Highlights
-
•
At-risk women receiving online skin self-examination education initiated mole checks.
-
•
Women receiving skin self-examination education sought health care when necessary.
-
•
Provided education increased confidence in mole checking and did not affect anxiety.
-
•
Women trained to do skin checks had fewer appointments for benign mole than controls.
-
•
Detection of early melanomas was facilitated by skin self-examination education.
Keywords: Melanoma, Screening, COVID-19, Rural, Women, Randomized controlled trial, Telemedicine, Skin self-examination, Online patient education
Abbreviations: EMR, Electronic medical record; HCP, Health care practitioner; n/a, not applicable; PLA, Pigmented Lesion Assay; RCT, Randomized controlled trial; SSE, Skin self-examination; US, United States
Abstract
Secondary melanoma prevention remains crucial to reduce morbidity and mortality for the 200,000 people in the United States estimated to develop melanoma in 2021. This 3-month randomized controlled trial of online skin self-examination (SSE) education among 1000 at-risk women who received care at Northwestern Medicine in Illinois sought to determine SSE initiation and monthly performance, SSE anxiety and confidence, and health care practitioner (HCP) visits for concerning moles. Positive responses to a personal history of sunburn, a personal or family history of skin cancer, and/or having 10 or more lifetime indoor tanning sessions identified and informed women of their increased risk of melanoma. At one month, 96.2% of women receiving SSE education (SSE women) initiated SSE compared to 48.1% in the active control arm (control) (p < 0.001). More control women sought HCP visits (n = 107) than SSE women (n = 39). Control women seen by HCPs identified benign lesions, especially seborrheic keratosis, more often than SSE women. More atypical nevi (SSE 38.5%, control 8.4%) and melanomas (SSE 25.6%, control 4.7%) were visually identified by SSE women seeing HPCs (p < 0.001). There was no significant difference in SSE anxiety between the control and SSE arms. Confidence increased significantly in the SSE arm whereas there was no change in the control group (p < 0.001). Women checked someone else for concerning moles [315/ 494 (63.8%) of SSE women]. Targeting at-risk women for SSE education may help reduce melanoma mortality, especially in rural communities where incidence and mortality are greater than in urban areas.
1. Introduction
Early detection of melanoma remains crucial in reducing morbidity and mortality. Melanoma is the sixth most common cause of cancer in women in the United States with an estimated 100,350 new invasive cases, about 100,000 cases of melanoma in situ, and 6850 deaths in 2020 (Siegel et al., 2020). The visible pre-invasive phase of some melanomas makes them amenable to early detection via visual skin inspection by laypersons’, skin self-examination (SSE). Earlier stage diagnosis (thinner tumors), and reduced melanoma-related mortality were associated with SSE, which was performed more often by women than men (Avilés-Izquierdo et al., 2016, Pollitt et al., 2009, Paddock et al., 2016, Robinson et al., 2002).
Previously, we demonstrated women’s recognition of being at-risk to develop melanoma by their positive response to screening questions delivered in-person during screening mammography and SSE performance after receiving a brochure (Robinson et al., 2019, Rzepecki et al., 2017). The remaining research question was will online SSE education targeting at-risk women, who were informed of their risk, result in SSE initiation and monthly performance without excessive utilization of health care resources by women seeking appointments for concerning benign moles in comparison with controls (Geller et al., 2015, Swetter et al., 2012). The COVID-19 pandemic provided an additional impetus for studying online dissemination due to concern that skin cancer may have progressed because people avoided in-person skin cancer screening out of concerns about contracting COVID-19 (Cancino et al., 2020 Oct 29, Czeisler et al., 2020, Asabor et al., 2021). Furthermore, the higher melanoma mortality in rural communities attributed to limited access to dermatologists and socioeconomic barriers to health care may have been exacerbated by the COVID-19 pandemic. (Henley et al., 2017, Caldwell et al., 2016, Glazer et al., 2017, Aneja et al., 2012).
This randomized controlled trial (RCT) was performed among women living in urban, suburban, and rural locations in Illinois during the COVID-19 pandemic. Women had prior access to preventive health care as demonstrated by their having a mammogram in the previous year; however, access to health care providers (HCPs) may have been restricted by the COVID-19 pandemic. Based upon our previous research, we hypothesized that a) greater SSE performance would be observed in those receiving the SSE intervention than in those receiving the active control arm, b) SSE education and performance will not increase anxiety and will increase confidence, and c) HCP visits for benign conditions would occur more among controls than among those receiving the SSE intervention (Robinson et al., 2019, Robinson et al., 2020, Rzepecki et al., 2017). The feasibility of acquiring concerning mole images with a smartphone for telemedicine evaluation and self-sampling for gene assay with adhesive patches for the pigmented lesion assay (PLA) (DermTech, Inc. La Jolla, CA) was examined in the SSE arm (Robinson and Jansen, 2020, Fried et al., 2020).
2. Methods
2.1. Study population
During the COVID-19 pandemic, 1000 women with a history of having a screening mammogram in the past year (Jan 2, 2019 until Feb 28, 2020) were enrolled into either SSE intervention or control arms. From July to September 2020, women were recruited using the Enterprise Data Warehouse of Northwestern University, a repository of patients willing to participate in research obtained by searching the electronic medical records (EMR) of Northwestern Medicine. Stratified recruitment used home zip codes to assure sufficient representation of women living in rural locations in comparison with suburban and urban locations as defined by census tract estimation of how many people live within a 5-mile radius of the person (Shensa et al., 2018, Siegel et al., 2020, Solomon et al., 2020, Swetter et al., 2012, US Census Bureau, 2020). Women were eligible if they a) were adults, who had a screening mammogram in the past year, b) able to read English, c) capable of taking a picture of a mole, d) had internet access, e) were willing to participate in three monthly online surveys and receive monthly text messages to their mobile phone, f) had access to FedEx to receive and send mole self-sampling kits, and g) were willing to allow the research team to access their EMR regarding concerning mole results. Exclusion criteria were a) being under age 18, b) a history of breast cancer or stage 3 or 4 melanoma, and c) previous SSE research participation.
