Abstract
Background
Dysmenorrhoea (period pain) is a common condition with a substantial impact on the well‐being and productivity of women. Primary dysmenorrhoea is defined as recurrent, cramping pelvic pain that occurs with periods, in the presence of a normal uterus, ovaries and fallopian tubes. It is thought to be caused by uterine contractions (cramps) associated with a high level of production of local chemicals such as prostaglandins. The muscle of the uterus (the myometrium) responds to these high levels of prostaglandins by contracting forcefully, causing low oxygen levels and consequently pain. Nifedipine is a calcium channel blocker in widespread clinical use for preterm labour due to its ability to inhibit uterine contractions in that setting. This review addresses whether this effect of nifedipine also helps with relief of the uterine contractions during menstruation
Objectives
To assess the effectiveness and safety of nifedipine for primary dysmenorrhoea.
Search methods
We searched for all published and unpublished randomised controlled trials (RCTs) of nifedipine for dysmenorrhoea, without language restriction and in consultation with the Cochrane Gynaecology and Fertility Group (CGF) Information Specialist.
The following databases were searched to 25 November 2021: the Cochrane Gynaecology and Fertility Group (CGF) Specialised Register of Controlled Trials, CENTRAL, MEDLINE, Embase, PsycINFO, and CINAHL. Also searched were the international trial registers: ClinicalTrials.gov, and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal, the Web of Science, OpenGrey, LILACS database, PubMed and Google Scholar. We checked the reference lists of relevant articles.
Selection criteria
We included RCTs comparing nifedipine with placebo for the treatment of primary dysmenorrhoea.
Data collection and analysis
The primary outcomes to be assessed were pain, and health‐related quality of life. Secondary outcomes were adverse effects, satisfaction, and need for additional medication. The two review authors independently assessed the included trials. There were insufficient data to allow meaningful meta‐analysis.
Main results
The evidence assessed was of very low quality overall. We examined three small RCTs, with a total of 106 participants. Data for analysis could be extracted from only two of these trials (with a total of 66 participants); two trials were published in the 1980s, and the third in 1993. Nifedipine may be effective for "any pain relief" compared to placebo in women with primary dysmenorrhoea (odds ratio (OR) 9.04, 95% confidence interval (CI) 2.61 to 31.31; 2 studies, 66 participants; very low‐quality evidence). The evidence suggests that if the rate of pain relief using placebo is 40%, the rate using nifedipine would be between 64% and 95%. For the outcome of "good" or "excellent" pain relief, nifedipine may be more effective than placebo; the confidence interval was very wide (OR 43.78, 95% CI 5.34 to 259.01; 2 studies, 66 participants; very low‐quality evidence). We are uncertain if the use of nifedipine was associated with less requirement for additional analgesia use than placebo (OR 0.54, 95% CI 0.07 to 4.20, 1 study, 42 participants; very low‐quality evidence). Participants indicated that they would choose to use nifedipine over their previous analgesic if the option was available. There were similar levels of adverse effects and menstruation‐related symptoms in the placebo and intervention groups (OR 0.94, 95% CI 0.08 to 10.90; 1 study, 24 participants; very low‐quality evidence); if the chance of adverse effects with placebo is 80%, the rate using nifedipine would be between 24% and 98%. There were no results regarding formal assessment of health‐related quality of life.
Authors' conclusions
The evidence is insufficient to confirm whether nifedipine is a possible medical treatment for primary dysmenorrhoea. The trials included in this review had very low numbers and were of low quality. Notably, there was a large imbalance in numbers randomised between placebo and treatment groups in one of the two trials with data available for analysis. While there was no evidence of a difference noted in adverse effects between groups, more data from larger participant numbers are needed for this outcome. Larger, more well‐conducted trials are required to elucidate the potential role of nifedipine in the treatment of this common condition, as it could be a useful addition to the therapeutic options available if shown to be well tolerated and effective. The safety of nifedipine in women of reproductive age is well established from trials of its use in preterm labour, and clinicians are accustomed to off‐label use for this indication. The drug is inexpensive and readily available. Other options for relief of primary dysmenorrhoea are not suitable for all women; NSAIDs and the oral contraceptive pill (OCP) are contraindicated for some women, and the OCP is not suitable for women who are trying to conceive. In addition, the trials examined suggest there may be a participant preference for nifedipine.
Plain language summary
Nifedipine for primary dysmenorrhoea (period pain)
Review question
Is nifedipine safe and effective for the relief of pain associated with primary dysmenorrhoea (also known as period pain or menstrual cramps)?
Background
Primary dysmenorrhoea is pain associated with menstruation (periods) due to cramping of the uterus (womb). It is a common condition in women of reproductive age, and can have a significant impact on normal activities. Nifedipine is a medication that is effective at slowing contractions of the uterus in pregnant women with preterm labour. This review addresses whether nifedipine also helps with relieving uterine contractions during menstruation.
Study characteristics
We found three randomised controlled trials (experiments where each person has an equal chance of being chosen to receive the treatment or a comparator). They compared the use of nifedipine with placebo (dummy pill) for primary dysmenorrhoea. A total of 106 women were included in the trials; however only information from two trials containing a total of 66 women was available for analysis. In one of these trials, randomisation was very unbalanced between groups: only five women received placebo whereas 19 received nifedipine. Our search for trials was done on 31 January 2019, and repeated on 5 June 2020 and 25 November 2021. One trial was identified through discussion with colleagues.
Key results
Overall we are uncertain of the effectiveness of nifedipine for relief of pain in primary dysmenorrhoea. Nifedipine may be effective for overall pain relief, and for obtaining the subsets of "good" or "excellent" pain relief. Caution is needed in drawing conclusions from this as the analysis is based on very low participant numbers. In the study where the question was asked, women who received nifedipine were more likely to prefer to continue taking the medication for future cycles than women taking the placebo (12/19 women taking nifedipine, versus 0/5 women taking placebo). In one study, participants taking nifedipine who were usually severely incapacitated by periods had a significant improvement in the ability to carry out daily activities compared to those assigned to placebo. In both trials where adverse effects were assessed there was a high, and similar, rate of adverse physical symptoms associated with menstruation both in women taking nifedipine and those taking placebo.
Quality of the evidence
We rated the quality of the evidence as very low. There was generally inadequate reporting of study methods, and one trial did not report results in a way that could be analysed. Results for analysis were drawn from only two trials, which included a total of 66 women, one of which had unbalanced randomisation.
