Abstract
Avian orthoavulavirus 1 (AOaV-1) causes Newcastle disease, one of the most important and contagious infections in poultry, where migratory birds can play a key role as a reservoir. Seven hundred and seven serum samples were collected from five penguin species (King, Magellanic, Gentoo, Chinstrap and Adelie penguins) in the Antarctic and Sub-Antarctic zones. Using a competitive ELISA to detect antibodies against AOaV-1, we identified positive individuals in all penguin species. The Magellanic penguin showed the highest seropositivity rate (30.3%), suggesting it could be a natural reservoir of this virus. At the Antarctic zones, Chinstrap penguin showed the highest occurrence (7.5%). Interesting, positive sera was only obtained in Sub-Antarctic and Northern zones at the Antarctic peninsula, no seroreactivity was observed in Southern locations. Further studies are needed to establish the role of these penguin species in the epidemiology of the AOaV-1 and determine the effects of this virus in these populations.
Keywords: Antarctica, avulavirus, Newcastle disease, orthoavulavirus, penguins
1 |. INTRODUCTION
Avian orthoavulavirus 1 (AOaV-1), formerly known as Avian avulavirus 1 (AAvV-1), avian paramyxovirus 1 (APMV-1) and Newcastle disease virus (NDV), belongs to the family Paramyxoviridae, subfamily Avulavirinae, genus Orthoavulavirus (International Committee on Taxonomy of Viruses (ICTV), 2019). AOaV-1 causes Newcastle disease, one of the most important and contagious infections in poultry, which negatively affects production and trade (Paramyxoviridae & Pneumoviridae, 2017). Wild birds, including aquatic/migratory birds, may act as natural reservoir hosts of AOaV-1 and may play a remarkable role in the spread of the virus in the environment (Rahman et al., 2018).
Although there are studies that have evaluated the presence of avian paramyxoviruses in penguins, only a few reports have confirmed the presence of AOaV-1 in this species (Table S1). To date, there is evidence of AOaV-1 infecting Adélie (Pygoscelis adeliae) and Magellanic (Spheniscus magellanicus) penguins (Thomazelli et al., 2010; Uhart et al., 2020), but little or no information is available in other penguin species, such as King (Aptenodytes patagonicus), Gentoo (Pygoscelis papua) and Chinstrap (Pygoscelis antarcticus) penguins. Viral-pathogens surveillance in wildlife enables the determination of virus reference values, detection of potential reservoir hosts, and prevention of epidemics in wildlife and domestic animals. Accordingly, we sought to determine the occurrence and circulation of AOaV-1 in penguins from the Antarctic and sub-Antarctic regions.
2 |. MATERIALS AND METHODS
A total of 707 blood samples were obtained from five penguin species, which were collected from 14 locations in the Antarctic Peninsula (Antarctic region) and two locations in the continental area of the Magallanes region, Chile (sub-Antarctic region) (Table S1). A total of 636 samples were obtained from Chinstrap (n = 292), Gentoo (n = 263) and Adelie (n = 81) penguins in Antarctica during Chilean Antarctic Scientific Expeditions in 2017 (n = 92), 2018 (n = 248) and 2019 (n = 296), organized by the Chilean Antarctic Institute (INACH). The sampling locations were distributed in three Antarctic regions: Northwest, Midwest and Southwest of the Antarctic Peninsula. The Northwest region (Northwest Peninsula and South Shetland Islands) included eight sampling locations: Ardley Island, Harmony Point on Nelson Island, Barrientos Island, Cape Shirreff on Livingston Island, Hannah Point on Livingston Island, Lions Rump on King George Island, Low Island, and Bernardo O’Higgins General Base on the Antarctic Peninsula. The Midwest region included three sampling locations: Biscoe Point on Anvers Island, President Gabriel González Videla Base on Paradise Bay, and Yelcho Base on Doumer Island. The Southwest region included three sampling locations: Avian Island, Lagotellerie Island and Emperor Island (Table 1; Figure 1a). The remaining samples were obtained from King penguins (n = 38) at Bahía Inútil, Tierra del Fuego, between 2014 and 2018, and from Magellanic penguins (n = 33) on Magdalena Island, Strait of Magellan in 2011 (Table 2; Figure 1b).
TABLE 1.
