Abstract
Ferulic acid (FA) and vanillic acid (VA) are considered as major phenolic metabolites of cyanidin 3-glucoside, a polyphenol that widely exists in plants that possess a protective effect against oxidative stress and inflammation in our previous study. This study aimed to investigate the effect of FA and VA on inflammation, gut barrier function, and growth performance in a weaned piglet model challenged with lipopolysaccharide (LPS). Thirty-six piglets (PIC 337 × C48, 28 d of age) were randomly allocated into 3 treatments with 6 replicate pens (2 piglets per pen). They were fed with a basal diet or a diet containing 4,000 mg/kg of FA or VA. Dietary supplementation of VA significantly increased average daily gain (ADG) (P < 0.05). Both FA and VA decreased serum levels of thiobarbituric acid reactive substances (TBARS), interlukin (IL)-1β, IL-2, IL-6, and tumor necrosis factor (TNF)-α (P < 0.05), and enhanced the expression of tight junction protein oclaudin (P < 0.05). Analysis of gut microbiota indicated that both FA and VA increased the Firmicutes/Bacteroidetes ratio alongside reducing the relative abundance of the Prevotellaceae family including Prevotella 9 and Prevotella 2 genera, but enriched the Lachoiraceaea family including the Lachnospiraceae FCS020 group (P < 0.05). Moreover, VA reduced the relative abundance of Prevotella 7 and Prevotella 1 but enriched Lachnospira, Eubacterium eligens group, and Eubacterium xylanophilum group (P < 0.05), while FA showed a limited effect on these genera. The results demonstrated that both VA and FA could alleviate inflammation and oxidative stress, but only VA has a significant positive effect on the growth performance of LPS-challenged piglets potentially through modulating gut microbiota.
Keywords: Ferulic acid, Vanillic acid, Inflammation, Gut barrier function, Growth performance, Piglet
1. Introduction
Polyphenols are secondary metabolites in plant products with multiple activities such as antioxidant, anti-inflammatory, and anti-bacterial activities (Xie et al., 2020; Wu et al., 2017; Daglia, 2012), and thus have the potential to replace antibiotics in the breeding industry (Fiore et al., 2020). Extensive studies have suggested that polyphenols like epigallocatechin gallate (Yan et al., 2020), resveratrol (Chen et al., 2021) (He et al., 2019) and proanthocyanidins (Chen et al., 2019) are able to alleviate oxidative stress and inflammatory response in pigs and poultry (Hu et al., 2019). Our previous studies have also revealed that Lonicera caerulea L. polyphenols, mainly cyanidin 3-glucoside (C3G), can attenuate inflammation in both high-fat diet and lipopolysaccharide (LPS)-induced mouse models (Wu et al., 2017, 2018). However, most polyphenols can be quickly degraded after ingestion with a poor absorption rate, and monophenol or phenolic acid metabolized by gut flora are regarded as the main bioactive compounds that enter circulation (Tan et al., 2019) (Wan et al., 2021). More than 20 kinds of C3G metabolites have been identified in blood circulation (de Ferrars et al., 2014b, de Ferrars et al., 2014a), while vanillic acid (VA), protocatechuic acid (PCA), ferulic acid (FA) and their derivates are considered as the potential bioactive metabolites against inflammation (Wu et al., 2019; Amin et al., 2015).
In the natural world, FA is usually joined with polysaccharides by covalent bonds in various plant cell walls like cereal bran, and regarded as the main bioactive compound of angelica sinensis, chuanxiong rhizoma, and ferula (Rosa et al., 2018). Vanillic acid is an oxidized form of vanillin produced during the conversion of vanillin into FA, and is one of the major components of “natural vanilla” aroma as well as the main bioactive compound of rhizoma picrorhizae (Rosa et al., 2018; Zhao and Moghadasian, 2008). Studies in vitro have demonstrated that FA, VA and PCA possess antioxidant and anti-inflammatory properties by directly bonding with protein kinase repressing nuclear factor kappa-B (NF-κB) signaling and activation of nuclear factor E2-related factor 2 (Nrf2) signaling in cell models (Wang et al., 2010; Yin et al., 2019; Kim et al., 2010). Considering the low absorption of polyphenol and phenolic metabolites will reach the hindgut, the biological activity of polyphenol appears to be closely linked with modulating gut microbiota, which is an essential part of the intestinal microenvironment. It is well-known that gut microbiota-derived metabolic substances like short-chain fatty acids and bacteriocin can promote gut development and prevent diarrhea (Donia and Fischbach, 2015), whereas gut microbiota disturbance can induce intestinal inflammation and barrier damage (Guevarra et al., 2018). Our previous study found that PCA may attenuate intestinal inflammation by modulating gut microbiota (Hu et al., 2020), and thus we hypothesized that VA and FA may have a similar effect due to their chemical structures. Therefore, in the present study, we aimed to clarify the effect of FA and VA on inflammation, gut barrier function and growth performance from the aspect of gut microbiota in the LPS-challenged weaned piglet model.
2. Materials and methods
The animal experiment protocol used for the present study was approved by the Hunan Agricultural University Institutional Animal Care and Use Committee.
2.1. Materials and reagents
Antibodies against occludin, claudin-1, zonula occludens 1 (ZO-1), and corresponding secondary antibodies were purchased from Proteintech Group, Inc. (Chicago, IL, USA). Ferulic acid (≥98%) and vanillic acid (≥98%) were provided by Shanghai Yuanye Bio-Technology Co., Ltd (Shanghai, China). Lipopolysaccharide (Escherichia coli Serotype O55:B5) was purchased from Sigma–Aldrich (St. Louis, MO, USA). The ingredients for the basal diet were provided by Hunan Liuyanghe Feedstuff Co., Ltd (Changsha, Hunan, China).
2.2. Experimental design and diets
Thirty-six weaned piglets (Pig Improvement Company line 337 × C48, 28 d of age) were randomly allocated into 3 treatments with 6 replicate pens (2 barrows per pen). Piglets were housed in fully slatted pens and had free access to feed and water. Piglets in the control group (CTL) were fed with a basal diet according to NRC (2012) (Table S1), while the other 2 treatments were fed a diet supplemented with 4,000 mg/kg of FA (FA group) or 4,000 mg/kg of VA (VA group), respectively, for 21 d. All piglets were challenged with LPS (10 μg/kg BW) on d 14 and 21 (2 h before sacrifice) through intraperitoneal injection based on our previous study (Hu et al., 2020).
