Abstract
Purpose
Preserflo MicroShunt is a minimally-invasive glaucoma drainage micro-tube used to shunt aqueous humor from the anterior chamber to the subtenon space. The safety of the procedure was considered satisfactory with a majority of minor side effects.
Observation
We describe the 5 year endothelial cell loss after Preserflo implantation in 2 primary open angle glaucoma patients. The case 1 presented a device-cornea touch after a backward migration of the device. The case 2 presented a modified aspect of the device compatible with an inflammatory reaction. Both cases were explanted.
Conclusion
As described in Ahmed glaucoma valve, Xen gel stent and Cypass, Preserflo MicroShunt can lead to endothelial cell loss in some cases. A long-term prospective study with pre and postoperative endothelial cell count and AS-OCT or UBM evaluation of the device positioning would be of great interest to assess the real impact of Preserflo MicroShunt and risk factors for endothelial cell loss.
Keywords: Preserflo, Microshunt, Endothelial cell loss, Safety, MIGS
1. Introduction
Preserflo MicroShunt (Santen Inc., Miami, FL) which was CE marked in 2012 and FDA approved in July 2020, is a minimally-invasive glaucoma drainage micro-tube used to shunt aqueous humor from the anterior chamber to the subtenon space. It is made from an inert biocompatible biomaterial called poly (styrene-block-isobutylene-block-styrene) or SIBS with an 8.5 mm length and 70 μm lumen. The device does not require a scleral flap, sclerotomy and iridectomy and aims to standardize glaucoma procedures. Some studies assessed its efficacy and safety with a maximum follow-up of 5 years.1, 2, 3, 4, 5 After one year, a mean IOP reduction varying from 29 to 40% was observed with a mean medication changing from 3 to 4 to 0 eyedrop.3,4 The surgical success persisted after 2 years and up to 5 years, with a mean medication of 1 eyedrop.6,7 The safety of the procedure was considered satisfactory with a majority of minor side effects: 13–29% of transient hypotony, 5–8% of transient hyphaema, 7–13% of transient choroidal detachment, 5–10% of transient shallow anterior chamber and 5–12% of needling (with a mean delay of 2 months after surgery).3,4,7,8 The 5 year evaluation of side effects found 13% of device touching the iris but no case of corneal decompensation.7 We must note that only one study in the literature objectively assessed endothelial cell count during follow-up while the cornea can remain clear even below 500 cells/mm2.4
We implanted 16 patients with Preserflo MicroShunt between June, 2014 and June, 2015 in the Institut du Glaucome, Hôpital Saint Joseph, Paris. After the observation of endothelial cell loss (ECL) in the presented Case 1, 5 years after surgery, a systematic specular microscopy was prescribed to all implanted patients.
2. Case series
2.1. Case 1
2.1.1. Initial presentation
In this report, we describe the case of a 63 year old woman suffering from advanced primary open angle glaucoma (POAG) implanted in July 2014. She underwent a bilateral selective laser trabeculoplasty 5 years before. Her surgical history included a deep sclerectomy in the fellow eye 1 year before. The preoperative implanted (left) eye presentation was as follow: 19 mmHg intraocular pressure (IOP) under 1 medication and 526 μmpachymetry, cup to disc ratio 0.9 and 66 μm average RNFL thickness. Further management options were explored and a Preserflo MicroShunt implantation in the left eye was decided.