Penguin species, sampling locations and serological results against NDV in the Antarctic Peninsula
|
P. adelia
|
P. antarcticus
|
P. papua
|
Total |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Location name | (+) | N | % | (+) | N | % | (+) | N | % | (+) | N | % |
| Northwest | Ardley Island | 1 | 14 | 7.1 | 3 | 104 | 2.9 | - | 4 | 118 | 3.4 | ||
| Harmony Point (Nelson Island) | - | 1 | 29 | 3.4 | 3 | 16 | 18.8 | 4 | 45 | 8.9 | |||
| Barrientos Island | - | 2 | 12 | 16.7 | - | 13 | - | 2 | 25 | 8.0 | |||
| Cape Shirreff (Livingston Island) | - | 13 | 112 | 11.6 | 1 | 37 | 2.7 | 14 | 149 | 9.4 | |||
| Hannah Point (Livingston Island) | - | 1 | 14 | 0.1 | 1 | 14 | 7.1 | 2 | 28 | 7.1 | |||
| Lions Rump (King George Island) | - | 5 | - | - | 4 | - | 4 | 69 | 5.8 | 4 | 78 | 5.1 | |
| Low Island | - | 2 | 16 | 0.1 | - | 2 | 16 | 12.5 | |||||
| Bernardo O’Higgins Base | - | 1 | - | - | 1 | - | 2 | 42 | 4.8 | 2 | 44 | 4.5 | |
| Midwest | Biscoe Point (Anvers Island) | - | 6 | - | - | - | - | 6 | - | ||||
| GGV Basea (Paradise Bay) | - | - | - | 37 | - | - | 37 | - | |||||
| Yelcho Base (Doumer Island) | - | 1 | - | - | - | 35 | - | - | 36 | - | |||
| Southwest | Avian Island | - | 43 | - | - | - | - | 43 | - | ||||
| Emperor Island | - | 7 | - | - | - | - | 7 | - | |||||
| Lagotellerie Island | - | 4 | - | - | - | - | 4 | - | |||||
| Total | 1 | 81 | 1.2 | 22 | 292 | 7.5 | 11 | 263 | 4.2 | 34 | 636 | 5.4 | |
Note: (+) seropositive samples, N samples total, % relative frequency.
Gabriel González Videla Base.
FIGURE 1.
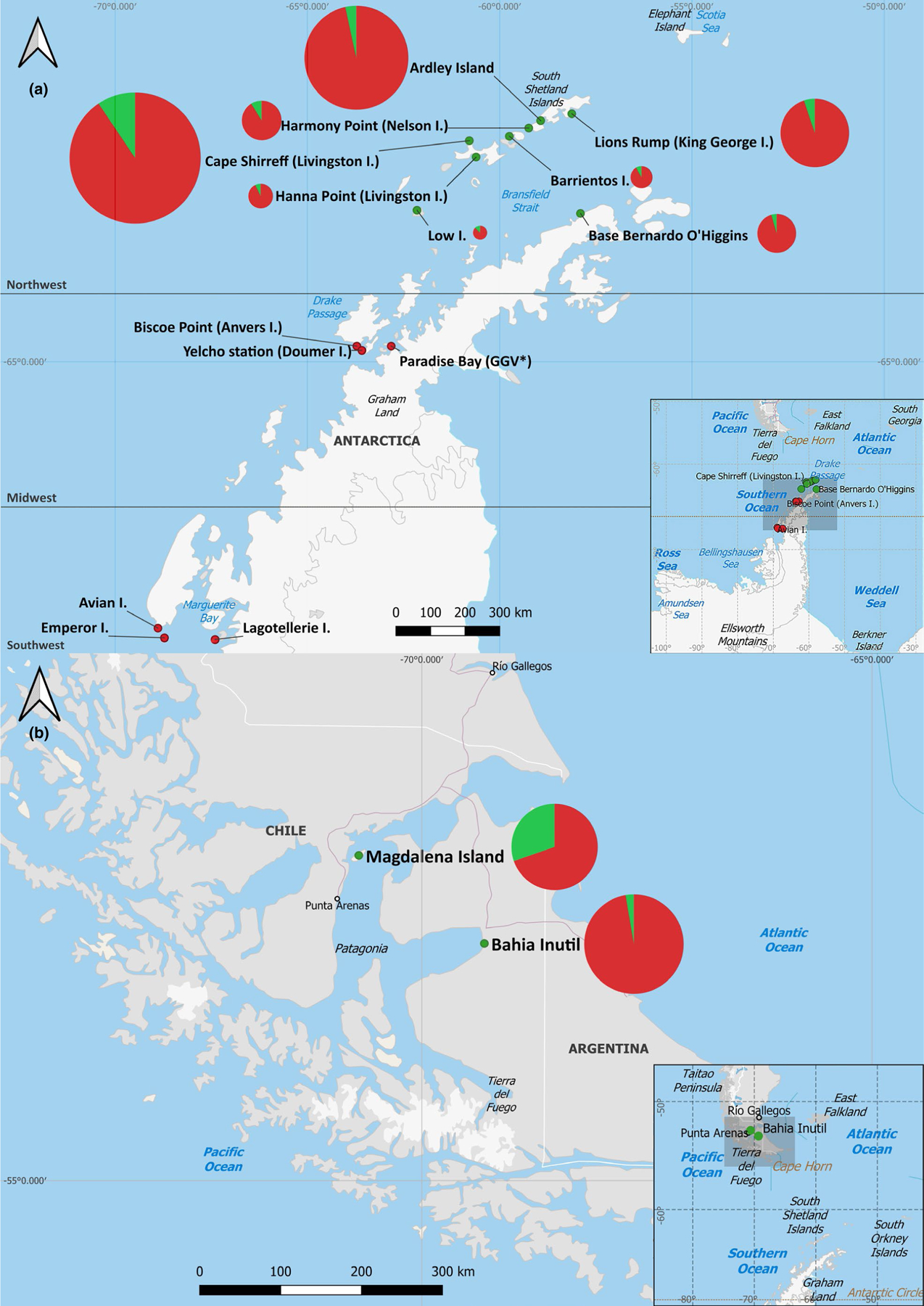
Map of sampling sites on the Antarctic Peninsula (a) and continental area of the Magallanes region (b). Sampling locations are shown in coloured dots, with green dots representing NDV positive sites and in red the negative. Seropositivity pie charts are shown for each location using the same colour code, and their size is proportional to the sample number
TABLE 2.
Penguin species, sampling locations and serological results against NDV in the continental area of the Magallanes region, by penguin species
|
A. patagonicus
|
S. magellanicus
|
Total |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Region | (+) | N | % | (+) | N | % | (+) | N | % |
| Continental Magallanes | 1 | 38 | 2.63 | 10 | 33 | 30.30 | 11 | 71 | 15.49 |
