Abstract
The pathogenesis of varicella and zoster and the effects of antiviral treatment were investigated using real-time PCR for varicella-zoster virus (VZV) DNA in skin lesions and peripheral blood. A higher occurrence of viremic VZV DNA was observed in varicella than in zoster. Acyclovir treatment resulted in marked suppression of viremia in varicella.
Varicella-zoster virus (VZV) is the causative agent of chicken pox (varicella) and shingles (zoster). In zoster, skin lesions are usually limited to the area of a single dermatome, reflecting restricted transneuronal spread. In varicella, however, VZV is disseminated via a hematogenous route. The viremic phase of VZV is not fully understood because a very small proportion of circulating peripheral blood cells carries the virus (1, 2, 5). Recently, we established a quantitative system to measure genome copies of VZV using a real-time PCR assay (4). This assay enables us to quantify VZV DNA accurately and reproducibly over a wide linear range (4). To further understand the nature of viremia and the impact of antiviral treatments, we quantified viral loads in the peripheral blood and skin lesions of patients with varicella using two real-time PCR assays and compared the results with those for patients with zoster.
Nineteen children with clinically diagnosed varicella (1 to 7 years old; median, 3 years) were enrolled in this study. All but one were otherwise healthy children. At the time of entry, six of the patients had already received oral acyclovir therapy, which was started at 80 mg/kg of body weight/day within 24 h of onset and continued for at least 48 h. Ten patients with zoster (2 to 15 years old; median, 8.5 years) were also enrolled. Five were healthy children, and the others had underlying diseases, such as leukemia. Three had already received acyclovir therapy at the time of entry. As presumptive negative controls, 28 children who had had either varicella or vaccination at least 6 months previously were tested.
All the specimens were obtained after informed consent. Blood samples were taken during the acute phase, when the patients had clinical indications. The numbers of days from onset to sampling were 2 to 5 days (mean, 3.5 days) in the varicella group and 2 to 7 days (mean, 3.6 days) in the zoster group. Vesicle fluid was obtained from three patients each with varicella and zoster. A vesicle with a 4-mm diameter was punctured, and all the fluid was aspirated. DNA was extracted from either 2 × 106 peripheral blood mononuclear cells (PBMC) or the vesicle fluid using a QIAamp Blood Kit (QIAGEN GmbH, Hilden, Germany). The real-time quantitative PCR assay was performed by using a TaqMan PCR Kit and a model 7700 Sequence Detector (PE Applied Biosystems, Foster City, Calif.) as previously described (4). Two genes, one encoding glycoprotein B (gB) and ORF62, were selected for quantification (7). To normalize the amount of VZV DNA in PBMC, human β-actin DNA was quantified by real-time PCR, and then the numbers of copies of VZV genomes were determined using the gB and ORF62 assays and were expressed per 105 cells (7). VZV DNA in vesicle fluid was determined using the gB and ORF62 assays and was expressed per milliliter of vesicle fluid.
