Abstract
Introduction
The adjuvant treatment of patients with resected lung adenocarcinoma (LUAD) remains unstandardized. We analyzed the survival outcomes of these patients based on EGFR mutation status and adjuvant chemotherapy treatment.
Methods
This noninterventional real-world study (ICAN) enrolled Chinese patients with resected stages I to III LUAD from April 8, 2010, to December 31, 2010. Tumor EGFR mutation status and 3-year disease-free survival (DFS) were determined. The extension phase provided long-term follow-up with overall survival (OS) as the primary end point. Secondary end points included DFS and prognostic factors of survival. Survival outcomes based on adjuvant chemotherapy treatment, EGFR mutation status, and postoperative stage were analyzed post hoc.
Results
Among 568 patients in the ICAN cohort, 472 continued to the extension phase and remained eligible. The 3-year DFS rate was 58.8%. In the extension cohort, 260 patients (55.1%) had EGFR-mutant disease and 207 (43.9%) received adjuvant chemotherapy. At a median follow-up of 109.0 (95% confidence interval [CI]: 106.6–111.4) months, median OS and DFS were 103.3 (95% CI: 101.7–104.9) and 67.4 (95% CI: 49.7–85.2) months, respectively. The 5-year OS and DFS rates were 68.9% (95% CI: 64.3–73.6) and 52.9% (95% CI: 48.2–57.7), respectively. EGFR wild-type disease was a significant independent predictor of worse OS (HR = 1.24, 95% CI: 1.07–1.44, p= 0.004) based on the Cox regression analysis of common factors. Post hoc subgroup analysis revealed that survival outcomes were not significantly different with adjuvant chemotherapy regardless of EGFR mutation status across all postoperative stages.
Conclusions
EGFR mutations are common in operable LUAD, and recurrence and mortality after resection were considerable. Adjuvant chemotherapy did not improve survival outcomes, regardless of EGFR mutation status and postoperative stage.
Keywords: Lung adenocarcinoma, Surgical resection, Prognostic factors, EGFR mutation, Adjuvant chemotherapy
Introduction
Lung cancer remains the leading cause of cancer-related mortality worldwide.1 In the People's Republic of China, lung cancer is prevalent and accounts for approximately a quarter of all cancer-related deaths.1 More than 80% of all lung cancers are NSCLCs, in which lung adenocarcinoma (LUAD) is the most common histologic subtype.2,3 In early and locally advanced (stages I–III) NSCLC, which comprises approximately one-third of diagnosed NSCLCs, surgical resection is recommended as an option by clinical guidelines.4, 5, 6, 7 Nevertheless, many patients with resected NSCLC experience recurrence (13.2%–41.5% depending on disease stage and length of follow-up).8, 9, 10, 11, 12, 13 Postrecurrence survival after surgery is poor (median overall survival [OS]: ∼26 mo), which presents a clinical unmet need.8,11
EGFR is a crucial oncogenic driver in NSCLC and is often mutated in patients with LUAD.2,3 Classical EGFR-activating mutations, namely exon 19 (Ex19) deletion and exon 21 (Ex21) L858R mutation, predict sensitivity to EGFR tyrosine kinase inhibitors (TKIs), which are the first-line therapies for EGFR-mutant advanced NSCLC.14, 15, 16 Recently, adjuvant EGFR TKI therapy was also found to have significant improvements in disease-free survival (DFS) in patients with EGFR-mutant resected NSCLC.17, 18, 19
Currently, adjuvant cisplatin-based chemotherapy is widely recommended for patients with resected stages II to IIIA NSCLC and select patients with stage IB disease, regardless of EGFR mutation status.20,21 The Lung Adjuvant Cisplatin Evaluation, a meta-analysis of five large trials (N = 4585), found that adjuvant chemotherapy led to a limited 5-year survival benefit of 5.4% among patients with resected NSCLC, regardless of EGFR mutation status.22 Despite being the recommended therapy after surgical resection of NSCLC, adjuvant chemotherapy brought on limited improvement in survival outcomes and significant toxicity.22 Particularly, with the favorable outcomes of EGFR TKIs in patients with EGFR-mutant resected NSCLC,17, 18, 19 the role of adjuvant chemotherapy in these patients remains inconclusive.
The noninterventional real-world (ICAN) study (NCT01106781) investigated the association of EGFR mutation status with 3-year DFS rate in Chinese patients with completely resected LUAD.23,24 We report here the final results of the ICAN study, with its extension phase, to provide follow-up data on the long-term survival and associated prognostic factors of the participants in the real-world setting. The effect of adjuvant chemotherapy and EGFR mutation status on long-term survival outcomes of these patients was analyzed across postoperative stages.
Materials and Methods
Study Design and Patients
This noninterventional study, conducted from April 8, 2010, to December 11, 2019, was designed to investigate EGFR mutation status, survival outcomes, and associated risk factors in Chinese patients with completely resected LUAD in the real-world setting. Eligible patients with stages I to III histologically diagnosed LUAD that was completely resected were enrolled across 26 sites in the People’s Republic of China. In the extension phase, eligible patients in 24 of the initial sites were included.
The study was conducted in accordance with the Declaration of Helsinki and Guidelines for Good Clinical Practice and was independently approved by the ethics committees of each participating center. All patients provided written informed consent before enrollment. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines.
Study Outcomes
Postoperative EGFR mutation status was determined for all patients using amplification-refractory mutation system, polymerase chain reaction (PCR) direct sequencing, mutant-enriched PCR, xTAG liquid chip, and branched DNA liquid chip.24
Patient clinical data and survival outcomes were collected in 3 years for the ICAN study.23 In the long-term extension phase (approximately 9 y of follow-up), the primary end points were EGFR mutation status and OS, defined as the length of time from informed consent to death or last confirmed survival owing to any cause. Secondary end points included DFS, defined as the length of time from informed consent to recurrence, metastasis, or death; treatment after tumor recurrence; and OS prognostic factors, including EGFR mutation status, age, sex, smoking status, postoperative stage, and adjuvant chemotherapy. The effect of adjuvant chemotherapy on survival outcomes according to EGFR mutation status and postoperative stage was analyzed post hoc.