Note: (+) seropositive samples, N samples total, % relative frequency.
Blood samples were obtained by venipuncture in the common plantar digital vein. To achieve this, the penguins were caught with a net, the eyes covered with a black cloth, and gently restrained in a prone position to expose the feet. The samples were centrifuged for 10 min at 800 g and the resulting sera were stored at −20°C until use. Antibodies against AOaV-1 nucleoprotein were detected using a multispecies competitive ELISA commercial kit (ID Screen® Newcastle Disease Competition, IDVet), following manufacturer’s recommendations. ELISA results were expressed as percent inhibition values (PI), according to manufacturer’s instructions, where a PI greater than 40 was considered positive, between 30 and 40 suspect and lower than 30 negative. Suspect samples were considered as negative in the seropositivity calculation. According to a Shapiro–Wilk test, the data do not follow a normal distribution. Overall results of PI (antibody levels) were compared by species and location using a Kruskal–Wallis test followed by Conover’s multiple comparisons test. To determine differences between year of collection for each location, Kruskal–Wallis or Mann–Whitney U test were performed. In addition, chi-square and Fisher’s exact test were performed to determine the association between region and species, with the purpose of collinearity, confounding factor checking and logistic regression model building approach. Subsequently, a simple logistic regression analysis was performed to assess for the effect of the specie as potential risk factors to NDV positivity (Dohoo et al., 2010). Goodness-of-fit was assessed using the Hosmer and Lemeshow Test (Hosmer et al., 2013). Statistically significant differences were set at p-value < .05. The statistical analyses were performed using R version 4.0.2 (R Core Team, 2020) and GraphPad Prism version 8.0.0 for Mac, GraphPad Software. Finally, the specificity of this kit was evaluated using positive antisera against novel Avian orthoavulaviruses (AOaV-17, AOaV-18, and AOaV-19), which have been widely detected previously in Chinstrap, Gentoo, and Adelie penguins from the Antarctic Peninsula, demonstrating the no cross-reactivity with them (Neira et al., 2017; Olivares et al., 2019; Wille et al., 2019).
