Abstract
Objective
To investigate the potential benefits of 3 therapeutic instrumental music performance (TIMP)-based interventions in rehabilitation of the affected upper-extremity (UE) for adults with chronic poststroke hemiparesis.
Design
Randomized-controlled pilot study.
Setting
University research facility.
Participants
Community-dwelling volunteers (N=30; 16 men, 14 women; age range, 33-76 years; mean age, 55.9 years) began and completed the protocol. All participants had sustained a unilateral stroke more than 6 months before enrollment (mean time poststroke, 66.9 months).
Intervention
Two baseline assessments, a minimum of 1 week apart; 9 intervention sessions (3 times/week for 3 weeks), in which rhythmically cued, functional arm movements were mapped onto musical instruments; and 1 post-test following the final intervention. Participants were block-randomized to 1 of 3 conditions: group 1 (45 minutes TIMP), group 2 (30 minutes TIMP, 15 minutes metronome-cued motor imagery [TIMP+cMI]), and group 3 (30 minutes TIMP, 15 minutes motor imagery without cues [TIMP+MI]). Assessors and investigators were blinded to group assignment.
Main Outcome Measures
Fugl-Meyer Upper-Extremity (FM-UE) and Wolf Motor Function Test- Functional Ability Scale (WMFT-FAS). Secondary measures were motor activity log (MAL)–amount of use scale and trunk impairment scale.
Results
All groups made statistically significant gains on the FM-UE (TIMP, P=.005, r=.63; TIMP+cMI, P=.007, r=.63; TIMP+MI, P=.007, r=.61) and the WMFT-FAS (TIMP, P=.024, r=.53; TIMP+cMI, P=.008, r=.60; TIMP+MI, P=.008, r=.63). Comparing between-group percent change differences, on the FM-UE, TIMP scored significantly higher than TIMP+cMI (P=.032, r=.57), but not TIMP+MI. There were no differences in improvement on WMFT-FAS across conditions. On the MAL, gains were significant for TIMP (P=.030, r=.54) and TIMP+MI (P=.007, r=.63).
Conclusion
TIMP-based techniques, with and without MI, led to significant improvements in paretic arm control on primary outcomes. Replacing a physical training segment with imagery-based training resulted in similar improvements; however, synchronizing internal and external cues during auditory-cMI may pose additional sensorimotor integration challenges.
Keywords: Music, Rehabilitation, Stroke, Upper extremity
List of abbreviations: cMI, cued motor imagery; FM-UE, Fugl-Meyer–Upper Extremity; MAL, motor activity log; Mdn, median; MI, motor imagery; TIMP, therapeutic instrumental music performance; UE, upper extremity; WMFT-FAS, Wolf Motor Function Test–Functional Ability Scale
Among all neurologic disorders, stroke contributes the largest proportion of disability-adjusted life-years (42.2%).1 As millions of individuals cope with functional health loss, the economic burden owing to poststroke care continues to rise.2 People living with the effects of stroke score consistently low on life satisfaction, perceived health, and health-related quality of life.3
Although a stroke may generate a number of disabling conditions, the most common sequela is motor impairment.4 Volitional movements are often segmented, slow, and indirect.5 If a functional threshold of recovery is not achieved, the affected individual may resort to compensatory movements,6 and paretic learned nonuse may ensue.7
Rehabilitation is believed to modulate motor recovery by interacting with underlying biological processes primarily during the first 6 months after a stroke.8 Many individuals at the chronic stage no longer receive rehabilitation services; however, evidence of significant treatment benefits during this phase is growing.9, 10, 11 It is critical for individuals at the chronic stage to continue to engage in rehabilitation that focuses on effective learning and training strategies, because behavioral experience has been shown to modify functional alterations in spared regions of the brain.12 These strategies may include enhanced movement feedback and opportunities for more independent training allowing for higher intensity rates. The music and imagery-based techniques in this study have been shown to address these needs13,14 but have not been researched in integrated applications. Our study attempts to address this gap by studying music-based interventions alone and in combination with imagery-based motor training.
Studies have shown beneficial effects of music interventions on motor control. The provision of structured temporal auditory information has been shown to lend significant stability to kinematic parameters during hemiparetic arm reaching.15,16 Studies in mapping functional movements on musical instruments found significant gains in hemiparetic arm rehabilitation.13 The Neurologic Music Therapy technique17 used in this study, therapeutic instrumental music performance (TIMP), was developed through 2 research streams: rhythmic auditory stimulation, which provides predictable anticipatory rhythmic cues to entrain movement,18,19 and sonification, which provides augmented auditory feedback by mapping sound on kinematic parameters.20
Motor imagery (MI) activates motor regions in the brain similar to physical practice21 and is widely used in sports training, but applications in neurorehabilitation have been more limited. For example, researchers found enhanced treatment efficacy when modified constraint-induced therapy was followed by MI practice.14,22 MI training offers many advantages, including more autonomous training time, which potentially increases the rate and intensity of therapy applications without additional physical load. However, a frequently reported shortcoming refers to reduced MI abilities associated with decline in cognitive function. Thus, effective MI may need to be paired with a physically active component that produces a robust and stable representation of movement retainable during mental rehearsal. Combining a novel, music-based motor intervention with MI presents a potentially attractive means to enhance and extend the effectiveness of rehabilitation. TIMP may be such an intervention owing to its augmented spatiotemporal structure and its rich sensory-based feedback-feedforward environment.