2.3. Sample collections
On d 21 of the trial, 6 piglets (one from each replicate pen) from each group were sacrificed after being anesthetized by sodium pentobarbital (50 mg/kg BW). Blood samples (10 mL) were collected from the jugular vein into anticoagulant-free vacuum tubes and centrifuged at 1,500 ×g for 10 min to get the serum after standing at room temperature for 30 min. Cecal contents were collected immediately into sterile tubes and snap-frozen in liquid nitrogen before storage at −80 °C for further DNA extraction. Two ileum segments (around 2 cm long) were sampled and gently washed in normal saline; one was used for collecting mucosal scrapings for detecting the mRNA expressions of tight junction proteins, and another was immediately fixed in phosphate-buffered paraformaldehyde (4%, pH 7.6) for immunohistochemical staining.
2.4. Measurement of inflammatory cytokines and antioxidant indices in serum
The antioxidant indices and inflammatory cytokines were measured as described before (Yuan et al., 2020) (Wu et al., 2015). Briefly, serum levels of interleukin (IL)-1β, IL-2, IL-6, and tumor necrosis factor-α (TNF-α) were measured with respective ELISA kits (Elabscience Biotechnology Co., Ltd, Wuhan, Hubei, China) according to the manufacturer's instructions, while the activity of total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px), total antioxidant capacity (T-AOC) and the level of thiobarbituric acid reactive substances (TBARS) were determined in serum by using respective assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instructions.
2.5. Real-time PCR
The mRNA expression of tight junction proteins including ZO-1, occludin, and claudin-1 was determined in ileum mucosa by real-time quantitative PCR as described previously (Hu et al., 2020), β-actin was used as a housekeeping gene to normalize target gene transcript levels and the primers of genes (Sangon Biotech, Shanghai, China) are shown in Table S2. The PCR reactions were performed in a 20-μL total reaction volume, which included 10 μL of 2 × SybrGreen qPCR Master Mix (Thermo Scientific), 0.4 μL each of the forward and reverse primers (10 μmol/L), 2 μL of cDNA template, and 7.2 μL of sterilized water. The PCR was carried out on a LightCycler480 Real-Time PCR system (Rotkreuz, Switzerland). The thermal cycler parameters were as follows: 3 min at 95 °C, 45 cycles for 5s at 95 °C, 30 s at 6 °C. The stability of the β-actin genes was evaluated by measuring the fluctuation range of the Ct values.
2.6. Immunohistochemical staining
The immunohistochemical staining of ileum segments was performed as described in our previous study (Hu et al., 2020). In brief, sections of ileum were incubated with specific primary antibody (occludin, claudin-1 or ZO-1 with a dilution rate of 1:100) at 4 °C overnight, followed by incubation with corresponding HRP-conjugated secondary antibody at 37 °C for 30 min, and then immunostained with DAB chromagen before being counterstained with Hematoxylin (Sigma–Aldrich, St. Louis, MO, USA). Stained sections were observed by using a Motic BA210T microscope (Motic China Group Co. Ltd., Xiamen, Fujian, China).
2.7. DNA extraction and 16S rRNA sequencing
The extraction of DNA from cecal contents and 16S rRNA gene sequencing was performed as described previously (Hu et al., 2020; Yuan et al., 2020). The V3-4 hypervariable region of the bacterial 16S rRNA gene was amplified with the primers 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). For each cecum content, a 10-digit barcode sequence was added to the 5′ end of the forward and reverse primers (provided by Allwegene Technology Inc., Beijing, China). The PCR was carried out on a Mastercycler Gradient (Eppendorf, Germany) using 25 μL reaction volumes, containing 12.5 μL KAPA 2G Robust Hot Start Ready Mix, 1 μL Forward Primer (5 μmol/L), 1 μL Reverse Primer (5 μmol/L), 5 μL DNA (total template quantity is 30 ng), and 5.5 μL H2O. Cycling parameters were 95 °C for 5 min, followed by 28 cycles of 95 °C for 45 s, 55 °C for 50 s and 72 °C for 45 s with a final extension at 72 °C for 10 min. The PCR products were purified using a QIAquick Gel Extraction Kit (QIAGEN, Germany), quantified using Real Time PCR, and sequenced on Miseq platform at Allwegene Technology Inc., Beijing, China. After the run, image analysis, base calling and error estimation were performed using Illumina Analysis Pipeline Version 2.6, and then the dataset was analyzed using QIIME (Version 1.8.0).
2.8. Statistical analysis
Results were expressed as means ± SD. The significant differences between groups were analyzed by one-way analysis of variance tests, followed by Fisher's least significant difference (LSD) and Duncan's multiple range tests with the SPSS statistical program (SPSS19, IBM Corp., Armonk, NY, USA). Correlations were analyzed by using linear regression model and spearman correlation analysis. A probability of P < 0.05 was considered significant.
3. Results
3.1. The effect of FA and VA on growth performance of piglets
As shown in Table 1, supplementation of VA significantly increased the final body weight (BW) and ADG of piglets (P = 0.004), while FA showed no significant change, as compared with the CTL group. Both VA and FA showed no significant effect on the average daily feed intake (ADFI) and feed-to-gain ratio (F:G) of the piglets.
Table 1.
The effect of ferulic acid and vanillic acid on growth performance of lipopolysaccharide (LPS)-induced weaned piglets.
| Item | Treatments1 |
P-value | ||
|---|---|---|---|---|
| CTL | FA | VA | ||
| Initial BW, kg | 8.9 ± 0.15 | 8.85 ± 0.08 | 8.86 ± 0.04 | 0.680 |
| Final BW, kg | 17.17 ± 0.53b | 17.7 ± 0.51b | 18.39 ± 0.49a | 0.003 |
| ADG, g | 413.75 ± 30.64b | 442.92 ± 24.31b | 476.25 ± 24.02a | 0.004 |
| ADFI, g | 598.71 ± 5.48 | 615.46 ± 47.39 | 642.38 ± 21.78 | 0.449 |
| F:G | 1.45 ± 0.11 | 1.39 ± 0.08 | 1.35 ± 0.06 | 0.157 |
ADG = average daily gain; ADFI = average daily feed intake; F:G = feed-to-gain ratio.
a,b Data were shown as means ± SD (n = 12), values with different letters differ significantly (P < 0.05).
