Abstract
Background
Thus far, the hip revision surgery has been widely used and promoted, and the technology has been constantly innovated, such as tissue engineering, 3D printing prosthesis, etc. However, traditional standardized prosthesis, allograft, autograft, bone cement and reinforcing ring are still the main treatment methods in the mainstream pelvic defects classification systems for hip revision. In addition, the mainstream classification systems are still mainly focus on the peri-acetabulum, but less on the large-scale complex pelvic defects that widely affecting the regions far away from the acetabulum, which also have a significant impact on the holistic biomechanical properties of pelvis.
Methods
After integrating the design experience of custom prostheses and the understanding of biomechanical properties of pelvis, an innovative pelvic defects classification for custom revision was preliminarily proposed, and was practiced in surgeries. Some typical cases were chosen for elucidation in this study, and two observers each evaluated their CT data independently twice. Intraobserver and interobserver agreement were calculated using the kappa statistic to evaluate the reliability. The pelvis defects were classified into five types and two subtypes. The corresponding reconstruction principles, as the main basis to support the classification, were also described in detail. Prosthesis position examination and Harris hip score were utilized to evaluate the clinical outcome.
Results
The installed prostheses resulted in high concordance with preoperative position planning, significantly improved Harris score, low postoperative complication rate and no re-revision case. In addition, The interobserver and intraobserver agreement were both excellent.
Conclusion
The presenting revision system for complex pelvic defects utilizing 3D-printed custom prosthesis and corresponding classification of pelvic defects can preliminarily guide patients’ grouping and prosthesis design, and may potentially provide an innovative, feasible, and efficient basis for complex total hip arthroplasty (THA) revision.
Translational potential statement
This study provides a novel method for prosthetic revision of peri-acetabular pelvic defects, and is expected to systematically improve the efficiency of prosthesis design and surgery in clinical practice.
Keywords: Hip revision, 3D-printed, Custom design, Pelvic defects, Classification system
1. Introduction
The developments in THA have led to a sharp increase in the demand for revision surgeries, which is anticipated to double by 2026 [1]. Traditional revision methods are usually unable to completely adjust its own structure according to the details of the patient's bone defects, as a result of which the focus shifted to 3D-printed individualized custom hip prostheses, a flexible, efficient, precise, and innovative technology [2]. In comparison with the traditional standardized prostheses, these individualized prostheses can flexibly modify their structure to adapt to the bone, preserving the patient's bone stock while enhancing its compatibility [3]. These prostheses can even help fine-tune screw insertion according to the patient's bone quality to further increase stability [4,5].
Despite the medical value, thus far, a targeted classification of pelvic defects for 3D-printed custom hip revision remains unavailable. As the two most commonly used classification systems at present, the Paprosky and American Academy of Orthopaedic Surgeons (AAOS) classification systems all mainly focus on the peri-acetabulum, but less on the large-scale complex pelvic defects which widely affect the ala of ilium, superior pubic ramus, and other pelvic regions. Although these structures are far away from the acetabulum, they still have a significant impact on the biomechanical properties of the hip joint. In view of the fact that 3D-printed custom prosthesis is often used in large-scale and complex THA revision patients, the corresponding pelvic defect classification system should focus on the whole pelvic structure and cover the above regions far away from the acetabulum. In addition, the two classification systems are based on the traditional revision methods, such as cages, bone graft, standardized prosthesis and so on. With the update of revision methods and the development of custom 3D-printed prosthesis, these current mainstream defect classification systems can not fully meet the needs of individualized revision. Furthermore, the Paprosky classification classifies most complex pelvic defects requiring individualized design prostheses into types IIIA and IIIB, similar to the AAOS classification, suggesting that these mainstream classification systems can adequately identify patients with such pelvic defects as a whole, but their further classification ability is compromised [[6], [7], [8]].
Thus, the present study comprehensively integrated the treatments and independent prosthesis designs from 2014 to 2019, and innovatively proposed a pelvic defect classification system based on 3D-printed individualized custom revision prostheses. The prosthesis design principles under each classification were also listed in detail, both as a basis to support this classification and to guide the specific 3D-printed prosthesis design. The study aims to furnish preliminary guidelines and provide a practical classification approach for the management of complex THA custom revision.
2. Materials and methods
Inclusion criteria: 1. Pelvic bone defect patients needing 3D-printed custom hip revision surgery; 2. A follow-up time of no less than 12 months and with complete follow-up data available.
Exclusion criteria: 1. Patients with pelvic bone tumor before revision, including benign and malignant tumors. 2. Refuse to carry out post-operative follow-up, or cannot complete the follow-up due to personal reasons. 3. Abnormal structure or function of hip joint due to other reasons that were unrelated to revision prostheses and surgery (tumor, immune system diseases such as ankylosing spondylitis, and central nervous system diseases such as stroke) after operation.
Of the 44 patients who underwent 3D-printed individualized prostheses with the instruction of the presenting revision system, 11 patients were excluded according to the above exclusion criteria. In view of the large and complex workload of subsequent three-dimensional evaluation of postoperative prostheses positions, we randomly selected 12 of the remaining patients to elucidate the revision system described in this study. The material of 3D-printing was titanium alloy and electron beam melting (EBM) technology was adopted. The inner diameter of the printed prosthesis was about 49 mm. This study was approved by the corresponding ethics committee and conducted in accordance with the World Medical Association Declaration of Helsinki (JBJS 79A:1089-98,1997). Patient confidentiality was protected according to the U.S. Health Insurance Portability and Accountability Act (HIPAA). Informed consent was also obtained before the experimentation for these patients.
2.1. Radiographic evaluation
Thin-slice computed tomography (CT) data of both legs were collected before and after operation, ranging from the lumbar vertebrae to the feet. The CT data of pelvis were reconstructed by Mimics Medical 20.0 (Materialise, Belgium) software. Observers evaluated the bone defects range according to the results of 3D reconstruction, and further classified each case into one specific category.
