et al., 2012). In livestock and poultry production, antibiotics in subtherapeutic doses are used as feed additives to control diseases and improve growth performance (Smith, 2019). However, antibiotics can not only kill harmful bacteria but also inhibit the growth and colonization of beneficial bacteria in intestine, resulting in the imbalance of intestinal microbiota (Alagawany et al., 2018). The overuse of antibiotics in the livestock and poultry industry has induced the emergence of many drug-resistant pathogens. In addition, the accumulation of antibiotic residues can contaminate livestock and poultry products such as meat and eggs (Chowdhury et al., 2018; Suresh et al., 2018), which threatens human health. Therefore, new and effective feed additives are urgently needed to replace antibiotics in livestock and poultry production.
The Food and Agriculture Organization of the United Nations and the World Health Organization define probiotics as live microorganisms that confer a health benefit on the host when administered in adequate doses (Hill et al., 2014). Probiotics, such as Lactobacillus (Incharoen et al., 2019), Bacillus (Al-Fataftah and Abdelqader, 2014), Bifidobacterium (Wang et al., 2020a), Aspergillus niger (Lin et al., 2020), and Aspergillus oryzae (Zahirian et al., 2019), commonly used in livestock and poultry, have many beneficial effects, mainly including killing or suppressing pathogenic bacteria, promoting nutrient utilization, enhancing intestinal barrier function, regulating immune response, and improving intestinal microbiota. Traditional Chinese medicine (TCM) has been regarded as an invaluable source of therapeutic agents and played an important role in maintaining both human and animal health (Mishra and Tiwari, 2011). Some TCMs with low toxicity, like Galla Chinensis (Xiang et al., 2015), Andrographis paniculata (Worasuttayangkurn et al., 2019), Arctii Fructus (Xu et al., 2015; Bok et al., 2017), Glycyrrhizae Radix (Jiang et al., 2020a), and Schizonepeta tenuifolia (Fung and Lau, 2002), contain various active ingredients, such as terpenoids, phenols, and volatile oils, which can not only improve growth performance, immune function, and intestinal microbiota of livestock and poultry, but also have antioxidant and anti-inflammatory activities (Samuel et al., 2017; Li et al., 2018; Pirgozliev et al., 2019; Jiang et al., 2020b; Shi et al., 2020). Compared with antibiotics, TCMs have many advantages including less toxicity and side effects, and reduced residues in body and risk of inducing drug resistance (Abdallah et al., 2019). The application of TCMs can effectively solve a series of problems caused by long-term use of antibiotics in livestock and poultry breeding. With the cross development of different subjects, such as microecology, fermentation engineering, and pharmacy, a kind of novel microecologics, Chinese medicine–probiotic compound microecological preparation (CPCMP), has sprung up. This formulation can make plenty use of both the efficacy of TCMs and probiotics through their synergistic actions (Liang et al., 2021). CPCMP may have the potential to be developed into a substitute for antibiotics in poultry production.
The integrity of intestinal barrier and the composition and structure of intestinal microbiota play a vital role in host's health. The intestinal barrier can prevent the invasion of exogenous pathogenic bacteria, toxins, and other harmful substances, help maintain intestinal homeostasis, and protect health (Yang et al., 2019a). Intestinal microflora participates in the biotransformation of nutrients, contributes to the clearance of the pathogens, and interacts with the gut-associated immune system (Kogut, 2018). It is considered as an internalized environmental factor, which is closely related to the occurrence and development of many diseases (Borda-Molina et al., 2018). Thus, once either the intestinal barrier or intestinal microflora becomes dysfunctional, health will be seriously threatened.
Previous studies have shown that fermented TCMs have advantageous effects on growth performance, meat quality, and immune function in cattle (Wang et al., 2017), pigs (Zhou et al., 2015), and ducks (Liu et al., 2017). The relevant studies in broilers also focus on their influence on growth performance and antioxidant activity (Niu et al., 2017; Niu et al., 2019; Liu et al., 2020). The reports on the effects of fermented TCMs and the compound formulation of fermented TCMs and probiotics on intestinal barrier and intestinal microbiota of broilers are numbered (Zhang et al., 2015; Qiao et al., 2018).
In our study, 5 kinds of TCMs (Galla Chinensis, A. paniculata, Arctii Fructus, Glycyrrhizae Radix, and S. tenuifolia) fermented by Aspergillus niger were combined with a kind of compound probiotics (a mixture of Lactobacillus plantarum A37 and L. plantarum MIII) to obtain a CPCMP. As mentioned above, these TCMs and probiotics have beneficial effects, such as enhancing immunity and improving intestinal health. The objective of this study was to examine the effects of CPCMP in broilers on growth performance, serum biochemical and antioxidant parameters, immune function, intestinal barrier, and intestinal microflora and explore whether the combined use of TCMs and probiotics shows the synergistic effects.
MATERIALS AND METHODS
Preparation of Feed Additives
Fermented TCMs Preparation
Five Chinese herbs, Galla Chinensis, A. paniculata, Arctii Fructus, Glycyrrhizae Radix, and S. tenuifolia, were purchased from Wuhan Renyitang Pharmacy Co., Ltd. (Wuhan, Hubei, China). After being dried and pulverized, they were passed through 80 mesh filters to obtain fine powders. The powders of Galla Chinensis, A. paniculata, Arctii Fructus, Glycyrrhizae Radix, and S. tenuifolia were mixed in a mass ratio of 3:3:3:1:1 (The ratio was screened and determined using orthogonal test and range analysis method by analyzing bacteriostatic effect on pathogenic bacteria in our previous study; Liang et al., 2021). Then the mixture was soaked in equal-mass water overnight and sterilized at 121°C for 30 min. After being naturally cooled to room temperature, the TCM mixture was inoculated with 109 colony-forming units (CFU)/mL of Aspergillus niger (which was obtained from the China Center of Industrial Culture Collection; NO:2033) and fermented at 26°C for 4 d. Subsequently, the ferment was inactivated by autoclave sterilization and ground into a powder as fermented TCMs for further use.
Compound Probiotics Acquisition
In our previous work, 2 probiotic strains, Lactobacillus plantarum A37 and L. plantarum MIII, were isolated from the cecal contents of healthy broilers, genetically identified, selected for their beneficial properties (such as inhibition to common pathogens [e.g., S. aureus, S. Epidermidis, Salmonella, and E. coli] and tolerance to high temperature, high bile salts, and acidic environment), and preserved in China Center for Type Culture Collection with the number of M2019559 and M2019560, respectively. They were both activated in de Man, Rogosa, and Sharpe (MRS) agar (Hopebio Co., Ltd, Qingdao, China) at 37°C for 24 h. Then, the single colonies were selected and fermented in MRS broth (pH 5.7) (Hopebio Co., Ltd) for 14 h. After centrifugation (Microfuge 20R, Beckman Coulter, Miami, FL, Germany) of the bacterial cultures at 4,000 rpm at 4°C for 15 min, the supernatants were discarded. The bacterial cell pellets were added with the protective agent (sterile water added with 12.0% trehalose, 15.0% skim milk, 3.0% sucrose, and 2.5% gelatin; the formula of which was determined using orthogonal test and range analysis method in our previous study) and freeze-dried using a commercial freeze dryer (Biocool, Beijing, China) at −60°C for 24 h. Then, the bacterial cells were ground into powders in a sterile environment. Finally, the powders of L. plantarum A37 and L. plantarum MIII were evenly mixed in a 1:1 mass ratio (The ratio was screened and determined in our previous study) to obtain the compound probiotics (> 1010 CFU/g solids) for further use.
CPCMP Preparation
The fermented TCMs and the compound probiotics were uniformly mixed at a mass ratio of 6:1 (The ratio was screened and determined in our previous study) to obtain the CPCMP (>1010 CFU/g solids), which was stored at 4°C for further use.
Animals and Treatment
A total of 450 one-day-old male Arbor Acres broilers with average initial body weight of 33.86 ± 0.57 g were purchased from Jingzhou Zhengkang Poultry Co., Ltd. (Jingzhou, Hubei, China). They were randomly assigned into 6 treatment groups with 5 replicates (cages) per treatment and 15 birds per replicate. Treatments were as followed (Table 1): A) blank control group, B) CPCMP group, C) positive control group, D) commercial CPCMP group, E) TCM group, and F) probiotics group. Experimental diets were formulated to meet or exceed nutritional requirements of broilers reported by the National Research Council (NRC, 1994). The composition of the basal diets during the 2 growth periods (1–21 and 22–42 d) is shown in Supplementary Table 1. All birds were housed in stainless steel cages (200 cm long × 100 cm wide × 40 cm high) with concrete floors of 0.133 m2 per bird, and had ad libitum access to water and feed during the whole 42-d experimental period. The temperature of the room was kept at 35°C for the first week and decreased to 24°C until the end of the study. All animal experiments were performed according to the protocols approved by the Hubei Provincial Animal Care and Use Committee of China under supervision of a licensed poultry veterinarian.
Table 1.
The group design of animal experiment.
| Group number | Group name | Treatment |
|---|---|---|
| A | Blank control group | A basal diet supplemented with no extra additives |
| B | CPCMP group | A basal diet supplemented with 0.2% Chinese medicine–probiotic compound microecological preparation (CPCMP) |
| C | Positive control group | A basal diet supplemented with 0.0035% chlortetracycline1 |
| D | Commercial CPCMP group | A basal diet supplemented with 0.2% commercially available CPCMP2 |
| E | TCM group | A basal diet supplemented with 0.2% fermented traditional Chinese medicines |
| F | Probiotics group | A basal diet supplemented with 0.2% compound probiotics |
Chlortetracycline was purchased from Charoen Pokphand Group Co., Ltd. (Harbin, Heilongjiang, China).
Commercially available CPCMP was purchased from Henan Yue's loyalty Technology Co., Ltd. (Xuchang, Henan, China).
Growth Performance Determination
Every week during the trial, all broilers were individually weighed after 4 h of feed deprivation. The body weight and feed consumption were recorded per replicate during different experimental periods to calculate the average body weight (ABW), average daily feed intake (ADFI), average daily gain (ADG), and feed:gain (F:G; the ratio of ADFI:ADG) (Zhang et al., 2021).