2.2. Study design
Women were recruited for this two-arm, parallel randomized control trial with an email stating the research wanted to determine the helpfulness of SSE education for women who are at-risk for getting melanoma, a deadly skin cancer, by having a personal or family history of skin cancer, several sunburns, and or 10 or more lifetime indoor tanning sessions. If there was no response to the initial recruitment email, then the potential participant received a telephone call. If there was no answer to the telephone call, a scripted voicemail provided a call back number. Women clicked on a link to a survey in the recruitment email that asked them to determine if they were at-risk to develop melanoma based on their responses to the same three items assessing risk provided in the recruitment email and used in the prior study (Rzepecki et al., 2017). The enrolled at-risk women were directed to the electronic database REDCap (Research Electronic Data Capture) to complete a web-based survey (Harris et al., 2009). Women underwent (1:1) randomization in blocks of 4, 6, or 8 to receive the SSE intervention [SSE brochure (see below for further description), monthly text messages, and goal setting] or control intervention materials (healthy living brochure with content devoted to healthy eating, physical activity, and sleep strategies, monthly text messages derived from each content area, and goal setting). Randomization was done within home domicile and stratified by three home domiciles (urban, suburban, rural). The biostatistical team (EG and MK) provided the randomization sequence in REDCap and allocation was concealed to those performing recruitment, electronic medical record (EMR) abstraction, and data analysis.
Participants in both arms received the same schedule of online assessments: the initial baseline survey and 3 monthly surveys (August- December 2020) assessing SSE initiation and monthly follow-up performance, anxiety, and confidence. If concerning mole(s) were found, then participants in both arms entered the location of concerning moles, and whether HCPs appointments were scheduled. Participants in the SSE arm responded to survey items affirming reading the brochure, skin check partner assistance, extent of SSE by selecting among 14 provided body locations, scores for mole features and location for one mole per month, and management decisions (Supplementary Table 1). Participants’ EMRs with scheduled HCPs appointments for concerning mole(s) were abstracted for clinical diagnosis and pathology for eight weeks after the final online survey (December 2020- Feb 2021).
The Northwestern University Institutional Review Board approved the research protocol. Participants provided written consent and were offered a $65 gift card after completing the final survey.
2.3. Skin self-examination intervention
The SSE brochure summarized border, color, and diameter scores each as 1 if normal, 2 if unsure, and 3 if abnormal (Robinson et al., 2014, Robinson et al., 2016) (Supplementary Fig. SSE brochure). The decision about seeking healthcare for a concerning mole was based upon the sum of the preceding scores: a) 3 = benign, stop checking the mole; b) 4–7 = check the mole in one month; and c) 8–9 = schedule HCP appointment to have the mole checked in 2–3 weeks (Robinson et al., 2016, Robinson et al., 2020). Women chose among the following options: a) watch the mole for change(s) monthly, b) upload a picture of the mole and have the dermatologist (JKR) recommend taking a sample at home with the PLA kit containing four adhesive patches to apply to the mole’s surface or c) schedule an HCP appointment to check the mole. Participants received instructions for taking pictures of moles with their smartphones. Store-and-forward teledermatology reduced the need for broadband internet access that was constrained in rural communities (Solomon et al., 2020).
The dermatologist (JKR) reviewed the submitted pictures and determined if the sharpness of the focus and lighting were adequate to assess the concerning lesion, and informed participants if the mole was benign or concerning. When the dermatologist determined the mole was concerning for melanoma, a PLA kit was sent to their residence by FedEx (DermTech, Inc. La Jolla, CA.). Participants reviewed the directions contained in the kit and, if needed, the dermatologist was available via Zoom to supervise non-invasive sample acquisition with the four provided adhesive patches. Participants received genomic analysis results and care recommendations from the dermatologist.
Three SSE intervention steps provided the antecedents to behavioral change defined by Green et al. (2005): 1) Women identified their predisposing risk factors in their responses to screening questions in the recruitment email and during registration. 2) The brochure provided the following enabling factors: color illustrations for a) scoring the features of the mole and b) practicing skin checks with a partner. 3) Reinforcement was provided through three monthly text reminders to perform SSE and score the mole’s features. After the first month, a goal was selected from those offered (Supplementary text).
2.4. Outcomes
The primary outcome was initiation and monthly follow-up SSE performance. Components of SSE were extent of body surface examined, partner assistance with mole examination, scoring features and location of the mole, and management decision regarding seeking care for a concerning mole. We transformed the extent of the body surface examined alone or with a partner into a single, scalar (0–14) outcome by assigning a point for each body surface examined (Supplementary table 1. Outcome Measures). If SSE was not performed, the reasons for not doing SSE were selected from the provided list.
The secondary outcomes, SSE anxiety and confidence, were assessed by all participants responding to 6 items assessing SSE anxiety (Likert scale sum 6–30) (Shensa et al., 2018) and seven confidence items (Likert scale sum 7–35) with five assessing self-efficacy for perceived ability to identify concerning moles (Robinson et al., 2008, Robinson et al., 2016) and two assessing the ability to carry out SSE. Anxiety and confidence were transformed into a single scale. Covariates of intervention effects (SSE performance/extent, and HCP appointment) by demographic characteristics e.g. age, household income, health insurance, domicile location, skin type, and risk factors, etc. were examined.
The tertiary outcome was the ratio of benign to malignant lesions (as defined in Table 4) identified by participants and diagnosed by HCPs, which was obtained from the EMR. An exploratory analysis was the location of the concerning moles. For the SSE intervention group addtional exploratory analyses of included the ability of the dermatologist to make a recommendation based on the submitted picture of the concerning mole (sharpness of focus and lighting of the submitted photograph) and obtaining a usable sample of the concerning mole, and checking the moles of others.
Table 4.
Clinical and pathological diagnoses of concerning moles abstracted from participants’ electronic medical records.
| Variable | Skin self-examination n = 494 | Control n = 495 | p-value (chi-square) |
|---|---|---|---|
| Physician appointments | 39 | 107 | n/a |
| Clinical diagnosis with visual inspection (%) | <0.001 | ||
| Benign nevus | 12 (30.8) | 38 (35.5) | |
| Seborrheic keratosis | 0 (0) | 40 (37.4) | |
| Cherry angioma | 0 (0) | 6 (5.6) | |
| Lentigo | 1 (2.6) | 8 (7.5) | |
| Dermatofibroma | 1 (2.6) | 1 (0.9) | |
| Atypical (dysplastic) nevus | 15 (38.5) | 9 (8.4) | |
| Melanoma | 10 (25.6) | 5 (4.7) | |
| Biopsy performed (%) | <0.001 | ||
| No | 17 (43.6) | 93 (86.9) | |
| Yes | 22 (56.4) | 14 (13.1) | |
| Pathologic diagnosis of skin biopsy (%) | 0.459 | ||
| Benign nevus | 0 | 1 (7.1) | |
| Seborrheic keratosis | 0 | 0 | |
| Cherry angioma | 0 | 0 | |
| Lentigo | 0 | 0 | |
| Dermatofibroma | 0 | 0 | |
| Atypical (dysplastic) nevus | 13 (59.1) | 6 (42.9) | |
| Melanoma | |||
| Melanoma in situ (Stage 0) | 5 (22.7) | 3 (21.4)* | |
| Stage 1A melanoma | 4 (18.2) | 3 (21.4) | |
| Stage 1B melanoma | 0 | 1 (7.1) | |
| Visual inspection ratio of benign: atypical nevi + melanoma | 14/25 (0.56) | 93/14 (6.64) | <0.001 |
One lesion diagnosed clinically by visual inspection as an atypical nevus was determined to be a melanoma in situ by the dermatopathologist.
