Highlights
-
•
CEDI research has the potential to improve health inequities.
-
•
QuitSMART Utah used a multi-component community engagement process.
-
•
The partnership had shared goals, resources, data, and mutual respect of expertise.
Keywords: Community-engaged research, Research-practice partnerships, Health equity, Implementation science
Abstract
Community engagement is critical to accelerate and improve implementation of evidence-based interventions to reduce health inequities. Community-engaged dissemination and implementation research (CEDI) emphasizes engaging stakeholders (e.g., community members, practitioners, community organizations, etc.) with diverse perspectives, experience, and expertise to provide tacit community knowledge regarding the local context, priorities, needs, and assets. Importantly, CEDI can help improve health inequities through incorporating unique perspectives from communities experiencing health inequities that have historically been left out of the research process. The community-engagement process that exists in practice can be highly variable, and characteristics of the process are often underreported, making it difficult to discern how engagement of community partners was used to improve implementation. This paper describes the community-engagement process for a multilevel, pragmatic randomized trial to increase the reach and impact of evidence-based tobacco cessation treatment among Community Health Center patients; describes how engagement activities and the resulting partnership informed the development of implementation strategies and improved the research process; and presents lessons learned to inform future CEDI research.
1. Introduction
Implementation and sustainment of multilevel evidence-based interventions (EBIs) has the potential to improve population health and reduce health inequities (Paskett et al., 2016). However, implementation of EBIs can be challenging and often requires coordination across stakeholders from multiple levels within an organization (e.g., healthcare system leadership, clinicians, patients) and across organizations (e.g., healthcare systems, public health systems, academic systems) (Glasgow et al., 1999, Glasgow et al., 2004, Green et al., 2009). Furthermore, adapting EBIs to fit the local context has been promoted for successful EBI implementation, and the engagement of individuals with tacit community knowledge is critical to the adaptation process (Chambers and Norton, 2016). Consequently, successful implementation of EBIs is dependent on a range of factors including effective engagement and collaboration among a diverse group of stakeholders.
Numerous disciplines and methods (e.g., action research, community-based participatory research [CBPR], team science, community engagement) (Wallerstein et al., 2017, Minkler and Wallerstein, 2011, Wallerstein and Duran, 2006, Stokols et al., 2008, Emmons et al., 2008, Lewin, 1946) have emphasized the importance of incorporating multiple stakeholder perspectives in research and practice to solve complex problems like those of implementing EBIs. Action research (Lewin, 1946) emphasized the importance of group participation and partnership to solve societal problems dating back to the 1930s. Community-based participatory research (CBPR) is a “collaborative approach to research” that emphasizes equitable sharing of power between academic and community partners (Minkler and Wallerstein, 2011, Wallerstein and Duran, 2006, Wallerstein and Duran, 2010). Team science is transdisciplinary research that emphasizes bringing together collaborators with a “combined set of expertise that is uniquely suited to address particular scientific problems in innovative and effective ways.” (Stokols et al., 2008, Vogel et al., 2013, Stokols, 2006) Community engagement is broadly defined as “the process of working collaboratively with and through groups of people affiliated by geographic proximity, special interest, or similar situations to address issues affecting the well-being of those people.” (Clinical and Translational Science Awards Consortium, 2011) The Centers for Disease Control and Prevention (CDC) and the National Institutes of Health (NIH) have identified nine principles of community engagement to assist researchers and practitioners in the engagement process, and categorized them as key components to consider prior to beginning engagement, necessary components for engagement to occur, and components to consider for engagement to be successful. Recently, the field of dissemination and implementation science has emphasized the need for community engagement in implementation science (Gopalan et al., 2020, Holt and Chambers, 2017, Chambers and Azrin, 2013) and defined Community-Engaged Dissemination and Implementation Research (CEDI) as “research involving dissemination or implementation of evidence-based health interventions within clinical or community-based settings using community-engaged processes or partnerships, including but not limited to community-based participatory research.” (Holt and Chambers, 2017) CEDI emphasizes engaging stakeholders (e.g., community members, practitioners, community organizations, etc.) with diverse perspectives, experience, and expertise to provide tacit community knowledge regarding the local context, priorities, needs, and assets. Importantly, CEDI can help improve health inequities through incorporating unique perspectives from communities experiencing health inequities that have historically been left out of the research process (Wallerstein and Duran, 2010).
Community engagement and consequently CEDI can exist along a spectrum of involvement and shared distribution of power among stakeholders (Clinical and Translational Science Awards Consortium, 2011, Ovretveit et al., 2014). Explicit description of the community-engagement process (e.g., when and how to engage community stakeholder partners; the various roles community stakeholders play in implementation research) is important to understand what processes and partnerships contribute to implementation success, and provide information on the generalizability of implementation research to other settings. Therefore, the purpose of this paper is to describe the community-engagement process used to develop and implement a multilevel, pragmatic randomized trial to increase the reach and impact of evidence-based tobacco cessation treatment among Community Health Center (CHC) patients across the state of Utah (QuitSMART Utah) (Fernandez et al., 2020).