2.2. Description of classification system with corresponding reconstruction principles
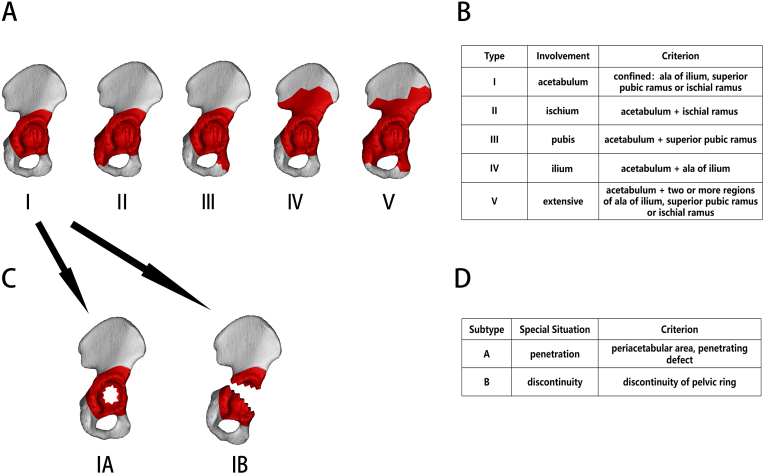
The pelvic defects were classified into types I–V and subtypes A–B according to their ranges (Fig. 1). The subtype was considered null if the bone defects did not meet the subtype A or B definition. A type and a subtype together form a complete category.
Figure 1.
Classification of the bone defect range. A, Schematic diagram of the bone defect range in types I–V; B, definitions used in the bone defect type classification; C, schematic diagram of bone defects in subtypes A and B; D, definitions used in bone defect subtype classification.
Type I defect refers to a relatively small range of bone defects around the acetabulum. They are not necessarily confined exactly to the acetabular rim. Even if the defect range is beyond the acetabular rim, as long as the ala of the ilium, superior pubic ramus, and ischial ramus are not involved, it will still be classified as a type I defect. This is because although the acetabulum is composed of three different structures, namely, the ischium, pubis, and ilium, a defect inside or around the acetabulum has no significant impact on the mechanical properties of these three structures and can therefore be classified into just one type. Conversely, once the bone defect involves the ala of the ilium, superior pubic ramus, or ischial ramus, the designs for augments, flanges, and screws will be fundamentally altered, necessitating a classification system to distinguish these situations.
The reason for this classification is that type I pelvic defect has a relatively limited range and shows satisfactory bone mass preservation. Flange placements of the ilium and pubis are viable options. For some patients with adequate bone mass preservation, a single acetabular cup of pure or approximate spherical shape can be firmly fixed through screwing to the anterior and posterior iliac spines, ischial ramus, and superior pubic ramus (Fig. 2). As a structure with excellent bone mass, the acetabulum rim can also provide rigid fixation by setting screws in its preserved part [9].
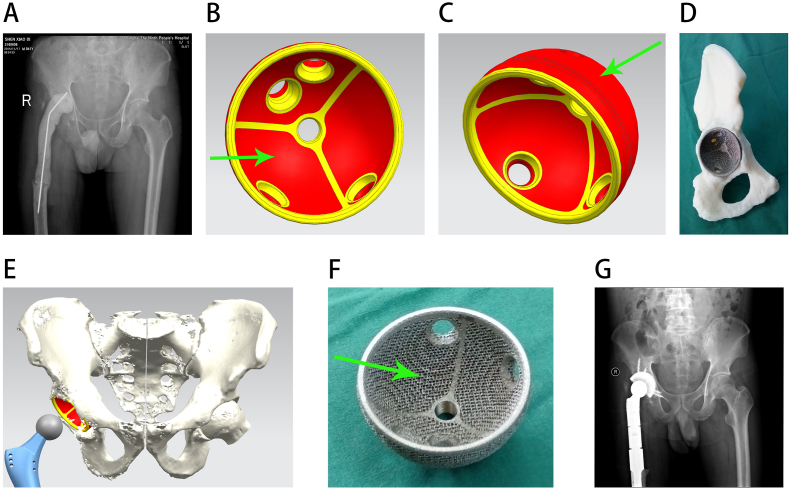
Figure 2.
Reconstruction of a type I pelvic defect. A, preoperative radiograph; B, internal surface of the acetabular cup prosthesis, with the red area showing the porous surface area and the green arrow indicating that the porous structure has also been added to the internal surface of the acetabular cup, which facilitated bone cement bonding; C, lateral surface of the acetabular cup, with the green arrow indicating the porous structure; D, installation diagram of the acetabular cup prosthesis; E, 3D design diagram of the preoperative prosthesis; F, an actual picture of the acetabular cup, with the green arrow showing that the porous structure has also been added to the inner side; G, postoperative radiograph.
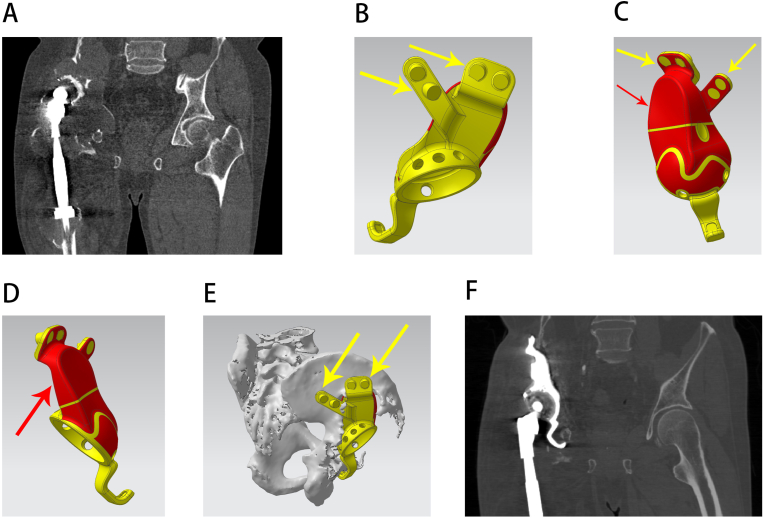
Type II pelvic defects are specifically defined by the involvement of the ischial ramus. Since the original bone mass of the ischial ramus is relatively satisfactory, it serves as the ideal target region for fixation. The posterior and lateral parts of the ischial tuberosity are adaptive for flanges, especially the posterior part, which is capable of bearing relatively longer screws [10]. Nevertheless, the ischium flange is considered to be the easiest one for pulling out in triflange acetabular components, which can be attributed to the lateralization due to gravity and the pelvic structure, resulting in increased shear force [9].