Summary of findings
Summary of findings 1. Nifedipine compared to placebo for primary dysmenorrhoea.
| Nifedipine compared to placebo for primary dysmenorrhoea | ||||||
| Patient or population: primary dysmenorrhoea Setting: outpatient clinic Intervention: nifedipine Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with nifedipine | |||||
| Pain relief (any) | 400 per 1,000 | 858 per 1,000 (635 to 954) | OR 9.04 (2.61 to 31.31) | 66 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1,2 | |
| Good or excellent pain relief | 0 per 1,000 | 0 per 1,000 (0 to 0) | OR 43.78 (5.34 to 359.01) | 66 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1,2 | |
| Health‐related quality of life | Not reported in any study | |||||
| Total adverse effects | 800 per 1,000 | 790 per 1,000 (242 to 978) | OR 0.94 (0.08 to 10.90) | 24 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2,3 | |
| Bothersome adverse effects | 400 per 1,000 | 265 per 1,000 (45 to 737) | OR 0.54 (0.07 to 4.20) | 24 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2,3 | |
| Requirement for additional medication | 800 per 1,000 | 219 per 1,000 (74 to 561) | OR 0.07 (0.02 to 0.32) | 42 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2, 4 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded 2 levels for imprecision; data from two small trials and thus likely underpowered to make reliable conclusions about outcomes 2Downgraded 1 level for serious risk of bias; very uneven allocation to intervention and control groups in one trial 3Downgraded 2 levels for imprecision; data only from one small trial and thus likely underpowered to make reliable conclusions about outcome 4Downgraded 1 level for serious risk of bias; unexplained high attrition (8/50 participants)
Background
Description of the condition
Dysmenorrhoea (period pain) is a common condition that has a substantial impact on the well‐being and productivity of women. When the pain is not due to a medical condition such as endometriosis, but thought to be related to cramping of the uterus, it is referred to as primary dysmenorrhoea.
Primary dysmenorrhoea is defined as recurrent, cramping pelvic pain that occurs with periods, in the presence of a normal uterus, ovaries and fallopian tubes. It is thought to be caused by uterine contractions (cramps) associated with a high level of production of local chemicals such as prostaglandins. The muscle of the uterus (the myometrium) responds to these high levels of prostaglandins by contracting forcefully, causing low oxygen levels and consequently pain. Menstrual pain affects up to 90% of menstruating women presenting to primary care (Jamieson 1996). An Australian study, in which women of reproductive age from randomly chosen households were interviewed by phone, showed prevalence of dysmenorrhoea of 71.7%, with severe pain being reported by 15% of these women (Pitts 2008). Primary dysmenorrhoea is often minimised or disregarded as a 'normal' part of having periods. However, it has a significant negative impact on quality of life and dysmenorrhoea is a common cause of absenteeism from both education and work environments in women ( Coco 1999; Iacovides 2015; Burnett 2017).
Description of the intervention
The uterus is mainly composed of smooth muscle tissue, which is a type of muscle in the body that is not under voluntary control. Smooth muscle cells need calcium to contract. Calcium channel blockers, such as nifedipine, reduce the amount of calcium that passes into the muscle cells and so prevent them from contracting. It is therefore suggested that administration of nifedipine may decrease menstrual pain by inhibiting uterine contractions (Proctor 2006).
Nifedipine is widely used to decrease uterine muscle contractions in clinical practice in obstetrics, where it is used to suppress preterm labour. Doses of nifedipine 10 mg to 20 mg are given orally, sublingually (under the tongue), or via both routes of administration, initially at 30‐minute intervals and then at approximately three‐hour intervals. Nifedipine is also used to treat hypertension in pregnancy. Although the available data for the first trimester are limited, the use of nifedipine in pregnancy has not been associated with birth defects in human studies (Smith 2000). There are no current uses in gynaecology.
Nifedipine is generally well tolerated by women of reproductive age. Adverse effects commonly reported include facial flushing, headache, symptomatic tachycardia (e.g. palpitations) and symptomatic hypotension (faintness) (Childress 1994). Other common adverse effects include rash, peripheral oedema (swelling), abdominal pain, and gingival hyperplasia (increase in gum tissue). Infrequent side effects include pulmonary oedema (fluid in lung tissue and air spaces), chest pain, dyspepsia (indigestion), constipation, muscle cramps, paraesthesia (pins and needles), and polyuria (frequency in urination) (AMH 2017).
Effective medical therapeutic options for primary dysmenorrhoea include nonsteroidal anti‐inflammatory drugs (NSAIDs) (Marjoribanks 2015) and the combined oral contraceptive pill (COCP) (Wong 2009). NSAIDs are very effective for pain relief associated with primary dysmenorrhoea, due to their inhibitory action on prostaglandin formation. NSAIDs appear to be more effective in this setting than paracetamol (Marjoribanks 2015). However, NSAIDs are associated with significant adverse effects. The COCP is used widely in clinical practice to improve period pain, heaviness of bleeding, and regularity. However, a Cochrane Review demonstrated only limited evidence for relief of pain associated with COCP (Wong 2009).
The medications known to be effective (NSAIDs and COCP) are, however, not suitable for all women with primary dysmenorrhoea. An example is that women who are trying to conceive would not wish to use the oral contraceptive pill. NSAIDs are not suitable for all women because use can be detrimental for some medical conditions. For example, NSAIDs are a well‐recognised trigger for asthma and should be avoided in women with this sensitivity. Women with underlying kidney problems generally should avoid NSAIDs as they can worsen kidney function. There are no medication contraindications to nifedipine in these groups of women (AMH 2017). Even women who are trying to conceive and wishing to avoid taking medication as much as possible could be reassured of no exposure, given that ovulation and subsequent conception occurs 10 to 14 days after the beginning of the period, which is the time at which nifedipine would be taken. Nifedipine has a half life of approximately 1.7 to 3.4 hours (Nifepidine product information).
How the intervention might work
Laboratory results on the effects of nifedipine on uterine muscle cells show inhibition of both spontaneous and induced myometrial contractility (Forman 1979; Moynihan 2008). Nifedipine is first‐line therapy for suppression of uterine contractions to prolong pregnancy in women presenting with threatened preterm labour, and has shown to be more effective for this indication than placebo and other drugs used for this purpose (Flenady 2014). Nifedipine may therefore also be effective for treatment of women with primary dysmenorrhoea. It is logical that this effect may decrease myometrial contraction‐related pain in women with primary dysmenorrhoea. To date, nifedipine for this potential indication has not been summarised in the literature, nor is it used in clinical practice for this indication.
Why it is important to do this review
There are effective interventions for primary dysmenorrhoea, including hormonal suppression of menses, and NSAIDs. However, these methods are not suitable for all women, such as those who wish to conceive, or who have kidney impairment or NSAIDs‐sensitive asthma. If nifedipine can be shown to be effective and safe for the treatment of primary dysmenorrhoea, women will have an additional option for management of this common and sometimes disabling condition. Nifedipine could potentially be used alone or in combination with other medical therapy.
Objectives
To assess the effectiveness and safety of nifedipine for primary dysmenorrhoea.
Methods
Criteria for considering studies for this review
Types of studies
Published and unpublished randomised controlled trials (RCTs) were eligible for inclusion. We excluded non‐randomised studies (e.g. studies with evidence of inadequate sequence generation, such as use of alternate days or patient numbers) because they are associated with a high risk of bias. We planned to include cross‐over trials, because this is a valid design in this context.
Types of participants
Trials with women with primary dysmenorrhoea were eligible for inclusion. Primary dysmenorrhoea was defined as painful uterine cramps associated with menstrual periods, in the absence of known pelvic pathology.
Types of interventions
Trials comparing nifedipine with any other pharmacological intervention or placebo were eligible for inclusion. We also planned to include trials comparing the addition of nifedipine to other pharmacological agents; for example 'drug X plus nifedipine versus drug X alone'. Nifedipine could have been administered by any route. Nifedipine had not been included in other published Cochrane Reviews regarding dysmenorrhoea as a comparator.
