Abstract
Background
The use of surgical drains is a very common practice after pancreatic surgery. The role of prophylactic abdominal drainage to reduce postoperative complications after pancreatic surgery is controversial. This is the third update of a previously published Cochrane Review to address the uncertain benifits of prophylactic abdominal drainage in pancreatic surgery.
Objectives
To assess the benefits and harms of routine abdominal drainage after pancreatic surgery, compare the effects of different types of surgical drains, and evaluate the optimal time for drain removal.
Search methods
In this updated review, we re‐searched CENTRAL, MEDLINE, Embase, Science Citation Index Expanded, and the Chinese Biomedical Literature Database (CBM) on 08 February 2021.
Selection criteria
We included all randomised controlled trials (RCTs) that compared abdominal drainage versus no drainage in people undergoing pancreatic surgery. We also included RCTs that compared different types of drains and different schedules for drain removal in people undergoing pancreatic surgery.
Data collection and analysis
Two review authors independently identified the studies for inclusion, collected the data, and assessed the risk of bias. We conducted the meta‐analyses using Review Manager 5. We calculated the risk ratio (RR) for dichotomous outcomes and the mean difference (MD) or standardized mean difference (SMD) for continuous outcomes with 95% confidence intervals (CI). For all analyses, we used the random‐effects model. We used GRADE to assess the certainty of the evidence for important outcomes.
Main results
We identified a total of nine RCTs with 1892 participants.
Drain use versus no drain use
We included four RCTs with 1110 participants, randomised to the drainage group (N = 560) and the no drainage group (N = 550) after pancreatic surgery. Low‐certainty evidence suggests that drain use may reduce 90‐day mortality (RR 0.23, 95% CI 0.06 to 0.90; two studies, 478 participants). Compared with no drain use, low‐certainty evidence suggests that drain use may result in little to no difference in 30‐day mortality (RR 0.78, 95% CI 0.31 to 1.99; four studies, 1055 participants), wound infection rate (RR 0.98, 95% CI 0.68 to 1.41; four studies, 1055 participants), length of hospital stay (MD ‐0.14 days, 95% CI ‐0.79 to 0.51; three studies, 876 participants), the need for additional open procedures for postoperative complications (RR 1.33, 95% CI 0.79 to 2.23; four studies, 1055 participants), and quality of life (105 points versus 104 points; measured with the pancreas‐specific quality of life questionnaire (scale 0 to 144, higher values indicating a better quality of life); one study, 399 participants). There was one drain‐related complication in the drainage group (0.2%). Moderate‐certainty evidence suggests that drain use probably resulted in little to no difference in morbidity (RR 1.03, 95% CI 0.94 to 1.13; four studies, 1055 participants). The evidence was very uncertain about the effect of drain use on intra‐abdominal infection rate (RR 0.97, 95% CI 0.52 to 1.80; four studies, 1055 participants; very low‐certainty evidence), and the need for additional radiological interventions for postoperative complications (RR 0.87, 95% CI 0.40 to 1.87; three studies, 660 participants; very low‐certainty evidence).
Active versus passive drain
We included two RCTs involving 383 participants, randomised to the active drain group (N = 194) and the passive drain group (N = 189) after pancreatic surgery. Compared with a passive drain, the evidence was very uncertain about the effect of an active drain on 30‐day mortality (RR 1.23, 95% CI 0.30 to 5.06; two studies, 382 participants; very low‐certainty evidence), intra‐abdominal infection rate (RR 0.87, 95% CI 0.21 to 3.66; two studies, 321 participants; very low‐certainty evidence), wound infection rate (RR 0.92, 95% CI 0.44 to 1.90; two studies, 321 participants; very low‐certainty evidence), morbidity (RR 0.97, 95% CI 0.53 to 1.77; two studies, 382 participants; very low‐certainty evidence), length of hospital stay (MD ‐0.79 days, 95% CI ‐2.63 to 1.04; two studies, 321 participants; very low‐certainty evidence), and the need for additional open procedures for postoperative complications (RR 0.44, 95% CI 0.11 to 1.83; two studies, 321 participants; very low‐certainty evidence). There was no drain‐related complication in either group.
Early versus late drain removal
We included three RCTs involving 399 participants with a low risk of postoperative pancreatic fistula, randomised to the early drain removal group (N = 200) and the late drain removal group (N = 199) after pancreatic surgery. Compared to late drain removal, the evidence was very uncertain about the effect of early drain removal on 30‐day mortality (RR 0.99, 95% CI 0.06 to 15.45; three studies, 399 participants; very low‐certainty evidence), wound infection rate (RR 1.32, 95% CI 0.45 to 3.85; two studies, 285 participants; very low‐certainty evidence), hospital costs (SMD ‐0.22, 95% CI ‐0.59 to 0.14; two studies, 258 participants; very low‐certainty evidence), the need for additional open procedures for postoperative complications (RR 0.77, 95% CI 0.28 to 2.10; three studies, 399 participants; very low‐certainty evidence), and the need for additional radiological procedures for postoperative complications (RR 1.00, 95% CI 0.21 to 4.79; one study, 144 participants; very low‐certainty evidence). We found that early drain removal may reduce intra‐abdominal infection rate (RR 0.44, 95% CI 0.22 to 0.89; two studies, 285 participants; very low‐certainty evidence), morbidity (RR 0.49, 95% CI 0.30 to 0.81; two studies, 258 participants; very low‐certainty evidence), and length of hospital stay (MD ‐2.20 days, 95% CI ‐3.52 to ‐0.87; three studies, 399 participants; very low‐certainty evidence), but the evidence was very uncertain. None of the studies reported on drain‐related complications.
Authors' conclusions
Compared with no drain use, it is unclear whether routine drain use has any effect on mortality at 30 days or postoperative complications after pancreatic surgery. Compared with no drain use, low‐certainty evidence suggests that routine drain use may reduce mortality at 90 days. Compared with a passive drain, the evidence is very uncertain about the effect of an active drain on mortality at 30 days or postoperative complications. Compared with late drain removal, early drain removal may reduce intra‐abdominal infection rate, morbidity, and length of hospital stay for people with low risk of postoperative pancreatic fistula, but the evidence is very uncertain.
Keywords: Humans, Abdomen, Abdomen/surgery, Drainage, Length of Stay, Pancreas, Pancreatic Fistula
Plain language summary
Drain use after pancreatic surgery
Review question
Can the use of a drain reduce postoperative complications after pancreatic surgery?
Background
The use of surgical drains is a very common practice after pancreatic surgery. However, the role of a drain in reducing complications after pancreatic surgery (called postoperative complications) is controversial.
Search date
The evidence is current to February 2021.
Study characteristics
We searched for all relevant, well‐conducted studies to February 2021. We included nine randomised controlled studies (an experiment in which participants are randomly allocated to two or more interventions, possibly including a control intervention or no intervention, and the results are compared). The nine studies included 1892 participants who underwent pancreatic surgery. Four of the nine studies randomised 1110 participants to drain use (number of participants = 560) or no drain use (N = 550). Two studies randomised 383 participants to an active drain (drains with low or high pressure suction, N = 194) and a passive drain (drains without suction, N = 189). Three studies randomised 399 participants with a low risk of postoperative pancreatic fistula (an abnormal communication between the pancreas and other organs due to leakage of pancreatic juice containing digestive enzymes from damaged pancreatic ducts) to early drain removal (N = 200) or late drain removal (N = 199).
Study funding sources
Five of the nine included studies were sponsored by non‐commercial grants. Two studies did not receive any funding. The other two studies did not report funding sources.
Key results
Drain use may reduce death at 90 days. Compared with no drain use, the evidence suggested that drain use may result in little to no difference in death at 30 days, wound infections, duration of hospitalization, the need for additional open procedures for postoperative complications, and quality of life. There was one drain‐related complication (the drainage tube was broken) in the drainage group (0.2%). Drain use probably resulted in little to no difference in overall complications. The evidence was very uncertain about the effect of drain use on infections in the abdomen and the need for additional radiological interventions for postoperative complications.
Compared with a passive drain, the evidence was very uncertain about the effect of an active drain on death at 30 days, infections in the abdomen, wound infections, overall complications, duration of hospitalization, and the need for additional open procedures for postoperative complications. There was no drain‐related complication in either group.
The evidence was very uncertain about the effect of early drain removal on death at 30 days, wound infections, hospital costs, and the need for additional open or radiological procedures for postoperative complications. We found that early drain removal may reduce infections in the abdomen, overall complications, and duration of hospitalization, but the evidence was very uncertain. None of the studies reported on drain‐related complications.
It is unclear whether routine drain use had any effect on death at 30 days or complications after surgery, compared with no drain use. Routine drain use may reduce death at 90 days compared with no drain use. The evidence is very uncertain about the effect of an active drain on death at 30 days or postoperative complications, compared with a passive drain. Compared with late drain removal, early drain removal appears to reduce infections in the abdomen, overall complications, duration of hospitalization for people with a low risk of postoperative pancreatic fistula, but the evidence is very uncertain.
Certainty of the evidence
All nine studies had weaknesses that potentially affected the reliability of the results. Overall, the certainty of the evidence ranged from very low to moderate.
Summary of findings
Summary of findings 1. Drain use versus no drain use following pancreatic surgery.
| Drain use versus no drain use following pancreatic surgery | ||||||
| Patient or population: people undergoing elective pancreatic resections Setting: hospital Intervention: drain use Comparison: no drain use | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no drain use | Risk with drain use | |||||
|
Mortality Follow‐up: 30 days |
23 per 1000 | 18 per 1000 (7 to 46) |
RR 0.78 (0.31 to 1.99) | 1055 (4 studies) | ⊕⊕⊝⊝ Lowa,b | Low‐certainty evidence suggested that drain use may result in little to no difference in 30‐day mortality compared with no drain use. |
|
Mortality Follow‐up: 90 days |
42 per 1000 | 10 per 1000 (3 to 38) |
RR 0.23 (0.06 to 0.90) | 478 (2 studies) | ⊕⊕⊝⊝ Lowc,d | Low‐certainty evidence suggested that drain use may result in a slight reduction in 90‐day mortality compared with no drain use. |
|
Intra‐abdominal infection Follow‐up: 30 days |
82 per 1000 | 80 per 1000 (43 to 148) |
RR 0.97 (0.52 to 1.80) | 1055 (4 studies) | ⊕⊝⊝⊝ Very lowa,b,e | The evidence was very uncertain about the effect of drain use on intra‐abdominal infection rate compared with no drain use. |
|
Wound infection Follow‐up: 30 days |
99 per 1000 | 97 per 1000 (68 to 140) |
RR 0.98 (0.68 to 1.41) | 1055 (4 studies) | ⊕⊕⊝⊝ Lowa,b | Low‐certainty evidence suggested that drain use may result in little to no difference in wound infection rate compared with no drain use. |
|
Drain‐related complications Follow‐up: 30 days |
See comment | See comment | Not estimable | 179 (1 study) | ⊕⊕⊝⊝ Lowf,g | Low‐certainty evidence suggested that drain use may result in little to no difference in drain‐related complications compared with no drain use. There was 1 drain‐related complication in the drainage group. The drainage tube was broken. |
|
Morbidity Follow‐up: 30 days |
597 per 1000 | 614 per 1000 (561 to 674) |
RR 1.03 (0.94 to 1.13) | 1055 (4 studies) | ⊕⊕⊕⊝ Moderate a | Moderate‐certainty evidence suggested that drain use probably resulted in little to no difference in morbidity compared with no drain use. |
|
Length of hospital stay Follow‐up: 30 days |
The mean length of hospital stay in the no drain groups was 11.3 days | The mean length of hospital stay in the drain groups was 0.14 days lower (0.79 lower to 0.51 higher) | MD ‐0.14 (‐0.79 to 0.51) | 876 (3 studies) | ⊕⊕⊝⊝ Lowh | One study including 179 participants reported the median and range values which were not suitable for pooling. This study reported no difference in the length of hospital stay between groups. Low‐certainty evidence suggested that drain use may result in little to no difference in length of hospital stay compared with no drain use. |
| * The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the control group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty. We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty. We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty. Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty. We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level for serious risk of bias: three studies with high risk of incomplete outcome data bDowngraded one level for serious imprecision: few events and a confidence interval that includes both potential benefit and potential harm from the intervention cDowngraded one level for serious risk of bias: two studies with high risk of incomplete outcome data dDowngraded one level for serious imprecision: few events eDowngraded one level for serious unexplained inconsistency: substantial heterogeneity I²= 52% fNot downgraded for risk of bias because this is considered an objective outcome that is unlikely to be affected by selection bias or performance bias gDowngraded two levels for very serious imprecision: small sample size and very few events hDowngraded two levels for very serious risk of bias: three studies with high risk of incomplete outcome data, all studies with high risk of performance bias, and this outcome was primarily determined by the surgeons
Summary of findings 2. Active drain versus passive drain following pancreatic surgery.
| Active drain versus passive drain following pancreatic surgery | ||||||
| Patient or population: people undergoing elective pancreatic resections Setting: hospital Intervention: active drain Comparison: passive drain | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with passive drain | Risk with Active drain | |||||
|
Mortality Follow‐up: 30 days |
16 per 1000 | 20 per 1000 (5 to 80) | RR 1.23 (0.30 to 5.06) | 382 (2 studies) | ⊕⊝⊝⊝ Very lowa,b | The evidence was very uncertain about the effect of an active drain on 30‐day mortality compared with passive drain. |
|
Mortality Follow‐up: 90 days |
Not reported | |||||
|
Intra‐abdominal infection Follow‐up: 30 days |
101 per 1000 | 88 per 1000 (21 to 368) | RR 0.87 (0.21 to 3.66) | 321 (2 studies) | ⊕⊝⊝⊝ Very lowa,b | The evidence was very uncertain about the effect of an active drain on intra‐abdominal infection rate compared with passive drain. |
|
Wound infection Follow‐up: 30 days |
88 per 1000 | 81 per 1000 (39 to 167) | RR 0.92 (0.44 to 1.90) | 321 (2 studies) | ⊕⊝⊝⊝ Very lowa,b | The evidence was very uncertain about the effect of an active drain on wound infection rate compared with passive drain. |
|
Drain‐related complications Follow‐up: 30 days |
See comment | See comment | Not estimable | 223 (1 study) |
⊕⊝⊝⊝ Very Lowa,b | There were no drain‐related complications in either group. |
|
Morbidity Follow‐up: 30 days |
370 per 1000 | 359 per 1000 (196 to 656) | RR 0.97 (0.53 to 1.77) | 382 (2 studies) | ⊕⊝⊝⊝ Very lowa,c,d | The evidence was very uncertain about the effect of an active drain on morbidity compared with passive drain. |
|
Length of hospital stay Follow‐up: 30 days |
The mean length of hospital stay in the passive drain group was 14.5 days | The mean length of hospital stay in the active drain group was 0.79 days lower (2.63 days lower to 1.04 days higher) | MD ‐0.79 (‐2.63 to 1.04) | 321 (2 studies) | ⊕⊝⊝⊝ Very lowc,e,f | The evidence was very uncertain about the effect of an active drain on length of hospital stay compared with passive drain. |
| * The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the control group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty. We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty. We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty. Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty. We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level for serious risk of bias: one study with unclear risk of incomplete outcome data bDowngraded two levels for very serious imprecision: small sample size, few events, and wide confidence intervals cDowngraded one level for serious imprecision: small sample size and a confidence interval that includes both potential benefit and potential harm from the intervention dDowngraded one level for serious unexplained inconsistency: substantial heterogeneity I²= 76% eDowngraded two levels for very serious risk of bias: one study with high risk of incomplete outcome data, both studies with high risk of performance bias, and this outcome was primarily determined by the surgeons fDowngraded one level for serious unexplained inconsistency: substantial heterogeneity I²= 72%
Summary of findings 3. Early versus late drain removal following pancreatic surgery.
| Early versus late drain removal following pancreatic surgery | ||||||
| Patient or population: people undergoing elective pancreatic resections Setting: hospital Intervention: early drain removal Comparison: late drain removal | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with late drain removal | Risk with early drain removal | |||||
|
Mortality Follow‐up: 30 days |
5 per 1000 | 5 per 1000 (0 to 78) | RR 0.99 (0.06 to 15.45) | 399 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,g | The evidence was very uncertain about the effect of early drain removal on 30‐day mortality compared with late drain removal. |
|
Mortality Follow‐up: 90 days |
Not reported | |||||
|
Intra‐abdominal infection Follow‐up: 30 days |
162 per 1000 | 71 per 1000 (36 to 144) | RR 0.44 (0.22 to 0.89) | 285 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,c,g | The evidence suggested that early drain removal may reduce intra‐abdominal infection rate compared with late drain removal but the evidence was very uncertain. |
|
Wound infection Follow‐up: 30 days |
35 per 1000 | 46 per 1000 (16 to 136) | RR 1.32 (0.45 to 3.85) | 285 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b,g | The evidence was very uncertain about the effect of early drain removal on wound infection rate compared with late drain removal. |
|
Drain‐related complications Follow‐up: 30 days |
Not reported | |||||
|
Morbidity Follow‐up: 30 days |
659 per 1000 | 323 per 1000 (198 to 534) | RR 0.49 (0.30 to 0.81) | 258 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,d,f,g | The evidence suggested that early drain removal may reduce morbidity compared with late drain removal but the evidence was very uncertain. |
|
Length of hospital stay Follow‐up: 30 days |
The mean length of hospital stay in the late drain removal group was 15.4 days | The mean length of hospital stay in the early drain removal group was 2.2 days lower (3.52 days lower to 0.87 days lower) | MD ‐2.2 (‐3.52 to ‐0.87) | 399 (3 RCTs) | ⊕⊝⊝⊝ Very lowe,f,g | The evidence suggested that early drain removal may reduce length of hospital stay compared with late drain removal but the evidence was very uncertain. |
| * The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the control group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty. We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty. We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty. Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty. We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
aNot downgraded for risk of bias because this is considered an objective outcome that is unlikely to be affected by performance bias or detection bias bDowngraded two levels for very serious imprecision: small sample size, few events, and wide confidence intervals cDowngraded two levels for very serious imprecision: small sample size and few events dDowngraded one level for serious unexplained inconsistency: substantial heterogeneity I²= 67% eDowngraded two levels for very serious risk of bias: all studies with high risk of performance bias, and this outcome was primarily determined by the surgeons fDowngraded one level due to serious imprecision: total population size was less than 400 gDowngraded one level for indirectness: different time points for early drain removal, and different definitions of low risk of postoperative pancreatic fistula
Background
Description of the condition
See 'Glossary' for an explanation of terms (Appendix 1).
Pancreatic cancer ranks fourteen of the most common cancers, and seven of the causes of cancer death from a global viewpoint (Bray 2018; Ferlay 2019). Regional differences exist in the number of new cases diagnosed per year (Kamisawa 2016; Maisonneuve 2019; Siegel 2019). The overall incidence of pancreatic cancer is approximately six cases per 100,000 people per year (Bray 2018; Dragovich 2020). The most common cause of pancreatic cancer is heavy tobacco usage (Kamisawa 2016; Maisonneuve 2019).
Although the exact worldwide incidence of chronic pancreatitis is unknown, the estimated incidence of chronic pancreatitis is six cases per 100,000 people per year in Europe, and probably in all western countries (Gupte 2018; Lew 2017; Singh 2019). The prevalence of chronic pancreatitis per 100,000 people is 3 cases in the UK, 4 in Japan, 26 in France, 41 in the USA, and 114 to 200 cases in south India (Bornman 2001; Braganza 2011; Garg 2004; Machicado 2017; Yadav 2011). The most common cause of chronic pancreatitis is alcohol abuse (Kleeff 2017; Lew 2017; Singh 2019).
Pancreatic surgery is performed to treat various pancreatic and extra‐pancreatic diseases, including pancreatic cancers, chronic pancreatitis, and biliary and duodenal malignancies (Cheng 2017; Cheng 2019; Deng 2020; Gurusamy 2013a). Although mortality due to pancreatic surgery has been reduced to less than 5%, the overall morbidity is still high, ranging from 30% to 60% (Bassi 2017; Gurusamy 2013a; Wente 2007a; Wente 2007b). The most common complications after pancreatic surgery include delayed gastric emptying (19% to 23%; Wente 2007b), postoperative pancreatic fistula (2% to 30%; Bassi 2017; Deng 2020; Hackert 2011; Wente 2007a; Wente 2007b), intra‐abdominal abscess (9% to 10%; Wente 2007a; Wente 2007b), wound infection (5% to 15%; Andrén‐Sandberg 2011; Halloran 2002), and postoperative bleeding (1% to 8%; Wente 2007a).
Description of the intervention
As a measure to reduce postoperative complications after pancreatic resections, prophylactic drains are traditionally placed in the subhepatic space near both the biliary and pancreatic anastomoses (Fisher 2018). Abdominal drainage has been in use for over 1000 years (Memon 2001).
Surgical drains are artificial tubes used to remove blood, pus, or other body fluids from wounds (Durai 2009). There are two main types of surgical drains: open and closed (Gurusamy 2007a; Li 2018; Wang 2015). An open drain communicates with the atmosphere (e.g. corrugated drain, Penrose drain, sump drain; Gurusamy 2007a; Li 2018; Wang 2015). A closed drain consists of a tube that drains into a collection bag or bottle, where the contents are not exposed to the atmosphere (Gurusamy 2007a; Li 2018; Wang 2015). Closed drains may be either active (suction drains under low or high pressure, e.g. Jackson‐Pratt drain, Redivac) or passive (drains without suction, e.g. Robinson drain, Pigtail drain; Gurusamy 2007a; Li 2018; Wang 2015).
How the intervention might work
Surgeons have routinely used drains after pancreatic surgery because of the possible collection of bile, pancreatic juice, or blood, which may require additional procedures. The primary reasons for placing abdominal drains after pancreatic resections are: (1) drainage of established intra‐abdominal collections (e.g. bile, pancreatic juice, pus); (2) prevention of further fluid accumulation; and (3) identification and monitoring of any fistula or bleeding. Theoretically, abdominal drainage has the potential to prevent or control postoperative complications (e.g. intra‐abdominal abscess, pancreatic or biliary fistula, bleeding; Bassi 2010; Conlon 2001; Van Buren 2014; Van Buren 2017; Adham 2013; Correa‐Gallego 2013; Fisher 2011; Giovinazzo 2011; Heslin 1998; Jeekel 1992; Kawai 2006; Lim 2013; Mehta 2013; Paulus 2012). The use of surgical drains is a very common practice after pancreatic surgery since the mid‐1930s (Allen 2011).
However, some surgeons have argued that abdominal drainage may fail to reduce postoperative complications, because a drain may become sealed and ineffective within a few days after pancreatic surgery (Heslin 1998; Paulus 2012). The drain itself appears to act as a foreign body, and may interfere with wound healing (Correa‐Gallego 2013; Fisher 2011; Paulus 2012). The drainage tube creates a pathway for contamination, and may increase the risk of postoperative infectious complications (Inoue 2011; Jeekel 1992). In addition, the use of a drain may be associated with an increased length of hospital stay (Fisher 2011; Mehta 2013; Paulus 2012; Van Buren 2017). Abdominal drainage may also be associated with rare adverse events, such as bowel perforation, hernia, and bleeding (Cameron‐Strange 1985; Henkus 1999; Makama 2010; Nomura 1998; Reed 1992; Sahu 2008; Srivastava 2007; Van Hee 1983). Studies have suggested that the routine placement of prophylactic abdominal drains may be unnecessary, and may be associated with an increased complication rate (Adham 2013; Correa‐Gallego 2013; Fisher 2011; Giovinazzo 2011; Heslin 1998; Jeekel 1992; Lim 2013; Mehta 2013; Paulus 2012).
Why it is important to do this review