Therefore, the central goal of this study was to investigate whether TIMP paired with MI and TIMP paired with cued MI (cMI), in which a rhythmic auditory cue used during active training was retained during imagery, showed better motor outcomes than TIMP alone. The effectiveness of all 3 conditions against no training was investigated by comparing intervention outcomes with a stable baseline control, determined by comparing 2 baseline measurements before interventions commenced. Based on previous research findings (see Park14 and Page et al22), it was anticipated that there would be greater reductions in impairment and improvements in functional capacity using MI in conjunction with active TIMP practice. It was also anticipated that cMI, retaining an auditory timing cue, would yield superior results to MI without an external cue.
Methods
Participants
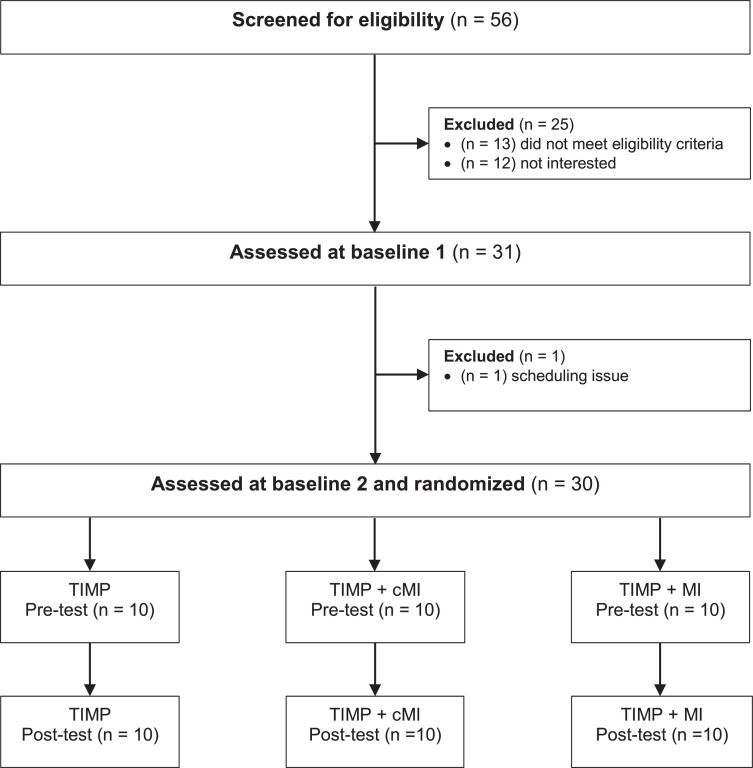
Thirty community-dwelling adults (16 men/14 women; age range, 33-76 years; mean age, 55.9 years) began and completed the protocol (fig 1). All participants had sustained a hemispheric stroke, ischemic or hemorrhagic, more than 6 months before enrollment (average time poststroke, 66.9 months; standard deviation [SD], 14.4 months). The study was approved by the participating university and hospital research ethics committees. Informed consent was obtained prior to participation, in accordance with the Declaration of Helsinki. Recruitment was primarily through posters. A few volunteers were referred by previous participants; the study was also registered at ClinicalTrials.gov (NCT03246217). Participants were recruited from a highly multicultural metropolitan region, and the study sample was reflective of this diversity. Inclusion criteria were hemiparesis, with at least minimal volitional movement of the affected limb; age ranging from 30 to 79 years; and adequate English language comprehension, as well as neurocognitive function, to understand and follow simple instructions. Participants were excluded if they were currently enrolled in an upper extremity (UE) rehabilitation program, had a comorbid neurologic disorder, or had been given injections for spasticity less than 3 months before training. Demographic data are provided in table 1. Data that support the findings of this study are available from the corresponding author upon reasonable request.
Fig 1.
Consort diagram for participant screening and enrollment.
Table 1.