CTL, a basal diet; FA, a basal diet containing 4,000 mg/kg ferulic acid; VA, a basal diet supplemented with 4,000 mg/kg vanillic acid.
3.2. FA and VA attenuated inflammation and oxidative stress in piglets
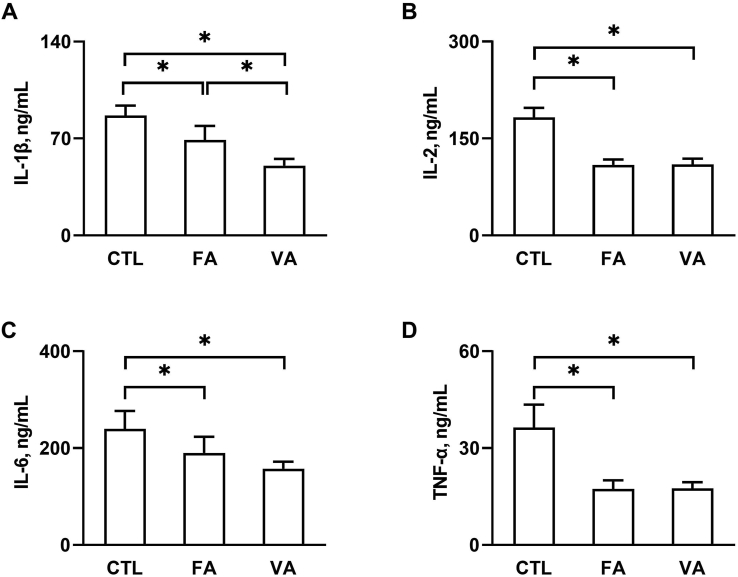
Serum cytokines including IL-1β, IL-2, IL-6 and TNF-α were measured to assess the inflammatory status in piglets. Fig. 1 shows that both FA and VA significantly decreased serum levels of IL-1β (Fig. 1A), IL-2 (Fig. 1B), IL-6 (Fig. 1C) and TNF-α (Fig. 1D) (P < 0.05 compared to the CTL group). There was no significant difference in the levels of IL-2, IL-6 and TNF-α between the VA and FA group, but the IL-1β level of the VA group was significantly lower than that of the FA group (P < 0.05).
Fig. 1.
The effect of ferulic acid (FA) and vanillic acid (VA) on the production of inflammatory cytokines. Serum levels of (A) IL-1β, (B) IL-2, (C) IL-6 and (D) TNF-α were measured by using ELISA kits. CTL, a basal diet; FA, a basal diet containing 4,000 mg/kg ferulic acid; VA, a basal diet supplemented with 4,000 mg/kg vanillic acid. Data were shown as means ± SD (n = 6) Single asterisk (∗) refers to a significant difference (P < 0.05).
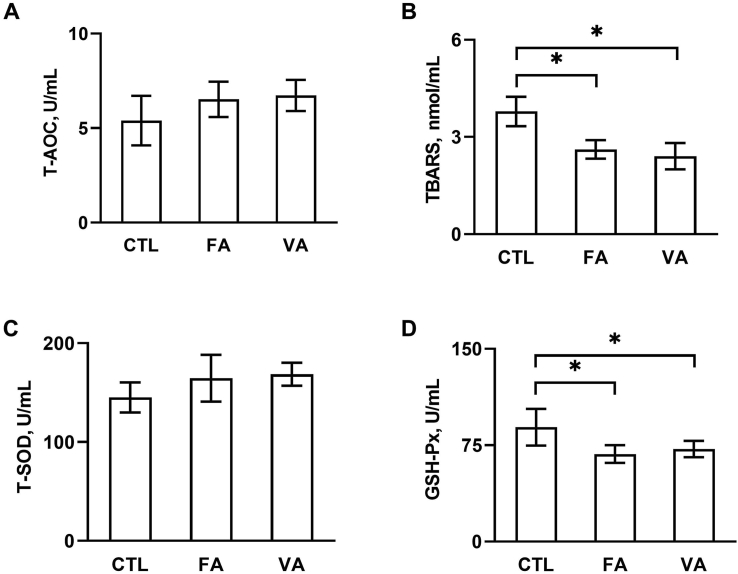
Antioxidant indicators including T-AOC, GSH-Px, T-SOD and TBARS, an end-product of lipid peroxidation, were measured to evaluate the oxidative status of piglets. As shown in Fig. 2, supplementation of FA or VA decreased the level of TBARS (Fig. 2B) and the activity of GSH-Px (Fig. 2D) (P < 0.05 compared to CTL group), however, both FA and VA showed no significant effect on T-AOC level (Fig. 2A) and T-SOD activity (Fig. 2C).
Fig. 2.
The Effect of ferulic acid (FA) and vanillic acid (VA) on antioxidant indicators. Serum levels of indicators including (A) T-AOC, (B) TBARS, (C) T-SOD and (D) GSH-Px were determined by using respective kits. CTL, a basal diet; FA, a basal diet containing 4,000 mg/kg ferulic acid; VA, a basal diet supplemented with 4,000 mg/kg vanillic acid. Data were shown as means ± SD (n = 6), Single asterisk (∗) refers to a significant difference (P < 0.05). T-AOC = total antioxidant capacity; TBARS = thiobarbituric acid reactive substances; T-SOD = total superoxide dismutase; GSH-Px = glutathione peroxidase.