The reason for this classification was that although the ischium has excellent mechanical properties, its flanges usually require high bone quality, and its exposure can cause unnecessary tissue damage, which collectively indicate that adding a flange on the ischium is not requisite [11]. In addition, the contribution of the ischium to the holistic stability of the pelvic ring is not as significant as that of the pubis; thus, for type II patients, reconstruction of the ischium is not routine. If necessary, a custom-made augment for ischium defects with screws through it can provide extra stability against lateralization (Fig. 3).
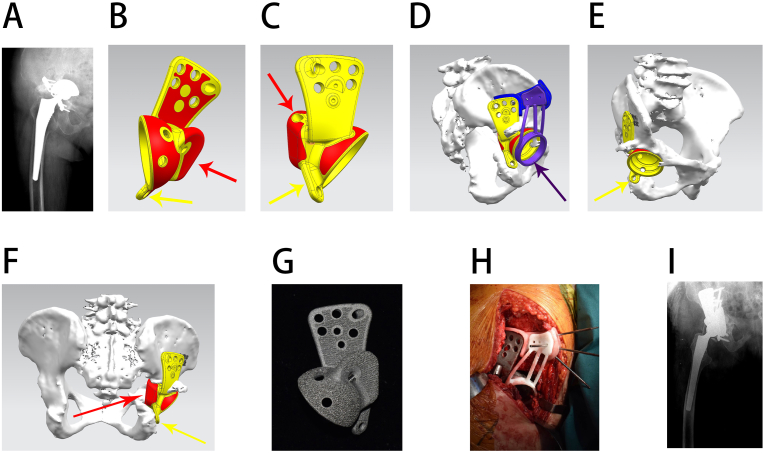
Figure 3.
Reconstruction of a type II pelvic defect. A, preoperative radiograph; B, side view of the prosthesis, with the red area showing the porous structure surface and the red and yellow arrows showing an individualized custom ischium augment and flange respectively; C, another side view of the prosthesis, with the red and yellow arrows showing the individualized custom ischium augment and flange, respectively; D, 3D design of a custom installation guide plate before operation, with the violet arrow showing that the custom guide plate can precisely attach to the bone and prosthesis to assist installation; E, 3D design of the prosthesis before the operation, with the yellow arrow showing an individualized custom ischium flange. F, back view of the prosthesis 3D design, with the yellow arrow showing an individualized custom ischium flange and the red arrow showing that the individualized custom ischium augment can reconstruct the ischium defect; G, an actual picture of the prosthesis; H, image of the intraoperative prosthesis installation; I, postoperative radiograph.
The type III pelvic defect refers to a pubis-involving bone defect, with superior pubic ramus damage being the classification criterion. The pubic flange does not always require screws, but its presence is critical for preventing internal rotation of the acetabular component [9,10]. Meanwhile, loosening of the fixed screws inside the superior pubic ramus can be critical for consequent prosthesis lateralization and may result in failure, indicating the need for determining pubis involvement before prosthesis design [12].
The reason for this classification was that the pubic flanges were the second most commonly used structure after the ilium in this study. In type III patients, the pubic flange value is compromised because of the bone defect; however, the pubis can still be reconstructed by a custom-made augment, and a screw can be further inserted into the residual pubis through the augment, which can effectively prevent lateralization of the acetabular component as a result of the stress of gravity (Fig. 4).
Figure 4.
Reconstruction of a type III pelvic defect. A, preoperative CT; B, side view of the prosthesis, with the red area showing the porous structure surface and the red arrow showing the individualized custom pubic augment; C, back view of the prosthesis, with the red arrow showing the individualized custom pubic augment; D, 3D design diagram of the prosthesis before the operation, with the red arrow showing that the individualized custom pubic augment can reconstruct the pubic defect; E, preoperative simulation of the 3D-printed model of the prosthesis only; F, preoperative simulation of the 3D-printed model of the installed prosthesis and bone; G, image of the intraoperative prosthesis installation; H, postoperative CT.
Type IV represents bone defects involving the ala of the ilium. The bone stock of the ala of the ilium, especially at the iliac spine, is usually well preserved to some extent. Its position above the acetabular prosthesis also ensures that it can effectively resist the upward stress to bear the weight of the trunk, making it a pivotal region for fixing flanges and screws.
The reason for this classification is that with respect to the flanges on custom prostheses, the ones on the ilium predominantly contribute to the stability of the components. Hence, once the ala of the ilium is involved, the setting position of the ilium flanges must be flexibly adjusted according to the defect area (Fig. 5). The area of ilium flanges can be reduced, and the number can be increased. In cases involving serious defects, it can be difficult to choose a reasonable fixation position even if the length of the screws required is relatively short. In such cases, internal screwing becomes the main choice for prosthesis fixation. The paths from the acetabular cup to the iliac spines are common routes for screwing, suggesting that if the defect involves the above regions, these optimal screwing routes will be disturbed and adequate fixation from the direction of the ilium may no longer be viable. In this case, the number of screw holes in the pubic flange can be increased, the obturator hook should be added, and the fixation of the cup toward the acetabulum bone must also be strengthened. If necessary, inserting the screw from the prosthesis to the sacrum is also an option.
Figure 5.
Reconstruction of a type IV pelvic defect. A, preoperative CT; B, diagram of the iliac bone flange, with the yellow arrows showing the custom iliac bone flanges design; C, back view of the prosthesis, with the red area showing the porous surface area, the yellow arrows showing the custom iliac bone flanges design, and the red arrow showing the custom iliac augment; D, side view of the prosthesis, with the red arrow showing the custom iliac augment; E, 3D design of the prosthesis before operation, with the yellow arrows showing the custom ilium flanges design, which can avoid the defect area and ensure the best use of the remaining ilium; F, postoperative CT.
Type V refers to extensive bone defects around the acetabulum, which should not be considered as a simple combination of the aforementioned types.
The reason for this classification was that for type V bone defects with a large volume, it is difficult to completely fill all defects by augments, which means that if the ilium flange can be set, the number of screw holes should be properly increased. The length of the screws can be obviously longer in type V, and if needed, screws from the prosthesis to the sacrum are also reasonable considering the lack of structural stability of this type (Fig. 6, Supplementary materials).
