Abstract
The gene bifocal (bif), required for photoreceptor morphogenesis in the Drosophila compound eye, encodes a protein that is shown to interact with protein phosphatase 1 (PP1) using the yeast two-hybrid system. Complex formation between Bif and PP1 is supported by coprecipitation of the two proteins. Residues 992 to 995 (RVQF) in the carboxy-terminal region of Bif, which conform to the consensus PP1-binding motif, are shown to be essential for the interaction of Bif with PP1. The interaction of PP1 with bacterially expressed and endogenous Bif can be disrupted by a synthetic peptide known to block interaction of other regulatory subunits with PP1. Null bif mutants exhibit a rough eye phenotype, disorganized rhabdomeres (light-gathering rhodopsin-rich microvillar membrane structures in the photoreceptor cells) and alterations in the actin cytoskeleton. Expression of wild-type bif transgenes resulted in significant rescue of these abnormalities. In contrast, expression of transgenes encoding the Bif F995A mutant, which disrupts binding to PP1, was unable to rescue any aspect of the bif phenotype. The results indicate that the PP1-Bif interaction is critical for the rescue and that a major function of Bif is to target PP1c to a specific subcellular location. The role of the PP1-Bif complex in modulating the organization of the actin cytoskeleton underlying the rhabdomeres is discussed.
Reversible protein phosphorylation catalyzed by protein kinases and protein phosphatases regulates the majority of cellular functions and therefore might also be predicted to play key roles in the regulation of many developmental processes. One of the most abundant eukaryotic protein phosphatases that dephosphorylate serine and threonine residues is protein phosphatase 1 (PP1), which exhibits pleiotropic functions (8, 11, 38). The known diverse actions of PP1 reside in the ability of the catalytic subunit of PP1 (PP1c) to associate with different regulatory subunits in vivo, which may target the catalytic subunit to specific subcellular locations and often modify its substrate specificity. The activities of the various PP1 complexes may thus be regulated differentially by intra- and extracellular signals acting upon the different subunits. Over 25 different regulatory subunits of PP1c have now been identified. For example, in mammals, glycogen binding subunits target PP1c to regulate the enzymes of glycogen metabolism and myosin binding subunits enable PP1c to regulate myofibrillar contractility (26, 27, 40). Binding of PP1c to scaffold proteins may modulate ion channel activity (44), while at neuronal synapses, neurabins I and II (also termed spinophilin) localize PP1c to the actin cytoskeleton at the plasma membrane (1, 32, 33, 37). Interaction of subunits with PP1c is mutually exclusive, an observation explained by the discovery that a short motif—(R/K)(V/I)X(F/W)—present in the majority (but not in all) of these subunits is sufficient for binding to PP1c (18, 27, 47). PP1c also binds to a number of small cytosolic inhibitor proteins, including inhibitor 1 (I-1) and I-2, which inhibit PP1c activity at nanomolar concentrations (reviewed in references 11 and 43). In Drosophila melanogaster, a widely distributed I-2 protein, as well as a testis-specific I-2-like protein, has been identified (7, 22, 25). A further PP1c-associated protein, KLP38B, a mitotic kinesin-related protein which plays a role in mitotic chromosome condensation in proliferating cells, has also been reported in this species (2), although it does not have a discernible PP1c-binding motif.
Four isoforms of PP1c exist in D. melanogaster (14, 16). They are encoded at chromosomal loci 87B, 96A, 9C, and 13C. PP1-87B null mutants exhibit a lethal phenotype at the larval stage, failing to exit mitosis and showing overcondensed chromatin (4, 13), while PP1-87B mutants with some residual activity are viable and exhibit dominant suppression of position effect variegation, indicating that PP1-87B also modulates chromosome condensation in interphase (6, 15). In contrast, PP1-9C (flapwing) mutants are viable but flightless due to defects in indirect flight muscles (35). These diverse phenotypes suggest that PP1c in D. melanogaster will be regulated by a variety of regulatory subunits comparable to those identified in mammals. In this communication we identify D. melanogaster Bifocal (Bif), required for the normal morphogenesis of the Drosophila compound eye, as a protein that interacts with PP1-87B via a PP1 consensus binding motif.