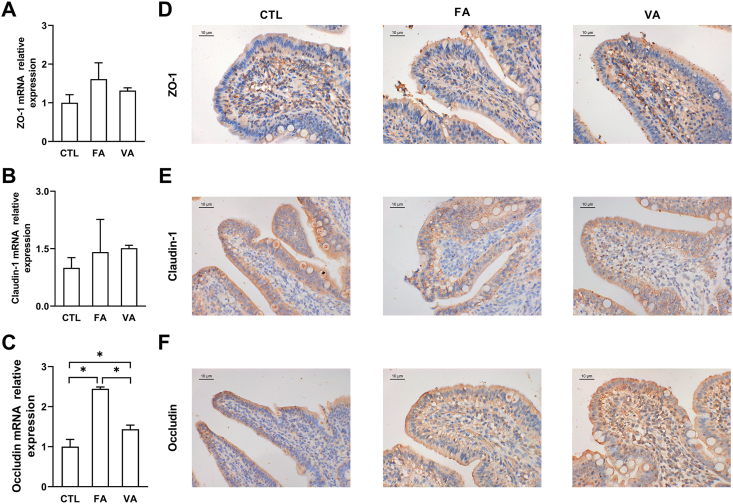
3.3. FA and VA enhanced the expression of tight junction protein occludin
The expression of tight junction proteins including ZO-1, occludin and claudin-1 were measured by Real-time PCR and immunohistochemical staining to assess the intestinal mechanical barrier function. As shown in Fig. 3, supplementation of FA or VA increased the mRNA (Fig. 3C) and protein (Fig. 3F) expression of occluding (P < 0.05), as compared with the CTL group. However, both FA and VA showed a limited effect on the expression of ZO-1 (Fig. 3A and D) and claudin-1 (Fig. 3B and E).
Fig. 3.
The effect of ferulic acid (FA) and vanillic acid (VA) on the expression of tight junction proteins. The mRNA expression of (A) zonula occludens 1 (ZO-1), (B) occludin, and (C) claudin-1 was quantitated by real-time PCR, and the protein expression of (D) ZO-1, (E) occludin, and (F) claudin-1 was detected by immunohistochemical staining. In real-time PCR, the data were shown as means ± SD (n = 6) of mRNA relative expressions. In immunohistochemical staining, the pictures shown were the representative of 6 individual stained sections photographed at 100× magnification, and tight junction proteins were stained yellow or brown-yellow. Scale bar, 10 μm. CTL, a basal diet; FA, a basal diet containing 4,000 mg/kg ferulic acid; VA, a basal diet supplemented with 4,000 mg/kg vanillic acid. Data were shown as means ± SD (n = 6). Single asterisk (∗) refers to a significant difference (P < 0.05).
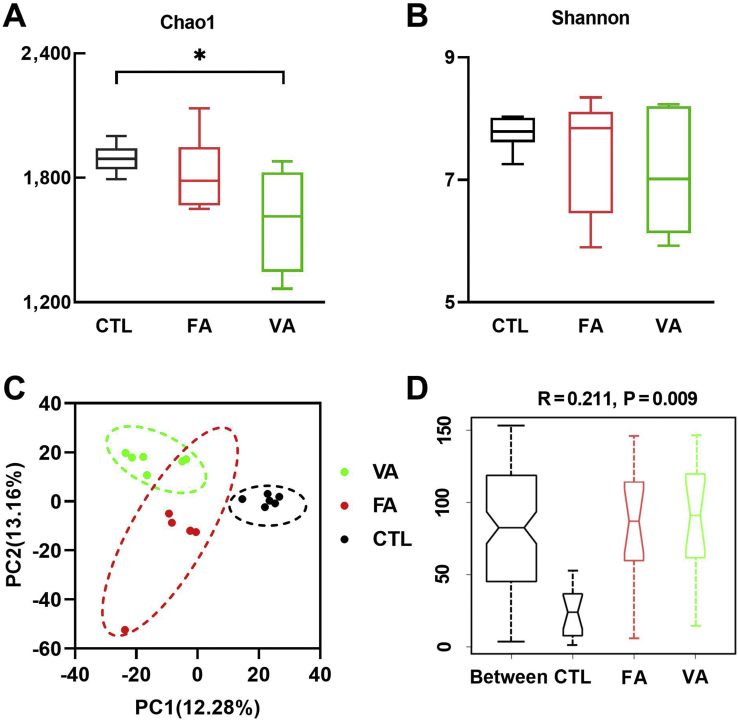
3.4. Modulation of gut microbiota by FA and VA
As microbes play a significant role in gut health and barrier function, cecal microbiota was characterized using 16S rRNA gene sequencing. As shown in Fig. 4, VA significantly decreased the Chao1 index (Fig. 4A) as compared with the CTL group (P < 0.05), while both VA and FA showed no significant effect on the Shannon index (Fig. 4B). However, the principal component analysis (Fig. 4C) and analysis of similarities (ANOSIM) (Fig. 4D) revealed that VA and FA had a significant effect on the composition of cecal microbiota, and there was an apparent difference between the clusters of the FA, VA and CTL groups.
Fig. 4.
The effect of ferulic acid (FA) and vanillic acid (VA) on cecal microbial community. The effect of FA and VA on (A) the Chao1 index and (B) Shannon index. (C) Principal component analysis ordination plots of microbial communities in the CTL, FA and VA groups based on the Bray–Curtis distance metric. (D) Analysis of similarities (ANOSIM) between CTL, FA and VA groups. CTL, a basal diet; FA, a basal diet containing 4,000 mg/kg ferulic acid; VA, a basal diet supplemented with 4,000 mg/kg vanillic acid. Data were shown as means ± SD (n = 6). Single asterisk (∗) refers to a significant difference (P < 0.05).