Subtype A specifically refers to the penetration defect in the area confined below the ala of the ilium and above the superior pubic ramus and ischial ramus. This area serves as the main fixation and stress antagonistic region of the acetabular component, which means that a penetrating defect involving it not only indicates the need for filling augments, but also a strong demand for additional rigid fixation provided by other surrounding structures.
Subtype A can be superimposed over any type of pelvic defect from types I–V, that is, on the basis of the corresponding range of bone defects, penetrating injuries can occur around the acetabulum. The reason for this classification is that for subtype A, as a result of the penetration, only part of the acetabular cup and augment can be in contact with the residual bone, while the other part directly faces the pelvic cavity, suggesting that the range of porous surfaces can also be reduced to the corresponding contact zone rather than the whole surface. Furthermore, special reverse augments, which extend into the pelvic cavity and contact the residual bone from the inside out, can also be employed in this subtype (Fig. 7, Supplementary materials). Through confrontation between the reinforcement block itself and the bone, resistance to the lateralization can be achieved. With two-way clamping of the residual bone by the main body of the prosthesis and these reverse augments, stability can be secured while the bone defect is filled.
Subtype B refers to bone defects with pelvic ring discontinuity. Once the discontinuity occurs, the revision objective should not be confined to accomplishing prosthesis fixation firmness but should also take into account a holistic improvement in the mechanical properties of the pelvic ring.
In order to achieve symmetry of the rotation center, instead of using the acetabular cup itself, an augment attached to the said cup was innovatively used to connect the discontinuity, while the main body of the acetabular cup was still freely set at the symmetrical acetabulum center (Fig. 8, Supplementary materials). The bone reaming scope can be appropriately expanded in subtype B to prevent weakened fixation due to bone retention with poor quality. The pubis remarkably contributes to the holistic stability of the pelvic ring, making internal screws with large diameters a common choice. Inserting screws from the acetabular cup to the sacrum can also directly transmit the stress to the spine, which can address the compromised mechanical properties of discontinued pelvic rings.
2.3. Statistical analysis: intraobserver and interobserver agreement
For these typical cases, the pelvic radiographs were examined by two of the authors. Each observer classified each case into one of the categories (type + subtype). In cases with intraobserver agreement, each observer performed a final classification more than 8 weeks later. The second classification was also used to evaluate interobserver agreement. A kappa value lower than 0.40 was considered as low agreement; values between 0.40 and 0.75 were considered as medium agreement; and values more than 0.75 indicated high agreement [13].
2.4. Examination of preoperative and postoperative prosthesis position accordance
As shown in Fig. 9 (Supplementary materials), the PRT files of preoperative prosthesis design (including the preoperative 3D pelvic reconstruction and corresponding 3D prosthesis structure) were imported into the UG NX 9.0 software (Siemens, Germany). The most anterior point of the pubic tubercle on both sides was manually selected by senior doctors. Take the middle point of these two points as marker point A. The most anterior point of the anterior-superior iliac spine on both sides was also selected as marker points B and C. Take the plane containing the foregoing three marker points as the reference plane (the anterior pelvic plane, or coronal plane) [14]. Connect the marker points B and C with a straight line. Take the middle point of the straight line as the origin, and the straight line itself was the X axis (horizontal axis). In the reference plane, a straight line perpendicular to the X axis through the origin was taken as the Y axis (vertical axis). So far, a rectangular coordinate system had been established.
The postoperative CT data of the pelvis (including the patient's bone and the installed prosthesis) were reconstructed using Mimics Medical 20.0 (Materialise, Belgium), exported as STL files, and further imported into the aforementioned PRT files of preoperative prosthesis design. Using the “point-to-point alignment” function of the software, multiple marker points (at least 10) were selected on the two pelvises (preoperative and postoperative), and they became further aligned (overlapped). After the pelvic bone alignment, the position deviation between the installed prosthesis and the preoperative design was shown.
Four points that were far from each other were selected on the inner surface of the installed prosthesis’ cup. A spherical surface was determined by the four points, and the spherical center of the spherical surface was recorded as the rotation center point of the prosthesis after operation. Because the inner surface of the cup in the preoperative prosthesis design was a standard spherical surface, the spherical center of the cup can be directly determined as the expected rotation center point of the preoperative prosthesis by the UG NX 9.0 (Siemens, Germany). The position difference between the preoperative expected rotation center point and the postoperative actual rotation center point was projected into the aforementioned rectangular coordinate system, and the displacement in the horizontal and vertical directions was obtained.
2.5. Evaluation of clinical outcome: hip function score, complication rate and Re-revision
The average follow-up period of these typical cases was 2.8 years (range, 1.1–5.9 years). Preoperative and postoperative Harris scores were calculated [15]. T-test was utilized for statistical analysis and p < 0.05 were considered significant. Re-revision rate and postoperative complications including prosthesis dislocation, aseptic loosening, periprosthetic infection, nerve palsy, etc. were recorded.
3. Results
3.1. Interobserver and intraobserver agreement
The interobserver and intraobserver agreement were both promising using the classification system proposed in the present study. The kappa scores for intraobserver agreement were 0.901 and 0.803, and the kappa scores for interobserver agreement was 0.797. The highest percentage of erroneous classification was for types IV, V and subtype A (Table 1).
Table 1.
Brief demographic data with results of classification for interobserver and intraobserver agreement evaluation.
| Case Number | Gender | Age | Observer Aa | Observer Ba | Observer Ab | Observer Bb |
|---|---|---|---|---|---|---|
| 1 | Female | 83 | VA | VA | VA | VA |
| 2 | Male | 47 | I | I | I | I |
| 3 | Male | 50 | III | III | III | III |
| 4 | Male | 66 | IVA | IVA | IVA | VA |
| 5 | Female | 60 | VA | V | VA | V |
| 6 | Male | 50 | III | IV | III | III |
| 7 | Female | 85 | V | V | V | V |
| 8 | Male | 55 | VA | VA | VA | VA |
| 9 | Male | 65 | IB | IB | IB | IB |
| 10 | Female | 60 | IIIA | IIIA | IIIA | IIIA |
| 11 | Female | 73 | IIA | IIA | IIA | IIA |
| 12 | Female | 65 | V | VA | VA | VA |
Classification in the first time; bThe final classification 8 weeks later.