Demographic characteristics
| TIMP (n=10) | TIMP+cMI (n=10) | TIMP+MI (n=10) | |
|---|---|---|---|
| Age, mean (SD), y | 54.7 (10.76) | 55.5 (15.01) | 57.6 (11.14) |
| Sex | Men: 5 Women: 5 |
Men: 5 Women: 5 |
Men: 6 Women: 4 |
| Years of education, mean (SD) | 15.4 (3.24) | 16 (1.83) | 17.4 (2.68) |
| MoCA score, mean (SD) | 26.8 (3.74) | 26.8 (2.90) | 26.8 (3.01) |
| Music training* | A: 6 B: 3 C: 2 |
A: 6 B: 2 C: 2 |
A: 1 B: 5 C: 4 |
| Months since stroke onset, mean (SD) | 71.7 (69.13) | 50.7 (45.53) | 78.3 (67.46) |
| Type of stroke | Hemorrhagic: 3 Ischemic: 7 |
Hemorrhagic: 3 Ischemic: 7 |
Hemorrhagic: 5 Ischemic: 5 |
| Side of lesion | Right: 7 Left: 3 |
Right: 7 Left: 3 |
Right: 3 Left: 7 |
| Location of lesion | Frontal lobe: 3 Brainstem: 2 MCA: 4 Basal ganglia: 1 |
Frontal lobe: 2 Brainstem: 0 MCA: 4 Basal ganglia: 2 Left thalamus, left occipital, right cerebellum: 1 Lacunar stroke in right corona radiata: 1 |
Frontal lobe: 2 Brainstem: 4 MCA: 2 Basal ganglia: 1 M1, PMC, IFG: 1 |
Abbreviations: IFG, inferior frontal gyrus; M1, primary motor cortex; MCA, middle cerebral artery; MoCA, Montreal Cognitive Assessment; PMC, premotor cortex.
Musical Background: A, none; B, limited, informal; C, some formal training (>1y).
Study design
This was a 3-armed, parallel, randomized controlled pilot with blinded assessment of outcome. Participants were randomly assigned to 1 of 3 intervention conditions. Randomization procedures were carried out by a blinded independent allocator drawing a group number from an opaque box, using blocked randomization to ensure that equal numbers were assigned to each group. The blinded independent allocator informed the participant's trainer of the condition assignment and provided the trainer with a unique identifying code for the participant. This identifying code was also provided to the independent assessors who were blinded to treatment allocation. At the conclusion of the trial, data entry was carried out by research assistants blinded to group assignment.
A power analysis indicated the following target sample size: the Fugl-Meyer–Upper Extremity (FM-UE) has a standardized response mean of 1.42 (95% confidence interval, 1.19-1.80), and the Wolf Motor Function Test–Functional Ability Scale (WMFT–FAS) has a standardized response mean of 1.3 (95% confidence interval, 1.03-1.67).23 At a P value of 0.05 and 80% power, 8 participants were deemed to be required per arm to detect a standardized difference for the FM-UE and 9 for the WMFT–FAS.
Procedures
Assessments and interventions were delivered in a university rehabilitation research facility. All participants were assessed at 2 baselines, a minimum of 1 week apart, to ascertain stability of impairments prior to treatment. A final assessment was administered immediately after the last intervention. Training sessions, facilitated by qualified rehabilitation clinicians, were scheduled 3 times a week for 3 weeks. Group 1 participants received 9 individual 45-minute sessions of TIMP. Group 2 received the same number of sessions with 30 minutes of TIMP followed by 15 minutes of auditory-cMI (TIMP+cMI). Auditory-cMI involved listening to a metronome cue, set to the participant's preferred pace for each previously practiced exercise, while engaging in kinesthetic and visual MI. Group 3 participants had 30 minutes of TIMP training followed by 15 minutes of MI without external cues (TIMP+MI).
TIMP exercises were designed to facilitate retraining of functional movement patterns involving proximal and distal control by mapping rhythmically cued movements onto acoustic and digital instruments, including a specially designed programmable digital interactive sound tablet with 32 surface key squares in 4 rows producing musical sounds on touch (sonification arm training apparatus).24 An external rhythmic auditory cue, set at the participant's preferred tempo, provided a consistent template for gauging movement trajectories in accordance with spatiotemporal constraints, emphasizing accuracy over speed. Beats per minute for slower tempi were subdivided to facilitate entrainment and promote auditory-motor coupling.25 Instruments and devices, selected according to participant need and the training goal, were placed as movement endpoints for shoulder abduction and adduction, elbow flexion and extension, wrist pronation and supination, selective finger dexterity, and upper trunk rotation exercises. Therapists provided 1-person assist if required, with a focus on movement quality rather than compensatory movements. Each session was subdivided into 3 15-minute repetition blocks, with sequence of foci continuously changed to avoid rote repetition. The MI groups practiced the third block in MI.
Assessments
UE impairment was assessed using the motor domain portion of the FM-UE,26 which assesses shoulder/elbow/forearm, wrist, hand/finger, and coordination/speed. It has been shown to have strong psychometric properties and is recommended for use in clinical trials of stroke rehabilitation.27 Arm motor function was assessed by means of the WMFT-FAS,28 evaluated as having excellent psychometric properties and clinical utility, which support its use in research and clinical contexts.29 The motor activity log (MAL),30 shown to be a reliable and valid measure of real-world use,31 was selected to provide self-reports on perceived use of the affected limb. Upper trunk impairment was assessed using the trunk impairment scale; analysis of clinimetric parameters supports use of this scale in clinical and research contexts.32
Data analysis
All participants completed the protocol as assigned. As the data did not meet assumptions required for parametric analyses, nonparametric equivalents were used. Differences between pretests 1 and 2, as well as differences between pretest 2 and post-test were assessed using the Wilcoxon signed-rank or sign test. The Kruskal-Wallis H test was used to assess between-group differences. A Bonferroni correction was applied for pairwise comparisons. In addition, correlation analyses were run using Kendall's tau-b to ascertain possible associations between and within subsections of outcome measures. The 0.05 level of probability was established as statistically significant. SPSS, version 26,a was used in analyses.