Statistical Analysis
On the basis of an estimated EGFR mutation rate of 40% to 50% in Asian patients with advanced LUAD,25,26 we enrolled a sample size of 571 patients to obtain 250 to 300 patients with EGFR mutations to provide power for further analysis. Assuming a median OS of 60 months and 370 events (approximately 65% of patients), the 95% confidence interval (CI) for median OS was estimated to be 54.2 to 66.4 months.27 In the long-term extension cohort, the full analysis set (FAS) comprised patients who remained eligible, had provided informed consent, and had at least one documented survival follow-up visit. The post hoc subgroup analysis of survival outcomes in patients based on adjuvant chemotherapy treatment and EGFR mutation status across postoperative stages was exploratory. Statistical analyses were performed using the Statistical Analysis System 9.4 software. All statistical tests were two-sided with a significance level of 0.05.
OS and DFS were estimated by the Kaplan–Meier method. If the date of a patient’s death was unknown, the death was recorded as the last date of contact; if no death occurred, survival was taken as the last date at which survival was confirmed. Similarly, if no recurrence, metastasis, or death occurred, the date of the last tumor evaluation was used for the calculation of truncated DFS time. Patients lost to follow-up were recorded under missing data. Using the R software (version 3.6.0), the worst outcome and multiple imputation methods were performed for sensitivity analyses of right-censored survival data of these patients.
Suitability of the prognostic variables for factor analysis was evaluated by collinearity diagnostics, the Kaiser-Meyer-Olkin test, and the Bartlett’s test of sphericity. Factor analysis was used to explore interdependencies among the prognostic variables and to extract them into independent common factors. The Cox proportional hazards model was then used to evaluate the effect of different common factors on survival outcomes.
Results
Patient Characteristics and Disposition
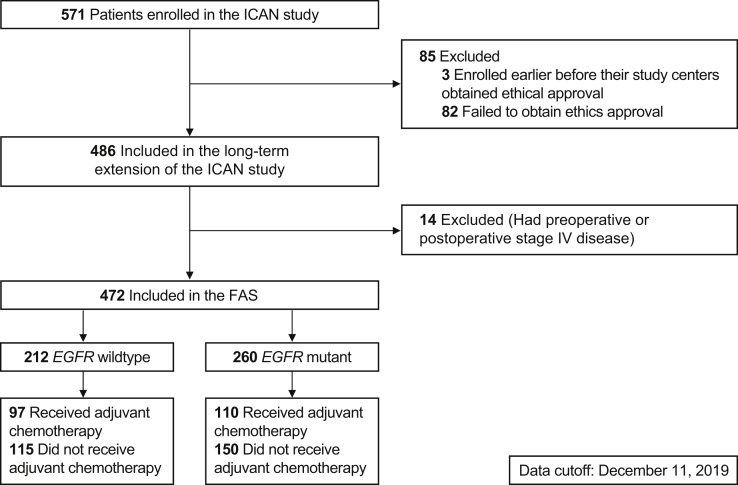
Among the 571 patients enrolled in the ICAN trial (from April 8, 2010, to December 31, 2010), 568 were included in the final analysis (three patients did not meet the ethics approval). A total of 486 patients continued to the extension phase, of whom, 14 had stage IV disease preoperatively or postoperatively and were excluded from the FAS (N = 472; Fig. 1).
Figure 1.
Flowchart of the study participants. FAS, full analysis set.
The demographics and clinical characteristics of the ICAN extension-phase cohort (FAS) were similar to those of the initial ICAN cohort (Table 1 and Supplementary Table 1). Median age of patients in the FAS was 60 (range: 34–88) years, and approximately half of the patients were male (230 [48.7%]; Table 1). Most patients underwent lobectomy (432 [91.5%]). In the postoperative phase, 273 (57.8%) and 199 (42.2%) patients had stage I and stages II to III disease, respectively; 207 patients (43.9%) received adjuvant chemotherapy, which was similar to that in the initial ICAN cohort (45.4%).23
Table 1.
Demographics and Clinical Characteristics of Patients in the ICAN Extension Phase (FAS)
| Parameters | ICAN Extension Phase (FAS) (N = 472) |
|---|---|
| Sex | |
| Male | 230 (48.7) |
| Female | 242 (51.3) |
| Median age (range), y | 60 (34–88) |
| Smoking status | |
| Nonsmoker | 308 (65.3) |
| Ex-smoker | 103 (21.8) |
| Smoker | 61 (12.9) |
| Preoperative performance status score | |
| 0 | 410 (86.9) |
| 1 | 54 (11.4) |
| 2 | 8 (1.7) |
| Preoperative stage | |
| Stage IA | 160 (33.9) |
| Stage IB | 151 (32.0) |
| Stage II | 45 (9.5) |
| Stage IIIA | 100 (21.2) |
| Stage IIIB | 9 (1.9) |
| Unknown | 7 (1.5) |
| Surgery type | |
| Lobectomy | 432 (91.5) |
| Local resection | 26 (5.5) |
| Pneumonectomy | 14 (3.0) |
| Postoperative stage | |
| Stage IA | 152 (32.2) |
| Stage IB | 121 (25.6) |
| Stage II | 61 (13.0) |
| Stage IIIA | 132 (28.0) |
| Stage IIIB | 6 (1.3) |
| Neoadjuvant therapy | |
| None | 464 (98.3) |
| Radiotherapy | 1 (0.2) |
| Chemotherapy | 7 (1.5) |
| Paclitaxel + cisplatin or carboplatina | 3 (0.6) |
| Taxotere + cisplatin or carboplatina | 2 (0.4) |
| Docetaxel + carboplatina | 1 (0.2) |
| Pemetrexed disodium for injection + nedaplatina | 1 (0.2) |
| Adjuvant therapy | |
| None | 261 (55.3) |
| Chemotherapy alone or in combination with radiotherapyb | 207 (43.9) |
| Targeted therapy | 4 (0.8) |
Note: Data are presented as number (%) of patients unless otherwise indicated.
FAS, full analysis set.
Calculated as percentages of the total number of patients in the ICAN extension phase (N = 472).
In the ICAN extension phase, 25 patients received chemotherapy in combination with radiotherapy.