The D. melanogaster eye is an excellent model system for study of the developmental processes at the cellular and subcellular levels. The adult compound eye comprises ∼800 repeats of a basic unit referred to as an ommatidium, each of which contains eight photoreceptor neurons (R cells) and an invariant array of nonneuronal accessory cells. R-cell development begins in the third-instar larval eye disc and is completed by the end of the third-instar larval stage (36). In the midpupal stage (∼48 h post-puparium formation), each R cell projects to the center of an ommatidium, a microvillar stack of membranes rich in rhodopsin (called the rhabdomere). The position of each rhabdomere depends on the class of R cell from which it is produced (45). R7 projects to the center of the ommatidium and contacts surrounding rhabdomeres of other R cells. R3 builds its rhabdomere against the stalk of R2 and R4, whereas R4 forms contacts with the rhabdomeres of R2 and R7. Rhabdomere development is essentially completed at 110 h of pupation (prior to eclosion), by which stage the rhabdomeres retract from the center of the retina, leaving behind an interretinal space (45). At the subcellular level, the rhabdomeral microvilli are supported by an axial actin cytoskeleton comprising at least two actin filaments per microvillus (3). The barbed ends of the actin filaments are located at the distal ends of the microvilli, and the pointed ends project into the cytoplasm of the R cells.
The gene bifocal (bif) at chromosomal locus 10D was previously identified in a P-element transposition screen; null mutations in bif give rise to a rough eye phenotype in the adult (5). Externally, bif mutant eyes exhibit occasional fusion of adjacent ommatidia and loss or duplication of bristles. More detailed examination revealed alterations in normal rhabdomere development; they become enlarged and frequently split. At the subcellular level, disorganization of the actin cytoskeleton was evident; actin staining becomes disordered and defective and the interretinal space is absent. In this paper, we show that an intact PP1c-binding site in Bif is required for rescue of the bif null mutant phenotypes, demonstrating a role for PP1 in regulating the organization of the actin cytoskeleton and the morphology of the rhabdomeres during development of the eye.
MATERIALS AND METHODS
General methods and the yeast two-hybrid analysis.
Microbial strains and methods for the yeast two-hybrid screen using human PP1γ1 have been described (17, 24, 25). In order to isolate proteins capable of interacting with PP1, the yeast strain Y190 containing a pAS2-PP1γ1 plasmid was transformed with DNA from a D. melanogaster third-instar larval cDNA library in pACT. Ten colonies (representing 0.008% of the cells transformed) were obtained on selective media, from which pACT plasmids were recovered into Escherichia coli, and their cDNA inserts were sequenced.
Oligonucleotides were synthesized by Audrey Gough (University of Dundee) on an Applied Biosystems model 394 DNA synthesizer. DNA sequencing was performed using Taq dye terminator cycle sequencing on PE Biosystems automated DNA sequencers.
Bacterial expression of Bif fused to glutathione S-transferase (GST) or maltose binding protein.
To construct the GST-Bif fusion vector, a 1,931-bp BglII fragment from the pACT-D2 construct, encoding residues 761 to 1235 of the Bif protein, was ligated into the BamHI site of the vector pGEX-3X (Pharmacia). The construct was verified by DNA sequencing and was then transformed into E. coli BL21(DE3) (pLysS) for expression. A 500-ml culture of E. coli containing the pGEX-Bif expression construct was grown at 37°C to an A600 of 0.5 and was then induced with a final concentration of 100 μM isopropyl-β-d-thiogalactopyranoside (IPTG) for 3 h at 37°C. The cells were harvested by centrifugation, resuspended in 25 ml of ice-cold lysis buffer (50 mM Tris-HCl [pH 7.5], 0.1 mM EGTA, 0.1% [vol/vol] 2-mercaptoethanol, 0.02% [wt/vol] Brij 35, 5% [vol/vol] glycerol, 1 mM EDTA, 200 mM NaCl) containing 0.1 mM phenylmethylsulfonyl fluoride and 1 mM benzamidine, and lysed by sonication. The lysate was centrifuged at 45,000 × g for 20 min, and the supernatant was added to 3 ml of glutathione-Sepharose (Pharmacia) equilibrated in lysis buffer. After incubation with mixing at 4°C for 1 h, the resin was washed three times with 20 ml of lysis buffer and was then transferred to an Econopac (Bio-Rad) column. Following further washing, the bound GST-Bif protein was eluted from the column with lysis buffer containing 20 mM free reduced glutathione. The peak protein fractions were pooled, concentrated by centrifugation through a Centricon 30 device (Amicon Inc.), and stored in aliquots at −80°C.
To construct the MBP-Bif fusion vector, the pACT-D2 BglII fragment (see above) was cloned into the BamHI site of plasmid pMAL-HA (plasmid pMAL-c2 [New England Biolabs, Inc.] modified to contain a hemagglutinin tag and additional restriction sites). After DNA sequencing, the plasmid was transformed into E. coli BL21(DE3) (pLysS) for expression. Expression and purification were performed as for the pGEX-Bif construct (see above), except that amylose resin was used and elution was performed with 20 mM maltose.
Mutagenesis of the Bif protein.
The pGEX-Bif construct was mutated to encode GST-Bif in which F995 was replaced by A using the Quickchange mutagenesis system (Stratagene) and the complementary oligonucleotides CCGCGTGCAGGCTAACGACACGC and GCGTGTCGTTAGCCTGCACGCGG (mutated codon in boldface). After verification that the sequence was correct, the mutated GST-Bif protein was expressed and purified as described above for the wild-type (wt) protein.