2. Community-engagement process
2.1. Project overview
Tobacco use remains the leading cause of preventable death and disability in the United States, (US Department of Health Human Services, Centers for Disease Control Prevention, National Center for Chronic Disease Prevention and Health Promotion.. The health consequences of smoking—50 years of progress: a report of the Surgeon General., 2014) yet over 50 million adults still use tobacco (Cornelius et al., 2020). Tobacco use is disproportionately concentrated in low socioeconomic status (SES), rural, (Roberts et al., 2016) and some racial/ethnic minority groups, and contributes substantially to inequities in mortality (Drope et al., 2018, Jha et al., 2006). Consequently, reducing tobacco use among these populations is critical to improving population health, and interventions that specifically target disadvantaged groups are recommended to improve health inequities (Hill et al., 2014). QuitSMART Utah is a pragmatic, multilevel Sequential Multiple Assignment Randomized Trial (SMART) conducted in 11 Community Health Center (CHC) systems and 32 clinics across the state of Utah. The primary goals of the study are to increase the reach and impact of evidence-based tobacco cessation treatment delivered via Quitlines. Quitlines are telephone or web-based cessation services that provide behavioral and pharmacotherapy interventions to interested tobacco-users.
The study consists of three clinic and patient level implementation strategies to increase the reach (Glasgow et al., 2019) of Quitlines: 1) Ask – Advise – Connect (AAC), (Vidrine et al., 2013, Vidrine et al., 2013, Piñeiro et al., 2020) a clinic level implementation strategy at the point of care that uses the electronic health record (EHR) to prompt the clinic practice team to systematically ask all patients about tobacco use, advise tobacco using patients to quit, and connect interested patients directly and electronically to the Utah Tobacco Quit Line (hereafter referred to as Quit Line); 2) Text messaging, a patient facing implementation strategy to provide additional opportunities to enroll in the Quit Line; and 3) Motivation And Problem Solving (MAPS) coaching calls, a patient facing implementation strategy to address patients’ motivation and barriers to engaging with the Quit Line and quitting tobacco. Additional information on QuitSMART Utah has been provided elsewhere (Fernandez et al., 2020, Gibson et al., 2021). The procedures for QuitSMART Utah are approved by the University Institutional Review Board (#00111985).
The community-engagement process for QuitSMART Utah followed principles of community engagement outlined by the CDC and NIH (Clinical and Translational Science Awards Consortium, 2011) and recently applied to CEDI (Shea et al., 2017). Prior to the engagement process: 1) be clear about the engagement process and the communities you want to engage; 2) be knowledgeable about community dynamics. For engagement to occur: 3) establish relationships and trust with formal and informal leaders, seek commitment from the community; and 4) remember that the community empowers itself. For successful engagement: 5) partnering with the community is necessary to create change and improve health; 6) all aspects of community engagement must recognize and respect diversity of the community throughout the planning, designing, and implementing community engagement approaches; 7) to sustain engagement, community assets and strengths must be identified and mobilized, and the community capacity and resources must be developed; 8) teams that engage community must be willing to release control to the community and be flexible to meet the communities’ changing needs; 9) collaboration requires a long-term commitment (Clinical and Translational Science Awards Consortium, 2011, Shea et al., 2017).
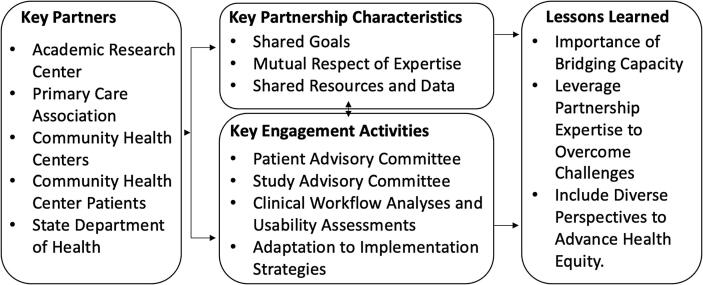
The community-engagement process for QuitSMART Utah consisted of the following activities: 1) development of a research-practice partnership including the creation of an “embedded” CHC Liaison, 2) formation of Patient and Study Advisory Committees, 3) clinical workflow and usability assessments with clinic staff, and 4) developing and adapting implementation strategies based on local context, prior empirical work, (Vinci et al., 2021) social cognitive theory, (Bandura, 2001, Bandura, 1986) and the Consolidated Framework for Implementation Research (Damschroder et al., 2009). A brief summary of the engagement process is depicted in Fig. 1.
Fig. 1.
Community-engagement process summary.
2.2. Key partners
A key component of our community-engagement process was the establishment of a research-practice partnership among Utah’s Primary Care Association and 32 clinics within 11 CHCs across the state; the Utah Department of Health; and an academic research center funded by the Clinical Translational Science Award and a National Cancer Institute Comprehensive Cancer Center (Huntsman Cancer Institute) that brought together diverse scientific expertise.
The Association for Utah Community Health is the federally designated Primary Care Association for the state of Utah, and represents Utah’s 13 CHC organizations and 53 clinics. Utah’s 13 CHC organizations provide comprehensive primary and preventive healthcare services to over 155,000 patients annually – 49% are Hispanic, 9% are American Indian/Alaska Native, 37% are best served in a language other than English, 66% are living under the poverty level, and 48% are uninsured ([37]). Eleven of the 13 CHCs volunteered for participation in QuitSMART Utah.
The Utah Department of Health provides the infrastructure for public health in Utah, and houses multiple programs for preventing and controlling chronic disease, including the Tobacco Prevention and Control Program. The Tobacco Prevention and Control Program assesses tobacco use in Utah, coordinates tobacco control efforts across the state, and manages the Quit Line in Utah (Utah Tobacco Quit Line). The Utah Tobacco Quit Line provides evidence-based interventions including both pharmacotherapy and behavioral interventions for tobacco cessation via phone counseling, online platforms, and text messaging.