More than half of the patients in the FAS (260 [55.1%]) were positive for EGFR mutation, the most common being Ex19 deletion (121 [46.5%]) and Ex21 L858R mutation (122 [46.9%]), with four patients (1.5%) having concomitant mutations. Uncommon mutations were exon 18 G719X and exon 20 insertion or T790M in 13 patients (5.0%). The ICAN cohort had a similar prevalence (55.1%) and distribution of EGFR mutations.23,24 EGFR mutations were observed in 63.2%, 55.4%, 54.1%, and 48.5% of patients with postoperative stage IA, IB, II, and IIIA disease, respectively; all six patients with stage IIIB disease had wild-type EGFR (Supplementary Table 2). Of the 207 patients who were receiving adjuvant chemotherapy, 97 (46.9%) had wild-type EGFR, 55 (26.6%) had EGFR Ex19 deletion, 50 (24.2%) had EGFR Ex21 L858R mutation, and five (2.4%) had uncommon EGFR mutations.
OS and DFS Outcomes
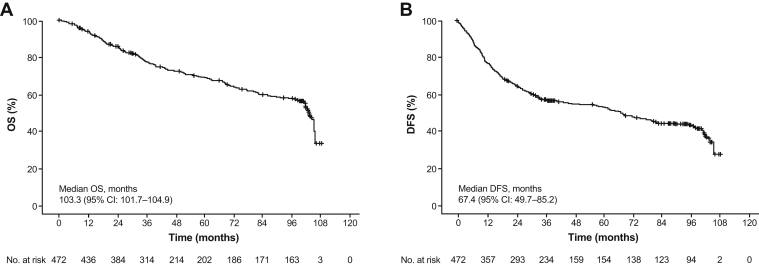
At the median follow-up time of 109.0 (95% CI: 106.6–111.4) months, 179 of 472 patients (37.9%) in the FAS experienced OS events. Median OS was 103.3 (95% CI: 101.7–104.9) months (Fig. 2A). The cumulative 1-, 2-, 3-, 5-, and 8-year OS rates were 93.8% (95% CI: 91.6–96.0), 85.8% (95% CI: 82.6–88.9), 77.2% (95% CI: 73.3–81.1), 68.9% (95% CI: 64.3–73.6), and 57.5% (95% CI: 52.2–62.8), respectively.
Figure 2.
Kaplan–Meier curves for (A) OS and (B) DFS in the full analysis set. CI, confidence interval; DFS, disease-free survival; OS, overall survival.
In the ICAN cohort, the 3-year DFS rate was 58.8%.23 At data cutoff in the extension cohort, overall median DFS was 67.4 (95% CI: 49.7–85.2) months (Fig. 2B). The cumulative 1-, 2-, 3-, 5-, and 8-year DFS rates were 77.1% (95% CI: 73.3–81.0), 64.7% (95% CI: 60.4–69.1), 56.6% (95% CI: 52.0–61.1), 52.9% (95% CI: 48.2–57.7), and 42.8% (95% CI: 37.7–47.9), respectively. Among the 252 patients with disease recurrence, 53 (21.0%) received subsequent chemotherapy (30 [11.9%]) or targeted therapy (23 [9.1%]).
Post Hoc Subgroup Analyses
Although the ICAN study revealed a significantly higher 3-year DFS in patients with mutant-EGFR compared with those with wild-type EGFR (65.6% versus 56.8%, p = 0.0347),23 no significant difference in the median DFS for patients with different EGFR mutation status across all postoperative stages was observed in the extension cohort (Supplementary Table 3). Similarly, classical EGFR-activating mutations were not significantly associated with OS across the postoperative stages, except for an OS benefit with Ex19 deletion in stage III disease; patients with uncommon EGFR mutations in stage I disease tended to have poorer OS, although the small sample size (n = 8) warrants further investigation (Supplementary Table 4).
Subgroup analysis for the FAS revealed that patients who did not receive adjuvant chemotherapy had a significantly higher median DFS (98.2 versus 27.2 mo, hazard ratio [HR] = 0.51, 95% CI: 0.40–0.65, p < 0.001) and median OS (not reached versus 101.5 mo, HR = 0.66, 95% CI: 0.49–0.88, p = 0.005) than those who did (Supplementary Fig. 1). To account for potential confounding factors, we compared the demographics and clinicopathologic characteristics of patients who received adjuvant chemotherapy versus those who did not (Supplementary Table 5). A higher disease stage was a potential confounder significantly associated with indication of adjuvant chemotherapy; adjusted odds ratios were 0.14 (95% CI: 0.08–0.25), 0.35 (95% CI: 0.20–0.60), and 0.96 (95% CI: 0.49–1.86) for stage IA, IB, and II disease, respectively, versus stage III disease. Adjusting for postoperative stage, median DFS and OS were not significantly different between patients who received adjuvant chemotherapy and those who did not across all disease stages (Supplementary Tables 3 and 4).