3 |. RESULTS AND DISCUSSION
Forty-five out of 707 samples (6.4%) were positive for antibodies against AOaV-1; 34 out of 636 (5.3%) from Antarctic locations and 11 out of 71 (15.5%) from sub-Antarctic locations (Figure 2a). All penguin species reported at least one positive sample (Figure 2b). In the Antarctic region, 22 out of 292 (7.5%) Chinstrap penguins were seropositive to AOaV-1, whereas 11 out of 263 (4.2%) Gentoo penguin and 1 out of 81 (1.2%) Adelie penguin showed positive serology. Of the 8 locations sampled, we found positive Chinstrap penguins at 6 locations, Gentoo penguins at 5 locations and only one location with one positive Adelie penguin (Table 1). In the sub-Antarctic region, 10 out of 33 (30.3%) samples were positive in Magellanic penguins from Magdalena Island and 1 sample out of 38 (2.6%) was positive in a King penguin from Bahía Inútil (Table 2).
FIGURE 2.
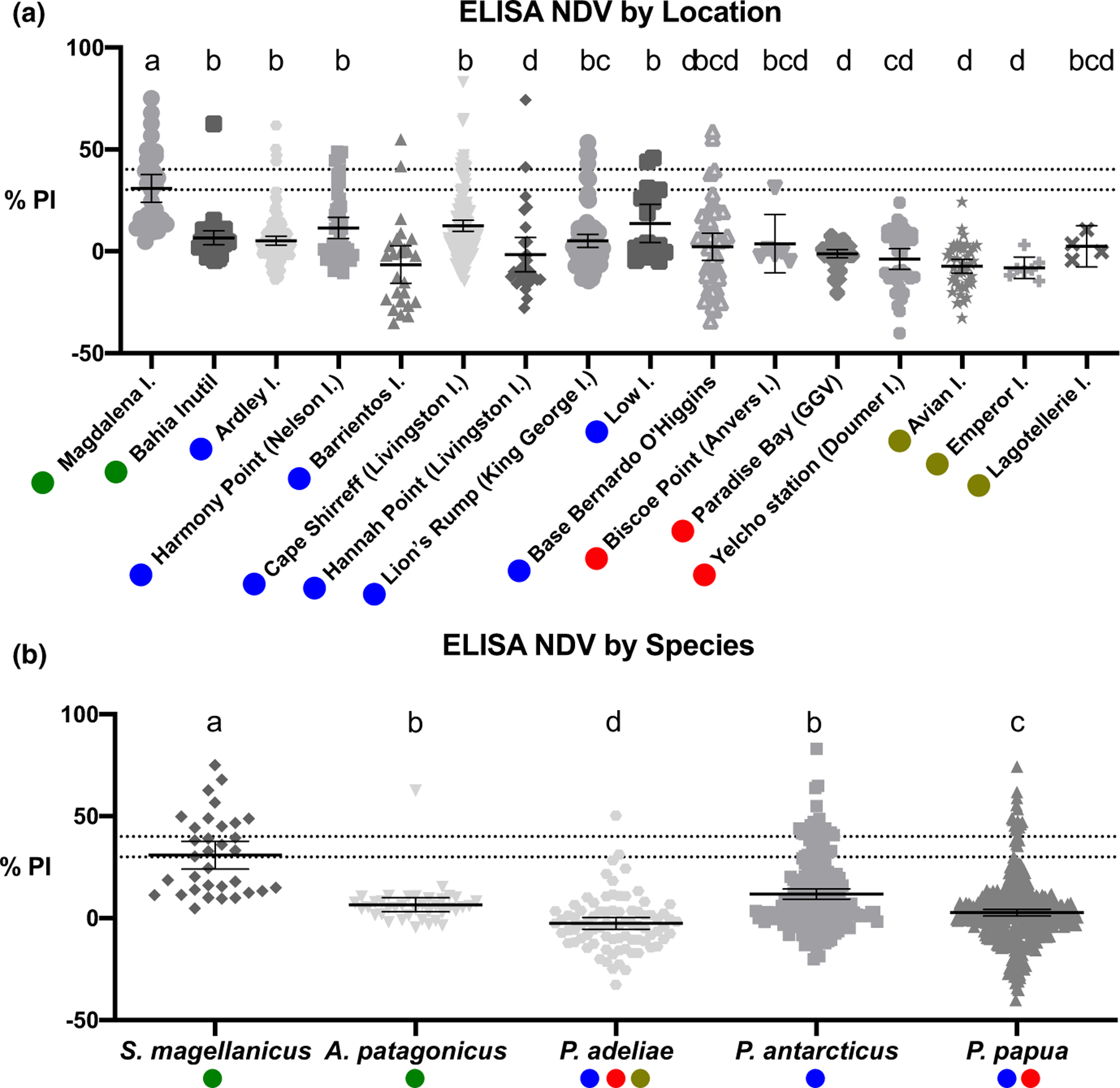
Identification of NDV seropositive positive penguins from Sub-Antarctic and Antarctic zones. Scatter plot of mean percent inhibition values (PI) with 95% CI, indicating antibody levels of penguin samples by location (a) and by of 30% and 40% (PI value ≥40: positive; 30–40: suspect; <30: negative). Green circles indicate samples taken in the continental area of the Magallanes region (sub-Antarctic locations); blue circles for samples collected from the Northwest Antartic region; red from Midwest; and brown for Southwest. Different letters above the columns indicate statistical difference (p < .05) [Colour figure can be viewed at wileyonlinelibrary.com]
Among Antarctic and sub-Antarctic regions, the Magellanic penguin and, therefore, Magdalena Island, had the highest antibody levels of all the species (H = 109.17, df = 4, p < .05, ε2 = 0.155) (Figure 2). In the Antarctic Peninsula, positive samples were only detected in the Northwest region, where the highest antibody levels were found in Low Island. However, there were no significant differences with other locations except for Barrientos Island and Hannah Point, which had the lowest antibody levels (p < .05) according to the multiple comparison test as shown in Figure 2a. The AOaV-17, AOaV-18 and AOaV-19 antisera were negative for the AOaV-1-ELISA; thus, there was no cross-reactivity between these viruses. Despite the low or nor detection of AOaV-1 antibodies, statistically significant differences were observed between years of sampling in Bernardo O’Higgins General Base (2018–2019, p < .05), Yelcho Base (2017–2018–2019; p < .05) and Avian Island (2017–2018; p < .05). The chi-square and Fisher’s exact tests confirmed the association between region and specie (p-value < .001), indicating that simple logistic regression should be performed, avoiding collinearity and the effect of potential confounding factors. The logistic regression evidence that S. magellanicus present a higher risk of positivity to NDV when compared to A. patagonicus (OR = 16.087; 95% CI = 1.930–134.087; p-value = .010); however, the other species sampled in this study did not show a significant difference (p > .05) (Table S3). Goodness-of-fit show a good adjustment between data and the model (p-value = .345).
