Abstract
Introduction
The prevalence of Primary ovarian insufficiency (POI) is estimated to be 1–2%, resulting from many causes, including, iatrogenic causes which are becoming more common.
There is no precise treatment to restore fertility in POI patients. However, new treatments -such as ovarian rejuvenation using platelet-rich autologous plasma (PRP)- are being tested and have shown promising results. We report using a new PRP injection protocol to manage a Syrian patient with ovarian insufficiency.
Case presentation
A 35-year-old woman with five years of primary infertility presented with decreased anti-mullerian hormone (AMH) after she underwent a laparoscopy one year ago. Where the AMH dropped from 1.07 to 0.39 ng/mL after it. She underwent an ovarian rejuvenation using PRP. Half mL of PRP was injected into every ovary, 2 mL were injected into the cervix, 7 mL intra-uterus, and 7 mL were injected intramuscularly to the patient's legs. Fifteen days following the operation, the patient's new AMH level was 0.94 ng/mL. The patient was placed on ovarian stimulation, and five days later the ultrasonography showed the development of six follicles in each ovary.
Discussion
Managing POI using ovarian rejuvenation is the best alternative treatment; When the donor eggs programs are not acceptable. The use of PRP in ovarian rejuvenation has been reported to be effective.
Conclusion
The use of PRP was beneficial in restoring fertility in a Syrian patient with ovarian insufficiency. Furthermore, the new PRP injection protocol was successful. However, more studies are needed to confirm this result.
Keywords: Platelet-rich plasma, Iatrogenic, Primary ovarian insufficiency, Treatment, Diminished ovarian reserve, Premature ovarian failure, Infertility, Ovarian insufficiency
Highlights
-
•
Primary ovarian insufficiency effect 1–2% of all women.
-
•
It is a major problem in countries where donor eggs programs are not acceptable.
-
•
Ovarian rejuvenation using platelet-rich plasma showed promising results.
-
•
The combination of generalized and localized effects may enhance the outcomes.
1. Background
Primary ovarian insufficiency (POI), an early occurrence of menopause, is defined as the premature disturbance of menstruation and ovulation before the age of 40 [1]. The prevalence of POI is estimated to be 1–2% [2,3], and most of these cases are idiopathic [4]. Other causes include; Genetic, iatrogenic, and autoimmune causes. Moreover, iatrogenic causes such as surgery are becoming more common due to the increased use of laparoscopy [4].The high level of follicle-stimulating hormone (FSH) in the blood can confirm the diagnosis [4]. In POI patients, anti-mullerian hormone (AMH) is usually low, reflecting the eggs’ reservoir in the ovaries [5].
As far as we know, there is no precise treatment to restore fertility in POI patients [4]. However, many studies suggested treatments with promising results; Such as ovarian rejuvenation using either stem cell therapy [6] or platelet-rich autologous plasma (PRP) [7]. Nevertheless, the donor eggs programs are still the best choice for POI patients who wish to become pregnant [4].
PRP is a plasma separated from peripheral blood by centrifugation; it contains a high concentration of platelets [8]. When the platelets are activated, they release growth factors that induce proliferation, growth, and differentiation [9]. PRP was used to treat several medical cases such as musculoskeletal injuries and illnesses [10,11]. On the other hand, it was found to have the ability to activate the follicles in POI patients [12].
The injection of PRP directly to the ovaries through laparoscopy was reported to be beneficial, as the serum level of AMH significantly increased [13]. However, further studies are still required. Nevertheless, this study reports the first attempt to manage a Syrian patient with ovarian insufficiency using a new PRP injection protocol. This case report has been reported in line with the SCARE Criteria [14].
1.1. Case presentation
A 35-year-old patient presented to our fertility clinic at the University Hospital of Obstetrics and Gynecology in Damascus Syria complaining of primary infertility for five years, with recurrent pregnancy loss, and many previous ovulation induction attempts. She has a regular 28 days menstrual cycle. The patient had a history of well-tolerated systemic lupus erythematosus (SLE), along with well-managed hypothyroidism since January 2021. She was diagnosed with idiopathic tubal adhesions one year ago and underwent laparoscopy as a treatment for the adhesions with no further information about the procedure. Before the laparoscopy, her AMH level was 1.07 ng/mL, with no further relevant information in the patient's medical history.