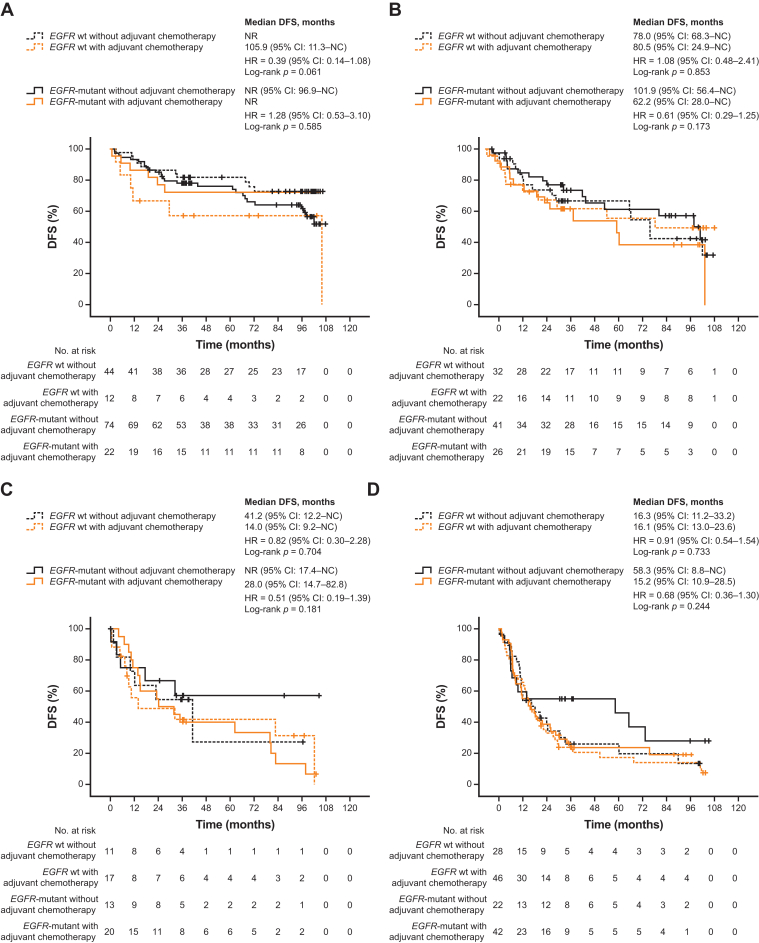
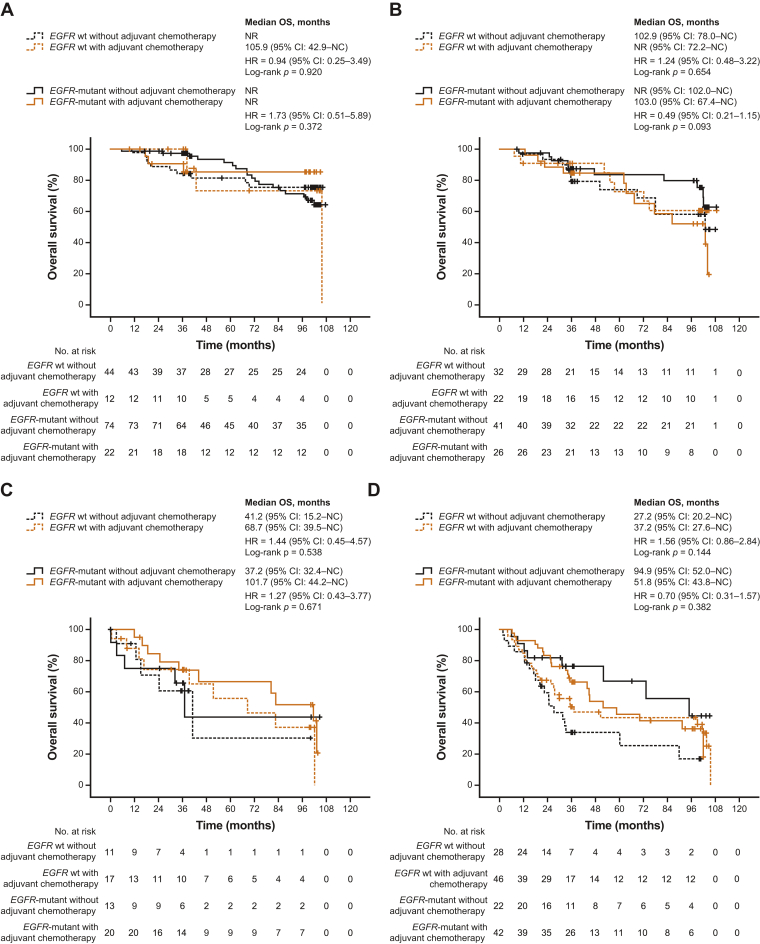
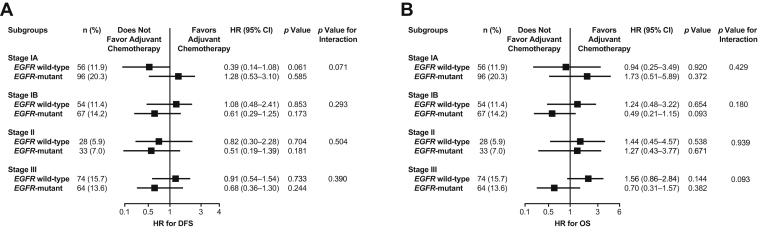
Furthermore, across all postoperative stages, patients who received adjuvant chemotherapy did not have significantly different DFS and OS versus those who did not, regardless of EGFR mutation status (Figure 3, Figure 4 and 5A and B and Supplementary Tables 6 and 7). Although not statistically significant, patients with EGFR-mutant stages IB to III disease who received adjuvant chemotherapy tended to have worse DFS versus those who did not. There was also a non-significant trend toward worse OS in patients with EGFR-mutant stage IB or III disease who received adjuvant chemotherapy versus those who did not, but this was not observed in patients with stage IA or II disease.
Figure 3.
Kaplan–Meier curves for DFS by adjuvant chemotherapy in subgroups defined by EGFR mutation status across postoperative stages (A) IA, (B) IB, (C) II, and (D) III. CI, confidence interval; DFS, disease-free survival; HR, hazard ratio; NC, not calculable; NR, not reached; wt, wild-type.
Figure 4.
Kaplan–Meier curves for OS by adjuvant chemotherapy in subgroups defined by EGFR mutation status across postoperative stages (A) IA, (B) IB, (C) II, and (D) III. CI, confidence interval; HR, hazard ratio; OS, overall survival; NC, not calculable; NR, not reached; wt, wild-type.
Figure 5.
Forest plot of the hazard ratios for (A) DFS and (B) OS of the patients based on the treatment with adjuvant chemotherapy in subgroups defined by EGFR mutation status and postoperative stage. CI, confidence interval; DFS, disease-free survival; HR, hazard ratio; OS, overall survival.
In patients who received adjuvant chemotherapy, DFS and OS were not significantly different by EGFR mutation status, and this was consistently observed across postoperative stages (Supplementary Fig. 2 and Supplementary Tables 8 and 9). In patients who did not receive adjuvant chemotherapy, DFS and OS were not significantly different by EGFR mutation status across postoperative stages, except for a significant OS benefit in patients with stage III EGFR-mutant versus wild-type disease (Supplementary Fig. 3 and Supplementary Tables 10 and 11).
Sensitivity Analysis
The demographics and clinical characteristics of patients lost to follow-up were similar to those with complete follow-up (Supplementary Table 12). Sensitivity analysis revealed that median DFS was 37.6 (95% CI: 33.1–55.9) months with a Harrell’s C-index of 0.691 (Supplementary Table 13) and median OS was 72.2 (95% CI: 60.2–96.9) months with a Harrell’s C-index of 0.723 (Supplementary Table 14). Median OS among patients lost to follow-up was 36.4 (interquartile range: 31.1–37.3) months; on the basis of this right-censored survival data, the worst-outcome median OS was 39.0 (95% CI: 33.2–44.8) months.