Sample Collection
Both at 21 and 42 days of age, 10 broilers (2 broilers per replicate, 5 replicates/treatment, n = 5) were randomly selected from each of the blank control, CPCMP, positive control, commercial CPCMP, TCM, and probiotics groups for sample collection. Blood samples were individually collected from the wing vein and placed in vacuum blood collection tubes. After the samples were allowed to stand at room temperature for 2 h, they were centrifuged at 3,000 rpm at 4°C for 10 min and the supernatants (serum samples) were stored at −80°C until further analysis. After blood collection, the broilers were anesthetized by injecting sodium pentobarbital (Sigma-Aldrich, St. Louis, MO) intravenously at a dose of 30 mg/kg (Mohammed et al., 2019) and decapitated. The abdominal cavity of broilers was quickly opened, and the spleen and bursa were removed for evaluation of immune organ indices. Afterward, the entire small intestine was rapidly removed and separated from the mesentery and connective tissue. Approximately 2 cm of the mid-duodenum, mid-jejunum, and mid-ileum from each broiler were obtained and fixed in 4% paraformaldehyde solution for histological examination. The remaining jejunum segments were washed with 0.9% saline to remove intestinal contents and then partially placed in a cryotube for quantitative real-time polymerase chain reaction (qRT-PCR). Part of the ileal mucosa was carefully scraped using a sterile glass microscope slide and collected into a cryotube for further analysis. Then, the cecal contents were collected from randomly selected 5 broilers (1 broiler per replicate, 5 replicates/treatment, n = 5) of each group for 16S rRNA gene sequencing. All tissue samples were kept in liquid nitrogen before further analysis.
Determination of Immune Organ Indices
After the spleen and bursa of each broiler were removed, the surrounding adipose tissue was cut off, and the 2 immune organs were immediately weighed. Immune organ index (mg/g) was calculated as the immune organ fresh weight (mg)/broiler weight (g) before slaughter.
Histological Measurement
After fixation in 4% paraformaldehyde solution for 24 to 48 h, the collected segments of the small intestine were dehydrated with increasing concentrations of alcohol, embedded in paraffin, sectioned into 5-μm-thick slides, and finally stained with hematoxylin and eosin (H&E). Ten complete crypt–villus units were randomly selected from each section (1 section per brolier, 2 broiler/replicate, 5 replicates/treatment). The villus height (VH) or crypt depth (CD) was defined as the vertical distance from the villus tip or from the crypt base to the villus–crypt junction, respectively (De Grande et al., 2020). The sections were observed using a light microscope (Leica DMi8, Winzer, Germany) with 10 × magnification, and color images were captured by computer-assisted image analysis system (Leica Application Suite X 3.4.2, Winzer, Germany). The mean VH and CD were calculated to obtain the VH-to-CD ratio (VH:CD).
Serum Biochemical Analysis
The contents of albumin (ALB, cat# abx350538) and triglycerides (TG, cat# abx257659) in the serum were quantified using commercial enzyme-linked immunosorbent assay (ELISA) kits (Abbexa Ltd., Cambridge, UK) in accordance with the manufacturer's instructions. The concentration of total cholesterol (TC, cat# BC1985) in the serum was determined using a TC assay kit (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) in accordance with the manufacturer's protocols.
Assessment of the Antioxidant Activity
The enzyme activity of glutathione peroxidase (GSH-Px, cat# abx357063) and the content of malondialdehyde (MDA, cat# abx257171) in the serum were tested using commercial ELISA kits (Abbexa Ltd.). All procedures were performed in accordance with the manufacturer's instructions.
Detection of Serum Inflammatory Cytokines
The contents of interleukin (IL)-1 beta (1β) (cat# abx250062), IL-6 (cat# abx250068), and IL-10 (cat# abx250055) in the serum were determined using the corresponding commercial ELISA kits (Abbexa Ltd.) in accordance with the manufacturer's instructions. The concentration of endotoxin (cat# 88282) in the serum was quantified using a Pierce LAL Chromogenic endotoxin Quantitation Kit (Thermo Fisher Scientific Inc., Waltham, MA) in accordance with the manufacturer's protocols.
Determination of Serum and Mucosal Antibodies
Approximately 0.1 g of ileal mucosa from each broiler was homogenized with 1 mL of phosphate buffer solution on ice and then centrifuged at 8,000 rpm at 4°C for 15 min to obtain the supernatants. The contents of secretory immunoglobulin (Ig) A (sIgA, cat# abx053627) in the serum and in the supernatants of the ileal mucosa homogenates and IgG (cat# abx150024) in the serum were measured using commercial ELISA kits (Abbexa Ltd.) in accordance with the manufacturer's instructions.
qRT-PCR Analysis
Total RNA of jejunum segment samples was isolated using TRIzol reagent (cat# abs9331, Absin Bioscience Inc., Shanghai, China) following the manufacturer's protocols. The integrity of isolated RNA was examined by 2% agarose gel electrophoresis, and the concentration and purity of RNA were determined from OD260/280 readings using a NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific Inc.). The RNA samples were diluted with diethyl pyrocarbonate-treated water. Subsequently, 1 μg of total RNA was immediately reverse-transcribed into complementary DNA using ReverTra Ace qPCR RT Master Mix with gDNA Remover (cat# FSQ-301, Toyobo Co., Ltd., Osaka, Japan) in accordance with the manufacturer's instructions. The reverse transcription condition was set up as follows: 15 min at 37°C and 5 min at 98°C. Finally, qRT-PCR was conducted on a CFX96 Real-Time PCR system (BioRad, CA) with a QuantiNova SYBR Green PCR Kit (cat# 208054, Qiagen GmbH, Hilden, Germany) following the manufacturer's protocols. The reaction mixture comprised 1.8 μL of complementary DNA, 1.4 μL each of the forward and reverse primers, 10 μL of SYBR Green PCR Master Mix, and 5.4 μL of RNase-free water. The PCR procedures consisted of a PCR initial heat activation at 95°C for 2 min and 40 cycles of denaturation at 95°C for 5 s, followed by a combined annealing/extension step at 60°C for 10 s. The melting curve conditions were as follows: 65°C for 10 s and an increase in temperature from 65 to 95°C with a heating rate of 0.5°C/s. The primer sequences for the target (occludin [OCLN] and zonula occludens-1 [ZO-1]) and reference (β-actin) genes are listed in Table 2. Each sample was tested in triplicate, and the relative mRNA expression levels of all target genes were calculated using the 2−ΔΔCT method (Livak and Schmittgen, 2001). The values in the blank control group were used as a calibrator.
Table 2.
Sequences for real-time PCR primers.
| Gene1 | Gene bank ID | Forward primers (5′-3′) | Reverse primers (5′-3′) | Length (bp) |
|---|---|---|---|---|
| OCLN | NM_205128.1 | TCATCGCCTCCATCGTCTAC | TCTTACTGCGCGTCTTCTGG | 240 |
| ZO-1 | XM_413773.4 | AAGTGTTTCGGGTTGTGGAC | GCTGTCTTTGGAAGCGTGTA | 160 |
| β-actin | NM_205518.1 | ACCGCAAATGCTTCTAAACC | ATAAAGCCATGCCAATCTCG | 100 |
Abbreviations: OCLN, occludin; ZO-1, zonula occludens-1.
Microbial Community Analysis by 16S rRNA Gene Sequencing
Total DNA was extracted from 0.5 g of cecal contents using a Magnetic Soil and Stool DNA Kit (cat# DP712, TIANGEN Biotech Co., Ltd., Beijing, China) in accordance with the manufacturer's protocols. The concentration and purity of the extracted DNA were determined using a Qubit 2.0 spectrophotometer (Invitrogen, Carlsbad, CA) and 1% (w/v) agarose gel electrophoresis. The DNA samples were diluted with sterile water to a concentration of 1 ng/μL and stored at −20°C before analysis. Then, quantitative PCR was used to amplify the V3–V4 hypervariable regions of extracted DNA with specific full length universal forward and reverse primers (515F, 806R). Subsequently, a QIAquick Gel Extraction Kit (cat# 28706, Qiagen GmbH) and a Thermo Scientific GeneJET Gel Extraction Kit (cat# K0692, Thermo Fisher Scientific Inc.) were used to further purify the PCR products. Simultaneously, the purity of the PCR mixture was evaluated using a Qubit 2.0 dsDNA HS Assay Kit (cat# Q32854, Invitrogen). The 16S rRNA gene sequencing was performed to analyze the cecal microbial community structures using the NovaSeq 6000 platform (Illumina, San Diego, CA) in Novogene Bioinformatics Co., Ltd. (Tianjin, China).
Statistical Analysis
Data are shown as mean values ± standard error. The IBM SPSS Statistics 21 software (International Business Machines Corp., Armonk, NY) was employed for statistical analysis. Multiple comparisons were conducted using one-way analysis of variance followed by Tukey's multiple comparisons test. A P-value < 0.05 was considered significant.
RESULTS
Growth Performance
The results of the effects of CPCMP on the growth performance of broilers were shown in Table 3 and Supplementary Table 2. Dietary supplementation with CPCMP markedly increased the ABW (P < 0.05) compared with the blank control (d 7, 14, 21, 28, 35, and 42), TCM (d 7, 14, 21, 28, 35, and 42), positive control (d 14, 21, and 28), commercial CPCMP (d 7, 14, 21, 28, and 42), or probiotics (d 7, 14, 21, 35, and 42) group. The ADG in the CPCMP group was notably higher (P < 0.05) than that of the blank control, commercial CPCMP, TCM, probiotics (d 1–21, 22–42, and 1–42, for all the 4 groups), or positive control (d 1–21) group. The ADFI in the CPCMP group was higher (P < 0.05) than that of the commercial CPCMP, TCM, probiotics (d 22–42 and 1–42, for all the 3 groups), or positive control (d 22–42) group, while it was lower than that of the blank control group (d 1–21; P < 0.05). The CPCMP group exhibited substantially decreased F:G ratio (P < 0.05) compared with the blank control (d 1–21, 22–42, and 1–42), TCM (d 1–21 and 1–42), commercial CPCMP (d 22–42 and 1–42), or probiotics (d 1–21) group. No difference in F:G ratio between the CPCMP and positive control groups was observed.
Table 3.
