Abstract
Introduction
Tracking progress in reaching global targets for reducing premature mortality from non-communicable diseases (NCDs) requires accurately collected population based longitudinal data. However, most African countries lack such data because of weak or non-existent civil registration systems. We used data from the Nairobi Urban Health and Demographic Surveillance System (NUDSS) to estimate NCD mortality trends over time and to explore the determinants of NCD mortality.
Methods
Deaths identified in the NUHDSS were followed up with a verbal autopsy to determine the signs and symptoms preceding the death. Causes of death were then assigned using InSilicoVA algorithm. We calculated the rates of NCD mortality in the whole NUHDSS population between 2008 and 2017, looking at how these changed over time. We then merged NCD survey data collected in 2008, which contains information on potential determinants of NCD mortality in a sub-sample of the NUHDSS population, with follow up information from the full NUHDSS including whether any of the participants died of an NCD or non-NCD cause. Poisson regression models were used to identify independent risk factors (broadly categorized as socio-demographic, behavioural and physiological) for NCD mortality, as well as non-NCD mortality.
Results
In the total NUHDSS population of adults age 18 and over, 23% were assigned an NCD as the most likely cause of death. There was evidence that NCD mortality decreased over the study period, with rates of NCD mortality dropping from 1.32 per 1000 person years in 2008–10 (95% CI: 1.13–1.54) to 0.93 per 1000 person years in 2014–17 (95% CI: 0.80–1.08). Of 5115 individuals who participated in the NCD survey in 2008, 421 died during the follow-up period of which 43% were attributed to NCDs. Increasing age, lower education levels, ever smoking and having high blood pressure were identified as independent determinants of NCD mortality in multivariate analyses.
Conclusion
We found that NCDs account for one-quarter of mortality in Nairobi slums, although we document a reduction in the rate of NCD mortality over time. This may be attributed to increased surveillance and introduction of population-wide NCD interventions and health system improvements from research activities in the slums. To achieve further decline there is a need to strengthen health systems to respond to NCD care and prevention along with addressing social factors such as education.
Keywords: NCD mortality trends, Risk factors, Informal settlements, Kenya
Introduction
Modelled estimates suggest that low- and middle-income countries (LMICs) face a particularly high burden of mortality from non-communicable diseases (NCDs). In 2014, for example, 80% of the global NCD deaths were predicted to come from these settings [1]. Within LMICs, there are pockets of the population that are particularly vulnerable to NCD mortality. The urban poor living in informal settlements in many LMIC cities face a high risk of developing some NCDs, due to increased exposure to some risk factors including tobacco use, poor diets, excessive alcohol consumption, and environmental pollution [[2], [3], [4]]. The case-fatality from NCDs is also likely to be higher in these populations due to poor access to quality healthcare services for screening, preventive and curative services for NCDs [[5], [6], [7]]. The urban population is increasing globally with 66% of the world's population projected to be urban by 2050 and countries of sub-Saharan Africa could account for more than half of the growth between 2019 and 2050 [8]. The urban slums in Africa are growing disproportionately. For example in Nairobi 60% of the population live in slums and 75% of the urban population growth is absorbed into slums [9].The importance of tackling the growing burden of non-communicable diseases (NCDs) has been recognized through the wide endorsement of the United Nations' global target to reduce premature mortality from NCDs by 25% by 2025 [10]. However, tracking progress towards this goal has been hampered by a lack of empirical data, particularly in vulnerable populations. Most African countries lack data to track mortality trends because of weak or non-existent civil registration systems, and consequently country-level NCD mortality estimates rely on mathematical models which depend heavily on assumptions and data from health facility records [[11], [12], [13]]. Health facility records available through program monitoring have limited value because they systematically omit population groups that are not in contact with the health system, who could be at a greater risk of NCD mortality [14].
Although limited in coverage, Health and Demographic Surveillance Systems (HDSS) can provide essential longitudinal data on levels of, and risk factors for, NCD mortality. The INDEPTH network undertook a comparative analysis of data from 21 HDSS sites (12 countries in Africa) and Asia and found that, between 2006 and 2012, urban sites recorded a higher level of all-cause mortality compared with more rural sites [15]. For HDSS sites where trends could be shown, there was evidence of an increase in NCD mortality over time; however in the HDSS in Nairobi slums, no change in NCD mortality was observed between 2003 and 2011 [16].
This paper provides a timely update of NCD mortality rates in the Nairobi HDSS, given the change in the landscape of NCD services, including the scale-up of services such as screening for NCDs, the development and rollout of treatment guidelines for cardiovascular diseases and increasing the breadth of health insurance cover for NCDs [17]. Additionally, in the Nairobi HDSS site, a community based intervention for prevention of cardiovascular disease was implemented from 2012 to 2014 [18]. The objective of this study is to update the estimates of NCD mortality in the Nairobi HDSS for the time period 2008 to 2017 using verbal autopsy (VA) data, and to explore the socio-demographic, behavioural and biological determinants of NCD mortality in this setting.
Material and methods
Context and population
The data were extracted from the Nairobi Urban Health and Demographic Surveillance System (NUHDSS) established in two informal settlements (Viwandani and Korogocho), approximately 10 km from the central business district of Nairobi city and with a total population of approximately 90,000 people in 2019 [19]. The African Population and Health Research Center (APHRC) has maintained regular individual and household demographic and socio-economic surveillance in this area through a quarterly census since 2002. The NUHDSS was set up to provide a platform for conducting socio-epidemiological studies among Nairobi's urban poor and for evaluating intervention programs.
Three studies capturing data on NCDs and their risk factors have been conducted within the NUHDSS. Firstly, between 2008 and 2009, a population-based NCD survey for NCD risk factors was conducted among adults (aged 18 years and above), randomly selected from the NUHDSS [20]. Between 2012 and 2014, a prospective quasi-experimental study in which an entire community (Korogocho) with approximately 35,000 adults was exposed to an intervention package for primary prevention of cardiovascular disease that comprised awareness campaigns, household screening for cardiovascular diseases risk factors, and referral and treatment of people with high cardiovascular diseases risk at a primary health clinic was conducted. The other community (Viwandani) was treated as a control group. During this study, primary healthcare facilities received training and equipment to enhance their capacity to deliver NCD services. A substantial reduction of population mean blood pressure was observed in both intervention and control arms [18]. Finally, in 2014–2015, 2000 adults aged 40–60 years were recruited for five years follow-up to understand the cardio metabolic disease risk, and explore gene–gene and gene–environment interactions that contribute to disease risk [21].
Data sources
This study draws on two main sources of data to provide information on the levels and determinants of NCD mortality in the NUHDSS population (1) NUHDSS data from all adults age 18 and over from 2008 through to the end of 2017 which is used to ascertain levels of NCD mortality in this population and; (2) data from the population-based NCD survey conducted in 2008/09 which provides information on potential risk factors for NCD mortality.