Prognostic Factors of Survival
The only prognostic factor of DFS identified in the ICAN study was postoperative stage.23 In the extension cohort, collinearity diagnostics revealed the presence of collinearity between adjuvant chemotherapy and other prognostic factors (eigenvalue: 0.017; condition index: 17.922; constant variance proportion: 0.99; variance proportions: <0.7; Supplementary Table 15). This finding, together with a Kaiser-Meyer-Olkin value of 0.538 and a Bartlett’s test of sphericity p value less than 0.001, suggested that a factor analysis of the prognostic variables was appropriate. Unlike the post hoc subgroup analyses, factor analyses considered the existing interdependencies among the prognostic variables studied, allowing for the identification of independent prognostic factors of survival.
Extraction of four common factors objectively explains 83.4% of the total variance (Supplementary Table 16). From the rotated factor matrix, the first common factor was smoking status (load: 0.905), the second common factors were postoperative stage (load: 0.867) and adjuvant chemotherapy (load: 0.736), the third was age (load: 0.958), and the fourth was EGFR mutation status (load: 0.982) (Supplementary Table 17).
Cox regression analysis of the four independent common factors revealed that patients who were current smokers (HR = 1.15, 95% CI: 1.03–1.30, p = 0.017) or had postoperative stages II to III disease and received adjuvant chemotherapy (HR = 1.79, 95% CI: 1.58–2.02, p < 0.001) had a higher risk of tumor recurrence and metastases (Supplementary Table 18). Patients who were current smokers (HR = 1.29, 95% CI: 1.12–1.49, p < 0.001), had postoperative stages II to III disease and received adjuvant chemotherapy (HR = 1.63, 95% CI: 1.41–1.87, p < 0.001), aged greater than or equal to 65 years (HR = 1.16, 95% CI: 1.01–1.34, p = 0.039), or had EGFR wild-type disease (HR = 1.24, 95% CI: 1.07–1.44, p = 0.004) had a higher risk of mortality (Supplementary Table 19).
Discussion
This extended ICAN study reports the EGFR mutation rates and long-term survival outcomes of Chinese patients with resected stages I to III LUAD in the real-world setting. Survival outcomes were analyzed by EGFR mutation status and adjuvant chemotherapy treatment. More than half of the cohort had EGFR-mutant disease, and more than 40% received adjuvant chemotherapy. Approximately 50% of the cohort had recurrent disease, with a mortality rate of approximately 30% within 5 years of surgery. EGFR mutation status was an independent prognostic factor of OS, whereas adjuvant chemotherapy did not significantly affect survival outcomes regardless of EGFR mutation status across all postoperative stages.
In the extension cohort, 55.1% of patients with resected LUAD were positive for EGFR mutations, which was consistent with previous reports,28,29 and similar to the prevalence in advanced disease.25,26,30 In a systemic review and meta-analysis of 16 studies in patients with resected NSCLC (N = 3337), the prevalence of EGFR-mutant disease was 19.2% to 59.5% in Asian studies (specifically 28.7%–52.5% in studies conducted in the People’s Republic of China), but it was lower in Western studies (3.4%–20.2%).29 Another systematic review of 151 LUAD studies worldwide (N = 33,162) revealed that the Asia-Pacific subgroup had the highest EGFR mutation frequency at 47%, compared with 12% to 22% in Western populations.28
Given the high prevalence of EGFR mutations in resected LUAD, determining their prognostic value is of importance. We found EGFR mutation status to be an independent predictor of OS (p = 0.004), although post hoc subgroup analyses did not reveal significant differences in survival outcomes with different EGFR mutations, except for an OS benefit observed with Ex19 deletion in stage III disease. Similarly, previous studies and meta-analyses investigating the association of EGFR mutations with survival outcomes in patients with resected NSCLC yielded conflicting results, with some supporting EGFR mutations as a favorable prognostic factor31, 32, 33, 34, 35, 36, 37, 38, 39 and others reporting either no significant association or association with negative prognosis.29,40, 41, 42 The association of EGFR mutations with favorable prognostic factors, including being female and never or light smoking, may influence these observations.34,42 Further studies are required to confirm the prognostic effects of different EGFR mutation types.
On the basis of this study and previous reports, the rates of recurrence and mortality after surgical resection of NSCLC remained high.8, 9, 10, 11, 12 Hence, the postsurgical management of NSCLC for improvement in survival outcomes is an urgent unmet need. Although adjuvant chemotherapy is the current standard of care for patients with resectable NSCLC, previous evidence on its use in these patients on the basis of EGFR mutation status is scarce, and limited benefit has been observed.20,21 In clinical practice, approximately 48% to 57% of patients with resected stages IB to IIIA NSCLC received adjuvant chemotherapy regardless of EGFR mutation status, with increased use at higher disease stages.7,43, 44, 45 Yet, the 5-year survival benefit with chemotherapy was only 5.4%,22 consistent with our observation that adjuvant chemotherapy had no significant benefit in patients with resected LUAD regardless of EGFR mutation status and postoperative stage. Across all postoperative stages, EGFR mutation status was not associated with survival outcomes regardless of adjuvant chemotherapy use, except for a significant OS benefit with stage III EGFR-mutant versus wild-type disease in patients who did not receive adjuvant chemotherapy. Nevertheless, different patient characteristics may exist between the EGFR-mutant and wild-type subgroups that potentially confound the analysis; this warrants further studies that account for such confounding factors to confirm whether EGFR mutations are associated with survival outcomes in patients who received adjuvant chemotherapy and those who did not.