Production of antibodies.
Antisera were raised against the GST-Bif(761–1235) protein and bacterially expressed PP1-87B (25) in sheep at Diagnostics Scotland (Carluke, Lanarkshire, United Kingdom). Anti-GST-Bif antibodies were affinity purified on a column matrix of MBP-Bif(761-1235) linked to CNBr-Sepharose (Pharmacia, Uppsala, Sweden) and were then coupled to protein G-Sepharose (1 mg of immunoglobulin G [IgG] per ml of resin) using dimethylpimelimidate (19). Anti-PP1-87B antibodies were similarly affinity purified on a column matrix of PP1-87B and coupled to protein G-Sepharose.
Immunoprecipitation and glutathione-Sepharose sedimentation.
Mixed male and female D. melanogaster adults were homogenized in lysis buffer (detailed above) containing complete protease inhibitor cocktail (Roche Diagnostics). After centrifugation at 15,000 × g for 10 min, the lysate was diluted to 1 mg/ml and precleared with preimmune IgG coupled to protein G-Sepharose for 1 h at 4°C. The supernatant was then incubated for 1 h at 4°C with anti-Bif IgG coupled to protein G-Sepharose. The immunoprecipitates were washed three times with lysis buffer and were then either subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) or washed once with protein phosphatase assay buffer and used in protein phosphatase assays. For SDS-PAGE, 100 μg of extract was used with 10 μl of beads for each immunoprecipitate. For assays, 30 μg of extract was used with 5 μl of beads for each immunoprecipitate.
Glutathione-Sepharose sedimentation experiments were performed in a manner similar to that for immunoprecipitations using purified proteins in lysis buffer containing 0.1 mg of bovine serum albumin (BSA)/ml to limit nonspecific interactions.
Protein phosphatase assays.
32P-labeled rabbit skeletal muscle glycogen phosphorylase was prepared by H. Y. L. Tung. Phosphatase assays were performed in the presence of 0.5 mM Mn2+ (PP1γ1 and PP1-87B) or in the absence of divalent cations (endogenous PP1) as previously described (12).
Immunoprecipitation-phosphatase assays were performed in a similar manner, except that a shaking incubator was used. One unit of phosphatase activity was that amount of enzyme that catalyzed the release of 1 μmol of [32P]phosphate/min from [32P]phosphorylase a in the assay.
Transgenic flies and fly stocks.
In order to produce transgenic flies, full-length bif cDNAs were cloned into the pUAST vector (9) and injected into embryos using standard procedures. F0 flies were crossed to yw flies, and w+ transformant progeny were collected and balanced. To ensure that these transgenes are capable of producing a Bif protein, several bif transformant lines were tested for expression by crossing to a mesodermal driver line, 24B-GAL4. The line 24B-GAL4 drives expression in the muscles where Bif is not normally expressed. Bif expression in embryos was visualized using anti-Bif antibody raised in sheep followed by a secondary antibody conjugated to horseradish peroxidase. To drive Bif expression in the eye, the pGMR-GAL4 driver, which allows Bif expression in many of the different cell types of the eye (20), was used. In our experiments, the strain used for expression of the transgenes contains the bif R47 allele (5). This allele possesses an internal deletion that deletes 3 kb from coding exon 3, resulting in the absence of detectable Bif protein expression with anti-Bif antibody.
Immunostaining of eye discs and electron microscopy.
Larval eye discs were dissected in accordance with previously published protocols with slight modifications (46). Wandering third-instar larval eye discs were dissected in phosphate-buffered saline and fixed in 4% paraformaldehyde for 30 min on ice. The dissected discs were then washed, blocked with BSA, stained with anti-Bif primary antibody, and then treated with fluorescein isothiocyanate-labeled anti-sheep secondary antibody. The larval eye discs were mounted in Vecta-shield and were visualized using confocal microscopy. Pupal eye discs were dissected as previously described (10) and were fixed with 4% paraformaldehyde for 30 min on ice. The dissected discs were then washed and stained with tetramethyl rhodamine isothiocyanate-phalloidin and mounted in Vecta-shield medium for confocal microscopy. A Bio-Rad 1024 confocal microscope was used to visualize the images. For transmission electron microscopy, adult heads were embedded in Epon resin and processed in accordance with published protocols (41), and ultrathin sections were counterstained with lead citrate and uranyl acetate. The sections were then visualized under a transmission electron microscope. For scanning of adult eyes, the heads were fixed, washed, and dehydrated with ethanol in accordance with the protocol described in reference 28 with minor modifications. These fly eyes were then visualized under a scanning electron microscope.