Through the regular household surveillance, deaths occurring in the NUHDSS have been identified and followed up with a VA interview conducted by trained interviewers who visit a household within three months of a death, to interview a credible close attendant (i.e. spouse or other close family member) on the circumstances surrounding the death. The VA is conducted using a semi-structured questionnaire that enables probing for symptoms and signs that the deceased had suffered before death. The details of the VA procedure are described in detail elsewhere [22]. Causes of death were assigned using the InSilicoVA algorithm [23] that assigns the cause of death from data obtained by VA interviews using probabilistic modelling to provide the probability of each potential cause of death for each death with a VA [24]. We classified deaths as attributable to an NCD if the cause of death with the highest probability fell into one of the following categories: cancers, cardiovascular diseases, and other NCD (including severe anaemia, severe malnutrition, diabetes, chronic obstructive pulmonary disease, asthma, acute abdomen, liver cirrhosis, renal failure, epilepsy and other/unspecified NCDs). If the cause of death with the highest probability was not an NCD death, then it was classified as other non-NCD mortality. This included deaths that were assigned to causes such as HIV/AIDs, TB, other infectious causes, external causes of death (injuries) and direct obstetric deaths. If no cause of death had a probability greater than 0.4 for an individual, then the cause of death was assigned as indeterminate.
We used NCD survey data collected to provide exposure information on the potential determinants of NCD mortality. The 2008 NCD survey was conducted between May 2008 and April 2009, a population-based cross sectional study among adults (aged 18 years and above) randomly selected from the NUHDSS [20]. Briefly, a stratified sampling strategy adapted from the WHO STEPwise protocol was used to randomly select individuals according to slum of residence (Korogocho and Viwandani), gender and age group ((18–29, 30–39, 40–49, 50–59, 60 years and over), to constitute 20 strata each with 250 respondents [25]. Eligible participants were approached in their homes and data on socio-demographic factors such as age, sex, ethnicity, education, and socioeconomic status (SES), and behavioural risk factors including unhealthy diet, physical inactivity, smoking, and harmful alcohol consumption were collected using a structured questionnaire developed based on framework by Wong et al. [26]. During the home visit, anthropometric measurements were taken (weight, height, waist and hip circumference), blood pressure was measured and a drop of blood from a finger prick was taken to test for glucose and total cholesterol levels using the combined ACCU-CHEK™ Glucose, Cholesterol and Triglycerides (GCT) digital meter. All these procedures were performed according to standardized protocols described before [4,27].
Data preparation
Two datasets were created, one of which included residency, mortality and cause of death information on all adults in the NUHDSS aged 18 and over between 2008 and 2017. Individuals who died but did not have a VA were dropped from this study. This dataset was then merged with the population-based NCD survey data (conducted in only a sub-sample of the full NUHDSS) and only residents which had participated in the NCD survey were retained in this second dataset for analysis.
Potential determinants of NCD mortality were divided into three groups using a hierarchical framework [28]: socio-demographic characteristics, behavioural risk factors, and physiological risk factors.
The sociodemographic risk factors of interest are as follows: sex; age (18–49, 50–59, 60–69, 70–79. 80+); education (below primary, primary and above) and; socio-economic status. SES was categorized into tertiles and was computed based on ownership or possession of various household items including a television, radio, refrigerator, cooker, sofa set, microwave, home computer, mobile phone, landline, land/plot, livestock, and a vehicle using the principal component analysis method commonly used by the Demographic and Health Surveys (DHS) Program [29].
Tobacco, alcohol use and physical activity, as reported in the 2008 survey, were considered potential behavioural risk factors. Use of smoking tobacco products, use of smokeless tobacco such as chewing tobacco or snuff tobacco and alcohol consumption were categorized as never used or ever used. Total moderate-vigorous physical activity (MVPA) in minutes per week were calculated from the accumulation of occupation, travel-related and leisure time physical activity and categorized into low, moderate and high according to WHO classification [30].
Physiological variables from the 2008 survey included in this analysis were: abdominal obesity, high blood pressure, diabetes and high cholesterol. Both waist circumference and Waist-Hip Ratio (WHR) were used as measures of abdominal obesity (obesity defined as waist circumference >80 cm in women and >94 cm in men; and WHR >0.80 in women and >0.95 in men) [31]. High blood pressure was defined as systolic blood pressure of at least 140 mmHg, or diastolic blood pressure at least 90 mmHg, or self-reported previous diagnosis and current use of antihypertensive medication. Participants were categorized as diabetic if their random blood glucose was found to be ≥11.1 mmol/L or if they had reported a prior diagnosis of diabetes by a health-care professional [32]. High cholesterol was defined as total cholesterol ≥5.2 mmol/L [33].
Statistical analyses
All data analysis was performed using Stata version 15 [34]. Total person years, numbers of deaths attributed to NCDs and NCD mortality rates were estimated overall and over three different time periods (2008–10; 2011–13; 2014–17) using data from all adults resident in the NUHDSS area. Residents stopped contributing time if they out-migrated and resumed contributing if they re-entered the NUHDSS area. We used the age distribution of the WHO Standard Population [35] to calculate age-standardized NCD mortality rates.
Deaths and person-years under observation in the NUHDSS were aggregated for the sub-set of individuals who participated in the 2008/2009 NCD survey for the period from 2008 to 2017. We calculated the age-adjusted NCD mortality rate per 1000 person-years both overall and stratified by individuals' socio-demographic characteristics and behavioural and physiological risk factors. Parametric regression survival-time models, with an exponential distribution function, were used to assess the strength of association between the potential risk factors and NCD mortality. The selection of the factors was guided by a conceptual framework as proposed by Victora, with potential risk factors organised in levels from distal to most proximate [28]. The initial model included only the sociodemographic risk factors. Socio-demographic factors associated with NCD mortality in univariate analyses were included in a multivariable model; those remaining independently associated were retained in a core model. At the next level of the hierarchical framework, behavioural risk factors were added one by one to the core socio-demographic model. Those behavioural risk factors which remained associated with NCD mortality in the adjusted model were included in the multivariable model. Associations with the physiological risk factors were determined in a similar way. This risk factor analysis was repeated for looking at non-NCD mortality, which includes all deaths that were not attributed to NCDs.
Ethical considerations
The NUHDSS protocol and consent procedures, including surveillance and VA, were approved by the Kenya Medical Research Institute/National Ethical Review Committee (NON-SSC Protocol No.339). Household heads provided written informed consent for all household-level data collection, while for individual interviews, adults consented for their own interviews and other measurements. Named data are securely stored in a MS-SQL database and only authorized data personnel have access rights. Datasets analysed by scientists are stripped of names to protect identity. Participants identified with medical conditions during surveys were offered counselling and referred for care to health facilities supported by the research program in the NUHDSS. Ethical approval for the analysis of this data was also granted by the London School of Hygiene and Tropical Medicine ethics committee (reference number 22520).
Results
Characteristics of study participants
Between 2008 and the end of 2017, there were 123,789 adults aged 18 and over in the NUHDSS contributing a total of 429,741 person years. There were 2544 deaths recorded in the NUHDSS, of which 88.7% (N = 2257) had a VA. Supplementary Table 1 shows the number of deaths in the NUHDD that received a VA by sex, age and calendar period. There was little evidence that coverage varied by age or sex; however, 80% of deaths identified in the NUHDSS received a VA in 2008–10, increasing to 92% of deaths in 2014–2017. While supplementary table 2 shows that the population characteristics of all deaths and those with VA are not different. Dropping those individuals that did not have a VA from the study population gave a final sample of 429,004 person years of follow-up in the full NUHDSS. In total, 5162 NUHDSS participants had data available from recruitment into the NCD survey in 2008. There were a total of 32,576 person years of follow up and 468 deaths recorded among the participants of the NCD survey by the end of 2017. Of these deaths to participants of the NCD survey, 90.0% (N = 421) had a VA (with a total of 5115 individuals providing 32,455 person years of observation).