The Center for Health Outcomes and Population Equity (HOPE) at the University of Utah and Huntsman Cancer Institute brings researchers from across the entire University and community partners together to create long-term solutions for chronic and infectious disease prevention among underserved populations, with the goal of eliminating health inequities in the Mountain West. Established at the University of Utah in 2017, the Center serves as a bridge between researchers and community, houses multiple researchers examining chronic and infectious disease prevention and control among underserved populations, and collaborates with a transdisciplinary group of researchers, including experts in biomedical informatics, sociotechnical design, implementation science, and statistical analysis.
2.2.1. Partnership development
The Center for HOPE met with the Association for Utah Community Health and the Tobacco Prevention and Control Program to understand priorities and current initiatives for each organization across Utah. Both partners identified tobacco cessation as a top priority, and the Tobacco Prevention and Control Program identified increasing engagement with evidence-based tobacco cessation treatment delivered via the Quit Line. The Association for Utah Community Health and the Tobacco Prevention and Control Program had an existing relationship, and were collaborating to increase patient engagement with the Quit Line by implementing AAC (Vidrine et al., 2013) in clinical care in CHCs across Utah. AAC has extensive empirical support and is a recommended “best practice” for tobacco control by the CDC (Center for Disease Control and Prevention. Best practices for comprehensive tobacco control programs—, 2014). Given the Center for HOPE leadership’s role in developing AAC, a partnership was created to align expertise and efforts. The partnership spent a considerable amount of time investigating referral pathways and Quit Line metrics by examining data from CHCs and the Quit Line. Discussions with Association for Utah Community Health and CHCs indicated that a lack of Health Information Technology (HIT) expertise in CHCs was a major barrier to implementation of AAC. The partners looked at opportunities for securing resources and identified the Patient Centered Outcomes Research Institute (PCORI) Pragmatic Clinical Trial program, at which time the partnership applied and was awarded funding.
2.2.2. Community health center liaison
A unique and integral component of the partnership was the creation of an “embedded” CHC Liaison. Since the onset of the project, an Association for Utah Community Health employee has been embedded at the Center for HOPE to serve as the bridge (Long et al., 2013) among the Center, Association for Utah Community Health, Tobacco Prevention and Control Program, and each of the CHC systems and clinics. The Liaison had worked with each CHC system on various projects for Association for Utah Community Health, had a history of working with Tobacco Prevention and Control Program for improving tobacco cessation at CHCs, and worked at one of the participating CHC clinics for 10 years. The Liaison is housed in the Center for HOPE four days a week, and provides expert knowledge of CHC operations and capacity for all phases of the research (e.g., development, implementation, dissemination). Funding for the Liaison is shared among Association for Utah Community Health, Tobacco Prevention and Control Program, and project funds from QuitSMART Utah.
2.3. Key partnership characteristics
Informed by community-engagement literature, the principles of community engagement, and core competencies for CEDI (Emmons et al., 2008, Clinical and Translational Science Awards Consortium, 2011, Shea et al., 2017, Matthews et al., 2018) our team has identified three key characteristics that contributed to partnership success for the QuitSMART Utah project (Fig. 1): 1) shared goals; 2) mutual respect of expertise, and 3) shared resources and data.
2.3.1. Shared goals
Identifying a common purpose or goal that is mutually defined by partners and provides both scientific and community benefit is recommended for establishing effective partnerships (Emmons et al., 2008, Wallerstein and Duran, 2010). As described above, the partnership is predicated on a shared goal – to improve tobacco related health inequities by decreasing tobacco use rates among underserved populations. Importantly, these goals were shared by each partner organization prior to the initiation of the project (Matthews et al., 2018). The research aligns with Association for Utah Community Health’s mission to enhance services for their CHC members, and to provide high-quality preventive health services to patients regardless of financial and geographic barriers; with Tobacco Prevention and Control Program’s focus to develop strategies and implement activities to alleviate the negative health effects of tobacco use, and to eliminate tobacco-related health disparities; and the Center for HOPE’s mission to decrease health inequities in the Mountain West through academic – community partnerships.
2.3.2. Mutual respect of expertise
Mutual respect and acknowledgement of each partners’ expertise is critical for conducting transdisciplinary research and creating partnerships, (Wallerstein and Duran, 2010, Stokols, 2006, Clinical and Translational Science Awards Consortium, 2011, Matthews et al., 2018) and entails identifying the community partner as an expert in local assets, needs, and culture (Emmons et al., 2008). The Association for Utah Community Health has intimate knowledge of each CHC system, and provides the research team extensive information on CHC system needs, preferences, and current conditions. The CHC Liaison provides real-time feedback on day-to-day project procedures, and ensures that CHC interests are consistently at the forefront of project priorities. Additionally, the CHC Liaison identified a champion at each CHC system to serve on the study advisory committee, and frequently consults the champions for study related input and suggestions; these CHC staff provide expertise on the needs of patients, routine clinical workflows, and facilitators and barriers to project implementation.
The Tobacco Prevention and Control Program provides critical expertise regarding all aspects of the Quit Line and other tobacco control efforts in Utah. The Tobacco Prevention and Control Program worked with the Quit Line vendor to create relationships between the research team and the vendor to configure the technical components of AAC, and manage the enrollment and outcome data collected from the Quit Line. The Tobacco Prevention and Control Program also has intimate knowledge of the regulatory approvals necessary to share data between study partners, and has managed the completion of the data use agreements and Institutional Review Board (IRB) approval required for the Utah Department of Health.