A whole new assessment was carried out, the physical and gynecological exams were within normal range with a regular menstrual cycle, the Ultrasonography of the ovaries showed no remarkable features, and the Hysterosalpingography showed open fallopian tubes. The lab test showed an AMH of 0.39 ng/mL; other lab tests were within the normal range including the FSH and Estradiol (E2), lupus anticoagulant (LAC), anti-cardiolipin antibodies, anti-β2-glycoprotein antibodies, and no male factor was found.
After explaining the procedure and taking the patient's consent, she underwent an ovarian rejuvenation using PRP. Twenty cm of blood were extracted from the patient and placed on two stages of centrifugation. The first one was on 1500 revolutions per minute (RPM) for 15 minutes, and the second one was on 2000 RPM for 10 minutes. Following that, the PRP was treated with multiple vitamins and other supplements, and about 20 mL of PRP was collected. The patient underwent a laparoscopy in which 0.5 mL of PRP was injected into each ovary, 2 mL were injected into the cervix, and 7 mL intra-uterus. Furthermore, 7 mL was injected intramuscularly into the patient's thighs. The operation was performed by Dr. Haitham Abbassi, and it went well, with no complications.
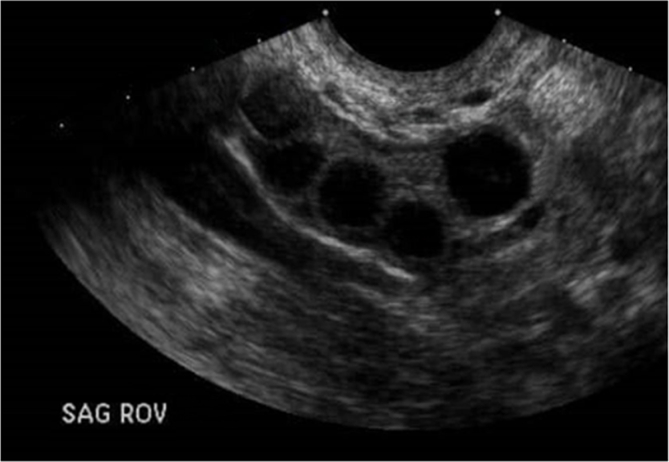
After the operation, a close follow-up was done. Fifteen days following the operation, the patient's new AMH level reached 0.94 ng/mL. After that, the patient was placed on ovarian stimulation using 100 Mg of clomiphene citrate. Five days after the stimulation, the Ultrasonography showed the development of six follicles in each ovary (Fig. 1). A planed in nitro fertilization (IVF) was planed. However, we lost contact with the patient.
Fig. 1.
Transvaginal ultrasound of the Right ovary.
Fig. 1 legend: the ecography shows the development of four follicles ranged between 1.3 and 1.5 cm in diameter.
2. Discussion
POI is the inability of the ovaries to produce enough estrogen or release eggs before the age of 40 [15]. The prevalence of POI is about 1–2% [2,3]. POI occurs due to; Genetic causes like Turner syndrome and Fragile X syndrome, autoimmune diseases, toxins, and iatrogenic causes, while the rest are considered idiopathic [4,16]. The iatrogenic causes are becoming more common due to the increase in the use of laparoscopy and Chemo-radiotherapy, as they reached up to 37% of POI cases in New Zealand [4].
Women with POI usually complain of infertility and other symptoms related to the drop in estrogens level, like irregular or skipped periods, hot flashes, vaginal dryness, and other symptoms [15]. However, women in the early stage of POI may only complain of unexplained infertility or repeated in vitro fertilization (IVF) failures along with normal menses (occult POI) [17]. In our case, the patient's POI was probably iatrogenic induced since the AMH levels dropped dramatically after the procedure. However, a laparoscopy for lysing adhesions won't cause an iatrogenic POI, but since no information about the procedure is available we can not be sure if the POI is iatrogenic or not. The patient complained only of five-year infertility which might be SLE induced. Nevertheless, the rapid drop in AMH levels can not be attributed to the patient's SLE.
