Abstract
Graded quantities of 1.38, 2.76 and 4.14 g/kg L-methionine were included in a control diet formulated to contain 3.07 g/kg digestible methionine. Each of the 4 dietary treatments was offered to 6 replicate cages (initially 8 birds per cage) from 1 to 21 d post–hatch. The parameters assessed included growth performance, nutrient utilisation (apparent metabolizable energy [AME], AME:GE ratios, N retention, N-corrected apparent metabolizable energy [AMEn]), apparent digestibility coefficients and disappearance rates of amino acids in the distal ileum. They also included free amino concentrations in systemic plasma (brachial vein) at 20 d post–hatch and in hepatic tissue at 14 and 21 d post–hatch. Graded L-methionine inclusions quadratically influenced weight gain (r = 0.688; P = 0.001) and FCR (r = 0.780; P < 0.001). It may be deduced from the quadratic regressions that 3.43 g/kg L-methionine supported maximum weight gain of 1,036 g/kg and 3.50 g/kg L-methionine minimum FCR of 1.193, from 1 to 21 d post–hatch. The control diet contained specified levels of 3.07 g/kg digestible methionine and 13.0 g/kg digestible lysine. Thus, an inclusion of 3.465 g/kg L-methionine corresponded to a total of 6.535 g/kg methionine or a methionine-to-lysine ratio of 50.3, which is higher than standard recommendations. The implications of this and other outcomes of the present study are reported and discussed.
Keywords: Amino acid, Broiler chicken, Methionine
1. Introduction
Typically, methionine is the first limiting acid in diets for broiler chickens (Bunchasak, 2009) and, consequently, inclusions of synthetic methionine in broiler diets were commercially adopted in the 1960s as they became increasingly cost-effective (Kidd et al., 2013). Such inclusions are now routinely practised and substantial proportions of requirements are met by synthetic methionine, which are likely to increase even further with the development and acceptance of reduced crude protein diets for chicken-meat production (Chrystal et al., 2020). Numerous assessments of methionine requirements for broiler chickens have been completed over many decades but they are continually made redundant by the advances in broiler production performance generated by genetics and breeding programs (Tavárez and de los Santos, 2016). There are various forms of synthetic methionine; whereas, L-methionine is a product of fermentation. Thus, the primary objective of this experiment was to determine the optimum inclusion level of L-methionine in diets based on wheat and soybean meal to support broiler growth performance from 1 to 21 d post–hatch. The control diet, to which graded L-methionine inclusions were added at 1.38, 2.76 and 4.14 g/kg, contained a specified level of 3.07 g/kg digestible methionine. In addition, the impacts of graded L-methionine inclusions on apparent digestibility and disappearance rates of amino acids in the terminal ileum were determined, as were the impacts on concentrations of free amino acids in systemic plasma and hepatic tissue. The relevance of apparent amino acid digestibilities and free amino acid concentrations in plasma and liver in relation to protein adequacy was assessed by Fernández- Figares et al. (1993), but this experiment was not based on practical diets as is the case in the present study. It is also relevant that determinations of ileal amino acid digestibilities and free amino acid concentrations are not usally completed when methionine requirements for broiler chickens are assessed.
2. Materials and methods
All experimental procedures were approved by the Animal Research Authority of the University of Sydney (Project number 2018/1448).
2.1. Experimental design and diet preparation
A control diet based on wheat, soybean meal, and canola meal with a digestible methionine specification of 3.07 g/kg was supplemented with 1.38, 2.76 and 4.14 g/kg L-methionine to generate 4 dietary treatments (Table 1). The L-methionine inclusions were based on 2019 Aviagen recommendations for Ross 308 broiler chickens. The recommended concentration of digestible methionine + cysteine from 1 to 21 d post–hatch is 9.08 g/kg so control diet with 6.37 g/kg was deficient (70%) and graded inclusions of 1.38, 2.76 and 4.14 g/kg L-methionine increased digestible methionine + cysteine to 85%, 101% and 116% of Aviagen recommendations. Given the digestible lysine specification of 13.0 g/kg, the methionine-to-lysine ratios increased from 24 to 34, 45 and 55 across the 4 dietary treatments. The 4 experimental diets were formulated to recommended guidelines and were iso-energetic (12.34 MJ/kg) and contained the same dietary levels of amino acids, other than methionine. Celite (Celite, Celite Corporation. Lompoc, CA), was included in the finisher diets as a source of acid insoluble ash (AIA) to serve as an inert dietary marker. All diets contained standard inclusions of exogenous phytase and xylanase and diets were steam-pelleted at a conditioning temperature of 80 °C and then crumbled. Diets were initially offered to birds as crumble from 1 to 14 d and subsequently in pellet form from 15 to 21 d post–hatch.
Table 1.
Composition and nutrient specifications of control diet (as-fed basis, g/kg).
| Feed ingredient | Content | Specification | Content |
|---|---|---|---|
| Wheat | 495 | Metabolizable energy, MJ/kg | 12.34 |
| Soybean meal | 315 | Crude protein | 236.9 |
| Canola meal | 75.0 | Calcium | 10.50 |
| Soy oil | 50.0 | Total phosphorus | 6.46 |
| L-Lysine HCl | 3.21 | Phytate phosphorus | 2.49 |
| L-Threonine | 1.61 | Non-phytate phosphorus | 3.97 |
| L-Valine | 0.90 | Sodium | 1.80 |
| L-Arginine | 0.74 | Potassium | 10.03 |
| L-Isoleucine | 0.39 | Chloride | 2.50 |
| Sodium chloride | 2.17 | DEB, mEq/kg | 264 |
| Sodium bicarbonate | 1.66 | Crude fat | 73.5 |
| Limestone | 11.14 | Crude fibre | 26.9 |
| Dicalcium phosphate | 15.38 | Digestible lysine | 13.0 |
| Digestible TSAA | 6.37 | ||
| Xylanase | 0.05 | Digestible methionine | 3.07 |
| Phytase | 0.05 | Digestible threonine | 8.58 |
| Choline chloride 60% | 1.00 | Digestible tryptophan | 2.68 |
| Celite | 20.0 | Digestible isoleucine | 8.84 |
| Sand1 | 4.14 | Digestible leucine | 14.78 |
| Vitamin-mineral premix2 | 3.00 | Digestible valine | 10.27 |
DEB = dietary electrolyte balance; TSAA = total sulphur amino acid.
L-Methionine was incorporated into the control diet at the expense of sand at appropriate inclusion rates to generate the balance of dietary treatments.
The vitamin-mineral premix supplied per tonne of feed: [MIU] retinol 12, cholecalciferol 5, [g] tocopherol 50, menadione 3, thiamine 3, riboflavin 9, pyridoxine 5, cobalamin 0.025, niacin 50, pantothenate 18, folate 2, biotin 0.2, copper 20, iron 40, manganese 110, cobalt 0.25, iodine 1, molybdenum 2, zinc 90, selenium 0.3.