The Center for HOPE and University of Utah provide the scientific expertise for the conduct of the project (e.g., study design methodology, statistical analysis); expertise in the design of a systematic process to adapt interventions based on input from CHC system leadership, CHC clinic staff, and patient and study advisory committees; and Biomedical Informatics expertise to design and implement AAC within each CHCs’ electronic health record (EHR) systems and electronic referrals between the EHRs and the Quit Line.
2.3.3. Shared resources and data
Sharing resources and data across partners is critical to creating trust, empowering each partner to create a shared sense of ownership of the partnership, and ensuring that each partner has mutual benefits from the project (Emmons et al., 2008). In QuitSMART Utah, all partner organizations have contributed resources to accomplish the goals of the project. The extramural funding received for QuitSMART Utah provided the funding for most project activities, including costs for implementing AAC in the EHR and for a yearly financial incentive to participating clinics. As funding for the project covered the costs related to implementing AAC, the Tobacco Prevention and Control Program reallocated funds they had originally planned to spend on AAC to other initiatives, including a program for free tobacco cessation medication to uninsured patients seen at CHCs. Notably, the funding for the Liaison is shared by the contract funding awarded for QuitSMART Utah, as well as the Tobacco Prevention and Control Program. The Tobacco Prevention and Control Program provides Quit Line services free of charge to all Utah residents, ensuring that all CHC patients – regardless of insurance status – can receive free evidence-based tobacco cessation treatment, and covered the costs for establishing closed-loop referrals between CHC EHRs and the Quit Line vendor. The partnership also shares data across study partners using an integrated data system. The data used to evaluate project outcomes come from existing data sources at the Association for Utah Community Health and the Tobacco Prevention and Control Program, and the Association for Utah Community Health and the Tobacco Prevention and Control Program are the brokers of the data. All Quit Line enrollment data from the Quit Line vendor are shared with the Tobacco Prevention and Control Program; similarly, all CHC clinic data are shared by the individual clinics with Association for Utah Community Health. Then the Association for Utah Community Health and the Tobacco Prevention and Control Program share relevant data with the University of Utah. The data system required extensive coordination among the partners, including the execution of 11 data sharing agreements and two institutional review board approvals.
2.4. Key engagement activities
2.4.1. Patient and study advisory committees
Patient and Study Advisory Committees were created to engage patients and other study stakeholders (e.g., CHCs, Association for Utah Community Health, and Utah Department of Health) and incorporate their input throughout the entire research process (i.e., planning, conducting, disseminating, and reporting). The Patient Advisory Committee consists of four patient representatives from diverse geographic locations of Utah (50% rural/frontier). The Study Advisory Committee includes representatives from each of the participating CHCs, Association for Utah Community Health, and the Tobacco Prevention and Control Program. The committees meet four times per year. During the planning stage, the Study Advisory Committee focused on discussing, identifying barriers to, refining, and making decisions on the following research topics/activities: participant recruitment and consent procedures, clinical data collection procedures, EHR adaptation, clinic practice team training, and text messaging content and procedures. During the planning stage, the Patient Advisory Committee discussed, refined, and made decisions on many of the same issues as the Study Advisory Committee, but had a specific emphasis on the following: participant recruitment and consent procedures, data collection procedures, EHR adaptation, clinic practice team training, text messaging content and procedures, MAPS content and procedures, and abstinence and quality-of-life outcomes.
2.4.2. Clinical workflow analyses and usability assessments
Using principles of user-centered design, (Clegg, 2000) the research team conducted clinical workflow analyses to identify current workflows for addressing tobacco use to inform the design of the AAC implementation strategy (Gibson et al., 2021). Briefly, the team sought to understand current workflows for asking patients about tobacco use, advising patients to quit using tobacco, and referring tobacco-using patients to the Quit Line. Using information collected from the workflow analyses and technical evaluations of the three different clinic EHR systems, the team designed preliminary versions of AAC. Adaptations were made to the design of AAC following usability assessments with CHC clinic staff. Detailed information regarding the clinical workflow analyses and usability assessments has been presented elsewhere (Gibson et al., 2021).
2.4.3. Adaptations to implementation strategies
The community-engagement activities (e.g., meetings with project partners, Study Advisory Committee/Patient Advisory Committee meetings, clinical workflow analyses and usability assessments) were used to inform the selection, development, and adaptation of the multilevel implementation strategies for QuitSMART Utah.
2.5. Discussion and lessons learned
Community engagement exists across a continuum of shared involvement, decision making, and power distribution among partners. One conceptualization of this spectrum places community-engagement in five different categories: outreach, consult, involve, shared leadership/participatory, and community driven (Clinical and Translational Science Awards Consortium, 2011, Yuen et al., 2015). This spectrum can be used to describe the overall engagement approach of an individual project, as well as the evolution of a partnership along the continuum of engagement. For the purpose of this paper, we also used this spectrum to categorize the types of engagement activities that were used in QuitSMART Utah. The overall community-engagement level for QuitSMART Utah can be categorized as Shared Leadership/Participatory, however the activities spanned the spectrum of engagement (Table 1). Importantly, the overall engagement approach for this partnership has evolved to Community Driven. For example, after funding was awarded for QuitSMART Utah, the community partners have identified numerous priority areas that that they would like to address and directed the partnership to pursue extramural funding in those areas.