The distribution of key socio-demographic characteristics of the NCD study sample compared to the adult population in the NUHDSS in 2008, 2012 and 2016 is shown in Table 1. The sex distribution and SES tertiles were similar in the study sample and the NUHDSS sample. There was a higher percentage of men than women and approximately one third of the participants were in each SES tertile. The population sampled in NCD survey in 2008 had a higher proportion of older people (above 50 years) and had lower percentage with secondary or more education compared to the entire NUHDSS.
Table 1.
Baseline characteristics of study sample compared with those in the NUHDSS (age 18+).
| NCD Study sample (N = 5115) in 2008 | Adults in NUHDSS (N = 47,554) in 2008 | Adults in NUHDSS (N = 51,961) in 2012 | Adults in NUHDSS (N = 54,441) in 2016 | |
|---|---|---|---|---|
| Sex | ||||
| Men | 2806 (54.9%) | 28,100 (59.1%) | 30,174 (58.1%) | 30,392 (55.8%) |
| Women | 2309 (45.1%) | 19,454 (40.9%) | 21,787 (41.9%) | 24,049 (44.2%) |
| Age group | ||||
| 18–49 | 3293 (64.4%) | 44,056 (92.6%) | 47,433 (91.3%) | 49,191 (90.4%) |
| 50–59 | 1145 (22.4%) | 2357 (5%) | 2972 (5.7%) | 3551 (6.5%) |
| 60–69 | 462 (9.0%) | 779 (1.6%) | 1084 (2.1%) | 1188 (2.2%) |
| 70–79 | 145 (2.8%) | 256 (0.5%) | 321 (0.6%) | 367 (0.7%) |
| 80+ | 70 (1.4%) | 106 (0.2%) | 151 (0.3%) | 144 (0.3%) |
| Educationab | ||||
| Below primary | 684 (13.4%) | 9190 (19.3%) | 9424 (18.1%) | 7911 (14.5%) |
| Primary | 3332 (65.1%) | 14,972 (31.5%) | 16,890 (32.5%) | 13,795 (25.3%) |
| Secondary + | 1097 (21.5%) | 16,068 (33.8%) | 21,345 (41.1%) | 21,929 (40.3%) |
| Don't know/missing | 2 (0.04%) | 7324 (15.4%) | 4302 (8.3%) | 10,806 (19.8%) |
| SES Tertilec | ||||
| Lowest | 1226 (24.0%) | 13,693 (28.8%) | 17,247 (33.2%) | 13,249 (24.3%) |
| Middle | 1572 (30.7%) | 14,298 (30.1%) | 16,175 (31.1%) | 15,479 (28.4%) |
| Highest | 2307 (45.1%) | 17,414 (36.6%) | 17,041 (32.8%) | 17,365 (31.9%) |
| Don't know/missing | 10 (0.2%) | 2149 (4.5%) | 1498 (2.9%) | 8348 (15.3%) |
Below primary = None & Primary Incomplete; Primary = Primary Complete; Secondary+ = Secondary & Higher.
Linked in from NUHDSS data so missing for ten individuals in 2008, but may have (or not have) data at later time points.
SES- Socioeconomic status are relative SES standards within a very poor community (e.g. highest tertile by no means reflects households that are materially well-off, but rather have a highest SES relative to households in the same community).
Trends in NCD mortality over time
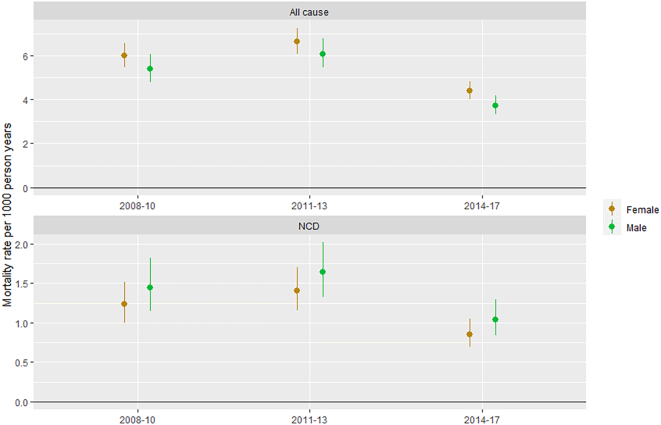
Overall, 23.0% of deaths (N = 520) in the NUHDSS between 2008 and 2017 were attributed by InSilicoVA to NCDs. As shown in Fig. 1, the percentage was lower in younger adults, and there was a relatively equal distribution of these deaths between cancer, cardiovascular disease and other NCDs. The overall crude rate of NCD mortality in the NUHDSS was 1.2 per 1000 person years [95% confidence interval (CI): 1.1–1.3], with the age-standardized rate much lower at 0.4 per 1000 person years (95% CI: 0.3–0.4). The NCD mortality rate per 1000 person years remained quite steady between 2008 and 10 (rate = 1.32, 95% CI: 1.13–1.54) and 2011–2013 (rate = 1.50, 95% CI: 1.30–1.73), but then dropped in 2014–17 (rate = 0.93, 95% CI: 0.80–1.08). As shown in Fig. 2. This pattern is consistent in both men and women.
Fig. 1.
Percentage of deaths in NUHDSS attributed by InSilicoVA to specific causes of death, by age group and sex (2008–2017).
Fig. 2.
All cause and NCD mortality rates in the NUHDSS cohort, by calendar year and sex.
Of the 421 deaths recorded among the participants of the NCD survey, 181 were attributed by InsilicoVA to NCDs (43.0%). The overall NCD mortality rate in this study population was 5.6 per 1000 person years (95% CI: 4.8–6.5), and the age standardized rate was 4.7 per 1000 person years (95% CI 4.0–5.4). The non NCD mortality rate was 7.4 per 1000 person years (95% CI: 6.5–8.4), dropping slightly to 7.1 per 1000 person years when standardizing to the WHO standard population (95% CI: 6.2–8.1).
Socio-demographic determinants of NCD mortality
Table 2 shows the age-standardized NCD mortality rates by key socio-demographic characteristics. There is strong evidence for an association between age and NCD mortality with an increasing rate of NCD mortality with increasing age in both crude and adjusted analyses. Those with primary education or above were found to have approximately 34% lower rates of NCD mortality after adjusting age compared to those with less than primary education [Rate ratio (RR) = 0.66, 95% CI = 0.47–0.92]. After adjusting for age and education, there was no evidence of an association between SES and NCD mortality. There was no evidence for an association between sex and NCD mortality after adjusting for the other socio-demographic determinants (RR = 0.93 for women compared with men, 95% CI = 0.68–1.27).
Table 2.