2.2. Bird management
Each of the 4 dietary treatments was offered to 6 replicates with initially 8 birds per replicate or a total 192 off-sex (parent line) male Ross 308 chicks from 1 to 14 d post–hatch and then 6 birds per replicate from 15 to 21 d post–hatch. On the first day, birds were individually identified (wing-tags), weighed and allocated into bioassay cages on the basis of body weights so that cage body weight means and variations were almost identical. Cage dimensions were 750 mm in width, 750 mm in depth and 500 mm in height. Thereafter, birds were offered experimental diets to 21 d post–hatch. Birds had unlimited access to feed and water under a ‘18-h-on-6-h-off’ lighting regime in an environmentally controlled facility. An initial room temperature of 32 ± 1 °C was maintained for the first week, which was gradually decreased to 22 ± 1 °C by the end of the second week and maintained at this temperature for the duration of the feeding study. Body weights were determined at d 0, 14 and 21, and feed intakes were recorded from which feed conversion ratios (FCR) were calculated. The incidence of dead or culled birds was recorded daily and their body weights used to adjust feed intake and FCR calculations.
2.3. Sample collection and chemical analysis
Total excreta were collected from 18 to 20 d post–hatch from each cage to determine parameters of nutrient utilisation which included apparent metabolizable energy (AME), AME-to-gross energy ratios (AME:GE ratios), nitrogen (N) retention and N-corrected apparent metabolizable energy (AMEn). Excreta were dried in a forced-air oven at 80 °C for 24 h and the GE of excreta and diets were determined using an adiabatic bomb calorimeter (Parr 1281 bomb calorimeter, Parr Instruments Co., Moline, IL, USA). The AME values of the diets were calculated on a dry matter basis from the following equation:
N contents of diets and excreta were determined using a nitrogen determinator (Leco Corporation, St Joseph, MI) and N retentions calculated from the following equation:
N-corrected AME (AMEn MJ/kg DM) values were calculated by correcting N retention to zero using the factor of 36.54 kJ/g N retained in the body (Hill and Anderson, 1958).
At 14 d post–hatch, 2 birds selected at random from each pen were euthanased to collect liver samples for free amino acid analyses and this procedure was repeated at 21 d post–hatch. Liver samples were freeze-dried and anlysed for free amino acid concentrations. At d 20, 3 birds at random were selected from each cage and blood samples were taken from the brachial vein. Blood samples were then centrifuged and the decanted plasma samples were then kept at −80 °C before analysis. Concentrations of 20 proteinogenic amino acids in plasma taken from the brachial veins and liver were determined using precolumn derivatisation amino acid analysis with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC; Waters AccQTag Ultra; www.waters.com) followed by separation of the derivatives and quantification by reversed phase ultra-performance liquid chromatography. All amino acids were detected by UV absorbance.
On d 21, the 6 remaining birds were euthanised by intravenous injection of sodium pentobarbitone and digesta samples were collected in their entirety from the distal ileum to determine apparent ileal digestibility coefficients of amino acids and protein (N). Small intestines were removed from euthanised birds and samples of digesta were gently expressed from the distal ileum in their entirety and pooled for each cage. Distal ileal samples were taken from below the mid-point between Meckel's diverticulum and the ileocaecal junction. The digesta samples were freeze-dried to determine apparent digestibilities of crude protein (N) and amino acids using AIA as the inert dietary marker. N concentrations were determined as already stated and AIA concentrations were determined by the method of Siriwan et al. (1993). Amino acid concentrations in feed and digesta were analysed by the following procedures. Approximately 70 mg of sample was hydrolysed in 20% HCl for 24 h at 110 °C. An internal standard (norvaline and α amino butyric acid; Nva/AABA) was added to each sample following hydrolysis. Following a 1:25 dilution in ultra-pure water, 10 μL of the solution was derivatised using an AccQ-Tag Ultra Derivatization Kit (Waters Corporation, Milford, Mass. USA) following suppliers recommended procedures. The use of HCl as the hydrolysis reagent converted asparagine and glutamine to their acid forms, aspartic acid and glutamic acid, respectively. In the presence of HCl, the amino acid tryptophan was destroyed while cysteine/cystine were partially destroyed. Therefore, quantitation for these amino acids was not undertaken by this hydrolysis method. Subsequently, amino acid analysis was based on the method of Cohen (2001) but adapted for use with an ACQUITY Ultra Performance LC (UPLC; Waters, Australia) system (Truong et al., 2015).
The apparent digestibility coefficients for protein (N) and amino acids in distal ileal were calculated from the following equation:
The apparent disappearance rates (g/bird per day) of amino acids were calculated from the following equation:
| Disappearance rate = Dietary concentration (g/kg) × Daily feed intake (kg/day) × Digestibility coefficient . |
2.4. Statistical analyses
Experimental data were analysed using JMP Pro 14.0 (SAS Institute Inc. JMP Software. Cary, NC). One-way analyses of variance were performed and linear and quadratic regressions were established where relevant. Mean cage values were the experimental and the significant 5% level of probability was determined using a Student's t-test.
3. Results
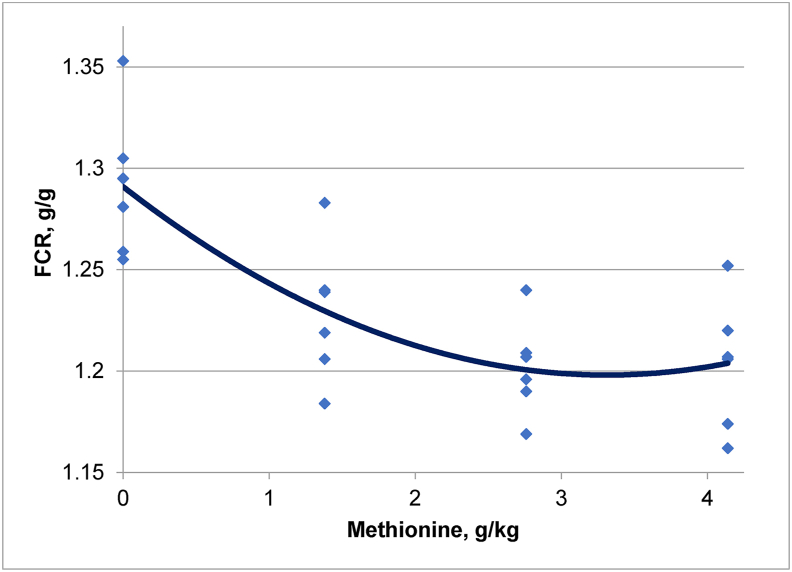
The effects of dietary treatments on growth performance from 1 to 14, 15 to 21 and 1 to 21 d post–hatch are shown in Table 2 where graded L-methionine inclusions improved weight gain (P < 0.005) and FCR (P < 0.001) from 1 to 21 d post–hatch. The addition of 4.14 g/kg L-methionine to the control diet significantly increased weight gain by 13.7% (1,036 versus 911 g/day) and enhanced FCR by 6.74% (1.204 versus 1.291) from 1 to 21 d post–hatch. Graded L-methionine dietary inclusions from 0 to 4.14 g/kg improved weight gain (r = 0.687; P = 0.001) and FCR (r = 0.847; P < 0.001) in a quadratic manner from 1 to 21 d post–hatch. These relationships are illustrated in Fig. 1, Fig. 2, respectively. It may be deduced from the relevant quadratic regressions that an inclusion of 3.43 g/kg L-methionine would generate the maximum weight gain of 1,036 g/bird and an inclusion of 3.50 g/kg L-methionine would generate the minimum FCR of 1.193 from 1 to 21 d post–hatch.