Although this study suggested that there was no significant benefit of adjuvant chemotherapy in patients with EGFR wild-type or EGFR-mutant resected NSCLC across all postoperative stages, recent evidence suggests a role for EGFR TKIs in the treatment of EGFR-mutant NSCLC after resection.17, 18, 19,46, 47, 48 Meta-analyses consistently revealed that adjuvant EGFR TKIs significantly improved DFS in patients with resected EGFR-mutant NSCLC compared with chemotherapy,49,50 and this was similarly observed in a retrospective study.51 To the best of our knowledge, ADJUVANT/CTONG1104 was the only phase 3 study performed in Chinese patients with early stage, EGFR-mutant NSCLC, where adjuvant gefitinib significantly improved DFS compared with chemotherapy.19
Furthermore, osimertinib is the first EGFR TKI to be approved for adjuvant treatment of patients with EGFR-mutant NSCLC after resection on the basis of the ADAURA study.17 Osimertinib resulted in a significantly lower recurrence rate versus placebo in patients with resected stages IB to IIIA EGFR-mutant NSCLC.17 Consistent with previous studies and clinical practice, approximately 60% of the ADAURA cohort received adjuvant chemotherapy; the proportions being 26%, 71%, and 80% for stage IB, II, and IIIA, respectively.17,43,44,47 Notably, osimertinib had similar DFS benefit in the subgroups with and without previous adjuvant chemotherapy across different disease stages.17,47 In contrast, the benefit of previous adjuvant chemotherapy in the ADJUVANT cohort was limited and requires further confirmation. Although it may be argued that the benefit of adjuvant osimertinib may be overestimated in patients who did not receive previous chemotherapy,52 the present real-world study revealed that adjuvant chemotherapy did not significantly affect survival outcomes in patients with EGFR-mutant LUAD across all postoperative stages. In addition, in the ADUARA trial, patients in the placebo arm who received background adjuvant chemotherapy had a higher disease recurrence rate versus those who did not.17,47 This poor survival outcome may be due to a higher proportion of patients with stage II/IIIA disease among those who received adjuvant chemotherapy versus those who did not (85.5% versus 41.9%).17,47
Besides EGFR TKI monotherapy, EGFR TKI in combination with chemotherapy resulted in superior survival benefits versus chemotherapy alone in resected EGFR-mutant NSCLC.50,51 Nevertheless, the benefit of EGFR TKI and chemotherapy combination did not significantly improve DFS compared with EGFR TKI alone.51 Taken together, current evidence supports that adjuvant EGFR TKI, rather than chemotherapy, should preferably be indicated for patients with resectable EGFR-mutant NSCLC in clinical practice, and the sequencing of EGFR TKI after adjuvant chemotherapy may be considered according to the ADAURA trial.17,47
This study has several limitations. First, in this real-word analysis, not all relevant survival risk factors had available data to adjust for confounding. This should be considered when interpreting the association between survival outcomes and the prognostic factors evaluated. Second, the subgroup analyses were post hoc and exploratory in nature, and further confirmation of the observations is warranted. The sample sizes for the subgroup analyses, particularly for patients with postoperative stage II or III disease, were relatively small. Furthermore, although the confounding effect of postoperative staging has been accounted for, differences in other patient characteristics between subgroups may exist that confound the analyses. Third, this study only included patients from the People’s Republic of China, which may not be globally representative. Considering ethnic differences, the significance of EGFR mutation status on survival outcomes in patients from Western countries is expected to be weaker.28,41 Last, the effect of genetic alterations in other oncogenic drivers, including KRAS mutation and ALK rearrangement, on survival outcomes with adjuvant chemotherapy was not studied. A comprehensive tumor genomic analysis of resected EGFR-mutant NSCLC (mainly LUAD) specimens suggested that additional predictive biomarkers may be useful to guide personalized adjuvant therapy within patients with resected EGFR-mutant NSCLC.53,54
In this real-word cohort of patients with completely resected stages I to III LUAD, EGFR mutations were common. Long-term follow-up yielded median OS and DFS of 103.3 and 67.4 months, respectively. Adjuvant chemotherapy did not improve survival outcomes, regardless of EGFR mutation status and postoperative stage. Smoking and higher postoperative disease stage with adjuvant chemotherapy treatment predicted for both worse DFS and OS; older age and EGFR wild-type disease predicted for worse OS alone.
CRediT Authorship Contribution Statement
Xue-Ning Yang, Yi-Long Wu: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing—original draft, Writing—review and editing.
Hong-Hong Yan: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Visualization, Writing—original draft, Writing—review and editing.
Jun Wang, Xiang-Yang Chu, Zhi-Dong Liu, Yi Shen, Hai-Tao Ma, Xiang-Ning Fu, Jian Hu, Nai-Kang Zhou, Yong-Yu Liu, Xin-Ming Zhou, Jing-Song Li, Kang Yang, Jian Li, Lin Xu, Si-Yu Wang, Qun Wang, Lun-Xu Liu, Shun Xu, Zhong-Yuan Chen, Hong-He Lou, Chang-Li Wang, Ying Cheng, Si-Yang Liu, Xu-Chao Zhang, Wen-Zhao Zhong: Data curation, Investigation, Resources, Writing—review and editing.
Acknowledgments
This study was funded by AstraZeneca and the Chinese Thoracic Oncology Group (study number: CTONG1802). Medical writing support, funded by AstraZeneca, was provided by Qing Yun Chong, Alice Carruthers, Tim Stentiford, Henry Chung, and Rachael Profit (Nucleus Global, Shanghai, People's Republic of China) in accordance with the Good Publication Practice 3 guidelines.
Footnotes
Drs. XN Yang and HH Yan contributed equally to this work.
Disclosure: Prof. Wu reports being on the advisory boards of AstraZeneca, Boehringer Ingelheim, Novartis, and Takeda; receiving honorarium from AstraZeneca, BeiGene, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Merck Sharp & Dohme Corp., Pfizer, and Roche for promotional activities and from Sanofi for nonpromotional activities; and receiving contract support and/or research grants from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Hengrui Therapeutics, and Roche. Dr. Zhong declares receiving speaker fees from AstraZeneca and Roche. The remaining authors declare no conflict of interest.
Cite this article as: Yang XN, Yan HH, Wang J, et al. Real-world survival outcomes based on EGFR mutation status in Chinese patients with lung adenocarcinoma after complete resection: results from the ICAN Study. JTO Clin Res Rep. 2022;3:100257.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2021.100257.
