Abstract
Secretory Immunoglobulin A (sIgA) builds the first line of the human immune defense. It is not clear whether the power of this defence line is constant across the 24-h day, depends on sleep pressure levels and can be influenced by external lighting conditions. Thus, in 10 healthy young volunteers, we retrospectively analyzed saliva samples for sIgA levels under strictly controlled laboratory conditions across 40 h of extended wakefulness under two lighting conditions (dim light 8 lx and blue-enriched light 250 lx, 9000 K) to test for circadian and homeostatic sleep-wake influences. We compared the temporal profile of sIgA with the circadian time course of melatonin and cortisol along with subjective sleepiness levels, assessed in the same study by Gabel et al. (2017). The 40-h time course of sIgA exhibited a clear circadian modulation with peak values in the mornings coinciding with the individuals' habitual rise-time. In addition, sIgA levels progressively increased throughout the 40 h of extended wakefulness and were temporally correlated with subjective sleepiness but not with subjective ratings of tension and discomfort. In contrast to the circadian profile of melatonin and cortisol, sIgA levels were not significantly altered by the lighting conditions. Unexpectedly, sIgA levels in the morning after recovery sleep from 40 h of extended wakefulness rose considerably by more than an order of magnitude (10 times more) compared to morning levels after baseline sleep. We have evidence that diurnal sIgA levels in humans are regulated by the circadian timing system, and challenging the status of the sleep-wake homeostat (i.e. extended wakefulness) boosts human sIgA levels. Thus, besides a person's circadian phase position, the first line of human immune defense also strongly depends on the person's sleep-wake history and actual sleepiness levels. In sum, the fight against pathogenic microorganisms by a key immunological component (sIgA) is modulated by two fundamental processes implicated in human sleep-wake regulation.
Keywords: Constant routine protocol, Sleep pressure, Secretory IgA, Melatonin, Cortisol, Sleepiness, Cross-correlation, Radioimmunoassay, Enzyme-linked immunosorbent assay
1. Introduction
Secretory immunoglobulin A (sIgA) is the most frequently produced immunoglobulin. It is actively secreted through mucosal epithelia and thus can be found in high concentrations on all mucosal surfaces of the human body serving as a first line of defense against invading organisms (Kobayashi et al., 1987; Phalipon et al., 2002). For this reason, sIgA plays an important role as an antibody against respiratory and intestinal pathogenic germs (Lamm, 1998; Murphy et al., 2017; Mestecky and Russell, 2009; Pietrzak et al., 2020). The antibodies bind pathogens and thus prevent their entry into tissues and cells (neutralization), but they can also alter their surface by binding to surface proteins, which makes pathogens recognizable to phagocytosing cells. Besides its mostly ‘passive’ or anti-inflammatory role, IgA has also become known as an inducer of ‘active’ immunity by controlling cytokine and chemokine production (Hansen et al., 2019). The most common manifestations of a sIgA deficiency are infections of the upper respiratory tract, gastrointestinal complaints, allergic signs, neoplasms and other immunodeficiency syndromes (Zimmermann, 2005). Altogether, sIgA plays a key role in prevention of infectious disease.
When salivary sIgA levels are measured in healthy volunteers under standard conditions, the normal range occurs at approx. 60.3 ± 3.5 μg/mL (D'Amelio et al., 1982). Serum and saliva IgA levels are lower in females and increase in both genders with age (Zimmermann, 2005; Weber-Mzell et al., 2004). Environmental influences such as light levels, or time of day, and sleep or wake state have only been scarcely investigated so far. In particular, data on diurnal sIgA rhythms are not univocal. In contrast to Hennig (1994) (Hennig and Henning, 1994), who reported that sIgA in saliva was not subject to a diurnal rhythm but fluctuated unsystematically over the course of the day, other authors published contrary results. Stiller-Winkler et al. (1998) (Stiller-Winkler, 1998) found that sIgA concentrations peak upon morning awakening and are characterized by a steady decrease in concentration over the subsequent 4 h, afterwards, sIgA levels remained constant. The peak levels in the morning appeared to be less dependent on time of day than on the time of awakening. In addition, the morning fall in sIgA correlated with the morning increase in salivary cortisol (Hucklebridge et al., 1998; Shirakawa et al., 2004). Time of day variations with high morning sIgA levels were also observed in volunteers under conditions of prolonged physical exercise (cycling) (Li and Gleeson, 2004). To best of our knowledge, there have been only two studies assessing a full 24-h profile of sIgA in humans so far with conflicting results (Shirakawa et al., 2004; Park and Tokura, 1999). One study reported a 24-h rhythm for sIgA with low values during the day and peak values during the night, whereas the other study reported sIgA peak values to occur at 6:50 in the morning. Furthermore, nightly sIgA levels tended to increase after exposure to bright light of 5000lx compared to 200lx from 06:30–19:30 concomitant with an increase in night-time melatonin levels (Park and Tokura, 1999).
Based on these previous studies it is not clear whether sIgA levels show diurnal rhythms, which are related to sleep-wake rhythms, the volunteers' sleep inertia levels in the morning, or are controlled by the circadian timing system or reveal a combined circadian and sleep-wake related modulation. In addition, since sIgA levels have been repeatedly shown to depend on a person's stress level and are considered a stress marker (for a review see (Giacomello et al., 2020)), this factor needs to be controlled for when assessing diurnal influences on sIgA profiles. Thus, here we retrospectively analyzed diurnal sIgA profiles from our previous study (Gabel et al., 2017), which comprised a modified constant routing protocol to study circadian rhythms controlling for external influences (i.e. light, temperature, food, sleep, wakefulness etc.) and keeping internal influences such as stress levels as constant as possible. First, we aimed at testing whether sIgA levels show circadian and sleep-wake homeostatic influences during 40 h of extended wakefulness (i.e. constant routine) and compared its time course with established markers of the human circadian timing system such as salivary melatonin and salivary cortisol. Second, we aimed at testing whether differential illumination levels during the 40-h extended wakefulness period affect the time course of sIgA. Third, we tested whether sIgA levels differ after one recovery night from 40 h of wakefulness compared to a baseline night after 16 h of wakefulness. Fourth, we tested whether subjective stress levels across the 40-h of extended wakefulness modulate the diurnal time course of sIgA.