2.1.2. MicroShunt implantation and follow-up
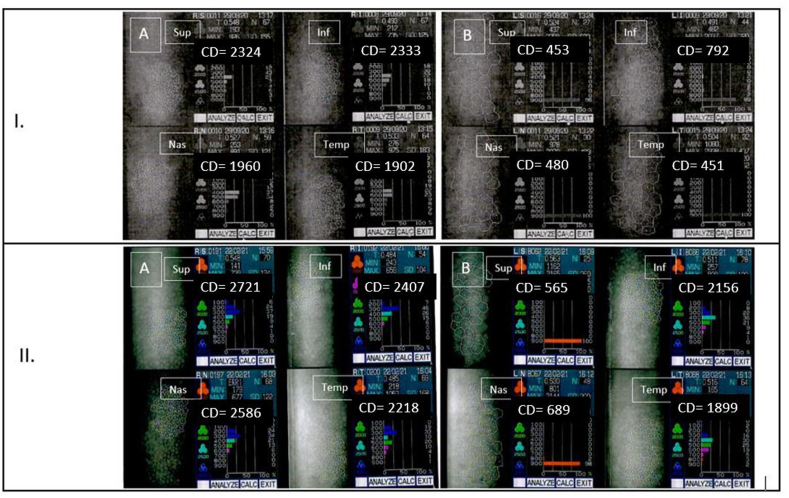
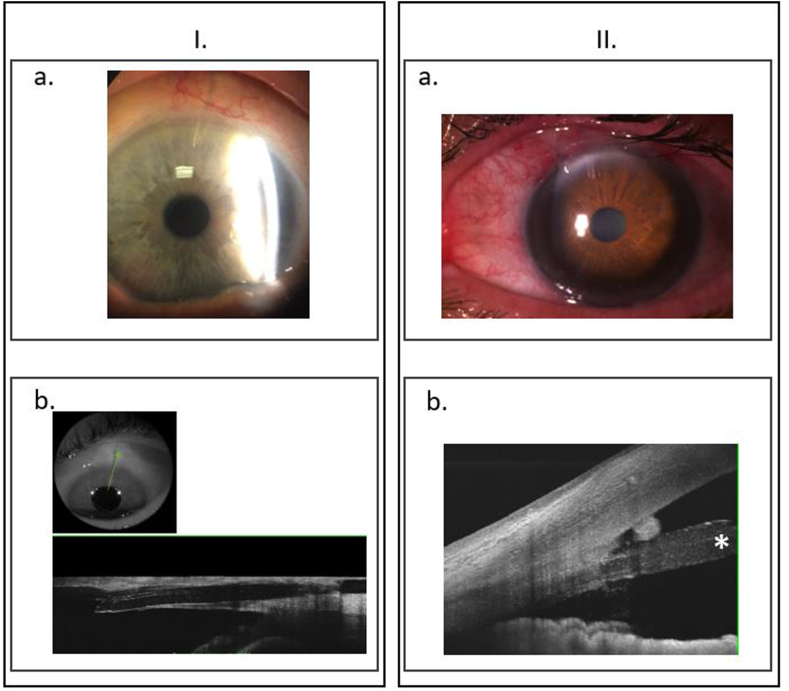
She underwent a standard Preserflo implantation with MMC 0.2 mg/mL for 2 minutes and IOP normalized to 13 mmHg without glaucoma medication on the day 1 postoperative. Six months after surgery, the IOP raised to 20 mmHg with failure of 3 needlings thus a 1 drop medication was prescribed. The patient underwent a phacoemulsification in both eyes one year after Preserflo implantation. The IOP remained controlled under 1 drop medication during 5 years but a device-corneal touch was then observed without any corneal edema nor guttata and a pachymetry of 520 μm. An endothelial cell count with specular microscopy was performed and found low endothelial cell count in the implanted eye compared to fellow eye with a central endothelial cell count of 549 cells/mm2 and 2559 cells/mm2, respectively (Fig. 1I). Fig. 2 presents the biomicroscopy and AS-OCT aspects of the implanted eye where we can see a long corneal trajectory of the device with a very short intracameral portion of it, probably because of a backward movement of the device with time.
Fig. 1.
Endothelial cell count with specular microscopy after Preserflo implantation. Fig. 1.I. Patient 1, pseudophakic. A/Right eye = fellow eye; B/Left eye = eye implanted with Preserflo microshunt, the endothelial cell loss affects mostly superior, nasal and temporal quadrants. Fig. 1.II. Patient 2, phakic. A/Right eye = fellow eye; B/Left eye = eye implanted with Preserflo microshunt, the endothelial cell loss concerns superior and nasal quadrants while inferior and temporal are subnormal.
Fig. 2.
Biomicroscopy and AS-OCT trajectory of the Preserflo MicroShunt.Fig. 2.I. Patient 1. a. Biomicroscopy of the left eye, the microshunt is located superiorly, no corneal edema. b. AS-OCT visualizing a horizontal and corneal trajectory of the microshunt. The intracameral portion of the microshunt is very short.
Fig. 2.II. Patient 2. a. Biomicroscopy of the left eye, the microshunt is located nasal-superiorly, no corneal edema. b. AS-OCT visualizing a satisfactory trajectory of the microshunt in the cornea and a suficient portion of the shunt in the anterior chamber, far from the cornea and the iris. We must note a hyperreflective structure between the shunt and the endothelium that can be an inflammatory reaction. We can also see a swollen intracameral part of the shunt (*).
2.1.3. MicroShunt removal
Preserflo MicroShunt was removed because of the ECL and the conjunctiva was sewed to limbus. The postoperative IOP was 4 mmHg with a diffuse filtering bleb and no seidel. At 1 month, the IOP was 14 mmHg with no glaucoma medication but topical dexamethasone phosphate and a clear cornea. Six months after Preserflo removal, the endothelial cell count remained relatively stable with 493 cells/mm2 centrally.