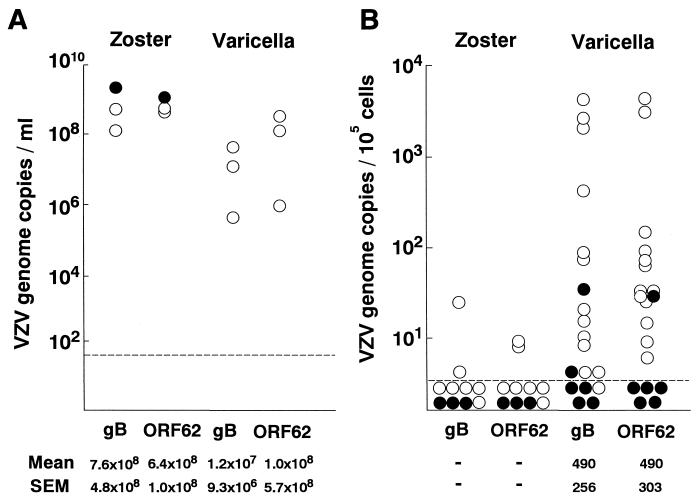
First, we quantified the viral loads in skin lesions of three patients each with varicella and zoster. In both patient groups, VZV DNA was readily detected (Fig. 1A). A large amount of VZV DNA was detected even in a patient with zoster who was receiving acyclovir therapy at the time of sampling. Then, we quantified the viral loads in PBMC from 19 patients with varicella and 10 with zoster in the acute phase. VZV DNA was detected in PBMC from only two patients with zoster, both of whom had no indications of being immunocompromised (Fig. 1B). PBMC from 28 control children were negative for VZV DNA. In contrast, most varicella patients had detectable VZV DNA in their PBMC (Fig. 1B). Of the six varicella patients receiving oral acyclovir therapy, four were negative for the gB gene (P = 0.01, in comparison with the results for untreated patients; Fisher's exact test) and five were negative for the ORF62 gene (P = 0.0004). Since the distributions of sampling days were somewhat different between the treated and untreated groups, we selected patients whose samples were obtained from days 3 to 5 and compared the viral loads in PBMC. The number of VZV genome copies in the untreated group was significantly larger than that in the acyclovir-treated group (Table 1). These results suggest that the acyclovir therapy suppressed the viremia in these patients.
FIG. 1.
Quantitation of VZV DNA in vesicles and PBMC. Copies of VZV genomes determined using the gB and ORF62 genes were quantified by real-time PCR. The means and standard errors of the means (SEM) of VZV genome copies in the PCR-positive samples are tabulated below the graphs. (A) Vesicle fluid obtained from three patients with zoster or varicella. (B) PBMC obtained from 10 patients with zoster and 19 patients with varicella. Symbols: open circles, patients without acyclovir therapy; closed circles, patients with acyclovir therapy. Samples below the dotted line were negative for VZV DNA.
TABLE 1.
Comparison of viral loads in PBMC between acyclovir-treated and untreated groups
| Treatment | No. of patientsa | Days after onset (mean ± SEM) | No. of VZV genomes (mean ± SEM/105 cells) determined with:
|
|
|---|---|---|---|---|
| gB gene | ORF62 | |||
| Acyclovir | 5 | 4.0 ± 0.3 | 7 ± 7b | 5 ± 5c |
| None (untreated) | 10 | 3.7 ± 0.3 | 510 ± 354b | 382 ± 339c |
Patients whose samples were obtained from days 3 to 5.
P = 0.04, as determined by the Mann-Whitney U test.
P = 0.008, as determined by the Mann-Whitney U test.
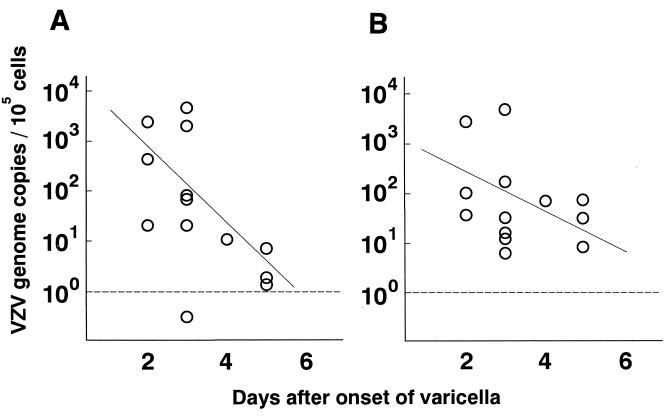
The amount of VZV DNA determined using the gB gene was plotted against the day after onset (Fig. 2A). The days after onset and the VZV DNA copy numbers were inversely correlated (r = 0.72, P < 0.01). The same plotting was done with the ORF62 gene (Fig. 2B). The VZV DNA copy number decreased with time, although the correlation was not statistically significant (r = 0.32, P = 0.28). No specimens were available between days 6 and 14; however, later in the convalescent phase, from 14 to 24 days after onset, PBMC were obtained from seven postvaricella patients. Significant levels of VZV DNA were not detected in these samples. These results show that VZV DNA was generally cleared by 14 days after the onset of varicella in our patients.