The typical diagnosis of POI is usually made after four to six months of amenorrhea in women under the age of 40 years, along with the changes in estradiol and FSH [4]. On the other hand, measuring AMH in diagnosing POI is becoming more common, since it has a high sensitivity to assess the ovarian reservoir. It is usually assessed before and after the surgery, or chemotherapy as a screening procedure for POI [5,17,18]. Our patient underwent a curative laparoscopy, in which she suffered from a significant drop of AMH afterward. Even though she did not meet the diagnosis criteria of POI, she was managed as POI according to her AMH level since it can detect the early stages of POI; In which the FSH and E2 are still within the normal range.
Patients with POI still have a 5–10% chance to conceive spontaneously [19]. On the other hand, there are no guidelines that address the fertility management of POI [4]. Many studies assessed the efficacy of clomiphene, gonadotropins, gonadotropin-releasing hormone (GnRH) agonists and antagonists, glucocorticoids, and menopause hormone therapy [20]. While other studies tested the efficacy of ovarian rejuvenation using stem cell therapy [6,21], and PRP [7]. Nevertheless, the use of donor eggs is still the first-line treatment so far [4,15,22]. In our case, the donor egg was not an option due to religious rejection of such a procedure. After discussing the possible options with the patient, she agreed to go with ovarian rejuvenation with PRP.
PRP is plasma with high platelet concentration, it is created from a patient's autologous blood by centrifugation [23]. It was first known in the 1970s [24], where thrombocytes were found to have a role in cell proliferation [25]. Many centrifugation methods were mentioned in the literature [25], and a similar centrifugation method was used on this patient blood.
The use of PRP injections is effective and safe [7], and many studies pointed at the efficacy of using PRP in ovarian rejuvenation, where it successfully activated the follicles [26,27]. Other studies reported the benefit of directly injecting the ovaries with PRP through laparoscopically assisted technique [27,28]. In our case, we used a similar technique, where we inject the ovaries directly with the PRP through laparoscopy. Moreover, we enhanced the procedure by injecting the PRP into the cervix, intrauterine, and two intramuscular injections. We found this protocol to be effective, where the patient's AMH significantly increased. Six follicles in each ovary were successfully developed, which can not be attributed to the 5–10% chance of spontaneous follicular development in POI patients.
As far as we know this is the first time this protocol is used, so there is no data reported and more research is needed to confirm our results. We think that generalized admission enhanced the localized effects of PRP and gives better results. However, more studies with proper design are needed to confirm this theory. Furthermore, we were unable to access the effect of this method on the pregnancy outcome, because we lost contact with the patient.
In conclusion, the use of PRP offers a good chance to restore fertility in POI patients. It would be more acceptable treatment than donor eggs programs in countries with religious beliefs. Furthermore, the new PRP injection protocol that we conducted on this patient was effective.
Availability of data and materials
Not applicable.
Funding
No funding was received.
Ethical approval
N/A.
Author contribution
RS, RT, DK drafted the manuscript. HA did the procedure and supervised the project. All authors have read and approved the final manuscript.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Guarantor
Professor Haitham Abbassi.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgment
The authors thank Albaraa Bara, MD, Kheder kheder, MD, and Luma Haj Kassem, MD for their help in reviewing the paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2021.103149.