Types of outcome measures
Primary outcomes
-
Pain. Eligible measures of pain included:
0 to 10 numeric pain rating scale (preferred);
visual analogue scale;
binary pain measures (i.e. yes/no); and
other measures of pain as reported in the included studies.
Health‐related quality of life, using a validated tool such as SF‐36 (preferred measure).
Secondary outcomes
-
Adverse effects (including the following examples):
any adverse effect;
headache;
facial flushing;
dizziness/faintness/symptomatic hypotension; and
palpitations/symptomatic tachycardia.
Satisfaction rate (as defined by trial authors).
Requirement for additional medication. This could be measured by yes/no answer, including different types and doses of other medications.
We anticipated that studies may have reported outcomes at multiple time points. We planned to use data from the longest period of follow‐up for our primary and secondary outcomes.
Search methods for identification of studies
We searched for all published and unpublished RCTs of nifedipine for dysmenorrhoea, without language restriction and in consultation with the Cochrane Gynaecology and Fertility Group (CGF) Information Specialist. The search date was 25 November 2021.
Electronic searches
We searched the following electronic databases, trial registers and websites:
The Cochrane Gynaecology and Fertility Group (CGF) Specialised Register of Controlled Trials; ProCite platform, searched 25 November 2021 (Appendix 1);
CENTRAL via the Cochrane Register of Studies Online (CRSO); web platform, searched 25 November 2021 (Appendix 2);
MEDLINE; OVID platform (Appendix 3) searched from 1946 to 25 November 2021;
Embase; OVID platform (Appendix 4) searched from 1980 to 25 November 2021;
PsycINFO; OVID platform (Appendix 5) searched from 1806 to 25 November 2021;
CINAHL; EBSCO platform (Appendix 6) searched from 1961 to 5 June 2020. Any later CINAHL search output is contained in the 2021 CENTRAL search output.
The MEDLINE and Embase searches were limited by the Cochrane search strategy filters for identifying randomised trials, which appear in the Cochrane Handbook of Systematic Reviews of Interventions (Chapter 6, 6.4.11; Lefebvre 2011). We combined the CINAHL search with a trial filter developed by the Scottish Intercollegiate Guidelines Network (SIGN) (www.sign.ac.uk/what-we-do/methodology/search-filters/).
Other sources of trial searches included the international trial registers: ClinicalTrials.gov, a service of the USA National Institutes of Health, and the WHO ICTRP search portal, the Web of Science, OpenGrey, LILACS database. We also searched PubMed and Google Scholar to check for any recent trials that were not yet indexed in the major databases.
Searching other resources
We handsearched reference lists of articles retrieved by the search and discussed the subject area with colleagues to obtain any additional trials.
Data collection and analysis
Selection of studies
After an initial screen of titles and abstracts retrieved by the search, conducted by RAE, we retrieved the full text of all potentially eligible studies. Two review authors (RAE and RMG) independently examined these full‐text articles for compliance with the inclusion criteria and selected studies eligible for inclusion in the review. Disagreements about study eligibility were resolved by discussion. We documented the selection process in a PRISMA flow chart.
Data extraction and management
The two review authors (RAE and RMG) independently extracted data from eligible studies using a data extraction form they designed and pilot‐tested. Any disagreements were resolved by discussion. Data extracted included study characteristics and outcome data.
Assessment of risk of bias in included studies
The review authors, independently of each other, used the Cochrane 'Risk of bias' tool to assess bias related to: selection (random sequence generation and allocation concealment); performance (blinding of participants and personnel); detection (blinding of outcome assessors); attrition (incomplete outcome data); reporting (selective reporting); and other bias (Higgins 2011). Disagreements were resolved by discussion. We described all judgements fully and presented the conclusions in 'Risk of bias' tables, which were incorporated into the interpretation of review findings by means of sensitivity analyses (see Sensitivity analysis).
We anticipated that attrition bias may be a challenge for this review, because dropping out is likely to be associated with lack of effect. We had planned to rate studies as being at high risk of bias if dropouts exceed 10%, or where the rate of attrition between the arms differed by over 10%. Given the subjective nature of the outcomes, unblinded trials were rated as being at high risk of bias.
Measures of treatment effect
For dichotomous data, we planned to use the numbers of events in the control and intervention groups of each study to calculate Mantel‐Haenszel odds ratios (ORs). For continuous data (e.g. pain score), if all studies reported exactly the same outcomes we planned to calculate the mean difference (MD) between treatment groups. If similar outcomes were reported on different scales we planned to calculate the standardised mean difference (SMD). An SMD of 0.2 would have been interpreted as representing a small effect, 0.5 as a moderate effect and 0.8 as a large effect. We planned to reverse the direction of effect of individual studies, if required, to ensure consistency across trials. We planned to treat ordinal data (e.g. quality‐of‐life scores) as continuous data. We planned to present 95% confidence intervals for all outcomes. Where data to calculate OR or MD were not available, we planned to use the most detailed numerical data available that may facilitate similar analyses of included studies (e.g. test statistics, P values). We planned to compare the magnitude and direction of effect reported by studies with how they are presented in the review, taking account of legitimate differences.
Unit of analysis issues
The primary analysis was to be per woman randomised. Statistical advice would have been sought regarding the analysis of cross‐over trials, to facilitate the appropriate inclusion of cross‐over data in meta‐analyses. Our approach of first choice was to incorporate cross‐over trials in the meta‐analysis and analyse as if the trial was a parallel‐group trial. Standard errors and CIs for these trials would have been adjusted to account for the paired design.
Dealing with missing data
We planned to analyse only the available data (i.e. not to apply any imputation). As noted above, if there was a large amount of missing data we recognised there would be a high risk of bias.
Assessment of heterogeneity
We planned to consider whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We would have assessed statistical heterogeneity by the measure of the I² statistic. An I² measurement greater than 50% would have been taken to indicate substantial heterogeneity.
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimise the potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. If we had been able to include 10 or more studies in an analysis, we planned to construct a funnel plot to explore the possibility of small‐study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
If we included a sufficient number of studies and they were similar, we planned to combine data using a fixed‐effect model in the following comparisons:
nifedipine versus placebo, stratified by dose;
nifedipine versus alternative active therapy, with separate analyses for each comparison;
active therapy plus nifedipine versus the same active therapy alone.
If the studies were insufficiently similar to pool, the evidence was to be summarised in a narrative synthesis.
An increase in the odds of a particular outcome, which may be beneficial (e.g. improved quality‐of‐life scores) or detrimental (e.g. adverse effects), is displayed graphically in the forest plots to the right of the centre line; and a decrease in the odds of an outcome is displayed to the left of the centre line. Statistical analysis was performed using Review Manager 5.3 (Review Manager 2014).
Subgroup analysis and investigation of heterogeneity
We planned to consider whether the clinical and methodological characteristics of the included trials were sufficiently similar for meta‐analysis to provide a clinically meaningful summary, and to assess statistical heterogeneity with the I² statistic, with an I² measurement greater than 50% being used to indicate high levels of heterogeneity.
In the event of substantial heterogeneity (I² greater than 50%), we planned to explore possible explanations in subgroup analyses (e.g. different populations) and sensitivity analyses (e.g. differing risk of bias), or both. We would have taken any statistical heterogeneity into account when interpreting the results, especially if there was any variation in the direction of effect. There were no pre‐planned subgroup analyses.