2. Methods
The here reported retrospective analyses were based on our previously published study. Thus, for more details on the methods, please be referred to Gabel et al. (2017).
Ethical approval
We sought written informed consent from all participants prior inclusion in the study. The study protocol, the screening procedures and consent forms followed the Declaration of Helsinki and were approved by the local ethics committee (EKBB/Ethikkommission beider Basel, Switzerland, Project identification code: 247/11).
2.1. Study volunteers
For the original study, healthy young and older male and female adults between 19 and 35 and between 55 and 75 years, respectively, were selected (Gabel et al., 2017). Only participants fulfilling all inclusion criteria, assessed by different questionnaires (for more information please see (Gabel et al., 2017)), were selected for a medical screening and a test night in our chronobiology laboratory letting them sleep with the entire polysomnographic setup. After a negative pregnancy test, all young female participants completed the study during the luteal phase of their menstrual cycle. One week prior laboratory admission, volunteers were asked to abstain from excessive alcohol and caffeine consumption. During this week they were also enquired to keep regular sleep-wake rhythms (i.e., a sleep duration of 8 h at night within a regular bedtime ± 30 min and no daytime napping) to ensure proper circadian entrainment between the sleep-wake and light-dark cycle. Volunteer's compliance was verified via wrist actigraphs (Actiwatch L, Cambridge Neurotechnologies, Cambridge, UK) and self-reported sleep logs. Thirty-eight healthy volunteers finally met all inclusion criteria out of an initial 650 potential participants. Out of the 38 study volunteers included in the initial study we selected only the young participants whose stored saliva samples were of good quality and still had enough saliva content for our retrospective analysis. We found a total of 10 volunteers (7 males, 3 females, mean age 23.8 ± 1.4 years) whose saliva samples fulfilled our quality criteria.
2.2. Study design and light settings
The entire in-laboratory part of the study included a 6-h baseline evening episode, followed by an 8-h baseline night sleep episode (BL), a 40-h total sleep deprivation (SD) ending with an 8-h recovery sleep episode (RC). The sleep-wake times were scheduled according to the individual's usual bedtime, which was calculated from the actigraphy and sleep-log data collected in the week prior to the in-laboratory part. The individual chronobiology suites for each participant were windowless, sound-attenuated, and temperature and humidity controlled without any access to time-of-day information. Participants could use the bathroom via a corridor outside the bedroom under dim light conditions (<8 lux) wearing blackened googles. After the scheduled 8-h baseline night at lights on, the light treatment started with a 40-h fluorescent white light exposure under 3 different conditions: a control dim light (DL: <8 lux, 2800 K) condition, a white light (WL: 250 lux, 2800 K) and a blue enriched white light (BL: 250 lux, 9000 K) condition (for more details on the light settings and lamp types see (Cajochen et al., 2019)). The irradiance in the DL condition was 0.0024 mW/cm2; photon irradiance: 6.58863 × 1016 photons/m2s, in the WL condition: 0.07 mW/cm2; photon irradiance: 2.00 × 1018 photons/m2s, and in the BL condition: 0.087 mW/cm2; photon irradiance: 2.30 × 1018 photons/m2s, this corresponds to a photoreceptor weighted irradiance for melanopsin of 0.4 μW/cm2 (2.93 melanopic equivalent daylight illuminance; melanopic EDI) for DL, 12.1 μW/cm2 (91.61 melanopic EDI) and 32.6 μW/cm2 (246 melanopic EDI) for BL.
Study volunteers were asked to participate in at least two light conditions, but one of the light condition was always the control DL condition. In order to have maximal contrast in melanopic EDI (2.93 vs. 246 melanopic EDI), we only selected participants who successfully completed the DL and BL condition for the here reported analyses. The use of any light-emitting electronic devices such as smartphones, tablets or laptops during the entire stay in the laboratory was not allowed. Standardized isocaloric meals were provided every 2 h during scheduled wakefulness. Participant's physical activity in their room was reduced to a minimum. They took scheduled computer tests (illuminance due to screen usage <10 lx) in regular time intervals and bathroom visits. In between the computer tests during scheduled wakefulness they were allowed reading, listening to music, writing or drawing, knitting, doing puzzles, and talking to the study helpers.
2.3. Collection of saliva for sIgA, melatonin, and cortisol
The participants were asked to take saliva samples at regular time intervals during scheduled wakefulness to later assess sIgA, melatonin and cortisol levels. Sampling rates dynamically changed with circadian phase, such that sampling frequency was decreased during the biological day when melatonin secretion is low (one sample every hour), and increased during the biological evening, night and early morning hours (one sample every 30 min). Salivary samples were immediately frozen and kept at −20 °C until the assays were conducted.
2.4. sIgA
Secretory IgA was measured by a direct salivary enzyme-linked immunosorbent assay (ELISA) (Salimetrics, Carlsbad, CA, USA). The minimum detectable dose was 2.5 μg/mL and the intra-assay coefficients of variation (CV) were 7% at 805 μg/mL, 5.3% at 336 μg/mL and 4.5% at 91 μg/mL.
2.5. Melatonin
The BÜHLMANN direct double-antibody radioimmunoassay was used for the melatonin assay (validated by gas chromatography–mass spectroscopy with an analytical least detectable dose of 0.65 pg/mL (Weber et al., 1997); distributed by NovoLytiX GmbH, Witterswil, Switzerland). The minimum detectable dose of melatonin (analytical sensitivity) was determined to be 0.2 pg/mL and the interassay coefficients of variation (CVs) were 20.1% at 0.60 pg/mL, 2.6% at 7.2 pg/mL, and 4.8% at 24.4 pg/mL (Weber et al., 1997).
2.6. Cortisol
Cortisol was measured using a direct salivary enzyme-linked immunosorbent assay (ELISA) (ALPCO Diagnostics, Salem, NH, USA), for quantitative determination of cortisol. The sensitivity was determined to be 1.0 ng/mL and the intra-assay coefficient of variance (CV) amounted to 10.3% for baseline values at 6.6 ng/mL.