RESULTS
Identification of Bif as a PP1-binding protein.
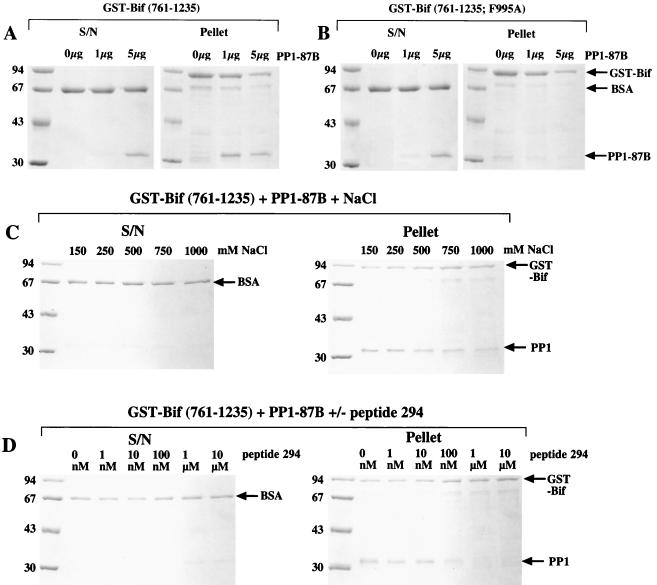
In order to identify proteins capable of interacting with PP1, a yeast two-hybrid screen of a D. melanogaster third-instar larval library was performed with human PP1γ1 as bait (24). Ten clones, able to activate transcription of both the his3 gene (permitting auxotrophic selection) and the lacZ reporter gene in the presence of the bait, were identified (25). One of these contained a cDNA, termed PP1D2, that encoded the 474 carboxy-terminal amino acids of the protein Bif (5). Residues 992 to 995 (RVQF) of Bif were seen to conform to the consensus PP1-binding motif of -(R/K)(V/I)XF- found in many regulatory subunits of PP1 (18). We therefore decided to test whether the protein encoded by the PP1D2 clone could bind the major form of D. melanogaster PP1 encoded at locus 87B in vitro. Figure 1A shows that a GST-Bif(761-1235) fusion protein is capable of interacting with D. melanogaster PP1-87B in the gluthione-Sepharose sedimentation assay. The interaction is stable to 1 M salt (Fig. 1C) and is not seen with GST alone (data not shown) or mutated GST-Bif (Fig. 1B; see description below). To investigate the PP1-Bif(761-1235) complex formation further, we utilized the synthetic peptide SP294, which comprises residues 773 to 810 of the human 53BP2 protein. This peptide contains the PP1-binding site of 53BP2 (18, 21) and has previously been shown to disrupt the interaction of PP1c with a number of its regulatory subunits (18, 22, 23, 29). The peptide was capable of dissociating GST-Bif(761-1235) from PP1-87B (Fig. 1D). SP294 at a concentration of 100 nM in the assay considerably reduced interaction, and 1 μM SP294 completely blocked interaction.
FIG. 1.
Glutathione-Sepharose sedimentation experiments. PP1-87B catalytic subunit was incubated with either GST-Bif(761-1235) or GST-Bif(761-1235; F995A) and 5 μl of glutathione-Sepharose in 100 μl of lysis buffer (containing 0.1 mg of BSA/ml) at 4°C with shaking for 1 h. Following washing with lysis buffer (three times, 1 ml), the entire pellet fraction and 1/10 of the supernatant (S/N) fraction were separated by SDS-PAGE (10%) and the proteins were visualized with Coomassie blue (R250) stain. (A) Two micrograms (approximately 1 μg of nondegraded protein) of GST-Bif(761-1235) was incubated with 0, 1, or 5 μg of PP1-87B catalytic subunit. (B) The procedure described for panel A was followed, except that GST-Bif(761-1235; F995A) was used. (C) Two micrograms (approximately 1 μg of nondegraded protein) of GST-Bif(761-1235) was incubated with 1 μg of PP1-87B catalytic subunit in lysis buffer containing 150 (standard lysis buffer), 250, 500, 750, or 1,000 mM NaCl. Washes also contained these concentrations of NaCl. (D) The procedure described for panel C was followed, except that the standard lysis buffer contained 0 nM, 1 nM, 100 nM, 100 nM, 1 μM, or 10 μM peptide 294. Washes did not contain peptide. Marker proteins are in the first lane of each gel, and sizes are shown in kilodaltons.