Routine use of prophylactic abdominal drainage in people undergoing pancreatic surgery is controversial. This is an update of a Cochrane Review assessing the role of prophylactic abdominal drainage for pancreatic surgery (Zhang 2018). We conducted this review to explore uncertainty arising from conflicting results from a number of studies in this area.
Objectives
To assess the benefits and harms of routine abdominal drainage after pancreatic surgery, compare the effects of different types of surgical drains, and evaluate the optimal time for drain removal.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs), regardless of sample size, language, or publication status, which compared (1) drain use versus no drain use, (2) different types of drains, or (3) different schedules for drain removal in people undergoing pancreatic surgery. We excluded quasi‐randomized studies, in which the allocation was performed on the basis of a pseudo‐random sequence (e.g. odd or even hospital number or date of birth, alternation, and non‐randomized studies) because of the potential for bias (Reeves 2021).
Types of participants
We included people, regardless of age, sex, or race, who underwent elective pancreatic resections (open or laparoscopic) for any pancreatic or extra‐pancreatic disease.
Types of interventions
Drain use versus no drain use
One type of drain versus another
Early versus late drain removal (up to four days versus more than four days)
Types of outcome measures
Primary outcomes
-
Mortality
30‐day mortality
90‐day mortality
-
Infectious complications
intra‐abdominal infection (measured at 30 days)
wound infection (measured at 30 days)
Drain‐related complications (measured at 30 days)
Secondary outcomes
Morbidity, as defined by study authors (measured at 30 days). We classified morbidity by the Clavien‐Dindo classification of surgical complications (Clavien 2009).
Length of hospital stay
Hospital costs (measured at 30 days)
-
Additional procedures for postoperative complications
open procedures (measured at 30 days)
radiological interventions (radiological drainage requiring insertion of drain or percutaneous aspiration; measured at 30 days)
Pain (measured at 30 days)
Quality of life (measured at 30 days)
The main reason to justify abdominal drainage was the assumption that it would reduce the infectious complication rate, and subsequent mortality and morbidity rates. We chose other clinical outcomes to assess whether abdominal drainage resulted in earlier discharge from hospital, fewer reoperations, and improvement in health‐related quality of life and cost effectiveness.
Reporting of the outcomes listed here was not an inclusion criterion for the review.
Search methods for identification of studies
Before searching, we designed the search strategies with the help of the Cochrane Gut Group Information Specialist. We placed no restrictions on the language of publication when searching the electronic databases, or reviewing reference lists in identified studies.
Electronic searches
This is the third update of a Cochrane Review originally published in 2015 (Peng 2015), and previously updated in 2016 (Cheng 2016), and 2018 (Zhang 2018).
For this updated review, we updated the search strategies and re‐searched the following electronic databases, with no language or date of publication restrictions:
Cochrane Central Register of Controlled Trials Ovid (CENTRAL; 2021, Issue 2) in the Cochrane Library (searched 08 February 2021; see Appendix 2);
MEDLINE Ovid (1946 to 08 February 2021; see Appendix 3);
Embase Ovid (1974 to 08 February 2021; see Appendix 4);
Science Citation Index Expanded Web of Science (1900 to 08 February 2021; see Appendix 5); and
Chinese Biomedical Literature Database (CBM; 1978 to 08 February 2021; see Appendix 6).
Searching other resources
We checked reference lists of all primary studies and relevant review articles that were identified during the search for RCTs, for additional references. We contacted authors of identified studies, and asked them to identify other published and unpublished studies.
We searched PubMed for errata or retractions of eligible studies, and reported the date this was done (www.ncbi.nlm.nih.gov/pubmed; accessed 08 February 2021). We also searched meeting abstracts of the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES; www.sages.org/; accessed 08 February 2021), and Conference Proceedings Citation Index, to explore further relevant clinical studies.
Clinical trials registers/trial result registers
We searched the following databases to identify ongoing studies (accessed 08 February 2021):
World Health Organization International Clinical Trials Registry Platform search portal (apps.who.int/trialsearch/);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov/);
Current Controlled Trials (www.controlled-trials.com/);
EU Clinical Trials Register (www.clinicaltrialsregister.eu/);
Chinese Clinical Trial Register (www.chictr.org/).
Data collection and analysis
We conducted this review according to the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2021).
Selection of studies
Two review authors (SH, WZ) independently screened the titles and abstracts of all the reports we identified as a result of the search, and coded them as 'retrieve' (eligible, potentially eligible, or unclear) or 'do not retrieve'. We retrieved the full‐text study reports and publications of identified reports, and two review authors (SH, WZ) independently screened the full text, identified studies for inclusion, and identified and recorded reasons to exclude the ineligible studies. We resolved any disagreements through discussion, or if required, we consulted a third review author (YC). We identified and excluded duplicates and collated multiple reports of the same study, so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and a 'Characteristics of excluded studies' table (Moher 2009).
Data extraction and management
We used a standard data collection form for study characteristics and outcome data, which we piloted on at least one study in the review. Two review authors (ML, ZL) extracted the following study characteristics from included studies:
methods: study design, total duration of study and run in, number of study centres and location, study setting, withdrawals, and date of study;
participants: number (N), mean age, age range, gender, severity of condition, diagnostic criteria, inclusion criteria, and exclusion criteria;
interventions: intervention, comparison;
outcomes: primary and secondary outcomes specified and collected, time points reported;
notes: funding for trial, notable conflicts of interest of trial authors.
Two review authors (ML, ZL) independently extracted outcome data from included studies. We noted in the 'Characteristics of included studies' table if outcome data were not reported in a usable way. We resolved disagreements by consensus, or by involving a third review author (YC). One review author (SH) copied the data from the data collection form into Review Manager 5 (Review Manager 2020). We double‐checked that the data were entered correctly by comparing the study reports with the data in the review. A second review author spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (SH, JX) independently assessed risk of bias for each study, using the original Cochrane risk of bias tool for randomised trials (RoB 1), as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We resolved any disagreements by discussion, or by involving a third review author (YC). We assessed the risk of bias according to the following domains:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting;
other bias.
We graded each potential source of bias as high, low, or unclear risk, and provided a quote from the study report together with a justification for our judgement in the risk of bias table. We summarized the risk of bias judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes where necessary (e.g. for unblinded outcome assessment, risk of bias for 30‐day mortality may be different than for a participant‐reported pain scale). Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the risk of bias table. We considered a trial to be at low risk of bias if we assessed the trial at low risk of bias across all domains. We considered trials at high risk of bias when one or more domains were at unclear or high risk of bias. We resolved any difference in opinion by discussion.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Assessment of bias in conducting the review
We conducted the review according to the published protocol, and reported any deviations from it in the 'Differences between protocol and review' section of the review (Cheng 2013).
Measures of treatment effect
We analysed dichotomous data as risk ratios (RR) and continuous data as mean differences (MD) with 95% confidence intervals (CI). For continuous data with different measurement scales in different randomised clinical trials, we calculated standardized mean differences (SMD) with 95% CI. We ensured that higher scores for continuous outcomes had the same meaning for the particular outcome, explained the direction to the reader, and reported where the directions were reversed, if this was necessary.
We undertook meta‐analyses only when this was meaningful, that is, if the treatments, participants, and underlying clinical question were similar enough for pooling to make sense.
A common way that trialists indicate when they have skewed data is by reporting medians and interquartile ranges. When we encountered this, we noted that the data were skewed, and considered the implication of this.
When multiple trial arms were reported in a single trial, we included only the relevant arms. If two comparisons (e.g. drug A versus placebo and drug B versus placebo) were entered into the same meta‐analysis, we halved the control group to avoid double‐counting.
Unit of analysis issues
The unit of analysis was the individual participant. We did not find any cross‐over or cluster‐randomized studies.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and request missing numerical outcome data, when needed (e.g. when a study was identified as abstract only). We did not get any responses. Thus, we only used the available data in the analyses.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the studies in each analysis (Higgins 2003). When we identified substantial heterogeneity (greater than 50%), we explored it by prespecified subgroup analysis, and we interpreted summary effect measures with caution.
Assessment of reporting biases
We had planed to use funnel plots to assess reporting biases. However, we did not generate any funnel plots because we included fewer than 10 studies (Higgins 2021).
Data synthesis
We performed the meta‐analyses using Review Manager 5 software (Review Manager 2020). For all analyses, we used a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We intended to perform the following subgroup analyses:
RCTs with low risk of bias versus RCTs with high risk of bias;
different etiologies (pancreatic cancer, chronic pancreatitis, and others);
type of operation (proximal, distal, or central pancreatectomy).
We conducted subgroup analyses for the following outcomes.
30‐day mortality;
90‐day mortality;
intra‐abdominal infection;
wound infection;
drain‐related complications.
Sensitivity analysis
We intended to perform sensitivity analyses to assess the robustness of our conclusions. The sensitivity analyses included:
using a fixed‐effect model rather than a random‐effects model;
calculating risk differences (RD), and odds ratios (OR) for dichotomous outcomes, as well as RR;
calculating SMD for continuous data with same measurement scales in different RCTs and calculating MD for continuous data with different measurement scales in different RCTs;
conducting worst‐case (the events happened in the experimental group but did not happen in the control group for missing participants) and best‐case (the events happened in the control group but did not happen in the experimental group for missing participants) scenario analyses for missing data.
If the results did not change, we considered them to be robust. If the results changed, we considered them to be less robust.
We planned sensitivity analyses for the following outcomes:
30‐day mortality;
90‐day mortality;
intra‐abdominal infection;
wound infection;
drain‐related complications.
Reaching conclusions
We based our conclusions only on findings from the quantitative or narrative synthesis of included studies for this review. We avoided making recommendations for practice, and tried, in our implications for research, to give the reader a clear sense of where the focus of any future research in the area should be, and the remaining uncertainties.
Summary of findings and assessment of the certainty of the evidence
We included these outcomes in our summary of findings tables: 30‐day mortality, 90‐day mortality, intra‐abdominal infection, wound infection, drain‐related complications, morbidity, and length of hospital stay. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence based on the studies that contributed data to the meta‐analyses for each outcome. We used the methods and recommendations described in Chapter 14 of the CochraneHandbookfor Systematic Reviews of Interventions (Schünemann 2021), and GRADEpro GDT software to develop and display the summary of findings tables (GRADEpro GDT). We justified all decisions to downgrade the certainty of the evidence using footnotes, and made comments to aid the reader's understanding of the review, where necessary.
We defined the certainty of the evidence as follows:
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
See 'Characteristics of included studies', 'Characteristics of excluded studies', and Characteristics of ongoing studies tables.
Results of the search
For this updated review, we identified 7959 records through the electronic searches of Cochrane Central Register of Controlled Trials Ovid (CENTRAL; 765 records), MEDLINE Ovid (1636 records), Embase Ovid (2993 records), Science Citation Index Expanded Web of Science (2080 records), and Chinese Biomedical Literature Database (CBM; 485 records). We did not identify any records by scanning the reference lists of the identified randomised controlled trials (RCTs). We excluded 1812 duplicates and 6127 clearly irrelevant records by screening the titles and abstracts. We retrieved the full text of the remaining twenty records for further assessment. We excluded 16 studies for the reasons listed in the 'Characteristics of excluded studies' table, and identified one ongoing study (Kaiser 2019). Three new RCTs fulfilled the inclusion criteria for this update. We show the study flow diagram in Figure 1.
1.
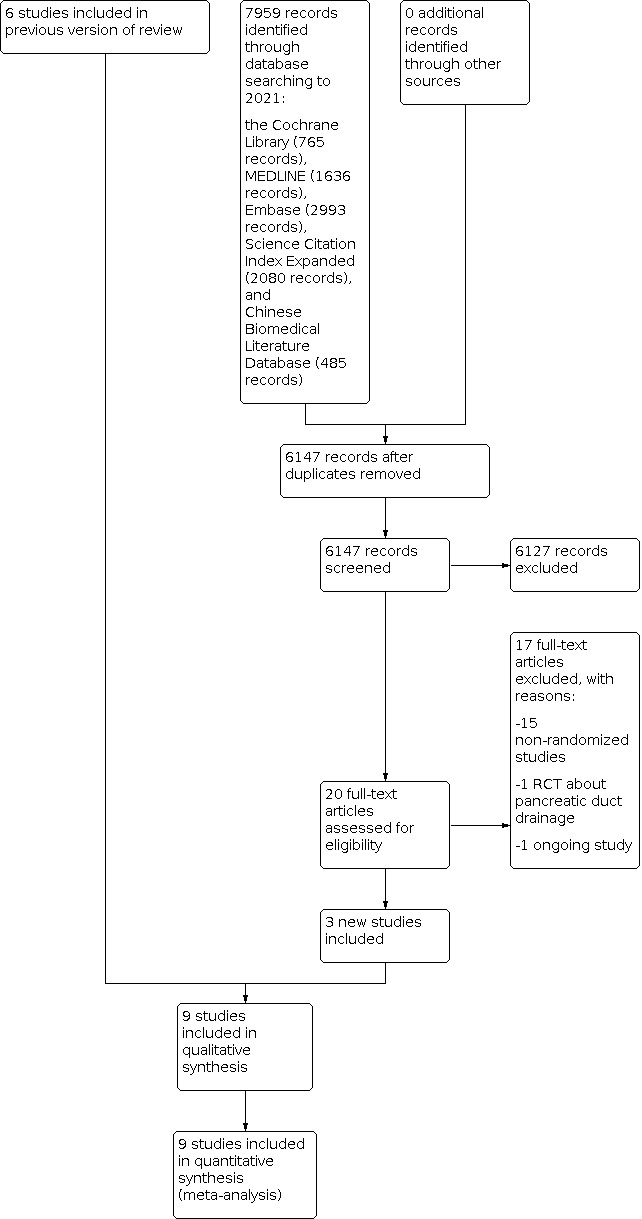
Study flow diagram: 2021 review update
Included studies
The last published version of this review included six RCTs, published between 2001 and 2016 (Bassi 2010; Conlon 2001; Jiang 2016; Van Buren 2014; Van Buren 2017; Witzigmann 2016). We added three recently published RCTs to this update (Čečka 2018; Dai 2020; Dembinski 2019). Therefore, we included nine RCTs (1892 participants) in this update, all of which provided data for the analyses. We listed details of the studies in the 'Characteristics of included studies' table.
Drain use versus no drain use
Four studies randomised 1110 participants who underwent elective pancreatic resections (604 pancreaticoduodenectomy, 439 distal pancreatectomy, and 67 other pancreatic surgery) to two groups: those who had drainage tubes inserted postoperatively (N = 560), and those who did not (N = 550 (Conlon 2001; Van Buren 2014; Van Buren 2017; Witzigmann 2016)). Two of the RCTs (163 participants) included participants who underwent laparoscopic pancreatic resections (Van Buren 2014; Van Buren 2017). All four RCTs (947 participants) included participants who underwent open pancreatic resections (Conlon 2001; Van Buren 2014; Van Buren 2017; Witzigmann 2016). Three of these studies were conducted in the USA (Conlon 2001; Van Buren 2014; Van Buren 2017), and one was conducted in Germany (Witzigmann 2016). The sample size calculation was described in three of the four RCTs (Van Buren 2014; Van Buren 2017; Witzigmann 2016). All of the studies used multiple surgeons to perform the surgeries, regardless of the number of study centres. Two RCTs were multi‐centre studies (Van Buren 2014; Van Buren 2017), one RCT was a dual‐centre study (Witzigmann 2016), and the other RCT was a single‐centre study (Conlon 2001). One study was terminated after an interim analysis because of excess mortality in the group with no drainage (Van Buren 2014). For all four trials, the mean age was 63.9 years; one or two drainage tubes were placed near the pancreatic anastomoses or pancreatic stumps; and the measured outcomes were mortality, morbidity, wound infection, intra‐abdominal infection, various postoperative complications, reoperation, additional radiological intervention, length of hospital stay, and quality of life.
One type of drain versus another
Two studies randomised 383 participants undergoing elective pancreatic resections (321 pancreaticoduodenectomy and 62 distal pancreatectomy) to the active drain group (N = 194) or the passive drain group (N = 189 (Čečka 2018; Jiang 2016)). All 383 participants in the two RCTs underwent open pancreatic resections. One RCT was conducted in China (Jiang 2016), the other in Czech Republic (Čečka 2018). Both RCTs described the sample size calculation. One RCT was a dual‐centre study involving many surgeons (Čečka 2018), while the other RCT was a single‐centre study involving one surgeon (Jiang 2016). For both trials, the mean age was 62.1 years; two drainage tubes were placed near both the biliary and pancreatic anastomoses and one drainage tube was placed near the pancreatic stump after distal pancreatectomy; and the measured outcomes were mortality, morbidity, wound infection, intra‐abdominal infection, various postoperative complications, reoperation, operation time, and length of hospital stay.
Early versus late drain removal
Three studies randomised 399 participants with low risk of postoperative pancreatic fistula, who were undergoing elective pancreatic resections (320 pancreaticoduodenectomy and 79 distal pancreatectomy) to the early removal group (N = 200), or the late removal group (N = 199 (Bassi 2010; Dai 2020; Dembinski 2019)). The definition of low risk of postoperative pancreatic fistula was not uniform among RCTs. Bassi 2010 defined low risk of postoperative pancreatic fistula as "an amylase value in drains on postoperative day 1 < 5000 IU/L". Dai 2020 defined it as "drain amylase on postoperative day 1 and 3 less than 5000 U/L, and drain output within postoperative day 3 less than 300 mL/d". Dembinski 2019 defined it as "drain amylase on postoperative day 3 less than 3 times the serum amylase activity". Two RCTs included 53 participants who underwent laparoscopic pancreatic resections (Dai 2020; Dembinski 2019). The other 346 participants in the three RCTs underwent open pancreatic resections. One RCT was conducted in Italy (Bassi 2010), one in China (Dai 2020), and the other in France (Dembinski 2019). Two RCTs described the sample size calculation (Bassi 2010; Dembinski 2019). All three RCTs were single‐centre studies involving many surgeons. For all three trials, the mean age was 59.5 years; two drainage tubes were placed near both the biliary and pancreatic anastomoses; and one drainage tube was placed near the pancreatic stump after distal pancreatectomy. In the early removal group, drains were removed on postoperative day 3 in two RCTs (Bassi 2010; Dai 2020), and postoperative day 4 in the third RCT (Dembinski 2019). In the late removal group, drains were removed on postoperative day 5 or later in all three RCTs. The three RCTs reported outcomes of mortality, wound infection, intra‐abdominal infection, postoperative pancreatic fistula, abdominal complications, pulmonary complications, reoperation, length of hospital stay, hospital readmission, morbidity, and hospital costs.
Excluded studies
We listed the details for the 16 excluded studies in the 'Characteristics of excluded studies' table. We excluded one RCT because it focused on pancreatic duct drainage (Lee 2009); the remaining studies were excluded because we identified them as non‐randomized studies (Adham 2013; Behrman 2015; Correa‐Gallego 2013; Fisher 2011; Giovinazzo 2011; Heslin 1998; Jeekel 1992; Kawai 2006; Kawaida 2021; Kunstman 2017; Lemke 2021; Lim 2013; Mehta 2013; Paulus 2012; Zaghal 2020).
Ongoing studies
We identified one ongoing study (Kaiser 2019). They plan to include 252 participants for open or minimally invasive distal pancreatectomy for pancreatic disease, and will randomize them to the drainage group or no drainage group. This trial started in March 2018. It is currently recruiting participants in Germany. The primary outcome is morbidity. The secondary outcomes are mortality, various postoperative complications, reoperation rate, operation time, length of hospital stay, duration of intensive care unit stay, and readmission rate. See the 'Characteristics of ongoing studies' table for more details.
Risk of bias in included studies
Figure 2 and Figure 3 summarize the risk of bias of the included studies. All nine studies were at high risk of bias.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
We judged random sequence generation at low risk of bias in eight studies, in which participants were randomised using computer‐generated numbers (Bassi 2010; Čečka 2018; Dai 2020; Dembinski 2019; Jiang 2016; Van Buren 2014; Van Buren 2017; Witzigmann 2016), and unclear risk of bias in one study, as it did not provide any specific information on the randomization process, and we did not receive a response from the authors (Conlon 2001). We judged allocation concealment at low risk of bias in three studies that used sealed opaque envelopes (Čečka 2018; Witzigmann 2016), or central allocation (Dai 2020), to conceal the allocation, and unclear risk of bias in the remaining six studies, which provided no information.
Blinding
We judged blinding of participants and personnel as unclear risk of bias in one study, which did not describe whether adequate blinding of participants and personnel was achieved, and we did not receive a response from the authors (Jiang 2016). We judged blinding of participants and personnel at high risk of bias in the six studies that stated 'open label' in the protocols (Bassi 2010; Čečka 2018; Dai 2020; Dembinski 2019; Van Buren 2014; Van Buren 2017). For the remaining two studies, the surgeons could not be blinded to group allocation (drain use versus no drain use (Conlon 2001; Witzigmann 2016)). Therefore, we judged both studies at high risk of blinding of participants and personnel.
We judged blinding of outcome assessment at low risk of bias in one study (Čečka 2018), unclear risk of bias in three studies, since they provided no information (Conlon 2001; Jiang 2016; Witzigmann 2016), and high risk of bias in the remaining five studies which stated 'open label' in the protocols (Bassi 2010; Dai 2020; Dembinski 2019; Van Buren 2014; Van Buren 2017).
Incomplete outcome data
There were no post‐randomisation dropouts in four studies (Bassi 2010; Conlon 2001; Dai 2020; Jiang 2016). Although there were seven dropouts (5.0%) in one study, the study authors analysed the data on an intention‐to‐treat basis (Dembinski 2019). We considered these five studies at low risk of attrition bias (Bassi 2010; Conlon 2001; Dai 2020; Dembinski 2019; Jiang 2016). There were some dropouts in three studies, but the data were not analysed on an intention‐to‐treat basis, therefore, we judged these three studies at high risk of bias for incomplete outcome data (Van Buren 2014; Van Buren 2017; Witzigmann 2016). There was one dropout in a study, it was not clear whether there was a substantial departure from the intervention in this study that would cause a per‐protocol analysis to differ significantly from an intention‐to‐treat analysis, therefore, we judged this study at unclear risk of bias for incomplete outcome data (Čečka 2018).
Selective reporting
The study protocols were available for seven studies (Bassi 2010; Čečka 2018; Dai 2020; Dembinski 2019; Van Buren 2014; Van Buren 2017; Witzigmann 2016). All of the studies reported their prespecified outcomes. Thus, we considered these seven studies to be free of selective reporting. The study protocols were not available for two studies, but hey fully reported on all expected outcomes, so we judged both studies to be free of selective reporting (Conlon 2001; Jiang 2016).
Other potential sources of bias
We judged baseline imbalance at low risk of bias in all nine studies.
Five of the nine studies were sponsored by non‐commercial grants (e.g. university grant, national cancer control program, charitable funding (Čečka 2018; Conlon 2001; Dai 2020; Van Buren 2014; Van Buren 2017)). Two studies did not receive any funding (Dembinski 2019; Witzigmann 2016). The other two studies provided no information about funding sources (Bassi 2010; Jiang 2016). Six of the nine studies declared no conflict of interest (Dai 2020; Dembinski 2019; Jiang 2016; Van Buren 2014; Van Buren 2017; Witzigmann 2016). The other three studies did not report on conflicts of interest (Bassi 2010; Čečka 2018; Conlon 2001).
Effects of interventions
See: Table 1; Table 2; Table 3
Drain use versus no drain use
Four studies (1110 participants) compared drain use with no drain use (Conlon 2001; Van Buren 2014; Van Buren 2017; Witzigmann 2016). Five hundred and sixty participants were randomised to the drainage group, and 550 participants to the no drainage group. See Table 1.
Mortality (30 days)
Four studies (1055 participants: 532 drain use, 523 no drain) reported on 30‐day mortality (Conlon 2001; Van Buren 2014; Van Buren 2017; Witzigmann 2016). There were eight deaths in the drainage group, and 12 deaths in the no drainage group. The evidence suggested that drain use may result in little to no difference in 30‐day mortality compared with no drain use (risk ratio (RR) 0.78, 95% confidence interval (CI) 0.31 to 1.99; four studies, 1055 participants; low‐certainty evidence; Analysis 1.1). We downgraded the certainty of the evidence one level for serious risk of bias because three studies with high risk of incomplete outcome data contributed 77.2% of the weight towards this effect estimate (Van Buren 2014; Van Buren 2017; Witzigmann 2016). We downgraded one level for serious imprecision because there were few deaths in either group, and the confidence interval included both potential benefit and potential harm from the intervention.
1.1. Analysis.