2.7. Subjective levels of sleepiness, tension and discomfort
Volunteers rated their subjectively perceived levels of sleepiness, tension and discomfort on visual analogue scales (VAS) at exact the same time points of saliva collection (i.e. during the biological day in hourly intervals, during the biological evening, night and early morning hours in half-hourly intervals). The extremes on the right of the 100 mm VAS scales were: “extremely sleepy”, “extremely tensed”, “extreme (bodily) discomfort”.
2.8. Statistical analysis
For analyses of time courses, all the variables were collapsed into 2.5-hourly time bins on an individual level by taking the average per bin and per light condition. For graphical illustrations we averaged individual data across volunteers per bin and light condition. Two out of ten volunteers were excluded from further analyses, since they had incomplete data for at least on lighting condition. A mixed-model analysis of variance for repeated measures (PROC MIXED, statistical package SAS [version 9.1; SAS Institute, Cary, NC, USA]) with the within factors “light condition” (dim vs. blue-enriched), and “time of day” (16 time bins) was calculated for the following endpoints: sIgA, melatonin, cortisol, subjective sleepiness, subjective tension, and subjective discomfort. The factor “study participant” was defined as random and a compound symmetry or an autoregressive model [ar (1)] for equidistant time series was chosen as a covariance structure. The Least squares means statement was applied for post-hoc comparisons. Statistical differences were assumed to be significant at p < 0.05.
In addition, day 1 (i.e. first 16 h of scheduled wakefulness) vs. day 2 (last 16 h of scheduled wakefulness) were compared for the above mentioned endpoints by a Wilcoxon signed rank test.
All variables for which a cross-correlation analysis was performed were binned in 2.5-h intervals and aligned with respect to elapsed time awake. For each volunteer, cross correlations over sixteen 2.5-h time lags were performed. The individual correlation coefficients (r values) for each time lag interval were Fisher's z-transformed before averaging across volunteers. The resulting mean r values were retransformed for each time lag bin. Correlations that extended beyond ±2 SE were considered statistically significant.
3. Results
3.1. Circadian and sleep-wake homeostatic regulation of sIgA levels
Fig. 1 depicts the time course of the classical circadian markers melatonin and cortisol as well as sIgA under the dim light and blue-enriched light condition across the 40-h constant routine protocol. While the visual inspection of the time course of melatonin and cortisol confirmed a clear-cut circadian modulation for both of these circadian markers, the time course of sIgA yielded a combination of a circadian modulation superimposed on a homeostatic increase in sIgA levels across the duration of extended wakefulness. The highest sIgA levels were attained in the first time bin after waking up from the baseline night in the morning and at the same time bin in the morning the next day after being awake for 24 h. This is indicative of a circadian modulation of sIgA levels. In general, sIgA levels were significantly higher during the second day of extended wakefulness as compared to the first day (t = 2.99; p = 0.021). This is indicative of sleep-wake homeostatic modulation of sIgA levels.
Fig. 1.
Time course of salivary melatonin, cortisol and sIgA across the 40 h of extended wakefulness under constant routine conditions in dim and blue-enriched light conditions (mean values ± SEM). Black bars near the abscissas indicate the timing of habitual bedtime under non-sleep deprivation conditions. All data were binned in 2.5-intervals and plotted relative to the average habitual bedtime. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2. Effects of moderate light (246 vs. 2.93 melanopic EDI) on sIgA levels
We could confirm the melatonin and cortisol suppressing effect of blue-enriched light as reported in Gabel et al., (2019) (Gabel et al., 2017) also in this subsample of the original cohort. For both, a significance for the factor “light condition” was found (Table 1). For sIgA, the factor “light condition” did not yield significance. The significance for the factor “time of day” indicated a time-dependent modulation of sIgA across the 40 h of extended wakefulness (Table 1). In contrast to melatonin and cortisol, the time course of sIgA was not affected by our light regime. The significant interaction term “light condition” x “time of day” for melatonin was most likely due to the later melatonin onset in the evening observed after blue-enriched light, while melatonin offset was not affected (Fig. 1).
Table 1.
Results of the mixed model analysis of variance for melatonin, cortisol and sIgA. In bold results with p < 0.05.
| Variable | Light | Time of day | Light x Time of Day |
|---|---|---|---|
| Melatonin | F1,217 = 11.3; p = 0.009 | F15,217 = 19.0; p < 0.0001 | F15,217 = 1.9; p = 0.027 |
| Cortisol | F1,207 = 18.5; p < 0.001 | F15,201 = 22.1; p < 0.0001 | F15,201 = 0.6; p = 0.853 |
| sIgA | F1,217 = 1.7; p = 0.201 | F15,217 = 4.8; p < 0.0001 | F15,217 = 0.73; p = 0.756 |
3.3. Effect of recovery sleep from extended wakefulness (40 h) on sIgA levels
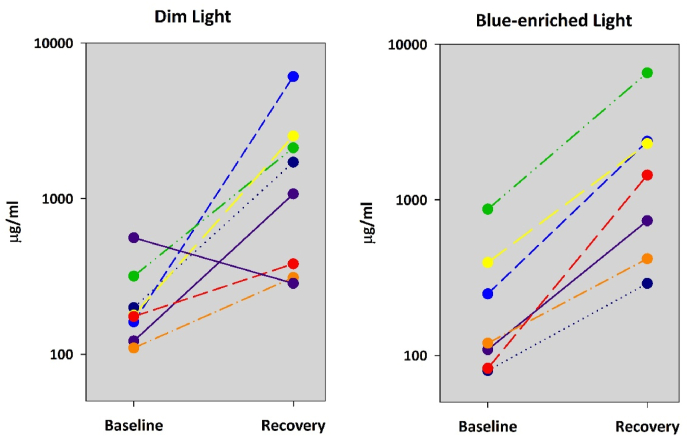
In order to focus on the morning values of sIgA, we also analyzed the first two saliva samples taken after the recovery night following the 40-h of extended wakefulness. Table 2 lists the collapsed values after the baseline and recovery night for the dim and blue-enriched light conditions. In both conditions, sIgA increased significantly after the recovery night from 40-h of extended wakefulness in comparison to the baseline night after 16 h of prior wakefulness, by a factor of 8.0 for the dim and a factor of 6.9 for the blue-enriched light condition respectively. Indeed, all volunteers, except one in the dim light condition, showed this strong sIgA increase in unison (Fig. 2). The same analysis for cortisol did not yield changes from the baseline to the recovery night, while for melatonin an increase in melatonin levels after the recovery compared to the baseline night was found only for the blue-enriched light condition (Table 2).