Socio-demographic, behavioural and physiological determinants of NCD mortality.
| Number of NCD deaths | Person years of follow-up | Age-standardized NCD mortality rate (95% CI) | Age-adjusted rate ratio | Adjusted rate ratioa | |
|---|---|---|---|---|---|
| Sociodemographic modela | |||||
| Sex | |||||
| Men | 94 | 17,290 | 4.9 (3.8–6.0) | 1 | 1 |
| Women | 87 | 15,164 | 4.6 (3.6–5.5) | 1.03 (0.77–1.38) | 0.93 (0.68–1.27) |
| Age group | |||||
| 18–49 | 19 | 17,342 | 1.1 (0.7–1.7) | 1 | 1 |
| 50–59 | 37 | 8001 | 4.6 (3.4–6.4) | 4.22 (2.43–7.34) | 4.02 (2.31–7.01) |
| 60–69 | 51 | 4826 | 10.6 (8.0–13.9) | 9.65 (5.70–16.33) | 8.62 (5.04–14.74) |
| 70–79 | 40 | 1614 | 24.8 (18.2–33.8) | 22.62 (13.10–39.06) | 18.90 (10.71–33.37) |
| 80+ | 34 | 673 | 50.6 (36.1–70.7) | 46.14 (26.32–80.89) | 34.76 (18.83–64.16) |
| Educationb | |||||
| Below primary | 79 | 4786 | 5.1 (4.0–6.3) | 1 | 1 |
| Primary + | 102 | 27,650 | 4.6 (3.5–5.7) | 0.66 (0.47–0.92) | 0.66 (0.47–0.92) |
| SES tertileb | |||||
| Lowest | 69 | 9372 | 4.7 (3.6–5.9) | 1 | 1 |
| Middle | 54 | 9884 | 5.1 (3.7–6.5) | 0.96 (0.67–1.38) | 0.99 (0.69–1.42) |
| Highest | 57 | 12,865 | 4.7 (3.4–6.0) | 0.86 (0.60–1.24) | 0.90 (0.63–1.29) |
| Behavioural modela | |||||
| Smokingc | |||||
| Never | 130 | 26,349 | 4.4 (3.6–5.2) | 1 | 1 |
| Ever | 51 | 6097 | 6.1 (4.2–8.0) | 1.28 (0.93–1.77) | 1.32 (0.95–1.83) |
| Physical activityc | |||||
| Low | 72 | 8087 | 5.0 (3.8–6.3) | 1 | 1 |
| Moderate | 56 | 8958 | 5.8 (4.2–7.4) | 1.01 (0.71–1.45) | 1.00 (0.70–1.43) |
| High | 52 | 15,367 | 3.3 (2.3–4.3) | 0.69 (0.48–1.00) | 0.73 (0.50–1.06) |
| Physiological modela | |||||
| High BPd | |||||
| No | 106 | 26,241 | 4.1 (3.3–5.0) | 1 | 1 |
| Yes | 75 | 6205 | 5.2 (3.9–6.6) | 1.45 (1.07–1.98) | 1.50 (1.10–2.04) |
| High waist-hip ratio | |||||
| No | 52 | 14,146 | 4.2 (3.0–5.4) | 1 | 1 |
| Yes | 129 | 18,309 | 5.0 (4.1–5.9) | 1.17 (0.84–1.62) | 1.15 (0.83–1.60) |
| High cholesterold | |||||
| No | 117 | 23,987 | 4.5 (3.7–5.4) | 1 | 1 |
| Yes | 50 | 5961 | 5.6 (3.8–7.4) | 1.11 (0.80–1.55) | 1.13 (0.81–1.58) |
Sociodemographic model adjusted for age and education; behavioural model adjusted for age, education and smoking; physiological model adjusted for age, education, smoking and high blood pressure.
Education missing for two persons representing no NCD deaths and 19 person years; SES missing completely for seven individuals and partially for others representing one NCD death and 333 person years.
Information on smoking missing from 35 subjects, giving a total of 215 person years at risk and one NCD death; information on physical activity missing for five persons, contributing 43 years of follow-up and one NCD death.
Information on cholesterol missing from 426 subjects, giving a total of 2507 person years at risk and 14 NCD deaths; information on blood pressure missing for one person, contributing nine years of follow-up and no NCD death.
Behavioural determinants of NCD mortality
After adjusting for only age, there was some evidence of decreased rates of NCD mortality among those who had high levels of physical activity compared to those with low levels (RR = 0.69, 95% CI = 0.93–1.77); however this association was attenuated after adjusting further for education and smoking (RR = 0.73, 95%CI = 0.50–1.06) (Table 2). There was weak evidence, after adjusting for age and education, that individuals who had ever smoked had just over 30% higher rates of NCD mortality compared with those who never smoked (RR = 1.32, 95% CI = 0.95–1.85). Alcohol consumption could not be included in the model because there were only 18 NCD deaths documented among the 853 individuals who reported any alcohol consumption (17% of NCD survey respondents), negating age-standardization.
Physiological determinants of NCD mortality
As shown in Table 2, in both crude and adjusted analyses there was no evidence of increased NCD mortality rates with high cholesterol or high waist-hip ratio. However, there was good evidence of increased mortality rates with high blood pressure after adjusting for age, education and smoking; those with high blood pressure in the NCD survey had 50% higher rates of NCD mortality compared to those who did not have high blood pressure (RR = 1.50, 95% CI = 1.10–2.04). There were 102 individuals who reported diabetes (2%), with 17 NCD deaths documented; due to the small numbers negating age-standardization, this was not included.
Determinants for non-NCD mortality
There are several notable differences in the risk factors for non-NCD mortality when compared with the determinants of NCD mortality (Table 3). With respect to the socio-demographic determinants, there is an association between increasing age and increasing rates of non-NCD mortality: however, unlike for NCD mortality, there is no evidence of an association between education and non-NCD mortality after adjusting for independent socio-demographic determinants, with a rate ratio of 1.03 when comparing those with primary education and those with no primary education (95% CI = 0.71–1.49). There is instead evidence for an association between SES and non-NCD mortality, with the wealthier having over 50% lower rates of non-NCD mortality compared with those in the lowest SES tertile (RR for highest versus lower tertile = 0.43, 95% CI = 0.31–0.58). We also find an association between sex and non-NCD mortality, with women having nearly 40% lower rates of NCD mortality compared with men (RR = 0.62, 95% CI = 0.48–0.81).
Table 3.