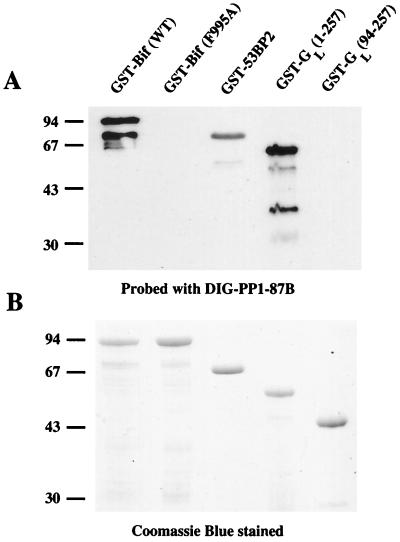
To determine if the interaction was occurring through the consensus PP1-binding sequence within Bif, we mutated F995 to A. The analogous mutation has previously been shown to abrogate binding of other PP1-interacting proteins to PP1c (18, 22, 23, 42) and, as expected, mutation of F995 in the Bif protein completely blocked binding of GST-Bif(761-1235) to PP1-87B (Fig. 1B). To analyze the interaction further, we performed a digoxigenin (DIG)-labeled PP1γ1 overlay of GST-Bif variants. Figure 2A shows that wild-type GST-Bif(761-1235) is able to bind DIG-PP1γ1 exceedingly well, whereas the ability of GST-Bif(761-1235; F995A) to bind DIG-PP1γ1 is much reduced. GST-53BP2(715-1005) and GST-GL(1-257) also bind DIG-PP1γ1 well, whereas GST-GL(94-257), which does not contain its PP1-binding site, is unable to bind to DIG-PP1γ1. When higher amounts are loaded on the gel, GST-Bif(761-1235; F995A) can be observed to retain weak PP1-binding capacity in the overlay assay (data not shown), which may be explained by the very strong interaction of the wt GST-Bif(761-1235) with PP1. This strong complex formation is consistent with residues other than F995, probably in or near the consensus PP1 motif of Bif, contributing to the overall binding.
FIG. 2.
Interaction of GST-Bif proteins with DIG-labeled PP1-87B. GST-Bif(761-1235) (0.5 μg), GST-Bif(761-1235; F995A) (0.5 μg), GST-53BP2(715-1005) (1 μg), GST-GL(1-257) (1 μg), and GST-GL(94–257) (1 μg) were separated on duplicate SDS–12.5% PAGE gels. One gel was transferred to nitrocellulose membrane and processed to identify proteins capable of interacting with DIG-labeled PP1-87B (A). The other gel was stained with Coomassie blue (R250) to visualize all the proteins (B). Marker sizes are in kilodaltons. WT, wild type.
The Bif protein modulates PP1 phosphatase activity in vitro.
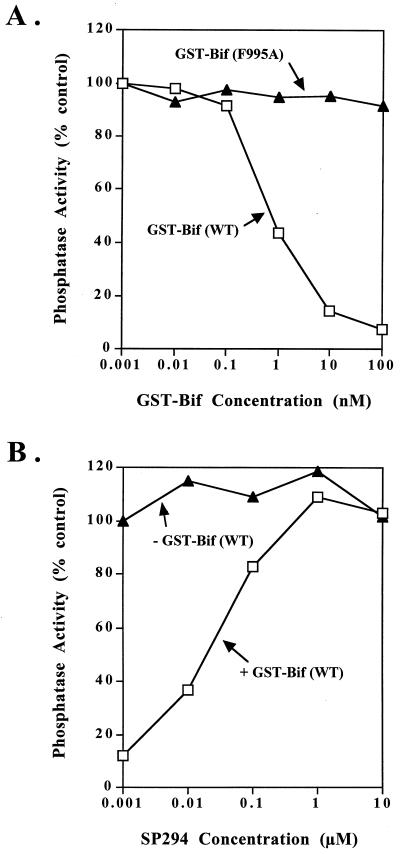
Demonstration of an interaction of D. melanogaster Bif protein with PP1c raised the question of whether Bif would affect the activity of PP1c. Since human PP1γ1 was used to isolate the Bif protein in the yeast two-hybrid screen, we used both D. melanogaster PP1-87B and human PP1γ1 to perform the assays. Wild-type GST-Bif(761-1235) dramatically inhibits the phosphorylase phosphatase activity of both PP1-87B (Fig. 3A) and PP1γ1 (data not shown), with a 50% inhibitory concentration of approximately 1 nM. Mutation of F995 to A within the consensus PP1-binding motif of Bif totally abolishes this inhibition unless very high concentrations of the protein are used (Fig. 3A), indicating that the inhibition is due to Bif and not to a contaminating protein. Previous results have shown that the SP294 peptide can relieve inhibition of PP1c activity caused by PP1-binding proteins (18, 22, 23, 29). We therefore examined the effect of SP294 peptide on the activity of the PP1–GST-Bif(761-1235) complex. Figure 3B shows that the peptide relieved the inhibition of PP1-87B caused by GST-Bif(761-1235) at a peptide concentration similar to that found to relieve inhibition of PP1c by other PP1-binding proteins under similar conditions. As expected, the peptide has no effect on PP1-87B activity in the absence of the GST-Bif(761-1235) protein (Fig. 3B). The inhibition of PP1γ1 by GST-Bif(761-1235) was similarly relieved by peptide 294 (data not shown).