Supplementary Data
References
- 1.Feng R.M., Zong Y.N., Cao S.M., Xu R.H. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond) 2019;39:22. doi: 10.1186/s40880-019-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fois S.S., Paliogiannis P., Zinellu A., Fois A.G., Cossu A., Palmieri G. Molecular epidemiology of the main druggable genetic alterations in non-small cell lung cancer. Int J Mol Sci. 2021;22:612. doi: 10.3390/ijms22020612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuan M., Huang L.L., Chen J.H., Wu J., Xu Q. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduct Target Ther. 2019;4:61. doi: 10.1038/s41392-019-0099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network NCCN clinical practice guidelines in oncology for non-small cell lung cancer version 3. 2020. https://www2.tri-kobe.org/nccn/guideline/lung/english/non_small.pdf
- 5.Chiari R., Sidoni A., Metro G. Early stage resectable non-small cell lung cancer: is neoadjuvant immunotherapy the right way forward? J Thorac Dis. 2018;10(suppl 33):S3890–S3894. doi: 10.21037/jtd.2018.10.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majem M., Juan O., Insa A., et al. SEOM clinical guidelines for the treatment of non-small cell lung cancer (2018) Clin Transl Oncol. 2019;21:3–17. doi: 10.1007/s12094-018-1978-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Postmus P.E., Kerr K.M., Oudkerk M., et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv1–iv21. doi: 10.1093/annonc/mdx222. [DOI] [PubMed] [Google Scholar]
- 8.Mizuno T., Arimura T., Kuroda H., Sakakura N., Yatabe Y., Sakao Y. Current outcomes of postrecurrence survival in patients after resection of non-small cell lung cancer. J Thorac Dis. 2018;10:1788–1796. doi: 10.21037/jtd.2018.01.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugimura H., Nichols F.C., Yang P., et al. Survival after recurrent nonsmall-cell lung cancer after complete pulmonary resection. Ann Thorac Surg. 2007;83:409–418. doi: 10.1016/j.athoracsur.2006.08.046. [DOI] [PubMed] [Google Scholar]
- 10.Taylor M.D., Nagji A.S., Bhamidipati C.M., et al. Tumor recurrence after complete resection for non-small cell lung cancer. Ann Thorac Surg. 2012;93:1813–1821. doi: 10.1016/j.athoracsur.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 11.Wang C., Wu Y., Shao J., Liu D., Li W. Clinicopathological variables influencing overall survival, recurrence and post-recurrence survival in resected stage I non-small-cell lung cancer. BMC Cancer. 2020;20:150. doi: 10.1186/s12885-020-6621-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams B.A., Sugimura H., Endo C., et al. Predicting postrecurrence survival among completely resected nonsmall-cell lung cancer patients. Ann Thorac Surg. 2006;81:1021–1027. doi: 10.1016/j.athoracsur.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Hung J.J., Hsu W.H., Hsieh C.C., et al. Post-recurrence survival in completely resected stage I non-small cell lung cancer with local recurrence. Thorax. 2009;64:192–196. doi: 10.1136/thx.2007.094912. [DOI] [PubMed] [Google Scholar]
- 14.Castellanos E., Feld E., Horn L. Driven by mutations: the predictive value of mutation subtype in EGFR-mutated non-small cell lung cancer. J Thorac Oncol. 2017;12:612–623. doi: 10.1016/j.jtho.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Shen P., Zhong W. Adjuvant EGFR TKI therapy for resectable non-small cell lung cancer: new era for personalized medicine. J Thorac Dis. 2018;10:1364–1369. doi: 10.21037/jtd.2018.03.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Y.L., Planchard D., Lu S., et al. Pan-Asian adapted Clinical Practice Guidelines for the management of patients with metastatic non-small-cell lung cancer: a CSCO–ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30:171–210. doi: 10.1093/annonc/mdy554. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y.L., Tsuboi M., He J., et al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2020;383:1711–1723. doi: 10.1056/NEJMoa2027071. [DOI] [PubMed] [Google Scholar]
- 18.Zhong W.Z., Wang Q., Mao W.M., et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol. 2018;19:139–148. doi: 10.1016/S1470-2045(17)30729-5. [DOI] [PubMed] [Google Scholar]
- 19.Zhong W.Z., Wang Q., Mao W.M., et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC: final overall survival analysis of CTONG1104 phase III trial. J Clin Oncol. 2021;39:713–722. doi: 10.1200/JCO.20.01820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsao A.S., Jolly S., Lee J.M. Updates in local-regionally advanced non–small cell lung cancer. Am Soc Clin Oncol Educ Book. 2019:553–562. doi: 10.1200/EDBK_237839. [DOI] [PubMed] [Google Scholar]
- 21.Artal Cortés Á., Calera Urquizu L., Hernando Cubero J. Adjuvant chemotherapy in non-small cell lung cancer: state-of-the-art. Transl Lung Cancer Res. 2015;4:191–197. doi: 10.3978/j.issn.2218-6751.2014.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pignon J.P., Tribodet H., Scagliotti G.V., et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y., Chu X., Wang J., et al. EGFR mutation and survival outcomes in patients with completely resected lung adenocarcinoma – a multiple centers, non-interventional study (ICAN trial) Ann Oncol. 2014;25(suppl 4):iv410. [Google Scholar]
- 24.Zhang X., Wu Y.L., Wang J., et al. A prospective comparison study on EGFR mutations by direct sequencing and ARMS in completely resected Chinese non-small cell lung cancer with adenocarcinoma histology (ICAN) J Clin Oncol. 2013;31(suppl 15) 1547–1547. [Google Scholar]
- 25.Han B., Tjulandin S., Hagiwara K., et al. EGFR mutation prevalence in Asia-Pacific and Russian patients with advanced NSCLC of adenocarcinoma and non-adenocarcinoma histology: the IGNITE study. Lung Cancer. 2017;113:37–44. doi: 10.1016/j.lungcan.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 26.Shi Y., Li J., Zhang S., et al. Molecular epidemiology of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology—Mainland China subset analysis of the PIONEER study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0143515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collett D. 3rd ed. Chapman & Hall/CRC; New York, NY: 2015. Modelling Survival Data in Medical Research. [Google Scholar]