Table 2.
Effects of dietary treatments on growth performance from 1 to 14, 15 to 21, and 1 to 21 d post–hatch.
| Item | 1 to 14 d post–hatch |
15 to 21 d post–hatch |
1 to 21 d post–hatch |
||||||
|---|---|---|---|---|---|---|---|---|---|
| FI, g/bird | WG, g/bird | FCR | FI, g/bird | WG, g/bird | FCR | FI, g/bird | WG, g/bird | FCR | |
| L-Methionine, g/kg | |||||||||
| 0 | 527 | 458 | 1.154 | 648 | 453b | 1.439a | 1,175 | 911a | 1.291a |
| 1.38 | 543 | 485 | 1.119 | 694 | 522a | 1.335b | 1,237 | 1,007b | 1.229b |
| 2.76 | 551 | 491 | 1.121 | 671 | 526a | 1.279b | 1,222 | 1,017b | 1.202b |
| 4.14 | 565 | 505 | 1.119 | 681 | 531a | 1.286b | 1,246 | 1,036b | 1.204b |
| SEM | 11.9 | 11.7 | 0.0174 | 16.6 | 19.3 | 0.0320 | 22.8 | 21.8 | 0.0130 |
| Significance (P = ) | 0.186 | 0.061 | 0.416 | 0.285 | 0.030 | 0.007 | 0.164 | 0.003 | <0.001 |
| Quadratic (r = ) | 0.455 | 0.580 | 0.342 | 0.300 | 0.580 | 0.666 | 0.414 | 0.687 | 0.847 |
| (P = ) | 0.088 | 0.014 | 0.270 | 0.373 | 0.014 | 0.002 | 0.140 | 0.001 | <0.001 |
FI = feed intake; WG = weight gain; SEM = standard error of the mean.
a, b Mean values within columns not sharing a common superscript are significantly different at the 5% level of probability.
Fig. 1.
Quadratic relationship (r = 0.688; P = 0.001) between dietary inclusions of L-methionine and weight gain from 1 to 21 d post–hatch where. Weight gain = 915.4 + 70.21 × Methionine – 10.22 × Methionine2.
Fig. 2.
Quadratic relationship (r = 0.780; P < 0.001) between dietary inclusions of L-methionine and FCR from 1 to 21 d post–hatch where. FCR = 1.291 – 0.056 × Methionine +0.008 × Methionine2.
The effects of dietary treatments on free amino acid concentrations in plasma at 20 d post–hatch are shown in Table 3. Increasing L-methionine supplementation from 0 to 4.14 g/kg quadratically decreased concentrations of histidine, lysine, threonine, valine, glycine and serine to significant extents; whereas, in contrast, methionine concentrations were increased by a four-fold factor (22.8 versus 5.7 μg/mL).
Table 3.
Effects of dietary treatments on free amino acid concentrations (μg/mL) in systemic plasma at 20 d post–hatch.
| L-Methionine, g/kg | Arginine | Histidine | Isoleucine | Leucine | Lysine | Methionine | Phenylalanine | Threonine | Tryptophan | Valine |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 86.5 | 25.8a | 27.3 | 34.8 | 137.3a | 5.7c | 25.5 | 144.0a | 7.0 | 51.8 |
| 1.38 | 84.3 | 16.3b | 25.5 | 33.7 | 71.2b | 11.7b | 26.2 | 99.5b | 6.8 | 45.8 |
| 2.76 | 90.0 | 14.5b | 25.8 | 33.3 | 61.0b | 16.8b | 26.5 | 93.3b | 7.7 | 45.3 |
| 4.14 | 89.7 | 13.2b | 24.2 | 31.2 | 59.3b | 22.8a | 25.7 | 95.0b | 7.0 | 43.0 |
| SEM | 4.91 | 1.74 | 1.56 | 2.14 | 7.33 | 1.82 | 1.43 | 9.76 | 0.43 | 2.31 |
| Significance (P = ) | 0.823 | <0.001 | 0.566 | 0.679 | <0.001 | <0.001 | 0.957 | 0.004 | 0.541 | 0.077 |
| Quadratic (r = ) | 0.492 | 0.777 | 0.279 | 0.261 | 0.879 | 0.841 | 0.118 | 0.683 | 0.154 | 0.516 |
| (P = ) |
0.055 |
<0.001 |
0.425 |
0.480 |
<0.001 |
<0.001 |
0.859 |
0.001 |
0.778 |
0.039 |
| L-Methionine, g/kg |
Alanine |
Asparagine |
Aspartic acid |
Cysteine |
Glutamine |
Glutamic acid |
Glycine |
Proline |
Serine |
Tyrosine |
| 0 | 85.7 | 54.2 | 16.8 | 18.0 | 230.3 | 88.2 | 82.0 | 89.5 | 101.7a | 27.8 |
| 1.38 | 93.8 | 48.7 | 12.5 | 19.0 | 193.5 | 89.7 | 71.8 | 77.5 | 76.5b | 31.8 |
| 2.76 | 113.8 | 52.2 | 11.3 | 18.8 | 209.7 | 70.3 | 64.5 | 81.0 | 66.0b | 34.8 |
| 4.14 | 99.7 | 47.7 | 15.0 | 20.2 | 201.5 | 77.8 | 63.7 | 79.5 | 70.7b | 30.0 |
| SEM | 7.07 | 3.59 | 1.69 | 0.98 | 15.80 | 15.06 | 5.02 | 4.85 | 4.45 | 2.01 |
| Significance (P = ) | 0.065 | 0.557 | 0.126 | 0.495 | 0.412 | 0.778 | 0.063 | 0.341 | <0.001 | 0.123 |
| Quadratic (r = ) | 0.472 | 0.214 | 0.492 | 0.307 | 0.281 | 0.167 | 0.548 | 0.336 | 0.811 | 0.473 |
| (P = ) | 0.071 | 0.611 | 0.055 | 0.355 | 0.422 | 0.739 | 0.024 | 0.284 | <0.001 | 0.070 |
a, b Mean values within columns not sharing a common superscript are significantly different at the 5% level of probability.
The effects of graded L-methionine inclusions on apparent ileal digestibility coefficients of amino acids and protein (N) are shown in Table 4. Methionine digestibility coefficients were quadratically increased by up to 5.19% (0.933 versus 0.887). In contrast, digestibility coefficients of the balance of 15 amino acids were compromised in a quadratic manner to highly significant extents. Indeed, the addition of 4.14 g/kg L-methionine to the control diet depressed the average digestibility coefficient of these 15 amino acids by 5.04% (0.754 versus 0.794). Graded L-methionine inclusions significantly influenced apparent amino acid disappearance rates (g/bird per day) as shown in Table 5. Methionine disappearance rates increased by more than a two-fold factor (0.223 versus 0.100); whereas, the disappearance rates of the majority of the balance of 15 amino acids were retarded. The disappearance rates of arginine, threonine, aspartic acid, glycine, serine and tyrosine were quadratically retarded to significant extents.