Effects of dietary CPCMP supplementation on the growth performance of broilers.
| Item1 | BC | CPCMP | PC | Commercial CPCMP | TCM | Probiotics |
|---|---|---|---|---|---|---|
| ABW (g/bird) | ||||||
| 1d | 34.18 ± 0.072 | 33.66 ± 0.43 | 34.26 ± 0.25 | 33.24 ± 0.38 | 32.96 ± 0.39 | 32.88 ± 0.76 |
| 21d | 809.52 ± 3.44c | 852.16 ± 5.78a | 831.07 ± 3.29b | 825.65 ± 3.30bc | 831.00 ± 4.99b | 827.72 ± 4.33bc |
| 42d | 2,363.57 ± 22.80c | 2,629.60 ± 30.69a | 2,597.09 ± 8.18a | 2,476.45 ± 19.48b | 2,486.35 ± 9.07b | 2,462.41 ± 6.95b |
| ADG (g/bird) | ||||||
| 1–21 d | 36.89 ± 0.15c | 39.07 ± 0.24a | 37.95 ± 0.16b | 37.81 ± 0.11b | 37.68 ± 0.22bc | 37.82 ± 0.21b |
| 22–42 d | 73.98 ± 1.09c | 84.66 ± 1.46a | 84.10 ± 0.39a | 78.74 ± 0.95b | 78.90 ± 0.44b | 77.85 ± 0.33b |
| 1–42 d | 55.46 ± 0.54c | 61.81 ± 0.73a | 60.99 ± 0.20a | 58.23 ± 0.48b | 58.38 ± 0.22b | 57.85 ± 0.17b |
| ADFI (g/bird) | ||||||
| 1–21 d | 51.28 ± 0.48a | 48.74 ± 0.32bc | 47.93 ± 0.54c | 47.66 ± 0.41c | 50.24 ± 0.32ab | 49.77 ± 0.37ab |
| 22–42 d | 168.56 ± 0.49a | 167.02 ± 0.67a | 162.01 ± 1.83b | 160.11 ± 0.86b | 158.47 ± 1.24bc | 154.81 ± 0.37c |
| 1–42 d | 110.17 ± 1.14a | 107.80 ± 0.37ab | 105.07 ± 0.68bc | 103.89 ± 0.45c | 104.26 ± 0.63c | 102.49 ± 0.45c |
| F:G (g:g) | ||||||
| 1–21 d | 1.40 ± 0.02a | 1.25 ± 0.01d | 1.28 ± 0.01cd | 1.27 ± 0.01cd | 1.34 ± 0.01b | 1.33 ± 0.01bc |
| 22–42 d | 2.27 ± 0.01a | 1.96 ± 0.01cd | 1.94 ± 0.02d | 2.03 ± 0.01b | 2.01 ± 0.01bc | 1.99 ± 0.01bc |
| 1–42d | 1.98 ± 0.02a | 1.74 ± 0.01cd | 1.72 ± 0.01d | 1.79 ± 0.01b | 1.79 ± 0.01b | 1.77 ± 0.01bc |
BC, blank control, basal diet; CPCMP, basal diet supplemented with 0.2% Chinese medicine–probiotic compound microecological preparation; PC, positive control, basal diet supplemented with 0.0035% chlortetracycline; commercial CPCMP, basal diet supplemented with 0.2% commercially available CPCMP; TCM, basal diet supplemented with 0.2% fermented traditional Chinese medicines; Probiotics, basal diet supplemented with 0.2% compound probiotics.
Means with different superscripts in the same row differ significantly (P < 0.05).
Abbreviations: ABW, average body weight; ADG, average daily gain; ADFI, average daily feed intake; F:G, feed:gain.
Data are mean values ± standard error of 5 replicates per treatment (15 broilers per replicate, n = 5).
Immune Organ Indices
The influence of dietary CPCMP addition on the indices of these 2 organs is presented in Table 4. Compared with the blank control, commercial CPCMP, TCM, or probiotics group, CPCMP supplementation augmented the spleen and bursa indices at 21 days of age (P < 0.05). The 2 indices at 42 days of age in the CPCMP group were both higher (P < 0.05) than those in the blank control or commercial CPCMP group.
Table 4.
Effects of dietary CPCMP supplementation on the immune organ indices of broilers.
| Item | BC | CPCMP | PC | Commercial CPCMP | TCM | Probiotics |
|---|---|---|---|---|---|---|
| Spleen index (mg/g) | ||||||
| 21 d | 0.73 ± 0.021,b | 0.97 ± 0.04a | 0.82 ± 0.03ab | 0.77 ± 0.01b | 0.72 ± 0.08b | 0.79 ± 0.02b |
| 42 d | 0.97 ± 0.09b | 1.23 ± 0.06a | 1.06 ± 0.04ab | 0.95 ± 0.04b | 1.03 ± 0.02ab | 1.05 ± 0.04ab |
| Bursa index (mg/g) | ||||||
| 21 d | 2.14 ± 0.03b | 2.55 ± 0.07a | 2.20 ± 0.11ab | 2.07 ± 0.05b | 2.12 ± 0.07b | 2.13 ± 0.09b |
| 42 d | 0.78 ± 0.05d | 1.29 ± 0.07a | 1.25 ± 0.06ab | 0.99 ± 0.05cd | 1.00 ± 0.05bcd | 1.07 ± 0.06abc |
BC, blank control, basal diet; CPCMP, basal diet supplemented with 0.2% Chinese medicine–probiotic compound microecological preparation; PC, positive control, basal diet supplemented with 0.0035% chlortetracycline; commercial CPCMP, basal diet supplemented with 0.2% commercially available CPCMP; TCM, basal diet supplemented with 0.2% fermented traditional Chinese medicines; Probiotics, basal diet supplemented with 0.2% compound probiotics.
Means with different superscripts in the same row differ significantly (P < 0.05).
Data are mean values ± standard error of 5 replicates per treatment (2 broilers per replicate, n=5).
Intestinal Morphology
The results of the effects of CPCMP on the intestinal morphology of broilers were shown in Table 5 and Supplementary Table 3. At 21 d, CPCMP supplementation significantly increased VH (P < 0.05) compared with the blank control (in the duodenum, jejunum, and ileum), positive control (in the jejunum and ileum), commercial CPCMP (in the jejunum and ileum), TCM (in the jejunum and ileum), or probiotics (in the duodenum, jejunum, and ileum) group. The VH:CD in the CPCMP group was higher (P < 0.05) than that in the blank control (in the duodenum, jejunum, and ileum), positive control (in the duodenum and ileum), commercial CPCMP (in the ileum), or TCM (in the jejunum) group. At 42 d, relative to the blank control group, the CPCMP group exhibited elevated VH (in the duodenum, jejunum, and ileum) and VH:CD (in the duodenum and jejunum) (P < 0.05) and decreased duodenal CD (P < 0.05). The duodenal VH in the CPCMP group was higher (P < 0.05) than that in the positive control, commercial CPCMP, TCM, or probiotics group. Moreover, CPCMP supplementation induced higher (P < 0.05) VH:CD (in the duodenum and jejunum) compared with the commercial CPCMP, TCM, or probiotics group.
Table 5.
Effects of dietary CPCMP supplementation on the jejunum morphology of broilers.
| Item1 | BC | CPCMP | PC | Commercial CPCMP | TCM | Probiotics |
|---|---|---|---|---|---|---|
| 21 d | ||||||
| VH (μm) | 362.18 ± 33.382,d | 820.95 ± 27.27a | 684.18 ± 4.34b | 673.48 ± 4.52b | 570.90 ± 10.24c | 549.80 ± 4.73c |
| CD (μm) | 136.68 ± 6.82 | 118.57 ± 19.86 | 115.16 ± 11.75 | 104.22 ± 9.25 | 126.51 ± 15.00 | 113.51 ± 7.88 |
| VH:CD | 2.61 ± 0.20c | 7.71 ± 1.11a | 5.97 ± 0.65ab | 6.31 ± 0.60ab | 4.36 ± 0.61bc | 4.94 ± 0.30abc |
| 42 d | ||||||
| VH (μm) | 437.50 ± 9.61cd | 518.89 ± 5.83ab | 553.66 ± 6.63a | 457.82 ± 6.60cd | 408.25 ± 14.11d | 488.06 ± 24.41bc |
| CD (μm) | 123.83 ± 5.52a | 99.83 ± 4.19ab | 79.17 ± 7.61b | 99.50 ± 5.06ab | 123.62 ± 8.47a | 120.54 ± 5.70a |
| VH:CD | 3.58 ± 0.16b | 5.23 ± 0.24a | 6.30 ± 0.44a | 3.90 ± 0.33b | 3.28 ± 0.21b | 4.05 ± 0.25b |
BC, blank control, basal diet; CPCMP, basal diet supplemented with 0.2% Chinese medicine–probiotic compound microecological preparation; PC, positive control, basal diet supplemented with 0.0035% chlortetracycline; commercial CPCMP, basal diet supplemented with 0.2% commercially available CPCMP; TCM, basal diet supplemented with 0.2% fermented traditional Chinese medicines; Probiotics, basal diet supplemented with 0.2% compound probiotics.
Means with different superscripts in the same row differ significantly (P < 0.05).
Abbreviations: CD, crypt depth; VH, villus height.
Analysis based on 10 measurements per section per broiler (2 broilers per replicate, 5 replicates per treatment). Data are mean values ± standard error.
Serum Biochemical Indicators
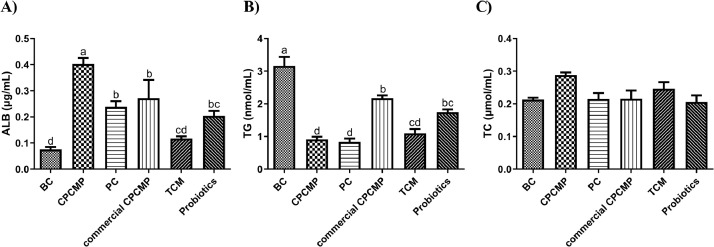
The CPCMP group had significantly higher ALB content (P < 0.05) than the blank control, positive control, commercial CPCMP, TCM, or probiotics group at 42 days of age (Figure 1). Supplementation with CPCMP reduced the concentration of TG (P < 0.05) compared with the blank control, commercial CPCMP, or probiotics group at the same age, but no difference was observed in the TG level (P > 0.05) between the groups of CPCMP and positive control, as well as CPCMP and TCM (Figure 1). The TC level in the CPCMP group was close to that in any other group (P > 0.05, Figure 1).
Figure 1.
Effects of dietary CPCMP supplementation on the serum biochemistry of broilers at 42 d. BC, blank control, basal diet; CPCMP, basal diet supplemented with 0.2% Chinese medicine–probiotic compound microecological preparation; PC, positive control, basal diet supplemented with 0.0035% chlortetracycline; commercial CPCMP, basal diet supplemented with 0.2% commercially available CPCMP; TCM, basal diet supplemented with 0.2% fermented traditional Chinese medicines; Probiotics, basal diet supplemented with 0.2% compound probiotics. (A) Albumin (ALB). (B) Triglycerides (TG). (C) Total cholesterol (TC). Results are presented as mean ± standard error (n = 5; 5 replicates/treatment, 2 broilers/replicate). a-dMeans that do not share common letters differ significantly (P < 0.05).