Socio-demographic, behavioural and physiological determinants of non-NCD mortality.
| Number of non NCD deaths | Person years of follow-up | Age-standardized non-NCD mortality rate (95% CI) | Age-adjusted rate ratio | Adjusted rate ratioa | |
|---|---|---|---|---|---|
| Sociodemographic modela | |||||
| Age group | |||||
| 18–49 | 99 | 17,342 | 5.7 (4.7–7.0) | 1 | 1 |
| 50–59 | 52 | 8001 | 6.5 (5.0–8.5) | 1.14 (0.81–1.59) | 1.09 (0.78–1.54) |
| 60–69 | 48 | 4826 | 9.9 (7.5–13.2) | 1.74 (1.23–2.46) | 1.59 (1.12–2.25) |
| 70–79 | 24 | 1614 | 14.9 (10.0–22.2) | 2.61 (1.67–4.07) | 2.41 (1.54–3.78) |
| 80+ | 17 | 673 | 25.3 (15.7–40.7) | 4.43 (2.65–7.41) | 3.78 (2.25–6.35) |
| Sex | |||||
| Men | 149 | 17,290 | 8.5 (7.1–10.0) | 1 | 1 |
| Women | 91 | 15,164 | 5.7 (4.5–6.9) | 0.68 (0.53–0.89) | 0.62 (0.48–0.81) |
| Educationb | |||||
| Below primary | 49 | 4786 | 6.6 (3.2–10.1) | 1 | 1 |
| Primary + | 191 | 27,650 | 7.0 (6.0–8.1) | 1.06 (0.74–1.52) | 1.03 (0.71–1.49) |
| SES tertileb | |||||
| Lowest | 116 | 9372 | 11.5 (9.2–13.8) | 1 | 1 |
| Middle | 56 | 9884 | 5.5 (4.0–7.0) | 0.49 (0.36–0.68) | 0.48 (0.35–0.66) |
| Highest | 65 | 12,865 | 5.3 (3.9–6.7) | 0.45 (0.33–0.61) | 0.43 (0.31–0.58) |
| Behavioural modela | |||||
| Smokingc | |||||
| Never | 166 | 26,349 | 6.2 (5.2–7.1) | 1 | 1 |
| Ever | 73 | 6097 | 11.7 (8.7–14.8) | 1.74 (1.32–2.30) | 1.56 (1.16–2.11) |
| Physical activityc | |||||
| Low | 80 | 8087 | 8.2 (6.1–10.2) | 1 | 1 |
| Moderate | 59 | 8958 | 6.6 (4.8–8.5) | 0.75 (0.53–1.06) | 0.68 (0.48–0.97) |
| High | 101 | 15,367 | 6.5 (5.2–7.9) | 0.80 (0.59–1.08) | 0.72 (0.52–0.98) |
| Physiological modela | |||||
| High BPd | |||||
| No | 174 | 26,241 | 6.7 (5.6–7.7) | 1 | 1 |
| Yes | 66 | 6205 | 9.0 (6.0–11.9) | 1.27 (0.94–1.72) | 1.31 (0.96–1.80) |
| High waist-hip ratio | |||||
| No | 94 | 14,146 | 6.7 (5.3–8.2) | 1 | 1 |
| Yes | 146 | 18,309 | 7.7 (6.3–9.1) | 1.04 (0.80–1.36) | 1.11 (0.83–1.47) |
| High cholesterold | |||||
| No | 182 | 23,987 | 7.5 (6.3–8.6) | 1 | 1 |
| Yes | 38 | 5961 | 5.9 (3.7–8.1) | 0.73 (0.51–1.04) | 0.67 (0.46–0.97) |
Sociodemographic model adjusted for age, sex and SES tertile, Behavioural model adjusted for: age, sex, SEStertile, smoking and physical activity, physiological model adjusted for: age, sex, SES tertile, smoking, physical activity, blood pressure and cholesterol.
SESmissing for 206 persons representing three non NCD deaths and 333 person years; education missing for two persons representing no deaths and 19 person years.
Information on smoking missing from 35 subjects, representing 215 person years at risk and one non-NCD death; information on physical activity missing for five persons, contributing 43 years of follow-up and no non-NCD deaths.
Information on cholesterol missing from 426 subjects, giving a total of 2507 person years at risk and 20 non-NCD deaths; information on blood pressure missing for one persons, contributing nine years of follow-up and no non-NCD death.
For behavioural factors, there is evidence for an association between both smoking and lower physical activity and increased rates of non-NCD mortality, after adjusting for the socio-demographic determinants. Ever smokers had over 50% higher rates of non-NCD mortality compared with never smokers (RR = 1.56, 95% CI = 1.16–2.11) and those with high physical activity had nearly 30% lower rates of non NCD-mortality compared with those with low physical activity (RR = 0.72, 95% CI = 0.52–0.98).
Physiological variables such as high blood pressure showed a weak evidence for an association with increased rates of non-NCD mortality after adjusting for the other independent determinants of non-NCD mortality (RR = 1.31, 95% CI = 0.96–1.80). There was good evidence that those with high cholesterol had lower odds of non-NCD mortality compared to those without high cholesterol (RR = 0.67, 95% CI = 0.46–0.97). There was no evidence of an association between having a high waist-hip ratio and non-NCD mortality.
Discussion
Our study indicates that approximately one quarter deaths of adults aged 18 years and over in this urban slum population in Kenya were due to NCDs between 2008 and 2017. This is slightly less than that reported in the recent publications in Kenya and other African Health and Demographic Surveillance Systems that have consistently shown that one-third of deaths are attributable to NCDs [15]. The difference might be, at least in part, due to our study covering a more recent time period in which there was evidence for a decrease in mortality due to NCDs. It is encouraging to note for the first time, a reduction in NCD mortality was observed in the Nairobi slums after 2013. An earlier observation by Oti et al. in the same population had revealed no substantial change in NCD mortality with NCD deaths per 1000 years fluctuating from 1.7 in 2003 to 5.6 in 2005, dropping to 2.7 in 2009, and finally rising to 3.7 in 2011 [16]. The analysis by Oti and colleagues only included adults from the age of 35 years which likely explains the higher mortality compared with our study. This is further illustrated in our own sub-analysis of a relatively older population who participated the 2008/9 NCD survey with a higher mortality rate of 4.7 per 1000 person years.
The decline in NCD mortality that we have observed for the first time in this population may be attributed to population-based interventions and improvements in health services resulting from implementation research conducted in the study population by the African Population and Health Research Center (APHRC), particularly from a population based intervention conducted between August 2012 and February 2014. A community based intervention was delivered through trained community health volunteers who led awareness campaigns, household visits for screening, and offered referral and treatment of people with hypertension leading to a substantial reduction in systolic blood pressure at the population level and to an increased fruit and vegetable consumption in the communities [18]. The intervention also included training of nurses and clinical officers, providing primary care guidelines for hypertension management, equipment supply, opening a new clinic at a central location in the slum, and also opening clinics on the weekends to increase access to care for daily labourers who work during weekdays. Additional, regular community engagement in NCD research, including dissemination of study findings and offering referrals for care increases the level of awareness and knowledge of prevention strategies to reduce the burden of NCDs. This finding suggests that it is possible the achieve the 25% reduction in NCD mortality if population-level NCD interventions and improvements in the primary health care system are implemented in the informal settlements.
We also examined the extent to which NCD risk factors measured in 2008 influenced mortality over time. In summary, age, lack of formal education, tobacco use and high blood pressure were associated with higher NCD mortality. There was no evidence that any of the following factors were associated with NCD mortality over the nine-year study period: sex, SES index, alcohol consumption, physical inactivity, obesity, cholesterol levels.