Table 4.
Effects of dietary treatments on apparent amino acid and protein digestibility coefficients in distal ileum at 21 d post–hatch.
| L-Methionine, g/kg | Protein (N) | Arginine | Histidine | Isoleucine | Leucine | Lysine | Methionine | Phenylalanine | Threonine |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.755a | 0.839a | 0.807a | 0.798a | 0.797a | 0.843a | 0.887c | 0.813a | 0.749a |
| 1.38 | 0.758a | 0.804b | 0.781ab | 0.781ab | 0.782ab | 0.822ab | 0.916b | 0.794ab | 0.721b |
| 2.76 | 0.727b | 0.788b | 0.749c | 0.756b | 0.755c | 0.799c | 0.929ab | 0.770c | 0.690c |
| 4.14 | 0.751a | 0.802b | 0.767bc | 0.768b | 0.766bc | 0.812bc | 0.933a | 0.784bc | 0.700bc |
| SEM | 0.0077 | 0.0065 | 0.0077 | 0.0075 | 0.0073 | 0.0056 | 0.0052 | 0.0073 | 0.0085 |
| Significance (P = ) | 0.041 | <0.001 | <0.001 | 0.005 | 0.004 | <0.001 | <0.001 | 0.004 | <0.001 |
| Quadratic (r = ) | 0.329 | 0.795 | 0.746 | 0.649 | 0.660 | 0.756 | 0.844 | 0.652 | 0.744 |
| (P = ) |
0.034 |
<0.001 |
<0.001 |
0.003 |
0.003 |
<0.001 |
<0.001 |
0.003 |
<0.001 |
| L-Methionine, g/kg |
Valine |
Alanine |
Aspartic acid |
Glutamic acid |
Glycine |
Proline |
Serine |
Tyrosine |
Total |
| 0 | 0.787a | 0.767a | 0.754a | 0.857a | 0.724a | 0.803a | 0.773a | 0.795a | 0.799a |
| 1.38 | 0.764a | 0.741ab | 0.716b | 0.836b | 0.691b | 0.782ab | 0.742b | 0.774a | 0.778b |
| 2.76 | 0.740b | 0.709c | 0.680c | 0.816c | 0.653c | 0.755c | 0.717c | 0.744b | 0.753c |
| 4.14 | 0.751ab | 0.726bc | 0.694bc | 0.829bc | 0.677bc | 0.772bc | 0.717c | 0.739b | 0.765bc |
| SEM | 0.0079 | 0.0089 | 0.0088 | 0.0065 | 0.0093 | 0.0074 | 0.0079 | 0.0073 | 0.0070 |
| Significance (P = ) | 0.003 | 0.001 | <0.001 | 0.002 | <0.001 | 0.002 | 0.003 | <0.001 | 0.001 |
| Quadratic (r = ) | 0.684 | 0.701 | 0.802 | 0.695 | 0.745 | 0.684 | 0.780 | 0.801 | 0.715 |
| (P = ) | 0.002 | <0.001 | <0.001 | 0.001 | <0.001 | 0.001 | <0.001 | <0.001 | 0.001 |
SEM = standard error of the mean.
a, b, c Mean values within columns not sharing a common superscript are significantly different at the 5% level of probability.
Table 5.
Effects of dietary treatments on apparent amino acid and protein disappearance rates (g/bird per day) in distal ileum at 21 d post–hatch.
| L-Methionine, g/kg | Protein (N) | Arginine | Histidine | Isoleucine | Leucine | Lysine | Methionine | Phenylalanine | Threonine |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 11.55a | 0.649c | 0.245b | 0.424b | 0.692b | 0.627 | 0.100a | 0.476b | 0.377b |
| 1.38 | 11.97ab | 0.624bc | 0.242b | 0.421b | 0.691b | 0.624 | 0.142b | 0.474b | 0.368ab |
| 2.76 | 11.43a | 0.591a | 0.224a | 0.394a | 0.642a | 0.587 | 0.196c | 0.442a | 0.349a |
| 4.14 | 12.50b | 0.609ab | 0.234ab | 0.410ab | 0.669ab | 0.604 | 0.223d | 0.463ab | 0.354a |
| SEM | 0.0224 | 0.0111 | 0.0042 | 0.0072 | 0.0117 | 0.0110 | 0.0034 | 0.0084 | 0.0072 |
| Significance (P = ) | 0.011 | 0.010 | 0.008 | 0.034 | 0.023 | 0.058 | <0.001 | 0.037 | 0.049 |
| Quadratic (r = ) | 0.435 | 0.570 | 0.462 | 0.388 | 0.379 | 0.397 | 0.976 | 0347 | 0.507 |
| (P = ) |
0.110 |
0.016 |
0.080 |
0.180 |
0.197 |
0.165 |
<0.001 |
0.261 |
0.044 |
| L-Methionine, g/kg |
Valine |
Alanine |
Aspartic acid |
Glutamic acid |
Glycine |
Proline |
Serine |
Tyrosine |
Total |
| 0 | 0.467 | 0.335c | 0.822c | 2.103b | 0.318c | 0.579 | 0.419c | 0.223c | 8.854 |
| 1.38 | 0.456 | 0.328bc | 0.789bc | 2.110b | 0.310bc | 0.587 | 0.409bc | 0.208b | 8.782 |
| 2.76 | 0.437 | 0.303a | 0.712a | 1.974a | 0.281a | 0.547 | 0.381a | 0.199ab | 8.260 |
| 4.14 | 0.451 | 0.318ab | 0.750ab | 2.062ab | 0.297ab | 0.576 | 0.391ab | 0.191a | 8.601 |
| SEM | 0.077 | 0.0057 | 0.0154 | 0.0350 | 0.0058 | 0.0102 | 0.0077 | 0.0036 | 0.1522 |
| Significance (P = ) | 0.073 | 0.005 | <0.001 | 0.048 | 0.001 | 0.060 | 0.010 | <0.001 | 0.053 |
| Quadratic (r = ) | 0.425 | 0.496 | 0.629 | 0.290 | 0.549 | 0.173 | 0.542 | 0.827 | 0.355 |
| (P = ) | 0.123 | 0.052 | 0.005 | 0.399 | 0.023 | 0.727 | 0.026 | <0.001 | 0.243 |
SEM = standard error of the mean.
a, b, c Mean values within columns not sharing a common superscript are significantly different at the 5% level of probability.
The effects of dietary treatments on parameters of nutrient utilisation are shown in Table 6. Graded L-methionine inclusions enhanced N retention by up to 5.04 percentage units (71.08% versus 66.04%) at 2.76 g/kg L-methionine and the quadratic effect was significant (P < 0.005). Alternatively, graded L-methionine inclusions depressed efficiency of energy utilisation quadratically as AME:GE ratios declined by up to 5.22% (0.635 versus 0.670) at an L-methionine inclusion of 4.14 g/kg L-methionine. Inclusions of L-methionine quadratically depressed N-corrected AME (P = 0.045) and tended to depress AME (P = 0.051).