2.5. Statistical analysis
To detect a difference between 80% of SSE in the control group and 86.6% in the intervention group using an independent test of proportions at a type I error rate of 5% and 80% power, a total sample size of 1000 was required. Additionally, using a McNemar’s test, samples of 500 per group provided 80% power to detect uptake of SSE of 7%, while 3% cease SSE when comparing baseline to follow-up. Since previous data suggested that approximately 14% of women should contact HCP for an appointment, our sample of 1000 women provided 80% power to detect a rate in the control group as small as 21% (Robinson et al., 2019).
Demographic characteristics were summarized using median and interquartile range (IQR) for age, and counts and percentages for other characteristics, including risk factors. The primary outcome, SSE performance was compared at each time between groups using a chi-square test of proportions, to assess the consistency of exams over time, McNemar’s test of paired proportions was used. Extent of SSE performance was compared using Wilcoxon rank-sum tests. The impact of demographic and risk factors on performance of SSE and extent of SSE was examined by testing main effects of those factors using generalized linear models with logit link (SSE), or log link (extent of SSE), after adjusting for randomization group. These potential covariates included age, skin type, income, domicile location, history of skin cancer, history of sunburn, history of tanning, and family history of skin cancer. If a main effect was detected, further models were fit to determine if the covariate moderated the association between randomization group and outcome by including interactions. Estimated anxiety and confidence regarding SSE were compared across time points using repeated measures analysis of variance (RM-ANOVA) models, and reporting using means and SDs. All analyses were run using R 3.6.0 (Vienna, Austria), at a nominal type I error rate of 5% (R core team 2020).
3. Results
3.1. Participant characteristics
Among the 1000 randomized women, 804 completed the study (80.4% retention) (Fig. 1). There was no difference in demographic characteristics between the two arms (Table 1). A personal history of sunburn (SSE intervention 98.4%; control 99.2%) was the greatest identified risk, followed by a personal history of indoor tanning (55.1%; 58.0%), and a family history of skin cancer (48.1%; 51.5%).
Fig. 1.
CONSORT Study Flow Diagram. CONSORT indicates Consolidated Standards of Reporting Trials.
Table 1.
Description of the population.
| Variable | Skin self-examination n = 494 |
Control n = 495 |
|---|---|---|
| Age (median [IQR]), y | 47.0 [41.2, 54.0] | 47.0 [41.0, 53.5] |
| Race (%) | ||
| White | 485 (96.8) | 480 (96.2) |
| Asian | 3 (0.6) | 3 (0.6) |
| African American | 1 (0.2) | 1 (0.2) |
| Mixed race | 1 (0.2) | 5 (1.0) |
| Prefer not to answer | 3 (0.6) | 6 (1.2) |
| Ethnicity (%) | ||
| Hispanic | 37 (7.5) | 36 (7.3) |
| Non-Hispanic | 457 (92.5) | 459 (92.7) |
| Highest level of education (%) | ||
| Some high school | 3 (0.6) | 2 (0.4) |
| High school graduate | 22 (4.5) | 28 (5.7) |
| Some post-high school education | 69 (14.0) | 63 (12.7) |
| College graduate | 215 (43.5) | 213 (43.0) |
| Graduate degree | 185 (37.4) | 189 (38.2) |
| Occupational status (%) | ||
| Work part-time | 81 (16.4) | 81 (16.4) |
| Work full-time | 277 (56.1) | 284 (57.4) |
| Unemployed | 35 (7.1) | 36 (7.3) |
| Student | 3 (0.6) | 2 (0.4) |
| Retired | 31 (6.3) | 37 (7.5) |
| Disabled | 4 (0.8) | 6 (1.2) |
| Homemaker | 63 (12.8) | 49 (9.9) |
| Annual household income (%) (US $) | ||
| 10–19,999 | 4 (0.8) | 8 (1.6) |
| 20–34,999 | 16 (3.2) | 23 (4.6) |
| 35–50,999 | 50 (10.1) | 50 (10.1) |
| 51–100,000 | 96 (19.4) | 76 (15.4) |
| Over 100,000 | 251 (50.8) | 259 (52.3) |
| Prefer not to answer | 77 (15.6) | 79 (16.0) |
| Health insurance (%) | ||
| Private/ other insurance | 437 (88.5) | 425 (85.9) |
| Medicaid/Medicare | 38 (7.7) | 46 (9.3) |
| No insurance | 19 (3.8) | 24 (4.8) |
| Location of home (%) | ||
| Urban | 227 (45.3) | 227 (45.5) |
| Suburban | 121 (24.2) | 121 (24.2) |
| Rural | 153 (30.5) | 151 (30.3) |
| Skin type (%) | ||
| I (always sunburn, never tan) | 29 (5.9) | 22 (4.4) |
| II (usually sunburn, tan minimally) | 213 (43.1) | 230 (46.5) |
| III (sometimes sunburn, tan moderately) | 216 (43.7) | 200 (40.4) |
| IV (rarely sunburn, tan deeply) | 36 (7.3) | 43 (8.7) |
| Risk factors | ||
| Personal history of skin cancer | ||
| No | 420 (85.0) | 435 (87.8) |
| Yes | 74 (15.0) | 60 (12.2) |
| Personal history of ever getting a sunburn | ||
| No | 8 (1.6) | 4 (0.8) |
| Yes | 486 (98.4) | 491 (99.2) |
| Personal history of 10 or more indoor tanning sessions in a lifetime | ||
| No | 222 (44.9) | 208 (42.0) |
| Yes | 272 (55.1) | 287 (58.0) |
| Family history of skin cancer | ||
| No | 256 (51.9) | 240 (48.5) |
| Yes | 238 (48.1) | 255 (51.5) |
Data reported by participants prior to randomization and receiving the intervention, thus, statistical analysis is not needed.