Table 1.
Description of levels of engagement (Clinical and Translational Science Awards Consortium, 2011, Long et al., 2013) and examples from QuitSMART Utah.
| Level of engagement | Definition | Example |
|---|---|---|
| Outreach | Communication flows from the research partner to the community to share/inform; research partner provides the community with information. | Research team provided clinic practice team members information on the free resources provided by the Quit Line during the Patient and Study Advisory Committee meetings. |
| Consult | Research partner gets information/feedback from the community to inform the project. | Clinic practice team members were observed during clinical workflow analyses to inform development of AAC. Clinic practice team members provided feedback on AAC design through interviews and usability assessments. |
| Involve | Communication is bidirectional from the research partner and community; research partner seeks participation from the community on issues. | Patient Advisory Committee members disclosed feeling uncomfortable disclosing tobacco use status with clinic practice members during the clinical encounter. Though not originally planned by the research team, a section on effective patient-provider communication was subsequently added to the planned clinic practice team training. |
| Shared Leadership/ Participatory | Strong bidirectional relationship; research partner and community have strong partnership from development to solutions for project. | The research and community partners identified shared priorities (i.e., improving tobacco use among underserved populations) and collaboratively identified solutions (i.e., implementation of AAC in CHC systems) that were the basis of the research project. After funding was awarded, the state department of health made the decision to use funding originally earmarked for implementation of AAC to partially fund a Liaison between CHCs, Association for Utah Community Health, and the University. |
| Community Driven | Strong community leadership; final decision making is at the community level. | After being awarded funding for the QuitSMART Utah project, the community partners identified additional priority areas (HPV vaccination, colorectal cancer screening) and the research team and partners subsequently sought out and were awarded funding for projects in these priority areas. |
AAC = Ask – Advise – Connect; CHC = Community Health Center.
Throughout the conduct of QuitSMART Utah, the research team has capitalized on the community-engagement process to address project challenges, improve implementation strategy design, and to leverage the partnership for additional projects addressing CHC, Association for Utah Community Health, and Utah Department of Health priorities. From this process, our team has identified numerous ‘lessons learned’ that may inform conduct of CEDI for other researchers (Gopalan et al., 2020).
2.5.1. Importance of bridging capacity
At the time of the QuitSMART Utah project development, the research team was new to Utah and had no existing relationship with the CHC systems. The Association for Utah Community Health and the CHC Liaison were critical to gaining buy-in for the project from CHC systems prior to project funding. Since the project onset, the CHC Liaison has been a conduit through which information flows among partners regarding project activities, partner priorities, and partner challenges (Rogers, 2010). Importantly, the Liaison had existing relationships with the CHC systems through numerous other capacities (e.g., the Liaison is also the Emergency Preparedness Coordinator for Association for Utah Community Health, among other roles) prior to the project start. These relationships were capitalized on to build trust between the CHC systems and the University (e.g., facilitating opportunities for interaction among CHC system leadership/staff and the research team) and to accomplish project tasks (e.g., signing memorandum of agreements, getting approval to conduct clinical workflow assessments). The Liaison has been essential to obtaining engagement from the CHC system, and has been an invaluable resource to the research team to ensure CHC priorities are at the top of the research team priorities.
2.5.2. Leverage partnership expertise to overcome challenges
The QuitSMART Utah project has not been immune to the challenges that come with conducting pragmatic clinical trials in real world settings, and the partnership has been critical to overcoming these challenges. For example, the initial protocol for the project included consenting tobacco-using patients to participate in the study during the clinical encounter, which would have required the clinic practice team to obtain and document consent of each patient. In addition to complicating the clinic practice team’s workflow, this process would have also added the requirement that each clinic practice team member ‘engage’ in research, and therefore require each clinic practice team member to complete training in the responsible conduct of human subject research. After consulting with the University IRB, the partnership decided to share data already being obtained by Association for Utah Community Health and Utah Department of Health to ultimately eliminate the requirement for individual patient consent and additional burden on clinic staff. The process of developing this integrated data system required extensive coordination among the partners, including the development and execution of 11 data sharing agreements and IRB approvals from the Utah Department of Health and the University. Ultimately, the commitment and trust of the partners enabled the partnership to capitalize on partner expertise and existing resources to advance the shared goal to help tobacco-using patients engage in evidence-based cessation treatment, despite challenges. Given the real-world challenges that often arise when conducting dissemination and implementation research, creating strong research-practice partnerships can enable innovative problem solving during project conduct (Gopalan et al., 2020).
2.5.3. Include diverse perspectives to advance health equity
The primary aim of the QuitSMART Utah project is to increase the reach and impact of evidence-based tobacco cessation treatment among CHC patients, a population with a disproportionate burden of tobacco use. The community-engagement process enabled diverse perspectives from CHC systems and CHC patients to be included in the design and conduct of the trial. These perspectives were critical throughout the implementation strategy adaptation process, and our implementation strategies were designed and adapted according to CHC staff and patient preferences. For example, the ‘Advise’ component of AAC implementation strategy was originally designed to read:
‘Quitting tobacco is the best thing you can do for your health. Do I have your permission to share your information with the Utah Tobacco Quit Line?’
After review of the statement, our Patient Advisory Committee members gave constructive feedback to modify the statement to show more support to the patient, to explicitly state the treatment options at the Quit Line, and to highlight that the Quit Line treatment is free. We subsequently changed the design of the ‘Advise’ component of AAC to read:
‘We would like to help you quit. We partner with the Utah Tobacco Quit Line. They offer free coaching and nicotine replacement. Do I have your permission to have the Quit Line contact you?’