The association of NCD mortality with increasing age is similar to recent findings in a rural health and demographic surveillance site in Ethiopia in which a 5-year increase in age was associated with 35% higher hazard of mortality [36]. The Global Burden of Disease (GBD) estimates in 2015 reported aging to be the most important factor for the increased occurrence of NCD mortality [37]. This is not surprising since NCD risk factors are shown to accumulate with increasing age [15,38]. As observed in our own study population, the proportion of older people has increased (from 7.1% in 2008 to 9.3%- Table 1) and this trend is happening globally. This suggests the need for NCD prevention programs to start at relatively young ages to prevent mortality in later life. Similar to findings from an urban DSS in Ouagadougou in Burkina Faso [39], the risk of mortality was lower among adults who had a primary level of education compared to those with no education. Education level mediates better health outcomes mainly through two ways. Firstly, it increases health care utilization, thus reducing the risk of mortality [40,41]. Secondly, education facilitates the adoption of healthy behaviours and the avoidance of unhealthy habits averting mortality from NCDs [42].
Studies mainly from high income countries have shown that tobacco smoking is associated with 2–3 fold higher relative risk of coronary heart disease, 1.5 times for stroke, 1.4 times for chronic obstructive pulmonary disease (COPD) and 12 fold risks for lung cancer [43,44]. In our study, we found that individuals who had ever smoked had over 30% higher rates of NCD mortality compared with those who never smoked. Reducing tobacco use is one of the WHO “best buys” recommended for preventing NCDs [45]. Integrating tobacco control in the slum population is crucial to achieve a reduction in NCD mortality in this setting.
Among the physiological factors investigated, we found high blood pressure conferred 50% higher rates of NCD mortality. Hypertension is the largest contributor to global burden of disease, accounting for 7% of global disability adjusted life years (DALYS) and is widely acknowledged as number one risk factor for mortality [46,47]. The GBD 2017 analysis revealed that from 1990 to 2017, while deaths due to high blood pressure decreased in 98 countries, many LMICs experienced an increase [48]. Tackling hypertension is a global priority which requires timely screening and treatment to achieve optimal blood pressure control and prevent NCD mortality. In the NUHDSS (where our study sample was drawn) only 19.5% of participants with hypertension were reported to be aware that they had it while hypertension control among all hypertensives was below 3% in 2008 [20]. The increased risk of death among patients with long-standing uncontrolled hypertension is commonly attributable to myocardial infarction, cardiac failure, renal insufficiency, stroke and left ventricular hypertrophy [49]. Thus early population level screening and prompt treatment for hypertension is needed in this vulnerable population to prevent mortality.
These findings may have an important public health implication with respect to preventing adult mortality from NCDs. In the 2011 UN General Assembly political declaration [10], among the commitments member states made to reducing NCD mortality by 25% by 2025 were targets for reducing six NCD risk factors including tobacco use and raised blood pressure that we have found to be leading causes of mortality in this population. The other NCD risk factors such as raised blood glucose and diabetes, and physical inactivity and harmful alcohol use did not stand out as a major driver of mortality in our study population. The reasons for lack of association with NCD mortality are unclear, however these could be attributed to the smaller number of people engaging in these risk factors and few mortality events observed during the study period and reliance on one baseline measurement in 2008, yet these are time-changing covariates.
Since mortality due to non-NCD causes (mainly communicable diseases) continues to be the predominant in the slums, we also examined factors for non-NCD mortality. We found that increasing age, being male, ever smoking and lower physical activity were associated with a higher rates of non-NCD mortality. While higher SES was associated with lower risk of non-NCD mortality, there was rather a contradicting evidence showing that those with high cholesterol levels had lower odds of non-NCD mortality.
These results largely agree with other published literature. Physical activity has previously been shown to reduce non-NCD mortality in other settings [50], with a likely pathway that physical activity is potent stimulus of immune function [51]. Mechanisms by which smoking increases the risk of infections include structural changes in the respiratory tract and a decrease in immune response thus increasing the risk of infections such as invasive pneumococcal disease, Influenzas and tuberculosis. The greatest public health impact of smoking on infection is the increased risk of tuberculosis, a particular problem in low-income countries where smoking rates are increasing rapidly [52]. Income inequality in the Nairobi is a major driver of non-NCD mortality. Wealth has been shown to reduce mortality in other settings [53]. For the case of Nairobi slums, where HIV and TB have been cited as major causes of non-NCD mortality, poorer residents are unable to access health services since private informal healthcare facilities are the main source of services. Financial barriers are also a major obstacle in access to services since less than 10% of the population in the slums have health insurance [53]. The negative relationship between cholesterol and non-NCD mortality is in agreement with the Framingham study findings which showed an inverse relationship between cholesterol level and non-NCD mortality, beginning at age 50 years [54]. This could be explained by decreases in cholesterol level observed in severely ill patients [55].
Some of the limitations to this study include; a short follow-up period to estimate NCD mortality and a small number of events could not permit us to further break down NCD mortality trends into specific cause sub-groups. Secondly, verbal autopsy is an imprecise tool of assigning cause of death and this is likely to misclassify some causes of death particularly for NCD deaths which comprise a diverse set of causes of death and a diverse set of symptoms. Some deaths did not receive a VA, and while there was no evidence for a systematic difference in who did not receive a VA by age or sex, we did note some changes over time with VA coverage increasing over time; this is likely to have led to an underestimate in the reduction of NCD mortality over time as deaths in the earliest time period were most likely to not have a cause assigned leading to an underestimate of the rates in this time period. Lastly, NCD risk factors were measured at baseline in a subset of participants whose characteristics were different from the overall adult population, and the time varying covariates were not measured during follow-up, yet these could have changed over time. However, this paper makes an important contribution using longitudinal data on levels of, and risk factors for, NCD mortality in a vulnerable population where there are no reliable civil registration and health records.
Conclusion
From our findings, it is clear that the NUHDSS population has experienced NCD mortality decline between 2008 and 2017. The basic mortality pattern is different from the previous stagnant trend reported in the same population but coincides with increased surveillance and introduction of population-wide NCD interventions and health system improvements arising from research activities in the NUHDSS. The mortality analysis contributes to the understanding of the progress towards the achievement of some of the sustainable development goals and the goal of the national NCD strategy in Kenya. Most importantly, the decline in mortality after the period of intense community-based NCD interventions highlights the importance of population-wide interventions and an understanding of the risk factors that drive NCD mortality. Mortality from communicable diseases is still evident with a shared set of risk factors with NCD mortality in the same community.
Public health recommendations
There is a need to consider taking practical approaches to integrated health systems strengthening targeting both communicable and NCDs to achieve further mortality declines in the slums. Focusing on community based interventions such as sensitizing populations on timely screening for hypertension and referral for care and increasing the breadth of health insurance coverage to enable access to quality services will be critical in reducing mortality. There is also a need address primary NCD risk factors such as strengthening the implementation of tobacco control policies in slum communities. Further research is needed on measurements of harmful alcohol consumption and physical inactivity as risk factors as the methods used in this study were subjective and not adequately powered.
Acknowledgements and funding
We are most grateful to community leaders and residents of the Korogocho and Viwandani slums who continuously volunteer to provide data for the NUHDSS. This work was also made possible by core funding for APHRC from The William and Flora Hewlett Foundation (grant no. 2009–40510), the Swedish International Cooperation Agency (SIDA) (grant no. 2011–001578), and the Rockefeller Foundation (grant no. 2009SCG302), the Wellcome Trust (UK) and Melinda Gates Foundation (USA). The analysis for this manuscript was supported by African Non-communicable Disease Longitudinal data Alliance (ANDLA) workshops held in Lilongwe and Johannesburg for harmonization and analysis of longitudinal population-level non-communicable diseases (NCD) multi-site data for investigating the burden, distribution, progression and aetiology of NCD in Africa funded by MRC (MR/P023851/1). The InSilicoVA algorithm work was supported by grant R01 HD086227 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The funders had no part in the design, execution, or interpretation of the work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gloepi.2021.100049.