Table 2.
Results of the pairwise comparisons (Wilcoxon signed rank test) for melatonin (in pg/mL), cortisol (in ng/mL) and sIgA (in μg/mL) levels between the morning after the baseline night and the morning after the recovery night for the dim and blue-enriched light condition. In bold results with p < 0.05.
| Variable | Light | Baseline morning | Recovery morning | Baseline vs. Recovery |
|---|---|---|---|---|
| Melatonin | Dim | 4.4 ± 1.1 | 5.8 ± 2.1 | n.s. |
| Blue | 3.6 ± 0.9 | 10.2 ± 2.6 | p = 0.031 | |
| Cortisol | Dim | 24.8 ± 2.1 | 21.5 ± 1.4 | n.s. |
| Blue | 22.5 ± 1.9 | 23.4 ± 1.1 | n.s. | |
| sIgA | Dim | 227.7 ± 52.5 | 1813.4 ± 683.3 | p = 0.039 |
| Blue | 271.6 ± 94.2 | 1868.9 ± 726.4 | p = 0.008 |
Fig. 2.
Individual sIgA levels in the morning after the baseline night and in the morning after the recovery night. Prior scheduled wakefulness was 16 h prior the baseline night, and 40 h prior the recovery night. Note: to better illustrate the sIgA values were plotted on a log scale.
3.4. Association of subjective sleepiness, tension, and discomfort with sIgA levels
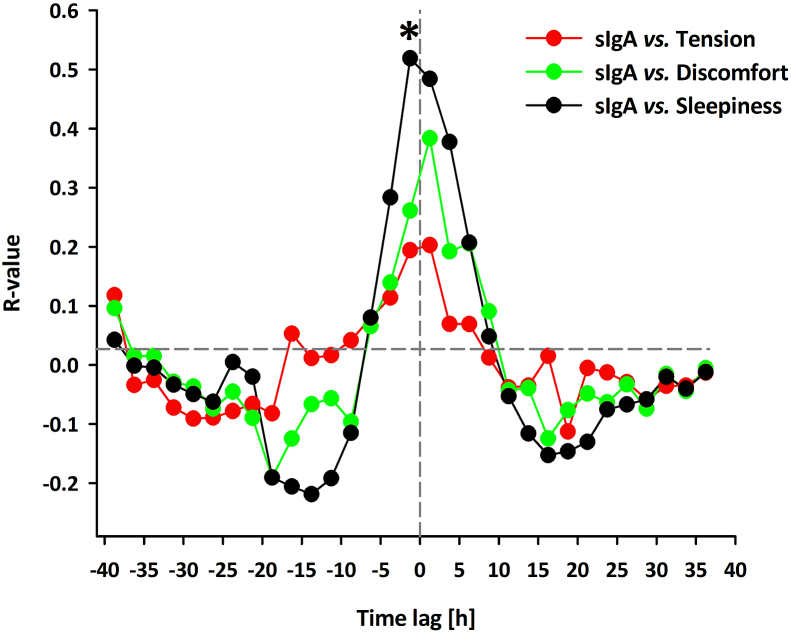
Fig. 3 depicts the time course of subjective sleepiness, tension, and discomfort levels across the 40 h of extended wakefulness under both light conditions. We could confirm a circadian and sleep-wake homeostatic modulation for subjective sleepiness also in this subsample of the young cohort as reported by Gabel et al., (2017) (Gabel et al., 2017). Moreover, we could replicate a significant alerting effect of blue-enriched light. The circadian and sleep-wake dependent pattern was less pronounced in the time course for subjective tension and discomfort. Although the factor ‘time of day’ yielded significance for both these measures (Table 3). This most likely came about a steady increase in both tension and discomfort across the 40 h of extended wakefulness. In addition, the factor light condition also yielded significance with lower levels of tension and discomfort during the blue-enriched light condition than during the dim light condition. Since the visual inspection of the 40-h time course of sIgA and the subjective markers of tension, discomfort and sleepiness revealed similarities, we computed cross-correlations between sIgA and the subjective markers on an individual level for time lags ranging from 40 to 0 h (Fig. 4). Peak correlations (i.e. highest r values) for the temporal association of sIgA and the subjective measures were found at time lags -1 to 1 (Fig. 4). In general, the sIgA levels correlated better with subjective sleepiness than with subjective tension and discomfort. However, none of the r-values surpassed the threshold for significance, except for the correlation between sIgA and subjective sleepiness at time lag -1 (r = 0.52 ± 0.25, p < 0.05).
Fig. 3.
Time course of subjectively perceived levels of sleepiness, tension, and discomfort across the 40 h of extended wakefulness under constant routine conditions in dim and blue-enriched light conditions (mean values ± SEM). Black bars near the abscissas indicate the timing of habitual bedtime under non-sleep deprivation conditions. All data were binned in 2.5-intervals and plotted relative to the average habitual bedtime. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Table 3.
Results of the mixed model analysis of variance for subjectively perceived sleepiness, tension and discomfort. In bold results with p < 0.05.
| Variable | Light | Time of day | Light x Time of Day |
|---|---|---|---|
| Sleepiness | F1,217 = 8.8; p = 0.003 | F15,217 = 19.2; p < 0.0001 | F15,217 = 0.52; p = 0.926 |
| Tension | F1,217 = 28.7; p < 0.001 | F15,217 = 2.15; p = 0.009 | F15,217 = 0.9; p = 0.565 |
| Discomfort | F1,217 = 30.9; p < 0.001 | F15,217 = 3.0; p = 0.0002 | F15,217 = 0.39; p = 0.98 |
Fig. 4.
Mean cross-correlation coefficients (after Fisher's z transformation; n = 8, average values) between sIgA vs. subjective tension, sIgA vs. subjective discomfort, and sIgA vs. subjective sleepiness. Cross correlation at time lag 0 represents the standard Pearson correlation coefficient. Positive lags indicate a later phase than sIgA (1 lag = 2.5 h). Data were binned in 2.5-h intervals for all variables; data are expressed with respect to elapsed time since scheduled waketime. Asterisks indicates time lags with significant correlation coefficients.