Results
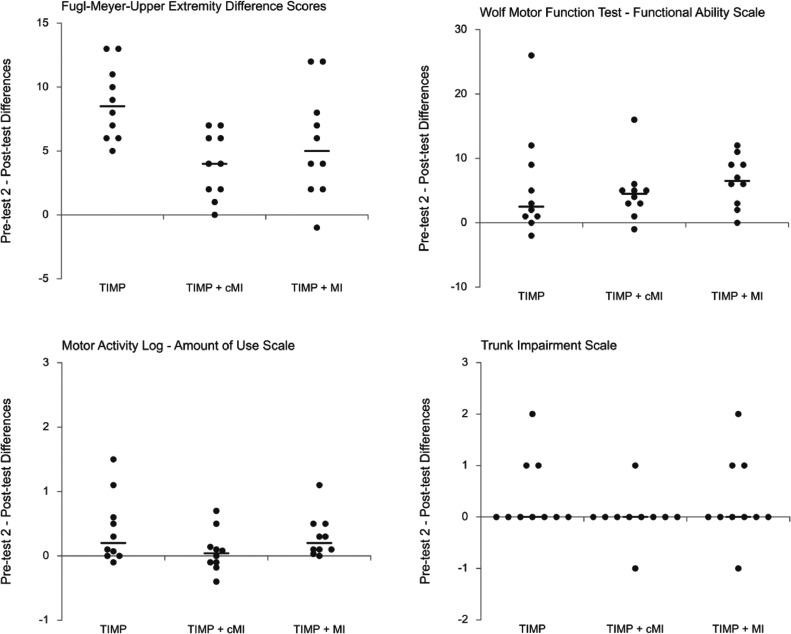
No statistically significant between-group differences were found on any demographic variables. The 2 pretest measures did not differ significantly, indicating a stable baseline (table 2). Subsequently, pretest 2 was used for statistical comparison with post-tests. The pretest 2 and post-test means, SDs, and probability values for each group are reported in table 3. For median FM-UE data, there was a statistically significant improvement between pretest 2 and post-test for each group: TIMP pretest 2 (median [Mdn], 29.50), post-test (Mdn, 35.00), difference (Mdn, 8.50), z=2.81, P=.005**, r=.63; TIMP+cMI pretest 2 (Mdn, 28.00), post-test (Mdn, 31.00), difference (Mdn, 4.00), z=2.68, P=.007**, r = .63; TIMP+MI pretest 2 (Mdn, 37.50), post-test (Mdn, 46.00), difference (Mdn, 5.00), z=2.71, P=.007**, r=.61. Figure 2 shows group medians, individual data points, and difference scores.33 Median increases for TIMP and TIMP+MI were above the clinically important difference estimate of 4.25 assessed by Page et al using a less impaired sample.34
Table 2.
Baseline measures
| Pretest 1 | Pretest 2 | P Value | |
|---|---|---|---|
| Fugl-Meyer Upper Extremity | 31.80 (14.67) | 31.73 (14.76) | .727 |
| Wolf Motor Function Test–Functional Ability Scale | 33.07 (16.96) | 32.97 (15.58) | .634 |
| Wolf Motor Function Test–Weight | 4.30 (4.74) | 4.23 (4.57) | .479 |
| Wolf Motor Function Test–Grip Strength | 8.2 (9.24) | 8.78 (9.85) | .328 |
| Trunk Impairment Scale* | 9.70 (1.44) | 10.13 (1.07) | .061 |
NOTE. Wilcoxon signed-rank test using pooled group of 30 participants. Data are presented as mean (SD), 2-sided asymptotic probability.
Sign test.
Table 3.