3.2. Comparison of SSE performance by intervention and controls
There was a significant increase in SSE initiation among SSE participants from 43.9% (95% Confidence interval (CI): 39.5%, 48.4%) prior to the intervention to 96.2% (95% CI: 94.1%, 97.7%) in the first month after the intervention (McNemar’s chi-square test with continuity correction = 0.058, df = 1) (Table 2). In comparison with controls, SSE women were two times more likely to perform SSE at one-month (Risk Ratio and 95% CI: (2.00 (1.82, 2.20), chi-square, p < 0.001) and younger women and women with a history of indoor tanning were more likely to initiate SSE (logistic regression parameter (β) estimates (standard errors (SE)): 0.98 (0.009), 1.49 (0.17), respectively; (Likelihood ratio tests, p < 0.05). At 2- or 3-months after the intervention, SSE performance was more likely for those receiving the SSE intervention (β (SE): 4.77 (0.15), Likelihood ratio test, p < 0.001) and in women with a history of skin cancer (β (SE): 1.71 (0.24); Likelihood ratio test, p < 0.05). There were no significant relationships with other covariates (domicile location, income, etc.), nor did age, history of tanning, or history of skin cancer moderate the relationship between randomization group and SSE.
Table 2.
Skin self-examination performance by women randomized to both study arms.
| Variable | Skin self-examination n = 494 |
Control n = 495 |
p-value (chi-Square) |
|---|---|---|---|
| 3 days read the brochure (%) | n/a | ||
| No | 7 (1.4) | n/a | n/a |
| Yes | 487 (98.6) | n/a | n/a |
| Baseline SSE performance | |||
| SSE ever prior to intervention (%)a | n/a | ||
| No | 277 (56.1) | 253 (51.1) | n/a |
| Yes | 217 (43.9) | 242 (48.9) | n/a |
| 1-month performed SSE (%) | |||
| No | 19 (3.8) | 257 (51.9) | <0.001 |
| Yes | 475 (96.2) | 238 (48.1) | |
| Partner assistance | |||
| No | 112 (23.6) | n/a | |
| Yes | 363 (76.4) | n/a | |
| Score of mole(s) identified in the last month | |||
| Did not score mole(s) | 42 (8.8) | n/a | n/a |
| No concerning mole(s) (score 3) | 283 (59.1) | n/a | |
| Found concerning mole(s) (score 4–9) | 150 (31.4) | n/a | |
| Concerning mole identified by general comprehension without scoring | |||
| No concerning mole reported | 282 (62.9) | 174 (73.1) | <0.001 |
| Concerning mole reported | 166 (37.1) | 64 (26.9) | |
| Extent of exam alone or with partner (14 body locations (median [IQR])b (Wilcoxon rank-sum test) | 12 [8,13] | 0 [0,7] | <0.001 |
| Management decision about concerning mole (%)c | |||
| Will make healthcare appointment | 22 (10.3) | n/a | n/a |
| Submitted picture of concerning mole | 50 (23.3) | n/a | n/a |
| Watch mole for change | 142 (66.4) | n/a | n/a |
| Made an appointment with HCP(%) | |||
| No | 139 (88.5) | 20 (31.3) | <0.001 |
| Yes | 18 (11.5) | 44 (68.7) | |
| 2-months performed SSE (%) | |||
| No | 115 (24.0) | 255 (52.9) | <0.001 |
| Yes | 355 (76.0) | 228 (47.1) | |
| Partner assistance | |||
| No | 185 (52.0) | n/a | n/a |
| Yes | 170 (48.0) | ||
| Score of mole(s) identified in the last month | |||
| Did not score mole(s) | 10 (2.8) | n/a | n/a |
| No concerning mole(s) (score 3) | 296 (82.8) | n/a | n/a |
| Found concerning mole(s) (score 4–9) | 51 (14.4) | n/a | |
| Concerning mole identified by general comprehension without scoring | |||
| No concerning mole reported | 305 (85.3) | 175 (78.9) | 0.032 |
| Concerning mole reported | 52 (14.7) | 49 (22.1) | |
| Extent of exam alone or with partner (14 body locations) (median [IQR]) b (Wilcoxon rank-sum test) | 5 [1,11] | 0 [0,8] | <0.001 |
| Management decision about concerning mole (%)c | |||
| Will make healthcare appointment | 3 (5.8) | n/a | n/a |
| Submitted picture of mole | 1 (1.9) | n/a | n/a |
| Watch mole for change | 48 (92.3) | n/a | n/a |
| Made an appointment with HCP (%) | |||
| No | 50 (96.2) | 2 (4.1) | <0.001 |
| Yes | 2 (3.8) | 47 (95.9) | |
| 3-months performed SSE (%) | |||
| No | 42 (10.8) | 203 (49.0) | <0.001 |
| Yes | 348 (89.2) | 211 (51.0) | |
| Partner assistance | |||
| No | 112 (32.5) | n/a | |
| Yes | 233 (67.5) | n/a | |
| Score of mole(s) identified in the last month | |||
| Did not score mole(s) | 127 (36.9) | n/a | n/a |
| No concerning mole(s) (score 3) | 157 (45.6) | n/a | n/a |
| Found concerning mole(s) (score 4–9) | 60 (17.5) | n/a | |
| Concerning mole identified by general comprehension without scoring | |||
| No concerning mole reported | 284 (82.6) | 180 (85.3) | 0.465 |
| Concerning mole reported | 60 (17.4) | 31 (14.7) | |
| Extent of exam alone or with partner (14 body locations) median [IQR])b (Wilcoxon rank-sum test) | 10 [6,12] | 1 [0,9] | <0.001 |
| Management decision about concerning mole (%)c | |||
| Will make healthcare appointment | 12 (20) | n/a | n/a |
| Submitted picture of mole | 12 (20) | n/a | n/a |
| Watch mole for change | 36 (60) | n/a | |
| Made an appointment with HCP | |||
| No | 47 (78.3) | 8 (25.8) | <0.001 |
| Yes | 13 (21.7) | 23 (74.2) | |
P values were determined by chi-square with the exception of extent of exam, which was determined with Wilcoxon rank-sum test.
Reported by participants prior to randomization and receiving the intervention, thus, statistical analysis is not needed.
Reported by participants as body locations checked by either the participant or by their skin check partner.
Participants were allowed one or more choices (e.g. a participant could make a healthcare appointment and submit a picture).