4. Discussion
Here we re-measured saliva samples collected in a previous study for sIgA concentrations across 40 h of extended wakefulness and in the morning after a recovery night. SIgA concentrations showed a strong circadian modulation with peak values in the morning at habitual rise times. The values decreased afterwards, reaching minimal values in the afternoon. We also found a strong influence of the homeostatic sleep-wake system, as indexed by a progressive increase of sIgA levels with the duration of scheduled wakefulness superimposed on the circadian modulation. Further, the circadian and homeostatic time course of sIgA was temporally associated with the time course of subjective sleepiness levels across the 40 h of extended wakefulness. Recovery after sleep deprivation triggered a massive increase in sIgA levels, which were one order of magnitude higher than the reported values in the normal range for healthy adults (D'Amelio et al., 1982).
4.1. Circadian modulation of sIgA
Previous studies have shown time of day effects for sIgA levels in humans (Hucklebridge et al., 1998; Shirakawa et al., 2004; Park and Tokura, 1999; Otsuki et al., 2004). However, whether these diurnal changes were driven by the circadian timing system, the sleep-wake cycle, or subjective alterations in perceived stress levels is still not clear. Our constant routine protocol has permitted control or elimination of major external masking effects (i.e. light, sound, temperature, social interactions) and has kept internal masking effects either at a reduced level (i.e. motor activity) or relatively constant (i.e. regular food intake) (Rietveld et al., 1993). Under such unmasking conditions, it is assumed that the circadian timing system modulates observed changes across time and not masking factors (Rietveld et al., 1993; Czeisler et al., 1985). Thus, we have strong evidence that the observed sIgA modulation is under circadian control, which corroborates findings in mice showing circadian clock-dependent changes in salivary IgA secretion (Wada et al., 2017). Salivary IgA secretion was highest during the middle of the light phase in these nocturnal animals, which corresponds to the middle of the dark/sleep phase in humans. Notably, the rhythm of salivary IgA secretion in these mice was lost after lesioning the central circadian pacemaker located in the suprachiasmatic nuclei (SCN) and after a clock gene mutation. In contrast to the study by Wada et al., 2017 (Wada et al., 2017) in animals and to the study by Park and Tokura, 1999 (Park and Tokura, 1999) in humans, sIgA values in our study did not peak in the middle of the rest/sleep period, but at usual rise time in the morning. This confirms previous studies, which reported peak values in the morning but did not measure sIgA levels at night (Hucklebridge et al., 1998; Shirakawa et al., 2004; Otsuki et al., 2004). In both the mice and human study, sleep was allowed and only interrupted for collecting saliva in the human study. Thus, it could be that sleep per se elicited a stimulatory effect on sIgA secretion, which we could not test in our constant routine protocol since our volunteers were not allowed to sleep for 40 h. However, we have evidence that sIgA levels have already started to increase before usual bedtime concomitantly with the evening onset of melatonin secretion. Accordingly, circulating concentrations of interleukin-6 (IL-6)showed a periodicity, with low values during the daytime and maximal values at night (Bauer et al., 1994). Similar to the sIgA levels in our study, IL-6 levels started to increase with the onset of melatonin (Redwine et al., 2000). Thus, besides actively opening the sleep gate in the late evening around the onset of melatonin (Shochat et al., 1998), the circadian pacemaker may also prepare the human immune response system in anticipation of the night-sleep period. Furthermore, it has been shown that sympathetic activation affects IgA secretion with strongest effects during the light phase in nocturnal animals (Wada et al., 2017). Since human sympathetic activation is also under circadian control (Viola et al., 2008; Scheer et al., 2010), it could very well be that the circadian-controlled sympathetic activation in our constant protocol elicited increases in sIgA levels. Interestingly, circadian regulated sympathetic activation starts before the usual rise time with peak values 2 h thereafter as indexed by a steep increase in heat production in the early morning (Krauchi and Wirz-Justice, 1994). This could explain the observed similarities in the time course of morning peak cortisol and sIgA levels in our study and a previous report (Hucklebridge et al., 1998). Indeed, also in nocturnal animals, the strongest sympathetic activation of salivary IgA was reported at Zeitgeber time 6, which corresponded to 6 h prior to the onset of the active phase of mice in their study (Wada et al., 2017).
4.2. Sleep-wake homeostatic modulation of sIgA
Although circadian rhythms can be evaluated and quantified in a constant routine protocol, a separation of circadian and homeostatic influence is impossible. Thus, any temporal dynamic of any variable measured in a constant routine protocol reflects an interacting modulation of the circadian timing and sleep-wake homeostatic system. This combined modulation was clearly seen in sIgA levels across the 40 h of scheduled wakefulness. Thus, besides a circadian modulation, we have strong evidence that the sleep-wake homeostatic system impacts sIgA levels. In other words, with the duration of being awake, sIgA levels rose progressively and yielded significantly higher values during the second day of extended wakefulness than during the first day. Unfortunately, human data on the impact of sleep deprivation on concentrations of immunoglobulins are scarce. We are aware of only one study measuring parameters of humoral immunity, including serum IgG, IgA, IgM and C3, C4, using an automatic analysis system under baseline and sleep deprivation conditions (Hui et al., 2007). Hui et al., 2007 (Hui et al., 2007) showed that the levels of IgG, IgA, IgM and C3, C4 were all higher in a sleep-deprived group than in a non-sleep deprived control group.
There are several comparable studies applying total sleep deprivation (40 h) under similarly controlled laboratory conditions as in our study. However, these studies focused on cytokines, particularly TNF-alpha and IL-6 (Redwine et al., 2000; Chennaoui et al., 2011; Frey et al., 2007; Irwin et al., 2016). Overall, results from these studies yielded mixed outcomes (i.e. increases, decreases and no effects across the sleep deprivation period). Apart from methodological issues among studies, these mixed results may be related to both pro- and anti-inflammatory action of IL-6 (Xing et al., 1998) and TNF-alpha. B cells (B Lymphocytes) are due to their well-characterized role in antibody production related to immunoglobulins (Tarlinton, 2019). A recent study found that even after a single and brief deprivation of sleep (6 h) (Korin et al., 2020), there was a significant increase in the B cell population in the mouse brain. In contrast, a human sleep deprivation study reported no change in leukocytes, lymphocytes, and B cells counts (Heiser et al., 2000).