Motor assessment outcomes
| TIMP | TIMP+cMI | TIMP+MI | |
|---|---|---|---|
| Fugl-Meyer Upper Extremity | |||
| Pretest 2 | 28.20 (12.07) | 32.20 (16.21) | 34.80 (16.40) |
| Post-test | 37.00 (11.87) | 36.10 (16.11) | 40.40 (15.04) |
| P value | .005⁎⁎ | .007⁎⁎ | .007⁎⁎ |
| Wolf Motor Function Test–Functional Ability Scale | |||
| Pretest 2 | 26.8 (11.02) | 33.8 (16.52) | 38.3 (17.67) |
| Post-test | 32.5 (18.01) | 38.5 (19.63) | 44.8 (17.48) |
| P value | .024* | .008⁎⁎ | .008⁎⁎ |
| Wolf Motor Function Test–Weight | |||
| Pretest 2 | 2.10 (3.32) | 4.80 (5.57) | 5.80 (4.13) |
| Post-test | 2.40 (4.06) | 5.30 (5.68) | 6.00 (4.32) |
| P value | .655 | .180 | .705 |
| Wolf Motor Function Test – Grip Strength | |||
| Pretest 2 | 4.22 (3.94) | 8.44 (8.80) | 13.66 (13.05) |
| Post-test | 4.38 (3.73) | 7.78 (9.97) | 13.52 (9.73) |
| P value | .779 | .441 | .594 |
| Motor Activity Log–Amount Scale | |||
| Pretest | .683 (.89) | .696 (.96) | 1.300 (1.15) |
| Post-test | 1.090 (1.38) | .770 (1.14) | 1.603 (1.38) |
| P value | .030* | .677 | .007⁎⁎ |
| Trunk Impairment Scale | |||
| Pretest 2 | 10.10 (1.20) | 10.50 (.85) | 9.80 (1.14) |
| Post-test | 10.50 (.85) | 10.50 (.71) | 10.10 (.99) |
| P value | .250 | 1.000 | .625 |
NOTE. Data presented as mean (SD).
P<.05.
P<.01 (2-sided asymptotic).
Fig 2.
Motor outcome change scores with group medians.
To determine if there were between-group differences, the Kruskal-Wallis H test was run based on percent change scores between pretest 2 and post-test. The mean ranks of percent change scores were statistically significantly different between intervention groups (H(2)=6.731, P=.035*). Pairwise comparisons were performed with a Bonferroni correction for multiple comparisons. Post hoc analyses revealed significant change score differences between TIMP (mean rank, 21.05) and TIMP+cMI (mean rank, 11.00) (P=.032*, r =.57). There was no significant difference between TIMP and TIMP+MI (mean rank, 14.45). A Kendall's tau-b correlation determined a significant positive association between change scores on the FM-UE subscales between Part A Shoulder/Arm and Part D Coordination/Speed (τ=.329, P=.029*) (table 4).
Table 4.
Correlation analyses between and within outcome measures
| Paired Difference Scores | Kendall's Tau-b | P Value |
|---|---|---|
| FM-UE part A, part B | τ=.208 | P=.174 |
| FM-UE part A, part C | = .149 | P=.310 |
| FM-UE part A, part D | τ= .329 | P=.029* |
| FM-UE part B, part C | τ= –.071 | P=.650 |
| FM-UE part B, part D | τ= .156 | P=.333 |
| FM-UE part C, part D | τ= .072 | P=.640 |
| FM-UE, WMFT-FAS | τ= .187 | P=.170 |
| FM-UE, MAL-AS | τ= .233 | P=.084 |
| WMFT-FAS, MAL-AS | τ= .388 | P=.004† |
P<.05.
P<.01.
Similar to the FM-UE results, WMFT-FAS showed statistically significant improvements between pretest 2 and post-test for each group: TIMP pretest 2 (Mdn, 22.50), post-test (Mdn, 23.50), difference (Mdn, 2.50), z=2.26, P=.024*, r=.53; TIMP+cMI pretest 2 (Mdn, 28.00), post-test (Mdn, 33.00), difference (Mdn, 4.50), z=2.66, P=.008**, r=.60; TIMP+MI pretest 2 (Mdn, 35.00), post-test (Mdn, 42.50), difference (Mdn, 6.50), z=2.67, P=.008**, r=.63. Means and SDs are provided in table 3. Lin et al35 reported a mean change score clinically important difference range of 0.2 to 0.4. Increases for TIMP (M=0.38), TIMP+cMI (M=0.31), and TIMP+MI (M=0.43) were all within this range. A Kruskal-Wallis H test showed no significant change score differences between the 3 conditions (H(2)=1.27, P=.531).
There was no significant post–pre-test difference on the MAL–how well scale, which assesses participants’ perceived quality of movement. However, a statistically significant increase in perceived MAL–amount of use was found for participants in TIMP (z=2.17, P=.030*, r=0.54) and TIMP+MI (z=2.68, P=.007**, r=0.63) and no significant differences in TIMP+cMI (z=.42, P=.677, r=0.10). Between-group differences were not significant (H(2)=2.45, P=.294). MAL-amount of use results correlated significantly with WMFT-FAS (τ=.388, P=.004**) (table 4).
Related-samples sign test revealed no significant pre- to post-test differences in each condition for the upper trunk portion of the trunk impairment scale. Similarly, there was no statistically significant difference between groups as assessed by an independent-samples Kruskal-Wallis test.
Discussion
The purpose of our research was to assess the effects of 3 TIMP-based interventions, with and without MI, on UE motor outcomes in a sample of 30 chronic poststroke participants. Results were clinically and statistically significant for all 3 groups on the WMFT-FAS and for TIMP and TIMP+MI on the FM-UE. Statistically significant results were obtained for TIMP+cMI on the FM-UE.