FIG. 3.
Effect of GST-Bif and SP294 on phosphorylase phosphatase activity of PP1-87B. (A) GST-Bif protein was included at the concentrations shown in a standard phosphorylase phosphatase assay with PP1-87B. Squares, GST-Bif(761-1235); triangles, GST-Bif(761-1235; F995A). (B) Phosphorylase phosphatase assays were performed with PP1-87B in the presence (squares) or absence (triangles) of 10 nM GST-Bif(761-1235) and with the concentrations of peptide 294 shown. In all assays the amount of PP1-87B was kept constant and such that (in the absence of other factors) 10% of the counts were released from phosphorylase during the assay. WT, wild type.
Interaction of endogenous Bif protein and PP1 in D. melanogaster extracts.
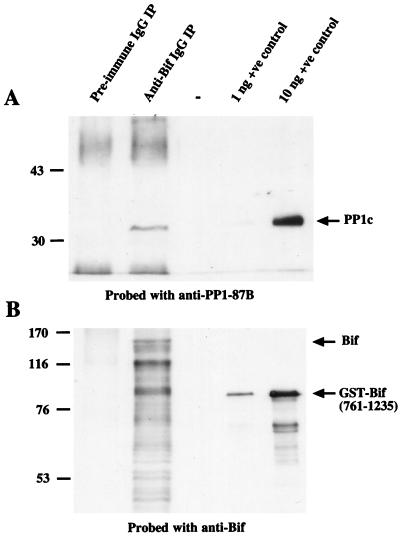
In order to be certain that interaction of bacterially expressed PP1-87B with the GST fusion of the carboxy-terminal half of the Bif protein was not an in vitro artifact, we examined endogenous D. melanogaster proteins for the presence of Bif. Figure 4A shows that the anti-Bif antibody is capable of immunoprecipitating a band from embryo D. melanogaster extracts that is the same size as the PP1-87B catalytic subunit and is recognized by the anti-PP1-87B antibody. This band is only seen in the postimmune (anti-Bif IgG immunoprecipitate) track and is therefore highly likely to represent endogenous PP1c. The low abundance of the endogenous Bif protein in D. melanogaster extracts made its detection difficult following SDS-PAGE and necessitated the use of biotinylated anti-Bif antibody followed by avidin-horseradish peroxidase. This method visualizes endogenous Bif proteins migrating at approximately 150 kDa in anti-Bif immunoprecipitates (Fig. 4B). In addition many smaller immunoreactive fragments are detected, suggesting that Bif is extensively degraded in anti-Bif immunoprecipitates, despite the presence of protease inhibitors. These immunoreactive bands are seen only in the postimmune IgG (anti-Bif immunoprecipitate) and therefore are very likely to represent endogenous Bif fragments. Note that the bacterially expressed GST-Bif(761-1235) also contains some degraded fragments.
FIG. 4.
Coimmunoprecipitation of PP1 with Bif from D. melanogaster extracts. Two hundred micrograms of D. melanogaster embryo extract was immunoprecipitated with either preimmune IgG or anti-Bif IgG coupled to protein G-Sepharose and separated by SDS-PAGE with 1 or 10 ng of the positive control proteins. (A) Samples were separated by SDS–10% PAGE and were then transferred to nitrocellulose membrane; the PP1-87B protein was visualized using anti-PP1-87B antibody. Positive control protein is PP1-87B. (B) Samples were separated by SDS–7.5% PAGE and transferred to nitrocellulose membranes, and the Bif protein was visualized using anti-Bif antibody. Positive control protein is GST-Bif(761-1235). Marker sizes are given in kilodaltons, and arrows indicate the position of proteins of interest. Note that the heavy and light IgG bands from the immunoprecipitating antibody are seen in panel A (preimmune and anti-Bif immunoprecipitates) and that the Bif protein undergoes extensive degradation, resulting in many immunoreactive bands with sizes below that of the full-length protein (B, anti-Bif immunoprecipitate). IP, immunoprecipitate. +ve, positive. −, blank lane.