4.3. Temporal association between subjective sleepiness, tension and discomfort in relation to sIgA
Many studies have reported that perceived stress levels modulate sIgA concentrations (Otsuki et al., 2004; Engeland et al., 2016; Deinzer et al., 2000; Takatsuji et al., 2008; Phillips et al., 2006; Trueba et al., 2012). Studies on acute stress have typically shown increases in sIgA levels (Takatsuji et al., 2008; Trueba et al., 2012), while evidence from chronic stress studies points to decreased sIgA levels (Deinzer et al., 2000; Phillips et al., 2006). As a proxy for stress levels in our study, subjective feelings of tension and discomfort were moderate (below 50 on the VAS scale from 0 to 100) but also increased across the 40 h of scheduled wakefulness. In contrast, cortisol, a well-known stress marker, did not increase across the 40 h, confirming fairly stress-free experimental conditions. Our cross-correlations analyses indicate that the dynamics in subjective sleepiness were associated with sIgA levels rather than stress. However, it is difficult to tease apart the influence of tension, discomfort and sleepiness, since all these variables may not behave independently from each other. However, the increased sleepiness levels due to elevated sleep pressure levels should be considered as a modulatory factor in clinical settings measuring parameters of humoral immunity.
4.4. Large increase in sIgA levels after recovery sleep from sleep deprivation
The massive increase in sIgA levels in the morning after the recovery night from 40-h of scheduled wakefulness cannot be explained by time of day effects or other factors since the conditions were exactly the same after the baseline and recovery night. As morning cortisol levels did not change from baseline to recovery (Table 2), we exclude stress effects. The only plausible explanation is that the challenge (i.e. 40 h of extended wakefulness) in sleep-wake homeostasis triggered this sIgA increase. This could again be related to sympathetic activation during the recovery night since higher sympathetic activation has been shown during sleep in recovery nights from total sleep deprivation (Viola et al., 2008; Cajochen, 1993). Notably, during recovery sleep after 40 h of sleep deprivation, parasympathetic indexes decreased and sympathetic predominance increased (Viola et al., 2008). Thus, from the perspective of autonomic control of the heart, recovery sleep from sleep deprivation did not show restoration (i.e., low sympathetic predominance). Concurrent measurements of heart rate parameters and sIgA levels during recovery sleep would have shed light on the association between sympathetic activation and the massive elevation of sIgA observed in our study.
4.5. Effects of 40-h exposure to dim vs. moderate blue-enriched light on sIgA
Gabel et al. (2017) reported that blue-enriched light suppressed cortisol and melatonin, particularly during the melatonin secretory phase compared to dim light, which was also the case in our sub-cohort of the original sample. In contrast, we do not have evidence that blue-enriched light of moderate brightness (250 photopic lx) affected sIgA levels. At first glance, our finding contradict Park and Tokura (14), who found increased nighttime sIgA levels concomitant with increased melatonin levels after daytime exposure to bright polychromatic light of 5000 lx. Besides the different light intensities in our and their study, the timing of light exposure was also different (40 h of light exposure in our study vs daytime light exposure from 06:30–19:30 in their study). Daytime exposure to bright light has been reported to increase circadian amplitude in many studies, which could explain the reported increase in both melatonin and sIgA levels in their study. However, it still needs to be tested whether sIgA levels acutely respond higher than 250 lx light levels at night.
4.6. Limitations and strengths
Limitations of our study comprise its retrospective nature of re-analysing saliva samples from a study initially designed for other purposes and the modest number of study participants. Thus, we formulated the hypotheses post-hoc, but prior to the analysis of the saliva samples for sIgA. Despite these limitations, the present study has several strengths. In contrast to the previous studies on sIgA, data collection was conducted under stringently controlled laboratory conditions controlling food intake, light exposure, and posture change during the assessment phase in the sleep laboratory. Finally, measures of subjective sleepiness, tension, and discomfort were assessed repeatedly across the entire study protocol. Finally, the dynamics of these variables were compared with the dynamics of sIgA and the classical markers of the circadian timing system (i.e. melatonin and cortisol) for the first time.
4.7. Clinical implications
Although we conducted our study with healthy participants in a very controlled laboratory environment without testing a specific clinical hypothesis, several potential clinical implications can be drawn from our observations. First, assessing sIgA levels when diagnosing IgA deficiencies in patients may depend on the time of the clinical visit. Since sIgA levels in the early morning are higher due to circadian influences, an IgA deficiency might be less likely to be captured in the morning compared to the afternoon. This is in line with a recent study (Holtkamp et al., 2021) showing that immune function is highest in the resting phase, shortly before activity resumes, which is in the early morning in humans. These and our results may suggest that when administering vaccines or immunotherapies against cancer, the time of day should possibly be taken into account to increase their effectiveness. Second, clinicians should ask their patients about their actual sleep-wake behaviour before diagnosing their immune status. As shown in our study, lack of sleep and increased sleepiness levels both boost sIgA levels considerably. Since lack of sleep and circadian misalignments are very common in our modern society, sIgA may be chronically enhanced in a significant proportion of our population. Third, it is not clear which immunological function of IgA is implicated in the circadian-related increase in sIgA levels in the morning and the massive increase after one recovery night from total sleep deprivation. It could either reflect IgA's function that binds to and neutralizes pathogens to prevent infection at mucosal sites of the body, or IgA that can contribute to the initiation of inflammation actively (Hansen et al., 2019). Thus, we are not sure whether our sleep deprived volunteers initiated a mild inflammation. Data suggest that acute sleep loss triggers inflammatory processes (Mullington et al., 2010). At least a single night of recovery sleep after 40 h of extended wakefulness was not enough to return to baseline sIgA levels. Studies in a clinical setting are required to shed more light on whether sIgA levels are elevated in chronically sleep-restricted patients and whether they return to normal levels after sufficient sleep.