3.3. Three components of SSE performance
3.3.1. Body surface examined alone and with partner assistance
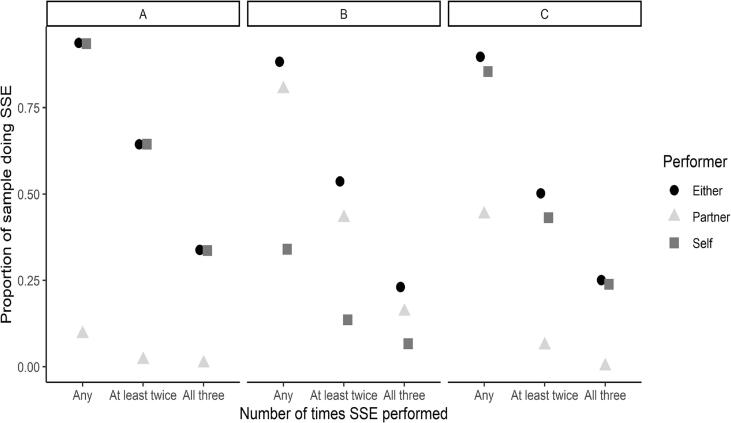
The extent of the body surface checked was greatest at 1 month (12 of 14 possible locations) (Table 2). Self-checking of any location was performed a) at least once by 100% of women, b) 2–3 times by 80% and c) all three times by 60%. Partner assistance in checking any location was performed a) at least once by 80%, b) 2–3 times by 50%, and c) all three times by 25%. Women checked areas they could see alone, e.g., chest, face, anterior neck, arms (Fig. 2A). Partner assistance was greatest in the first month and decreased in subsequent months. Partners assisted with checking the back and shoulders, which was similar to checking the posterior neck and scalp (Fig. 2B). Other locations, e.g. dorsum of feet, were checked more by oneself; however, some women recruited partners to assist (Fig. 2C). No covariates were found to be associated with the extent of SSE at either time point.
Fig. 2.
Proportion of body checked at any time, at least twice, and all three times by self-examination, partner checking, or either. a) Chest, b) Back and shoulders. c) Dorsum of the feet. Any = skin checked at any time during the three months (1, 2 or3 times). At least twice = skin checked 2 or 3 times. All three times = skin checked all 3 times.
3.3.2. Scoring moles and management decisions of women with concerning moles
Among the SSE women, 226 (57.%) elected to follow moles for change(s), 63 submitted pictures (16.2%) and 37 (9.5%) intended to make appointments with HCPs. Concerning moles, for which women scheduled HCP appointments, were scored in the range of 8–9. Concerning moles submitted as pictures were given scores of 4–6 by participants. The 63 concerning mole pictures reviewed by the dermatologist (JKR) were determined to be in focus with sufficient lighting and given scores of 4–6 by the dermatologist. The dermatologist determined that 53 were benign lesions and 10 were clinically suspicious for malignancy
Since it is not always possible to distinguish between atypical nevi and melanoma with visual inspection, biopsies are usually required. In this research, participants used the PLA kit to self-sample the 10 clinically suspicious moles. The PLA test was used to rule-out melanoma because of the high negative predictive value (>99%) (Fried et al., 2020). All 10 PLA specimens were adequate and showed benign lesions.
3.4. Reasons for not doing SSE
The most common reason for not performing SSE was “too busy to do SSE each month”, which was expressed significantly more at two months (88/113; 77%) than at other months (one-month 3/19, 15.8%; three-months 13/42, 31.0% (p < 0.001). Forgetting to perform SSE was the next most frequent reason (45/176, 25.6%). Having regular appointments with a dermatologist (23/174, 13.2%) was the least common reason.
3.5. SSE anxiety and confidence
For the secondary outcome, there was no significant difference in SSE anxiety between the control and SSE arms. The combined mean of anxiety for both arms at baseline was 1.4 [SD 0.5]. The combined mean for all follow-up months was 1.6 [0.5]. The combined confidence mean at baseline was 2.3 [0.5]. Confidence significantly increased to 4.6 [0.4] in the SSE arm whereas there was no change in the control group with a mean of 2.0 [0.5] (chi-square, p < 0.001).
3.6. Ratio of benign to malignant diagnoses by physician visual inspection
Since atypical nevi cannot be distinguished visually from melanoma in situ, the clinical diagnosis of atypical (dysplastic) nevus was grouped with melanoma to report the ratio of benign to malignant diagnosis (the tertiary outcome) (Table 4). More biopsies of clinically concerning atypical nevi were performed in the SSE arm (p < 0.001). The ratio of benign to malignant diagnosis by visual inspection demonstrated a significant difference in HCP visits for benign lesions, especially seborrheic keratosis, among controls (6.64) vs SSE women (0.56) (chi-square, p < 0.001).
4. Exploratory analyses
4.1. Location of concerning moles
The common locations for concerning moles were the back and shoulders (SSE, 22.3%; control 11.1%) and legs (SSE, 19.8%; control 20.1%) (Table 3). Partners’ assistance was demonstrated by participants in the SSE arm finding twice the number of concerning moles on back and shoulders as controls, who were not advised to enlist the help of a partner. Controls identified more concerning moles on locations easily seen without partner assistance: chest and face.
Table 3.
Location of concerning moles identified by women in both study arms*
| Body Location | Skin self-examination n = 278 (%) |
Control n = 144 (%) |
|---|---|---|
| Back and shoulders | 62 (22.3) | 16 (11.1) |
| Legs | 55 (19.8) | 29 (20.1) |
| Arms | 44 (15.8) | 27 (18.8) |
| Chest | 23 (8.3) | 24 (16.7) |
| Face | 23 (8.3) | 20 (13.9) |
| Neck (front) | 23 (8.3) | 13 (9.0) |
| Abdomen | 20 (7.2) | 3 (2.1) |
| Neck (back) | 10 (3.6) | 1 (0.7) |
| Hands | 5 (1.8) | 2 (1.4) |
| Dorsum feet | 4 (1.4) | 4 (2.8) |
| Scalp | 4 (1.4) | 0 (0.0) |
| Ears | 3 (1.1) | 2 (1.4) |
| Buttocks | 2 (0.7) | 2 (1.4) |
| Soles of feet |
|
1 (0.7) |
Participants reported one or more concerning moles in each monthly survey.
4.2. Checking moles of others
In total, 315/494 (63.8%) of SSE women checked someone else for concerning moles, most often during the first month. The spouse was checked most often (287/315, 91.1%), followed by the child (37/315, 11.7%), sibling (5/315, 1.6%), and parent (4/315, 1.3%).