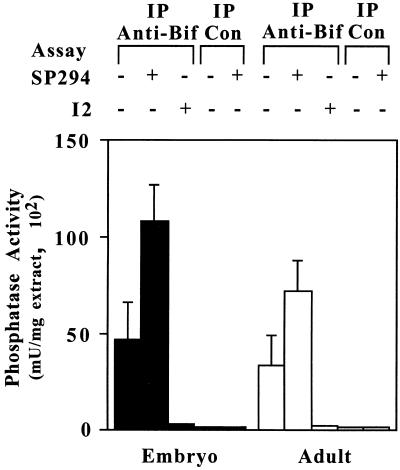
Further evidence that a complex is formed between endogenous Bif and PP1 was obtained by assaying the protein phosphatase activity associated with Bif immunoprecipitates from D. melanogaster extracts. Figure 5 shows that significant phosphatase activity is immunoprecipitated specifically from both embryo and adult D. melanogaster extracts with the anti-Bif antibody but not with the preimmune IgG. This activity is totally inhibited by the PP1-specific inhibitor I-2 protein, demonstrating that the activity is due to PP1 in the precipitate. Addition of peptide SP294 substantially increases the protein phosphatase activity detected in the anti-Bif immunoprecipitates but not in the preimmune IgG immunoprecipitates. The results indicate that endogenous Bif protein and PP1c are present in a complex in D. melanogaster extracts.
FIG. 5.
Coimmunoprecipitation assay of PP1 phosphatase activity from D. melanogaster extracts. Fifteen micrograms of D. melanogaster embryo or adult extract was immunoprecipitated with either anti-Bif IgG or control (preimmune) IgG coupled to protein G-Sepharose. After washing to remove unbound proteins, phosphatase activity associated with the beads was determined in a phosphatase assay using phosphorylase a as the substrate and including 10 μM SP294 or 100 nM I-2 where indicated. Con, control. +, presence of substance. −, absence of substance.
Wild-type (wt) Bif but not mutated BifF995A can rescue the eye phenotypes of bif null mutants.
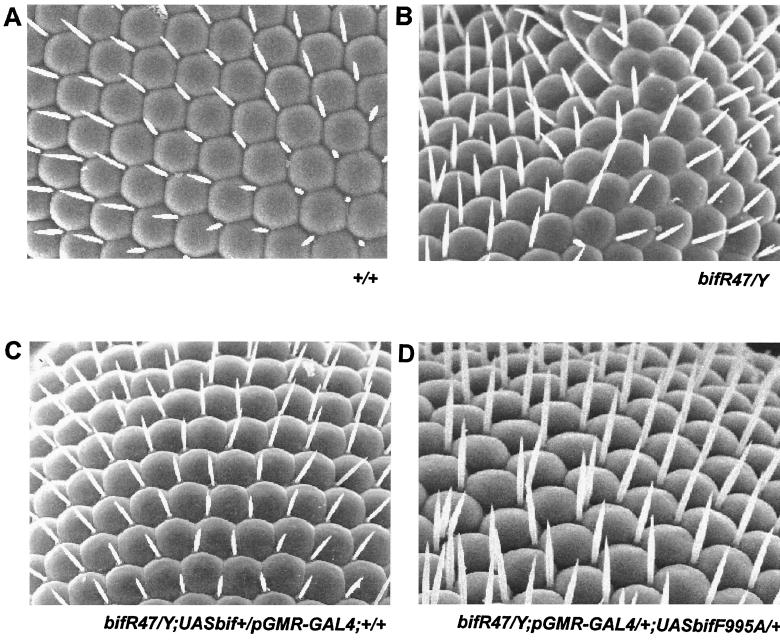
Our in vitro data and sedimentation assays have shown direct binding of Bif with PP1-87B and indicated that Bif and PP1 are part of a complex in vivo. To elucidate the functional significance of this interaction in vivo, both wt bif+ and an F995A mutated form of bif (bifF995A), which does not bind PP1 in vitro, were introduced as transgenes in the pUAST vector (9) under the control of the GAL4-UAS into bif mutant flies. Homozygous and hemizygous deletions of the X-linked bif gene affect the morphology of the compound eye in Drosophila (5). Externally, the wt eye is comprised of ∼800 ommatidia arranged in hexagonal shapes with bristles projecting from alternate ommatidial vertices (45) (Fig. 6A). In the bifR47 null allele, which deletes 3 kb from coding exon 3 of bif (5), adult eyes show bristles that are short, missing, or duplicated (Fig. 6B) and adjacent ommatidia show fusion at a low frequency. Expression of a wt UAS-bif+ transgene in many of the cells of the ommatidia, using the pGMR-GAL4 driver (20), rescues this bif mutant phenotype (Fig. 6C); in contrast, expression of the UAS-bifF995A in a bif null mutant could not effect this rescue (Fig. 6D). In these experiments the levels of the mutant and wt transgene expression are similar (compare Fig. 6I and J).