Table 6.
Effects of dietary treatments on parameters of nutrient utilisation in broiler chickens from 18 to 20 d post–hatch.
| L-Methionine, g/kg | AME, MJ/kg | AME:GE ratio | N retention, % |
AMEn, MJ/kg |
|---|---|---|---|---|
| 0 | 12.59 | 0.670a | 66.04b | 11.31 |
| 1.38 | 12.43 | 0.655ab | 70.33a | 11.03 |
| 2.76 | 12.39 | 0.654ab | 71.08a | 11.04 |
| 4.14 | 12.14 | 0.635b | 70.08a | 10.77 |
| SEM | 0.123 | 0.0080 | 0.988 | 0.135 |
| Significance (P = ) | 0.105 | 0.0048 | 0.008 | 0.071 |
| Quadratic (r = ) | 0.497 | 0.541 | 0.661 | 0.507 |
| (P = ) | 0.051 | 0.026 | 0.002 | 0.045 |
AME = apparent metabolizable energy; GE = gross energy; AMEn = nitrogen corrected AME; SEM = standard error of the mean.
a, b Mean values within columns not sharing a common superscript are significantly different at the 5% level of probability.
The effects of graded L-methionine inclusions on free amino acid concentrations in liver samples taken at 14 and 21 d post–hatch are displayed in Table 7, Table 8, respectively. On d 14, the transition of dietary L-methionine inclusions from 0 to 4.14 g/kg increased methionine hepatic concentrations (P = 0.05) but depressed hepatic concentrations of histidine, threonine, aspartic acid, glycine, proline, and serine (P < 0.02). Dietary L-methionine inclusions quadratically influenced glycine (r = 0.552, P = 0.022) and aspartic acid (r = 0.528, P = 0.032) hepatic concentrations. On d 21, L-methionine inclusions significantly influenced hepatic concentrations of alanine (P = 0.005) and serine (P = 0.012); whereas, dietary L-methionine inclusions from 0 to 4.14 g/kg quadratically significantly reduced hepatic concentrations of histidine, lysine, threonine and aspartic acid.
Table 7.
Effect of dietary treatments on free amino acid concentrations (mg/g) in liver samples at 14 d post–hatch.
| L-Methionine, g/kg | Arginine | Histidine | Isoleucine | Leucine | Lysine | Methionine | Phenylalanine | Threonine | Tryptophan | Valine |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.252 | 0.227a | 0.103 | 0.167 | 0.430a | 0.037b | 0.102 | 0.560a | 0.062 | 0.168 |
| 1.38 | 0.260 | 0.158b | 0.102 | 0.168 | 0.203b | 0.055a | 0.110 | 0.397b | 0.060 | 0.162 |
| 2.76 | 0.242 | 0.143b | 0.090 | 0.147 | 0.163b | 0.060a | 0.093 | 0.330b | 0.057 | 0.142 |
| 4.14 | 0.238 | 0.130b | 0.090 | 0.147 | 0.157b | 0.072a | 0.095 | 0.322b | 0.055 | 0.137 |
| SEM | 0.0131 | 0.0182 | 0.0071 | 0.0092 | 0.0334 | 0.0060 | 0.0069 | 0.0425 | 0.0036 | 0.0113 |
| Significance (P = ) | 0.642 | 0.006 | 0.393 | 0.194 | <0.001 | 0.050 | 0337 | 0.003 | 0.558 | 0.175 |
| Quadratic (r = ) | 0.235 | 0.666 | 0.341 | 0.397 | 0.823 | 0.672 | 0.263 | 0.709 | 0.307 | 0.451 |
| (P = ) |
0.551 |
0.002 |
0.275 |
0.165 |
<0.001 |
0.002 |
0.471 |
<0.001 |
0.354 |
0.093 |
| L-Methionine, g/kg |
Alanine |
Asparagine |
Aspartic acid |
Glutamic acid |
Glutamine |
Glycine |
Proline |
Serine |
Tyrosine |
Total |
| 0 | 0.417 | 0.187 | 1.152 | 1.938 | 0.657 | 0.527 | 0.470a | 0.868a | 0.113 | 8.445 |
| 1.38 | 0.580 | 0.205 | 1.068 | 1.989 | 1.248 | 0.517 | 0.342b | 0.638b | 0.122 | 8.389 |
| 2.76 | 0.477 | 0.172 | 0.972 | 1.793 | 0.892 | 0.467 | 0.350b | 0.505bc | 0.107 | 7.202 |
| 4.14 | 0.488 | 0.177 | 1.013 | 1.767 | 0.968 | 0.417 | 0.347b | 0.405c | 0.115 | 7.143 |
| SEM | 0.0395 | 0.0199 | 0.0463 | 0.1080 | 0.1398 | 0.0293 | 0.0283 | 0.0746 | 0.0069 | 0.5075 |
| Significance (P = ) | 0.059 | 0.654 | 0.065 | 0.411 | 0.053 | 0.056 | 0.011 | 0.002 | 0.509 | 0.146 |
| Quadratic (r = ) | 0.377 | 0.167 | 0.528 | 0.315 | 0.382 | 0.552 | 0.616 | 0.720 | 0.069 | 0.439 |
| (P = ) | 0.200 | 0.737 | 0.032 | 0.333 | 0.191 | 0.022 | 0.007 | 0.001 | 0.952 | 0.105 |
SEM = standard error of the mean.
a, b, c Mean values within columns not sharing a common superscript are significantly different at the 5% level of probability.
Table 8.
Effect of dietary treatments on free amino acid concentrations (g/kg) in liver samples at 21 d post–hatch.