Abbreviations
- POI
primary ovarian insufficiency
- PRP
platelet-rich autologous plasma
- AMH
anti-mullerian hormone
- FSH
follicle-stimulating hormone
- RPM
revolutions per minute
- SLE
Systemic lupus erythematosus
- E2
Estradiol
- IVF
in vitro fertilization
- GnRH
gonadotropin-releasing hormone
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.National cancer institute at the national institutes of health. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/primary-ovarian-insufficiency
- 2.Lagergren K., Hammar M., Nedstrand E., Bladh M., Sydsjö G. The prevalence of primary ovarian insufficiency in Sweden; a national register study. BMC Wom.Health. 2018 Oct 25;18(1):175. doi: 10.1186/s12905-018-0665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qin Y., Jiao X., Simpson J.L., Chen Z.J. Genetics of primary ovarian insufficiency: new developments and opportunities. Hum. Reprod. Update. 2015;21(6):787–808. doi: 10.1093/humupd/dmv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fenton A.J. Premature ovarian insufficiency: pathogenesis and management. J Midlife Health. 2015;6(4):147–153. doi: 10.4103/0976-7800.172292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.La Marca A., Pati M., Orvieto R., Stabile G., Carducci Artenisio A., Volpe A. Serum anti-müllerian hormone levels in women with secondary amenorrhea. Fertil. Steril. 2006 May;85(5):1547–1549. doi: 10.1016/j.fertnstert.2005.10.057. Epub 2006 Apr 17. [DOI] [PubMed] [Google Scholar]
- 6.Gupta S., Lodha P., Karthick M.S., Tandulwadkar S.R. Role of autologous bone marrow-derived stem cell therapy for follicular recruitment in premature ovarian insufficiency: review of literature and a case report of world's first baby with ovarian autologous stem cell therapy in a perimenopausal woman of age 45 year. J. Hum. Reprod. Sci. 2018;11(2):125–130. doi: 10.4103/jhrs.JHRS_57_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melo P., Navarro C., Jones C., et al. The use of autologous platelet-rich plasma (PRP) versus no intervention in women with low ovarian reserve undergoing fertility treatment: a non-randomized interventional study. J. Assist. Reprod. Genet. 2020;37:855–863. doi: 10.1007/s10815-020-01710-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pietrzak W.S., Eppley B.L. Platelet rich plasma: biology and new technology. J. Craniofac. Surg. 2005;16:1043–1054. doi: 10.1097/01.scs.0000186454.07097.bf. [DOI] [PubMed] [Google Scholar]
- 9.Sundman E.A., Cole B.J., Karas V., Della Valle C., Tetreault M.W., Mohammed H.O., Fortier L.A. The anti-inflammatory and matrix restorative mechanisms of platelet-rich plasma in osteoarthritis. Am. J. Sports Med. 2014;42:35–41. doi: 10.1177/0363546513507766. [DOI] [PubMed] [Google Scholar]
- 10.Moraes V.Y., Lenza M., Tamaoki M.J., Faloppa F., Belloti J.C. Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst. Rev. 2013 Dec 23;(12):CD010071. doi: 10.1002/14651858.CD010071. [DOI] [PubMed] [Google Scholar]
- 11.Le A.D.K., Enweze L., DeBaun M.R., et al. Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med. 2018;11:624–634. doi: 10.1007/s12178-018-9527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosseini L., Shirazi A., Naderi M.M., Shams-Esfandabadi N., Borjian Boroujeni S., Sarvari A., Sadeghnia S., Behzadi B., Akhondi M.M. Platelet-rich plasma promotes the development of isolated human primordial and primary follicles to the preantral stage. Reprod. Biomed. Online. 2017;35:343–350. doi: 10.1016/j.rbmo.2017.04.007. 10.1016/j.rbmo.2017.04.007, Epub 2017 May 15. [DOI] [PubMed] [Google Scholar]
- 13.Sills E.S., Rickers N.S., Li X., Palermo G.D. First data on in vitro fertilization and blastocyst formation after intraovarian injection of calcium gluconate-activated autologous platelet-rich plasma. Gynecol. Endocrinol. 2018 Sep;34(9):756–760. doi: 10.1080/09513590.2018.1445219. Epub 2018 Feb 28. PMID: 29486615. [DOI] [PubMed] [Google Scholar]