4.8. Summary
Here we report evidence that sIgA levels depend on a person's circadian phase position (i.e. internal time) and sleepiness levels during wakefulness. Furthermore, challenging the sleep homeostat (i.e. sleep deprivation) leads to acute and substantial elevation of sIgA levels which manifests themselves particularly after recovery sleep from sleep deprivation. In sum, our data underline the importance of the circadian and sleep-wake process as critical modulators of an essential immunological component, sIgA, implicated in the defence against microbial and viral infections, which start at mucosal surfaces and comprise approximately 95% of all infections (Sato and Kiyono, 2012).
Author contributions
C.C. initiated writing of this paper and managed its execution as well as the final analysis. C.C. wrote an initial draft. V.G. carried out the study, was responsible for the study administration and the analysis of the melatonin, cortisol and subjective sleepiness data. A.F.E.was responsible for the sIgA measurements and J.W. supervised the sIgA analyses. C.C. served as supervisor of the study. K.K. J.W. and V.G. contributed equally and provided critical review of and revisions to the manuscript. All authors have approved the final version of this manuscript.
Funding
This research was supported in part by the Velux Foundation Switzerland, Philips, The Netherlands, and in part by the Swiss National Foundation (#310030_130689; P1BSP1-155209), and Toshiba Materials.
Declaration of competing interest
C.C. has had the following commercial interests in the last two years (2019–2020) related to lighting: honoraria, travel, accommodation and/or meals for invited keynote lectures, conference presentations or teaching from Toshiba Materials, Velux, Firalux, Lighting Europe, Electrosuisse, Novartis, Roche, Elite, Servier, and WIR Bank. C.C. is a member of the Daylight Academy. J.W. is the founder and A.F.E. employee of NovoLytiX GmbH. K.K. was an employee of Toshiba Materials, Japan and is now employed by Seoul Semiconductors. V.G does not report any conflict of interest.
Acknowledgments
We thank Dr. Krebs for medical screenings, Claudia Renz, Marie-France Dattler and all the helpers for their help in data acquisition, and the volunteers for participating in this demanding study.
References
- Bauer J., et al. Interleukin-6 serum levels in healthy persons correspond to the sleep-wake cycle. Clin. Invest. 1994;72(4):315. doi: 10.1007/BF00180048. [DOI] [PubMed] [Google Scholar]
- Cajochen C. ETH Zürich; Zürich: 1993. Heart Rate, Submental EMG and Core Body Temperature in Relation to EEG Slow-Wave Activity during Human Sleep: Effect of Light Exposure and Sleep Deprivation. [Google Scholar]
- Cajochen C., et al. Evidence that homeostatic sleep regulation depends on ambient lighting conditions during wakefulness. Clocks Sleep. 2019;1(4):517–531. doi: 10.3390/clockssleep1040040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chennaoui M., et al. Effect of one night of sleep loss on changes in tumor necrosis factor alpha (TNF-alpha) levels in healthy men. Cytokine. 2011;56(2):318–324. doi: 10.1016/j.cyto.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Czeisler C.A., et al. A clinical method to assess the endogenous circadian phase (ECP) of the deep circadian oscillator in man. Sleep. 1985;14:295. 295. [Google Scholar]
- D'Amelio R., et al. Serum and salivary IgA levels in normal subjects: comparison between tonsillectomized and non-tonsillectomized subjects. Int. Arch. Allergy Appl. Immunol. 1982;68(3):256–259. doi: 10.1159/000233108. [DOI] [PubMed] [Google Scholar]
- Deinzer R., et al. Prolonged reduction of salivary immunoglobulin A (sIgA) after a major academic exam. Int. J. Psychophysiol. 2000;37(3):219–232. doi: 10.1016/s0167-8760(99)00112-9. [DOI] [PubMed] [Google Scholar]
- Engeland C.G., et al. Psychological distress and salivary secretory immunity. Brain Behav. Immun. 2016;52:11–17. doi: 10.1016/j.bbi.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey D.J., Fleshner M., Wright K.P., Jr. The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav. Immun. 2007;21(8):1050–1057. doi: 10.1016/j.bbi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Gabel V., et al. Differential impact in young and older individuals of blue-enriched white light on circadian physiology and alertness during sustained wakefulness. Sci. Rep. 2017;7(1):7620. doi: 10.1038/s41598-017-07060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomello G., Scholten A., Parr M.K. Current methods for stress marker detection in saliva. J. Pharmaceut. Biomed. Anal. 2020;191:113604. doi: 10.1016/j.jpba.2020.113604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen I.S., Baeten D.L.P., den Dunnen J. The inflammatory function of human IgA. Cell. Mol. Life Sci. 2019;76(6):1041–1055. doi: 10.1007/s00018-018-2976-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiser P., et al. White blood cells and cortisol after sleep deprivation and recovery sleep in humans. Eur. Arch. Psychiatr. Clin. Neurosci. 2000;250(1):16–23. doi: 10.1007/pl00007534. [DOI] [PubMed] [Google Scholar]
- Hennig J. In: Die psychobiologische Bedeutung des sekretorischen Immunglobulin A im Speichel. Henning J., editor. Waxmann Verlag GmbH; Münster-New York: 1994. p. 262. [Google Scholar]
- Holtkamp S.J., et al. Circadian clocks guide dendritic cells into skin lymphatics. Nat. Immunol. 2021 doi: 10.1038/s41590-021-01040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucklebridge F., Clow A., Evans P. The relationship between salivary secretory immunoglobulin A and cortisol: neuroendocrine response to awakening and the diurnal cycle. Int. J. Psychophysiol. 1998;31(1):69–76. doi: 10.1016/s0167-8760(98)00042-7. [DOI] [PubMed] [Google Scholar]