4.3. Submitted pictures of concerning moles
The 63 submitted concerning mole pictures reviewed by the dermatologist (JKR) all had adequate focus and lighting and were interpreted as 53 benign lesions and 10 clinically suspicious for malignancy.
5. Discussion
Melanoma detection was achieved in this study by informing women about their risk of developing melanoma and providing online SSE education (Johansson et al., 2021). Since most melanomas occur in patients age 40 years and older, screening mammography, which also begins at this age, identified women with access to health care, who were screened for their risk of developing melanoma and informed of their risk. (Johnson et al, 2017). Women acted on their perceived risk of developing melanoma by performing SSE without substantially increasing physician appointments and biopsies for benign pigmented lesions in comparison to controls, thus, limiting overdiagnosis (Waldmann et al., 2012, Elder, 2018). Women, who submitted pictures of concerning moles, received feedback through teledermatology that the lesion was benign or potentially malignant. Women with moles deemed appropriate for biopsy performed self-sampling of moles further reducing in-person physician appointments and biopsies. Online recruitment and education in this study reached women who may not have been aware of their risk incurred by their prior indoor tanning. The program did not induce anxiety. Women used the brochure as a resource to check the moles of spouses and relatives.
While there are no national guidelines that recommend population-based skin cancer screening, screening people at-risk for melanoma was endorsed by the US Preventive Services Task Force (Wernli et al., 2016, Henrikson et al., 2018). Growing evidence indicates that time to presentation to health care and initial management of cancer are key determinants of patient outcomes (Neal et al., 2015). Melanoma has one of the longest delays in the median time to presentation for cancer care (Keeble et al., 2014). Melanoma stage is a proxy measure of the delay in presentation for care with a more advanced disease stage representing a longer duration of disease. In this study, cases were detected at an early stage (melanoma in situ or Stage 1A) or atypical nevi, which are benign clinical mimics of melanoma in situ and thin melanoma. Although most in situ and early melanoma will be indolent, approximately 15% of melanoma deaths result from metastases from early melanoma and it is not possible to know the biological behavior for any patient (Shain and Bastian, 2016, Gimotty et al., 2007).
Comparing this research with existing SSE literature was difficult because recent SSE trials focused on melanoma survivors with educational interventions delivered in-person with print (Coroiu et al., 2020, Körner et al., 2013, Robinson et al., 2014, Robinson et al., 2016) or web-based materials (Bowen et al., 2015, Loescher et al., 2010, Coups et al., 2016, Robinson et al., 2020). A 26–30% increase in SSE was reported among melanoma survivors with varying outcome measures of SSE thoroughness. The importance of self-efficacy in mediating SSE among melanoma survivors was demonstrated in two different populations of melanoma survivors; thus, in this study, increased confidence was important to continue SSE performance (Robinson et al., 2008, Coroiu et al., 2020). Prior SSE studies conducted in primary care physician offices of patients without a prior history of melanoma used patient completion of self-assessment of melanoma risk tools in HCPs’ offices, HCP examination, and counseling to demonstrate 20–31.4% improvement in SSE performance in comparison with controls (Rat et al., 2015, Walter et al., 2020, Weinstock et al., 2007). Our study expanded identification of at-risk women beyond HCPs’ offices. To our knowledge, this study is the only online melanoma detection RCT to target at-risk women. We demonstrated a significant 52.3% increase in SSE performance at one month in comparison with controls; however, the extent of SSE declined in subsequent months.
The study has limitations. Screening recruitment letters and consent may have encouraged women to perform SSE, indicating the Hawthorne effect whereby participants modified their behavior in response to being studied (McCambridge et al., 2014). There was less self-sampling of concerning moles than expected because many women elected to follow the mole for change rather than submit a picture. The need for broadband internet access may limit generalizability to rural populations. Furthermore, women in this study had access to health care, which may limit generalizability to the general population. While it was likely that patient outcomes were improved by SSE and early melanoma detection, assessing the effect of the intervention on mortality during the short period of this study was not possible.
6. Conclusion
The key components of the SSE intervention were a) participation of women with access to preventive health care, b) three readily identifiable risks were used to inform women of their increased risk, c) delivery of structured SSE education including management decision rules coupled with risk awareness, and d) teledermatology support. In comparison with controls, women receiving SSE education did not have an increased number of in-person appointments for benign moles and presented for health care early with melanoma in situ and atypical nevi. The feasibility of supporting SSE with teledermatology was demonstrated by participants reliably obtaining images of concerning moles with their own smartphones. With one-fifth of the US population residing in rural areas, but only one-tenth of all physicians practicing in rural areas, remote telehealth care delivery of melanoma detection targeting those at-risk may serve rural populations (Zahnd et al., 2018). If telehealth services remain covered beyond the COVID-19 pandemic and if rural communities gain greater access to broadband internet, incorporating SSE education for at-risk women into women’s health programs may help reduce melanoma mortality in rural communities (Welch et al., 2021).
CRediT authorship contribution statement
June K. Robinson: Conceptualization, Methodology, Supervision, Funding acquisition, Project administration, Writing – original draft, Writing – review & editing. Samer Wahood: Methodology, Investigation, Validation, Writing – review & editing. Sophia Ly: Methodology, Investigation, Validation, Writing – review & editing. Jessie Kirk: Methodology, Investigation, Validation, Writing – review & editing. Jamie Yoon: Investigation, Validation, Writing – review & editing. James Sterritt: Investigation, Data curation, Writing – review & editing. Elizabeth Gray: Software, Formal analysis, Writing – review & editing. Mary Kwasny: Formal analysis, Writing – review & editing.
Acknowledgments
Acknowledgments
The authors declare no conflicts of interest.
Financial interests
This work was supported in part by the National Cancer Institute of the National Institutes of Health [grant number P30CA060553 to the Robert H. Lurie Comprehensive Cancer Center]. This work was also supported by Gail Elden, in memory of Richard Elden.
Pigmented lesion assay testing was provided by DermTech Inc. (La Jolla, CA).
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, [JKR]. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2021.101532.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Self-reported outcome measures.
Choices for skin self-examination goals.
Brochure provided to women randomized to the skin self-examination intervention.
CONSORT checklist.