These results show evidence of AOaV-1 infection in different penguin species from Antarctic and sub-Antarctic zones. We highlight this is the first report of AOaV-1 in the continental area of the Magallanes region of Chile and the first report that shows evidence of AOaV-1 infection in King penguins. A previous study, based on hemagglutination inhibition (HI) assay, screened AOaV-1 in King penguins but they did not detect antibodies against this virus (Gauthier-Clerc et al., 2002). In addition, this study suggests that this virus might circulate in Magellanic, Adelie, Chinstrap and Gentoo penguins in nature. A previous serologic study of Adelie, Chinstrap and Gentoo penguins from King George Island, Antarctica, by HI against AOaV-1, using an NDV B1 strain, a Class II NDV vaccine strain, reported an overall positivity of 33.3%. However, this study does not specify which species were positive, nor the seropositivity rate by species (Thomazelli et al., 2010) and also HI reports cross-reactivity as a limitation (Nayak et al., 2012).
Penguin species, including Antarctic penguins, have been described as potential reservoirs for several avian orthoavulaviruses, which could have the potential to infect other avian hosts (Wille et al., 2019). Given the high seropositivity (30.3%) to AOaV-1 seen in Magellanic penguins, we speculate that this species could be a natural reservoir for this virus. This is supported by a recent report from Argentina, where breeding colonies of this penguin species distributed along the entire coast were positive to AOaV-1, with a seroprevalence of 44% (Uhart et al., 2020). Furthermore, unlike the other sampled penguin species in this study, Magellanic penguin nest on the coasts and islands of southern Argentina and Chile, and migrate north in winter, reaching the coasts of Uruguay and southeastern Brazil (Yamamoto et al., 2019). Additionally, for at least three decades, over 25 rehabilitation centres have received and kept in captivity Magellanic penguins, until recovery, releasing them back into the wild (García-Borboroglu et al., 2006). It has been previously suggested that AOaV-1 may spillover from poultry into the environment (Garcia et al., 2013). Thus, the extended geographical movement of this species in South America and closer contact with people might result in their exposure to other animal pathogens. The high seroprevalence in this species may be of concern for potential outbreaks that could affect this or other avian species and highlights the need for further long-term surveillance in the region.