Sensitivity analysis
We planned to conduct sensitivity analyses for the primary outcomes to determine whether the conclusions were robust to arbitrary decisions made regarding the eligibility and analysis.
These analyses would have considered whether the review conclusions would have differed if:
eligibility was restricted to studies without high risk of bias (defined as studies at low risk of bias in all or most domains, and not at high risk of bias in any domain);
outcomes were measured at the first follow‐up time point rather than at the longest follow‐up time point;
a random‐effects model had been adopted; and
the summary effect measure was the risk ratio rather than odds ratio.
Summary of findings and assessment of the certainty of the evidence
We prepared a summary of findings table to present the results of the meta‐analysis for the main comparison (nifedipine versus placebo), using GRADEpro GDT (GRADEpro GDT 2015) and Cochrane methods (Higgins 2011). No studies collected information about health‐related quality of life and this is noted in the summary of findings table. We were unable to perform a meta‐analysis for side effects or requirement for additional medication, so we have presented the results from the single study for which we could extract data for analysis for these outcomes in the summary of findings table.
We assessed the quality of the evidence using GRADE criteria, considering: risk of bias, consistency of effect, imprecision, indirectness, and publication bias. Judgements about the quality of the evidence (high, moderate, low, or very low) were made by two review authors working independently, with disagreements resolved by discussion. Judgements were justified, documented, and incorporated into reporting of results for each outcome. We extracted study data, formatted our comparisons in data tables, and prepared the summary of findings table before writing the results and conclusions of our review.
Results
Description of studies
Results of the search
The search identified 20 studies. After examination of titles, we excluded 12 for ineligibility. The abstracts were retrieved for further examination; of these, eight full‐text articles were obtained for assessment. We independently checked these for eligibility, and three studies were assessed as being eligible for inclusion (Figure 1).
1.
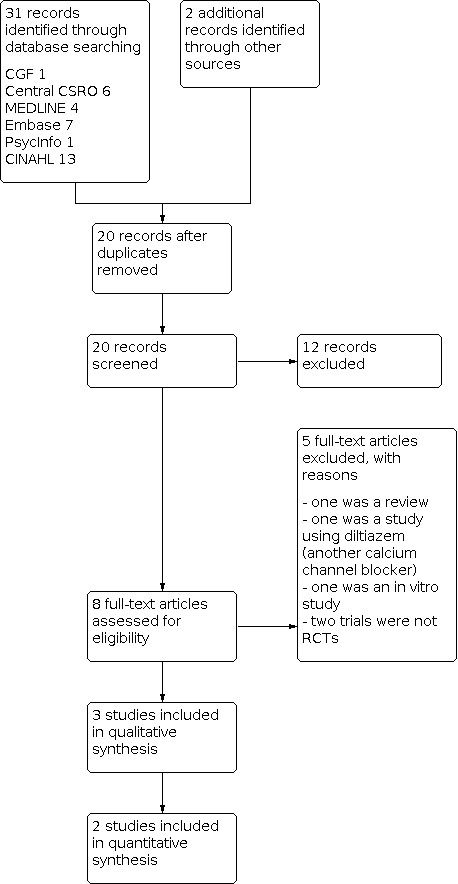
Study flow diagram.
Included studies
See: Characteristics of included studies.
Trial design and setting
The review includes three studies, all RCTs comparing nifedipine with placebo. Two were published in the mid‐1980s and the third in 1993. A total of 106 women were included. The study by Mondero was carried out in the USA, and examined use of nifedipine over one menstrual cycle; there were 24 participants in total (Mondero 1983). The study by Gavino and colleagues was published in Spanish and translated; it was conducted in Mexico, and studied 120 accumulated cycles for 40 women over three menstrual cycles (Gavino 1986). The third study was conducted in India and studied three consecutive menstrual cycles in 42 women (Kulshreshtha 1993).
Participants
All trials included women of reproductive age (15 to 35 years, 16 to 30 years, and 14 to 25 years). Primary dysmenorrhoea was clearly defined in each study; history and gynaecological examination were used to exclude secondary dysmenorrhoea. Participants had to be otherwise healthy. In one study, participants had to have pain that was significant enough to require the use of analgesics in previous cycles (Mondero 1983), and in another, information about the impact of dysmenorrhoea on daily activities was collected over the three cycles prior to the intervention, with a "severity score" calculated to reflect the impact (Kulshreshtha 1993). One study stated explicitly that participants were not using any intrauterine devices or oral contraceptives (Gavino 1986); the other studies did not include details regarding use of contraceptives or other hormones.
Interventions
All trials compared nifedipine to placebo. Nifedipine 10 mg capsules were used in two studies, but dosage regimens differed. In Mondero 1983, participants were instructed to take one capsule at the onset of cramping, and if no relief was obtained in 45 to 60 minutes, a repeat dose was taken. If there was no improvement after two hours, no further doses were to be taken. If there was some relief, participants could then take one capsule every four hours as needed, up to a maximum of four capsules per day. This could be repeated the following day if necessary. In Gavino 1986, one capsule was taken at the onset of dysmenorrhoea; this dose was taken every eight hours if there was not complete relief with the first dose. In the third study, 5mg of nifedipine could be taken every 8 hours as needed for up to three days (Kulshreshtha 1993).
Outcomes
All studies assessed the effectiveness of the medication by subjectively measuring the relief of dysmenorrhoea. All used categorical scales to record the degree of pain relief. The total dosages used were recorded except in the study by Kulshreshtha and colleagues. All studies collected some information about side effects. One study also collected information regarding heaviness of menstrual bleeding (Gavino 1986). The study by Mondero assessed satisfaction by asking participants to compare the study drug to the usual medication used for their dysmenorrhoea, and also asked participants to state whether they would choose to use the study drug monthly on an ongoing basis if it was available. Only one study collected information about additional analgesic use during the study period; this study also assessed the impact of the intervention on the ability to carry out normal daily activities in those participants who usually were "severely incapacitated" during their cycles by comparing severity scores before and during the intervention (Kulshreshtha 1993).
Excluded studies
Five studies were excluded from the review. Two studies were single‐arm trials of nifedipine in women with primary dysmenorrhoea, without a comparison group. Another was an RCT comparing placebo with an alternative calcium channel blocker, diltiazem, in women with dysmenorrhoea. This was only published as an abstract, in French, and was translated for assessment for eligibility. All other excluded studies were not relevant to women with dysmenorrhoea. See: Characteristics of excluded studies.
Risk of bias in included studies
The risk of bias for each study is summarised in Figure 2 and Figure 3.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
All studies stated that they were randomised. One stated this was done using a random number table and we considered this to hold a low risk of bias (Gavino 1986). The other studies were unclear on the details of randomisation. One stated randomisation had been done through the hospital pharmacy (Mondero 1983). Although this is likely to be at low risk of bias, given the lack of clarity or detail around this method we formally assessed this study to be at an unclear risk of bias. Randomisation was unbalanced between groups: five participants received placebo and 19 were allocated to the intervention. There was no information about the method of randomisation in the study by Kulshreshtha and colleagues so we rated this at unclear risk of bias.
Allocation concealment
This was not addressed specifically in any study, so we assessed all to be at unclear risk of this bias.
