1. Introduction
From 2017 onward, we have published extensive data demonstrating that sodium-glucose co-transporter 2 inhibitors (SGLT2is) inhibit the Na+/H+ exchanger-1 (NHE-1) activity in cardiomyocytes from rabbits and mice.1–3 Subsequent studies by other groups have further demonstrated that SGLT2is also inhibits the NHE-1 in rat endothelial and human atrial cells.4,5 Chung et al.6 have recently reported that they cannot replicate these results and they conclude that the SGLT2is do not inhibit the cardiac NHE-1. There are numerous methodological differences between the contradictory studies. Chung et al. suggest that some of these differences explain the different outcomes. Chung et al. apply higher extracellular pHo (7.4 vs. 7.2), use a different pH-buffering system (HEPES vs. combined HEPES-low bicarbonate buffering), use a lower dimethylsulfoxide (DMSO) concentration (0.01% vs. 0.02), do not pace the cells and subject the cells to high-flow superfusion. Therefore, we set out to test whether these methodological differences can indeed explain the lack of NHE-1 inhibition by empagliflozin (EMPA) in the paper by Chung et al.
2. EMPA and NHE-1 activity
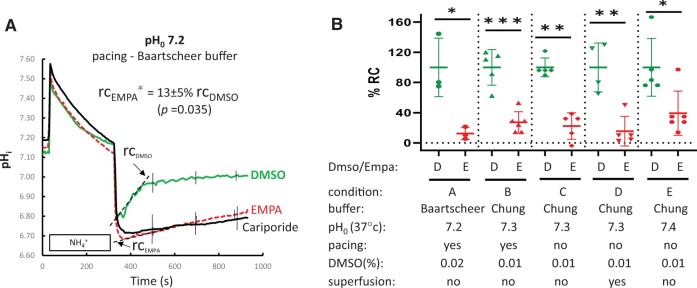
We tested the effect of EMPA under several conditions in our experimental set-up.1,2 For each condition, effects of control (0.01–0.02% DMSO), EMPA (1 µM), and the NHE-1 inhibitor cariporide (CARI; 10 µM) were tested on NHE-1 activity in brick-shaped, cross-striated, freshly isolated ventricular myocytes from 11 weeks old rabbits [male New Zealand White (Crl: KBL, n = 6), anaesthetized with i.p. 100 mg/kg ketamine and 20 mg/kg xylazine]. Rabbits were euthanized when hearts were excised for cell isolation in compliance with the approval of the Animal Care and Use Committee of our institution and following Directive 2010/63/EU. Activity of NHE-1 was analysed by the rate constant (rc) of a linear fit of ΔpHi over the first 100 s following withdrawal (see Figure 1A). First, we reconfirmed that EMPA strongly inhibited the cardiac NHE under our conditions ((pHo7.2 at 37° or pHo7.3 before heating), HEPES plus low HCO3, 0.02% DMSO, 2 Hz pacing, no flow, fast wash-out of NH4+ fluid by 2 mL): rcEMPA was only 13 ± 5% of rcDMSO (Figure 1A and B, condition A). Next, we selectively applied two of the following conditions from Chung et al.6 Only HEPES buffer, 0.01% instead of 0.02% DMSO at pHo 7.3 (condition B). Figure 1B shows that under these conditions EMPA still significantly inhibited the NHE-1: rcEMPA equalled 27 ± 6% of rcDMSO (P < 0.001). To examine whether adding another condition of Chung et al. may induce the loss of NHE-1 inhibition by EMPA, i.e., we either stopped pacing (condition C) or stopped pacing and added superfusion (condition D) to the conditions. In both conditions, inhibition on NHE-1 activity by EMPA remained significant and strong [rcEMPA was 16 ± 12% (P = 0.003)or 15 ± 9% (P = 0.002) of rcDMSO, respectively, Figure 1B, conditions C and D]. This indicating that differences in pacing and superfusion also do not explain the differences between our work and Chung et al. Finally, we increased pHo further from 7.3 to 7.4 (37°) as applied by Chung et al., leaving all other variables constant (condition E). Again, significant inhibition of EMPA on NHE-1 activity remained present (rcEMPA was 39 ± 12% of rcDMSO; P = 0.015) (Figure 1B, condition E). Thus, under all conditions used by Chung et al. NHE-1 inhibition by Empa remained intact in our experimental set-up.