References
- Aneja S., Aneja S., Bordeaux J.S. Association of increased dermatologist density with lower melanoma mortality. Arch. Dermatol. 2012;148(2):174–178. doi: 10.1001/archdermatol.2011.345. [DOI] [PubMed] [Google Scholar]
- Asabor, E.M., Bunick, C.G., Cohen, J.M., Patient perspectives on teledermatology at an academic dermatology department amid the COVID-19 pandemic. J Am Acad Dermatol. 2021; 84(1): 158-161. doi. 10.1016/j.jaad.2020.09.029. [DOI] [PMC free article] [PubMed]
- Avilés-Izquierdo J.A., Molina-López I., Rodríguez-Lomba E., Marquez-Rodas I., Suarez-Fernandez R., Lazaro-Ochaita P. Who detects melanoma? Impact of detection patterns on characteristics and prognosis of patients with melanoma. J. Am. Acad. Dermatol. 2016;75(5):967–974. doi: 10.1016/j.jaad.2016.07.009. [DOI] [PubMed] [Google Scholar]
- Bowen D.J., Burke W., Hay J.L., Meischke H., Harris J.N. Effects of web-based intervention on risk reduction behaviors in melanoma survivors. J. Cancer Surviv. 2015;9(2):279–286. doi: 10.1007/s11764-014-0412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J.T., Ford C.L., Wallace S.P., Wang M.C., Takahashi L.M. Intersection of living in a rural versus urban area and race/ethnicity in explaining access to health care in the United States. Am. J. Public Health. 2016;106(8):1463–1469. doi: 10.2105/AJPH.2016.303212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancino R.S., Su Z., Mesa R., Tomlinson G., Wang J. The impact of COVID-19 on cancer-screening: challenges and opportunities. JMIR Cancer. 2020;6(20) doi: 10.2196/21697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coroiu A., Moran C., Bergeron C., Drapeau M., Wang B., Kezouh A., Ernst J., Batist G., Körner A. Short and long-term barriers and facilitators of skin self-examination among individuals diagnosed with melanoma. BMC Cancer. 2020;20(1) doi: 10.1186/s12885-019-6476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coups, E.J., Manne, J.L., Stapleton, J.L., et al., Skin self-examination behaviors among individuals diagnosed with melanoma. Melanoma Res. 2016; 26(1): 71-6. doi: 10.1097/CMR.0000000000000204. [DOI] [PubMed]
- Czeisler M.É., Marynak K., Clarke K.E.N., Salah Z., Shakya I., Thierry J.M., Ali N., McMillan H., Wiley J.F., Weaver M.D., Czeisler C.A., Rajaratnam S.M.W., Howard M.E. Delay or avoidance of medical care because of COVID-19-related concerns-United States, June 2020. MMWR. 2020;69(36):1250–1257. doi: 10.15585/mmwr.mm6936a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder D.E. Melanoma screening and mortality. J. Natl. Cancer Inst. 2018;110(10):1135–1136. doi: 10.1093/jnci/djy056. [DOI] [PubMed] [Google Scholar]
- Fried L., Tan A., Bajaj s., et al. Technological advances for detection of melanoma. J. Am. Acad. Dermatol. 2020;83:996–1004. doi: 10.1016/j.jaad2020.03.122. [DOI] [PubMed] [Google Scholar]
- Geller A.C., Swetter S.M., Weinstock M.A. Focus on early detection to reduce melanoma deaths. J. Invest. Dermatol. 2015;135(4):947–949. doi: 10.1038/jid.2014.534. [DOI] [PubMed] [Google Scholar]
- Gimotty P.A., Elder D.E., Fraker D.L., et al. Identification of high-risk patients among those diagnosed with thin cutaneous melanomas. J. Clin. Oncol. 2007;25(9):1129–1134. doi: 10.1200/JCO.2006.08.1463. [DOI] [PubMed] [Google Scholar]
- Glazer, A.M., Farberg, A.S., Winkelmann, R.R., Rigel, D.S., Analysis of trends in geographic distribution and density of US dermatologists. JAMA Dermatol. 2017;153(4):322-5. doi: 10.1001/jamadermatol.2016.5411. [DOI] [PubMed]
- Green L.W., Kreuter M.W. 4th ed. McGraw-Hill; 2005. Health Program Planning: An Educational and Ecological Approach. [Google Scholar]
- Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley S.J., Anderson R.N., Thomas C.C., Massetti G., Peaker B., Richardson L.C. Invasive cancer incidence, 2004–2013, and deaths, 2006–2015, in nonmetropolitan and metropolitan counties-United States. MMWR Morb. Mortal. Wkly Rep. 2017;66(14):1–12. doi: 10.15585/mmwr.ss6614a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrikson N.B., Morrison C.C., Blasi P.R., Nguyen M., Shibuya K.C., Patnode C.D. Behavioral counseling for skin cancer prevention. Evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319(11):1143. doi: 10.1001/jama.2017.21630. [DOI] [PubMed] [Google Scholar]
- Johannson, M., Brodersen, J., Gotzsche, P.C., et al. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database of Systematic Reviews 2019, Issue 6. Art. No.:CD012352 Doi:10.1002/14651858.CD012352.pub2. Accessed Jan 3, 2021. [DOI] [PMC free article] [PubMed]
- Johnson M.M., Leachman S.A., Aspinwall L.G., et al. Skin cancer screening: recommendations for data-driven screening guidelines and a review of the USPHSTF controversy. Melanoma Manag. 2017;4:13–37. doi: 10.2217/mmt-2016-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeble S., Abel G.A., Saunders C.L., et al. Variation in promptness of presentation among 10,297 patients subsequently diagnosed with one of 18 cancers: evidence from a National Audit of Cancer Diagnosis in Primary Care. Int. J. Cancer. 2014;135(5):1220–1228. doi: 10.1002/ijc.v135.510.1002/ijc.28763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner A., Drapeau M., Thombs B.D., et al. Barriers and facilitators of adherence to medical advice on skin self-examination during melanoma follow-up care. BMC Dermatol. 2013;13(1) doi: 10.1186/1471-5945-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loescher L.J., Hibler E., Hiscox H., et al. An Internet-delivered video intervention for skin self-examination by patients with melanoma. Arch. Dermatol. 2010;146(8) doi: 10.1001/archdermatol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCambridge J., Witton J., Elbourne D.R. Systematic review of the Hawthorne effect: new concepts are needed to study research participant effects. J. Clin. Epidemiol. 2014;67(3):267–277. doi: 10.1016/j.jclinepi.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal R.D., Tharmanathan P., France B., et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? systematic review. Br. J. Cancer. 2015;112(S1):S92–S107. doi: 10.1038/bjc.2015.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock LE, Lu SE, Bandera EV, et al. Skin self-examination and long-term melanoma survival. Melanoma Res. 2016; 26:401–8. doi: 10.1097/CMR.0000000000000255. [DOI] [PubMed]