Blinding
The trials by Gavino and colleagues and Kulshreshtha and colleagues both explicitly stated that a double‐blind technique was used, and so we rated these studies as being at low risk of bias. The other trial was less clear; an identical‐looking placebo capsule was used but it was unclear as to whether the investigator was blinded to the treatment allocated at data collection (Mondero 1983). Data were collected contemporaneously by the participants, but these data and side‐effects were discussed with the investigator at the end of the study, so it is possible that bias could have been introduced at this point if the investigator was unblinded.
Incomplete outcome data
All women were accounted for in all trials, and so they were judged to be at low risk of bias.
Selective reporting
All reported outcomes were pre‐specified in the methods and so all were rated as being at low risk of bias.
Other potential sources of bias
No other sources of bias were identified.
Effects of interventions
See: Table 1
Nifedipine versus placebo
The overall participant numbers involved were very small, with a total of 106 women over the three trials. The study by Mondero included raw numbers in the results tables (number of participants (n) = 24), but Gavino 1986 only presented the P value for the difference between the groups for relief‐of‐pain data. We did not attempt to contact the authors to obtain raw data, as the study was conducted in Mexico over 30 years ago, and published in a Spanish language journal (Gavino 1986). In the third study, the total score for relief of pain over the three cycles was presented (Kulshreshtha 1993).
1.1 Pain relief
The Mexican study did not display the raw data for relief of pain, but reported that there was some improvement in pain in both the placebo and nifedipine groups after medication administration, with greater improvement in the nifedipine group (Gavino 1986). However, without the raw data, results from this trial cannot be independently assessed. The American study reported actual numbers, with 'relief obtained' divided into four categories (none, some, good, excellent) (Mondero 1983). Only 19 participants received nifedipine, and five received placebo; these data were entered for comparison. In the third study, using total scores from three cycles, we determined whether there was any pain relief based on the scale described in the study. Any participant who had a total score at least nine or over was taken as having some relief of pain over the cycles. The overall mean of the severity scores between the two groups was significantly different in the study statistics, with a lower mean score in the participants receiving nifedipine (p<0.001). Overall, nifedipine may have led to an improvement in "any pain relief" compared to placebo (OR 9.04, 95% CI 2.61 to 31.31; 2 studies, 66 participants; low quality evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: Nifedipine vs placebo, Outcome 1: Pain relief (any)
1.2 Good or excellent pain relief
More participants reported "good" or "excellent" relief of pain after administration of nifedipine (14/19 women, 74%) than after placebo (0/5 women) in the Mondero study. In this study, 'drug failure' was defined as no improvement two hours after the initial dose of one or two capsules. Using this definition, three women experienced drug failures, giving an overall 'response rate' of 84%. In those who responded to nifedipine, the average time for improvement was 40 minutes, and the average time for complete relief was 74 minutes. In the study by Kulshreshtha and colleagues, patients with a total pain relief score of 15 or over were considered to have good/excellent relief of pain over the three cycles; there were 13 of 22 participants taking nifedipine in this range, with none of the 20 participants assigned to placebo in this group. In this study, onset of action was reported to be 15‐20 minutes. Overall, nifedipine may have led to an improvement in "good" or "excellent" pain relief (OR 43.78, 95% CI 5.34 to 359.01; 2 studies, 66 participants; low quality evidence; Analysis 1.2).
1.2. Analysis.

Comparison 1: Nifedipine vs placebo, Outcome 2: Good or excellent pain relief
1.3 Health‐related quality of life
This outcome was not formally collected in any study. However, in the Indian study, women in the nifedipine group had a significantly lower total severity score than those in the placebo group (mean 5.4/18 vs 12.6/18, p <0.001). This score reflected the ability of participants to undertake normal daily activities, with a lower score reflecting less impairment in usual functioning (Kulshreshtha 1993).
1.4 Adverse effects
Side effects were reported in all included studies but data suitable for analysis was only available in the study by Mondero. In this study, 15 out of 19 participants treated with nifedipine experienced apparent drug‐related symptoms; headache (n = 8) and facial flushing were the most commonly reported. In the corresponding placebo group, 4 out of 5 participants noted advers effects; headaches were again common and were reported by three participants (Mondero 1983). Comparisons were done for "total adverse effects" (OR 0.94, 95% CI 0.08 to 10.90; 1 study, 24 participants; Analysis 1.3) and "bothersome adverse effects"(OR 0.54, 95% CI 0.07 to 4.20; 1 study, 24 participants; Analysis 1.4), and we are uncertain whether nifedipine has increased adverse effects compared to placebo. The study by Gavino and colleagues reported symptoms associated with menstruation and found no difference between placebo and nifedipine overall; however headache and vomiting were more frequently reported in the nifedipine group (Gavino 1986). In the third study, authors stated that side effects of nifedipine were reported in 5 of 22 participants, but that these were transient, lasting only a few minutes (Kulshreshtha 1993). There was no information provided about adverse effects in the placebo group in this study.
1.3. Analysis.

Comparison 1: Nifedipine vs placebo, Outcome 3: Total side effects
1.4. Analysis.

Comparison 1: Nifedipine vs placebo, Outcome 4: Bothersome side effects
1.5 Satisfaction rate
In one study, 12 of the 19 participants who received nifedipine thought that the substance used was better than their previous analgesic, and 12 reported that they would use the substance monthly if possible; none of the participants receiving placebo agreed with these statements (Mondero 1983). In this study, five participants found the side effects "bothersome", but three of these would still choose to continue to use nifedipine regardless, given the efficacy. No assessment of overall satisfaction was made in the other trials. In one study, among participants who were usually "severely incapacitated" during periods, more of those allocated to nifedipine were able to continue their daily activities compared to the placebo group.
1.6 Requirement for additional medication
This information was collected in only one study (Kulshreshtha 1993). In the nifedipine group 6/22 participants required additional analgesics, compared to 16/20 participants in the placebo group (p<0.001).
Discussion
Summary of main results
Efficacy of nifedipine
The relevant trials included only small numbers of participants, so only limited conclusions can be drawn. The evidence is of very low quality, but suggests that nifedipine may be effective for the relief of pain in primary dysmenorrhoea. There were no data available regarding health‐related quality of life. In the one study where it was assessed, participants assigned to nifedipine were less likely to use additional analgesia during the menstrual cycle (Kulshreshtha 1993). One study asked about ongoing use of nifedipine; the majority of participants who received the active drug said they would choose to use it again, and in preference to the analgesic they had previously used for this indication (Mondero 1983).
Adverse effects of nifedipine
Again, data are limited due to small numbers of participants and incomplete reporting, but overall nifedipine was tolerated at a similar level as placebo. There were high, and similar, levels of adverse effects/symptoms of menstruation in both the placebo and intervention groups in both studies. The most frequent adverse effect reported was headache, which is an expected finding with nifedipine use, however this was also frequently reported in the placebo group.
Overall completeness and applicability of evidence
The searches identified only a very small amount of available evidence. The RCTs identified were carried out in an appropriate cohort for assessing the impact of medication on primary dysmenorrhoea. The search did not identify any studies that used active comparators or adjunctive therapy.