Traditionally, stroke rehabilitation has focused primarily on subacute interventions, as initial research found that most recovery occurred in a critical window within the first 3 to 6 months during a period of heightened neuroplasticity. Chronic stroke rehabilitation interventions have been less clearly effective. However, recent evidence has shown that diligent rehabilitation in the chronic phase can lead to meaningful improvements.10,11 Our pilot results support such findings. Improvements were found in all 3 conditions on primary outcomes after a relatively short intervention period. These findings could have widespread implications for future chronic stroke investigations. Emphasis in training was on quality rather than speed of movement, with therapists providing 1-person assist when required to preserve movement quality and avoid compensatory motions.
Although all 3 conditions showed statistically significant pre- to post-test increases regardless of physical exercise protocols only or combined with MI, when comparing gains across groups on the FM-UE, TIMP was significantly better than TIMP+cMI but not TIMP+MI. There were no significant between-group differences on the WMFT-FAS. As primary study outcome, this leads us to suggest, from a preliminary clinical view, that reducing physical practice segments during motor therapy can be replaced with imagery-based segments without losing benefits. This may be clinically relevant because MI can provide unsupervised training time and increased time on task that may otherwise be restricted owing to physical fatigue.
Thus, our results may suggest that the augmented sensory-based feedback-feedforward structure of TIMP can be an effective practice model providing enriched and integrated auditory-temporal and visual-spatial inputs that build stable mental representations for effective imagery-based movement mapping. This finding may have considerable importance for assessing how to optimize effectiveness of imagery approaches in motor rehabilitation.
Contrary to predictions, TIMP+cMI showed lower improvement than TIMP and TIMP+MI. This was only found in the FM-UE between-group comparisons and MAL-AU post–pretest results. Sample sizes were small and heterogeneous; nevertheless, this may indicate some important considerations about effective imagery conditions. In the emulation theory of MI,36 efferent motor centers of the brain drive a body emulator, which includes the musculoskeletal system and relevant sensors. This emulator generates likely proprioceptive and kinesthetic signals that can be flexibly and dynamically compared with the efferent copy in the absence of movement-generated feedback. In TIMP+cMI, the provision of an external auditory cue may have caused additional sensory integration challenges in synchronizing with internally generated auditory imagery and MI, thereby reducing the flexible responsiveness of the emulator.
TIMP is based on sensorimotor learning, which involves acquiring new mappings of sensory and motor variables. The predictability of the rhythmic cue allows the brain to plan a movement within a fixed spatiotemporal constraint, adjusting velocity and acceleration parameters in accordance with the temporal template. Prior knowledge of the rhythmic period facilitates this mapping, allowing for greater control of the effector's movement in space, and modulating muscle activation patterns.37
Studies have shown that neural oscillations from auditory stimuli drive auditory-motor entrainment, with even brief periods of rhythmic auditory priming enhancing subsequent neural efficiency.38 Given the extensive connections between auditory and motor systems and rhythm processing in the brain,39 it is conceivable that training to a predictable pulse at their preferred rate induced entrainment of neural oscillations to the attended beat, facilitating a reduction in neuromotor noise that may negatively affect motor execution and planning.5 Individuals with stroke demonstrate greater reliance on feedback control,5 and TIMP exercises provide ongoing sensorimotor feedback. Most importantly for our study, these perceptual–motor processes appear not to be attenuated in the training conditions incorporating MI.
Finally, an additional critical component in the TIMP setup was the use of a large digital sound surface tablet sonification arm training apparatus, which participants played in a variety of functional movement repetitions. Desired sequences were programmable and visually displayed on the surface key squares which would facilitate independent practice for future use, even via remote programming, with movement parameters recorded and played back. The adaptation of music technology to neurorehabilitation may be an important research direction for providing higher intensity rates, more independence, remote interaction between therapist and patient, and data driven therapy.
Study limitations
Although our results are encouraging, the current study had a number of limitations. It was a small clinical pilot trial with only 10 participants per arm. There was variability in stroke location and type, and this variability contributed to heterogeneity between the 3 groups. Furthermore, there were no follow-up assessments to determine sustainability of results.
Conclusions
Our findings suggest that active TIMP segments may be partially replaced with TIMP-based MI without reducing improvements in motor performance. Imagery-based training, integrated into TIMP protocols, could therefore play an important role in motor rehabilitation. Synchronizing internal and external cues during cMI, however, may pose sensory integration challenges. Our data add to the growing evidence base to extend the recovery window and suggest that TIMP-based therapies, with and without MI, could play important roles in this extended window.
Supplier
-
a.
SPSS, version 26; IBM Corp.
Acknowledgments
Special thanks are owed to all research study participants, as well as to Shannon Duane, MScOT and Kyurim Kang, PhD, for administering assessments, and Nicole Richard, MA, Louise Ho, MA, Bing Li, MME, Chrystalla Paleshi, MA and Stéphanie K. Lavigne, MA, Neurologic Music Therapists, for providing training.