AOaV-1-positive penguins were detected in all sampled locations form the Northwest region of the Antarctic Peninsula, suggesting that at least at some point this virus circulated widely in this area. Thomazelli et al. (2010) also reported AOaV-1-positive penguins in this region (King George Island) (Thomazelli et al., 2010). Noteworthy, in our study no positive samples were detected in the Midwest and Southwest zones of the Peninsula. This might be a relevant finding due to the proximity of the Northwest zone of the Peninsula to South America and the possibility of migratory birds reaching this zone during the Southern Hemisphere summer. For example, South Polar Skuas (Stercorarius maccormicki), which have been found seropositive for AOaV-1 (Miller et al., 2008), migrate to the northern hemisphere and back in different times of the year. In fact, Yogui and Sericano (2009) suggest that skuas breeding at King George Island may winter in the northwestern Pacific Ocean (Yogui & Sericano, 2009). It would be important to confirm this hypothesis and determine the potential long-distance birds that might serve as carriers and introduce this virus to Antarctica or vice versa. Alternatively, penguins nesting in sub-Antarctic zones have been seen in Antarctica and vice versa, allowing the transmission of AOaV-1 from and to this region. For instance, King penguins have been seen in the north of the Antarctic Peninsula (Gryz et al., 2018; Juáres et al., 2017; Schiavini et al., 2005); as well as colonies of Gentoo penguins have been found on the south coast of Argentina (Schiavini et al., 2005). Finally, some species included in our study breed in mixed-species colonies or in proximity to other penguin species or other seabirds, which could facilitate the spread of the virus.
In general, serology has the advantage of showing the historical record of exposure to certain pathogens, so it is not necessary to detect animals during infection and shedding, which can be limited to a few days. This is especially important when asymptomatic or difficult to detect infections occurs, such as in penguins. Although the dynamics of AOaV-1 infection in penguins remain unknown, in poultry, the virus can be shed for up to 4 weeks, while antibodies can be detected within 6– 10 days of infection and persist for over a year (Paramyxoviridae & Pneumoviridae, 2017). The commercial ELISA kit used in this study is able to detect anti-AOaV-1 nucleoprotein antibodies in domestic and wild avian species. According to the manufacturer, this is a validated commercial with high sensitivity and specificity, earlier detection of seroconversion (7 dpi), detection of all AOaV-1 strains, and no cross-reactions with AOaV-3, which produces almost all the false positives in immunodiagnostic tests (Nayak et al., 2012; Rauw et al., 2020). Alternatively, we determine no cross-reactions with AOaV-17, AOaV-18, and AOaV-19, which have been widely detected in penguins from the Antarctic Peninsula (Neira et al., 2017; Olivares et al., 2019; Wille et al., 2019). However, the ELISA has some limitations, such as that it is not able to distinguish lineages and differentiate between Class I and Class II AOaV-1 strains. Therefore, we are not able to infer the potential virulence of the AOaV-1 strains that are infecting these penguin populations, which could have been achieved by an HI assay, using a specific AOaV-1 strain.
Another limitation is that the commercial ELISA kit has been not previously tested in penguins. However, the manufacturer recommended this kit for both, domestic and wildlife. The cut-off value for commercial ELISA kits may be optimized between species. Shriner et al. (2016), demonstrated different cut-off values in an ELISA Influenza kit for mallard ducks, using experimentally inoculated animals (Shriner et al., 2016). Additional studies aimed at viral isolation and sequencing are necessary to understand viral ecology and fully characterize the AOaV-1 strains circulating in Antarctic and sub-Antarctic penguins.
In conclusion, different penguin species and populations have been infected with AOaV-1. Further studies are necessary to determine the role of these penguin species in the ecology of the AOaV-1, as well as the pathogenic potential of this virus in these animal populations.