Antioxidant Performance
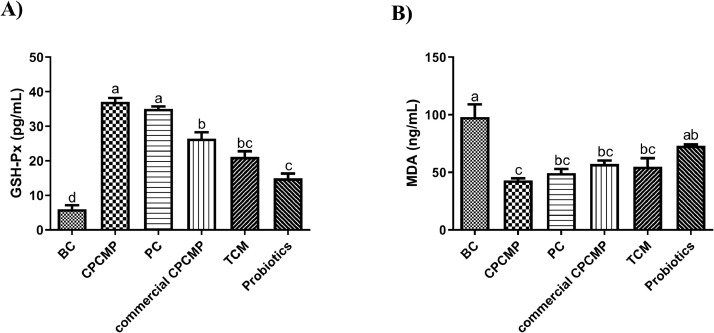
Relative to the blank control, commercial CPCMP, TCM, or probiotics group, dietary CPCMP clearly enhanced the activity of GSH-Px at 42 d (P < 0.05), whereas there was no difference in the GSH-Px activity between the CPCMP and positive control groups (P > 0.05, Figure 2). The MDA level in the CPCMP group was similar (P > 0.05) to that in the positive control, commercial CPCMP, or TCM group but substantially lower (P < 0.05) than that in the blank control or probiotics group (Figure 2).
Figure 2.
Effects of dietary CPCMP supplementation on the antioxidant performance of broilers at 42 d. BC, blank control, basal diet; CPCMP, basal diet supplemented with 0.2% Chinese medicine–probiotic compound microecological preparation; PC, positive control, basal diet supplemented with 0.0035% chlortetracycline; commercial CPCMP, basal diet supplemented with 0.2% commercially available CPCMP; TCM, basal diet supplemented with 0.2% fermented traditional Chinese medicines; Probiotics, basal diet supplemented with 0.2% compound probiotics. (A) The enzyme activity of GSH-Px and (B) content of MDA in the serum were determined. Results are presented as mean ± standard error (n = 5; 5 replicates/treatment, 2 broilers/replicate). a-cMeans that do not share common letters differ significantly (P < 0.05). Abbreviations: GSH-Px, glutathione peroxidase; MDA, malondialdehyde.
Inflammatory Cytokines
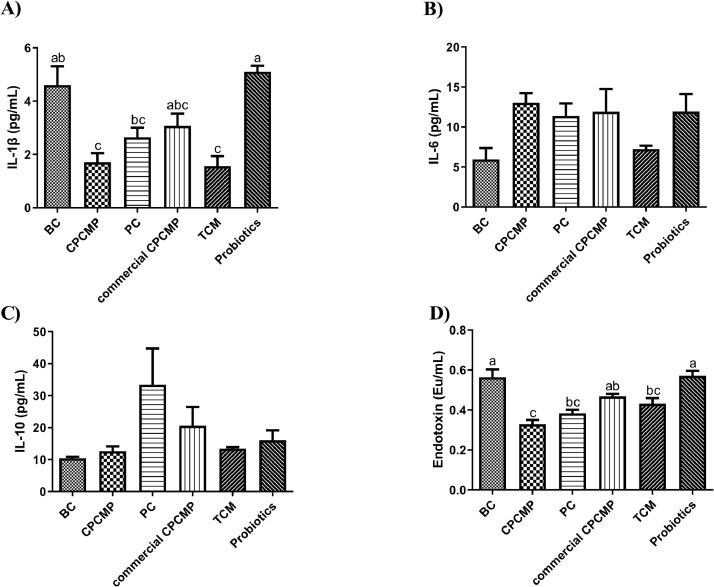
The IL-1β level in the CPCMP group was clearly decreased compared with that in the blank control or probiotics group at 42 d (P < 0.05) but was close (P > 0.05) to that in the positive control, commercial CPCMP, or TCM group (Figure 3). Supplementation with CPCMP distinctly decreased the content of endotoxin (P < 0.05) compared with the blank control, commercial CPCMP, or probiotics group (Figure 3). However, no significant difference was observed in the IL-6 or IL-10 levels between the CPCMP group and any other experimental group (P > 0.05, Figure 3).
Figure 3.
Effects of dietary CPCMP supplementation on the inflammatory cytokines of broilers at 42 d. BC, blank control, basal diet; CPCMP, basal diet supplemented with 0.2% Chinese medicine–probiotic compound microecological preparation; PC, positive control, basal diet supplemented with 0.0035% chlortetracycline; commercial CPCMP, basal diet supplemented with 0.2% commercially available CPCMP; TCM, basal diet supplemented with 0.2% fermented traditional Chinese medicines; Probiotics, basal diet supplemented with 0.2% compound probiotics. The contents of (A) interleukin (IL)-1 beta (1β), (B) IL-6, and (C) IL-10 in the serum were determined by ELISA. (D) Level of endotoxin in the serum. Results are presented as mean ± standard error (n = 5; 5 replicates/treatment, 2 broilers/replicate). a-cMeans that do not share common letters differ significantly (P < 0.05).
Antibody Levels in the Serum and Mucosa
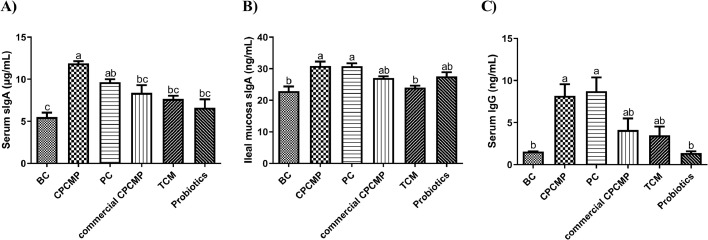
The CPCMP group showed clearly increased sIgA levels in the serum and ileal mucosa, compared with the blank control, commercial CPCMP, TCM, or probiotics group and with the blank control or TCM group, respectively (P < 0.05, Figure 4). The serum IgG level in the CPCMP group was also higher (P < 0.05) than that in the blank control or probiotics group (Figure 4).
Figure 4.
Effects of dietary CPCMP supplementation on the antibody levels in the serum and mucosa of broilers at 42 d. BC, blank control, basal diet; CPCMP, basal diet supplemented with 0.2% Chinese medicine–probiotic compound microecological preparation; PC, positive control, basal diet supplemented with 0.0035% chlortetracycline; commercial CPCMP, basal diet supplemented with 0.2% commercially available CPCMP; TCM, basal diet supplemented with 0.2% fermented traditional Chinese medicines; Probiotics, basal diet supplemented with 0.2% compound probiotics. The contents of sIgA in the serum (A) and ileal mucosa (B) and IgG in the serum (C) were determined by ELISA. Results are presented as mean ± standard error (n = 5; 5 replicates/treatment, 2 broilers/replicate). a-cMeans that do not share common letters differ significantly (P < 0.05).
Expressions of Tight Junction-Associated Genes
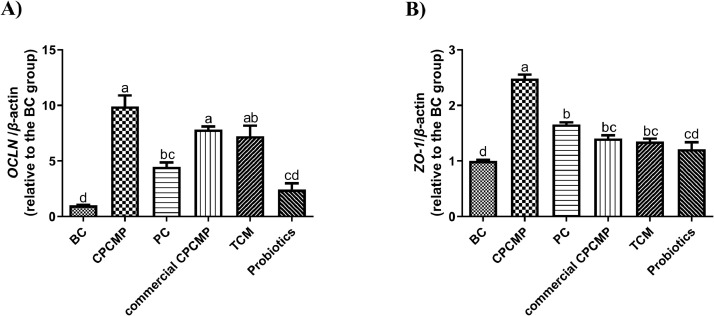
The mRNA expression of OCLN in the CPCMP group at 42 d was notably increased (P < 0.05) compared with that in the blank control, positive control, or probiotics group but was similar to that in the commercial CPCMP or TCM group (P > 0.05, Figure 5). The CPCMP group had the highest ZO-1 mRNA expression among all the 6 groups (P < 0.05, Figure 5).
Figure 5.
Effects of dietary CPCMP supplementation on the expression levels of tight junction-associated genes of broilers at 42 d. BC, blank control, basal diet; CPCMP, basal diet supplemented with 0.2% Chinese medicine–probiotic compound microecological preparation; PC, positive control, basal diet supplemented with 0.0035% chlortetracycline; commercial CPCMP, basal diet supplemented with 0.2% commercially available CPCMP; TCM, basal diet supplemented with 0.2% fermented traditional Chinese medicines; Probiotics, basal diet supplemented with 0.2% compound probiotics. The mRNA expression levels of (A) OCLN and (B) ZO-1 in the jejunal mucosa were determined by qRT-PCR. Results are presented as mean ± standard error (n = 5; 5 replicates/treatment, 2 broilers/replicate). a-dMeans that do not share common letters differ significantly (P < 0.05). Abbreviations: OCLN, occludin; ZO-1, zonula occludens-1.
Intestinal Microflora
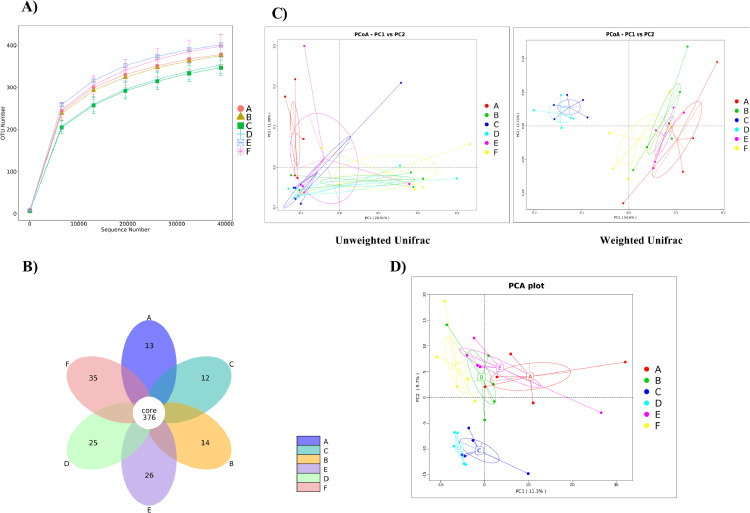
As shown in Figure 6A, the rarefaction curve of the number of operational taxonomic units (OTUs) tended to be flat, and the sequence number of each sample was between 35,000 and 40,000, which indicated that the sequencing data were reasonable and the sequencing depth met the needs of experimental analysis.
Figure 6.
Effects of dietary CPCMP supplementation on the intestinal microflora of broilers at 42 d. (A) Rarefaction curve analysis of the number of operational taxonomic units (OTUs). (B) Total OTU analysis. (C) Principal coordinates analysis (PCoA) based on the unweighted (left) and weighted (right) UniFrac metric. (D) Principal component analysis (PCA). A, BC (blank control) group (basal diet); B, CPCMP group (basal diet supplemented with 0.2% Chinese medicine–probiotic compound microecological preparation); C, PC (positive control) group (basal diet supplemented with 0.0035% chlortetracycline); D, commercial CPCMP group (basal diet supplemented with 0.2% commercially available CPCMP); E, TCM group (basal diet supplemented with 0.2% fermented traditional Chinese medicines); F, Probiotics group (basal diet supplemented with 0.2% compound probiotics). (n = 5; 5 replicates/treatment, 1 broiler/replicate).