3.2. Accordance evaluation of position between installation and preoperative design
Postoperative examination of position coincidence indicated that the prostheses highly corresponded with the preoperative location planning (Table 2). The average prosthesis displacement in the horizontal and vertical directions were 3.92 mm and 4.15 mm respectively, which preliminarily indicated that this classification system could commendably guide the design and operation of custom THA revision surgeries.
Table 2.
Results of postoperative displacement of prostheses in comparison with preoperative design.
| Number | Horizontal displacementa (mm) | Vertical displacementb (mm) |
|---|---|---|
| 1 | −5.605 | 5.752 |
| 2 | 4.530 | 3.536 |
| 3 | 1.028 | 1.588 |
| 4 | 4.783 | −8.788 |
| 5 | −6.818 | 2.451 |
| 6 | 1.761 | 8.712 |
| 7 | −0.695 | 1.708 |
| 8 | 3.396 | 2.163 |
| 9 | −0.026 | −5.380 |
| 10 | 8.189 | 0.250 |
| 11 | 5.550 | 5.553 |
| 12 | 4.680 | −3.933 |
In horizontal displacement, outward displacement is positive and inward displacement is negative.
in vertical displacement, downward displacement is positive and upward displacement is negative.
3.3. Evaluation of clinical outcome
As shown in Table 3, Harris scores were significantly improved after revision (from 39.19 ± 19.05 to 79.78 ± 8.44) (p < 0.001). Furthermore, no other complications occurred except for one case with postoperative periprosthetic infection and one case with hip dislocation, which received a non-surgical reduction. During the follow-up period, no patients underwent re-revision surgery.
Table 3.
Results of clinical follow-up: Harris hip score.
| pre-operation | post-operation | T | p |
|---|---|---|---|
| 39.19 ± 19.05 | 79.78 ± 8.44 | −8.440 | <0.001 |
4. Discussion
A revision surgery is bound to encounter various obstacles in comparison with primary prosthesis replacement surgery, whether it involves hip, knee or shoulder: extensive osteolysis or even fracture often exists around the prosthesis, failed prostheses are sometimes difficult to remove, and the poor quality of the residual bone also significantly threatens the holistic stability [[16], [17], [18], [19]]. These complexities all pose severe challenges to the field of hip joint revision.
The Paprosky and AAOS classification systems, as the two most commonly used classification systems in revision THA, all mainly focus on the peri-acetabulum, but less on the ala of ilium, superior pubic ramus, and other pelvic regions that are far away from the acetabulum. Clinical practice has shown that although these regions do not directly contact with the femoral head, but are still critical for the stability of hip prosthesis and the biomechanical properties of the whole pelvis, which means that the mainstream classification systems mentioned above can, but may not be entirely suitable for large-scale complex bone defects which need custom revision THA. In addition, the two classification systems are based on the traditional revision methods, such as standard prostheses, cages, bone graft and so on. With the update of revision methods and the development of custom 3D-printed prosthesis, these current mainstream defect classification systems can not fully meet the needs of 3D printing technology and custom revision. Furthermore, the Paprosky classification classifies most complex pelvic defects requiring individualized design prostheses into types IIIA and IIIB, similar to the AAOS classification, indicating that their ability to further subdivide large-scale complex bone defects can be improved.
In view of this situation, based on the experience in the design of 3D-printed hip prostheses and understanding of pelvic biomechanics, we preliminarly proposed a bone defect classification system for custom revision THA with large-scale complex pelvic defects, and the corresponding reconstruction principles. After that, revision surgeries using corresponding 3D-printed custom prosthesis were further carried out under the guidance of this classification system. Although some typical cases were included for elucidation in this study, it is important to point out that the classification is mainly based on our previous experience of prosthesis design and the understanding of pelvic biomechanics, rather than according to these cases, that is to say that this study is not a clinical trail, but a summary and integration based on the design concept.
In comparison with the traditional standard revision prostheses, the custom prostheses in the present study offered various unique advantages. These flexible integrated designs can be employed in custom prostheses to avoid the use of allografts, which further prevents allograft fracture, high operation times, use of additional fixation materials, unnecessary trauma, and disease transmission [5,8,20]. The custom prostheses in this study can adapt to the complex bone defect anatomy of patients undergoing revision surgeries, which can make installation convenient and precise and may also realize better immediate stability. The precise individualized design of these prostheses structure helps avoid bone reaming for prosthesis matching to the greatest extent and preserves precious bone mass at the revision site, thereby securing long-term stability and preserving bone stock foundation for the possible subsequent revision [4,9]. In the present study, the porous surface was designed on custom prostheses by 3D printing to promote bone ingrowth for long-term fixation instead of screws, better preventing stress shielding, deleterious bone remodeling, fibrous membrane formation, and even screw breakage and plate detachment [21,22]. In cases involving pelvic discontinuity, with the traditional reconstruction method, the jumbo cup requires a substantial amount of residual bone stock, and complete restoration of the symmetrical rotation center is challenging because it must be connected to the pelvic discontinuity site. In this study, we innovatively used individualized integrated prostheses that connected the discontinuity with its augments and restored the symmetrical center with its acetabular cup main body, effectively solving the dilemma of subtype B bone defects and maximizing postoperative joint function. With individualized design, the prostheses in the present study could avoid the anterior-superior and anterior-inferior quadrants of the acetabulum as far as possible when setting screws, thereby protecting the obturator nerve, obturator artery, and external iliac artery [8,23,24]. Obturator hooks can effectively resist the movement in the upper and outer directions and are suitable for most types of patients. Therefore, they were added in some custom prostheses in this study to provide a secondary secure attachment, especially when the fixation from the cup and the ilium was insufficient [6]. Using the innovative design of a porous surface on the inner surface of the cup, a small amount of bone cement can be used to connect the acetabular cup and the inner liner with much better adhesion. Based on the premise of accurate preoperative classification and individualized design, the custom acetabulum components in the present study can even be fixed directly on the sacrum through long screws to achieve integrated stress conduction from the spine to the prosthesis and lower limbs, demonstrating the unique advantages of custom prostheses that traditional standard ones might not achieve in cases involving pelvic ring discontinuity, acetabulum penetration, extensive pelvic defects, and other complex revisions.