Appendix A. Supplementary data
Supplementary material
References
- 1.World Health Organization . World Health Organization; Geneva: 2014. Global status report on noncommunicable diseases. [DOI] [PubMed] [Google Scholar]
- 2.Anand K., Shah B., Yadav K., Singh R., Mathur P., Paul E., et al. Are the urban poor vulnerable to non-communicable diseases? A survey of risk factors for non-communicable diseases in urban slums of Faridabad National. Med J India. 2007;20:115. [PubMed] [Google Scholar]
- 3.Ziraba A.K., Fotso J.C., Ochako R. Overweight and obesity in urban Africa: a problem of the rich or the poor? BMC Public Health. 2009;9:465. doi: 10.1186/1471-2458-9-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haregu T.N., Oti S., Egondi T., Kyobutungi C. Co-occurrence of behavioral risk factors of common non-communicable diseases among urban slum dwellers in Nairobi, Kenya. Glob Health Action. 2015;8:28697. doi: 10.3402/gha.v8.28697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayah R., Joshi M.D., Wanjiru R., Njau E.K., Otieno C.F., Njeru E.K., et al. A population-based survey of prevalence of diabetes and correlates in an urban slum community in Nairobi, Kenya. BMC Public Health. 2013;13:371. doi: 10.1186/1471-2458-13-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gowda M.J., Bhojani U., Devadasan N., Beerenahally T.S. The rising burden of chronic conditions among urban poor: a three-year follow-up survey in Bengaluru, India. BMC Health Serv Res. 2015;15:330. doi: 10.1186/s12913-015-0999-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yadav K., Krishnan A. Changing patterns of diet, physical activity and obesity among urban, rural and slum populations in North India. Obes Rev. 2008;9:400–408. doi: 10.1111/j.1467-789X.2008.00505.x. [DOI] [PubMed] [Google Scholar]
- 8.United Nations . United Nations Department of Economics and Social Affairs, Population Division; New York, USA: 2015. World urbanization prospects: The 2014 revision. [Google Scholar]
- 9.Da Cruz F. 2006. Nairobi urban sector profile: UN-HABITAT. [Google Scholar]
- 10.World Health Organization . World Health Organization; Geneva: 2012. Sixty-fifth World Health assembly, second report of Committee A. A65/54. [Google Scholar]
- 11.Setel P.W., Macfarlane S.B., Szreter S., Mikkelsen L., Jha P., Stout S., et al. Group MoVEw: a scandal of invisibility: making everyone count by counting everyone. Lancet. 2007;370:1569–1577. doi: 10.1016/S0140-6736(07)61307-5. [DOI] [PubMed] [Google Scholar]
- 12.Arudo J., Gimnig J.E., Ter Kuile F.O., Kachur S.P., Slutsker L., Kolczak M.S., et al. Comparison of government statistics and demographic surveillance to monitor mortality in children less than five years old in rural western Kenya. Am J Trop Med Hyg. 2003;68(4_suppl):30–37. [PubMed] [Google Scholar]
- 13.United Nations Statistics Division Demographic and social statistics. 2017. http://unstats.un.org/unsd/demographic/CRVS/CR_coverage.htm Available from:
- 14.Whiting D.R., Setel P.W., Chandramohan D., Wolfson L.J., Hemed Y., Lopez A.D. Estimating cause-specific mortality from community-and facility-based data sources in the United Republic of Tanzania: options and implications for mortality burden estimates. Bull World Health Organ. 2006;84:940–948. doi: 10.2471/blt.05.028910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim Streatfield P., Khan W.A., Bhuiya A., Hanifi S.M., Alam N., Bagagnan C.H., et al. Adult non-communicable disease mortality in Africa and Asia: evidence from INDEPTH health and demographic surveillance system sites. Glob Health Action. 2014;7:25365. doi: 10.3402/gha.v7.25365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oti S.O., van de Vijver S., Kyobutungi C. Trends in non-communicable disease mortality among adult residents in Nairobi’s slums, 2003–2011: applying InterVA-4 to verbal autopsy data. Glob Health Action. 2014;7:25533. doi: 10.3402/gha.v7.25533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otieno P.O., Wambiya E.O.A., Mohamed S.M., Mutua M.K., Kibe P.M., Mwangi B., et al. Access to primary healthcare services and associated factors in urban slums in Nairobi-Kenya. BMC Public Health. 2020;20:981. doi: 10.1186/s12889-020-09106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van de Vijver S., Oti S.O., Gomez G.B., Agyemang C., Egondi T., van Charante E.M., et al. Impact evaluation of a community-based intervention for prevention of cardiovascular diseases in the slums of Nairobi: the SCALE-UP study. Glob Health Action. 2016;9:30922. doi: 10.3402/gha.v9.30922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wamukoya M., Kadengye D.T., Iddi S., Chikozho C., System D.S. The Nairobi Urban health and demographic surveillance of slum dwellers, 2002–2019: value, processes, and challenges. Glob Epidemiol. 2020:100024. [Google Scholar]
- 20.van de Vijver S.J., Oti S.O., Agyemang C., Gomez G.B., Kyobutungi C. Prevalence, awareness, treatment and control of hypertension among slum dwellers in Nairobi, Kenya. J Hypertens. 2013;31:1018–1024. doi: 10.1097/HJH.0b013e32835e3a56. [DOI] [PubMed] [Google Scholar]
- 21.Ali S.A., Soo C., Agongo G., Alberts M., Amenga-Etego L., Boua R.P., et al. Genomic and environmental risk factors for cardiometabolic diseases in Africa: methods used for Phase 1 of the AWI-Gen population cross-sectional study. Glob Health Action. 2018;11(sup2):1507133. doi: 10.1080/16549716.2018.1507133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oti S.O., Kyobutungi C. Verbal autopsy interpretation: a comparative analysis of the InterVA model versus physician review in determining causes of death in the Nairobi DSS. Popul Health Metr. 2010;8:21. doi: 10.1186/1478-7954-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nichols E.K., Byass P., Chandramohan D., Clark S.J., Flaxman A.D., Jakob R., et al. The WHO 2016 Verbal autopsy instrument: an international standard suitable for automated analysis by InterVA, InSilicoVA, and tariff 2.0. PLoS Med. 2018;15:1. doi: 10.1371/journal.pmed.1002486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark S.J., Mc Cormick T., Li Z., Wakefield J. 2015. InSilicoVA: a method to automate cause of death assignment for verbal autopsy. arXiv preprint. arXiv:150402129. [Google Scholar]
- 25.STEPwise approach to chronic disease risk factor surveillance (STEPS) 2021. https://www.who.int/ncds/surveillance/steps/en/
- 26.Wong N.D., Black H.R., Gardin J.M. McGraw-Hill Professional; New York: 2005. Preventive cardiology: A practical approach. [Google Scholar]