According to the obtained abundance matrix of OTUs, the number of OTUs in each group was calculated using R software, and the proportions of shared and unique OTUs were intuitively presented in the flower figure (Figure 6B). A total of 376 shared OTUs were observed among all groups. The unique OTUs of the blank control, CPCMP, positive control, commercial CPCMP, TCM, and probiotics groups were 13, 14, 12, 25, 26, and 35, respectively.
Principal component analysis (PCA) and principal coordinates analysis (PCoA) revealed that the gut microbiota in the CPCMP group clearly differed from that in the blank control, positive control, commercial CPCMP, TCM, or probiotics group (Figures 6C and 6D). The results suggested that CPCMP remarkably altered the gut microbiota of broilers.
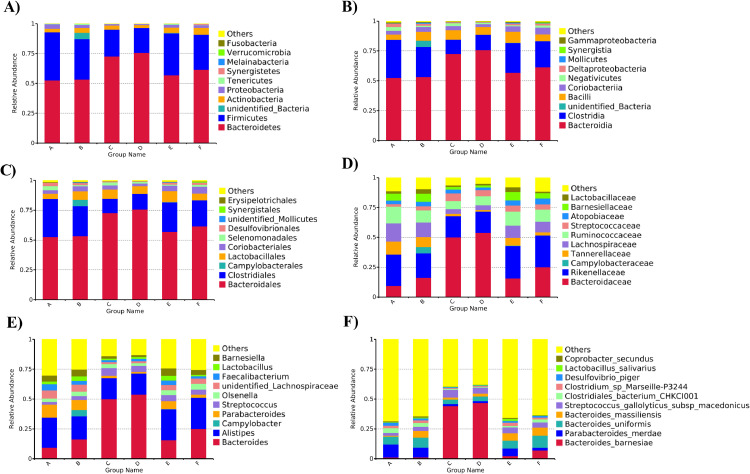
The histograms representing gut microbiota community structure displayed the microbial species and relative abundances of each group. Considering the bacterial composition at the phylum level (Figure 7A), 10 phyla were identified, including Bacteroidetes, Firmicutes, unidentified Bacteria, Actinobacteria, Proteobacteria, Tenericutes, Synergistetes, Melainabacteria, Verrucomicrobia, and Fusobacteria. The most abundant phyla among all groups were Bacteroidetes and Firmicutes. The relative abundances of unidentified Bacteria and Verrucomicrobia in the CPCMP group were obviously higher than those in the blank control, positive control, commercial CPCMP, TCM, or probiotics group. At the class level (Figure 7B), 10 classes, such as Bacteroidia and Clostridia, were found in all groups. Compared with the blank control, positive control, commercial CPCMP, TCM, or probiotics group, CPCMP supplementation augmented the proportions of unidentified Bacteria and Mollicutes and decreased the proportion of Negativicutes. Similarly, sequencing data identified 10 orders of microflora, of which Bacteroidales and Clostridiales were the most abundant among all groups (Figure 7C). The relative abundance of Selenomonadales or unidentified Mollicutes in the CPCMP group was down- or upregulated, respectively, compared with that of the blank control, positive control, commercial CPCMP, or probiotics group. As shown in Figure 7D, Bacteroidaceae, Rikenellaceae, Lachnospiraceae, and Ruminococcaceae accounted for the majority of the 10 families of microbiota. The CPCMP group exhibited the higher proportions of Barnesiellaceae and Lactobacillaceae, compared with the blank control, positive control, commercial CPCMP, or probiotics group. Campylobacteraceae was only identified in the CPCMP group. At the genus level, Campylobacter, Lactobacillus, and Barnesiella were more abundant in the CPCMP group than in the blank control, positive control, commercial CPCMP, or probiotics group, and the CPCMP group had higher abundance of Olsenella than the blank control, positive control, or commercial CPCMP group (Figure 7E). Ten species, such as Bacteroides barnesiae and Parabacteroides merdae, were identified in all groups (Figure 7F). The CPCMP group had higher levels of B. uniformis, B. massiliensis, Lactobacillus salivarius, and Coprobacter secundus compared with the blank control, positive control, or commercial CPCMP group. The proportion of B. uniformis or L. salivarius in the CPCMP group was more abundant than that in the TCM or probiotics group, respectively.
Figure 7.
Gut microbial community structure of broilers of all groups at the (A) phylum, (B) class, (C) order, (D) family, (E) genus, and (F) species levels. A, BC (blank control) group (basal diet); B, CPCMP group (basal diet supplemented with 0.2% Chinese medicine–probiotic compound microecological preparation); C, PC (positive control) group (basal diet supplemented with 0.0035% chlortetracycline); D, commercial CPCMP group (basal diet supplemented with 0.2% commercially available CPCMP); E, TCM group (basal diet supplemented with 0.2% fermented traditional Chinese medicines); F, Probiotics group (basal diet supplemented with 0.2% compound probiotics). (n = 5; 5 replicates/treatment, 1 broiler/replicate).
DISCUSSION
Accumulating evidence indicates that the intestinal barrier and microflora are critical in maintaining the host health (Xue et al., 2018; Pi et al., 2020). Many studies have shown that microecologics can effectively protect the intestinal barrier and regulate the intestinal microbiota in animals (Li et al., 2020; Sun et al., 2020). As a new Chinese medicine–probiotic formulation, CPCMP can make full use of the efficacy of TCMs and probiotics to improve livestock and poultry performance (Liang et al., 2021). At present, many researches have focused on the effects of fermented TCMs on the growth performance, immune function, and meat quality of animals, such as cattle (Wang et al., 2017), pigs (Zhou et al., 2015), ducks (Liu et al., 2017), and broilers (Niu et al., 2017; Niu et al., 2019; Liu et al., 2020). To our knowledge, there is a dearth of studies investigating the influence of fermented TCMs or the compound formulation of fermented TCMs and probiotics on the intestinal barrier and microbiota of broilers (Zhang et al., 2015; Qiao et al., 2018). In the present work, we prepared a CPCMP composed of 5 kinds of TCMs (Galla Chinensis, A. paniculata, Arctii Fructus, Glycyrrhizae Radix, and S. tenuifolia) fermented by Aspergillus niger and a kind of compound probiotics (a mixture of L. plantarum A37 and L. plantarum MIII), and evaluated its effects on the growth performance and antioxidant/immune status of broilers, as well as its influence on intestinal barrier and intestinal microflora of broilers.
The growth performance of broilers was improved by dietary CPCMP treatment. Its beneficial influence was superior to that of chlortetracycline, commercial CPCMP, TCMs, or probiotics. Moreover, the safety of the TCMs used in CPCMP is guaranteed. Galla Chinensis (Xiang et al., 2015) and A. paniculata (Worasuttayangkurn et al., 2019; Jiang et al., 2021) used in appropriate levels have been reported to be nontoxic. Glycyrrhizae Radix (also called as licorice) is a kind of TCM used both as medicine and food in China (Jiang et al., 2020a). Its usage at the upper-limit dose (2,000 mg/kg) and the long-term exposure to it both failed to cause adverse effects in male rats (Shin et al., 2008). Arctii Fructus which is the dried ripe fruit of Arctium lappa L. (Xu et al., 2015) was reported to be very safe in the assessment of toxicity of an oral administration in mice for 8 wk (Bok et al., 2017). S. tenuifolia (also called as catnip) used as an over-the-counter drug was found to be relatively safe (Fung and Lau, 2002; Satomi et al., 2009; Zhu et al., 2009). Furthermore, the additive amount of CPCMP in the basal diet (0.2%) was low, which further ensured the safety of CPCMP.
It was shown that CPCMP had the potential to be an ideal substitute for antibiotics in poultry production. The results also suggested that the combined use of probiotics and TCMs had synergistic effects. The possible explanations are as follows. On the one hand, probiotics have the ability to improve the bioavailability of TCMs (Chen et al., 2016). On the other hand, TCMs can in turn act as prebiotics to promote the growth of probiotics (Wang et al., 2017; Liao et al., 2020).
As the main immune organs of broilers, the bursa of Fabricius and spleen play an important role in host immune response against various pathogenic substances (Seidavi et al., 2017). The bursa of Fabricius, as a central immune organ for birds, is the site for the development and maturation of B-lymphocytes and serum-specific antibodies which are closely associated with humoral immunity (Laparidou et al., 2019). The spleen is a peripheral immune organ and is the core site for immune response initiated by lymphocytes toward antigen stimulation (Pozo et al., 2009). Therefore, the indices of these 2 organs are important indicators for evaluating the immune status of poultry. Our results showed that the spleen and bursa indices of broilers in the CPCMP group were increased, which indicated that CPCMP could promote the maturation of immune organs. The levels of serum and ileal mucosal sIgA and serum IgG in the CPCMP group were also increased. The improvement of these indicators may be attributed to the fact that most TCMs contained in the prepared CPCMP, such as A. paniculata, Arctii Fructus, Glycyrrhizae Radix, and S. tenuifolia, can enhance immunity by protecting the immune system from damage and suppressing the production of inflammatory cytokines (Yue et al., 2012; Yang et al., 2019b; Zhang et al., 2019a; Shi et al., 2020). Moreover, as a component of the CPCMP, Lactobacillus plantarum has been reported to show immunomodulatory properties (Vareille-Delarbre et al., 2019; Zhang et al., 2019b).
Diets supplemented with CPCMP affected the serum biochemical and antioxidant parameters of broilers. The levels of serum biochemical indicators ALB and TG in the CPCMP group were up- and downregulated, respectively. The improvement of these indicators may be attributed to the role of hydrolases in CPCMP, such as lipases and proteases. GSH-Px is an important peroxidase that balances redox status, and its activity represents the ability to scavenge peroxide. MDA is the main product of lipid peroxidation and exhibits cytotoxicity. Its content reflects the degree of lipid peroxidation and body damage (Wu et al., 2015). The GSH-Px activity and the MDA content in the CPCMP group were increased and decreased, respectively, consistent with previous studies (Zhao et al., 2019; Long et al., 2020). One possible explanation for the improvement of these 2 indicators is that most TCMs contained in the CPCMP, like Galla Chinensis, A. paniculata, Arctii Fructus, and Glycyrrhizae Radix, have many active ingredients with antioxidant properties, such as gallic acid, andrographolide, arctigenin, and glycyrrhetinic acid (Samuel et al., 2017; Mussard et al., 2019; Zhang et al., 2019c; Jiang et al., 2020b). But the concrete active ingredient or ingredients that act in the CPCMP are still unclear and need to be dissected in a future study. Lactobacillus plantarum in the CPCMP has also exerted antioxidant activity (Cui et al., 2019; Izuddin et al., 2020), which may contribute to the antioxidant efficacy of the CPCMP.