| L-Methionine, g/kg | Arginine | Histidine | Isoleucine | Leucine | Lysine | Methionine | Phenylalanine | Threonine | Tryptophan | Valine |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.214 | 0.099 | 0.078 | 0.135 | 0.168 | 0.039 | 0.070 | 0.403 | 0.049 | 0.141 |
| 1.38 | 0.210 | 0.083 | 0.075 | 0.133 | 0.127 | 0.047 | 0.074 | 0.330 | 0.050 | 0.136 |
| 2.76 | 0.201 | 0.063 | 0.074 | 0.135 | 0.104 | 0.046 | 0.071 | 0.283 | 0.050 | 0.130 |
| 4.14 | 0.221 | 0.065 | 0.077 | 0.139 | 0.110 | 0.050 | 0.074 | 0.268 | 0.049 | 0.135 |
| SEM | 0.0108 | 0.0111 | 0.0041 | 0.0075 | 0.0171 | 0.0036 | 0.0044 | 0.0376 | 0.0021 | 0.0063 |
| Significance (P = ) | 0.638 | 0.099 | 0.938 | 0.957 | 0.059 | 0.214 | 0.893 | 0.080 | 0.970 | 0.683 |
| Quadratic (r = ) | 0.239 | 0.503 | 0.020 | 0.123 | 0.551 | 0.406 | 0.102 | 0.530 | 0.109 | 0.254 |
| (P = ) |
0.578 |
0.047 |
0.812 |
0.852 |
0.022 |
0.150 |
0.896 |
0.031 |
0.882 |
0.497 |
| L-Methionine, g/kg |
Alanine |
Asparagine |
Aspartic acid |
Glutamic acid |
Glutamine |
Glycine |
Proline |
Serine |
Tyrosine |
Total |
| 0 | 0.562c | 0.086 | 1.412 | 1.981 | 0.396 | 0.478 | 0.471 | 0.609a | 0.117 | 7.509 |
| 1.38 | 0.591bc | 0.088 | 1.251 | 1.788 | 0.381 | 0.421 | 0.443 | 0.401ab | 0.126 | 6.758 |
| 2.76 | 0.704a | 0.069 | 1.115 | 1.650 | 0.311 | 0.386 | 0.405 | 0.228b | 0.129 | 6.165 |
| 4.14 | 0.672ab | 0.072 | 1.162 | 1.767 | 0.363 | 0.407 | 0.407 | 0.295b | 0.131 | 6.465 |
| SEM | 0.0278 | 0.0117 | 0.0762 | 0.1101 | 0.0487 | 0.029 | 0.0221 | 0.0767 | 0.0086 | 0.3892 |
| Significance (P = ) | 0.005 | 0.566 | 0.058 | 0.233 | 0.641 | 0.175 | 0.135 | 0.012 | 0.645 | 0.121 |
| Quadratic (r = ) | 0.612 | 0.252 | 0.547 | 0.425 | 0.221 | 0.460 | 0.477 | 0.636 | 0.278 | 0.491 |
| (P = ) | 0.007 | 0.503 | 0.024 | 0.121 | 0.592 | 0.082 | 0.066 | 0.004 | 0.430 | 0.055 |
SEM = standard error of the mean.
a, b, c Mean values within columns not sharing a common superscript are significantly different at the 5% level of probability.
4. Discussion
The key finding of the present study was that L-methionine inclusions of 3.43 and 3.50 g/kg supported maximum weight gain and minimum FCR, respectively. Moreover, these predicted outcomes are marginally superior to 2019 Aviagen performance objectives for gain (1,036 versus 975 g/bird) and FCR (1.193 versus 1.222) for male Ross 308 broilers from 1 to 21 d post–hatch. Given that the control diet contained specified levels of 3.07 g/kg digestible methionine and 13.0 g/kg digestible lysine, then the average inclusion of 3.465 g/kg L-methionine corresponds to a total of 6.535 g/kg methionine or a digestible methionine-to-lysine ratio of 50.3. This ratio is higher than the ratios of 45 and 40 recommended by Tillman and Dozier (2013), and Wu (2014a), respectively. Given that nearly 30 years ago Baker and Han (1994) recommended a methionine-to-lysine ratio of 36, it does appear that methionine requirements are increasing with advances in broiler performance parameters in response to selection programs (Tavarez and de los Santos, 2016).
Conventionally, methionine requirement is not considered solely in feed formulation. Instead, total Sulphur amino acid (TSAA) requirement, including both methionine and cysteine, is considered. In the present study, the predicted optimal digestible TSAA concertation for FCR was 9.87 g/kg which reflects a digestible TSAA-to-lysine ratio of 76. This is similar to 2019 Ross 308 Nutrient Specifications where an average digestible TSAA-to-lysine ratio of 75 was recommended for birds from 0 to 24 d post-hatch. Liu et al. (2019) reported male lysine requirement was higher than female and it is worth noting breeder nutrient specifications are often based on as-hatch birds. In practice, the requirement of TSAA is often met by supplementing methionine alone but a diet lacking cysteine may result in higher methionine supplementation (Pacheco et al., 2018). Digestible cysteine-to-TSAA ratio was recommended to be 0.46 by 2019 Ross 308 Nutrient Specifications regardless of growing phases; similarly, Rostagno et al. (2017) suggested digestible cysteine-to-TSAA ratio needs to be no lower than 0.45. The supplemented diets in the present study had lower digestible cysteine-to-TSAA ratios ranged from 0.32 to 0.43. The lower digestible cysteine levels in the supplemented diets may have contributed to higher methionine requirements observed in the present study.
Graded L-methionine inclusion levels quadratically enhanced N retention (r = 0.661; P = 0.002) which is to be expected given the inadequate methionine levels in the control diet. However, graded L-methionine inclusions quadratically compromised AME:GE ratios (r = 0.541; P = 0.026), or efficiency of energy utilisation. This outcome was not anticipated as Sekiz et al. (1975) reported that graded additions of synthetic methionine to an inadequate broiler diet did not significantly influence metabolizable energy. The possibility remains that the energetic costs arising from the deamination of unbalanced amino acids, detoxification of ammonia and excretion of uric acid (Selle et al., 2020) may have contributed to the variations in efficiency of energy utilisation observed in the present study.
The addition of 4.14 g/kg L-methionine to the control diet increased the ileal digestibility of methionine per se by 5.19% but decreased the digestibility of the 15 remaining amino acids by 5.04% (Table 4), which is a curious dichotomy. Non-bound methionine is notionally 100% digestible (Lemme et al., 2005) and accounted for about 57% of total methionine in the 4.14 g/kg L-methionine dietary treatment and this would have contributed to the 5.19% increase in digestibility. However, an explanation for the 5.04% decrease for the balance of amino acid is not as straightforward. It may be relevant that graded L-methionine inclusion levels numerically increased feed intakes and by up to 6.04% (1,246 versus 1,175 g/bird). Increased feed intakes imply increased rates of feed passage which would reduce the time available for protein to interact with digestive enzymes and for amino acids to interact with absorptive surfaces and intestinal uptake transport systems (Rochell et al., 2012). Teeter and Smith (1985) investigated the effects of 5 tiers of feed intakes on total tract protein (N) digestibility (adjusted for uric acid concentrations) by force-feeding broiler chickens. Increasing feed intakes compromised total tract protein (N) digestibility coefficients by up to 8.94% (0.866 versus 0.951). Also, Massuquetto et al. (2019) found that increasing feed intakes of broiler chickens compromised ileal protein (N) digestibility coefficients by 7.26% (0.818 versus 0.882) from 21 to 35 d post–hatch. Thus, both studies illustrate the negative impacts increased feed intakes may have on protein digestibility. Therefore, it is noteworthy that significant negative linear relationships between feed intakes and ileal digestibility coefficients were detected for 10 amino acids and negative linear relationships approached significance (P < 0.01) for a further 5 amino acids as shown in Table 9. Numerous factors could be involved but it appears that the negative impact L-methionine had on the digestibility of other amino acids may have been largely due to the increases in feed intakes generated by graded inclusions of L-methionine. As shown in Table 5, graded L-methionine inclusions increased methionine disappearance rate but reduced the disappearance rates of 12 amino acids where the exceptions were lysine, valine and proline. Clearly, the reduced disappearance rates of majority of amino acids were pursuant to reductions in their apparent digestibility coefficients.