Supplementary Material
ACKNOWLEDGEMENTS
This paper is dedicated to the memory of Dr. Daniel Gonzalez-Acuña, whose contributions to Wildlife and Antarctic Science, inspire and encourage the new generations of veterinarians and scientists in Chile. We are grateful to the staff of Instituto Antártico Chileno for all their support during the Antarctic expeditions, especially to Pablo Espinoza, Renato Borras, Patricio Barraza, Pamela Santibañez and Wendy Rubio. We thank the Chilean Navy, especially to the crews of the AP-46 Almirante Oscar Viel and AP-41 Aquiles navy ships and the personal support from Marine Corps and Naval Aviation, for their assistance in field sampling. We are grateful to all field assistants especially to Juan Mena from the Animal Virology Lab, Facultad de Ciencias Veterinarias y Pecuarias, Universidad de Chile and Luis Muñoz Peralta ranger from Parque Pingüino Rey. To Corporación Nacional Forestal (CONAF) for the work permits in Monumento Natural Los Pingüinos, the logistic support and their crew during all the field seasons. To Wildlife Conservation Society (WCS) Chile for laboratory centrifuge for use in Parque Pingüino Rey.
Funding information
This study was partially funded by the Animal Virology Laboratory, Faculty of Veterinary and Animal Sciences, Universidad de Chile and Programa Fondecyt N° 11170877 and N° 1211517 to V.N.; INACH RT_46–16, PIA ACT 1408 from CONICYT to V.N and R.A.M., and the Center for Research in Influenza Pathogenesis (CRIP), a National Institute of Allergy and Infectious Diseases– funded Center of Excellence in Influenza Research and Surveillance (CEIRS), contract number HHSN272201400008C to V.N. and R.A.M.; the Global Penguin Society and the King Penguin Park to C.G.
Footnotes
CONFLICT OF INTEREST
Authors have no conflict of interest to declare.
ETHICAL APPROVAL
All authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received. All sampling protocols were approved by the Ethical Scientific Committee for Animal and Environmental Care of the Pontifical Catholic University of Chile, (certificate number 150430002), and the Subsecretaría de pesca y Acuicultura de Chile permissions (certificate numbers 2549–2011, 3199–2014 y 1014–2017) and Corporación Nacional de Chile (CONAF) permission (certificate 453–2011).
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Cardenas Garcia S, Navarro Lopez R, Morales R, Olvera MA, Marquez MA, Merino R, Miller PJ, & Afonso CL (2013). Molecular epidemiology of Newcastle disease in Mexico and the potential spillover of viruses from poultry into wild bird species. Applied and Environmental Microbiology, 79(16), 4985–4992. 10.1128/AEM.00993-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohoo I, Martin W, & Stryhn H (2010). Logistic regression. In McPike CSM (Ed.), Veterinary epidemiologic research (2nd ed., pp. 396–422). AVC Inc. University of Prince Edward Island. [Google Scholar]
- García-Borboroglu P, Boersma PD, Ruoppolo V, Reyes L, Rebstock GA, Griot K, Heredia SR, Adornes AC, & da Silva RP (2006). Chronic oil pollution harms Magellanic penguins in the Southwest Atlantic. Marine Pollution Bulletin, 52(2), 193–198. 10.1016/j.marpolbul.2005.11.004 [DOI] [PubMed] [Google Scholar]
- Gauthier-Clerc M, Eterradossi N, Toquin D, Guittet M, Kuntz G, & Le Maho Y (2002). Serological survey of the king penguin, Aptenodytes patagonicus, in Crozet Archipelago for antibodies to infectious bursal disease, influenza A and Newcastle disease viruses. Polar Biology, 25(4), 316–319. 10.1007/s00300-001-0346-7 [DOI] [Google Scholar]
- Gryz P, Gerlée A, & Korczak-Abshire M (2018). New breeding site and records of king penguins (Aptenodytes patagonicus) on King George Island (South Shetlands, Western Antarctic). Polar Record, 54(4), 275–283. 10.1017/S0032247418000554 [DOI] [Google Scholar]
- Hosmer DW, Lemeshow S, & Sturdivant R (2013). Applied logistic regression (3rd ed.). Wiley. International Committee on Taxonomy of Viruses (ICTV) (2019). Virus taxonomy: 2019 release. [Google Scholar]
- Juáres MA, Ferrer F, Coria NR, & Santos MM (2017). Breeding events of king penguin at the South Shetland Islands: Has it come to stay? Polar Biology, 40(2), 457–461. 10.1007/s00300-016-1947-5 [DOI] [Google Scholar]