Comparison 1: Drain use versus no drain use, Outcome 1: Mortality (30 days)
Mortality (90 days)
Two studies (478 participants: 242 drain use, 236 no drain) reported on 90‐day mortality (Van Buren 2014; Van Buren 2017). There were two deaths in the drainage group and 10 deaths in the no drainage group. The evidence suggested that drain use may reduce 90‐day mortality compared with no drain use (RR 0.23, 95% CI 0.06 to 0.90; two studies, 478 participants; low‐certainty evidence; Analysis 1.2). We downgraded the certainty of the evidence one level for serious risk of bias because both studies were at high risk of incomplete outcome data; and one level for serious imprecision because there were few deaths in either group.
1.2. Analysis.

Comparison 1: Drain use versus no drain use, Outcome 2: Mortality (90 days)
Intra‐abdominal infection
Four studies (1055 participants: 532 drain use, 523 no drain) reported on intra‐abdominal infection (Conlon 2001; Van Buren 2014; Van Buren 2017; Witzigmann 2016). There were 42 intra‐abdominal infections in the drainage group, and 43 intra‐abdominal infections in the no drainage group. The evidence was very uncertain about the effect of drain use on intra‐abdominal infection rate compared with no drain use (RR 0.97, 95% CI 0.52 to 1.80; four studies, 1055 participants; very low‐certainty evidence; Analysis 1.3). We downgraded the evidence one level for serious risk of bias because three studies with high risk of incomplete outcome data contributed 80.6% of the weight towards this effect estimate (Van Buren 2014; Van Buren 2017; Witzigmann 2016). We downgraded the certainty of the evidence one level for serious imprecision, because there were few events in either group and confidence intervals included both potential benefit and potential harm from the intervention; and one level for serious inconsistency (unexplained statistical heterogeneity I² = 52%).
1.3. Analysis.

Comparison 1: Drain use versus no drain use, Outcome 3: Intra‐abdominal infection
Wound infection
Four studies (1055 participants: 532 drain use, 523 no drain) reported on wound infection. There were 52 wound infections in the drainage group, and 52 wound infections in the no drainage group (Conlon 2001; Van Buren 2014; Van Buren 2017; Witzigmann 2016). The evidence suggested that drain use may result in little to no difference in wound infection rate compared with no drain use (RR 0.98, 95% CI 0.68 to 1.41; four studies, 1055 participants; low‐certainty evidence; Analysis 1.4). We downgraded the certainty of the evidence one level for serious risk of bias because three studies with high risk of incomplete outcome data contributed 80.8% of the weight towards this effect estimate (Van Buren 2014; Van Buren 2017; Witzigmann 2016). We downgraded one level for serious imprecision, because there were few events in either group and the confidence interval included both potential benefit and potential harm from the intervention.
1.4. Analysis.

Comparison 1: Drain use versus no drain use, Outcome 4: Wound infection
Drain‐related complications
One study (179 participants: 91 drain use, 88 no drain) reported this outcome (Conlon 2001). There was one drain‐related complication (broken drain) in the drainage group, and no drain‐related complications in the no drainage group. The evidence suggested that drain use may result in little to no difference in drain‐related complications compared with no drain use (one study, 179 participants; low‐certainty evidence). We did not downgrade the certainty of the evidence for risk of bias because this is considered an objective outcome, which is unlikely to be affected by selection bias or performance bias in this study. We downgraded two levels for very serious imprecision because of the small sample size and very few events.
Morbidity
Four studies (1055 participants: 532 drain use, 523 no drain) reported on morbidity (Conlon 2001; Van Buren 2014; Van Buren 2017; Witzigmann 2016). There were 328 participants with complications in the drainage group, and 312 participants with complications in the no drainage group. The evidence suggested that drain use probably resulted in little to no difference in morbidity compared with no drain use (RR 1.03, 95% CI 0.94 to 1.13; four studies, 1055 participants; moderate‐certainty evidence; Analysis 1.5). We downgraded the certainty of evidence one level for serious risk of bias because three studies with high risk of incomplete outcome data contributed 84.4% of the weight towards this effect estimate (Van Buren 2014; Van Buren 2017; Witzigmann 2016).
1.5. Analysis.

Comparison 1: Drain use versus no drain use, Outcome 5: Morbidity
Length of hospital stay
Four studies (1055 participants: 532 drain use, 523 no drain) reported on length of hospital stay (Conlon 2001; Van Buren 2014; Van Buren 2017; Witzigmann 2016). One study reported the mean and standard deviation (SD (Witzigmann 2016)). Two studies reported the median and interquartile range (IQR) values (Van Buren 2014; Van Buren 2017). Assuming a normal distribution of the data, we imputed the mean and SD values using the formula (1) mean = median, and (2) SD = IQR / 1.35; according to the Cochrane Handbook (Higgins 2021). The evidence suggested that drain use may result in little to no difference in length of hospital stay compared with no drain use (MD ‐0.14 days, 95% CI ‐0.79 to 0.51; three studies, 876 participants; low‐certainty evidence; Analysis 1.6). One study including 179 participants reported the median and range values which were not suitable for pooling (Conlon 2001). Conlon 2001 reported no difference in the length of hospital stay between groups (Analysis 1.7). We downgraded the certainty of evidence two levels for very serious risk of bias, because three studies were at high risk of incomplete outcome data (Van Buren 2014; Van Buren 2017; Witzigmann 2016). In addition, all of the studies were at high risk of performance bias, and length of hospital stay was primarily determined by the surgeons.
1.6. Analysis.