Regarding prosthesis installation position evaluation, this study creatively utilized the postoperative pelvis CT data to display the 3D position of prosthesis and consistently calculated the deviation between the preoperative position planning and the postoperative actual position using a computer software from the perspective of prosthesis design. This method purely evaluated the role of the classification system in increasing the accuracy of the prosthesis installation while decreasing prosthesis displacement. In addition, some clinical outcome factors, such as the patient's pain, abilities to walk and dress are not only related to the prosthesis position, but also to differences in muscle condition, age, and physique. The position evaluation method used in this study avoided these differences and evaluated the prosthesis position more pertinently.
This study has some shortcomings. Initially, the classification system of this study needs three-dimensional reconstruction of bone, which makes CT a necessary examination rather than X-ray film, which is much cheaper and simpler. The three-dimensional reconstruction does play a positive role in the accurate planning of surgery and prosthesis design, but it undoubtedly increases the complexity of the diagnostic process and increases the medical cost; Secondly, although the 3D post-operative prosthesis position evaluation method used in this study could intuitively elucidate the accuracy of prosthesis installation in different directions, it required high skills for researchers. At the same time, it had a great computational load on computers, resulting in a great computational burden. As a result, the data of all 33 patients were not presented in this paper, Instead, some of them were randomly selected to illustrate the revision system. In future research, we will complete the measurement and calculation of the other patients in batches, and constantly improve this system. Thirdly, for type II patients, the degree of defect is closely related to the final prosthesis design. Since the ischium does not directly participate in the stress conduction of the pelvic ring, when the scope of the ischium defect is huge and it is difficult to completely fill the defect, sometimes the defect may not be forcibly reconstructed. This means that type II patients can be further subdivided according to the necessity of ischium reconstruction. However, considering the complexity of this classification system and the difficulty of popularization, this subdivision process has not been defined and implemented in the end.
5. Conclusion
In conclusion, the revision system for complex pelvic defects utilizing 3D-printed custom prosthesis resulted in satisfactory installation accuracy and excellent reliability. The classification can preliminarily guide patients’ grouping, prosthesis design and installation, and may preliminarily provide an innovative, feasible, and efficient approach for complex THA revision surgeries.
Authorship & conflicts of interest statement
The first three authors (Yongqiang Hao, Dinghao Luo, Junxiang Wu) contributed equally to this manuscript. We thank the funding support from the National Key R&D Program of China (2016YFC1100600), General program of NSFC (81972058) and Shanghai Key Clinical Specialty Construction Project - Biomedical Materials (matching funds), Three-year Action Plan of Shenkang Development Center (SHDC2020CR2019B), Huangpu District Industrial Support Fund (XK2020009) and National Key Science and Technology Infrastructure of Translational Medicine (Shanghai) Open Project (TMSZ-2020-207). Each author certifies that he or she has no commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Footnotes
Investigation performed at Shanghai Ninth People's Hospital & Shanghai Key Laboratory of Orthopaedic Implant, Shanghai, People's Republic of China.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2021.09.006.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Reconstruction of a type V pelvic defect. A, preoperative radiograph; B, back view of the prosthesis, with the red area showing the porous structure surface and the red arrows showing the individualized custom ilium, pubic, and ischium augments, respectively; C, side view of the prosthesis, with the red arrows showing the individualized custom ilium, pubic, and ischium augments, respectively; D, 3D design of the prosthesis before the operation, with the red arrows showing that the individualized custom augments can reconstruct the ilium, pubic, and ischium defects; E, 3D design of the custom installation guide plate before operation, with the violet arrow showing that the custom guide plate can clearly conduct the installation; F, an actual picture of the prosthesis; G, image of the intraoperative prosthesis installation; H, postoperative radiograph.
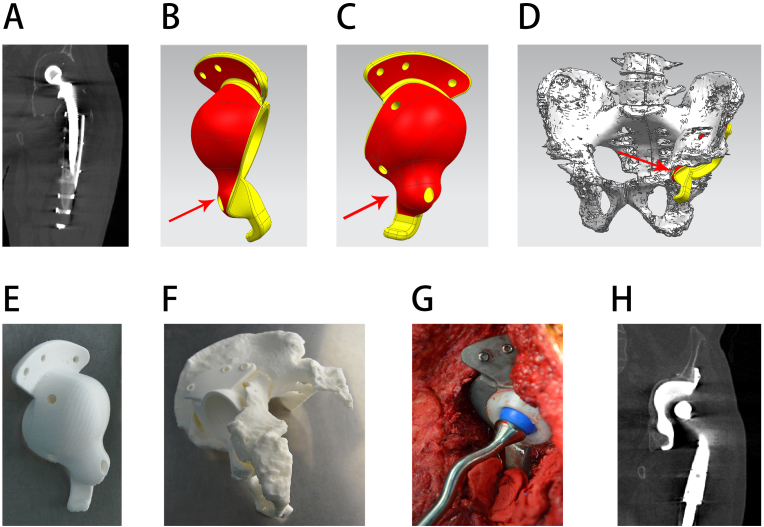
Reconstruction of a subtype A pelvic defect. A, preoperative radiograph; B, side view of the prosthesis, with the red area showing the porous structure surface and the red arrow showing an individualized augment that can clamp the bone with the iliac flange; C, side view of the prosthesis, with the red arrow showing an individualized augment that can clamp the bone with the iliac flange; D, 3D design of the prosthesis before the operation, with the red arrows showing that the individualized custom augment can reconstruct the ilium defect and the green arrow showing a long screw fixed directly to the sacrum. E, back view of the 3D design, with the red arrows showing that the individualized custom augment can clamp the bone with the iliac flange and the green arrow showing a long screw fixed directly to the sacrum; F, postoperative radiograph.
Reconstruction of a subtype B pelvic defect. A, preoperative radiograph; B, side view of the prosthesis, with the red area showing the porous structure surface, red arrows showing the individualized custom ilium augment, and the yellow arrow showing the main body of the acetabular cup, which can be freely set at the symmetrical acetabulum center; C, back view of the prosthesis, with the red area showing the porous structure surface, red arrows showing the individualized custom ilium augment, and the yellow arrow showing the main body of the acetabular cup; D, 3D design of the custom installation guide plate before the operation, with the violet arrow showing that the custom guide plate can precisely attach to the bone and prosthesis to assist installation; E, 3D design of the prosthesis before the operation, with the red arrows showing that the individualized custom augment can reconstruct the discontinuity, the green arrow showing a long screw fixed directly to the sacrum, and the yellow arrow showing the main body of the acetabular cup; F, 3D design of the prosthesis before the operation, with the red arrows showing that the individualized custom augment can reconstruct the discontinuity, the green arrow showing a long screw fixed directly to the sacrum, and the yellow arrow showing the main body of the acetabular cup; G, preoperative 3D-printed model of the bone; H, intraoperative prosthesis installation; I, postoperative radiograph.