- 27.Wekesah F.M., Klipstein-Grobusch K., Grobbee D.E., Kadengye D., Asiki G., Kyobutungi C.K. Determinants of mortality from cardiovascular disease in the slums of Nairobi, Kenya. Glob Heart. 2020;15:1. doi: 10.5334/gh.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Victora C.G., Huttly S.R., Fuchs S.C., Olinto M. The role of conceptual frameworks in epidemiological analysis: a hierarchical approach. Int J Epidemiol. 1997;26:224–227. doi: 10.1093/ije/26.1.224. [DOI] [PubMed] [Google Scholar]
- 29.Vyas S., Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan. 2006;21:459–468. doi: 10.1093/heapol/czl029. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization . World Health Organization; Geneva: 2010. Global recommendations on physical activity for health. [PubMed] [Google Scholar]
- 31.World Health Organization . World Health Organization; Geneva: 2011. Waist circumference and waist-hip ratio: report of a WHO expert consultation. [Google Scholar]
- 32.World Health Organization . World Health Organization; Geneva: 2006. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation. [Google Scholar]
- 33.Detection NCEPEPo . 2002. Adults ToHBCi: Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III): National Cholesterol Education Program, National Heart, Lung, and Blood. [PubMed] [Google Scholar]
- 34.StataCorp LLC . StataCorp LP; College Station, TX: 2017. Stata data analysis and statistical software: Release 15. [internet]https://www.stata.com Available from: [Google Scholar]
- 35.Ahmad O.B., Boschi-Pinto C., Lopez A.D., Murray C.J., Lozano R. Vol. 9. World Health Organization; Geneva: 2001. Inoue M. Age standardization of rates: a new WHO standard; p. 10. [Google Scholar]
- 36.Abera S.F., Gebru A.A., Biesalski H.K., Ejeta G., Wienke A., Scherbaum V., et al. Social determinants of adult mortality from non-communicable diseases in northern Ethiopia, 2009-2015: evidence from health and demographic surveillance site. PLoS One. 2017;12 doi: 10.1371/journal.pone.0188968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prince M.J., Wu F., Guo Y., Robledo L.M.G., O’Donnell M., Sullivan R., et al. The burden of disease in older people and implications for health policy and practice. Lancet. 2015;385:549–562. doi: 10.1016/S0140-6736(14)61347-7. [DOI] [PubMed] [Google Scholar]
- 38.Oti S.O., van de Vijver S.J., Agyemang C., Kyobutungi C. The magnitude of diabetes and its association with obesity in the slums of Naairobi, Kenya: results from a cross-sectional survey. Trop Med Int Health. 2013;18:1520–1530. doi: 10.1111/tmi.12200. [DOI] [PubMed] [Google Scholar]
- 39.Rossier C., Soura A.B., Duthé G., Findley S. Non-communicable disease mortality and risk factors in formal and informal neighborhoods, Ouagadougou, Burkina Faso: evidence from a health and demographic surveillance system. PLoS One. 2014;9:12. doi: 10.1371/journal.pone.0113780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ito S., Takachi R., Inoue M., Kurahashi N., Iwasaki M., Sasazuki S., et al. Education in relation to incidence of and mortality from cancer and cardiovascular disease in Japan. Eur J Public Health. 2008;18:466–472. doi: 10.1093/eurpub/ckn052. [DOI] [PubMed] [Google Scholar]
- 41.Chisumpa V.H., Odimegwu C.O., De Wet N. Adult mortality in sub-saharan Africa, Zambia: where do adults die? SSM-Popul Health. 2017;3:227–235. doi: 10.1016/j.ssmph.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rawshani A., Svensson A.-M., Rosengren A., Eliasson B., Gudbjörnsdottir S. Impact of socioeconomic status on cardiovascular disease and mortality in 24,947 individuals with type 1 diabetes. Diabetes Care. 2015;38:1518–1527. doi: 10.2337/dc15-0145. [DOI] [PubMed] [Google Scholar]
- 43.Peto R., Boreham J., Lopez A.D. Oxford University Press; Oxford: 1996. Mortality from smoking in developed countries. [Google Scholar]
- 44.Parish S., Collins R., Peto R., Youngman L., Barton J., Jayne K., et al. Cigarette smoking, tar yields, and non-fatal myocardial infarction: 14000 cases and 32000 controls in the United Kingdom. BMJ. 1995;311:471–477. doi: 10.1136/bmj.311.7003.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allen L.N., Pullar J., Wickramasinghe K.K., Williams J., Roberts N., Mikkelsen B., et al. Evaluation of research on interventions aligned to WHO ‘Best Buys’ for NCDs in low-income and lower-middle-income countries: a systematic review from 1990 to 2015. BMJ Glob Health. 2018;3:1. doi: 10.1136/bmjgh-2017-000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mathers C., Stevens G., Mascarenhas M. World Health Organization; Geneva: 2009. Global health risks: Mortality and burden of disease attributable to selected major risks. [Google Scholar]
- 47.Lim S.S., Vos T., Flaxman A.D., Danaei G., Shibuya K., Adair-Rohani H., et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jagannathan R., Patel S.A., Ali M.K., Narayan K.V. Global updates on cardiovascular disease mortality trends and attribution of traditional risk factors. Curr Diab Rep. 2019;19:44. doi: 10.1007/s11892-019-1161-2. [DOI] [PubMed] [Google Scholar]
- 49.Kannel W. Potency of vascular risk factors as the basis for antihypertensive therapy. Eur Heart J. 1992;13(suppl_G):34–42. doi: 10.1093/eurheartj/13.suppl_g.34. [DOI] [PubMed] [Google Scholar]
- 50.Trolle-Lagerros Y., Mucci L.A., Kumle M., Braaten T., Weiderpass E., Hsieh C.-C., et al. Physical activity as a determinant of mortality in women. Epidemiology. 2005:780–785. doi: 10.1097/01.ede.0000181312.35964.22. [DOI] [PubMed] [Google Scholar]
- 51.Nieman D.C., Wentz L.M. The compelling link between physical activity and the body’s defense system. J Sport Health Sci. 2019;8:201–217. doi: 10.1016/j.jshs.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arcavi L., Benowitz N.L. Cigarette smoking and infection. Arch Intern Med. 2004;164:2206–2216. doi: 10.1001/archinte.164.20.2206. [DOI] [PubMed] [Google Scholar]
- 53.Demakakos P., Biddulph J.P., Bobak M., Marmot M.G. Wealth and mortality at older ages: a prospective cohort study. J Epidemiol Community Health. 2016;70:346–353. doi: 10.1136/jech-2015-206173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kronmal R.A., Cain K.C., Ye Z., Omenn G.S. Total serum cholesterol levels and mortality risk as a function of age: a report based on the Framingham data. Arch Intern Med. 1993;153:1065–1073. [PubMed] [Google Scholar]
- 55.Noel M.A., Smith T.K., Ettinger W.H. Characteristics and outcomes of hospitalized older patients who develop hypocholesterolemia. J Am Geriatr Soc. 1991;39:455–461. doi: 10.1111/j.1532-5415.1991.tb02489.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material