The VH is associated with the function of the small intestine. A larger VH indicates a stronger ability of the small intestine to absorb nutrients (Jiang et al., 2019). Moreover, a high VH:CD manifests that the small intestine has improved digestion and absorption capacity (Jiang et al., 2019). Our results showed that CPCMP treatment augmented VH and VH:CD of broilers, indicating that dietary CPCMP could improve intestinal morphology and promote nutrient adsorption and utilization in broilers. It has been reported that Lactobacillus plantarum could protect the villi from pathogenic bacteria and promote the growth of the villi (Xu et al., 2020). Probiotics may also help boost the proliferation of crypt cells in the small intestine (He et al., 2019). Furthermore, Galla Chinensis, Arctii Fructus, and Glycyrrhizae Radix contained in the CPCMP have some active ingredients, such as gallic acid (Samuel et al., 2017), pectin (Li et al., 2019), and glycyrrhetinic acid (Chen et al., 2019), which can positively modulate the intestinal morphology and function. It is speculated that the aforementioned effects of CPCMP on intestinal morphology may be related to the combined action of probiotics and TCMs.
Occludin and ZO-1 are 2 important intestinal tight junction proteins that are closely related to the restoration of the intestinal barrier (Yang et al., 2017). Occludin is crucial for the stability of intestinal tight junction and gut barrier function (Saitou et al., 2000), while ZO-1 is a major protein associated with intestinal epithelial integrity and can be used as a marker of intestinal mechanical barrier (Peng et al., 2019). In our study, the mRNA expression levels of OCLN and ZO-1 in the jejunum in the CPCMP group were elevated. These results showed that dietary CPCMP could enhance the intestinal barrier function of broilers, which may be associated with the positive effect of probiotics on maintaining intestinal barrier (Wang et al., 2020b). Moreover, the inhibition of IL-1β secretion of broilers by CPCMP may lead to the upregulated mRNA expression of tight junction-related genes, thus preserving the intestinal tight junction barrier to some extent (Rawat et al., 2020). Further study is needed to elucidate the detailed mechanism.
Furthermore, the cecal microflora of broilers was determined by 16S rRNA gene sequencing. The results of PCA and PCoA showed that CPCMP significantly changed the diversity of gut microbiota. The intestinal microflora community in all groups was evaluated based on the following levels: phylum, class, order, family, genus, and species. Our results showed that Bacteroidetes and Firmicutes are the dominant phyla in the cecum of broilers, which is consistent with previous studies (Yu et al., 2019; Chang et al., 2020). The relative abundance of Bacteroidetes or Firmicutes in the CPCMP group was higher or lower than that in the blank control group, respectively. The proportions of Bacteroidetes and Firmicutes in the CPCMP group were substantially decreased and increased, respectively, compared with that in the positive control, commercial CPCMP, or probiotics group. These results are inconsistent with previous studies, in which researchers found up- and downregulated proportions of Bacteroidetes and Firmicutes with the supplementation of sodium butyrate (Zou et al., 2019) or selenium-enriched yeast (Yang et al., 2020), respectively. We speculate that it may be attributed to the difference in breeding environment and the complex composition and diverse effects of CPCMP on intestinal microbiota. Olsenella and Lactobacillus are beneficial bacteria that can produce short-chain fatty acids and have the ability to provide intestinal cells with energy and protect the intestinal barrier (Kong et al., 2019). Barnesiella is a recently discovered genus that can use glucose to produce butyrate and isobutyrate, and its abundance is positively correlated with the contents of short-chain fatty acids (Gu et al., 2019). Our results showed that CPCMP group had the higher relative abundances of Olsenella, Lactobacillus, and Barnesiella than most of the other groups, which is consistent with previous studies (Wu et al., 2019; Peng et al., 2020). These results indicated that CPCMP facilitated the growth and colonization of the intestinal beneficial bacteria, which might be related to the improvement of the growth performance and intestinal health.
CONCLUSIONS
Dietary CPCMP prepared in our study could boost the growth performance, regulate the serum parameters, promote the immune function, improve the intestinal morphology, enhance the intestinal barrier function, and modulate the intestinal microflora of broilers. CPCMP exhibited the synergistic effects of the combined use of TCMs and probiotics to some extent. This study may provide a realistic basis for the development and application of CPCMP as a novel ideal antibiotic substitute in the livestock and poultry industry.
Acknowledgments
ACKNOWLEDGMENTS
This study was supported by the National Key Research and Development Program of China (Nos. 2017YFD0501000, 2017YFD0501500) and the National Undergraduate Innovation and Entrepreneurship Training Program Project (No. 202010512019). We are also thankful for the technical assistance in 16S rRNA gene sequencing provided by the Tianjin Novogene Bioinformatics Co., Ltd.
DISCLOSURES
The authors have no conflicts of interest to report.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2021.101412.
Appendix. Supplementary materials
REFERENCES
- Abdallah A., Zhang P., Zhong Q.Z., Sun Z.W. Application of traditional Chinese herbal medicine by-products as dietary feed supplements and antibiotic replacements in animal production. Curr. Drug Metab. 2019;20:54–64. doi: 10.2174/1389200219666180523102920. [DOI] [PubMed] [Google Scholar]
- Alagawany M., Abd El-Hack M.E., Farag M.R., Sachan S., Karthik K., Dhama K. The use of probiotics as eco-friendly alternatives for antibiotics in poultry nutrition. Environ. Sci. Pollut. Res. Int. 2018;25:10611–10618. doi: 10.1007/s11356-018-1687-x. [DOI] [PubMed] [Google Scholar]
- Al-Fataftah A.R., Abdelqader A. Effects of dietary Bacillus subtilis on heat-stressed broilers performance, intestinal morphology and microflora composition. Anim. Feed Sci. Technol. 2014;198:279–285. [Google Scholar]
- Bok S.H., Cho S.S., Bae C.S., Park D.H., Park K.M. Safety of 8-weeks oral administration of Arctium lappa L. Lab. Anim. Res. 2017;33:251–255. doi: 10.5625/lar.2017.33.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borda-Molina D., Seifert J., Camarinha-Silva A. Current perspectives of the chicken gastrointestinal tract and its microbiome. Comput. Struct. Biotechnol. J. 2018;16:131–139. doi: 10.1016/j.csbj.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J., Wang T., Wang P., Yin Q.Q., Liu C.Q., Zhu Q., Lu F.S., Gao T.Z. Compound probiotics alleviating aflatoxin B1 and zearalenone toxic effects on broiler production performance and gut microbiota. Ecotoxicol. Environ. Saf. 2020;194 doi: 10.1016/j.ecoenv.2020.110420. [DOI] [PubMed] [Google Scholar]
- Chen F., Wen Q., Jiang J., Li H.L., Tan Y.F., Li Y.H., Zeng N.K. Could the gut microbiota reconcile the oral bioavailability conundrum of traditional herbs. J. Ethnopharmacol. 2016;179:253–264. doi: 10.1016/j.jep.2015.12.031. [DOI] [PubMed] [Google Scholar]
- Chen G., Bei B., Feng Y., Li X.Z., Jiang Z., Si J.Y., Qing D.G., Zhang J., Li N. Glycyrrhetinic acid maintains intestinal homeostasis via HuR. Front. Pharmacol. 2019;10:535. doi: 10.3389/fphar.2019.00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S., Mandal G.P., Patra A.K. Different essential oils in diets of chickens: 1. Growth performance, nutrient utilisation, nitrogen excretion, carcass traits and chemical composition of meat. Anim. Feed Sci. Technol. 2018;236:86–97. [Google Scholar]
- Cui K., Wang Q., Wang S.Q., Diao Q.Y., Zhang N.F. The facilitating effect of tartary buckwheat flavonoids and Lactobacillus plantarum on the growth performance, nutrient digestibility, antioxidant capacity, and fecal microbiota of weaned piglets. Animals (Basel). 2019;9:986. doi: 10.3390/ani9110986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Grande A., Leleu S., Delezie E., Rapp C., De Smet S., Goossens E., Haesebrouck F., Van Immerseel F., Ducatelle R. Dietary zinc source impacts intestinal morphology and oxidative stress in young broilers. Poult. Sci. 2020;99:441–453. doi: 10.3382/ps/pez525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung D., Lau C.B.S. Schizonepeta tenuifolia: chemistry, pharmacology, and clinical applications. J. Clin. Pharmacol. 2002;42:30–36. doi: 10.1177/0091270002042001003. [DOI] [PubMed] [Google Scholar]
- Gu J., Thomas-Ahner J.M., Riedl K.M., Bailey M.T., Vodovotz Y., Schwartz S.J., Clinton S.K. Dietary black raspberries impact the colonic microbiome and phytochemical metabolites in mice. Mol. Nutr. Food Res. 2019;63 doi: 10.1002/mnfr.201800636. [DOI] [PubMed] [Google Scholar]