Including the patient perspective in our project has enabled our team to make adaptations to our implementation strategies that incorporate the preferences of populations currently experiencing health inequities (McNulty et al., 2019). Additionally, partnering with CHC systems throughout the design and implementation of the project further illuminated the digital divide the CHC systems face to implementation of EBIs that use health information technology, and facilitated the adaptation of implementation strategies for use in safety-net healthcare systems (described in detail elsewhere31). Ultimately, CEDI has the potential to improve health inequities if researchers partner with organizations that reach underserved populations (McNulty et al., 2019) and include diverse perspectives throughout design and implementation.
2.5.4. Seek opportunities to sustain partnerships
Importantly, this partnership has now served as the infrastructure for multiple funded projects. Since being awarded funding for the QuitSMART Utah project, the partnership has been leveraged for three additional funded projects to implement EBIs in CHCs, including funding by the American Cancer Society to improve HPV vaccination rates, funding from the CDC to increase colorectal cancer screening rates, and funding from the NIH to increase COVID-19 screening and testing. The infrastructure of the partnership and community-engagement process has enabled development of projects that simultaneously address priorities of project partners and provide an opportunity to create generalizable information for dissemination and implementation of EBIs. These projects have provided additional opportunities to further align partner goals, build trust, and reinforce commitment to a sustained partnership.
2.6. Challenges and future directions
Conducting CEDI is not without challenges, (Gopalan et al., 2020) including the additional resources necessary to build relationships and obtain oversight approval from multiple entities, and maintaining the balance of expectations from community stakeholders and funders. However, as mentioned above, the infrastructure from QuitSMART Utah has been leveraged for multiple additional CEDI projects. Additionally, in the future this infrastructure can provide opportunities for testing innovative models of CEDI, such as the ‘inside-out’ model, that promotes changing clinical practice and policies from outside-in (e.g., meeting external policies/pressures) to inside-out (e.g., creating evidence-based practices from practice-based evidence) (Green, 2008, Miller et al., 2019).
3. Conclusions
In conclusion, CEDI research has the potential to improve dissemination and implementation of EBIs and advance implementation research . Incorporating expertise from diverse perspectives, including populations that experience health inequities and organizations serving populations experiencing health inequities, is critical to the design and conduct of implementation research to improve health inequities. The community-engagement process described above depicts a tangible example of how expertise, resources, and data can be shared across multisector partners to ultimately capitalize on assets of each partner to accomplish a shared goal of improving population health and eliminating health inequities.
4. Funding and disclaimers
This work was supported through a Patient Centered Outcomes Research Institute (PCORI) Project Program Award (PCS-2017C2-7613), the National Cancer Institute (P30CA042014), the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR002538), and the Huntsman Cancer Foundation.
5. Disclaimer
All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors, or Methodology Committee.
CRediT authorship contribution statement
Chelsey R. Schlechter: Conceptualization, Methodology, Project administration, Writing – original draft, Writing – review & editing. Guilherme Del Fiol: Conceptualization, Methodology, Funding acquisition, Writing – review & editing. Cho Y. Lam: Conceptualization, Methodology, Funding acquisition, Writing – review & editing. Maria E. Fernandez: Conceptualization, Methodology, Funding acquisition, Writing – review & editing. Tom Greene: Funding acquisition, Methodology, Writing – review & editing. Melissa Yack: Methodology, Writing – review & editing. Sandra Schulthies: Conceptualization, Resources, Writing – review & editing. Marci Nelson: Conceptualization, Resources, Writing – review & editing. Claudia Bohner: Conceptualization, Resources, Writing – review & editing. Alan Pruhs: Conceptualization, Resources, Funding acquisition, Writing – review & editing. Tracey Siaperas: Conceptualization, Resources, Writing – review & editing. Kensaku Kawamoto: Funding acquisition, Writing – review & editing. Bryan Gibson: Methodology, Writing – review & editing. Inbal Nahum-Shani: Funding acquisition, Writing – review & editing. Timothy J. Walker: Methodology, Writing – review & editing. David W. Wetter: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank the Association for Utah Community Health, Utah Department of Health, National Jewish Health, and all the Community Health Center organizations and patients who are partnering on this project.
References
- Bandura A. Prentice Hall; New Jersey: 1986. Social Foundations of Thought and Action. [Google Scholar]
- Bandura A. Social cognitive theory: an agentic perspective. Annu. Rev. Psychol. 2001;52(1):1–26. doi: 10.1146/annurev.psych.52.1.1. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. Best practices for comprehensive tobacco control programs—2014. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. 2014:162–169.