Quality of the evidence
Overall the evidence is of very low quality. The included trials were very small, and data from only two trials was available for analysis (n = 66). In one of the analysed studies, randomisation was very unbalanced between the placebo and intervention groups, with five participants allocated to placebo and 19 allocated to nifedipine. In regard to risk of bias, there were unclear or absent descriptions of randomisation, allocation concealment and blinding. One study did not report raw numbers for outcomes, including only a P value for efficacy for pain relief. One study clearly stated that participants rated pain relief as it occurred, but then discussion of the dosage, improvement, and side effects occurred at a follow‐up visit with the investigator, which could introduce bias depending on whether blinding was still present at this time.
Potential biases in the review process
We did not identify any sources of bias in the review process.
Agreements and disagreements with other studies or reviews
There are no other reviews assessing the use of nifedipine for dysmenorrhoea. Nifedipine is in routine clinical use in women of reproductive age for suppression of preterm contractions. It is generally well tolerated in clinical use for this indication, with a similar side effect profile as was reported in the included studies. It is reasonable to expect the same if used in a cohort of women of similar age and health status, apart from being non‐pregnant, for the relief of dysmenorrhoea.
The studies described are the only RCTs identified describing the use of nifedipine for primary dysmenorrhoea. There are two single‐arm trials, both of which were excluded as use of nifedipine was not compared to placebo or alternative active therapy, but they show a similar effect on pain, with similar side effects (Sandahl 1979; Ulmsten 1985). Also excluded was an abstract describing a small RCT evaluating another calcium channel blocker, diltiazem, which also showed a positive impact on pain scores in primary dysmenorrhoea (Audebert 1985). The included and excluded studies are all very old. In addition, we did not find any current ongoing studies examining the use of nifedipine for dysmenorrhoea. Given the widespread use of nifedipine for suppression of uterine contractions in preterm labour, it is curious that this medication has not received wider research interest for use in primary dysmenorrhoea.
The participants in the included trials are a similar cohort to patients seeking treatment for primary dysmenorrhoea. Many women with this condition are already using hormonal contraceptives or NSAIDs (or both) for treatment, both of which known to be are effective for primary dysmenorrhoea (Marjoribanks 2015; Burnett 2017; Wong 2009). Given that nifedipine does not interact with either of these categories of medication, as well as further studies regarding efficacy and tolerability of nifedipine as a single agent, it would be interesting to evaluate the effects of these different therapies in combination.
Authors' conclusions
Implications for practice.
The evidence available, although of low quality, suggests that nifedipine is a possible medical treatment for pain relief in women with primary dysmenorrhoea. Theoretically it has potential to be effective, and further trials would be useful. Consistent with this result, in the one study asking about ongoing use, the majority of participants who received the active drug would choose to use it again, and in preference to the analgesic they had previously used for this indication, compared to no participants in the placebo group. Although numbers are again very small, there was no difference in tolerability of nifedipine compared to placebo in this review. Adverse effects should be included as a primary outcome in future studies of nifedipine for primary dysmenorrhoea. Evidence in this regard may come from both randomised and observational studies.
Implications for research.
Larger and more well conducted trials are warranted to further examine the utility and safety of nifedipine for primary dysmenorrhoea. As well as efficacy, an effective and tolerable dosage schedule could be established. Comparison against placebo is vital, but nifedipine could be compared against medications shown to be effective for relief of primary dysmenorrhoea, especially nonsteroidal anti‐inflammatory drugs (NSAIDs), given they would be used in a similar episodic way around the time of menses. Outcomes in new trials could include relief of pain, need for additional medication as well as the trial medication, and preference for nifedipine over previously used medications. Examining adverse effects is vital (including any adverse effects and only those adverse effects that were serious enough to discourage use), as from our experience in clinical practice using nifedipine for women in preterm labour, some side effects are noticeable but tolerated by patients.
What's new
| Date | Event | Description |
|---|---|---|
| 10 January 2022 | Review declared as stable | Included reviews are more than 20 years old, and no ongoing studies are likely. Accordingly this review will not be updated. |
History
Protocol first published: Issue 12, 2017 Review first published: Issue 12, 2021
Acknowledgements
We wish to thank Helen Nagels, Managing Editor of Cochrane Gynaecology and Fertility Group, for her support and advice regarding the group's methods and procedures; and Marian Showell, Information Specialist, for her assistance with search strategies.
The authors thank Drs Rik van Eekelen and Jeppe Schroll for providing peer review feedback.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group (CGF) specialised register search
ProCite platform
Searched 25 November 2021
Keywords CONTAINS "dysmenorrhea" or "Dysmenorrhea‐Symptoms" or "dysmenorrhoea" or "pain‐dysmenorrhea" or "pain‐pelvic" or "pelvic pain" or "menstrual cramps" or "menstrual pain" or "primary dysmenorrhea" or "*Dysmenorrhea" or "menstrual distress" or "*menstrual pain" or "primary dysmenorrhea" or Title CONTAINS "dysmenorrhea" or "Dysmenorrhea‐Symptoms" or "dysmenorrhoea" or "pain‐dysmenorrhea" or "pain‐pelvic" or "pelvic pain" or "menstrual cramps" or "menstrual pain" or "primary dysmenorrhea" or "*Dysmenorrhea" or "menstrual distress" or "*menstrual pain" or "primary dysmenorrhea"
AND
Keywords CONTAINS "Nifedipine" or "calcium antagonists" or Title CONTAINS "Nifedipine" or "calcium antagonists"
(1 record)
Appendix 2. CENTRAL via the Cochrane Register of Studies Online (CRSO) search strategy
Web platform
Searched 25 November 2021
#1 MESH DESCRIPTOR Dysmenorrhea EXPLODE ALL TREES 655
#2 Dysmenorr* 2415
#3 (pain* period*):TI,AB,KY 88