Table 9.
Linear relationships between feed intakes and apparent ileal amino acid digestibility coefficients from 1 to 21 d post–hatch.
| Amino acid | Correlation coefficient | Significance |
|---|---|---|
| Arginine | r = −0.430 | P = 0.036 |
| Histidine | r = −0.420 | P = 0.041 |
| Isoleucine | r = −0.438 | P = 0.032 |
| Leucine | r = −0.429 | P = 0.036 |
| Lysine | r = −0.382 | P = 0.065 |
| Methionine | r = 0.218 | P = 0.305 |
| Phenylalanine | r = −0.391 | P = 0.059 |
| Threonine | r = −0.398 | P = 0.060 |
| Valine | r = −0.494 | P = 0.014 |
| Alanine | r = −0.458 | P = 0.024 |
| Aspartic acid | r = −0.357 | P = 0.086 |
| Glutamic acid | r = −0.467 | P = 0.021 |
| Glycine | r = −0.405 | P = 0.050 |
| Proline | r = −0.413 | P = 0.045 |
| Serine | r = −0.387 | P = 0.062 |
| Tyrosine | r = −0.454 | P = 0.026 |
| Total amino acids | r = −0.428 | P = 0.037 |
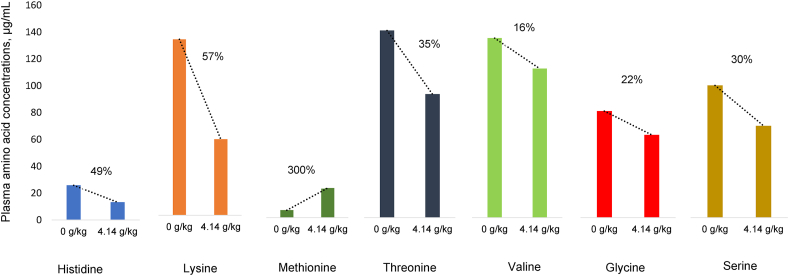
Free amino acid concentrations in systemic plasma illustrate the dynamic equilibrium between the post-enteral availability of amino acids and their utilisation (Fernández-Fígares et al., 2014). Fundamentally, amino acids are utilised for protein accretion but are also involved in numerous metabolic and functional roles (Wu, 2014b). Predictably, methionine plasma concentrations were increased in this experiment; however, lysine (56.8%), histidine (48.8%), threonine (35.2%) and serine (35.1%) were significantly decreased by graded L-methionine inclusions, where the percentage reductions from 4.14 g/kg L-methionine are shown in parentheses. Also, L-methionine quadratically depressed plasma concentrations of valine and glycine and these shifts in free plasma amino acid concentrations are illustrated in Fig. 3. Similar findings were reported by Fernández-Fígares et al. (2005) where the addition of 2 g/kg D,L-methionine to a soybean meal-based diet significantly increased free methionine plasma concentrations but significantly decreased concentrations of lysine, threonine, valine and leucine plus 8 non-essential amino acids including glycine and serine. Concentrations of free amino acids in systemic plasma may be predictive of limiting amino acids in broiler diets (Prieto et al., 1994). Interestingly, as shown in Table 10, weight gain was negatively correlated with plasma concentrations of 5 amino acids: lysine, histidine, threonine, serine and valine. The implication is that these amino acids were incorporated into protein in greater amounts relative to their post-enteral availability than the balance of amino acids. That weight gain was positively related to free methionine concentrations, which is consistent with the increasing dietary inclusions of L-methionine.
Fig. 3.
Percentage changes in plasma amino acid concentrations following an increase in dietary L-methionine inclusions from 0 to 4.14 g/kg.
Table 10.
Linear relationships weight gain to 21 d post–hatch and free amino acid concentrations in systemic plasma.
| Amino acid | Correlation coefficient | Significance |
|---|---|---|
| Arginine | r = 0.020 | P = 0.926 |
| Histidine | r = −0.532 | P = 0.008 |
| Isoleucine | r = −0.206 | P = 0.333 |
| Leucine | r = −0.099 | P = 0.647 |
| Lysine | r = −0.623 | P = 0.001 |
| Methionine | r = 0.427 | P = 0.038 |
| Phenylalanine | r = 0.047 | P = 0.826 |
| Threonine | r = −0.528 | P = 0.008 |
| Tryptophan | r = −0.304 | P = 0.149 |
| Valine | r = −0.404 | P = 0.050 |
| Alanine | r = 0.237 | P = 0.265 |
| Asparagine | r = −0.256 | P = 0.228 |
| Aspartic acid | r = −0.042 | P = 0.846 |
| Glutamine | r = −0.200 | P = 0.348 |
| Glutamic acid | r = 0.004 | P = 0.984 |
| Glycine | r = −0.307 | P = 0.145 |
| Proline | r = −0.256 | P = 0.228 |
| Serine | r = −0.514 | P = 0.010 |
| Tyrosine | r = 0.213 | P = 0.317 |
| Total amino acids | r = −0.343 | P = 0.101 |
Graded L-methionine inclusions increased hepatic methionine concentrations by up to 94.6% (0.072 versus 0.037 mg/g) at 14 d post–hatch but significantly reduced concentrations of lysine (63.5%), serine (53.3%), histidine (42.7%), threonine (42.5%), and proline (26.2%) where percentage reductions are shown in parentheses. However, the influence of L-methionine inclusions on concentrations of hepatic free amino acids is less pronounced at 21 d post–hatch. Somewhat similar outcomes were reported by Fernández-Fígares et al. (1997) where the dietary addition of 2.0 g/kg D,L-methionine decreased free hepatic concentrations of arginine, valine, leucine, phenylalanine and methionine but increased glycine concentrations. However, the relevance of free amino acid concentrations in the liver is questionable as there were not any significant relationships between weight gain and hepatic amino acid concentrations at 21 d post–hatch (data not shown), which was not the case with free amino acid plasma concentrations.
In conclusion, graded levels of L-methionine enhanced weight gain, feed intake and FCR in broiler chickens from 1 to 21 d post–hatch; an average inclusion level of 3.465 g/kg L-methionine supported the best weight gain and FCR. This corresponded to a digestible methionine-to-lysine ratio of 50.3, which is higher than present recommendations. Graded L-methionine inclusions increased digestibility coefficients, disappearance rates and systemic plasma concentrations of methionine but decreased these parameters for the balance of amino acids to varying extents. It appeared that depressed apparent amino acid digestibility coefficients may have been a consequence of increased voluntary feed intakes. Free amino acid concentrations in systemic plasma provided some indication of growth performance which did not appear to be the case for free amino acid hepatic concentrations.