- Chambers D.A., Azrin S.T. Research and services partnerships: partnership: a fundamental component of dissemination and implementation research. Psychiatric Serv. 2013;64(6):509–511. doi: 10.1176/appi.ps.201300032. [DOI] [PubMed] [Google Scholar]
- Chambers D.A., Norton W.E. The adaptome: advancing the science of intervention adaptation. Am. J. Prev. Med. 2016;51(4):S124–S131. doi: 10.1016/j.amepre.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg C.W. Sociotechnical principles for system design. Appl. Ergon. 2000;31(5):463–477. doi: 10.1016/s0003-6870(00)00009-0. [DOI] [PubMed] [Google Scholar]
- Clinical and Translational Science Awards Consortium . US Gov Printing Office; Rockville, MD: 2011. Principles of community engagement. [Google Scholar]
- Cornelius M.E., Wang T.W., Jamal A., Loretan C.G., Neff L.J. Tobacco product use among Adults—United States, 2019. Morb. Mortal. Wkly. Rep. 2020;69(46):1736–1742. doi: 10.15585/mmwr.mm6946a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damschroder L.J., Aron D.C., Keith R.E., Kirsh S.R., Alexander J.A., Lowery J.C. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement. Sci. 2009;4(1):50. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drope J, Liber AC, Cahn Z, et al. Who's still smoking? Disparities in adult cigarette smoking prevalence in the United States. 2018;68(2):106-115. [DOI] [PubMed]
- Emmons K.M., Viswanath K., Colditz G.A. The role of transdisciplinary collaboration in translating and disseminating health research: lessons learned and exemplars of success. Am. J. Prev. Med. 2008;35(2):S204–S210. doi: 10.1016/j.amepre.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Fernandez M.E., Schlechter C.R., Del Fiol G., Gibson B., Kawamoto K., Siaperas T., Pruhs A., Greene T., Nahum-Shani I., Schulthies S., Nelson M., Bohner C., Kramer H., Borbolla D., Austin S., Weir C., Walker T.W., Lam C.Y., Wetter D.W. QuitSMART Utah: an implementation study protocol for a cluster-randomized, multi-level Sequential Multiple Assignment Randomized Trial to increase Reach and Impact of tobacco cessation treatment in Community Health Centers. Implement. Sci. 2020;15(1) doi: 10.1186/s13012-020-0967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson B, Kramer, H., Weir, C., Del Fiol, G., Borbolla, D., Schlechter, CR., Lam, CY., Nelson, M., Bohner, C., Schulthies, S., Siaperas, T., Pruhs, A., Nahum-Shani, I., Fernandez, M., Wetter, DW. Workflow Analysis for Design of an Electronic Health Record based Tobacco Cessation Intervention in Community Health Centers. JAMIA Open. 2021. [DOI] [PMC free article] [PubMed]
- Glasgow R.E., Vogt T.M., Boles S.M. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am. J. Public Health. 1999;89(9):1322–1327. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow R.E., Klesges L.M., Dzewaltowski D.A., Bull S.S., Estabrooks P. The future of health behavior change research: What is needed to improve translation of research into health promotion practice? Ann. Behav. Med. 2004;27(1):3–12. doi: 10.1207/s15324796abm2701_2. [DOI] [PubMed] [Google Scholar]
- Glasgow R.E., Harden S.M., Gaglio B., Rabin B., Smith M.L., Porter G.C., Ory M.G., Estabrooks P.A. RE-AIM planning and evaluation framework: adapting to new science and practice with a 20-year review. Front. Public Health. 2019;7 doi: 10.3389/fpubh.2019.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalan G., Bunger A.C., Powell B.J. Skills for developing and maintaining community-partnerships for dissemination and implementation research in children’s behavioral health: implications for research infrastructure and training of early career investigators. Admin. Policy Mental Health Mental Health Serv. Res. 2020;47(2):227–243. doi: 10.1007/s10488-019-00930-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L.W. Making research relevant: if it is an evidence-based practice, where's the practice-based evidence? Fam. Pract. 2008;25(Supplement 1):i20–i24. doi: 10.1093/fampra/cmn055. [DOI] [PubMed] [Google Scholar]
- Green L.W., Glasgow R.E., Atkins D., Stange K. Making evidence from research more relevant, useful, and actionable in policy, program planning, and practice: slips “twixt cup and lip”. Am. J. Prev. Med. 2009;37(6):S187–S191. doi: 10.1016/j.amepre.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 20.Health Resources & Services Administration. Utah Health Center Data. 2020.
- Hill S., Amos A., Clifford D., Platt S. Impact of tobacco control interventions on socioeconomic inequalities in smoking: review of the evidence. Tobacco Control. 2014;23(e2):e89–e97. doi: 10.1136/tobaccocontrol-2013-051110. [DOI] [PubMed] [Google Scholar]
- Holt CL, Chambers DA. Opportunities and challenges in conducting community-engaged dissemination/implementation research. In: Oxford University Press; 2017. [DOI] [PMC free article] [PubMed]
- Jha P, Peto R, Zatonski W, Boreham J, Jarvis MJ, Lopez AD. Social inequalities in male mortality, and in male mortality from smoking: indirect estimation from national death rates in England and Wales, Poland, and North America. 2006;368(9533):367-370. [DOI] [PubMed]
- Lewin K. Action research and minority problems. J. Soc. Issues. 1946;2(4):34–46. [Google Scholar]