Figure 1.
Empagliflozin inhibits NHE-1 activity in all experimental conditions tested. (A) NHE-1 activity reflected by time course of pH recovery following wash-out for DMSO control, EMPA and CARI in isolated rabbit ventricular myocytes for condition A (pHo = 7.2, Baartscheer (HEPES + low HCO3)-buffer, 2 Hz pacing, 0.02% DMSO, no flow), NHE-1 activity index by rc (- - - -), the rate constant (Δ pH/Δ s) of linear fit over first 100 s of pH recovery. rcEMPA provided as % rcDMSO, all groups n = 3 cells/3 rabbits, data means ± standard error of the mean; for clarity error bars only provided for 500, 700, and 900 s. (B) Summary of individual % RC for DMSO and EMPA measurements for all five conditions. %RC relative to the normalized value for DMSO measurements for each condition. Condition A (DMSO and EMPA n = 3 cells/3 rabbits), condition B (DMSO n = 5 cells/3 rabbits, Empa n = 6 cells/3 rabbits), condition C (DMSO and EMPA n = 5 cells/4 rabbits), condition D (DMSO n = 4 cells/4 rabbits, EMPA n = 5 cells/4 rabbits), condition E (DMSO n = 5 cells/3 rabbits, Empa n = 6 cells/3 rabbits) *P < 0.05, ** P < 0.01, *** P < 0.001 rcEMPA vs. rcDMSO by two-tail, unpaired student’s t-test.
Chung et al. show a significant pH recovery in the presence of CARI. This indicates that other active pH neutralizing systems are present in their assay and that the assay used by Chung et al. is not 100% specific for NHE-1 activity. This is supported by the faster pH recovery under DMSO in their results compared to ours. The assay used by Chung et al. contrasts with our assay that shows CARI prevented pH recovery (Figure 1A and data not shown for conditions B–E).
Thus, our experiments indicate that the absence of NHE-1-inhibition by EMPA reported in Chung et al. cannot be explained by any of the deviating experimental conditions applied by Chung et al., as was partly suggested by these authors. We suggest that the non-specificity of their NHE-1 assay, together with a possible low sensitivity of their set-up to detect NHE-1 inhibition by EMPA, may, at least partly, explain why no inhibition by EMPA was detected. Further research will be necessary to elucidate this in more detail. We here demonstrate that the inhibitory effect of EMPA remains under each condition tested.
3. EMPA and intracellular sodium
Measurement of intracellular sodium ([Na+]i) is notoriously difficult. We have optimized these measurements by applying a dual emission mode to quantify small Δ[Na+]I in a small measurement chamber (30 µL).1 Chung et al.6 state that EMPA did not change baseline [Na+]I in isolated cardiomyocytes (their Fig. 3A), but they used a much larger chamber and a suboptimal excitation mode (emission ratio). We transformed their ratio-data into [Na+]I, by assuming a Rmin = 0.93, Kd = 25 and average rat [Na+]i of 12 mM. Both EMPA and CARI lowered [Na+]i to the same degree (14.2 ± 1.38 to 11.9 ± 0.58 and 16.5 ± 2.22 to 13.5 ± 0.70 mM, respectively, mean ± standard error of the mean). DMSO showed a small increase of [Na+]I by 0.9 mM. Thus, the data presented by Chung et al. do not deviate from ours.