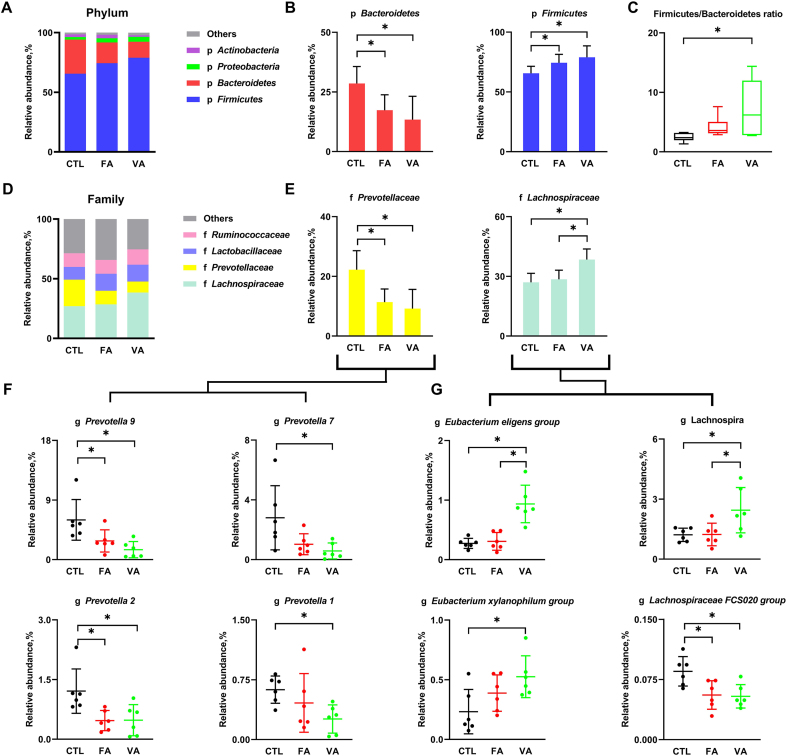
At the phylum level, both FA and VA decreased the relative abundance of Bacteroidetes and increased the relative abundance of Firmicutes as compared with the CTL group (P < 0.05) (Fig. 5A and B), but only VA had a significant positive effect on the Firmicutes to Bacteroidetes ratio (Fig. 5C). At the family level, both FA and VA decreased the relative abundance of Prevotellaceae (P < 0.05), whereas only VA, but not FA, increased the relative abundance of Lachnospirceae significantly (Fig. 5D and E). Further analysis at the genus level showed that the relative abundance of Prevotella 9, Prevotella 7, Prevotella 2 and Prevotella 1, which belong to the family of Prevotellaceae, were significantly down-regulated by VA, while only Prevotella 9 and Prevotella 2 were reduced significantly by FA (Fig. 5F). On the other hand, VA, but not FA, significantly up-regulated the relative abundance of genera belong to the family of Lachnospirceae including Eubacterium eligens group, Lachnospira and Eubacterium xylanophium group, although both VA and FA increased the relative abundance of Lachnospiraceae FCS020 group (P < 0.05) (Fig. 5G).
Fig. 5.
Modulation of gut microbiota by ferulic acid (FA) and vanillic acid (VA). (A) The relative abundance of cecal microbial phyla (mean of each group), (B) the effect of FA and VA on the relative abundance of Bacteroidetes and Firmicutes, and (C) the ratio of Firmicutes to Bacteroidetes in each group. (D) The relative abundance of cecal microbial family (mean of each group), and (E) the effect of FA and VA on the relative abundance of microbial family including Prevotellaceae and Lachoiraceaea, as well as genera including (F) prevotella 9, prevotella 7, prevotella 2 and prevotella 1 which belong to Prevotellaceae, and (G) Eubacterium eligens group, Lachnospira, Eubacterium xylanophium group, and Lachnospiraceae FC020 group which belong to Lachoiraceaea were analyzed by 16S rRNA gene sequencing. CTL, a basal diet; FA, a basal diet containing 4,000 mg/kg ferulic acid; VA, a basal diet supplemented with 4,000 mg/kg vanillic acid. Data were shown as means ± SD (n = 6). Single asterisk (∗) refers to a significant difference (P < 0.05).
3.5. Correlation analysis between serum indicators and altered microbiota
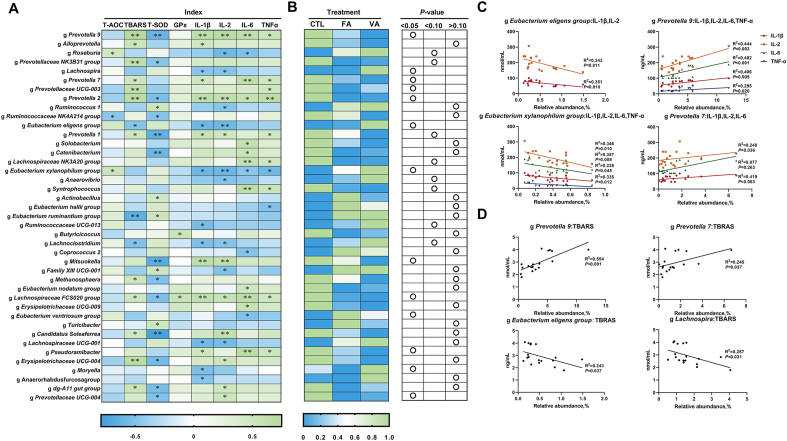
To further understand the role of whole gut microbiota in regulating oxidative and inflammatory status, genera that had a relative abundance of greater than 0.05% were chosen to process the correlation analysis with serum indicators. As shown in Fig. 6A, a total number of 50 microbial genera were significantly correlated with the levels of antioxidant indicators and cytokines. Based on the crosscheck with the ANOVA and spearman's correlation results (Fig. 6B), 13 genera were selected to perform linear regression analyses. As shown in Fig. 6C, E. eligens group had a negative linear correlation with IL-1β, IL-2, IL-6 and TNF-α (P < 0.05), E. xylanophium group had a negative linear correlation with IL-1β and IL-2 (P < 0.05), Prevotella 9 had a positive linear correlation with the IL-1β, IL-2, IL-6 and TNF-α (P < 0.05), while Prevotella 7 had a positive linear correlation with the IL-1β, IL-2 and IL-6 (P < 0.05). Moreover, Fig. 6D shows that Prevotella 9 and Prevotella 7 had a positive linear correlation with the TBARS level (P < 0.05), while E. eligens group and Lachnospira had a negative linear correlation with the TBARS level (P < 0.05).
Fig. 6.
Correlation analysis between serum indicators and altered microbiota. (A) Heatmap of Spearman's correlation between anti-oxidant indicators, inflammatory cytokines and gut microbiota. (B) Heatmap of the significant differences of genera correlated with the antioxidant indicators and cytokines levels. (C) Linear regression analyses between inflammatory cytokines and genera. (D) Linear regression analyses between anti-oxidant indicators and genera. Significant correlations were marked by ∗ P < 0.05, ∗∗ P < 0.01.