2.2. Case 2
2.2.1. Initial presentation
The second report describes the case of a 68 year old woman suffering from advanced POAG implanted in November 2014. Her surgical history included a trabeculectomy in the fellow eye 6 years before and a deep sclerectomy in the implanted eye the year before. The implanted (left) eye presentation was: 19 mmHg IOP under 2 medications and 503 μm pachymetry, cup to disc ratio 0.8 and 58 μm average peripapillary RNFL thickness. The implantation of a Preserflo MicroShunt in the left eye was thus decided.
2.2.2. MicroShunt implantation and follow-up
She underwent a standard Preserflo implantation with MMC 0.2 mg/mL for 2 minutes and IOP normalized to 10 mmHg without glaucoma medication on the day 1 postoperative. Six months after surgery, the IOP was still 10 mmHg without medication but it increased after 9 months to 17 mmHg with a flat non filtering bleb. After 5 years, the IOP was 13 mmHg under 2 medications. The cornea was clear and there was no device-corneal or device-iris touch but a routine endothelial cell count was performed. We observed a low endothelial cell density in the implanted eye compared to fellow eye with central endothelial cell count of 1139 cells/mm2 and 2366 cells/mm2, respectively (phakic in both eyes) (Fig. 1.II.). We must note that the endothelial cell loss mostly concerned the superior (565 cells/mm2) and nasal (689 cells/mm2) quadrants, regarding the device. Fig. 2 presents the biomicroscopy and AS-OCT aspects of the implanted eye visualizing a satisfactory trajectory of the microshunt in the cornea and a suficient portion of the shunt in the anterior chamber, far from the cornea and the iris. We must note a hyperreflective structure between the shunt and the endothelium that could be an inflammatory reaction. We did not observe tyndall nor synechiae when we examined the patient at 5 years postoperative. We can also guess a swollen and dedifferentiated intracameral part of the shunt that can be part of an inflammatory reaction.
2.2.3. MicroShunt removal
Five days after Preserflo removal, the postoperative IOP was 5 mmHg with a diffuse filtering bleb and no seidel. After 1 month, the IOP was 13 mmHg with no medication, a fine diffuse bleb and a clear cornea.
3. Discussion
The present case series reports endothelial cell loss 5 years after Preserflo MicroShunt implantation in 2 patients (17%) in a series of 16 implanted patients (10 patients with 5 year follow-up, 4 patients lost to follow-up). We hypothesized the reasons why ECL occurred: in case 1, showing 549 cells/mm2, the intracameral portion of the device was short with a very horizontal trajectory and corneal touch (probably after backward migration); in case 2, showing 1139 cells/mm2, a hyperreflective structure and a swollen intracameral portion of the device could be the traduction of local inflammation around the tube. The mean ± SD central endothelial cell count in the remaining 10 patients, 5 years after Preserflo implantation, was 1946 ± 480 vs 2095 ± 339 cells/mm2 in implanted and fellow eyes, respectively. The mean difference was of 149 ± 333 cells/mm2 between eyes (P t-test paired = 0.2).
In the literature, a huge majority of minor adverse events such as transient hypotony and hyphaema were described. Vision-threatening complications are uncommon and were reported in only 1% of Preserflo implantations in the 1 year results of a randomized clinical trial.4
The ECL induced by tubes was described in many other situations. Xen Gel Stent (Allergan INC, Dublin, Ireland) induced ECL, with a negative effect of blinking and rubbing the eye when the device was located near the endothelium.9 Endothelial cell count was also assessed 2 years after Ahmed Glaucoma valve implantation with a decrease of 15% at 12 months and 19% at 24 months.10 Another situation was the CyPass Micro-Stent (Alcon) device, withdrawn of the market place, because of a late observation of ECL, after 5 years.11 The hypotheses made by the authors were: the jet flow around the tube end caused by the heart beat, the inflammation in the anterior chamber, a foreign body reaction to the tube, contact between appearing rings and endothelium (i.e. implantation depth).
Furthermore the patient 2 presented in the case series had a history of filtering surgery and patient 1 underwent cataract surgery after Preserflo. Arnavielle et al. demonstrated an endothelial cell density decrease of 10% 12 months after trabeculectomy.12 Wirbelauer et al. found 11% endothelial cell density count decrease after standard cataract surgery13 The ECL in the present cases could be potentiated by the history of filtering and cataract surgery besides Preserflo implantation.
Another case of ECL 1 year after Preserflo implantation was described by Baker et al. in a recent study because of the proximity of the device to the cornea.4 However the mean endothelial cell density loss in the whole sample at 1 year postoperative did not differ between Preserflo and trabeculectomy groups (−5.2% vs −6.9% cell density compared to preoperative, respectively). We must note that these results are 1 year results while ECL can occur later as for Cypass device. The present paper describes major side effects at 5 years and highlights the need to follow implanted patients for several years.