Footnotes
Disclosures: none.
Clinical Trial Register No. (ClinicalTrials.gov): NCT03246217.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.arrct.2021.100162.
Appendix. Supplementary materials
References
- 1.GBD 2016 Neurology Collaborators Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajsic S, Gothe H, Borba HH, et al. Economic burden of stroke: a systematic review on post-stroke care. Eur J Health Econ. 2019;20:107–134. doi: 10.1007/s10198-018-0984-0. [DOI] [PubMed] [Google Scholar]
- 3.Usuba K, Li AKC, Nowrouzi-Kia B. Trend of the burden of chronic illnesses: using the Canadian Community Health Survey. Public Health. 2019;177:10–18. doi: 10.1016/j.puhe.2019.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol. 2009;8:741–754. doi: 10.1016/S1474-4422(09)70150-4. [DOI] [PubMed] [Google Scholar]
- 5.McCrea PH, Eng JJ. Consequences of increased neuromotor noise for reaching movements in persons with stroke. Exp Brain Res. 2005;162:70–77. doi: 10.1007/s00221-004-2106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schweighofer N, Han CE, Wolf SL, Arbib MA, Winstein CJ. A functional threshold for long-term use of hand and arm function can be determined: predictions from a computational model and supporting data from the extremity Constraint-Induced Therapy Evaluation (EXCITE) trial. Phys Ther. 2009;89:1327–1336. doi: 10.2522/ptj.20080402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taub E, Uswatte G, Mark VW, Morris DM. The learned nonuse phenomenon: implications for rehabilitation. Europa Medicophys. 2006;42:241–255. [PubMed] [Google Scholar]
- 8.Kwakkel G, Kollen BJ, Lindeman E. Understanding the pattern of functional recovery after stroke: facts and theories. Restor Neurol Neurosci. 2004;22:281–299. [PubMed] [Google Scholar]
- 9.Teasell R, Mehta S, Pereira S, et al. Time to rethink long-term rehabilitation management of stroke patients. Top Stroke Rehabil. 2012;19:457–462. doi: 10.1310/tsr1906-457. [DOI] [PubMed] [Google Scholar]
- 10.Ward NS, Brander F, Kelly K. Intensive upper limb neurorehabilitation in chronic stroke: outcomes from the Queen Square programme. J Neurol Neurosurg Psychiatry. 2019;90:498–506. doi: 10.1136/jnnp-2018-319954. [DOI] [PubMed] [Google Scholar]
- 11.Ballester BR, Maier M, Duff A, et al. A critical time window for recovery extends beyond one-year post-stroke. J Neurophysiol. 2019;122:350–357. doi: 10.1152/jn.00762.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nudo RJ. Postinfarct cortical plasticity and behavioral recovery. Stroke. 2007;2:840–845. doi: 10.1161/01.STR.0000247943.12887.d2. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Fornells A, Rojo N, Amengual JL, Ripolles P, Altenmuller E, Munte TF. The involvement of audio-motor coupling in the music-supported therapy applied to stroke patients. Ann N Y Acad Sci. 2012;1252:282–293. doi: 10.1111/j.1749-6632.2011.06425.x. [DOI] [PubMed] [Google Scholar]
- 14.Park JH. The effects of modified constraint-induced therapy combined with mental practice on patients with chronic stroke. J Phys Ther Sci. 2015;27:1585–1588. doi: 10.1589/jpts.27.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thaut MH, Kenyon GP, Hurt CP, McIntosh GC, Hoemberg V. Kinematic optimization of spatiotemporal patterns in paretic arm training with stroke patients. Neuropsychologia. 2002;40:1073–1081. doi: 10.1016/s0028-3932(01)00141-5. [DOI] [PubMed] [Google Scholar]
- 16.Kim J-r, Jung M-y, Yoo E-y, Park J-H, Kim S-H, Lee J. Effects of rhythmic auditory stimulation during hemiplegic arm reaching in individuals with stroke: an exploratory study. Hong Kong J Occup Ther. 2014;24:64–71. [Google Scholar]
- 17.Thaut MH, Hoemberg V, editors. Handbook of neurologic music therapy. Oxford University Press; Oxford, UK: 2014. [Google Scholar]
- 18.Thaut MH, Leins AK, Rice RR, et al. Rhythmic auditory stimulation improves gait more than NDT/Bobath training in near-ambulatory patients early poststroke: a single-blind, randomized trial. Neurorehabil Neural Repair. 2007;21:455–459. doi: 10.1177/1545968307300523. [DOI] [PubMed] [Google Scholar]