4. Discussion
Intestinal barrier injury, disturbed gut microbiota, inflammation and oxidative damage are severe problems around weaning and usually lead to impaired growth performance and increased mortality of weaning piglets (Campbell et al., 2013). The intestinal tract is perilously juxtaposed between the external environment and the host immune system, and can be easily influenced by dietary components (Fan and Pedersen, 2021). Intestinal epithelial damage induced by oxidative stress and inflammation is one of the leading causes of diarrhea in weaning piglets (Campbell et al., 2013; Smith et al., 2010). Our previous study found that PCA could relieve intestinal inflammation and oxidative damage in weaned piglets (Hu et al., 2020). Vanillic acid is a PCA methylation of the 4′-hydroxy before being converted to FA by microbial activity (Segura et al., 1999). Pharmacokinetics study revealed that VA and FA showed a much higher serum concentration than C3G and PCA after the consumption of C3G (de Ferrars et al., 2014a), which suggesting that VA and FA are metabolites that can be easily absorbed and are suitable for application. In vitro antioxidant and free radical scavenging assay results have shown that both FA and VA possess significant DPPH free radical scavenging abilities (Mathew et al., 2015). However, the present study indicated that supplementation of VA but not FA has a significant positive effect on the growth performance of weaned piglets. The higher ADFI of the VA group might be one of the potential reasons, as VA has a unique fragrance and its precursor in the chemical industry, known as vanillin, has been used as a phagostimulant in the swine industry (Chen et al., 2017). Serum indicators indicated that both FA and VA decreased the TBARS level, a biomarker of oxidative stress, but showed limited effects on T-AOC and T-SOD. Interestingly, the activity of GSH-Px, a major antioxidant enzyme, was significantly decreased by both VA and FA. It may have been caused by the changed gut microbiota, which has been considered to be closely correlated with the level of oxidative stress in the body (Cao, 2018), and our results revealed that Prevotella 2, a genus that significantly correlated with GSH-Px activity (Hu et al., 2020), was down-regulated by both FA and VA.
Weaning is associated with an early inflammatory response, which presents increased pro-inflammatory cytokines like IL-1β, IL-2, IL-6, and TNF-α after weaning (Pie et al., 2004). Both LPS and pro-inflammatory cytokines can trigger the activation of pro-inflammatory transcription factors such as NF-κB and exacerbate not only inflammatory reactions but also impair intestinal integrity (Dong et al., 2020). Our previous study found that C3G, the parent polyphenol of FA and VA, can directly bind to transforming growth factor β activated kinase-1 (TAK1) and inhibit the activation of NF-κB (Wu et al., 2017). Another in vitro study also revealed that FA could reduce LPS-induced pro-inflammatory cytokines (IL-1β, IL-2, TNF-α) by inhibiting NF-κB and mitogen-activated protein kinase (MAPK) signaling pathways in bovine endometrial epithelial cells (Yin et al., 2019). In this study, both FA and VA significantly decreased the levels of IL-1β, IL-2, IL-6, and TNF-α, which may partially explain the enhanced expression of Oclaudin since the overproduction of IL-6 and TNF-α was thought to have participated in enhancing intestinal epithelial permeability (Hu et al., 2013).
It is widely acknowledged that gut microbiota possesses enzymatic and metabolic activities that involve the body's metabolism and affect the health status of the host (Vrieze et al., 2012; Tremaroli and Backhed, 2012). A recent study has suggested that gut microbiota variation may be an essential mechanism leading to intestinal inflammation (Tilg et al., 2020). Gut microbiota changes dramatically after weaning (Guevarra et al., 2018), and disturbed gut microbiota is the main effect of weaning on piglets to cause intestinal system dysfunctions and result in intestinal barrier injury, intestinal inflammation, and oxidative damage (Campbell et al., 2013). Our previous studies have demonstrated that polyphenols may exhibit their biological function by regulating gut microbiota (Wu et al., 2018; Hu et al., 2020). In this study, VA and FA showed a different effect on the growth performance of piglets, although they have similar chemical structures. It might be due to their different influences on gut microbiota, in which only VA significantly increased the Firmicutes/Bacteroides ratio, an index that represents a highly efficient absorption of calories and subsequent weight gain due to Firmicutes, which are more effective as an energy source than Bacteroidetes (Koliada et al., 2017). Moreover, the family of Lachnospiraceae, one of the producers of short-chain fatty acids (Chen et al., 2019), was enriched by VA but not FA, although Prevotellaceae, a family dramatically increased after weaning (Guevarra et al., 2018), has been inhabited by both FA and VA. Based on correlation analysis at the genus level, genera that belong to the Lachnospiraceae are negatively correlated with the inflammatory cytokines (E. eligens group and Eubacterium xylanophilum group) and TBARS level (E. eligens group and Lachnospira). Other studies have also indicated that E. eligens group and E. xylanophilum group are considered as lactate-utilizing butyrate producers, which can promote the production of the anti-inflammatory cytokine IL-10 (Venkataraman et al., 2016; Sun et al., 2018), and Lachnospira has been shown to be in low abundance in Crohn's disease patients (Forbes et al., 2018). On the other hand, Prevotella 9 and Prevotella 7, belonging to the Prevotellaceae family, were positively correlated with inflammatory cytokines and TBARS level. Prevotella, which has been reported to be highly associated with levels of inflammation, was increased by a carbohydrate-based diet from nursed to weaned piglets (Zhang et al., 2019; Wu et al., 2011). These results suggested that the gut microbes up-regulated by FA and VA like Lachnospira, E. eligens group and E. xylanophilum group could have potential benefits to piglets, while the Prevotellaceae family might be a target for alleviation of weaning stress response in the piglet.
5. Conclusion
Dietary supplementation of VA has a positive effect on the growth performance of weaned piglets. Supplementation of FA or VA can alleviate oxidative stress (TBARS), reduce the level of pro-inflammatory cytokines (IL-1β, IL-2, IL-6 and TNF-α), and increase the expression of tight junction protein oclaudin in LPS-challenged weaned piglets. An analysis of gut microbiota reveals that VA enriched genera belong to the Lachnospiraceae family, including genera of Lachnospira, E. eligens group and E. xylanophilum group, which are negatively correlated with the inflammatory cytokines and TBARS levels. In addition, VA down-regulated the relative abundance of Prevotellaceae, including genera of Prevotella 9, Prevotella 7, Prevotella 2 and Prevotella 1, which are positively correlated with inflammatory cytokines and TBARS levels, whereas FA only down-regulated Prevotella 9 and Prevotella 2.