Examination of preoperative and postoperative prosthesis position accordance. A, schematic diagram of the establishment of the reference plane (the anterior pelvic plane, or coronal plane) and rectangular coordinate system, with the red arrows showing the anterior-superior iliac spine points, the yellow arrow showing the midpoint of the pubic tubercle. The blue rectangle is the determined anterior pelvic plane of the pelvis. The green arrow shows the midpoint of the two anterior-superior iliac spine points, that is, the origin of the rectangular coordinate system; B, schematic diagram of the determination of the preoperative expected rotation center, the green arrow indicates the spherical inner surface of the acetabular cup used to determine the spherical center, and the red arrow is the spherical shell center confirmed by the software, i.e. the preoperative expected rotation center; C, Schematic diagram determining the actual postoperative rotation center of prosthesis. The red points indicate the four points used to determine the inner surface of the sphere, and the green points indicate the center of the sphere, which is the actual postoperative rotation center; D, Accordance evaluation of position between installation and preoperative design. The red point is the preoperative expected rotation center, and the green point indicates the postoperative actual rotation center. The position difference between the two points is projected into the anterior plane of the pelvis (coronal plane) shown in the blue quadrilateral.
References
- 1.Gwam C.U., Mistry J.B., Mohamed N.S., Thomas M., Bigart K.C., Mont M.A., et al. Current epidemiology of revision total hip arthroplasty in the United States: national inpatient sample 2009 to 2013. J Arthroplasty. 2017;32(7):2088. doi: 10.1016/j.arth.2017.02.046. [DOI] [PubMed] [Google Scholar]
- 2.Henckel J., Holme T.J., Radford W., Skinner J.A., Hart A.J. 3D-printed patient-specific guides for hip arthroplasty. J Am Acad Orthop Surg. 2018;26(16):e342. doi: 10.5435/JAAOS-D-16-00719. [DOI] [PubMed] [Google Scholar]
- 3.Abdelaal O., Darwish S., El-Hofy H., Saito Y. Patient-specific design process and evaluation of a hip prosthesis femoral stem. Int J Artif Organs. 2019;42(6):271. doi: 10.1177/0391398818815479. [DOI] [PubMed] [Google Scholar]
- 4.Haglin J.M., Eltorai A.E., Gil J.A., Marcaccio S.E., Botero-Hincapie J., Daniels A.H. Patient-specific orthopaedic implants. Orthop Surg. 2016;8(4):417. doi: 10.1111/os.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen A.F., Hozack W.J. Component selection in revision total hip arthroplasty. Orthop Clin N Am. 2014;45(3):275. doi: 10.1016/j.ocl.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Jones C.W., Choi D.S., Sun P., Chiu Y.F., Lipman J.D., Lyman S., et al. Clinical and design factors influence the survivorship of custom flange acetabular components. Bone Joint J. 2019;101-b(6_Supple_B):68. doi: 10.1302/0301-620X.101B6.BJJ-2018-1455.R1. [DOI] [PubMed] [Google Scholar]
- 7.Paprosky W.G., Perona P.G., Lawrence J.M. Acetabular defect classification and surgical reconstruction in revision arthroplasty. A 6-year follow-up evaluation. J Arthroplasty. 1994;9(1):33. doi: 10.1016/0883-5403(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 8.D'Antonio J.A. Periprosthetic bone loss of the acetabulum. Classification and management. Orthop Clin N Am. 1992;23(2):279. [PubMed] [Google Scholar]
- 9.Barlow B.T., Oi K.K., Lee Y.Y., Carli A.V., Choi D.S., Bostrom M.P. Outcomes of custom flange acetabular components in revision total hip arthroplasty and predictors of failure. J Arthroplasty. 2016;31(5):1057. doi: 10.1016/j.arth.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Abdel M.P., Trousdale R.T., Berry D.J. Pelvic discontinuity associated with total hip arthroplasty: evaluation and management. J Am Acad Orthop Surg. 2017;25(5):330. doi: 10.5435/JAAOS-D-15-00260. [DOI] [PubMed] [Google Scholar]
- 11.Gladnick B.P., Fehring K.A., Odum S.M., Christie M.J., DeBoer D.K., Fehring T.K. Midterm survivorship after revision total hip arthroplasty with a custom triflange acetabular component. J Arthroplasty. 2018;33(2):500. doi: 10.1016/j.arth.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 12.Solomon L.B., Abrahams J.M., Callary S.A., Howie D.W. The stability of the porous tantalum components used in revision THA to treat severe acetabular defects: a radiostereometric analysis study. J Bone Joint Surg Am. 2018;100(22):1926. doi: 10.2106/JBJS.18.00127. [DOI] [PubMed] [Google Scholar]
- 13.Ippolito E., Farsetti P., Boyce A.M., Corsi A., De Maio F., Collins M.T. Radiographic classification of coronal plane femoral deformities in polyostotic fibrous dysplasia. Clin Orthop Relat Res. 2014;472(5):1558. doi: 10.1007/s11999-013-3380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins S.W., Spratley E.M., Boe R.A., Hayes C.W., Jiranek W.A., Wayne J.S. A novel approach for determining three-dimensional acetabular orientation: results from two hundred subjects. J Bone Joint Surg Am. 2014;96(21):1776. doi: 10.2106/JBJS.L.01141. [DOI] [PubMed] [Google Scholar]
- 15.Harris W.H. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51(4):737. [PubMed] [Google Scholar]
- 16.Mancuso F., Beltrame A., Colombo E., Miani E., Bassini F. Management of metaphyseal bone loss in revision knee arthroplasty. Acta Biomed : Atenei Parmensis. 2017;88(2s):98. doi: 10.23750/abm.v88i2-S.6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salem K.H., Lindner N., Tingart M., Elmoghazy A.D. Severe metallosis-related osteolysis as a cause of failure after total knee replacement. J Clin Ortho Trauma. 2020;11(1):165. doi: 10.1016/j.jcot.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauer C., Zaharia B., Galliot F., Parot J., Houfani F., Mayer J., et al. Management and results in periprosthetic tibial fracture after total knee arthroplasty: two-center 15-case retrospective series at 2 years' follow-up. Ortho Traumatol Surg Res : OTSR. 2020;106(3):449. doi: 10.1016/j.otsr.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Casp A.J., Montgomery S.R., Jr., Cancienne J.M., Brockmeier S.F., Werner B.C. Osteoporosis and implant-related complications after anatomic and reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2020;28(3):121. doi: 10.5435/JAAOS-D-18-00537. [DOI] [PubMed] [Google Scholar]