- He T.F., Long S.F., Mahfuz S., Wu D., Wang X., Wei X.M., Piao X.S. Effects of probiotics as antibiotics substitutes on growth performance, serum biochemical parameters, intestinal morphology, and barrier function of broilers. Animals (Basel) 2019;9:985. doi: 10.3390/ani9110985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., Calder P.C., Sanders M.E. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- Incharoen T., Charoensook R., Onoda S., Tatrakoon W., Numthuam S., Pechkong T. The effects of heat-killed Lactobacillus plantarum L-137 supplementation on growth performance, intestinal morphology, and immune-related gene expression in broiler chickens. Anim. Feed Sci. Technol. 2019;257 doi: 10.3390/vetsci10020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuddin W.I., Humam A.M., Loh T.C., Foo H.L., Samsudin A.A. Dietary postbiotic Lactobacillus plantarum improves serum and ruminal antioxidant activity and upregulates hepatic antioxidant enzymes and ruminal barrier function in post-weaning lambs. Antioxidants (Basel) 2020;9:250. doi: 10.3390/antiox9030250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Qi L., Lv Z.P., Jin S., Wei X.H., Shi F.X. Dietary stevioside supplementation alleviates lipopolysaccharide-induced intestinal mucosal damage through anti-inflammatory and antioxidant effects in broiler chickens. Antioxidants (Basel) 2019;8:575. doi: 10.3390/antiox8120575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M.Y., Zhao S.J., Yang S.S., Lin X., He X.G., Wei X.Y., Song Q., Li R., Fu C.M., Zhang J.M., Zhang Z. An “essential herbal medicine”—licorice: a review of phytochemicals and its effects in combination preparations. J. Ethnopharmacol. 2020;249 doi: 10.1016/j.jep.2019.112439. [DOI] [PubMed] [Google Scholar]
- Jiang L., Deng Y., Li W., Lu Y. Arctigenin suppresses fibroblast activity and extracellular matrix deposition in hypertrophic scarring by reducing inflammation and oxidative stress. Mol. Med. Rep. 2020;22:4783–4791. doi: 10.3892/mmr.2020.11539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M.Y., Sheng F.Y., Zhang Z., Ma X., Gao T.H., Fu C.M., Li P. Andrographis paniculata (Burm.f.) Nees and its major constituent andrographolide as potential antiviral agents. J. Ethnopharmacol. 2021;272 doi: 10.1016/j.jep.2021.113954. [DOI] [PubMed] [Google Scholar]
- Kogut M.H. The effect of microbiome modulation on the intestinal health of poultry. Anim. Feed Sci. Technol. 2018;250:32–40. [Google Scholar]
- Kong C., Gao R.Y., Yan X.B., Huang L.S., Qin H.L. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition. 2019;60:88175–88184. doi: 10.1016/j.nut.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Laparidou M., Schlickenrieder A., Thoma T., Lengyel K., Schusser B. Blocking of the CXCR4-CXCL12 interaction inhibits the migration of chicken B cells into the bursa of Fabricius. Front Immunol. 2019;10:3057. doi: 10.3389/fimmu.2019.03057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Liu T.Y., Yan C., Xie R.X., Guo Z.X., Wang S.N., Zhang Y.J., Li Z.X., Wang B.M., Cao H.L. Diammonium glycyrrhizinate protects against nonalcoholic fatty liver disease in mice through modulation of gut microbiota and restoration of intestinal barrier. Mol. Pharm. 2018;15:3860–3870. doi: 10.1021/acs.molpharmaceut.8b00347. [DOI] [PubMed] [Google Scholar]
- Li K.D., Zhu L.L., Li H., Zhu Y.P., Pan C., Gao X.D., Liu W. Structural characterization and rheological properties of a pectin with anti-constipation activity from the roots of Arctium lappa L. Carbohydr. Polym. 2019;215:119–129. doi: 10.1016/j.carbpol.2019.03.051. [DOI] [PubMed] [Google Scholar]
- Li N., Pang B., Li J.J., Liu G.W., Xu X.G., Shao D.Y., Jiang C.M., Yang B.W., Shi J.L. Mechanisms for Lactobacillus rhamnosus treatment of intestinal infection by drug-resistant Escherichia coli. Food Funct. 2020;11:4428–4445. doi: 10.1039/d0fo00128g. [DOI] [PubMed] [Google Scholar]
- Liang W.F., Li H.T., Zhou H.Y., Wang M., Zhao X., Sun X.H., Li C.T., Zhang X.M. Effects of Taraxacum and Astragalus extracts combined with probiotic Bacillus subtilis and Lactobacillus on Escherichia coli-infected broiler chickens. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W., Imran K., Huang G., Chen S., Liu L., Leong W.K., Li X.A., Wu J., Wendy Hsiao W.L. Bifidobacterium animalis: the missing link for the cancer-preventive effect of Gynostemmapentaphyllum. Gut Microbes. 2020;24 doi: 10.1080/19490976.2020.1847629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Ding B.A., Chen L.Y., Zhang Z.M., He H.L., Wang J.G., Wang X.Z., Zhang L.C., Ni X.M., Fronte B. The effect of Aspergillus niger as a dietary supplement on blood parameters, intestinal morphology, and gut microflora in Haidong chicks reared in a high altitude environment. Vet. World. 2020;13:2209–2215. doi: 10.14202/vetworld.2020.2209-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.Y., Cao G.J., Zhou J.L., Yao X., Fang B.H. The effects of Bacillus coagulans-fermented and non-fermented Ginkgo biloba on abdominal fat deposition and meat quality of Peking duck. Poult. Sci. 2017;96:2264–2273. doi: 10.3382/ps/pex017. [DOI] [PubMed] [Google Scholar]
- Liu S.S., Yan W.J., Ma C., Liu Y.J., Gong L.M., Levesque C., Dong B. Effects of supplemented culture media from solid-state fermented Isaria cicadae on performance, serum biochemical parameters, serum immune indexes, antioxidant capacity and meat quality of broiler chickens. Asian-Austr. J. Anim. Sci. 2020;33:568–578. doi: 10.5713/ajas.19.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Long L.N., Kang B.J., Jiang Q., Chen J.S. Effects of dietary Lycium barbarum polysaccharides on growth performance, digestive enzyme activities, antioxidant status, and immunity of broiler chickens. Poult. Sci. 2020;99:744–751. doi: 10.1016/j.psj.2019.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra B.B., Tiwari V.K. Natural products: an evolving role in future drug discovery. Eur. J. Med. Chem. 2011;46:4769–4807. doi: 10.1016/j.ejmech.2011.07.057. [DOI] [PubMed] [Google Scholar]
- Mohammed A.A., Jiang S., Jacobs J.A., Cheng H.W. Effect of a synbiotic supplement on cecal microbial ecology, antioxidant status, and immune response of broiler chickens reared under heat stress. Poult. Sci. 2019;98:4408–4415. doi: 10.3382/ps/pez246. [DOI] [PubMed] [Google Scholar]
- Mussard E., Cesaro A., Lespessailles E., Legrain B., Berteina-Raboin S., Toumi H. Andrographolide, a natural antioxidant: an update. Antioxidants (Basel) 2019;8:571. doi: 10.3390/antiox8120571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . 9th rev. ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Niu Y., Wan X.L., Zhang X.H., Zhao L.G., He J.T., Zhang J.F., Zhang L.L., Wang T. Effect of supplemental fermented Ginkgo biloba leaves at different levels on growth performance, meat quality, and antioxidant status of breast and thigh muscles in broiler chickens. Poult. Sci. 2017;96:869–877. doi: 10.3382/ps/pew313. [DOI] [PubMed] [Google Scholar]
- Niu Y., Wan X.L., Zhang L.L., Wang C., He J.T., Bai K.W., Zhang X.H., Zhao L.G., Wang T. Effect of different doses of fermented Ginkgo biloba leaves on serum biochemistry, antioxidant capacity hepatic gene expression in broilers. Anim. Feed Sci. Technol. 2019;248:132–140. [Google Scholar]
- Peng Y.J., Yan Y.M., Wan P., Chen D., Ding Y., Ran L.W., Mi J., Lu L., Zhang Z.J., Li X.Y., Zeng X.X., Cao Y.L. Gut microbiota modulation and anti-inflammatory properties of anthocyanins from the fruits of Lycium ruthenicum Murray in dextran sodium sulfate-induced colitis in mice. Free Radic. Biol. Med. 2019;136:96–108. doi: 10.1016/j.freeradbiomed.2019.04.005. [DOI] [PubMed] [Google Scholar]
- Peng Y.J., Yan Y.M., Wan P., Dong W., Huang K.Y., Ran L.W., Mi J., Lu L., Zeng X.X., Cao Y.L. Effects of long-term intake of anthocyanins from Lycium ruthenicum Murray on the organism health and gut microbiota in vivo. Food Res. Int. 2020;130 doi: 10.1016/j.foodres.2019.108952. [DOI] [PubMed] [Google Scholar]
- Pi Y., Mu C.L., Gao K., Liu Z., Peng Y., Zhu W.Y. Increasing the hindgut carbohydrate/protein ratio by cecal infusion of corn starch or casein hydrolysate drives gut microbiota-related bile acid metabolism to stimulate colonic barrier function. mSystems. 2020;5 doi: 10.1128/mSystems.00176-20. e00176–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirgozliev V., Mansbridge S.C., Rose S.P., Mackenzie A.M., Beccaccia A., Karadas F., Ivanova S.G., Staykova G.P., Oluwatosin O.O., Bravo D. Dietary essential oils improve feed efficiency and hepatic antioxidant content of broiler chickens. Animal. 2019;13:502–508. doi: 10.1017/S1751731118001520. [DOI] [PubMed] [Google Scholar]
- Pozo A.L., Godfrey E.M., Bowles K.M. Splenomegaly: investigation, diagnosis and management. Blood Rev. 2009;23:105–111. doi: 10.1016/j.blre.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Rawat M., Nighot M., Al-Sadi R., Gupta Y., Viszwapriya D., Yochum G., Koltun W., Ma T.Y. IL1B increases intestinal tight junction permeability by upregulation of MIR200C-3p, which degrades occludin mRNA. Gastroenterology. 2020;159:1375–1389. doi: 10.1053/j.gastro.2020.06.038. [DOI] [PubMed] [Google Scholar]
- Saitou M., Furuse M., Sasaki H., Schulzke J.D., Fromm M., Takano H., Noda T., Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol. Biol. Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H.X., Song Y.Z., Shi H.T., Bian C.Z. Fermented astragalus in diet altered the composition of fecal microbiota in broiler chickens. AMB Express. 2018;8:151. doi: 10.1186/s13568-018-0682-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel K.G., Wang J., Yue H.Y., Wu S.G., Zhang H.J., Duan Z.Y., Qi G.H. Effects of dietary gallic acid supplementation on performance, antioxidant status, and jejunum intestinal morphology in broiler chicks. Poult. Sci. 2017;96:2768–2775. doi: 10.3382/ps/pex091. [DOI] [PubMed] [Google Scholar]
- Satomi Y., Ohara K., Yazaki K., Ito M., Honda G., Nishino H. Production of the monoterpene limonene and modulation of apoptosis-related proteins in embryonic-mouse NIH 3T3 fibroblast cells by introduction of the limonene synthase gene isolated from Japanese catnip (Schizonepeta tenuifolia) Biotechnol. Appl. Biochem. 2009;52:185–190. doi: 10.1042/BA20080023. [DOI] [PubMed] [Google Scholar]
- Seidavi A., Dadashbeiki M., Alimohammadi-Saraei M.H., van den Hoven R., Payan-Carreira R., Laudadio V., Tufarelli V. Effects of dietary inclusion level of a mixture of probiotic cultures and enzymes on broiler chickens immunity response. Environ. Sci. Pollut. Res. Int. 2017;24:4637–4644. doi: 10.1007/s11356-016-8206-8. [DOI] [PubMed] [Google Scholar]