Author contributions
The overall feeding study was supervised by Shemil Macelline. Yangsu Kim and Yumin Bao conceptualised the trial design and Peter Chrystal formulated the diets. Leon McQuade and Bernard McInerney analysed free amino acid concentrations in systemic plasma and hepatic tissue and amino acid concentrations in diets and digesta. The manuscript was written by Shemil Macelline, Sonia Yun Liu and Peter Selle.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
We would like to thank Ms Joy Gill, Ms Kylie Warr, Mr Duwei Chen and Mr Peter Bird of the Peanut Research Foundation within the University of Sydney for their invaluable technical assistance. Also, we would like to acknowledge the financial support provided by Australian Government Research and Training Program International Scholarship (RTP) for the PhD candidature of Mr Shemil Macelline.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Baker D.H., Han Y. Ideal amino acid profile for chicks during the first three weeks post hatching. Poultry Sci. 1994;73:1441–1447. doi: 10.3382/ps.0731441. [DOI] [PubMed] [Google Scholar]
- Bunchasak C. Role of dietary methionine in poultry production. J Poultry Sci. 2009;46:169–179. [Google Scholar]
- Chrystal P.V., Selle P.H., Liu S.Y. Facilitating the acceptance of tangibly reduced-crude protein diets for chicken-meat production. Anim Nutr. 2020;6:247–257. doi: 10.1016/j.aninu.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S.A. Amino acid analysis protocols. Humana Press; 2001. Amino acid analysis using precolumn derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate; pp. 39–47. [DOI] [PubMed] [Google Scholar]
- Fernandez-Figares I., Lachica M., Pérez L., Nieto R., Aguilera J.F., Prieto C. The effect of dietary protein quality on free amino acids in plasma, muscle and liver of growing chickens. Anim Sci. 1993;57:309–318. [Google Scholar]
- Fernández-Figares I., Prieto C., Nieto R., Aguilera J.F. Free amino acid concentrations in plasma, muscle and liver as indirect measures of protein adequacy in growing chickens. Anim Sci. 1997;64:529–539. [Google Scholar]
- Fernández-Fígares I., Nieto R., Prieto C., Aguilera J.F. Plasma free amino acid profiles in growing chickens fed soyabean meal supplemented with DL-methionine. J Anim Feed Sci. 2005;14:283–296. [Google Scholar]
- Fernández-Fígares I., Nieto R., Aguilera J.F., Lachica M. Changes in tissue free amino acid pools in growing chickens fed thermally treated vetch diets. J Anim Physiol Anim Nutr. 2014;98:318–327. doi: 10.1111/jpn.12082. [DOI] [PubMed] [Google Scholar]
- Hill F.W., Anderson D.L. Comparison of metabolizable energy and productive energy determinations with growing chicks. J Nutr. 1958;64:587–603. doi: 10.1093/jn/64.4.587. [DOI] [PubMed] [Google Scholar]
- Kidd M.T., Tillman P.B., Waldroup P.W., Holder W. Feed-grade amino acid use in the United States: the synergetic inclusion history with linear programming. J Appl Poultry Res. 2013;22:583–590. [Google Scholar]
- Lemme A., Rostagno H.S., Petri A., Albino L.F. Standardised ileal digestibility of crystalline amino acids. Proceedings, European Symposium on Poultry Nutrition. Balatonfüred, Hungary. 2005;15:462–464. [Google Scholar]
- Liu S.Y., Rochell S.J., Maynard C.W., Caldas J.V., Kidd M.T. Digestible lysine concentrations and amino acid densities influence growth performance and carcass traits in broiler chickens from 14 to 35 days post-hatch. Anim Feed Sci Technol. 2019;255:114216. [Google Scholar]
- Massuquetto A., Panisson J.C., Marx F.O., Surek D., Krabbe E.L., Maiorka A. Effect of pelleting and different feeding programs on growth performance, carcass yield, and nutrient digestibility in broiler chickens. Poultry Sci. 2019;98:5497–5503. doi: 10.3382/ps/pez176. [DOI] [PubMed] [Google Scholar]
- Pacheco L.G., Sakomura N.K., Suzuki R.M., Dorigam J.C.P., Viana G.S., Van Milgen J., et al. Methionine to cystine ratio in the total sulfur amino acid requirements and sulfur amino acid metabolism using labelled amino acid approach for broilers. BMC Vet Res. 2018;14 doi: 10.1186/s12917-018-1677-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto C., Aguilera J.F., Fernández-Fígares I., Perez L., Nieto R., Ferrando G. The use of plasma free amino acids for predicting limiting amino acid(s) in diets for chickens. Anim Feed Sci Technol. 1994;47:151–164. [Google Scholar]
- Rochell S.J., Applegate T.J., Kim E.J., Dozier W.A. Effects of diet type and ingredient composition on rate of passage and apparent ileal amino acid digestibility in broiler chicks. Poultry Sci. 2012;91:1647–1653. doi: 10.3382/ps.2012-02173. [DOI] [PubMed] [Google Scholar]
- Rostagno H.S., Albino L.F.T., Hannas M.I., Donzele J.L., Sakomura N.K., Perazzo F.G., et al. Brazilian tables for poultry and swine: composition of feedstuffs and nutritional requirements. 2017. [Google Scholar]
- Sekiz S.S., Scott M.L., Nesheim M.C. The effect of methionine deficiency on bodyweight, food and energy utilization in the chick. Poultry Sci. 1975;54:1184–1188. doi: 10.3382/ps.0541184. [DOI] [PubMed] [Google Scholar]
- Selle P.H., Chrystal P.V., Liu S.Y. The cost of deamination in reduced-crude protein broiler diets. Proc, Aust Poult Sci Symp. Sydney. Australia. 2020;31:63–66. [Google Scholar]
- Siriwan P., Bryden W.L., Mollah Y., Annison E.F. Measurement of endogenous amino acid losses in poultry. Br Poultry Sci. 1993;34:939–949. doi: 10.1080/00071669308417654. [DOI] [PubMed] [Google Scholar]
- Tavárez M.A., de los Santos F.S. Impact of genetics and breeding on broiler production performance: a look into the past, present, and future of the industry. Anim Front. 2016;6:37–41. [Google Scholar]
- Teeter R.G., Smith M.O. Feed intake effects upon gain, carcass yield, and ration digestibility in broilers force fed five feed intakes. Poultry Sci. 1985;64:2155–2160. doi: 10.3382/ps.0642155. [DOI] [PubMed] [Google Scholar]
- Tillman P.B., Dozier W.A. Proceedings, Arkansas nutrition conference. Rogers, AK; 2013. Current amino acid considerations for broilers: responses ratios and economics. [Google Scholar]
- Truong H.H., Neilson K.A., McInerney B.V., Khoddami A., Roberts T.H., Liu S.Y., Selle P.H. Performance of broiler chickens offered nutritionally-equivalent diets based on two red grain sorghums with quantified kafirin concentrations as intact pellets or re-ground mash following steam-pelleting at 65 or 97 C conditioning temperatures. Anim Nutr. 2015;1:220–228. doi: 10.1016/j.aninu.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. Dietary requirements of synthesizable amino acids by animals: a paradigm shift in protein nutrition. J Anim Sci Biotechnol. 2014;5:1–12. doi: 10.1186/2049-1891-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. Functional amino acids in nutrition and health. Amino Acids. 2014;45:405–411. doi: 10.1007/s00726-013-1500-6. [DOI] [PubMed] [Google Scholar]