- 28.Midha A., Dearden S., McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII) Am J Cancer Res. 2015;5:2892–2911. [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Z., Wang T., Zhang J., et al. Prognostic value of epidermal growth factor receptor mutations in resected non-small cell lung cancer: a systematic review with meta-analysis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0106053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shigematsu H., Lin L., Takahashi T., et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 31.Kim H., Lee H.J., Hong H., et al. The prognostic implications of EGFR mutation and ALK rearrangement for the long-term outcomes of patients with resected lung adenocarcinomas. Thorac Cancer. 2019;10:1619–1627. doi: 10.1111/1759-7714.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isaka T., Nakayama H., Ito H., Yokose T., Yamada K., Masuda M. Impact of the epidermal growth factor receptor mutation status on the prognosis of recurrent adenocarcinoma of the lung after curative surgery. BMC Cancer. 2018;18:959. doi: 10.1186/s12885-018-4849-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayasaka K., Shiono S., Matsumura Y., et al. OA19. 07 Difference of postoperative survival due to the type of EGFR gene mutation in surgically resected lung adenocarcinomas. J Thorac Oncol. 2017;12:S320–S321. [Google Scholar]
- 34.Okamoto T., Kitahara H., Shimamatsu S., et al. Prognostic impact of EGFR driver mutations on postoperative disease recurrence in lung adenocarcinoma. Anticancer Res. 2016;36:3057–3063. [PubMed] [Google Scholar]
- 35.Kudo Y., Shimada Y., Saji H., et al. Prognostic factors for survival after recurrence in patients with completely resected lung adenocarcinoma: important roles of epidermal growth factor receptor mutation status and the current staging system. Clin Lung Cancer. 2015;16:e213–e221. doi: 10.1016/j.cllc.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Ohtaki Y., Shimizu K., Kakegawa S., et al. Postrecurrence survival of surgically resected pulmonary adenocarcinoma patients according to EGFR and KRAS mutation status. Mol Clin Oncol. 2014;2:187. doi: 10.3892/mco.2013.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Izar B., Sequist L., Lee M., et al. The impact of EGFR mutation status on outcomes in patients with resected stage I non-small cell lung cancers. Ann Thorac Surg. 2013;96:962–968. doi: 10.1016/j.athoracsur.2013.05.091. [DOI] [PubMed] [Google Scholar]
- 38.Tamiya A., Koh Y., Isa S.I., et al. Impact of somatic mutations on prognosis in resected non-small-cell lung cancer: the Japan Molecular Epidemiology for lung cancer study. Cancer Med. 2020;9:2343–2351. doi: 10.1002/cam4.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang S.M., Zhu Q.G., Ding X.X., et al. Prognostic value of EGFR and KRAS in resected non-small cell lung cancer: a systematic review and meta-analysis. Cancer Manag Res. 2018;10:3393–3404. doi: 10.2147/CMAR.S167578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He Q., Xin P., Zhang M., et al. The impact of epidermal growth factor receptor mutations on the prognosis of resected non-small cell lung cancer: a meta-analysis of literatures. Transl Lung Cancer Res. 2019;8:124–134. doi: 10.21037/tlcr.2019.03.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ito M., Miyata Y., Kushitani K., et al. Increased risk of recurrence in resected EGFR-positive pN0M0 invasive lung adenocarcinoma. Thorac Cancer. 2018;9:1594–1602. doi: 10.1111/1759-7714.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim Y.T., Seong Y.W., Jung Y.J., et al. The presence of mutations in epidermal growth factor receptor gene is not a prognostic factor for long-term outcome after surgical resection of non-small-cell lung cancer. J Thorac Oncol. 2013;8:171–178. doi: 10.1097/JTO.0b013e318277a3bb. [DOI] [PubMed] [Google Scholar]
- 43.Buck P.O., Saverno K.R., Miller P.J., Arondekar B., Walker M.S. Treatment patterns and health resource utilization among patients diagnosed with early stage resected non-small cell lung cancer at US community oncology practices. Clin Lung Cancer. 2015;16:486–495. doi: 10.1016/j.cllc.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 44.Chouaid C., Danson S., Andreas S., et al. Adjuvant treatment patterns and outcomes in patients with stage IB-IIIA non-small cell lung cancer in France, Germany, and the United Kingdom based on the LuCaBIS burden of illness study. Lung Cancer. 2018;124:310–316. doi: 10.1016/j.lungcan.2018.07.042. [DOI] [PubMed] [Google Scholar]
- 45.Kris M.G., Gaspar L.E., Chaft J.E., et al. Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non-small-cell lung cancers: American Society of Clinical Oncology/Cancer Care Ontario clinical practice guideline update. J Clin Oncol. 2017;35:2960–2974. doi: 10.1200/JCO.2017.72.4401. [DOI] [PubMed] [Google Scholar]
- 46.Huang Q., Li J., Sun Y., Wang R., Cheng X., Chen H. Efficacy of EGFR tyrosine kinase inhibitors in the adjuvant treatment for operable non-small cell lung cancer by a meta-analysis. Chest. 2016;149:1384–1392. doi: 10.1016/j.chest.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 47.Wu Y., John T., Grohe C., et al. OA06.04 postoperative chemotherapy use and outcomes from ADAURA: osimertinib as adjuvant therapy for resected EGFR mutated NSCLC. J Thorac Oncol. 2021 doi: 10.1016/j.jtho.2021.10.014. pii: S1556-0864(21)03285-8. [DOI] [PubMed] [Google Scholar]
- 48.Yue D., Xu S., Wang Q., et al. Erlotinib versus vinorelbine plus cisplatin as adjuvant therapy in Chinese patients with stage IIIA EGFR mutation-positive non-small-cell lung cancer (EVAN): a randomised, open-label, phase 2 trial. Lancet Respir Med. 2018;6:863–873. doi: 10.1016/S2213-2600(18)30277-7. [DOI] [PubMed] [Google Scholar]
- 49.Chen R.L., Sun L.L., Cao Y., et al. Adjuvant EGFR-TKIs for patients with resected EGFR-mutant non-small cell lung cancer: a meta-analysis of 1,283 patients. Front Oncol. 2021;11:629394. doi: 10.3389/fonc.2021.629394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang W., Li X., Xie X., et al. EGFR inhibitors as adjuvant therapy for resected non-small cell lung cancer harboring EGFR mutations. Lung Cancer. 2019;136:6–14. doi: 10.1016/j.lungcan.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 51.Wen M., Wang L., Wang X., et al. Optimal adjuvant therapy in resected stage IIIA-N2 non-small-cell lung cancer harboring EGFR mutations. Oncol Res Treat. 2020;43:686–693. doi: 10.1159/000506692. [DOI] [PubMed] [Google Scholar]
- 52.Kulkarni A.A., Naqash A.R., Puri S., Dienstmann R. Is it time to implement adjuvant targeted therapy in EGFR-mutant non–small-cell lung cancer? JCO Precis Oncol. 2021;5:408–414. doi: 10.1200/PO.20.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu Y.L., Liu S.Y., Wang Q., et al. A comprehensive model of genetic-features predicts outcome of personalized adjuvant treatment in resected EGFR-mutant stage II-IIIA NSCLC: results from a phase III trial (CTONG 1104-ADJUVANT) Ann Oncol. 2019;30(suppl 5):v586. [Google Scholar]
- 54.Liu S.Y., Bao H., Wang Q., et al. Genomic signatures define three subtypes of EGFR-mutant stage II–III non-small-cell lung cancer with distinct adjuvant therapy outcomes. Nat Commun. 2021;12:6450. doi: 10.1038/s41467-021-26806-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.