4. EMPA and NHE-1 inhibition in intact isolated heart
Chung et al. also provided data (cardiac mechanical function and energetics) showing that they were unable to find evidence of NHE-1-inhibition by EMPA in Langendorff-perfused healthy rat hearts, thereby reproducing our results in healthy mouse hearts.2 However, under pathological conditions, EMPA delayed contracture development during ischaemia in healthy mouse hearts3 and reduced lactate generation in diabetic hearts,7 both in an NHE-1 dependent fashion. Of note, EMPA, in contrast to Cariporide, was unable to reduce the development of infarct size during reperfusion.3 Thus, also in the intact heart, EMPA can have NHE-1 inhibitory effects particularly in pathological conditions.
5. Conclusions
In summary, we show that our data can be replicated and that the failure to replicate them by Chung et al. cannot be ascribed to some of the deviating experimental conditions between both studies, as was partly suggested by the authors. It cannot be excluded that the non-specificity of their NHE-1 assay contributes to some of the differences. Calculated [Na+]i suggests that NHE is also inhibited in the conditions applied by Chung et al. We therefore reconfirm that SGLT2i inhibits the cardiac NHE when measured under multiple experimental conditions. This has been confirmed by others in other cell types5 and in myocytes from humans.4
Conflict of interest: none declared.
Data availability
The data underlying this article will be shared upon reasonable request to the corresponding author.
References
- 1. Baartscheer A, Schumacher CA, Wüst RCI, Fiolet JWT, Stienen GJM, Coronel R, Zuurbier CJ.. Empagliflozin decreases myocardial cytoplasmic Na+ through inhibition of the cardiac Na+/H+ exchanger in rats and rabbits. Diabetologia 2017;60:568–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Uthman L, Baartscheer A, Bleijlevens B, Schumacher CA, Fiolet JWT, Koeman A, Jancev M, Hollmann MW, Weber NC, Coronel R, Zuurbier CJ.. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na+/H+ exchanger, lowering of cytosolic Na+ and vasodilation. Diabetologia 2018;61:722–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Uthman L, Nederlof R, Eerbeek O, Baartscheer A, Schumacher C, Buchholtz N, Hollmann MW, Coronel R, Weber NC, Zuurbier CJ.. Delayed ischaemic contracture onset by empagliflozin associates with NHE1 inhibition and is dependent on insulin in isolated mouse hearts. Cardiovasc Res 2019;115:1533–1545. [DOI] [PubMed] [Google Scholar]
- 4. Trum M, Riechel J, Lebek S, Pabel S, Sossalla ST, Hirt S, Arzt M, Maier LS, Wagner S.. Empagliflozin inhibits Na+/H+ exchanger activity in human atrial cardiomyocytes. ESC Heart Fail 2020;7:4429–4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cappetta D, De Angelis A, Ciuffreda LP, Coppini R, Cozzolino A, Miccichè A, Dell'Aversana C, D’Amario D, Cianflone E, Scavone C, Santini L, Palandri C, Naviglio S, Crea F, Rota M, Altucci L, Rossi F, Capuano A, Urbanek K, Berrino L.. Amelioration of diastolic dysfunction by dapagliflozin in a non-diabetic model involves coronary endothelium. Pharmacol Res 2020;157:104781. [DOI] [PubMed] [Google Scholar]
- 6. Chung YJ, Park KC, Tokar S, Eykin TR, Fuller W, Pavlovic D, Swietach P, Shattock MJ.. Off-target effects of sodium-glucose co-transporter 2 blockers: empagliflozin does not inhibit Na+/H+ exchanger-1 or lower [Na+]I in the heart. Cardiovasc Res 2021;117:2794–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang H, Uthman L, Bakker D, Sari S, Chen S, Hollmann MW, Coronel R, Weber NC, Houten SM, van Weeghel M, Zuurbier CJ.. Empagliflozin decreases lactate generation in an NHE-1 dependent fashion and increases α-ketoglutarate synthesis from palmitate in type II diabetic mouse hearts. Front Cardiovasc Med 2020;7:592233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.