- Long J.C., Cunningham F.C., Braithwaite J. Bridges, brokers and boundary spanners in collaborative networks: a systematic review. BMC Health Servi. Res. 2013;13(1):158. doi: 10.1186/1472-6963-13-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews A.K., Castillo A., Anderson E., Willis M., Choure W., Rak K., Ruiz R. Ready or not? Observations from a long-standing community engagement advisory board about investigator competencies for community-engaged research. J. Clin. Transl. Sci. 2018;2(3):129–134. doi: 10.1017/cts.2018.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty M., Smith J.D., Villamar J., Burnett-Zeigler I., Vermeer W., Benbow N., Gallo C., Wilensky U., Hjorth A., Mustanski B., Schneider J., Brown C.H. Implementation research methodologies for achieving scientific equity and health equity. Ethn. Dis. 2019;29(Suppl 1):83–92. doi: 10.18865/ed.29.S1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W.L., Rubinstein E.B., Howard J., Crabtree B.F. Shifting implementation science theory to empower primary care practices. Ann. Fam. Med. 2019;17(3):250–256. doi: 10.1370/afm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkler M., Wallerstein N. John Wiley & Sons; 2011. Community-Based Participatory Research for Health: From Process to Outcomes. [Google Scholar]
- Ovretveit J, Hempel S, Magnabosco JL, Mittman BS, Rubenstein LV, Ganz DA. Guidance for research-practice partnerships (R-PPs) and collaborative research. Journal of health organization and management. 2014. [DOI] [PubMed]
- Paskett E., Thompson B., Ammerman A.S., Ortega A.N., Marsteller J., Richardson DeJuran. Multilevel interventions to address health disparities show promise in improving population health. Health Aff. 2016;35(8):1429–1434. doi: 10.1377/hlthaff.2015.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñeiro B, Vidrine DJ, Wetter DW, et al. Implementation of Ask-Advise-Connect in a safety net healthcare system: quitline treatment engagement and smoking cessation outcomes. Translational Behavioral Medicine. 2018. [DOI] [PMC free article] [PubMed]
- Roberts M.E., Doogan N.J., Kurti A.N., Redner R., Gaalema D.E., Stanton C.A., White T.J., Higgins S.T. Rural tobacco use across the United States: how rural and urban areas differ, broken down by census regions and divisions. Health & Place. 2016;39:153–159. doi: 10.1016/j.healthplace.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers E.M. Simon and Schuster; 2010. Diffusion of Innovations. [Google Scholar]
- Shea C.M., Young T.L., Powell B.J., Rohweder C., Enga Z.K., Scott J.E., Carter-Edwards L., Corbie-Smith G. Researcher readiness for participating in community-engaged dissemination and implementation research: a conceptual framework of core competencies. Transl. Behav. Med. 2017;7(3):393–404. doi: 10.1007/s13142-017-0486-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokols D. Toward a science of transdisciplinary action research. Am. J. Community Psychol. 2006;38(1–2):79–93. doi: 10.1007/s10464-006-9060-5. [DOI] [PubMed] [Google Scholar]
- Stokols D., Hall K.L., Taylor B.K., Moser R.P. The science of team science: overview of the field and introduction to the supplement. Am. J. Prev. Med. 2008;35(2):S77–S89. doi: 10.1016/j.amepre.2008.05.002. [DOI] [PubMed] [Google Scholar]
- US Department of Health Human Services, Centers for Disease Control Prevention, National Center for Chronic Disease Prevention and Health Promotion.. The health consequences of smoking—50 years of progress: a report of the Surgeon General. 2014.
- Vidrine JI, Shete S, Cao Y, et al. Ask-Advise-Connect: a new approach to smoking treatment delivery in health care settings. 2013;173(6):458-464. [DOI] [PMC free article] [PubMed]
- Vidrine J.I., Shete S., Li Y., Cao Y., Alford M.H., Michelle Galindo-Talton R.N., Rabius V., Sharp B., Harmonson P., Zbikowski S.M., Miles L., Wetter D.W. The ask-advise-connect approach for smokers in a safety net healthcare system a group-randomized trial. Am. J. Prev. Med. 2013;45(6):737–741. doi: 10.1016/j.amepre.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidrine J.I., Shete S., Cao Y., Greisinger A., Harmonson P., Sharp B., Miles L., Zbikowski S.M., Wetter D.W. Ask-advise-connect a new approach to smoking treatment delivery in health care settings. JAMA Int. Med. 2013;173(6):458. doi: 10.1001/jamainternmed.2013.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinci C., Lam C., Schlechter C.R., Shono Y., Vidrine J.I., Wetter D.W. Increasing treatment enrollment among smokers who are not motivated to quit: a randomized clinical trial. Transl. Behav. Med. 2021 doi: 10.1093/tbm/ibab114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel A.L., Hall K.L., Fiore S.M., Klein J.T., Michelle Bennett L., Gadlin H., Stokols D., Nebeling L.C., Wuchty S., Patrick K., Spotts E.L., Pohl C., Riley W.T., Falk-Krzesinski H.J. The team science toolkit: enhancing research collaboration through online knowledge sharing. Am. J. Prev. Med. 2013;45(6):787–789. doi: 10.1016/j.amepre.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Wallerstein N.B., Duran B. Using community-based participatory research to address health disparities. Health Promot. Pract. 2006;7(3):312–323. doi: 10.1177/1524839906289376. [DOI] [PubMed] [Google Scholar]
- Wallerstein N., Duran B. Community-based participatory research contributions to intervention research: the intersection of science and practice to improve health equity. Am. J. Public Health. 2010;100(S1):S40–S46. doi: 10.2105/AJPH.2009.184036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallerstein N., Duran B., Oetzel J.G., Minkler M. John Wiley & Sons; 2017. Community-Based Participatory Research for Health: Advancing Social and Health Equity. [Google Scholar]
- Yuen T., Park A.N., Seifer S.D., Payne-Sturges D. A systematic review of community engagement in the US environmental protection agency’s extramural research solicitations: implications for research funders. Am. J. Public Health. 2015;105(12):e44–e52. doi: 10.2105/AJPH.2015.302811. [DOI] [PMC free article] [PubMed] [Google Scholar]