Comparison 1: Drain use versus no drain use, Outcome 6: Length of hospital stay (days)
1.7. Analysis.
Comparison 1: Drain use versus no drain use, Outcome 7: Length of hospital stay
| Length of hospital stay | ||||
| Study | Number in study | Comparison | Results | Comment |
| Conlon 2001 | 179 (91 versus 88) | Drain use versus no drain use | Drain use versus no drain use: median (range): 9 (3‐34) versus 9 (5‐44), without reporting the p value | Authors reported that there was no difference in the length of hospital stay between groups |
Hospital costs
None of the studies reported this outcome.
Additional open procedures for postoperative complications
Four studies (1055 participants: 532 drain use, 523 no drain) reported on this outcome (Conlon 2001; Van Buren 2014; Van Buren 2017; Witzigmann 2016). There were 50 participants who needed additional open procedures for postoperative complications in the drainage group, and 37 participants in the no drainage group. The evidence suggested that drain use may result in little to no difference in the need for additional open procedures for postoperative complications compared with no drain use (RR 1.33, 95% CI 0.79 to 2.23; four studies, 1055 participants; low‐certainty evidence; Analysis 1.8). We downgraded the certainty of evidence one level for serious risk of bias, because three studies with high risk of incomplete outcome data contributed 83.1% of the weight towards this effect estimate (Van Buren 2014; Van Buren 2017; Witzigmann 2016). We downgraded the certainty of the evidence one level for serious imprecision, because there were few events in either group and the confidence interval included both potential benefit and potential harm from the intervention.
1.8. Analysis.

Comparison 1: Drain use versus no drain use, Outcome 8: Additional open procedures for postoperative complications
Additional radiological interventions for postoperative complications
Three studies (660 participants (330 drain use, 330 no drain) reported on this outcome (Conlon 2001; Van Buren 2014; Van Buren 2017). There were 35 participants who needed additional radiological interventions for postoperative complications in the drainage group, and 40 participants in the no drainage group. The evidence was very uncertain about the effect of drain use on the need for additional radiological interventions for postoperative complications compared with no drain use (RR 0.87, 95% CI 0.40 to 1.87; three studies, 660 participants; very low‐certainty evidence; Analysis 1.9). We downgraded the certainty of evidence one level for serious risk of bias, because two studies with high risk of incomplete outcome data contributed 69.7% of the weight towards this effect estimate (Van Buren 2014; Van Buren 2017). We downgraded the certainty of the evidence one level for serious imprecision because there were few events in either group, and the confidence interval included both potential benefit and potential harm from the intervention; and one level for serious inconsistency (unexplained statistical heterogeneity I² = 64%).
1.9. Analysis.

Comparison 1: Drain use versus no drain use, Outcome 9: Additional radiological interventions for postoperative complications
Pain
None of the studies reported on pain.
Quality of life
One study (399 participants: 202 drain use, 197 no drain) reported this outcome (Van Buren 2017). Van Buren 2017 used the pancreas‐specific quality of life questionnaire (FACT‐PA) to assess quality of life. The FACT‐PA is a scale of 0 to 144, with higher values indicating better quality of life. The mean quality of life score at 30 days after pancreatic surgery was 105 points in the drainage and 104 points in the no drainage group. The study reported the mean quality of life score, without providing the standard deviation. The evidence suggested that drain use may result in little to no difference in quality of life compared with no drain use; low‐certainty evidence. We downgraded the certainty of the evidence one level for serious risk of bias, because the study was at high risk of incomplete outcome data; and one level for serious imprecision, because of the small sample size.
Active drain versus passive drain
Two studies (383 participants) compared active drain versus passive drain (Čečka 2018; Jiang 2016). One hundred and ninety‐four participants were randomised to the active drain group and 189 participants to the passive drain group. See Table 2.
Mortality (30 days)
Both studies (382 participants: 193 active drain, 189 passive drain) reported on 30‐day mortality. There were four deaths in the active drain group, and three deaths in the passive drain group. The evidence was very uncertain about the effect of an active drain on 30‐day mortality compared with a passive drain (RR 1.23, 95% CI 0.30 to 5.06; two studies, 382 participants; very low‐certainty evidence; Analysis 2.1). We downgraded the certainty of the evidence one level for serious risk of bias, because one study with unclear risk of incomplete outcome data contributed 80.3% of the weight towards this effect estimate (Čečka 2018). We downgraded two levels for very serious imprecision, because of the small sample size, few events, and wide confidence interval.
2.1. Analysis.

Comparison 2: Active drain versus passive drain, Outcome 1: Mortality (30 days)
Mortality (90 days)
Neither study reported this outcome.
Intra‐abdominal infection
Both studies (321 participants: 162 active drain, 159 passive drain) reported on intra‐abdominal infection. There were 17 intra‐abdominal infections in the active drain group, and 16 intra‐abdominal infections in the passive drain group. The evidence was very uncertain about the effect of an active drain on intra‐abdominal infection rate compared with a passive drain (RR 0.87, 95% CI 0.21 to 3.66; two studies, 321 participants; very low‐certainty evidence; Analysis 2.2). We downgraded the certainty of the evidence one level for serious risk of bias, because one study with unclear risk of incomplete outcome data contributed 81.8% of the weight towards this effect estimate (Čečka 2018). We downgraded two levels for very serious imprecision, because of the small sample size, few events, and wide confidence interval.
2.2. Analysis.

Comparison 2: Active drain versus passive drain, Outcome 2: Intra‐abdominal infection
Wound infection
Both studies (321 participants: 162 active drain, 159 passive drain) reported on wound infection. There were 13 wound infections in the active drain group, and 14 wound infections in the passive drain group. The evidence was very uncertain about the effect of an active drain on wound infection rate compared with a passive drain (RR 0.92, 95% CI 0.44 to 1.90; two studies, 321 participants; very low‐certainty evidence; Analysis 2.3). We downgraded the certainty of the evidence one level for serious risk of bias, because one study with unclear risk of incomplete outcome data contributed 55.6% of the weight towards this effect estimate (Čečka 2018). We downgraded two levels for very serious imprecision because of the small sample size, few events, and wide confidence interval.
2.3. Analysis.

Comparison 2: Active drain versus passive drain, Outcome 3: Wound infection
Drain‐related complications
One study (223 participants: 112 active drain, 111 passive drain) reported this outcome (Čečka 2018). There was no drain‐related complication in either group. We downgraded the certainty of the evidence one level for serious risk of bias, and two levels for very serious imprecision because of the small sample size and few events.
Morbidity
Both studies (382 participants: 193 active drain, 189 passive drain) reported on morbidity. There were 75 participants with complications in the active drain group, and 70 participants with complications in the passive drain group. The evidence was very uncertain about the effect of an active drain on morbidity compared with a passive drain (RR 0.97, 95% CI 0.53 to 1.77; two studies, 382 participants; very low‐certainty evidence; Analysis 2.4). We downgraded the certainty of the evidence one level for serious risk of bias because one study with unclear risk of incomplete outcome data contributed 56.3% of the weight towards this effect estimate (Čečka 2018). We downgraded one level for serious imprecision, because of the small sample size, and a confidence interval that included both potential benefit and potential harm from the intervention; and one level for serious inconsistency (unexplained statistical heterogeneity I² = 76%).
2.4. Analysis.

Comparison 2: Active drain versus passive drain, Outcome 4: Morbidity
Length of hospital stay
Both studies (321 participants: 162 active drain, 159 passive drain) reported on length of hospital stay. One study reported the mean and SD (Jiang 2016). The other study reported the median and interquartile range (IQR (Čečka 2018)). Assuming a normal distribution of the data, we imputed the mean and SD values using the formula (1) mean = median, and (2) SD = IQR / 1.35; according to the Cochrane Handbook (Higgins 2021). The evidence was very uncertain about the effect of an active drain on length of hospital stay compared with a passive drain (MD ‐0.79 days, 95% CI ‐2.63 to 1.04; two studies, 321 participants; very low‐certainty evidence; Analysis 2.5). We downgraded the certainty of the evidence two levels for very serious risk of bias, because one study was at unclear risk of incomplete outcome data (Čečka 2018) and both studies were at high risk of performance bias, and length of hospital stay was primarily determined by the surgeons. We downgraded one level for serious imprecision, because of the small sample size, and a confidence interval that included both potential benefit and potential harm from the intervention; and one level for serious inconsistency (unexplained statistical heterogeneity I² = 72%).
2.5. Analysis.

Comparison 2: Active drain versus passive drain, Outcome 5: Length of hospital stay (days)
Hospital costs
Neither study reported this outcome.
Additional open procedures for postoperative complications
Both studies (321 participants: 162 active drain, 159 passive drain) reported on this outcome. There were six participants who needed additional open procedures for postoperative complications in the active drain group, and 13 participants in the passive drain group. The evidence was very uncertain about the effect of an active drain on the need for additional open procedures for postoperative complications compared with a passive drain (RR 0.44, 95% CI 0.11 to 1.83; two studies, 321 participants; very low‐certainty evidence; Analysis 2.6). We downgraded the certainty of the evidence one level for serious risk of bias because one study with unclear risk of incomplete outcome data contributed 67.2% of the weight towards this effect estimate (Čečka 2018). We downgraded two levels for very serious imprecision, because of the small sample size, few events, and wide confidence interval; and one level for serious inconsistency (unexplained statistical heterogeneity I² = 39%).
2.6. Analysis.

Comparison 2: Active drain versus passive drain, Outcome 6: Additional open procedures for postoperative complications
Additional radiological interventions for postoperative complications
Neither study reported this outcome.
Pain
Neither study reported this outcome.
Quality of life
Neither study reported this outcome.
Early versus late drain removal
Three studies (399 participants with low risk of postoperative pancreatic fistula: 200 early drain removal, 199 late drain removal) compared early versus late drain removal (Bassi 2010; Dai 2020; Dembinski 2019). See Table 3.
Mortality (30 days)
Three studies reported on 30‐day mortality. There was one death in the early removal group, and one death in the late removal group. The evidence was very uncertain about the effect of early drain removal on 30‐day mortality compared with late drain removal (RR 0.99, 95% CI 0.06 to 15.45; three studies, 399 participants; very low‐certainty evidence; Analysis 3.1). We downgraded the certainty of the evidence two levels for very serious imprecision, because of the small sample size, few events, and wide confidence interval; and one level for indirectness, because of the different time points for early drain removal and different definitions of low risk of postoperative pancreatic fistula.
3.1. Analysis.

Comparison 3: Early versus late drain removal, Outcome 1: Mortality (30 days)
We did not downgraded the certainty of the evidence for risk of bias, because we considered this an objective outcome, which was unlikely to be affected by performance bias or detection bias.
Mortality (90 days)
None of the studies reported this outcome.
Intra‐abdominal infection
Two studies (285 participants: 143 early removal, 142 late removal) reported on intra‐abdominal infection (Dai 2020; Dembinski 2019). There were 10 intra‐abdominal infections in the early removal group, and 23 intra‐abdominal infections in the late removal group. The evidence suggested that early drain removal may reduce intra‐abdominal infection rate compared with late drain removal, but the evidence was very uncertain (RR 0.44, 95% CI 0.22 to 0.89; two studies, 285 participants; very low‐certainty evidence; Analysis 3.2). We downgraded the certainty of the evidence two levels for very serious imprecision, because of the small sample size and few events; and one level for indirectness, because of the different time points for early drain removal and different definitions of low risk of postoperative pancreatic fistula.
3.2. Analysis.

Comparison 3: Early versus late drain removal, Outcome 2: Intra‐abdominal infection
We did not downgraded the certainty of the evidence for risk of bias, because we considered this an objective outcome, which was unlikely to be affected by performance bias or detection bias.
Wound infection
Two studies (285 participants: 143 early removal, 142 late removal) reported on wound infection (Dai 2020; Dembinski 2019). There were 13 intra‐abdominal infections in the early removal group, and 14 intra‐abdominal infections in the late removal group. The evidence was very uncertain about the effect of early drain removal on wound infection rate compared with late drain removal (RR 1.32, 95% CI 0.45 to 3.85; two studies, 285 participants; very low‐certainty evidence; Analysis 3.3). We downgraded the certainty of the evidence two levels for very serious imprecision, because of the small sample size, few events, and wide confidence interval; and one level for indirectness because of the different time points for early drain removal and different definitions of low risk of postoperative pancreatic fistula.
3.3. Analysis.

Comparison 3: Early versus late drain removal, Outcome 3: Wound infection
We did not downgraded the certainty of the evidence for risk of bias, because we considered this an objective outcome, which was unlikely to be affected by performance bias or detection bias.
Drain‐related complications
None of the studies reported this outcome.
Morbidity
Two studies (258 participants: 129 early removal, 129 late removal) reported on morbidity (Bassi 2010; Dai 2020). There were 41 participants with complications in the early removal group, and 85 participants with complications in the late removal group. The evidence suggested that early drain removal may reduce morbidity, but the evidence was very uncertain (RR 0.49, 95% CI 0.30 to 0.81; two studies, 258 participants; very low‐certainty evidence; Analysis 3.4). We downgraded the certainty of the evidence two levels for very serious imprecision, because of the small sample size and few events; one level for serious inconsistency (unexplained statistical heterogeneity I² = 67%); and one level for indirectness, because of the different time points for early drain removal and different definitions of low risk of postoperative pancreatic fistula.
3.4. Analysis.

Comparison 3: Early versus late drain removal, Outcome 4: Morbidity
We did not downgraded the certainty of the evidence for risk of bias, because we considered this an objective outcome, which was unlikely to be affected by performance bias or detection bias.
Length of hospital stay
All three studies (399 participants: 200 early removal, 199 late removal) reported on length of hospital stay, providing the mean and standard deviation (SD). The evidence suggested that early drain removal may reduce length of hospital stay, but the evidence was very uncertain (MD ‐2.20 days, 95% CI ‐3.52 to ‐0.87; three studies, 399 participants; very low‐certainty evidence; Analysis 3.5). We downgraded the certainty of the evidence two levels for very serious risk of bias, because all three studies were at high risk of performance bias, and length of hospital stay was primarily determined by the surgeons; one level for serious imprecision, because of the small sample size; and one level for indirectness, because of the different time points for early drain removal and different definitions of low risk of postoperative pancreatic fistula.
3.5. Analysis.

Comparison 3: Early versus late drain removal, Outcome 5: Length of hospital stay (days)
Hospital costs
Two studies (258 participants: 129 early removal, 129 late removal) reported on hospital costs, but reported them using different measurement scales (Bassi 2010; Dai 2020). The evidence was very uncertain about the effect of early drain removal on hospital costs compared with late drain removal (SMD ‐0.22, 95% CI ‐0.59 to 0.14; two studies, 258 participants; very low‐certainty evidence; Analysis 3.6). We downgraded the certainty of the evidence one level for serious risk of bias, because both studies were at high risk of performance bias; one level for serious imprecision, because of the small sample size; one level for serious inconsistency (unexplained statistical heterogeneity I² = 54%); and one level for indirectness, because of the different time points for early drain removal and different definitions of low risk of postoperative pancreatic fistula.
3.6. Analysis.

Comparison 3: Early versus late drain removal, Outcome 6: Hospital costs
Additional open procedures for postoperative complications
All three studies (399 participants: 200 early removal, 199 late removal) reported this outcome. There were six participants who needed additional open procedures for postoperative complications in the early removal group, and eight participants in the late removal group. The evidence was very uncertain about the effect of early drain removal on the need of additional open procedures for postoperative complications compared with late drain removal (RR 0.77, 95% CI 0.28 to 2.10; three studies, 399 participants; very low‐certainty evidence; Analysis 3.7). We downgraded the certainty of the evidence two levels for very serious imprecision, because of the small sample size, few events, and wide confidence interval; and one level for indirectness, because of the different time points for early drain removal and different definitions of low risk of postoperative pancreatic fistula.
3.7. Analysis.

Comparison 3: Early versus late drain removal, Outcome 7: Additional open procedures for postoperative complications
We did not downgraded the certainty of the evidence for risk of bias, because we considered this an objective outcome, which was unlikely to be affected by performance bias or detection bias.
Additional radiological interventions for postoperative complications
One study (144 participants: 72 early removal, 72 late removal) reported this outcome (Dai 2020). There were three participants who needed additional radiological interventions for postoperative complications in the early removal group, and three participants in the late removal group. The evidence was very uncertain about the effect of early drain removal on the need for additional radiological procedures for postoperative complications compared with late drain removal (RR 1.00, 95% CI 0.21 to 4.79; one study, 144 participants; very low‐certainty evidence; Analysis 3.8). We downgraded the certainty of the evidence two levels for very serious imprecision, because of the small sample size, few events, and wide confidence interval; and one level for indirectness, because of the different time points for early drain removal and different definitions of low risk of postoperative pancreatic fistula.
3.8. Analysis.