A prospective study with long term follow-up combining pre and postoperative endothelial cell count and AS-OCT or UBM evaluation of the device positioning would be of great interest to assess the real impact of Preserflo MicroShunt and the major risk factors of ECL.
Patient consent
Written consent to publish this case has not been obtained. This report does not contain any personal identifying information.
Funding
No funding or grant support.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
The following authors have no financial disclosures: CC, SH, EB, YL.
Acknowledgements
None.
References
- 1.Riss I., Batlle J., Pinchuk L., Kato Y.P., Weber B.A., Parel J.-M. [One-year results on the safety and efficacy of the InnFocus MicroShuntTM depending on placement and concentration of mitomycin C] J Fr Ophtalmol. 2015 Nov;38(9):855–860. doi: 10.1016/j.jfo.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Batlle J.F., Fantes F., Riss I., et al. Three-year follow-up of a novel aqueous humor MicroShunt. J Glaucoma. 2016 Feb;25(2):e58–65. doi: 10.1097/IJG.0000000000000368. [DOI] [PubMed] [Google Scholar]
- 3.Schlenker M.B., Durr G.M., Michaelov E., Ahmed I.I.K. Intermediate outcomes of a novel standalone ab externo SIBS microshunt with mitomycin C. Am J Ophthalmol. 2020 Jul;215:141–153. doi: 10.1016/j.ajo.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 4.Baker N.D., Barnebey H.S., Moster M.R., et al. Ab-Externo MicroShunt versus trabeculectomy in primary open-angle glaucoma: one-year results from a 2-year randomized, multicenter study. Ophthalmology. 2021 May 27;(21) doi: 10.1016/j.ophtha.2021.05.023. S0161-6420. 00384-00385. [DOI] [PubMed] [Google Scholar]
- 5.Beckers H.J.M., Aptel F., Webers C.A.B., et al. Safety and effectiveness of the PRESERFLO® MicroShunt in primary open-angle glaucoma: results from a 2-year multicenter study. Ophthalmol Glaucoma. 2021 Jul 28;(21) doi: 10.1016/j.ogla.2021.07.008. S2589-4196. 00179-4. [DOI] [PubMed] [Google Scholar]
- 6.Scheres L.M.J., Kujovic-Aleksov S., Ramdas W.D., et al. XEN® Gel Stent compared to PRESERFLOTM MicroShunt implantation for primary open-angle glaucoma: two-year results. Acta Ophthalmol. 2021 May;99(3):e433–e440. doi: 10.1111/aos.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batlle J.F., Corona A., Albuquerque R. Long-term results of the PRESERFLO MicroShunt in patients with primary open-angle glaucoma from a single-center nonrandomized study. J Glaucoma. 2021 Mar 1;30(3):281–286. doi: 10.1097/IJG.0000000000001734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durr G.M., Schlenker M.B., Samet S., Ahmed I.I.K. One-year outcomes of stand-alone ab externo SIBS microshunt implantation in refractory glaucoma. Br J Ophthalmol. 2020 Oct 23;0:1–9. doi: 10.1136/bjophthalmol-2020-317299. [DOI] [PubMed] [Google Scholar]
- 9.Gillmann K., Bravetti G.E., Mermoud A., Mansouri K. Anterior chamber XEN gel stent movements: the impact on corneal endothelial cell density. J Glaucoma. 2019 Jun;28(6):e93–e95. doi: 10.1097/IJG.0000000000001200. [DOI] [PubMed] [Google Scholar]
- 10.Lee E.-K., Yun Y.-J., Lee J.-E., Yim J.-H., Kim C.-S. Changes in corneal endothelial cells after Ahmed glaucoma valve implantation: 2-year follow-up. Am J Ophthalmol. 2009 Sep;148(3):361–367. doi: 10.1016/j.ajo.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 11.Lass J.H., Benetz B.A., He J., et al. Corneal endothelial cell loss and morphometric changes 5 Years after phacoemulsification with or without CyPass micro-stent. Am J Ophthalmol. 2019 Dec;208:211–218. doi: 10.1016/j.ajo.2019.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Arnavielle S., Lafontaine P.O., Bidot S., Creuzot-Garcher C., D'Athis P., Bron A.M. Corneal endothelial cell changes after trabeculectomy and deep sclerectomy. J Glaucoma. 2007 May;16(3):324–328. doi: 10.1097/IJG.0b013e3180391a04. [DOI] [PubMed] [Google Scholar]
- 13.Wirbelauer C., Wollensak G., Pham D.T. Influence of cataract surgery on corneal endothelial cell density estimation. Cornea. 2005 Mar;24(2):135–140. doi: 10.1097/01.ico.0000141234.48967.e3. [DOI] [PubMed] [Google Scholar]