- Hui L., et al. Effects of sleep and sleep deprivation on immunoglobulins and complement in humans. Brain Behav. Immun. 2007;21(3):308–310. doi: 10.1016/j.bbi.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Irwin M.R., Olmstead R., Carroll J.E. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol. Psychiatr. 2016;80(1):40–52. doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K., et al. Resistance of normal serum IgA and secretory IgA to bacterial IgA proteases: evidence for the presence of enzyme-neutralizing antibodies in both serum and secretory IgA, and also in serum IgG. Microbiol. Immunol. 1987;31(11):1097–1106. doi: 10.1111/j.1348-0421.1987.tb01341.x. [DOI] [PubMed] [Google Scholar]
- Korin B., et al. Short-term sleep deprivation in mice induces B cell migration to the brain compartment. Sleep. 2020;43(2) doi: 10.1093/sleep/zsz222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauchi K., Wirz-Justice A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am. J. Physiol. 1994;267(3 Pt 2):R819–R829. doi: 10.1152/ajpregu.1994.267.3.R819. [DOI] [PubMed] [Google Scholar]
- Lamm M.E. Current concepts in mucosal immunity. IV. How epithelial transport of IgA antibodies relates to host defense. Am. J. Physiol. 1998;274(4):G614–G617. doi: 10.1152/ajpgi.1998.274.4.g614. [DOI] [PubMed] [Google Scholar]
- Li T.L., Gleeson M. The effect of single and repeated bouts of prolonged cycling and circadian variation on saliva flow rate, immunoglobulin A and alpha-amylase responses. J. Sports Sci. 2004;22(11–12):1015–1024. doi: 10.1080/02640410410001716733. [DOI] [PubMed] [Google Scholar]
- Mestecky J., Russell M.W. Specific antibody activity, glycan heterogeneity and polyreactivity contribute to the protective activity of S-IgA at mucosal surfaces. Immunol. Lett. 2009;124(2):57–62. doi: 10.1016/j.imlet.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullington J.M., et al. Sleep loss and inflammation. Best Pract. Res. Clin. Endocrinol. Metabol. 2010;24(5):775–784. doi: 10.1016/j.beem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K., et al. The humoral immune response. Janeway's Immunobiol. 2017:399–444. ninth ed. [Google Scholar]
- Otsuki T., et al. Secretory IgA in saliva and academic stress. Int. J. Immunopathol. Pharmacol. 2004;17(2 Suppl. l):45–48. doi: 10.1177/03946320040170S208. [DOI] [PubMed] [Google Scholar]
- Park S.J., Tokura H. Bright light exposure during the daytime affects circadian rhythms of urinary melatonin and salivary immunoglobulin A. Chronobiol. Int. 1999;16(3):359–371. doi: 10.3109/07420529909116864. [DOI] [PubMed] [Google Scholar]
- Phalipon A., et al. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity. 2002;17(1):107–115. doi: 10.1016/s1074-7613(02)00341-2. [DOI] [PubMed] [Google Scholar]
- Phillips A.C., et al. Stressful life events are associated with low secretion rates of immunoglobulin A in saliva in the middle aged and elderly. Brain Behav. Immun. 2006;20(2):191–197. doi: 10.1016/j.bbi.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Pietrzak B., et al. Secretory IgA in intestinal mucosal secretions as an adaptive barrier against microbial cells. Int. J. Mol. Sci. 2020;21(23) doi: 10.3390/ijms21239254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redwine L., et al. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J. Clin. Endocrinol. Metab. 2000;85(10):3597–3603. doi: 10.1210/jcem.85.10.6871. [DOI] [PubMed] [Google Scholar]
- Rietveld W.J., Minors D.S., Waterhouse J.M. Circadian rhythms and masking: an overview. Chronobiol. Int. 1993;10(4):306–312. doi: 10.1080/07420529309059713. [DOI] [PubMed] [Google Scholar]
- Sato S., Kiyono H. The mucosal immune system of the respiratory tract. Curr. Opin. Virol. 2012;2(3):225–232. doi: 10.1016/j.coviro.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Scheer F.A., et al. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc. Natl. Acad. Sci. U. S. A. 2010;107(47):20541–20546. doi: 10.1073/pnas.1006749107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa T., Mitome M., Oguchi H. Circadian rhythms of S-IgA and cortisol in whole saliva —compensatory mechanism of oral immune system for nocturnal fall of saliva secretion—. Pediatr. Dent. J. 2004;14(1):115–120. [Google Scholar]
- Shochat T., Haimov I., Lavie P. Melatonin--the key to the gate of sleep. Ann. Med. 1998;30(1):109–114. doi: 10.3109/07853899808999392. [DOI] [PubMed] [Google Scholar]
- Stiller-Winkler R. Speichel als alternatives Untersuchungsmaterial zur Bestimmung immunologischer Parameter in epidemiologischen Studien? Hyg. Umweltmed. 1998;201:63–64. [Google Scholar]
- Takatsuji K., et al. The effects of examination stress on salivary cortisol, immunoglobulin A, and chromogranin A in nursing students. Biomed. Res. 2008;29(4):221–224. doi: 10.2220/biomedres.29.221. [DOI] [PubMed] [Google Scholar]
- Tarlinton D. B cells still front and centre in immunology. Nat. Rev. Immunol. 2019;19(2):85–86. doi: 10.1038/s41577-018-0107-2. [DOI] [PubMed] [Google Scholar]
- Trueba A.F., et al. Effects of psychosocial stress on the pattern of salivary protein release. Physiol. Behav. 2012;105(3):841–849. doi: 10.1016/j.physbeh.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Viola A.U., et al. PER3 polymorphism and cardiac autonomic control: effects of sleep debt and circadian phase. Am. J. Physiol. Heart Circ. Physiol. 2008;295(5):H2156–H2163. doi: 10.1152/ajpheart.00662.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada M., et al. Circadian clock-dependent increase in salivary IgA secretion modulated by sympathetic receptor activation in mice. Sci. Rep. 2017;7(1):8802. doi: 10.1038/s41598-017-09438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J.M., et al. A direct ultrasensitive RIA for the determination of melatonin in human saliva: comparison with serum levels. J. Sleep Res. 1997;26:757. [Google Scholar]
- Weber-Mzell D., et al. Gender, age and seasonal effects on IgA deficiency: a study of 7293 Caucasians. Eur. J. Clin. Invest. 2004;34(3):224–228. doi: 10.1111/j.1365-2362.2004.01311.x. [DOI] [PubMed] [Google Scholar]
- Xing Z., et al. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J. Clin. Invest. 1998;101(2):311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M.R. Ernst-Moritz-Arndt-Universität; Greifswald: 2005. Zur Alters- und Geschlechtsabhängigkeit des sekretorischen Immunglobulin A im Parotis-, Submandibularis- und Mischspeichel, in Medical Faculty; p. 84. [Google Scholar]