- 19.McIntosh GC, Brown SH, Rice RR, Thaut MH. Rhythmic auditory-motor facilitation of gait patterns in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 1997;62:22–26. doi: 10.1136/jnnp.62.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaffert N, Janzen TB, Mattes K, Thaut MH. A review on the relationship between sound and movement in sports and rehabilitation. Front Psychol. 2019;10:244. doi: 10.3389/fpsyg.2019.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardwick RM, Caspers S, Eickhoff SB, Swinnen SP. Neural correlates of action: comparing meta-analyses of imagery, observation, and execution. Neurosci Biobehav Rev. 2018;94:31–44. doi: 10.1016/j.neubiorev.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Page SJ, Levine P, Khoury JC. Modified constraint-induced therapy combined with mental practice: thinking through better motor outcomes. Stroke. 2009;40:551–554. doi: 10.1161/STROKEAHA.108.528760. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh YW, Wi CY, Lin KC, Chang YF, Chen CL, Liu JS. Responsiveness and validity of three outcome measures of motor function after stroke rehabilitation. Stroke. 2009;40:1386–1391. doi: 10.1161/STROKEAHA.108.530584. [DOI] [PubMed] [Google Scholar]
- 24.Schaffert N, Janzen TB, Ploigt R, Schlueter S, Vuong V, Thaut MH. Development and evaluation of a novel music-based therapeutic device for upper extremity movement training: a pre-clinical, single-arm trial. PLoS One. 2020;15 doi: 10.1371/journal.pone.0242552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McAuley JD, Henry MJ, Tkach J. Tempo mediates the involvement of motor areas in beat perception. Ann N Y Acad Sci. 2012;1252:77–84. doi: 10.1111/j.1749-6632.2011.06433.x. [DOI] [PubMed] [Google Scholar]
- 26.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 27.Gladstone DJ, Danells CJ, Black SE. The Fugl-Meyer assessment of motor recovery after stroke: A critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16:232–240. doi: 10.1177/154596802401105171. [DOI] [PubMed] [Google Scholar]
- 28.Wolf SL, Lecraw DE, Barton LA, Jann BB. Forced use of hemiplegic upper extremities to reverse the effect of learned nonuse among chronic stroke and head-injured patients. Exp Neurol. 1989;104:125–132. doi: 10.1016/s0014-4886(89)80005-6. [DOI] [PubMed] [Google Scholar]
- 29.Morris DM, Uswatte G, Crago JE, Cook EW, Taub E. The reliability of the Wolf Motor Function Test for assessing upper extremity function after stroke. Arch Phys Med Rehabil. 2001;82:750–755. doi: 10.1053/apmr.2001.23183. [DOI] [PubMed] [Google Scholar]
- 30.Taub E, McCulluch K, Uswatte G, Morris DM. Motor activity log (MAL) manual. 2011. Available at: https://www.uab.edu/citherapy/images/pdf_files/CIT_Training_MAL_manual.pdf. Accessed November 5, 2017.
- 31.Uswatte G, Taub E, Morris D, Light K, Thompson PA. The Motor Activity Log-28: assessing daily use of the hemiparetic arm after stroke. Neurology. 2006;67:1189–1194. doi: 10.1212/01.wnl.0000238164.90657.c2. [DOI] [PubMed] [Google Scholar]
- 32.Verheyden G, Nieuwboer A, Mertin J, Preger R, Kiekens C, De Weerdt W. The Trunk Impairment Scale: a new tool to measure motor impairment of the trunk after stroke. Clin Rehabil. 2004;18:326–334. doi: 10.1191/0269215504cr733oa. [DOI] [PubMed] [Google Scholar]
- 33.Weissgerber TL, Garovic VD, Savic M, Winham SJ, Milic NM. From static to interactive: transforming data visualization to improve transparency. PLoS Biol. 2016;14 doi: 10.1371/journal.pbio.1002484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Page SJ, Fulk GD, Poyne P. Clinically important differences for the Upper-Extremity Fugl-Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Phys Ther. 2012;92:791–798. doi: 10.2522/ptj.20110009. [DOI] [PubMed] [Google Scholar]
- 35.Lin KC, Hsieh YW, Wu CY, Chen CL, Jang Y, Liu JS. Minimal detectable change and clinically important difference of the Wolf Motor Function Test in stroke patients. Neurorehabil Neural Repair. 2009;23:429–434. doi: 10.1177/1545968308331144. [DOI] [PubMed] [Google Scholar]
- 36.Grush R. The emulation theory of representation: motor control, imagery, and perception. Behav Brain Sci. 2004;27:377–442. doi: 10.1017/s0140525x04000093. [DOI] [PubMed] [Google Scholar]
- 37.Thaut MH. Entrainment and the motor system. Mus Ther Perspect. 2013;31:31–34. [Google Scholar]
- 38.Crasta JE, Thaut MH, Anderson CW, Davies PL, Gavin WJ. Auditory priming improves neural synchronization in auditory-motor entrainment. Neuropsychologia. 2018;117:102–112. doi: 10.1016/j.neuropsychologia.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 39.Braun Janzen T, Thaut MH. In: The Oxford handbook of music and the brain. Thaut MH, Hodges DA, editors. Oxford University Press; Oxford: 2019. Cerebral organization of music processing. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.