- Shi Y., Zhong L., Liu Y.L., Zhang J.Z., Lv Z., Li Y., Hu Y. Effects of dietary andrographolide levels on growth performance, antioxidant capacity, intestinal immune function and microbioma of rice field eel (Monopterus Albus) Animals (Basel) 2020;10:1744. doi: 10.3390/ani10101744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S., Jang J.Y., Choi B.I., Baek I.J., Yon J.M., Hwang B.Y., Park D., Jeon J.H., Nam S.Y., Yun Y.W., Kim Y.B. Licorice extract does not impair the male reproductive function of rats. Exp. Anim. 2008;57:11–17. doi: 10.1538/expanim.57.11. [DOI] [PubMed] [Google Scholar]
- Smith J.A. Broiler production without antibiotics: United States field perspectives. Anim. Feed Sci. Technol. 2019;250:93–98. [Google Scholar]
- Sun M.Y., Liu Y.J., Song Y.L., Gao Y., Zhao F.J.Z., Luo Y.H., Qian F., Mu G.Q., Tuo Y.F. The ameliorative effect of Lactobacillus plantarum-12 on DSS-induced murine colitis. Food Funct. 2020;11:5205–5222. doi: 10.1039/d0fo00007h. [DOI] [PubMed] [Google Scholar]
- Suresh G., Das R.K., Brar S.K., Rouissi T., Ramirez A.A., Chorfi Y., Godbout S. Alternatives to antibiotics in poultry feed: molecular perspectives. Crit. Rev. Microbiol. 2018;44:318–335. doi: 10.1080/1040841X.2017.1373062. [DOI] [PubMed] [Google Scholar]
- Vareille-Delarbre M., Miquel S., Garcin S., Bertran T., Balestrino D., Evrard B., Forestier C. Immunomodulatory effects of Lactobacillus plantarum on inflammatory response induced by Klebsiella pneumoniae. Infect. Immun. 2019;87 doi: 10.1128/IAI.00570-19. e00570–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Xie H.J., Liu F., Wang Y.H. Production performance, immunity, and heat stress resistance in Jersey cattle fed a concentrate fermented with probiotics in the presence of a Chinese herbal combination. Anim. Feed Sci. Technol. 2017;228:59–65. [Google Scholar]
- Wang Y.Y., Xie Q.H., Zhang Y., Ma W., Ning K., Xiang J.Y., Cui J.W., Xiang H.Y. Combination of probiotics with different functions alleviate DSS-induced colitis by regulating intestinal microbiota, IL-10, and barrier function. Appl. Microbiol. Biotechnol. 2020;104:335–349. doi: 10.1007/s00253-019-10259-6. [DOI] [PubMed] [Google Scholar]
- Wang M.J., Wu H.Q., Lu L.H., Jiang L., Yu Q.H. Lactobacillus reuteri promotes intestinal development and regulates mucosal immune function in newborn piglets. Front. Vet. Sci. 2020;7:42. doi: 10.3389/fvets.2020.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worasuttayangkurn L., Nakareangrit W., Kwangjai J., Sritangos P., Pholphana N., Watcharasit P., Rangkadilok N., Thiantanawat A., Satayavivad J. Acute oral toxicity evaluation of Andrographis paniculata-standardized first true leaf ethanolic extract. Toxicol. Rep. 2019;6:426–430. doi: 10.1016/j.toxrep.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q.J., Wang Z.B., Wang G.Y., Li Y.X., Qi Y.X. Effects of feed supplemented with fermented pine needles (Pinus ponderosa) on growth performance and antioxidant status in broilers. Poult. Sci. 2015;94:1138–1144. doi: 10.3382/ps/pev013. [DOI] [PubMed] [Google Scholar]
- Wu Y.Q., Wang Y.L., Yin D.F., Wu W., Sun X.Y., Zhang Y.Q., Guo X.R., Chen J., Yuan J.M. Effect of supplementation of nicotinamide and sodium butyrate on the growth performance, liver mitochondrial function and gut microbiota of broilers at high stocking density. Food Funct. 2019;10:7081–7090. doi: 10.1039/c9fo00904c. [DOI] [PubMed] [Google Scholar]
- Xiang F., Peng L.C., Yin Z.Q., Jia R.Y., Hu Z.Q., Li Z.W., Ni X.Q., Liang X.X., Li L.X., He C.L., Yin L.Z., Su G., Lv C. Acute and subchronic toxicity as well as evaluation of safety pharmacology of Galla chinensis solution. J. Ethnopharmacol. 2015;162:181–190. doi: 10.1016/j.jep.2014.12.021. [DOI] [PubMed] [Google Scholar]
- Xu Z.H., Gu C.C., Wang K., Ju J.X., Wang H.Y., Ruan K.F., Feng Y. Arctigenic acid, the key substance responsible for the hypoglycemic activity of Fructus Arctii. Phytomedicine. 2015;22:128–137. doi: 10.1016/j.phymed.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Xu T.Y., Chen Y., Yu L.F., Wang J., Huang M.X., Zhu N.H. Effects of Lactobacillus plantarum on intestinal integrity and immune responses of egg-laying chickens infected with Clostridium perfringens under the free-range or the specific pathogen free environment. BMC Vet. Res. 2020;16:47. doi: 10.1186/s12917-020-2264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M.L., Ji X.Q., Liang H., Liu Y., Wang B., Sun L.L., Li W.W. The effect of fucoidan on intestinal flora and intestinal barrier function in rats bearing breast cancer. Food Funct. 2018;9:1214–1223. doi: 10.1039/c7fo01677h. [DOI] [PubMed] [Google Scholar]
- Yang G., Bibi S., Du M., Suzuki T., Zhu M.J. Regulation of the intestinal tight junction by natural polyphenols: a mechanistic perspective. Crit. Rev. Food Sci. Nutr. 2017;57:3830–3839. doi: 10.1080/10408398.2016.1152230. [DOI] [PubMed] [Google Scholar]
- Yang L., Liu G., Lian K.X., Qiao Y.J., Zhang B.J., Zhu X.Q., Luo Y., Shang Y.X., Gu X.L. Dietary leonurine hydrochloride supplementation attenuates lipopolysaccharide challenge-induced intestinal inflammation and barrier dysfunction by inhibiting the NF-κB/MAPK signaling pathway in broilers. J. Anim. Sci. 2019;97:1679–1692. doi: 10.1093/jas/skz078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E.J., Song I.S., Song K.S. Ethanol extract of Glycyrrhizae Radix modulates the responses of antigen-specific splenocytes in experimental autoimmune encephalomyelitis. Phytomedicine. 2019;54:56–65. doi: 10.1016/j.phymed.2018.09.189. [DOI] [PubMed] [Google Scholar]
- Yang S.H., Li L., Yu L.H., Sun L., Li K., Tong C., Xu W.X., Cui G.Y., Long M., Li P. Selenium-enriched yeast reduces caecal pathological injuries and intervenes changes of the diversity of caecal microbiota caused by Ochratoxin-A in broilers. Food Chem. Toxicol. 2020;137 doi: 10.1016/j.fct.2020.111139. [DOI] [PubMed] [Google Scholar]
- Yu M., Li Z.M., Chen W.D., Wang G., Cui Y.Y., Ma X.Y. Dietary supplementation with citrus extract altered the intestinal microbiota and microbial metabolite profiles and enhanced the mucosal immune homeostasis in yellow-feathered broilers. Front Microbiol. 2019;10:2662. doi: 10.3389/fmicb.2019.02662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue G.G., Chan B.C., Kwok H.F., To M.H., Hon K.L., Fung K.P., Lau C.B., Leung P.C. Screening for anti-inflammatory and bronchorelaxant activities of 12 commonly used Chinese herbal medicines. Phytother Res. 2012;26:915–925. doi: 10.1002/ptr.3659. [DOI] [PubMed] [Google Scholar]
- Zaffiri L., Gardner J., Toledo-Pereyra L.H. History of antibiotics. From salvarsan to cephalosporins. J. Invest. Surg. 2012;25:67–77. doi: 10.3109/08941939.2012.664099. [DOI] [PubMed] [Google Scholar]
- Zahirian M., Seidavi A., Solka M., Nosrati M., Corazzin M. Dietary supplementation of Aspergillus oryzae meal and its effect on performance, carcass characteristics, blood variables, and immunity of broiler chickens. Trop. Anim. Health Prod. 2019;51:2263–2268. doi: 10.1007/s11250-019-01930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.H., Sun Z.Y., Cao F.L., Ahmad H., Yang X., Zhao L.G., Wang T. Effects of dietary supplementation with fermented ginkgo leaves on antioxidant capacity, intestinal morphology and microbial ecology in broiler chicks. Br. Poult. Sci. 2015;56:370–380. doi: 10.1080/00071668.2015.1030590. [DOI] [PubMed] [Google Scholar]
- Zhang J., Cao P., Gui J.J., Wang X., Han J., Wang Y.W., Wang G.D. Arctigenin ameliorates renal impairment and inhibits endoplasmic reticulum stress in diabetic db/db mice. Life Sci. 2019;223:194–201. doi: 10.1016/j.lfs.2019.03.037. [DOI] [PubMed] [Google Scholar]
- Zhang Z.W., Man C.X., Sun L.L., Yang X.Y., Li M.Y., Zhang W., Jiang Y.J. Short communication: complete genome sequence of Lactobacillus plantarum J26, a probiotic strain with immunomodulatory activity. J. Dairy Sci. 2019;102:10838–10844. doi: 10.3168/jds.2019-16593. [DOI] [PubMed] [Google Scholar]
- Zhang M., Chang Z., Zhao F., Zhang P., Hao Y.J., Yan L., Liu N., Wang J.L., Bo L., Ma P., Zhou W., Ma X., Xu Q.B., Zhou R. Protective effects of 18β-glycyrrhetinic acid on monocrotaline-induced pulmonary arterial hypertension in rats. Front. Pharmacol. 2019;10:13. doi: 10.3389/fphar.2019.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Zhong G., Shao D., Wang Q., Hu Y., Wu T.X., Ji C.J., Shi S.R. Dietary supplementation with Bacillus subtilis promotes growth performance of broilers by altering the dominant microbial community. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.R., Chen Y.P., Cheng Y.F., Qu H.M., Li J., Wen C., Zhou Y.M. Effects of dietary phytosterols on growth performance, antioxidant status, and meat quality in Partridge Shank chickens. Poult. Sci. 2019;98:3715–3721. doi: 10.3382/ps/pez059. [DOI] [PubMed] [Google Scholar]
- Zhou H., Wang C.Z., Ye J.Z., Chen H.X., Tao R. Effects of dietary supplementation of fermented Ginkgo biloba L. residues on growth performance, nutrient digestibility, serum biochemical parameters and immune function in weaned piglets. Anim. Sci. J. 2015;86:790–799. doi: 10.1111/asj.12361. [DOI] [PubMed] [Google Scholar]
- Zhu J.J., Zeng X.P., Berkebile D., DU H.J., Tong Y., Qian K. Efficacy and safety of catnip (Nepeta cataria) as a novel filth fly repellent. Med. Vet. Entomol. 2009;23:209–216. doi: 10.1111/j.1365-2915.2009.00809.x. [DOI] [PubMed] [Google Scholar]
- Zou X., Ji J., Qu H., Wang J., Shu D.M., Wang Y., Liu T.F., Li Y., Luo C.L. Effects of sodium butyrate on intestinal health and gut microbiota composition during intestinal inflammation progression in broilers. Poult. Sci. 2019;98:4449–4456. doi: 10.3382/ps/pez279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.