- 20.Sims L., Kulyk P., Woo A. Intraoperative culture positive allograft bone and subsequent postoperative infections: a retrospective review. Canadian J Surg J canadien de chirurgie. 2017;60(2):94. doi: 10.1503/cjs.008016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pakos E.E., Stafilas K.S., Tsovilis A.E., Vafiadis J.N., Kalos N.K., Xenakis T.A. Long term outcomes of total hip arthroplasty with custom made femoral implants in patients with congenital disease of hip. J Arthroplasty. 2015;30(12):2242. doi: 10.1016/j.arth.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 22.Mukherjee K., Gupta S. Bone ingrowth around porous-coated acetabular implant: a three-dimensional finite element study using mechanoregulatory algorithm. Biomech Model Mechanobiol. 2016;15(2):389. doi: 10.1007/s10237-015-0696-7. [DOI] [PubMed] [Google Scholar]
- 23.Kieser D.C., Ailabouni R., Kieser S.C.J., Wyatt M.C., Armour P.C., Coates M.H., et al. The use of an Ossis custom 3D-printed tri-flanged acetabular implant for major bone loss: minimum 2-year follow-up. Hip Int : J Clin Exper Res Hip Pathol Ther. 2018;28(6):668. doi: 10.1177/1120700018760817. [DOI] [PubMed] [Google Scholar]
- 24.Hitz O.F., Flecher X., Parratte S., Ollivier M., Argenson J.N. Minimum 10-year outcome of one-stage total hip arthroplasty without subtrochanteric osteotomy using a cementless custom stem for crowe III and IV hip dislocation. J Arthroplasty. 2018;33(7):2197. doi: 10.1016/j.arth.2018.02.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Reconstruction of a type V pelvic defect. A, preoperative radiograph; B, back view of the prosthesis, with the red area showing the porous structure surface and the red arrows showing the individualized custom ilium, pubic, and ischium augments, respectively; C, side view of the prosthesis, with the red arrows showing the individualized custom ilium, pubic, and ischium augments, respectively; D, 3D design of the prosthesis before the operation, with the red arrows showing that the individualized custom augments can reconstruct the ilium, pubic, and ischium defects; E, 3D design of the custom installation guide plate before operation, with the violet arrow showing that the custom guide plate can clearly conduct the installation; F, an actual picture of the prosthesis; G, image of the intraoperative prosthesis installation; H, postoperative radiograph.
Reconstruction of a subtype A pelvic defect. A, preoperative radiograph; B, side view of the prosthesis, with the red area showing the porous structure surface and the red arrow showing an individualized augment that can clamp the bone with the iliac flange; C, side view of the prosthesis, with the red arrow showing an individualized augment that can clamp the bone with the iliac flange; D, 3D design of the prosthesis before the operation, with the red arrows showing that the individualized custom augment can reconstruct the ilium defect and the green arrow showing a long screw fixed directly to the sacrum. E, back view of the 3D design, with the red arrows showing that the individualized custom augment can clamp the bone with the iliac flange and the green arrow showing a long screw fixed directly to the sacrum; F, postoperative radiograph.
Reconstruction of a subtype B pelvic defect. A, preoperative radiograph; B, side view of the prosthesis, with the red area showing the porous structure surface, red arrows showing the individualized custom ilium augment, and the yellow arrow showing the main body of the acetabular cup, which can be freely set at the symmetrical acetabulum center; C, back view of the prosthesis, with the red area showing the porous structure surface, red arrows showing the individualized custom ilium augment, and the yellow arrow showing the main body of the acetabular cup; D, 3D design of the custom installation guide plate before the operation, with the violet arrow showing that the custom guide plate can precisely attach to the bone and prosthesis to assist installation; E, 3D design of the prosthesis before the operation, with the red arrows showing that the individualized custom augment can reconstruct the discontinuity, the green arrow showing a long screw fixed directly to the sacrum, and the yellow arrow showing the main body of the acetabular cup; F, 3D design of the prosthesis before the operation, with the red arrows showing that the individualized custom augment can reconstruct the discontinuity, the green arrow showing a long screw fixed directly to the sacrum, and the yellow arrow showing the main body of the acetabular cup; G, preoperative 3D-printed model of the bone; H, intraoperative prosthesis installation; I, postoperative radiograph.
Examination of preoperative and postoperative prosthesis position accordance. A, schematic diagram of the establishment of the reference plane (the anterior pelvic plane, or coronal plane) and rectangular coordinate system, with the red arrows showing the anterior-superior iliac spine points, the yellow arrow showing the midpoint of the pubic tubercle. The blue rectangle is the determined anterior pelvic plane of the pelvis. The green arrow shows the midpoint of the two anterior-superior iliac spine points, that is, the origin of the rectangular coordinate system; B, schematic diagram of the determination of the preoperative expected rotation center, the green arrow indicates the spherical inner surface of the acetabular cup used to determine the spherical center, and the red arrow is the spherical shell center confirmed by the software, i.e. the preoperative expected rotation center; C, Schematic diagram determining the actual postoperative rotation center of prosthesis. The red points indicate the four points used to determine the inner surface of the sphere, and the green points indicate the center of the sphere, which is the actual postoperative rotation center; D, Accordance evaluation of position between installation and preoperative design. The red point is the preoperative expected rotation center, and the green point indicates the postoperative actual rotation center. The position difference between the two points is projected into the anterior plane of the pelvis (coronal plane) shown in the blue quadrilateral.