#4 ((menstrua* cramp*)):TI,AB,KY 72
#5 ((menstrua* adj3 distress*)):TI,AB,KY 112
#6 ((period* adj3 cramp*)):TI,AB,KY 6
#7 ((menstrua* adj3 pain*)):TI,AB,KY 701
#8 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 2789
#9 MESH DESCRIPTOR Nifedipine EXPLODE ALL TREES 2144
#10 Nifedipine*:TI,AB,KY 3974
#11 (calcium antagoni*):TI,AB,KY 2414
#12 (calcium channel blocker*):TI,AB,KY 4707
#13 Adalat*:TI,AB,KY 136
#14 Procardia*:TI,AB,KY 12
#15 cordipin*:TI,AB,KY 4
#16 corinfar*:TI,AB,KY 18
#17 fenigidin*:TI,AB,KY 2
#18 #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 9119
#19 #8 AND #18 6
Appendix 3. MEDLINE search strategy
OVID platform
Searched from 1946 until 25 November 2021
1 exp Dysmenorrhea/ (4234) 2 pain$ period$.tw. (225) 3 menstrua$ cramp$.tw. (172) 4 (period$ adj3 cramp$).tw. (30) 5 (menstrua$ adj3 pain$).tw. (1613) 6 Dysmenorr$.tw. (6422) 7 (menstrua$ adj3 distress$).tw. (279) 8 or/1‐7 (8818) 9 exp Nifedipine/ (15704) 10 Nifedipine$.tw. (19706) 11 calcium antagoni$.tw. (11504) 12 calcium channel blocker$.tw. (16126) 13 Adalat$.tw. (189) 14 Procardia$.tw. (47) 15 cordipin$.tw. (12) 16 corinfar$.tw. (184) 17 fenigidin$.tw. (15) 18 nifangin$.tw. (3) 19 or/9‐18 (45047) 20 randomized controlled trial.pt. (551043) 21 controlled clinical trial.pt. (94552) 22 randomized.ab. (541447) 23 randomised.ab. (107753) 24 placebo.tw. (229401) 25 clinical trials as topic.sh. (198197) 26 randomly.ab. (370406) 27 trial.ti. (251645) 28 (crossover or cross‐over or cross over).tw. (91324) 29 or/20‐28 (1481868) 30 exp animals/ not humans.sh. (4918677) 31 29 not 30 (1364111) 32 8 and 19 and 31 (4)
Appendix 4. Embase search strategy
OVID platform
Searched from 1980 until 25 November 2021
1 exp dysmenorrhea/ (12335) 2 pain$ period$.tw. (336) 3 Dysmenorr$.tw. (8566) 4 menstrua$ cramp$.tw. (253) 5 (period$ adj3 cramp$).tw. (48) 6 (menstrua$ adj3 pain$).tw. (2271) 7 (menstrua$ adj3 distress$).tw. (313) 8 or/1‐7 (15541) 9 exp nifedipine/ (49612) 10 Nifedipine$.tw. (23840) 11 calcium antagoni$.tw. (14069) 12 calcium channel blocker$.tw. (22429) 13 Adalat$.tw. (2599) 14 Procardia$.tw. (796) 15 cordipin$.tw. (29) 16 corinfar$.tw. (333) 17 fenigidin$.tw. (12) 18 nifangin$.tw. (9) 19 or/9‐18 (78802) 20 8 and 19 (72) 21 Randomized controlled trial/ (679533) 22 Controlled clinical study/ (464287) 23 Random$.ti,ab. (1709867) 24 randomization/ (91973) 25 intermethod comparison/ (277070) 26 placebo.ti,ab. (326185) 27 (compare or compared or comparison).ti. (529871) 28 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. (2386205) 29 (open adj label).ti,ab. (92233) 30 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. (242895) 31 double blind procedure/ (186607) 32 parallel group$1.ti,ab. (28341) 33 (crossover or cross over).ti,ab. (110826) 34 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. (363438) 35 (assigned or allocated).ti,ab. (428191) 36 (controlled adj7 (study or design or trial)).ti,ab. (388554) 37 (volunteer or volunteers).ti,ab. (255447) 38 human experiment/ (557373) 39 trial.ti. (336835) 40 or/21‐39 (5503216) 41 20 and 40 (7)
Appendix 5. PsycINFO search strategy
OVID platform
Searched from 1806 until 25 November 2021
1 exp Dysmenorrhea/ (246) 2 pain$ period$.tw. (50) 3 Dysmenorr$.tw. (458) 4 menstrua$ cramp$.tw. (27) 5 pelvi$ pain$.tw. (683) 6 (period$ adj3 cramp$).tw. (3) 7 (menstrua$ adj3 pain$).tw. (248) 8 (menstrua$ adj3 distress$).tw. (281) 9 or/1‐8 (1561) 10 exp Channel Blockers/ (1101) 11 calcium antagoni$.tw. (158) 12 calcium channel blocker$.tw. (693) 13 Adalat$.tw. (1) 14 nifedipine.tw. (506) 15 or/10‐14 (1939) 16 9 and 15 (1)
Appendix 6. CINAHL search strategy
EBSCO platform
Searched from 1961 until 5 June 2020. Later CINAHL output is included in the CENTRAL 25 November 2021 search output.
| # | Query | Results |
| S33 | S20 AND S32 | 13 |
| S32 | S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 | 1,603,234 |
| S31 | TX allocat* random* | 13,319 |
| S30 | (MH "Quantitative Studies") | 30,611 |
| S29 | (MH "Placebos") | 13,729 |
| S28 | TX placebo* | 71,500 |
| S27 | TX random* allocat* | 13,319 |
| S26 | (MH "Random Assignment") | 68,389 |
| S25 | TX randomi* control* trial* | 222,168 |
| S24 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 1,219,382 |
| S23 | TX clinic* n1 trial* | 295,506 |
| S22 | PT Clinical trial | 110,871 |
| S21 | (MH "Clinical Trials+") | 320,131 |
| S20 | S9 AND S19 | 25 |
| S19 | S10 OR S11 OR S12 OR S13 OR S14 OR S15 | 7,886 |
| S18 | TX corinfar* | 0 |
| S17 | TX cordipine* | 0 |
| S16 | TX cordipin* | 0 |
| S15 | TX Procardia* | 21 |
| S14 | TX Adalat* | 30 |
| S13 | TX calcium channel blocker* | 6,131 |
| S12 | TX (calcium antagoni*) | 1,293 |
| S11 | TX Nifedipine* | 1,615 |
| S10 | (MM "Nifedipine") | 579 |
| S9 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 | 11,001 |
| S8 | TX(menstrua* N3 distress*) | 158 |
| S7 | TX (menstrua* N3 pain*) | 796 |
| S6 | TX (period* N3 cramp*) | 27 |
| S5 | TX (pelvi* pain*) | 5,321 |
| S4 | TX (menstrua* N3 cramp*) | 149 |
| S3 | TX (pain* period*) | 3,317 |
| S2 | TX Dysmenorr* | 2,526 |
| S1 | (MM "Dysmenorrhea") | 1,057 |
Data and analyses
Comparison 1. Nifedipine vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Pain relief (any) | 2 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.04 [2.61, 31.31] |
| 1.2 Good or excellent pain relief | 2 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 43.78 [5.34, 359.01] |
| 1.3 Total side effects | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.08, 10.90] |
| 1.4 Bothersome side effects | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.07, 4.20] |
| 1.5 Requirement for additional medication | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 13.60 [3.09, 59.83] |
1.5. Analysis.

Comparison 1: Nifedipine vs placebo, Outcome 5: Requirement for additional medication
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Gavino 1986.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Women aged 16 to 30 years with primary dysmenorrhoea (n = 40) | |
| Interventions | Nifedipine 10 mg sublingual 8 hourly as needed versus placebo | |
| Outcomes | Improvement in pain, duration of menstruation, amount of bleeding, menstrual symptoms/side‐effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used random number table with double‐blind technique |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated used double‐blind technique |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated used double‐blind technique |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if all participants accounted for |
| Selective reporting (reporting bias) | Low risk | Questionnaire used for all participants to collect data |
| Other bias | Low risk | Nil identified |
Kulshreshtha 1993.