- Miller GD, Watts JM, & Shellam GR (2008). Viral antibodies in south polar skuas around Davis Station, Antarctica. Antarctic Science, 20(5), 455–461. 10.1017/S0954102008001259 [DOI] [Google Scholar]
- Nayak B, Dias FM, Kumar S, Paldurai A, Collins PL, & Samal SK (2012). Avian paramyxovirus serotypes 2–9 (APMV-2–9) vary in the ability to induce protective immunity in chickens against challenge with virulent Newcastle disease virus (APMV-1). Vaccine, 30(12), 2220–2227. 10.1016/j.vaccine.2011.12.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neira V, Tapia R, Verdugo C, Barriga G, Mor S, Ng TFF, García V, Del Río J, Rodrigues P, Briceño C, Medina RA, & González-Acuña D (2017). Novel avulaviruses in penguins, Antarctica. Emerging Infectious Disease Journal, 23(7), 1212–1214. 10.3201/eid2307.170054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares F, Tapia R, Gálvez C, Meza F, Barriga GP, Borras-Chavez R, Mena-Vasquez J, Medina RA, & Neira V (2019). Novel penguin Avian avulaviruses 17, 18 and 19 are widely distributed in the Antarctic Peninsula. Transboundary and Emerging Diseases, 66(6), 2227–2232. 10.1111/tbed.13309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paramyxoviridae and Pneumoviridae (2017). Fenner’s veterinary virology (pp. 327–356). 10.1016/b978-0-12-800946-8.00017-9 [DOI]
- R Core Team (2020). European environment agency. Retrieved from https://www.eea.europa.eu/data-and-maps/indicators/oxygen-consuming-substances-in-rivers/r-development-core-team-2006
- Rahman A-U, Habib M, & Shabbir MZ (2018). Adaptation of Newcastle disease virus (NDV) in feral birds and their potential role in interspecies transmission. The Open Virology Journal, 12(1), 52–68. 10.2174/1874357901812010052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauw F, Ngabirano E, Gardin Y, Palya V, & Lambrecht B (2020). Effectiveness of a simultaneous rHVT-F(ND) and rHVT-H5(AI) vaccination of day-old chickens and the influence of NDV- and AIV-specific MDA on immune response and conferred protection. Vaccines, 8(3), 1–22. 10.3390/vaccines8030536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavini A, Yorio P, Gandini P, Raya Rey A, & Dee Boersma YP (2005). Los pingüinos de las costas argentinas: estado poblacional y conservación. El Hornero Revista de Ornitología Neotropical, 20, 5–23. [Google Scholar]
- Shriner SA, VanDalen KK, Root JJ, & Sullivan HJ (2016). Evaluation and optimization of a commercial blocking ELISA for detecting antibodies to influenza A virus for research and surveillance of mallards. Journal of Virological Methods, 228, 130–134. 10.1016/j.jviromet.2015.11.021 [DOI] [PubMed] [Google Scholar]
- Thomazelli LM, Araujo J, Oliveira DB, Sanfilippo L, Ferreira CS, Brentano L, Pelizari VH, Nakayama C, Duarte R, Hurtado R, Branco JO, Walker D, & Durigon EL (2010). Newcastle disease virus in penguins from King George Island on the Antarctic region. Veterinary Microbiology, 146(1–2), 155–160. 10.1016/j.vetmic.2010.05.006 [DOI] [PubMed] [Google Scholar]
- Uhart M, Thijl Vanstreels RE, Gallo L, Cook RA, & Karesh WB (2020). Serological survey for select infectious agents in wild Magellanic penguins (Spheniscus magellanicus) in Argentina, 1994–2008. Journal of Wildlife Diseases, 56(1), 66. 10.7589/2019-01-022 [DOI] [PubMed] [Google Scholar]
- Wille M, Aban M, Wang J, Moore N, Shan S, Marshall J, González-Acuña D, Vijaykrishna D, Butler J, Wang J, Hall RJ, Williams DT, & Hurt AC (2019). Antarctic penguins as reservoirs of diversity for avian avulaviruses. Journal of Virology, 93(11), 1–12. 10.1128/JVI.00271-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Yoda K, Blanco GS, & Quintana F (2019). Female-biased stranding in Magellanic penguins. Current Biology, 29, R12–R13. 10.1016/j.cub.2018.11.023 [DOI] [PubMed] [Google Scholar]
- Yogui GT, & Sericano JL (2009). Levels and pattern of polybrominated diphenyl ethers in eggs of Antarctic seabirds: Endemic versus migratory species. Environmental Pollution, 157(3), 975–980. 10.1016/j.envpol.2008.10.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