Author contributions
Ruizhi Hu and Shusong Wu are the primary investigators in this study. Baizhen Li, Jijun Tan, Jiahao Yan and Ying Wang participated in the animal experiments. Zhiyi Tang, Ming Liu and Chenxing Fu participated in sample analysis and statistical data analysis. Hongfu Zhang and Jianhua He revised the manuscript. Shusong Wu designed this study and wrote the manuscript as corresponding author.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
This work was partially supported by the funds from the National Natural Science Foundation of China (31741115, 32102578), Fellowship of China Postdoctoral Science Foundation (2021T140715), Hunan Provincial Natural Science Foundation for Distinguished Young Scholars (2019JJ30012), and Scientific Research Fund of Hunan Provincial Education Department (18B098).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Appendix
The appendix to this article can be found online at https://doi.org/10.1016/j.aninu.2021.06.009.
Supplementary data
The following is the supplementary data to this article:
References
- Amin H., Czank C., Raheem S., Zhang Q., Botting N., Cassidy A., et al. Anthocyanins and their physiologically relevant metabolites alter the expression of il-6 and vcam-1 in cd40l and oxidized ldl challenged vascular endothelial cells. Mol Nutr Food Res. 2015;59:1095–1106. doi: 10.1002/mnfr.201400803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J., Crenshaw J., Polo J. The biological stress of early weaned piglets. J Anim Sci Biotechnol. 2013;4:4. doi: 10.1186/2049-1891-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S. Cellular stress responses and gut microbiota in inflammatory bowel disease. Gastroent Res Pract. 2018;2018:7192646. doi: 10.1155/2018/7192646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Wu P., Cai Z., Fang Y., Zhou H., Lasanajak Y., et al. Puerariae lobatae radix with chuanxiong rhizoma for treatment of cerebral ischemic stroke by remodeling gut microbiota to regulate the brain-gut barriers. J Nutr Biochem. 2019;65:101–114. doi: 10.1016/j.jnutbio.2018.12.004. [DOI] [PubMed] [Google Scholar]
- Chen M., Chen X., Nsor-Atin D., Masamba K., Ma J., Zhong F. Optimization of key aroma compounds for dog food attractant[J] Anim Feed Sci Technol. 2017;225:173–181. [Google Scholar]
- Chen X., Zeng Z., Huang Z., Chen D., He J., Chen H., et al. Effects of dietary resveratrol supplementation on immunity, antioxidative capacity and intestinal barrier function in weaning piglets. Anim Biotechnol. 2021;2:240–245. doi: 10.1080/10495398.2019.1683022. [DOI] [PubMed] [Google Scholar]
- Daglia M. Polyphenols as antimicrobial agents. Curr Opin Biotechnol. 2012;23:174–181. doi: 10.1016/j.copbio.2011.08.007. [DOI] [PubMed] [Google Scholar]
- de Ferrars R., Cassidy A., Curtis P., Kay C. Phenolic metabolites of anthocyanins following a dietary intervention study in post-menopausal women. Mol Nutr Food Res. 2014;58:490–502. doi: 10.1002/mnfr.201300322. [DOI] [PubMed] [Google Scholar]
- de Ferrars R., Czank C., Zhang Q., Botting N., Kroon P., Cassidy A., et al. The pharmacokinetics of anthocyanins and their metabolites in humans. Br J Pharmacol. 2014;171:3268–3282. doi: 10.1111/bph.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong N., Xue C., Zhang L., Zhang T., Wang C., Bi C., et al. Oleanolic acid enhances tight junctions and ameliorates inflammation in salmonella typhimurium-induced diarrhea in mice via the tlr4/nf-kappa b and mapk pathway. Food Funct. 2020;11:1122–1132. doi: 10.1039/c9fo01718f. [DOI] [PubMed] [Google Scholar]
- Donia M., Fischbach M. Small molecules from the human microbiota. Science. 2015;349 doi: 10.1126/science.1254766. 1254766-1254766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;1:55–71. doi: 10.1038/s41579-020-0433-9. [DOI] [PubMed] [Google Scholar]
- Fiore M., Messina M., Petrella C., D'Angelo A., Greco A., Ralli M., et al. Antioxidant properties of plant polyphenols in the counteraction of alcohol-abuse induced damage: impact on the mediterranean diet. J Funct Foods. 2020;71:104012. [Google Scholar]
- Forbes J., Chen C., Knox N., Marrie R., El-Gabalawy H., de Kievit T., et al. A comparative study of the gut microbiota in immune-mediated inflammatory diseases-does a common dysbiosis exist? Microbiome. 2018;6:221. doi: 10.1186/s40168-018-0603-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevarra R., Hong S., Cho J., Kim B., Shin J., Lee J., et al. The dynamics of the piglet gut microbiome during the weaning transition in association with health and nutrition. J Anim Sci Biotechnol. 2018;9:9. doi: 10.1186/s40104-018-0269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S., Li S., Arowolo M., Yu Q., Chen F., Hu R., et al. Effect of resveratrol on growth performance, rectal temperature and serum parameters of yellow-feather broilers under heat stress. Anim Sci J. 2019;90:401–411. doi: 10.1111/asj.13161. [DOI] [PubMed] [Google Scholar]
- Hu C., Xiao K., Luan Z., Song J. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J Anim Sci. 2013;91:1094–1101. doi: 10.2527/jas.2012-5796. [DOI] [PubMed] [Google Scholar]
- Hu R., He Z., Liu M., Tan J., Zhang H., Hou D., et al. Dietary protocatechuic acid ameliorates inflammation and up-regulates intestinal tight junction proteins by modulating gut microbiota in lps-challenged piglets. J Anim Sci Biotechnol. 2020;11:92. doi: 10.1186/s40104-020-00492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R., He Y., Arowolo M., Wu S., He J. Polyphenols as potential attenuators of heat stress in poultry production. Antioxidants. 2019;8:11. doi: 10.3390/antiox8030067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Kim M., Um J., Hong S. The beneficial effect of vanillic acid on ulcerative colitis. Molecules. 2010;15:7208–7217. doi: 10.3390/molecules15107208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliada A., Syzenko G., Moseiko V., Budovska L., Puchkov K., Perederiy V., et al. Association between body mass index and firmicutes/bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017;17:6. doi: 10.1186/s12866-017-1027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew S., Abraham T., Zakaria Z. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J Food Sci Tech Mys. 2015;52:5790–5798. doi: 10.1007/s13197-014-1704-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC . Nutrient requirements of swine. 11th ed. National Academic Press; Washington, DC: 2012. [Google Scholar]