| Study characteristics | ||
| Methods | Double blind randomised placebo controlled parallel trial | |
| Participants | 14‐25 year old females with primary dysmenorrhoea | |
| Interventions | Nifedipine 5mg 8 hourly as needed for up to 3 days (n = 22) versus placebo (n = 20) | |
| Outcomes | Relief score (ability to relieve symptoms), severity score (degree to which dysmenorrhoea interfered with activities), need for additional analgesics, side effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated in methods |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated used double‐blind technique |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Stated used double‐blind but not clearly described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8 out of total 50 patients dropped out of trial without stating reasons |
| Selective reporting (reporting bias) | Low risk | All remaining 42 participants' data included |
| Other bias | Low risk | Nil identified |
Mondero 1983.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Women aged 15 to 35 years with primary dysmenorrhoea (n = 24) | |
| Interventions | Nifedipine 10 mg capsules (n = 19) versus placebo (n = 5) | |
| Outcomes | Relief of pain, if substance was better than previous analgesic, if would use substance monthly, amount of relief, dosage schedule needed if more than one pill used, side effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Probably done but uncertain; "randomised through hospital pharmacy". Very uneven numbers in each group: nifedipine = 19; placebo = 5. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo capsules were identical‐looking. Not clearly stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clearly stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Unclear risk | Participants recorded reaction to the medications as they occurred but these and side effects were discussed with investigator at follow‐up visit |
| Other bias | Low risk | Nil identified |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andersson 1988 | Review article |
| Audebert 1985 | The intervention in this study was a different calcium channel blocker, ineligible for this review |
| Occhiuto 2009 | In vitro study of myometrial extracts |
| Sandahl 1979 | Not a randomised controlled trial (nifedipine given as intervention but no control) |
| Ulmsten 1985 | Not a randomised controlled trial (nifedipine given as intervention but no control) |
Differences between protocol and review
Contributions of authors
RAE drafted the review and RMG provided advice and systematic review expertise.
Sources of support
Internal sources
No formal sources of support, Other
External sources
No formal sources of support, Other
Declarations of interest
Rachel A Earl: none known.
Rosalie M Grivell: none known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Gavino 1986 {published data only}
- Gavino AS, Gavino GF, Ahued JRA. Use of a calcium antagonist in the management of primary dysmenorrhoea [Uso de un calcio-antagonista en el manejo de dismenorrea primaria]. Ginecologica y Obstetricia de Mexico 1986;54:208-10. [PubMed] [Google Scholar]
Kulshreshtha 1993 {published data only}
- Kulshreshtha S, Sharma P, Sharma AL, Agrawal S. Clinical trial of nifedipine in primary dysmenorrhoea. Indian Journal of Pharmacology 1993;25:88-90. [Google Scholar]
Mondero 1983 {published data only}
- Mondero NA. Nifedipine in the treatment of dysmenorrhoea. Journal of America Osteopathic Association May 1983;82(9 Supplement):704/19-708/23. [PubMed] [Google Scholar]
References to studies excluded from this review
Andersson 1988 {published data only}
- Andersson KE. Calcium antagonists and dysmenorrhoea. Annals of the New York Academy of Sciences March 1988;522(1):747-56. [DOI] [PubMed] [Google Scholar]
Audebert 1985 {published data only}
- Audebert AJM, Colle M, Coquelin JP, Emperaire JC. Study of a calcium inhibitor in dysmenorrhoea [Essai d'un inhibiteur calcique dans la dysmenorrhee]. La Presse Medicale 1985;14(3):163. [PubMed] [Google Scholar]
Occhiuto 2009 {published data only}
- Occhiuto F, Pino A, Palumbo DR, Samperi S, De Pasquale R, Sturlese E, et al. Relaxing effects of Valeriana officinalis extracts on isolated human non-pregnant uterine muscle. Journal of Pharmacy and Pharmacology 2009;61:251-6. [DOI] [PubMed] [Google Scholar]
Sandahl 1979 {published data only}
- Sandahl B, Ulmsten U, Andersson KE. Trial of the calcium antagonist nifedipine in the treatment of primary dysmenorrhoea. Archives of Gynecology 1979;227:147-51. [DOI] [PubMed] [Google Scholar]
Ulmsten 1985 {published data only}
- Ulmsten U. Calcium blockade as a rapid pharmacological test to evaluate primary dysmenorrhoea. Gynecologic and Obstetric Investigation 1985;20:78-83. [DOI] [PubMed] [Google Scholar]
Additional references
AMH 2017
- AMH. Australian Medicines Handbook. Adelaide: Australian Medicines Handbook Pty Ltd, 2017. [Google Scholar]
Burnett 2017
- Burnett M, Lemyre M. No. 345 - primary dysmenorrhea consensus guideline. Journal of Obstetrics and Gynaecology Canada 2017;39(7):585-95. [DOI] [PubMed] [Google Scholar]
Childress 1994
- Childress CH, Katz VL. Nifedipine and its indications in obstetrics and gynecology. Obstetrics and Gynecology 1994;83(4):616-24. [DOI] [PubMed] [Google Scholar]
Coco 1999
- Coco AS. Primary dysmenorrhea. American Family Physician 1999;60(2):489-96. [PubMed] [Google Scholar]
Flenady 2014
- Flenady V, Wojcieszek AM, Papatsonis DNM, Stock OM, Murray L, Jardine LA, et al. Calcium channel blockers for inhibiting preterm labour and birth. Cochrane Database of Systematic Reviews 2014, Issue 6. Art. No: CD002255. [DOI: 10.1002/14651858.CD002255.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Forman 1979
- Forman A, Andersson KE, Pesson CG, Ulmsten U. Relaxant effects of nifedipine on isolated, human myometrium. Acta Pharmacologica et Toxicologica 1979;45(2):81-6. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed prior to 5 December 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Iacovides 2015
- Iacovides S, Avidon I, Baker F. What do we know about primary dysmenorrhoea today: a critical review. Human Reproduction Update 2015;21(6):762-78. [DOI] [PubMed] [Google Scholar]
Jamieson 1996
- Jamieson DJ, Steege JF. The prevalence of dysmenorrhea, dyspareunia, pelvic pain, and irritable bowel syndrome in primary care practices. Obstetrics & Gynecology 1996;87:55-8. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s).. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Marjoribanks 2015
- Marjoribanks J, Ayeleke RO, Farquhar C, Proctor M. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database of Systematic Reviews 2015, Issue 7. Art. No: CD001751. [DOI: 10.1002/14651858.CD001751.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moynihan 2008
- Moynihan AT, Smith TJ, Morrison JJ. The relaxant effect of nifedipine in human uterine smooth muscle and the BK(Ca) channel. American Journal of Obstetrics and Gynecology 2008;198(2):237.e1-8. [DOI: 10.1016/j.ajog.2007.08.074] [DOI] [PubMed] [Google Scholar]
Nifepidine product information
- Bayer Australia. Adalat 10 and Adalat 20 tablets product information. www.bayerresources.com.au/resources/uploads/pi/file9302.pdf (accessed 30 November 2017).
Pitts 2008
- Pitts MK, Ferris JA, Smith AMA, Shelley JM, Richters J. Prevalence and correlates of three types of pelvic pain in a nationally representative sample of Australian women. Medical Journal of Australia 2008;189(3):138-43. [DOI] [PubMed] [Google Scholar]
Proctor 2006
- Proctor M, Farquhar C. Diagnosis and management of dysmenorrhoea. BMJ 2006;332(7550):1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Smith 2000
- Smith P. Nifedipine in pregnancy. British Journal of Obstetrics and Gynaecology 2000;107:299-307. [DOI] [PubMed] [Google Scholar]
Wong 2009
- Wong CL, Farquhar C, Roberts H, Proctor M. Oral contraceptive pill for primary dysmenorrhoea. Cochrane Database of Systematic Reviews 2009, Issue 4. Art. No: CD002120. [DOI: 10.1002/14651858.CD002120.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