Comparison 3: Early versus late drain removal, Outcome 8: Additional radiological interventions for postoperative complications
We did not downgraded the certainty of the evidence for risk of bias, because we considered this an objective outcome, which was unlikely to be affected by performance bias or detection bias.
Pain
None of the studies reported this outcome.
Quality of life
None of the studies reported this outcome.
Subgroup analysis
We did not perform any of the planned subgroup analyses because there too few studies included in each comparison.
Sensitivity analysis
We performed the following planned sensitivity analyses:
used a fixed‐effect model rather than a random‐effects model;
calculated RD and OR, as well as RR for dichotomous outcomes;
conducted worst‐case and best‐case scenario analyses for missing data.
We observed no change in the direction of the results between a fixed‐effect and a random‐effects model, by calculating the risk difference (RD) or odds ratio (OR) for dichotomous outcomes (Table 4).
1. Sensitivity analyses.
| Comparisons | Outcomes | Main analysis (random‐effects model) | Fixed‐effect model | RD for dichotomous outcomes | OR for dichotomous outcomes | worst‐case scenario and best‐case scenario analysis for missing data | |
| worst/best‐case | best/worst‐case | ||||||
| Drain use versus no drain use | Mortality (30 days) | RR 0.78 (0.31 to 1.99) | RR 0.67 (0.28 to 1.58) | RD ‐0.01 (‐0.03 to 0.01) | OR 0.77 (0.29 to 2.03) | RR 2.58 (0.41 to 16.09) | RR 0.17 (0.04 to 0.79) |
| Drain use versus no drain use | Mortality (90 days) | RR 0.23 (0.06 to 0.90) | RR 0.23 (0.06 to 0.90) | RD ‐0.05 (‐0.16 to 0.07) | OR 0.21 (0.05 to 0.87) | RR 2.27 (0.06 to 84.61) | RR 0.07 (0.00 to 1.23) |
| Drain use versus no drain use | Intra‐abdominal infection | RR 0.97 (0.52 to 1.80) | RR 0.97 (0.64 to 1.45) | RD ‐0.00 (‐0.06 to 0.05) | OR 0.96 (0.48 to 1.92) | RR 1.72 (0.59 to 4.96) | RR 0.45 (0.31 to 0.63) |
| Drain use versus no drain use | Wound infection | RR 0.98 (0.68 to 1.41) | RR 0.98 (0.68 to 1.41) | RD 0.01 (‐0.03 to 0.04) | OR 0.98 (0.65 to 1.48) | RR 1.79 (0.81 to 3.95) | RR 0.53 (0.29 to 0.95) |
| Drain use versus no drain use | Drain‐related complications | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Active drain versus passive drain | Mortality (30 days) | RR 1.23 (0.30 to 5.06) | RR 1.27 (0.32 to 5.07) | RD 0.01 (‐0.02 to 0.03) | OR 1.24 (0.29 to 5.28) | RR 1.51 (0.40 to 5.77) | RR 1.22 (0.30 to 5.02) |
| Active drain versus passive drain | Mortality (90 days) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Active drain versus passive drain | Intra‐abdominal infection | RR 0.87 (0.21 to 3.66) | RR 1.07 (0.58 to 1.96) | RD ‐0.00 (‐0.10 to 0.10) | OR 0.87 (0.19 to 4.07) | RR 0.88 (0.20 to 3.99) | RR 0.87 (0.21 to 3.57) |
| Active drain versus passive drain | Wound infection | RR 0.92 (0.44 to 1.90) | RR 0.91 (0.44 to 1.88) | RD ‐0.01 (‐0.07 to 0.05) | OR 0.91 (0.41 to 2.01) | RR 0.98 (0.48 to 2.01) | RR 0.91 (0.44 to 1.89) |
| Active drain versus passive drain | Drain‐related complications | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Early versus late drain removal | Mortality (30 days) | RR 0.99 (0.06 to 15.45) | RR 0.99 (0.06 to 15.45) | RD ‐0.00 (‐0.02 to 0.02) | OR 0.99 (0.06 to 16.08) | RR 0.99 (0.06 to 15.45) | RR 0.99 (0.06 to15.45) |
| Early versus late drain removal | Mortality (90 days) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Early versus late drain removal | Intra‐abdominal infection | RR 0.44 (0.22 to 0.89) | RR 0.43 (0.21 to 0.87) | RD ‐0.09 (‐0.17 to ‐0.02) | OR 0.39 (0.18 to 0.87) | RR 0.44 (0.22 to 0.89) | RR 0.44 (0.22 to 0.89) |
| Early versus late drain removal | Wound infection | RR 1.32 (0.45 to 3.85) | RR 1.35 (0.47 to 3.90) | RD 0.01 (‐0.02 to 0.05) | OR 1.35 (0.43 to 4.29) | RR 1.32 (0.45 to 3.85) | RR 1.32 (0.45 to 3.85) |
| Early versus late drain removal | Drain‐related complications | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
‐: not available; OR: odds ratios; RR: risk ratio; RD: risk differences
There were 102 post‐randomization dropouts in four studies (Čečka 2018; Van Buren 2014; Van Buren 2017; Witzigmann 2016). We observed no change in the direction of the results by changing between worst‐case and best‐case scenario analyses for missing data, except for the following outcomes for the comparison drain use versus no drain use: mortality (30 days), mortality (90 days), intra‐abdominal infection, and wound infection (Table 4). We considered these outcomes to be less robust.
In the worst‐case scenario analyses for missing data in the comparison drain use versus no drain use, the results became consistent with both significant benefit and significant harm for 90‐day mortality (RR 2.27, 95% CI 0.06 to 84.61).
In the best‐case scenario analyses for missing data in the comparison drain use versus no drain use, the results became consistent with significant benefit in drain use for 30‐day mortality (RR 0.17, 95% CI 0.04 to 0.79), intra‐abdominal infection (RR 0.45, 95% CI 0.31 to 0.63), and wound infection (RR 0.53, 95% CI 0.29 to 0.95); the results became consistent with both significant benefit and significant harm for 90‐day mortality (RR 0.07, 95% CI 0.00 to 1.23).
We did not perform the planned sensitivity analysis by calculating SMD for continuous data with same measurement scales in different RCTs or calculating MD for continuous data with different measurement scales in different RCTs, because we did not include any of the continuous outcomes in the sensitivity analyses.
Discussion
Summary of main results
Evidence from nine studies, with 1892 people undergoing pancreatic surgery, contributed data to the outcomes of interest for this review.
For the comparison of drain use versus no drain use (four studies, 1110 participants), we found low‐certainty evidence that drain use may reduce 90‐day mortality compared with no drain use. There was low‐certainty evidence suggesting that drain use may result in little to no difference in 30‐day mortality, wound infection rate, length of hospital stay, the need for additional open procedures for postoperative complications, and quality of life compared with no drain use. There was moderate‐certainty evidence that drain use probably resulted in little to no difference in morbidity compared with no drain use. There was very uncertain evidence about the effect of drain use on intra‐abdominal infection rate and the need for additional radiological interventions for postoperative complications compared with no drain use. One study reported one drain‐related complication in the drainage group compared to no events in the no drainage group.
Two studies (383 participants) compared the use of an active drain versus a passive drain. There was very uncertain evidence about the effect of an active drain on 30‐day mortality, intra‐abdominal infection rate, wound infection rate, morbidity, length of hospital stay, and the need for additional open procedures for postoperative complications compared with a passive drain. One study reported there were no drain‐related complications in either group.
Data comparing early versus late drain removal were available from three studies (399 participants). There was very uncertain evidence about the effect of early drain removal on 30‐day mortality, wound infection rate, hospital costs, and the need for additional or radiological open procedures for postoperative complications compared with late drain removal. We found that early drain removal may reduce intra‐abdominal infection rate, morbidity, and length of hospital stay compared with late drain removal, but the evidence was very uncertain. None of the studies reported on drain‐related complications.
A previous version of this review included six studies with 1384 people undergoing pancreatic surgery. However, most of the studies compared drain use versus no drain use (four studies,1110 participants). Only one small study (160 participants) compared the use of an active drain versus a passive drain and suggested the efficacy of an active drain on earlier discharge from hospital. With the addition of three recent studies to this review (Čečka 2018; Dai 2020; Dembinski 2019), the results changed from the previous publication (Zhang 2018).
Overall completeness and applicability of evidence
All of the studies included people who were undergoing elective pancreaticoduodenectomy (N = 1245, 65.8%), distal pancreatectomy (N = 580, 30.7%), or other pancreatic surgery (N = 67, 3.5%) for various pancreatic and extra‐pancreatic diseases, including pancreatic cancers, ampullary cancers, chronic pancreatitis, biliary and duodenal malignancy. The majority (N = 1113, 58.9%) of the participants had either pancreatic cancers (N = 936, 49.5%) or chronic pancreatitis (N = 177, 9.4%). Only four of the studies included participants (N = 216, 11.4%) who underwent laparoscopic pancreatic resections (Dai 2020; Dembinski 2019; Van Buren 2014; Van Buren 2017); therefore, the results of this review may not be applicable to people undergoing laparoscopic pancreatic resections. Therefore, the results of this review are mostly applicable to people undergoing elective open pancreaticoduodenectomy, distal pancreatectomy, or other pancreatic surgery for various pancreatic and extra‐pancreatic diseases, especially pancreatic cancers or chronic pancreatitis.
Quality of the evidence
For drain use versus no drain use, we rated the certainty of evidence for seven important outcomes. We found low‐certainty evidence for 30‐day mortality, 90‐day mortality, wound infection, drain‐related complications, and length of hospital stay; moderate‐certainty evidence for morbidity; and very low‐certainty evidence for intra‐abdominal infection. We did not downgrade the certainty of the evidence for drain‐related complications for risk of bias, because it was unlikely to be affected by selection bias or performance bias. We downgraded the certainty of the evidence for 30‐day mortality, 90‐day mortality, intra‐abdominal infection, and wound infection by one level for serious risk of bias, because there were incomplete outcome data in some studies. We downgraded the certainty of the evidence for length of hospital stay by two levels for very serious risk of bias, because there were incomplete outcome data and lack of blinding of participants and personnel, since length of hospital stay was primarily determined by the surgeons. We downgraded the certainty of the evidence for intra‐abdominal infection by one level for serious unexplained inconsistency (substantial heterogeneity). We downgraded the certainty of the evidence for 30‐day mortality, 90‐day mortality, intra‐abdominal infection, and wound infection by one level for serious imprecision (few events or the confidence intervals of pooled data were either very close to or crossed the line of no effect). We downgraded the certainty of the evidence for drain‐related complications two levels for very serious imprecision, because of the small sample size and very few events. There was no indirectness of evidence, as the included studies assessed the appropriate population, intervention, comparisons, and outcomes. We included too few studies in this comparison to assess publication bias (Table 1).
For active drain versus passive drain, we found very low‐certainty evidence for all outcomes. We downgraded the certainty of the evidence for 30‐day mortality, 90‐day mortality, intra‐abdominal infection, wound infection, drain‐related complications, and morbidity by one level for serious risk of bias, because of incomplete outcome data in one study (Čečka 2018). We downgraded the certainty of the evidence by two levels for length of hospital stay for very serious risk of bias, because of incomplete outcome data and lack of blinding of participants and personnel, since length of hospital stay was primarily determined by the surgeons. We downgraded the certainty of the evidence for morbidity and length of hospital stay by one level for serious unexplained inconsistency (substantial heterogeneity). We downgraded the certainty of the evidence for 30‐day mortality, 90‐day mortality, intra‐abdominal infection, wound infection, and drain‐related complications by two levels for very serious imprecision, because of the small sample sizes, few events, and wide confidence intervals. We downgraded the certainty of the evidence for morbidity and length of hospital stay by one level for serious imprecision, because of the small sample sizes, and because the confidence intervals of pooled data were either very close to or crossed the line of no effect. There was no indirectness of evidence, as the included studies assessed the appropriate population, intervention, comparisons, and outcomes. We were unable to assess publication bias as we included too few studies in this comparison (Table 2).
For the comparison between early and late drain removal, we found very low‐certainty evidence for all outcomes. We did not downgrade the certainty of the evidence for 30‐day mortality, intra‐abdominal infection, wound infection, and morbidity for risk of bias, because these outcomes were unlikely to be affected by selection bias or performance bias. We downgraded the certainty of the evidence for length of hospital stay by two levels for very serious risk of bias, because of lack of blinding of participants and personnel, as length of hospital stay was primarily determined by the surgeons. We downgraded the certainty of the evidence for morbidity by one level for serious unexplained inconsistency (substantial heterogeneity). We downgraded the certainty of the evidence for 30‐day mortality, intra‐abdominal infection, and wound infection by two levels for very serious imprecision, because of the small sample sizes and few events. We downgraded the certainty of the evidence for morbidity and length of hospital stay by one level for serious imprecision, because of the small sample sizes. We downgraded the certainty of the evidence for all outcomes assessed by one level for indirectness (different time points for early drain removal and different definitions of low risk of postoperative pancreatic fistula). We were unable to assess publication bias, since we included too few studies in this comparison (Table 3).
Potential biases in the review process
There were some unavoidable, potential biases of note in the review process. First, when we contacted some investigators to request further information, we did not get a reply. The missing data may introduce bias to this review. In addition, we could not explore publication bias, because there were too few studies included in each comparison, and we did not have access to protocols for two studies (Conlon 2001; Jiang 2016).
Agreements and disagreements with other studies or reviews
There is increasing evidence from Cochrane Reviews that routine abdominal drainage after various abdominal operations is not mandatory (Gurusamy 2007a; Gurusamy 2007b; Gurusamy 2013b; Li 2018; Rolph 2004; Wang 2015). The routine use of surgical drains has also been questioned in other areas, including thyroid, gynaecological, and orthopaedic surgeries (Charoenkwan 2017; Gates 2013; Parker 2007; Samraj 2007).
One systematic review that compared drain use with no drain use in people undergoing pancreatic resections concluded that the routine use of abdominal drains after pancreatic resection may result in a higher risk of major complications. Van der Wilt 2013 included three studies that we had considered for this review (Conlon 2001; Fisher 2011; Heslin 1998). Two of these studies were non‐randomized, so we did not include them in this review. Another systematic review also compared drain use with no drain use after pancreatic resections. Hüttner 2017 included three randomised controlled trials (RCTs), and concluded that drain use and no drain had similar results for mortality, morbidity, and re‐intervention (Conlon 2001; Van Buren 2014; Witzigmann 2016). One recent systematic review on this topic included six non‐randomized studies, and four RCTs (Conlon 2001; Van Buren 2014; Van Buren 2017; Witzigmann 2016). Lyu 2020 showed comparable outcomes for pancreatic resections with or without drainage. The results of this review update disagree with the findings of previous systematic reviews about mortality (Hüttner 2017; Lyu 2020; Van der Wilt 2013). We included the same four RCTs and used GRADE to assess the certainty of the evidence in this review update. We found that drain use reduce 90‐day mortality. With regard to other outcomes, this review update is consistent with the findings of Hüttner 2017 and Lyu 2020.
One recent systematic review compared the use of active drain with passive drain in people undergoing pancreatic resection, and included three RCTs (Čečka 2018; Jiang 2016; Lee 2009). One of these studies was a RCT about pancreatic duct drainage, so we did not include it in this review (Lee 2009). Gachabayov 2019 concluded that there was no difference in clinical outcomes between active drain and passive drain. For this review update, we included the two RCTs for this comparison and used GRADE to assess the certainty of the evidence. Our results agree with the findings of Gachabayov 2019.
Another systematic review compared early drain removal with late drain removal in people undergoing pancreatic resection and included two studies that we had considered for this review (Bassi 2010; Kawai 2006). One of the studies was not randomised, so we did not include it (Kawai 2006). Diener 2011 concluded that early drain removal seemed to be superior to late drain removal. For this review update, we included three RCTs for this comparison and used GRADE to assess the certainty of the evidence (Bassi 2010; Dai 2020; Dembinski 2019). Our results agree with the findings of Diener 2011.
Authors' conclusions
Implications for practice.
Compared with no drain use, it is unclear whether routine drain use has any effect on mortality at 30 days or on postoperative complications after pancreatic surgery. Low‐certainty evidence suggests that routine abdominal drainage may reduce mortality at 90 days, compared with no drain use. The evidence is very uncertain about the effect of an active drain on mortality at 30 days or postoperative complications compared with the use of a passive drain. Compared with late drain removal, early drain removal may reduce intra‐abdominal infection rate, morbidity, and length of hospital stay for people with a low risk of postoperative pancreatic fistula, but the evidence is very uncertain.
Implications for research.
More studies with an adequate sample size are necessary to assess the benefits and harms of abdominal drainage for people with high risk of postoperative pancreatic fistula. A sample size of 870 (435 in each group) would be required to detect an absolute reduction in the intra‐abdominal infection rate of 5% (from 10% to 5%), at 80% power and an alpha‐error set at 0.05 (Conlon 2001).
Future studies should report the rate and grade of the postoperative complication according to the Clavien‐Dindo Classification (Clavien 2009; Dindo 2004). Future studies should analyse the data on an intention‐to‐treat basis in the case of post‐randomization dropouts. More studies are needed to assess drain versus no drain use in laparoscopic pancreatic resections.
What's new
| Date | Event | Description |
|---|---|---|