- 14.Agha R.A., Franchi T., Sohrabi C., Mathew G., for the SCARE Group The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 15.Clinic Mayo. Primary ovarian insufficiency. https://www.mayoclinic.org/diseases-conditions/premature-ovarian-failure/symptoms-causes/syc-20354683
- 16.MedlinePlus Primary ovarian insufficiency. https://medlineplus.gov/primaryovarianinsufficiency.html
- 17.Visser J., Schipper I., Laven J., et al. Anti-Müllerian hormone: an ovarian reserve marker in primary ovarian insufficiency. Nat. Rev. Endocrinol. 2012;8:331–341. doi: 10.1038/nrendo.2011.224. [DOI] [PubMed] [Google Scholar]
- 18.Nelson S.M. Biomarkers of ovarian response: current and future applications. Fertil. Steril. 2013 Mar 15;99(4):963–969. doi: 10.1016/j.fertnstert.2012.11.051. Epub 2013 Jan 8. [DOI] [PubMed] [Google Scholar]
- 19.De Caro J.J., Dominguez C., Sherman S.L. Reproductive health of adolescent girls who carry the FMR1 premutation: expected phenotype based on current knowledge of fragile x-associated primary ovarian insufficiency. Ann. N. Y. Acad. Sci. 2008;1135:99–111. doi: 10.1196/annals.1429.029. [DOI] [PubMed] [Google Scholar]
- 20.Ben-Nagi J., Panay N. Premature ovarian insufficiency: how to improve reproductive outcome? Climacteric. 2014 Jun;17(3):242–246. doi: 10.3109/13697137.2013.860115. Epub 2013 Dec 16. PMID: 24341612. [DOI] [PubMed] [Google Scholar]
- 21.Lee H.J., Selesniemi K., Niikura Y., Niikura T., Klein R., Dombkowski D.M., Tilly J.L. Bone marrow transplantation generates immature oocytes and rescues long-term fertility in a preclinical mouse model of chemotherapy-induced premature ovarian failure. J. Clin. Oncol. 2007 Aug 1;25(22):3198–3204. doi: 10.1200/JCO.2006.10.3028. [DOI] [PubMed] [Google Scholar]
- 22.European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI, Webber L., Davies M., Anderson R., Bartlett J., Braat D., Cartwright B., Cifkova R., de Muinck Keizer-Schrama S., Hogervorst E., Janse F., Liao L., Vlaisavljevic V., Zillikens C., Vermeulen N. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum. Reprod. 2016 May;31(5):926–937. doi: 10.1093/humrep/dew027. Epub 2016 Mar 22. [DOI] [PubMed] [Google Scholar]
- 23.Dohan Ehrenfest D.M., Rasmusson L., Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF) Trends Biotechnol. 2009 Mar;27(3):158–167. doi: 10.1016/j.tibtech.2008.11.009. Epub 2009 Jan 31. PMID: 19187989. [DOI] [PubMed] [Google Scholar]
- 24.Andia I. In: Clinical Indications and Treatment Protocols with Platelet-Rich Plasma in Dermatology. Alves R., Grimalt R., editors. Barcelona, Ediciones Mayo; 2016. Platelet-rich plasma biology; pp. 3–15. [Google Scholar]
- 25.Harmon K., Hanson R., Bowen J., Greenberg S., Magaziner E., Vandenbosch J., et al. Guidelines for the use of platelet rich plasma. Int Cell Med Soc. 2013;41:356–364. [Google Scholar]
- 26.Panda S.R., Sachan S., Hota S. A systematic review evaluating the efficacy of intra-ovarian infusion of autologous platelet-rich plasma in patients with poor ovarian reserve or ovarian insufficiency. Cureus. 2020 Dec 12;12(12) doi: 10.7759/cureus.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cakiroglu Y., Saltik A., Yuceturk A., Karaosmanoglu O., Kopuk S.Y., Scott R.T., Tiras B., Seli E. Effects of intraovarian injection of autologous platelet-rich plasma on ovarian reserve and IVF outcome parameters in women with primary ovarian insufficiency. Aging (Albany NY) 2020 Jun 5;12(11):10211–10222. doi: 10.18632/aging.103403. Epub 2020 Jun 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petryk N., Petryk M. Ovarian rejuvenation through platelet-rich autologous plasma (PRP)-a chance to have a baby without donor eggs, improving the life quality of women suffering from early menopause without synthetic hormonal treatment. Reprod. Sci. 2020 Nov;27(11):1975–1982. doi: 10.1007/s43032-020-00266-8. Epub 2020 Jul 22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.