- Pie S., Lalles J., Blazy F., Laffitte J., Seve B., Oswald I. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J Nutr. 2004;134:641–647. doi: 10.1093/jn/134.3.641. [DOI] [PubMed] [Google Scholar]
- Rosa L., Jordao N., Soares N., de Mesquita J., Monteiro M., Teodoro A. Pharmacokinetic, antiproliferative and apoptotic effects of phenolic acids in human colon adenocarcinoma cells using in vitro and in silico approaches. Molecules. 2018;23:2569. doi: 10.3390/molecules23102569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura A., Bunz P., D'Argenio D., Ornston L. Genetic analysis of a chromosomal region containing vana and vanb, genes required for conversion of either ferulate or vanillate to protocatechuate in acinetobacter. J Bacteriol. 1999;181:3494–3504. doi: 10.1128/jb.181.11.3494-3504.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith F., Clark J., Overman B., Tozel C., Huang J., Rivier J., et al. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am J Physiol-Gastr L. 2010;298:G352–G363. doi: 10.1152/ajpgi.00081.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M., Wu W., Chen L., Yang W., Huang X., Ma C., et al. Microbiota-derived short-chain fatty acids promote th1 cell il-10 production to maintain intestinal homeostasis. Nat Commun. 2018;9:3555. doi: 10.1038/s41467-018-05901-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J., Li Y., Hou D., Wu S. The effects and mechanisms of cyanidin-3-glucoside and its phenolic metabolites in maintaining intestinal integrity. Antioxidants. 2019;8:479. doi: 10.3390/antiox8100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H., Zmora N., Adolph T., Elinav E. The intestinal microbiota fuelling metabolic inflammation. Nat Rev Immunol. 2020;20:40–54. doi: 10.1038/s41577-019-0198-4. [DOI] [PubMed] [Google Scholar]
- Tremaroli V., Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- Venkataraman A., Sieber J., Schmidt A., Waldron C., Theis K., Schmidt T. Variable responses of human microbiomes to dietary supplementation with resistant starch. Microbiome. 2016;4:33. doi: 10.1186/s40168-016-0178-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze A., Van N., Holleman F., Salojarvi J., Kootte R., Bartelsman J., et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–916. doi: 10.1053/j.gastro.2012.06.031. e7. [DOI] [PubMed] [Google Scholar]
- Wan M., Co V., El-Nezami H. Dietary polyphenol impact on gut health and microbiota. Crit Rev Food Sci Nutr. 2021;61:690–711. doi: 10.1080/10408398.2020.1744512. [DOI] [PubMed] [Google Scholar]
- Wang D., Wei X., Yan X., Jin T., Ling W. Protocatechuic acid, a metabolite of anthocyanins, inhibits monocyte adhesion and reduces atherosclerosis in apolipoprotein e-deficient mice. J Agric Food Chem. 2010;58:12722–12728. doi: 10.1021/jf103427j. [DOI] [PubMed] [Google Scholar]
- Wu G., Chen J., Hoffmann C., Bittinger K., Chen Y., Keilbaugh S., et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Hu R., Nakano H., Chen K., Liu M., He X., et al. Modulation of gut microbiota by lonicera caerulea l. Berry polyphenols in a mouse model of fatty liver induced by high fat diet. Molecules. 2018;23:3213. doi: 10.3390/molecules23123213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Hu R., Tan J., He Z., Liu M., Li Y., et al. Abstract wp534: cyanidin 3-glucoside and its metabolites protect against nonalcoholic fatty liver disease: crosstalk between serum lipids, inflammatory cytokines and mapk/erk pathway. Stroke. 2019;50 AWP534-AWP534. [Google Scholar]
- Wu S., Yano S., Chen J., Hisanaga A., Sakao K., He X., et al. Polyphenols from lonicera caerulea l. Berry inhibit lps-induced inflammation through dual modulation of inflammatory and antioxidant mediators. J Agric Food Chem. 2017;65:5133–5141. doi: 10.1021/acs.jafc.7b01599. [DOI] [PubMed] [Google Scholar]
- Wu S., He X., Wu X., Qin S., He J., Zhang S., et al. Inhibitory effects of blue honeysuckle (lonicera caerulea l) on adjuvant-induced arthritis in rats: crosstalk of anti-inflammatory and antioxidant effects. J Funct Foods. 2015;17:514–523. [Google Scholar]
- Xie K., He X., Chen K., Sakao K., Hou D. Ameliorative effects and molecular mechanisms of vine tea on western diet-induced nafld. Food Funct. 2020;11:5976–5991. doi: 10.1039/d0fo00795a. [DOI] [PubMed] [Google Scholar]
- Yan Z., Zhong Y., Duan Y., Chen Q., Li F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim Nutr. 2020;6:115–123. doi: 10.1016/j.aninu.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin P., Zhang Z., Li J., Shi Y., Jin N., Zou W., et al. Ferulic acid inhibits bovine endometrial epithelial cells against lps-induced inflammation via suppressing nk-kappab and mapk pathway. Res Vet Sci. 2019;126:164–169. doi: 10.1016/j.rvsc.2019.08.018. [DOI] [PubMed] [Google Scholar]
- Yuan X., Yan J., Hu R., Li Y., Wang Y., Chen H., et al. Modulation of gut microbiota and oxidative status by β-carotene in late pregnant sows. Front Nutr. 2020;7:612875. doi: 10.3389/fnut.2020.612875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Liu H., Wang S., Zhang W., Wang J., Tian H., et al. Fecal microbiota and its correlation with fatty acids and free amino acids metabolism in piglets after a lactobacillus strain oral administration. Front Microbiol. 2019;10:785. doi: 10.3389/fmicb.2019.00785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Moghadasian M. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: a review. Food Chem. 2008;109:691–702. doi: 10.1016/j.foodchem.2008.02.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.