| 8 February 2021 | New citation required and conclusions have changed | Searches rerun; three new RCTs with 508 participants identified and included in analyses; review updated accordingly. Conclusions changed for comparison of active drain versus passive drain. Author order changed. |
| 8 February 2021 | New search has been performed | Searches rerun; three additional trials found |
History
Protocol first published: Issue 6, 2013 Review first published: Issue 8, 2015
| Date | Event | Description |
|---|---|---|
| 15 November 2017 | New search has been performed | Searches rerun; one trial identified; new evidence incorporated; conclusions did not change |
Acknowledgements
We acknowledge the contribution of authors of previous version of this review: Wei Zhang, Sirong He, Yao Cheng, Jie Xia, Mingliang Lai, Nansheng Cheng, Zuojin Liu.
We acknowledge the help and support of the Cochrane Gut Group. The authors would also like to thank the following editors and peer referees who provided comments on the present review update: Claudio Bassi (Peer Reviewer), Huan Song (Editor), Yuhong Yuan (Information Specialist and previous Managing Editor), Teo Quay (Managing Editor), Frances Tse (Contact Editor), Grigorios Leontiadis (Sign‐Off Editor). We would also like to thank Victoria Pennick for copy‐editing this version of the review.
Yuhong Yuan (Information Specialist, Cochrane Gut Group) revised the search strategies in this updated version.
The Methods section of this review is based on a standard template provided by Cochrane Gut Group.
Appendices
Appendix 1. Glossary of terms
Abscess: a collection of pus that has built up within the tissues of the body
Active drain: drains that suction under low or high pressure
Adverse events: untoward side effects
Anastomosis: connection between two organs (e.g. stomach and small intestine) created by surgery
Biliary: related to the bile duct
Chronic pancreatitis: long‐standing inflammation of the pancreas
Delayed gastric emptying: a medical condition consisting of paresis (partial paralysis) of the stomach, resulting in food remaining in the stomach for an abnormally long time
Duodenal: related to the first section of the small intestine
Drainage: the process or system by which water or waste liquid flows away
Incidence: the rate at which something happens
Morbidity: the proportion of people with any postoperative complications
Mortality: the proportion of deaths (after surgery)
Pancreas: the organ in the body that produces insulin and a liquid that helps your body to use the food that you eat
Pancreatic: relating to the pancreas
Pancreatic anastomoses: the surgical connection of the bile‐pancreatic duct and gut to form a continuous channel
Pancreatic fistula: an abnormal communication between the pancreas and other organs due to leakage of pancreatic juice containing digestive enzymes from damaged pancreatic ducts
Pancreaticoduodenectomy: a major surgical operation involving the pancreas, duodenum, and other organs
Passive drain: drains without suction
Postoperative: relating to the time after someone has had a medical operation
Prevalence: the total number of people with an illness at a designated time
Prophylactic: protective or preventive
Randomized controlled trials: an experiment in which participants are randomly allocated to two or more interventions, possibly including a control intervention or no intervention, and the results are compared
Subhepatic: under the liver.
Appendix 2. Cochrane CENTRAL Ovid search strategy
exp Pancreas/
pancrea*.af.
or/1‐2
exp General Surgery/
(resect* or surger* or surgical or operat* or postoperat*).tw,kw.
exp Pancreatectomy/
pancreatectom*.tw,kw.
exp Pancreaticoduodenectomy/
(duodenopancreatectom* or pancreatoduodenectom* or pancreaticogastrostom*).tw.
whipple.tw.
exp Pancreaticojejunostomy/
(pancreatojejunostom* or pancreaticojejunostom*).tw,kw.
((pancrea* adj5 (duodenectom* or jejunostom* or gastrostom*)) or pancreaticojejunal anastomosis or jejunopancreatic anastomosis or jejuno‐pancreatic anastomosis).tw,kw.
or/4‐13
3 and 14
exp Drainage/
drain*.af.
(suction* or aspirat* or paracentesis).tw,kw.
or/16‐18
15 and 19
Appendix 3. MEDLINE Ovid search strategy
exp Pancreas/
pancrea*.af.
or/1‐2
exp General Surgery/
(resect* or surger* or surgical or operat* or postoperat*).tw,kw.
exp Pancreatectomy/
pancreatectom*.tw,kw.
exp Pancreaticoduodenectomy/
(duodenopancreatectom* or pancreatoduodenectom* or pancreaticogastrostom*).tw.
whipple.tw.
exp Pancreaticojejunostomy/
(pancreatojejunostom* or pancreaticojejunostom*).tw,kw.
((pancrea* adj5 (duodenectom* or jejunostom* or gastrostom*)) or pancreaticojejunal anastomosis or jejunopancreatic anastomosis or jejuno‐pancreatic anastomosis).tw,kw.
or/4‐13
3 and 14
exp Drainage/
drain*.af.
(suction* or aspirat* or paracentesis).tw,kw.
or/16‐18
15 and 19
randomized controlled trial.pt.
controlled clinical trial.pt.
random*.ab.
placebo.ab.
trial.ab.
groups.ab.
or/21‐26
exp animals/ not humans.sh.
27 not 28
20 and 29
Note:
Lines 21‐28. RCT filter: “Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐maximizing version (2008 revision); Ovid format”. We made the following minor revisions: we used “random*.ab.” instead of “randomized.ab” or “randomly.ab.” to capture word variations such as “randomised, randomization, random”; we removed “drug therapy.fs.” from the above filter as this review is not related to drug therapy.
Appendix 4. Embase Ovid search strategy
exp pancreas/
pancrea*.af.
or/1‐2
exp surgery/
(resect* or surger* or surgical or operat* or postoperat*).tw,kw.
exp pancreatectomy/
pancreatectom*.tw,kw.
exp pancreaticoduodenectomy/
(duodenopancreatectom* or pancreatoduodenectom* or pancreaticogastrostom*).tw.
whipple.tw.
exp pancreaticojejunostomy/
(pancreatojejunostom* or pancreaticojejunostom*).tw,kw.
((pancrea* adj5 (duodenectom* or jejunostom* or gastrostom*)) or pancreaticojejunal anastomosis or jejunopancreatic anastomosis or jejuno‐pancreatic anastomosis).tw,kw.
or/4‐13
3 and 14
exp surgical drainage/ or exp aspiration/ or exp suction/
drain*.af.
(suction* or aspirat* or paracentesis).tw,kw.
or/16‐18
15 and 19
random:.tw.
placebo:.mp.
double‐blind:.tw.
or/21‐23
exp animal/ not human.sh.
24 not 25
20 and 26
Note: Lines 21‐23. RCT filter. Hedge Best balance of sensitivity and specificity filter for identifying randomized trials in Embase. (https://hiru.mcmaster.ca/hiru/HIRU_Hedges_EMBASE_Strategies.aspx)
Appendix 5. Science Citation Index Expanded Web of Science search strategy
Topic=(pancrea*)
Topic=((surger* or operat* or operative therap* or resec*)) OR Topic=((pancreatectom* or pancreaticojejunostom* or pancreaticoduodenectom* or pancreaticogastrostom* or duodenopancreatectom*))
Topic=(drain* or suction* or aspirat* or paracentesis)
#3 AND #2 AND #1
Topic=(single blind* or double blind* or clinical trial* or placebo* or random* or controlled clinical trial* or research design or comparative stud* or controlled trial* or (follow up stud*) or prospective stud*)
#5 AND #4
Appendix 6. Chinese Biomedical Literature Database (CBM) search strategy
主题词:随机对照试验/全部树/全部副主题词
主题词:临床对照试验/全部树/全部副主题词
主题词:临床试验/全部树/全部副主题词
主题词:病例对照研究/全部树/全部副主题词
主题词:随机分配/全部树/全部副主题词
主题词:对比研究/全部树/全部副主题词
主题词:前瞻性研究/全部树/全部副主题词
主题词:安慰剂/全部树/全部副主题词
全部字段:随机
全部字段:单盲
全部字段:双盲
全部字段:盲法
(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12)
主题词:胰腺/全部树/SU
主题词:外科手术/全部树/全部副主题词
主题词:吻合术, 外科/全部树/全部副主题词
全部字段:手术治疗
全部字段:手术
全部字段:切除术
主题词:胰腺切除术/全部树/全部副主题词
主题词:胰十二指肠切除术/全部树/全部副主题词
全部字段:胰体尾切除术
全部字段:胰腺部分切除
全部字段:胰十二指肠吻合术
全部字段:胰头十二指肠吻合术
全部字段:Whipple术式
全部字段:Child术式
(#15 OR #16 OR #17 OR #18 OR #19 OR #20 OR#21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27)
主题词:引流/全部树/全部副主题词
主题词:抽吸/全部树/全部副主题词
全部字段:腹腔引流
全部字段:引流管
全部字段:手术引流
全部字段:伤口引流
(#29 OR #30 OR #31 OR #32 OR #33 OR #34)
(#13 AND # 14 AND #28 AND #35)
Data and analyses
Comparison 1. Drain use versus no drain use.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Mortality (30 days) | 4 | 1055 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.31, 1.99] |
| 1.2 Mortality (90 days) | 2 | 478 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.06, 0.90] |
| 1.3 Intra‐abdominal infection | 4 | 1055 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.52, 1.80] |
| 1.4 Wound infection | 4 | 1055 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.68, 1.41] |
| 1.5 Morbidity | 4 | 1055 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.94, 1.13] |
| 1.6 Length of hospital stay (days) | 3 | 876 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.79, 0.51] |
| 1.7 Length of hospital stay | 1 | Other data | No numeric data | |
| 1.8 Additional open procedures for postoperative complications | 4 | 1055 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.79, 2.23] |
| 1.9 Additional radiological interventions for postoperative complications | 3 | 660 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.40, 1.87] |
Comparison 2. Active drain versus passive drain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Mortality (30 days) | 2 | 382 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.30, 5.06] |
| 2.2 Intra‐abdominal infection | 2 | 321 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.21, 3.66] |
| 2.3 Wound infection | 2 | 321 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.44, 1.90] |
| 2.4 Morbidity | 2 | 382 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.53, 1.77] |
| 2.5 Length of hospital stay (days) | 2 | 321 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐2.63, 1.04] |
| 2.6 Additional open procedures for postoperative complications | 2 | 321 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.11, 1.83] |
Comparison 3. Early versus late drain removal.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Mortality (30 days) | 3 | 399 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.06, 15.45] |
| 3.2 Intra‐abdominal infection | 2 | 285 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.22, 0.89] |
| 3.3 Wound infection | 2 | 285 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.45, 3.85] |
| 3.4 Morbidity | 2 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.30, 0.81] |
| 3.5 Length of hospital stay (days) | 3 | 399 | Mean Difference (IV, Random, 95% CI) | ‐2.20 [‐3.52, ‐0.87] |
| 3.6 Hospital costs | 2 | 258 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.59, 0.14] |
| 3.7 Additional open procedures for postoperative complications | 3 | 399 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.28, 2.10] |
| 3.8 Additional radiological interventions for postoperative complications | 1 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.21, 4.79] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bassi 2010.
| Study characteristics | ||
| Methods | Randomized controlled trial | |
| Participants | Country: Italy, single‐centre Time of enrolment: March 2008 to April 2009 Number randomised: 114 Post‐randomization dropout: 0 (0%) Intention‐to‐treat analysis: yes Description of sample size calculation: yes Mean age: 56.6 years Females: 55 (48.2%) Pancreatic cancer: 56 (49.1%) Biliary cancer: 2 (1.8%) Ampullary cancer: 7 (6.1%) Chronic pancreatitis: 3 (2.6%) Other: 46 (40.4%) Pancreaticoduodenectomy: 75 (65.8%) Distal pancreatectomy: 39 (34.2%) Other pancreatic surgery: 0 (0%) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants (N = 114) were randomly assigned to 1 of 2 groups Group 1: early drain removal (postoperative day 3; N = 57) Group 2: late drain removal (postoperative day 5 or later; N = 57) |
|
| Outcomes | Postoperative pancreatic fistula, abdominal complications, pulmonary complications, reoperation, length of hospital stay, hospital readmission, postoperative mortality, morbidity, and hospital costs | |
| Notes | Two drainage tubes (Penrose drains) were placed in relation to the pancreatic and biliary anastomoses through separate skin incisions after pancreaticoduodenectomy. One drainage tube was placed in relation to the pancreatic stump through separate skin incisions after distal pancreatectomy. Funding: no information provided Conflicts of interest for the study authors: no information provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "eligible patients were randomised by a computer‐generated allocation schedule" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Masking: Open Label" in the protocol |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Masking: Open Label" in the protocol |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomization dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was available (NCT00931554). All of the study's prespecified outcomes were reported. |
| Other bias | Low risk | Comment: the study appeared to be free of other sources of bias |
Čečka 2018.
| Study characteristics | ||
| Methods | Randomized controlled trial | |
| Participants | Country: Czech Republic, dual‐centre Time of enrolment: November 2013 to April 2016 Duration of follow‐up period: 3 months postoperatively Number randomised: 223 Post‐randomization dropout: 1 (0.4%) Intention‐to‐treat analysis: no Description of sample size calculation: yes Mean age: 63.9 years Females: 113 (50.9%) Pancreatic cancer: 117 (52.7%) Biliary cancer: 10 (4.5%) Duodenal cancer: 0 (0%) Ampullary cancer: 15 (6.8%) Chronic pancreatitis: 15 (6.8%) Pancreaticoduodenectomy: 161 (72.5%) Distal pancreatectomy: 61 (27.5%) Other pancreatic surgery: 0 (0%) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants (N = 223) were randomly assigned to 1 of 2 groups Group 1: active drain (N = 112) Group 2: passive drain (N = 111) |
|
| Outcomes | Mortality, morbidity, wound infection, intra‐abdominal infection, various postoperative complications, reoperation, readmission, additional radiological intervention, and length of hospital stay | |
| Notes | Funding: Ministry of Health Czech Republic Conflicts of interest for the study authors: no information provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was accomplished using balanced permutation blocks by generation of random numbers to obtain homogeneity of the groups" |
| Allocation concealment (selection bias) | Low risk | Quote: "Opaque, sealed envelopes were produced and labeled with the randomization number containing a sheet that stated the group allocation for the patient" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Masking: Open Label" in the protocol Quote: "Obviously, operating surgeons, attending physicians, and nursing staff could not be blinded because the drains look different" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "outcome assessors were blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "One patient in group B was lost during follow‐up and thus 111 patients in each group were analyzed" Comment: there was one post‐randomization dropout. However, it is not clear whether there was a substantial departure from the intervention in this trial that would cause a per‐protocol analysis to differ significantly from an intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was available (NCT01988519). All of the study's prespecified outcomes were reported |
| Other bias | Low risk | Comment: the study appeared to be free of other sources of bias |
Conlon 2001.
| Study characteristics | ||
| Methods | Randomized controlled trial | |
| Participants | Country: USA, single‐centre Time of enrolment: unknown Number randomised: 179 Post‐randomization dropout: 0 (0%) Intention‐to‐treat analysis: yes Description of sample size calculation: no Mean age: 65.4 years Females: 90 (50.3%) Pancreatic cancer: 142 (79.3%) Biliary cancer: 3 (1.7%) Duodenal cancer: 10 (5.6%) Ampullary cancer: 24 (13.4%) Chronic pancreatitis: 0 (0%) Pancreaticoduodenectomy: 139 (77.7%) Distal pancreatectomy: 40 (22.3%) Other pancreatic surgery: 0 (0%) Open pancreatic resections: 179 (100%) Laparoscopic pancreatic resections: 0 (0%) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants (N = 179) were randomly assigned to 1 of 2 groups Group 1: drainage (N = 91) Group 2: no drainage (N = 88) |
|
| Outcomes | Mortality, morbidity, wound infection, intra‐abdominal infection, various postoperative complications, reoperation, additional radiological intervention, and length of hospital stay | |
| Notes | Two drainage tubes (Jackson‐Pratt closed suction drains) were placed in relation to the pancreatic and biliary anastomoses through separate skin incisions after pancreaticoduodenectomy. One drainage tube was placed in relation to the pancreatic stump through separate skin incisions after distal pancreatectomy. Funding: Milton and Bernice Stern Fund for Pancreatic Research Conflicts of interest for the study authors: no information provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were randomised during surgery by the envelope method" Comment: no information was provided whether the envelope was opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind performing surgeons |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomization dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but the published reports included all expected outcomes |
| Other bias | Low risk | Comment: the study appeared to be free of other sources of bias |
Dai 2020.
| Study characteristics | ||
| Methods | Randomized controlled trial | |
| Participants | Country: China, single‐centre Time of enrolment: June 2014 to July 2018 Number randomised: 144 Post‐randomization dropout: 0 (0%) Intention‐to‐treat analysis: yes Description of sample size calculation: no Mean age: 56.2 years Females: 65 (45.1%) Pancreatic cancer: 72 (50.0%) Biliary cancer: 6 (4.2%) Ampullary cancer: 9 (14.8%) Chronic pancreatitis: not mentioned Other: 57 (39.6%) Pancreaticoduodenectomy: 104 (72.2%) Distal pancreatectomy: 40 (27.8%) Other pancreatic surgery: 0 (0%) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants (N = 144) were randomly assigned to 1 of 2 groups Group 1: early drain removal (postoperative day 3; N = 72) Group 2: late drain removal (postoperative day 5 or later; N = 72) |
|
| Outcomes | Postoperative pancreatic fistula, abdominal complications, pulmonary complications, reoperation, length of hospital stay, hospital readmission, postoperative mortality, morbidity, and hospital costs | |
| Notes | Two non‐sucking drain tubes were placed underneath the pancreatic and biliary anastomoses or near the pancreatic remnant, and in the splenic space Funding: Beijing Municipal Science & Technology Commission Conflicts of interest for the study authors: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "they were randomly assigned into 2 groups by central randomization system, generated by the Medical Research & Biometrics Center in National Center for Cardiovascular Disease" |
| Allocation concealment (selection bias) | Low risk | Quote: "central randomization system" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Masking: Open Label" in the protocol |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Masking: Open Label" in the protocol |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomization dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was available (NCT02230436). All of the study's prespecified outcomes were reported |
| Other bias | Low risk | Comment: the study appeared to be free of other sources of bias |
Dembinski 2019.
| Study characteristics | ||
| Methods | Randomized controlled trial | |