- Pollitt R.A., Geller A.C., Brooks D.R., et al. Efficacy of skin self-examination practices for early melanoma detection. Cancer Epidemiol. Biomarkers Prev. 2009;18:3018–3023. doi: 10.1158/1055-9965. [DOI] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2020. R: A language and environment for statistical computing. https://www.R-project.org/ [Google Scholar]
- Rat C., Quereux G., Grimault C., Gaultier A., Khammari A., Dreno B., Nguyen J.M. Melanoma incidence and patient compliance in a targeted melanoma screening intervention. One-year follow-up in a large French cohort of high-risk patients. Eur. J. Gen. Pract. 2015;21(2):1–7. doi: 10.3109/13814788.2014.949669. [DOI] [PubMed] [Google Scholar]
- Robinson J.K., Fisher S.G., Turrisi R.J. Predictors of skin self-examination. Cancer. 2002;95:135–146. doi: 10.1002/cncr.10637. [DOI] [PubMed] [Google Scholar]
- Robinson J.K., Stapleton J., Turrisi R. Relationship and partner moderator variables increase self-efficacy of performing skin self-examination. J. Am. Acad. Dermatol. 2008;58(5):755–762. doi: 10.1016/j.jaad.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J.K., Gaber R., Hultgren B., Eilers S., Blatt H., Stapleton J., Mallett K., Turrisi R., Duffecy J., Begale M., Martini M., Bilimoria K., Wayne J. Skin self-examination education for early detection of melanoma: a randomized controlled trial of Internet, workbook, and in-person interventions. J. Med. Internet Res. 2014;16(1):e7. doi: 10.2196/jmir.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J.K., Wayne J.D., Martini M.C., Hultgren B.A., Mallett K.A., Turrisi R. Early detection of new melanomas by patients with melanoma and their partners using a structured skin self-examination skills training intervention: a randomized clinical trial. JAMA Dermatol. 2016;152(9):979–985. doi: 10.1001/jamadermatol.2016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, J.K., Perez, M., Abou-el-Seoud, D., et al. Targeted melanoma screening: risk self-assessment and skin self-examination education delivered during mammography of women. JNCI Cancer Spectrum. 2019, 3 (3):1-8. doi: 10.1093/jncics/pkz047. [DOI] [PMC free article] [PubMed]
- Robinson J.K., Reavy R., Mallett K.A., Turrisi R. Remote skin self-examination training of melanoma survivors and their skin check partners: a randomized trial and comparison with in-person training. Cancer Med. 2020;9(19):7301–7309. doi: 10.1002/cam4.v9.1910.1002/cam4.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JK, Jansen B. Caring for melanoma survivors with self-detected concerning moles during the COVID-19 restricted physician access: a cohort study. SKIN: The J Cutaneous Med. 2020; 4(3):248-251. doi.org/10.25251/skin.4.3.5.
- Rzepecki A.K., Jain N., Ali Y., Chavez L., Choi J., Schlosser B., Liko-Hazizi E., Friedewald S.M., Robinson J.K. Promoting early detection of melanoma during the mammography experience. Int. J. Women’s Dermatol. 2017;3(4):195–200. doi: 10.1016/j.ijwd.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shain A.H., Bastian B.C. From melanocytes to melanomas. Nat. Rev. Cancer. 2016;16(6):345–358. doi: 10.1038/nrc.2016.37. [DOI] [PubMed] [Google Scholar]
- Shensa A., Sidani J.E., Dew M.A., Escobar-Viera C.G., Primack B.A. Social media use and depression and anxiety symptoms: a cluster analysis. Am. J. Health Behav. 2018;42(2):116–128. doi: 10.5993/AJHB.42.2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Jemal A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- Solomon, Z.J., Ramachandran, V., Kohn, T.P., et al., The association of broadband internet access with dermatology practitioners: an ecologic study. J Am Acad Dermatol. 2020; 83(6):1767-1770. doi. 10.1016/j.jaad.2020.03.065. [DOI] [PubMed]
- Swetter S.M., Pollitt R.A., Johnson T.M., Brooks D.R., Geller A.C. Behavioral determinants of successful early melanoma detection: Role of self and physician skin examination. Cancer. 2012;118(15):3725–3734. doi: 10.1002/cncr.26707. [DOI] [PubMed] [Google Scholar]
- US Census Bureau. American Community Survey. https://www.census.gov/programs-surveys/acs. Accessed June 2020.
- Waldmann A., Nolte S., Geller A.C., et al. Frequency of excisions and yields of malignant skin tumors in a population-based screening intervention of 360,288 whole-body examinations. Arch. Dermatol. 2012;148:903–910. doi: 10.1001/archdermatol.2012.893. [DOI] [PubMed] [Google Scholar]
- Walter F.M., Pannebakker M.M., Barclay M.E., et al. Effect of skin self-monitoring smartphone application on time to physician consultation among patients with possible melanoma: a phase 2 randomized clinical trial. JAMA Network Open. 2020;3(2) doi: 10.1001/jamanetworkopen.2020.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock M.A., Risica P.M., Martin R.A., et al. Melanoma early detection with thorough skin self-examination: the “Check It Out” randomized trial. Am. J. Prev. Med. 2007;32(6):517–524. doi: 10.1016/j.amepre.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernli K.J., Henrikson N.B., Morrison C.C., Nguyen M., Pocobelli G., Blasi P.R. Screening for skin cancer in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316(4):436–447. doi: 10.1001/jama.2016.5415. [DOI] [PubMed] [Google Scholar]
- Zahnd W.E., James A.S., Jenkins W.D., Izadi S.R., Fogleman A.J., Steward D.E., Colditz G.A., Brard L. Rural-urban differences in cancer incidence and trends in the United States. Cancer Epidemiol. Biomarkers Prev. 2018;27(11):1265–1274. doi: 10.1158/1055-9965.EPI-17-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch H.G., Mazer B.L., Adamson A.S. The rapid rise in cutaneous melanoma diagnosis. N. Eng. J. Med. 2021;384(1):72–79. doi: 10.1056/NEJMsb2019760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Self-reported outcome measures.
Choices for skin self-examination goals.
Brochure provided to women randomized to the skin self-examination intervention.
CONSORT checklist.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, [JKR]. The data are not publicly available due to their containing information that could compromise the privacy of research participants.