FIG. 2.
Correlation between viral loads and days after onset in patients with varicella. VZV genome copies in PBMC from 13 patients not receiving acyclovir were determined using the gB (A) and ORF62 (B) genes and plotted against the day after the onset of varicella. The correlation between days after onset and the amounts of VZV DNA was calculated using regression analysis. Samples below the dotted line were negative for VZV DNA.
In this study, VZV DNA was detectable in PBMC from most patients with varicella. On the other hand, most patients with zoster lacked detectable VZV DNA in PBMC, despite the presence of high levels of VZV genomes in vesicular fluid. Recently, it was reported that 16% of patients with zoster had detectable VZV DNA in their blood (6). We quantified VZV DNA using two independent real-time PCR assays which offer increased sensitivity and accuracy compared with previous assays. Our results suggest that viremia, if present in zoster, does not occur with the same kinetics or magnitude as in varicella.
We showed that varicella patients receiving oral acyclovir therapy had undetectable or very small amounts of VZV DNA in their PBMC. Early administration of acyclovir reduces both the time to the cessation of new vesicle formation and the maximum number of skin lesions, suggesting that therapy suppresses viremia (3). Our results indicate that acyclovir therapy reduces the viral load in the peripheral blood of patients and thereby ameliorates varicella.
Acknowledgments
We thank Stephen E. Straus, National Institutes of Health, Bethesda, Md., for helpful suggestions.
This work was supported by a grant from the Japan Society for the Promotion of Science (JSPS-RFTF97L00703).
REFERENCES
- 1.Arvin A M. Varicella-zoster virus. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Straus S E, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2547–2585. [Google Scholar]
- 2.Asano Y, Itakura N, Kajita Y, Suga S, Yoshikawa T, Yazaki T, Ozaki T, Yamanishi K, Takahashi M. Severity of viremia and clinical findings in children with varicella. J Infect Dis. 1990;161:1095–1098. doi: 10.1093/infdis/161.6.1095. [DOI] [PubMed] [Google Scholar]
- 3.Dunkle L M, Arvin A M, Whitley R J, Rotbart H A, Feder H, Jr, Feldman S, Gershon A A, Levy M L, Hayden G F, McGuirt P V, Harris J, Balfour H H. A controlled trial of acyclovir for chickenpox in normal children. N Engl J Med. 1991;325:1539–1544. doi: 10.1056/NEJM199111283252203. [DOI] [PubMed] [Google Scholar]
- 4.Kimura H, Wang Y, Pesnicak L, Cohen J I, Hooks J J, Straus S E, Williams R K. Recombinant varicella-zoster virus glycoproteins E and I: immunologic responses and clearance of virus in a guinea pig model of chronic uveitis. J Infect Dis. 1998;178:310–317. doi: 10.1086/515638. [DOI] [PubMed] [Google Scholar]
- 5.Koropchak C M, Solem S M, Diaz P S, Arvin A M. Investigation of varicella-zoster virus infection of lymphocytes by in situ hybridization. J Virol. 1989;63:2392–2395. doi: 10.1128/jvi.63.5.2392-2395.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mainka C, Fuss B, Geiger H, Hofelmayr H, Wolff M H. Characterization of viremia at different stages of varicella-zoster virus infection. J Med Virol. 1998;56:91–98. doi: 10.1002/(sici)1096-9071(199809)56:1<91::aid-jmv15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Pevenstein S R, Williams R K, McChesney D, Mont E K, Smialek J E, Straus S E. Quantitation of latent varicella-zoster virus and herpes simplex virus genomes in human trigeminal ganglia. J Virol. 1999;73:10514–10518. doi: 10.1128/jvi.73.12.10514-10518.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]