| Participants | Country: France, single‐centre Time of enrolment: June 2011 to December 2015 Number randomised: 141 Post‐randomization dropout: 7 (5.0%) Intention‐to‐treat analysis: yes Description of sample size calculation: yes Mean age: 64.3 years Females: 90 (63.8%) Pancreatic cancer: 99 (70.2%) Biliary cancer: 8 (5.7%) Ampullary cancer: 9 (6.4%) Chronic pancreatitis: 5 (3.5%) Other: 20 (14.2%) Pancreaticoduodenectomy: 141 (100%) Distal pancreatectomy: 0 (0%) Other pancreatic surgery: 0 (0%) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants (N = 141) were randomly assigned to 1 of 2 groups Group 1: early drain removal (postoperative day 4; N = 71) Group 2: late drain removal (postoperative day 5 or later; N = 70) |
|
| Outcomes | Postoperative pancreatic fistula, abdominal complications, pulmonary complications, reoperation, length of hospital stay, hospital readmission, postoperative mortality, morbidity, and hospital costs | |
| Notes | Two drainage tubes were placed in relation to the pancreatic and biliary anastomoses through separate skin incisions after pancreaticoduodenectomy Funding: no funding Conflicts of interest for the study authors: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "by means of an electronic module integrated in an electronic case report form" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Masking: Open Label" in the protocol |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Masking: Open Label" in the protocol |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were seven post‐randomization dropouts. The study performed an intention‐to‐treat analysis which included the dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was available (NCT01368094). All of the study's prespecified outcomes were reported |
| Other bias | Low risk | Comment: the study appeared to be free of other sources of bias |
Jiang 2016.
| Study characteristics | ||
| Methods | Randomized controlled trial | |
| Participants | Country: China, single‐centre Time of enrolment: April 2010 to May 2015 Number randomised: 160 Post‐randomization dropout: 0 (0%) Intention‐to‐treat analysis: yes Description of sample size calculation: yes Mean age: 59.6 years Females: 42 (26.3%) Pancreatic cancer: 53 (33.1%) Biliary cancer: 36 (22.5%) Duodenal cancer: 28 (17.5%) Ampullary cancer: 33 (20.6%) Chronic pancreatitis: 5 (3.1%) Pancreaticoduodenectomy: 160 (100%) Distal pancreatectomy: 0 (0%) Other pancreatic surgery: 0 (0%) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants (N = 160) were randomly assigned to 1 of 2 groups Group 1: active drain (N = 82) Group 2: passive drain (N = 78) |
|
| Outcomes | Mortality, morbidity, wound infection, intra‐abdominal infection, various postoperative complications, reoperation, readmission, additional radiological intervention, and length of hospital stay | |
| Notes | Funding: no information provided Conflicts of interest for the study authors: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "we randomised our patients using a computer‐generated random number" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Patients were prospectively assigned a code and data were recorded in a database by two nurses" Comment: no information provided on whether the 2 nurses were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomization dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but the published reports included all expected outcomes |
| Other bias | Low risk | Comment: the study appeared to be free of other sources of bias |
Van Buren 2014.
| Study characteristics | ||
| Methods | Randomized controlled trial | |
| Participants | Country: USA, multi‐centre Time of enrolment: September 2011 to December 2012 Number randomised: 137 Post‐randomization dropout: 3 (2.2%) Intention‐to‐treat analysis: no Description of sample size calculation: yes Mean age: 63.2 years Females: 62 (45.3%) Pancreatic cancer: 67 (48.9%) Biliary cancer: not mentioned Duodenal cancer: not mentioned Ampullary cancer: 17 (12.4%) Chronic pancreatitis: 15 (10.9%) Pancreaticoduodenectomy: 137 (100%) Distal pancreatectomy: 0 (0%) Other pancreatic surgery: 0 (0%) Open pancreatic resections: 126 (92%) Laparoscopic pancreatic resections: 11 (8%) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants (N = 137) were randomly assigned to 1 of 2 groups Group 1: drainage (N = 68) Group 2: no drainage (N = 69) |
|
| Outcomes | Mortality, morbidity, wound infection, intra‐abdominal infection, various postoperative complications, reoperation, readmission, additional radiologic intervention, and length of hospital stay | |
| Notes | Funding: Rodrick A MacDonald Foundation, Cancer Prevention and Research Institute of Texas grant, and Dan L Duncan Cancer Center Support grant Conflicts of interest for the study authors: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using a computerized randomization system at the coordinating center" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Masking: open label" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Masking: open label" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "There were 3 cases for which the randomization group assignment was inadvertently not followed" Comment: there were three post‐randomization dropouts. The study did not conduct an intention‐to‐treat analysis that included the dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was available (NCT01441492). All of the study's prespecified outcomes were reported |
| Other bias | Low risk | Comment: the study appeared to be free of other sources of bias |
Van Buren 2017.
| Study characteristics | ||
| Methods | Randomized controlled trial | |
| Participants | Country: USA and Canada, multi‐centre Time of enrolment: September 2011 to November 2016 Number randomised: 399 Post‐randomization dropout: 55 (13.8%) Intention‐to‐treat analysis: no Description of sample size calculation: yes Mean age: 61.0 years Females: 205 (60.0%) Pancreatic cancer: 171 (49.7%) Biliary cancer: not mentioned Duodenal cancer: not mentioned Ampullary cancer: not mentioned Chronic pancreatitis: 33 (9.6%) Pancreaticoduodenectomy: 0 (0%) Distal pancreatectomy: 344 (100%) Other pancreatic surgery: 0 (0%) Open pancreatic resections: 247 (61.9%) Laparoscopic pancreatic resections: 152 (38.1%) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants (N = 399) were randomly assigned to 1 of 2 groups Group 1: drainage (N = 202) Group 2: no drainage (N = 197) |
|
| Outcomes | Mortality, morbidity, wound infection, intra‐abdominal infection, various postoperative complications, reoperation, readmission, additional radiologic intervention, length of hospital stay, and quality of life | |
| Notes | Funding: Rodrick A MacDonald Foundation Conflicts of interest for the study authors: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised preoperatively to DP with or without intraperitoneal drain placement using a computerized randomization system at the coordinating center" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Masking: open label" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Masking: open label" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "There were 55 patients who were excluded from the study after randomization and were not followed. Four patients, 2 in each group, were lost to follow‐up and excluded from the analysis". Comment: there were 59 post‐randomization dropouts. The study did not perform an intention‐to‐treat analysis that included the 59 dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was available (NCT01441492). All of the study's prespecified outcomes were reported |
| Other bias | Low risk | Comment: the study appeared to be free of other sources of bias |
Witzigmann 2016.
| Study characteristics | ||
| Methods | Randomized controlled trial | |
| Participants | Country: Germany, dual‐centre Time of enrolment: July 2007 to June 2015 Number randomised: 438 Post‐randomization dropout: 43 (2.2%) Intention‐to‐treat analysis: no Description of sample size calculation: yes Mean age: 63.4 years Females: 139 (35.2%) Pancreatic cancer: 159 (40.3%) Biliary cancer: 23 (5.8%) Duodenal cancer: 5 (1.3%) Ampullary cancer: 19 (4.8%) Chronic pancreatitis: 101 (25.6%) Pancreaticoduodenectomy: 328 (83.0%) Distal pancreatectomy: 0 (0%) Other pancreatic surgery: 67 (17.0%) Open pancreatic resections: 395 (100%) Laparoscopic pancreatic resections: 0 (0%) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants (N = 395) were randomly assigned to 1 of 2 groups Group 1: drainage (N = 202) Group 2: no drainage (N = 193) |
|
| Outcomes | Mortality, morbidity, wound infection, intra‐abdominal infection, various postoperative complications, reoperation, operation time, and length of hospital stay | |
| Notes | Funding: no funding Conflicts of interest for the study authors: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A random list was created by GWT‐TUD Ltd" |
| Allocation concealment (selection bias) | Low risk | Quote: "The random allocation sequence was implemented by the use of sequentially numbered opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind performing surgeons |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "A total of 438 patients were randomised. Forty‐three patients (9.8%) were excluded because no pancreatic resection with consecutive pancreaticojejunal anastomosis was performed. Thus, the intention‐to‐treat population consisted of 395 patients". Comment: there were 43 post‐randomization dropouts. The study did not perform an intention‐to‐treat analysis that included the 43 dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was available (ISRCTN04937707). All of the study's prespecified outcomes were reported |
| Other bias | Low risk | Comment: the study appeared to be free of other sources of bias |
DP: distal pancreatectomy; IU: international unit; N: number of participants
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adham 2013 | A non‐randomized study |
| Behrman 2015 | A non‐randomized study |
| Correa‐Gallego 2013 | A non‐randomized study |
| Fisher 2011 | A non‐randomized study |
| Giovinazzo 2011 | A non‐randomized study |
| Heslin 1998 | A non‐randomized study |
| Jeekel 1992 | Case series |
| Kawai 2006 | A non‐randomized study |
| Kawaida 2021 | A non‐randomized study |
| Kunstman 2017 | A non‐randomized study |
| Lee 2009 | Randomized controlled trial about pancreatic duct drainage |
| Lemke 2021 | A non‐randomized study |
| Lim 2013 | A non‐randomized study |
| Mehta 2013 | A non‐randomized study |
| Paulus 2012 | A non‐randomized study |
| Zaghal 2020 | A non‐randomized study |
ICU: intensive care unit
Characteristics of ongoing studies [ordered by study ID]
Kaiser 2019.
| Study name | Abdominal drainage versus no drainage after distal pancreatectomy: study protocol for a randomised controlled trial |
| Methods | Randomized controlled trial |
| Participants | Country: Germany
Number of enrolment: 252
Inclusion criteria: 1. Aged ≥ 18 years 2. People planned for open or minimally invasive distal pancreatectomy for pancreatic disease 3. Signed informed consent provided Exclusion criteria: 1. Indication for pancreatic resection with a pancreaticojejunal anastomosis 2. American Society of Anesthesiologists (ASA) physical status classification ≥ 4 3. Impaired mental state or language barriers impeding informed consent 4. Participation in another interventional trial with interference of intervention and outcome of this trial |
| Interventions | Participants were randomly assigned to two groups Group 1: drainage Group 2: no drainage |
| Outcomes | The primary outcome is morbidity. The secondary outcomes are mortality, various postoperative complications, reoperation rate, operation time, length of hospital stay, duration of ICU stay, and readmission rate. |
| Starting date | March 2018 |
| Contact information | Principal investigator: Thilo Hackert, University Hospital Heidelberg, Germany Email: thilo.hackert@med.uni‐heidelberg.de |
| Notes |
Differences between protocol and review
We updated the search strategies with the help of the Cochrane Gut Group Information Specialist.
We added time points of measurement for all outcomes.
Because overall infectious complications were not reported, we chose two types of infectious complication (intra‐abdominal infection and wound infection) for the summary of findings table.
We did not perform any subgroup analyses because of an insufficient number of studies were included in the meta‐analyses.
We did not create funnel plots to assess reporting biases because there were fewer than 10 included studies.
We did not perform the planned sensitivity analysis by calculating SMD for continuous data with same measurement scales in different RCTs or calculating MD for continuous data with different measurement scales in different RCTs, because none of the continuous outcomes were assessed in the sensitivity analyses. We performed the other planned sensitivity analyses for primary outcomes, including 30‐day mortality, 90‐day mortality, intra‐abdominal infection, wound infection, and drain‐related complications.
Contributions of authors
Conceiving the review: YC
Designing the review: SH
Co‐ordinating the review: JX
Designing search strategies: SH, WZ
Study selection: SH, WZ
Data extraction: ML, ZL
Writing the review: SH, JX
Providing general advice on the review: NC
Securing funding for the review: ZL
Performing previous work that was the foundation of the current study: YC
Sources of support
Internal sources
-
Chongqing Medical University, China
This review was supported by National Natural Science Foundation of China (Grant No. 81701950, 82172135), Medical Research Projects of Chongqing (Grant No. 2018MSXM132), and the Kuanren Talents Program of the second affiliated hospital of Chongqing Medical University (Grant No. KY2019Y002).
External sources
No sources of support provided
Declarations of interest
SH: None known
JX: None known
WZ: None known
ML: None known
NC: None known
ZL: None known
YC: None known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Bassi 2010 {published data only}
- Bassi C, Molinari E, Malleo G, Crippa S, Butturini G, Salvia R, et al. Early versus late drain removal after standard pancreatic resections: results of a prospective randomized trial. Annals of Surgery 2010;252(2):207-14. [DOI] [PubMed] [Google Scholar]
- NCT00931554. Randomized trial of early versus standard drainage removal after pancreatic resections [Early versus standard drainage removal after pancreatic resections: results of a prospective randomized clinical trial]. clinicaltrials.gov/ct2/show/NCT00931554 (first received 2 July 2009).
Čečka 2018 {published data only}
- Čečka F, Jon B, Skalický P, Čermáková E, Neoral Č, Loveček M. Results of a randomized controlled trial comparing closed-suction drains versus passive gravity drains after pancreatic resection. Surgery 2018;164(5):1057-63. [DOI] [PubMed] [Google Scholar]
- NCT01988519. Randomized controlled trial comparing closed-suction drain versus passive gravity drain following pancreatic resection (DRAPA) [Results of a randomized controlled trial comparing closed-suction drains versus passive gravity drains after pancreatic resection]. clinicaltrials.gov/ct2/show/NCT01988519 (first received 20 November 2013).
Conlon 2001 {published data only}
- Conlon KC, Labow D, Leung D, Smith A, Jarnagin W, Coit DG, et al. Prospective randomized clinical trial of the value of intraperitoneal drainage after pancreatic resection. Annals of Surgery 2001;234(4):487-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dai 2020 {published data only}
- Dai M, Liu Q, Xing C, Kleeff J, Liao Q, Guo J, et al. Early drain removal after major pancreatectomy reduces postoperative complications: a single-center, randomized, controlled trial. Journal of Pancreatology 2020;3(2):93-100. [Google Scholar]
- NCT02230436. Early versus late drain removal after pancreatectomy: a randomized prospective trial [Early drain removal after major pancreatectomy reduces postoperative complications: a single-center, randomized, controlled trial]. clinicaltrials.gov/ct2/show/NCT02230436 (first received 3 September 2014).
Dembinski 2019 {published data only}
- Dembinski J, Mariette C, Tuech JJ, Mauvais F, Piessen G, Fuks D, et al. Early removal of intraperitoneal drainage after pancreatoduodenectomy in patients without postoperative fistula at POD3: results of a randomized clinical trial. Journal of Visceral Surgery 2019;156(2):103-12. [DOI] [PubMed] [Google Scholar]
- NCT01368094. Early (4 days) versus standard drainage of the abdominal cavity after pancreaticoduodenectomy [Early removal of intraperitoneal drainage after pancreatoduodenectomy in patients without postoperative fistula at POD3: results of a randomized clinical trial]. clinicaltrials.gov/ct2/show/NCT01368094 (first received 7 June 2011).
Jiang 2016 {published data only}
- Jiang H, Liu N, Zhang M, Lu L, Dou R, Qu L. A randomized trial on the efficacy of prophylactic active drainage in prevention of complications after pancreaticoduodenectomy. Scandinavian Journal of Surgery 2016;105:215-22. [DOI] [PubMed] [Google Scholar]
Van Buren 2014 {published data only}
- McMillan MT, Fisher WE, Van Buren G, McElhany A, Bloomston M, Hughes SJ, et al. The value of drains as a fistula mitigation strategy for pancreatoduodenectomy: something for everyone? Results of a randomized prospective multi-institutional study. Journal of Gastrointestinal Surgery 2015;19(1):21-30. [DOI] [PubMed] [Google Scholar]
- McMillan MT, Malleo G, Bassi C, Butturini G, Salvia R, Roses RE, et al. Drain management after pancreatoduodenectomy: reappraisal of a prospective randomized trial using risk stratification. Journal of the American College of Surgeons 2015;221(4):798-809. [DOI] [PubMed] [Google Scholar]
- NCT01441492. Pancreas resection with and without drains [A randomized prospective multicenter trial of pancreaticoduodenectomy with and without routine intraperitoneal drainage]. clinicaltrials.gov/ct2/show/NCT01441492 (first received 27 September 2011).
- Van Buren G 2nd, Bloomston M, Hughes SJ, Winter J, Behrman SW, Zyromski NJ, et al. A randomized prospective multicenter trial of pancreaticoduodenectomy with and without routine intraperitoneal drainage. Annals of Surgery 2014;259(4):605-12. [DOI] [PubMed] [Google Scholar]
Van Buren 2017 {published data only}
- NCT01441492. Pancreas resection with and without drains [A randomized prospective multicenter trial of pancreas resection with and without routine intraperitoneal drainage]. clinicaltrials.gov/ct2/show/NCT01441492 (first received 27 September 2011).
- Van Buren G 2nd, Bloomston M, Schmidt CR, Behrman SW, Zyromski NJ, Ball CG, et al. A prospective randomized multicenter trial of distal pancreatectomy with and without routine intraperitoneal drainage. Annals of Surgery 2017;266(3):421-31. [DOI] [PubMed] [Google Scholar]
Witzigmann 2016 {published data only}
- ISRCTN04937707. Intra-abdominal drainage for two days versus no drainage following pancreas resection. isrctn.com/ISRCTN04937707 (first received 27 June 2007).
- Witzigmann H, Diener MK, Kienkötter S, Rossion I, Bruckner T, Werner B, et al. No need for routine drainage after pancreatic head resection: the dual-center, randomized, controlled PANDRA trial. Annals of Surgery 2016;264(3):528-37. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adham 2013 {published data only}
- Adham M, Chopin-Laly X, Lepilliez V, Gincul R, Valette PJ, Ponchon T. Pancreatic resection: drain or no drain? Surgery 2013;154(5):1069-77. [DOI] [PubMed] [Google Scholar]
Behrman 2015 {published data only}
- Behrman SW, Zarzaur BL, Parmar A, Riall TS, Hall BL, Pitt HA. Routine drainage of the operative bed following elective distal pancreatectomy does not reduce the occurrence of complications. Journal of Gastrointestinal Surgery 2015;19(1):72-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Correa‐Gallego 2013 {published data only}
- Correa-Gallego C, Brennan MF, Dʼangelica M, Fong Y, Dematteo RP, Kingham TP, et al. Operative drainage following pancreatic resection: analysis of 1122 patients resected over 5 years at a single institution. Annals of Surgery 2013;258(6):1051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fisher 2011 {published data only}
- Fisher WE, Hodges SE, Silberfein EJ, Artinyan A, Ahern CH, Jo E, et al. Pancreatic resection without routine intraperitoneal drainage. International Hepato-Pancreato-Biliary Association 2011;13(7):503-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giovinazzo 2011 {published data only}
- Giovinazzo F, Butturini G, Salvia R, Mascetta G, Monsellato D, Marchegiani G, et al. Drain management after pancreatic resection: state of the art. Journal of Hepato-biliary-pancreatic Sciences 2011;18(6):779-84. [DOI] [PubMed] [Google Scholar]
Heslin 1998 {published data only}
- Heslin MJ, Harrison LE, Brooks AD, Hochwald SN, Coit DG, Brennan MF. Is intra-abdominal drainage necessary after pancreaticoduodenectomy? Journal of Gastrointestinal Surgery 1998;2(4):373-8. [DOI] [PubMed] [Google Scholar]
Jeekel 1992 {published data only}
- Jeekel J. No abdominal drainage after Whipple's procedure. British Journal of Surgery 1992;79(2):182. [DOI] [PubMed] [Google Scholar]
Kawai 2006 {published data only}
- Kawai M, Tani M, Terasawa H, Ina S, Hirono S, Nishioka R, et al. Early removal of prophylactic drains reduces the risk of intra-abdominal infections in patients with pancreatic head resection: prospective study for 104 consecutive patients. Annals of Surgery 2006;244(1):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kawaida 2021 {published data only}
- Kawaida H, Kono H, Amemiya H, Hosomura N, Higuchi Y, Nakayama T, et al. Early drain removal regardless of drain fluid amylase level might reduce risk of postoperative pancreatic fistula. Anticancer Research 2021;41(1):403-8. [DOI] [PubMed] [Google Scholar]
Kunstman 2017 {published data only}
- Kunstman JW, Starker LF, Healy JM, Salem RR. Pancreaticoduodenectomy can be performed safely with rare employment of surgical drains. American Surgeon 2017;83(3):265-73. [PubMed] [Google Scholar]
Lee 2009 {published data only}
- Lee SE, Ahn YJ, Jang JY, Kim SW. Prospective randomized pilot trial comparing closed suction drainage and gravity drainage of the pancreatic duct in pancreaticojejunostomy. Journal of Hepato-biliary-pancreatic Surgery 2009;16(6):837-43. [DOI] [PubMed] [Google Scholar]
Lemke 2021 {published data only}
- Lemke M, Park L, Balaa FK, Martel G, Khalil JA, Bertens KA. Passive versus active intra-abdominal drainage following pancreaticoduodenectomy: a retrospective study using the American College of Surgeons NSQIP database. World Journal of Surgery 2021;45(2):554-61. [DOI] [PubMed] [Google Scholar]
Lim 2013 {published data only}
- Lim C, Dokmak S, Cauchy F, Aussilhou B, Belghiti J, Sauvanet A. Selective policy of no drain after pancreaticoduodenectomy is a valid option in patients at low risk of pancreatic fistula: a case-control analysis. World Journal of Surgery 2013;37(5):1021-7. [DOI] [PubMed] [Google Scholar]
Mehta 2013 {published data only}
- Mehta VV, Fisher SB, Maithel SK, Sarmiento JM, Staley CA, Kooby DA. Is it time to abandon routine operative drain use? A single institution assessment of 709 consecutive pancreaticoduodenectomies. Journal of the American College of Surgeons 2013;216(4):635-42. [DOI] [PubMed] [Google Scholar]
Paulus 2012 {published data only}
- Paulus EM, Zarzaur BL, Behrman SW. Routine peritoneal drainage of the surgical bed after elective distal pancreatectomy: is it necessary? American Journal of Surgery 2012;204(4):422-7. [DOI] [PubMed] [Google Scholar]
Zaghal 2020 {published data only}
- Zaghal A, Tamim H, Habib S, Jaafar R, Mukherji D, Khalife M. Drain or no drain following pancreaticoduodenectomy: the unsolved dilemma. Scandinavian Journal of Surgery 2020;109(3):228-37. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Kaiser 2019 {published data only}
- Kaiser J, Niesen W, Probst P, Bruckner T, Doerr-Harim C, Strobel O, et al. Abdominal drainage versus no drainage after distal pancreatectomy: study protocol for a randomized controlled trial. Trials 2019;20(1):332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Allen 2011
- Allen PJ. Operative drains after pancreatic resection - the Titanic is sinking. HPB: the Official Journal of the International Hepato Pancreato Biliary Association 2011;13(9):595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andrén‐Sandberg 2011
- Andrén-Sandberg A. Complications of pancreatic surgery. North American Journal of Medical Sciences 2011;3(12):531-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bassi 2017
- Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 2017;161(3):584-91. [DOI] [PubMed] [Google Scholar]
Bornman 2001
- Bornman PC, Beckingham IJ. ABC of diseases of liver, pancreas, and biliary system: chronic pancreatitis. BMJ 2001;322(7287):660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Braganza 2011
- Braganza JM, Lee SH, McCloy RF, McMahon MJ. Chronic pancreatitis. Lancet 2011;377(9772):1184-97. [DOI] [PubMed] [Google Scholar]
Bray 2018
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 2018;68(6):394-424. [DOI] [PubMed] [Google Scholar]
Cameron‐Strange 1985
- Cameron-Strange A, Horner J. Haemorrhage following insertion of continuous suction drains at appendectomy. Journal of the Royal College of Surgeons of Edinburgh 1985;30(4):271-2. [PubMed] [Google Scholar]
Charoenkwan 2017
- Charoenkwan K, Kietpeerakool C. Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy for the prevention of lymphocyst formation in women with gynaecological malignancies. Cochrane Database of Systematic Reviews 2017, Issue 6. Art. No: CD007387. [DOI: 10.1002/14651858.CD007387.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheng 2017
- Cheng Y, Briarava M, Lai M, Wang X, Tu B, Cheng N, et al. Pancreaticojejunostomy versus pancreaticogastrostomy reconstruction for the prevention of postoperative pancreatic fistula following pancreaticoduodenectomy. Cochrane Database of Systematic Reviews 2017, Issue 9. Art. No: CD012257. [DOI: 10.1002/14651858.CD012257.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheng 2019
- Cheng Y, He S, Xia J, Ding X, Liu Z, Gong J. Duct-to-mucosa pancreaticojejunostomy for the prevention of postoperative pancreatic fistula following pancreaticoduodenectomy. Cochrane Database of Systematic Reviews 2019, Issue 10. Art. No: CD013462. [DOI: 10.1002/14651858.CD013462] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clavien 2009
- Clavien PA, Barkun J, Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Annals of Surgery 2009;250(2):187-96. [DOI] [PubMed] [Google Scholar]
Deng 2020
- Deng Y, He S, Cheng Y, Cheng N, Gong J, Gong J, et al. Fibrin sealants for the prevention of postoperative pancreatic fistula following pancreatic surgery. Cochrane Database of Systematic Reviews 2020, Issue 3. Art. No: CD009621. [DOI: 10.1002/14651858.CD009621.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Diener 2011
- Diener MK, Tadjalli-Mehr K, Wente MN, Kieser M, Büchler MW, Seiler CM. Risk-benefit assessment of closed intra-abdominal drains after pancreatic surgery: a systematic review and meta-analysis assessing the current state of evidence. Langenbeck's Archives of Surgery 2011;396(1):41-52. [DOI] [PubMed] [Google Scholar]
Dindo 2004
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of Surgery 2004;240(2):205-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dragovich 2020
- Dragovich T, Erickson RA, Larson CR, Shabahang M. Pancreatic cancer, 2020. emedicine.medscape.com/article/280605-overview (accessed 30 January 2021).
Durai 2009
- Durai R, Mownah A, Ng PC. Use of drains in surgery: a review. Journal of Perioperative Practice 2009;19(6):180-6. [DOI] [PubMed] [Google Scholar]
Ferlay 2019
- Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. International Journal of Cancer 2019;144(8):1941-53. [DOI] [PubMed] [Google Scholar]
Fisher 2018
- Fisher WE. Intraperitoneal drainage and pancreatic resection. Advances in Surgery 2018;52(1):205-22. [DOI] [PubMed] [Google Scholar]
Gachabayov 2019
- Gachabayov M, Gogna S, Latifi R, Dong XD. Passive drainage to gravity and closed-suction drainage following pancreatoduodenectomy lead to similar grade B and C postoperative pancreatic fistula rates. A meta-analysis. International Journal of Surgery 2019;67(2019):24-31. [DOI] [PubMed] [Google Scholar]
Garg 2004
- Garg PK, Tandon RK. Survey on chronic pancreatitis in the Asia-Pacific region. Journal of Gastroenterology and Hepatology 2004;19(9):998-1004. [DOI] [PubMed] [Google Scholar]
Gates 2013
- Gates S, Anderson ER. Wound drainage for caesarean section. Cochrane Database of Systematic Reviews 2013, Issue 12. Art. No: CD004549. [DOI: 10.1002/14651858.CD004549.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT: GRADEpro Guideline Development Tool. McMaster University and Evidence Prime, 2021. Available from www.gradepro.org.
Gupte 2018
- Gupte A, Goede D, Tuite R, Forsmark CE. Chronic pancreatitis. BMJ 2018;361:k2126. [DOI] [PubMed] [Google Scholar]
Gurusamy 2007a
- Gurusamy KS, Samraj K, Davidson BR. Routine abdominal drainage for uncomplicated liver resection. Cochrane Database of Systematic Reviews 2007, Issue 3. Art. No: CD006232. [DOI: 10.1002/14651858.CD006232.pub2] [DOI] [PubMed] [Google Scholar]
Gurusamy 2007b
- Gurusamy KS, Samraj K. Routine abdominal drainage for uncomplicated open cholecystectomy. Cochrane Database of Systematic Reviews 2007, Issue 2. Art. No: CD006003. [DOI: 10.1002/14651858.CD006003.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurusamy 2013a
- Gurusamy KS, Koti R, Fusai G, Davidson BR. Somatostatin analogues for pancreatic surgery. Cochrane Database of Systematic Reviews 2013, Issue 4. Art. No: CD008370. [DOI: 10.1002/14651858.CD008370.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurusamy 2013b
- Gurusamy KS, Koti R, Davidson BR. Routine abdominal drainage versus no abdominal drainage for uncomplicated laparoscopic cholecystectomy. Cochrane Database of Systematic Reviews 2013, Issue 9. Art. No: CD006004. [DOI: 10.1002/14651858.CD006004.pub4] [DOI] [PubMed] [Google Scholar]
Hackert 2011
Halloran 2002
- Halloran CM, Ghaneh P, Bosonnet L, Hartley MN, Sutton R, Neoptolemos JP. Complications of pancreatic cancer resection. Digestive Surgery 2002;19(2):138-46. [DOI] [PubMed] [Google Scholar]
Henkus 1999
- Henkus HE. Complications of high-vacuum suction drainage. European Journal of Surgery 1999;165(8):813-4. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook/PDF/v5.2/chapter-08.
Higgins 2021
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Hüttner 2017
- Hüttner FJ, Probst P, Knebel P, Strobel O, Hackert T, Ulrich A, et al. Meta-analysis of prophylactic abdominal drainage in pancreatic surgery. British Journal of Surgery 2017;104(6):660-8. [DOI] [PubMed] [Google Scholar]
Inoue 2011
- Inoue M, Uchida K, Otake K, Koike Y, Okugawa Y, Kobayashi M, et al. Placement of prophylactic drains after laparotomy may increase infectious complications in neonates. Pediatric Surgery International 2011;27(9):975-9. [DOI] [PubMed] [Google Scholar]
ISRCTN04937707
- ISRCTN04937707. Intra-abdominal drainage for two days versus no drainage following pancreas resection. isrctn.com/ISRCTN04937707 (first received 27 June 2007).
Kamisawa 2016
- Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet 2016;388(10039):73-85. [DOI] [PubMed] [Google Scholar]
Kleeff 2017
- Kleeff J, Whitcomb DC, Shimosegawa T, Esposito I, Lerch MM, Gress T. Chronic pancreatitis. Nature Reviews Disease Primers 2017;3:17060. [DOI] [PubMed] [Google Scholar]
Lew 2017
- Lew D, Afghani E, Pandol S. Chronic pancreatitis: current status and challenges for prevention and treatment. Digestive Diseases and Sciences 2017;62(7):1702-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2018
- Li Z, Zhao L, Cheng Y, Cheng N, Deng Y. Abdominal drainage to prevent intra-peritoneal abscess after open appendectomy for complicated appendicitis. Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No: CD010168. [DOI: 10.1002/14651858.CD010168.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lyu 2020
- Lyu Y, Cheng Y, Wang B, Zhao S, Chen L. Peritoneal drainage or no drainage after pancreaticoduodenectomy and/or distal pancreatectomy: a meta-analysis and systematic review. Surgical Endoscopy 2020;34(11):4991-5005. [DOI] [PubMed] [Google Scholar]
Machicado 2017
- Machicado JD, Yadav D. Epidemiology of recurrent acute and chronic pancreatitis: similarities and differences. Digestive Diseases and Sciences 2017;62(7):1683-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maisonneuve 2019
- Maisonneuve P. Epidemiology and burden of pancreatic cancer. Presse Medicale 2019;48(3 Pt 2):e113-23. [DOI] [PubMed] [Google Scholar]
Makama 2010
- Makama JG, Ahmed A, Ukwenya Y, Mohammed I. Drain site hernia in an adult: a case report. West African Journal of Medicine 2010;29(6):429-31. [DOI] [PubMed] [Google Scholar]
Memon 2001
- Memon MA, Memon MI, Donohue JH. Abdominal drains: a brief historical review. Irish Medical Journal 2001;94(6):164-6. [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS 2009;3(3):12-130. [PMC free article] [PubMed] [Google Scholar]
NCT00931554
- NCT00931554. Randomized trial of early versus standard drainage removal after pancreatic resections [Early versus standard drainage removal after pancreatic resections: results of a prospective randomized clinical trial]. clinicaltrials.gov/ct2/show/NCT00931554 (first received 2 July 2009).
NCT01368094
- NCT01368094. Early (4 days) versus standard drainage of the abdominal cavity after pancreaticoduodenectomy [Early removal of intraperitoneal drainage after pancreatoduodenectomy in patients without postoperative fistula at POD3: results of a randomized clinical trial]. clinicaltrials.gov/ct2/show/NCT01368094 (first received 7 June 2011).
NCT01441492
- NCT01441492. Pancreas resection with and without drains [A randomized prospective multicenter trial of pancreas resection with and without routine intraperitoneal drainage]. clinicaltrials.gov/ct2/show/NCT01441492 (first received 27 September 2011).
NCT01988519
- NCT01988519. Randomized controlled trial comparing closed-suction drain versus passive gravity drain following pancreatic resection (DRAPA) [Results of a randomized controlled trial comparing closed-suction drains versus passive gravity drains after pancreatic resection.]. clinicaltrials.gov/ct2/show/NCT01988519 (first received 20 November 2013).
NCT02230436
- NCT02230436. Early versus late drain removal after pancreatectomy: a randomized prospective trial [Early drain removal after major pancreatectomy reduces postoperative complications: a single-center, randomized, controlled trial]. clinicaltrials.gov/ct2/show/NCT02230436 (first received 3 September 2014).
Nomura 1998
- Nomura T, Shirai Y, Okamoto H, Hatakeyama K. Bowel perforation caused by silicone drains: a report of two cases. Surgery Today 1998;28(9):940-2. [DOI] [PubMed] [Google Scholar]
Parker 2007
- Parker MJ, Livingstone V, Clifton R, McKee A. Closed suction surgical wound drainage after orthopaedic surgery. Cochrane Database of Systematic Reviews 2007, Issue 3. Art. No: CD001825. [DOI: 10.1002/14651858.CD001825.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reed 1992
- Reed MW, Wyman A, Thomas WE, Zeiderman MR. Perforation of the bowel by suction drains. British Journal of Surgery 1992;79(7):679. [DOI] [PubMed] [Google Scholar]
Reeves 2021
- Reeves BC, Deeks JJ, Higgins JPT, Shea B, Tugwell P, Wells GA. Chapter 24: Including non-randomized studies on intervention effects. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. The Cochrane Collaboration, 2020.
Rolph 2004
- Rolph R, Duffy JMN, Alagaratnam S, Ng P, Novell R. Intra-abdominal drains for the prophylaxis of anastomotic leak in elective colorectal surgery. Cochrane Database of Systematic Reviews 2004, Issue 4. Art. No: CD002100. [DOI: 10.1002/14651858.CD002100.pub2] [DOI] [Google Scholar]
Sahu 2008
- Sahu SK, Bahl DV, Husain M, Sachan PK. Drain erosion into bowel: an unusual complication. The Internet Journal of Surgery 2007;16(2).
Samraj 2007
- Samraj K, Gurusamy KS. Wound drains following thyroid surgery. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No: CD006099. [DOI: 10.1002/14651858.CD006099.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Siegel 2019
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: A Cancer Journal for Clinicians 2019;69(1):7-34. [DOI] [PubMed] [Google Scholar]
Singh 2019
- Singh VK, Yadav D, Garg PK. Diagnosis and management of chronic pancreatitis: a review. JAMA 2019;322(24):2422-34. [DOI] [PubMed] [Google Scholar]
Srivastava 2007
- Srivastava P, Srivastava S, Sahu M. Iatrogenic bowel perforation secondary to surgical drain after cholecystectomy: a case report with review of literature. Internet Journal of Surgery 2007;13(1).
Van der Wilt 2013
- Van der Wilt AA, Coolsen MM, De Hingh IH, Van der Wilt GJ, Groenewoud H, Dejong CH, et al. To drain or not to drain: a cumulative meta-analysis of the use of routine abdominal drains after pancreatic resection. HPB: the Official Journal of the International Hepato Pancreato Biliary Association 2013;15(5):337-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Hee 1983
- Van Hee R. Complications of drainage. Acta Chirurgica Belgica 1983;83(5):340-4. [PubMed] [Google Scholar]
Wang 2015
- Wang Z, Chen J, Su K, Dong Z. Abdominal drainage versus no drainage post-gastrectomy for gastric cancer. Cochrane Database of Systematic Reviews 2015, Issue 5. Art. No: CD008788. [DOI: 10.1002/14651858.CD008788.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wente 2007a
- Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007;142(1):20-5. [DOI] [PubMed] [Google Scholar]
Wente 2007b
- Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007;142(5):761-8. [DOI] [PubMed] [Google Scholar]
Yadav 2011
- Yadav D, Timmons L, Benson JT, Dierkhising RA, Chari ST. Incidence, prevalence, and survival of chronic pancreatitis: a population-based study. American Journal of Gastroenterology 2011;106(12):2192-9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Cheng 2013
- Cheng Y, Yang C, Lin Y, Lu J, Wu S, Zhou R, et al. Prophylactic abdominal drainage for pancreatic surgery. Cochrane Database of Systematic Reviews 2013, Issue 6. Art. No: CD010583. [DOI: 10.1002/14651858.CD010583] [DOI] [Google Scholar]
Cheng 2016
- Cheng Y, Xia J, Lai M, Cheng N, He S. Prophylactic abdominal drainage for pancreatic surgery. Cochrane Database of Systematic Reviews 2016, Issue 10. Art. No: CD010583. [DOI: 10.1002/14651858.CD010583.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peng 2015
- Peng S, Cheng Y, Yang C, Lu J, Wu S, Zhou R, et al. Prophylactic abdominal drainage for pancreatic surgery. Cochrane Database of Systematic Reviews 2015, Issue 8. Art. No: CD010583. [DOI: 10.1002/14651858.CD010583.pub2] [DOI] [PubMed] [Google Scholar]
Zhang 2018
- Zhang W, He S, Cheng Y, Xia J, Lai M, Cheng N, et al. Prophylactic abdominal drainage for pancreatic surgery. Cochrane Database of Systematic Reviews 2018, Issue 6. Art. No: CD010583. [DOI: 10.1002/14651858.CD010583.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]