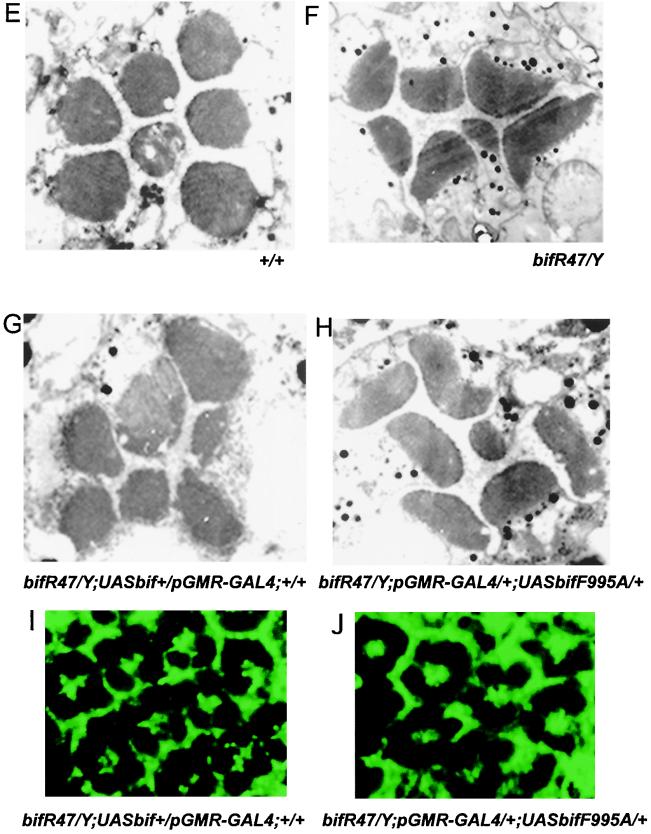
FIG. 6.
Rescue of the adult eye phenotypes of bif mutants. (A to D) Scanning micrographs of adult eyes show the organization of ommatidia and bristles. (A) Region of a wt eye with its normal ommatidial and bristle organization. (B) Homozygous mutant bifR47 eye showing loss and multiplication of bristle phenotypes. (C) Rescue of the bif bristle phenotype when expression of a bif+ transgene is driven by the pGMR-GAL4 driver in bifR47 mutant animals. The expression of the bif+ transgene restored the number of bristles in the mutant to near-wt levels. (D) Ectopic expression of the F995A mutant form of bif under control of the pGMR-GAL4 driver in the mutant flies. The bifF995A mutant form of bif fails to significantly rescue any of the bif bristle phenotypes; note that although the ommatidial fusion phenotype is not seen in this panel, it is still present at the same frequency as in the mutant. (E to H) Electron micrographs of sections of adult eyes. A representative ommatidium is shown in each panel. (E) Rhabdomeres of a wt ommatidium with their normal roundness and ordered organization. (F) Mutant ommatidium from homozygous bifR47 allele. Note that essentially all bif mutant rhabdomeres have abnormal shapes and that mutant ommatidia essentially all show irregular patterns in the spatial organization of their rhabdomeres. (G) Rescue of the bif mutant rhabdomere phenotype by expression of a UAS-bif+ transgene with the pGMR-GAL4 driver; the spatial organization of the mutant rhabdomeres within each mutant ommatidium reverts to a near-wt pattern (in 29 of 30 ommatidia the trapezoidal pattern of rhabdomeres is partially restored); in terms of rhabdomere shape, only 20% of the rhabdomeres have obviously abnormal shapes (n = 210). (H) Expression of the bifF995A mutant form fails to rescue the bif rhabdomere phenotypes (n = 210). Of the rhabdomeres, 100% show defective shapes (n = 210), while 97% of the ommatidia show defects in the pattern of their rhabdomeres' spatial organization (n = 30). (I and J) Expression of bif+ and bifF995A transgenes driven by the pGMR-GAL4 driver in a bifR47 background. The panels show regions of eye discs from wandering third-instar larvae, stained with anti-Bif antibody (green) at the plane of some of the photoreceptor preclusters. As can be seen, both transgenes express Bif protein of similar levels. In this plane of section, Bif is expressed only in the cells that also show Bif expression in the wt; Bif expression in other regions, like the cone cells, is not shown in this figure.
Internally, the wt ommatidia contain eight photoreceptor cells (R1 to R8), each of which projects into the center a light-gathering organelle called the rhabdomere. Rhabdomeres are round. The rhabdomeres for R1 to R7 are organized in an asymmetric trapezoidal pattern, with the R7 rhabdomere located at the center; the R8 rhabdomere lies under the R7 rhabdomere and is not visible at this plane of section (45) (Fig. 6E). In bif null mutants, the majority of rhabdomeres are elliptical; in addition, the trapezoidal organization is also disrupted (Fig. 6F). These defects could be partially rescued by pGMR-GAL4-driven expression of UAS-bif+ (Fig. 6G), but pGMR-GAL4-mediated expression of UAS-bifF995A cannot rescue these defects (Fig. 6H).
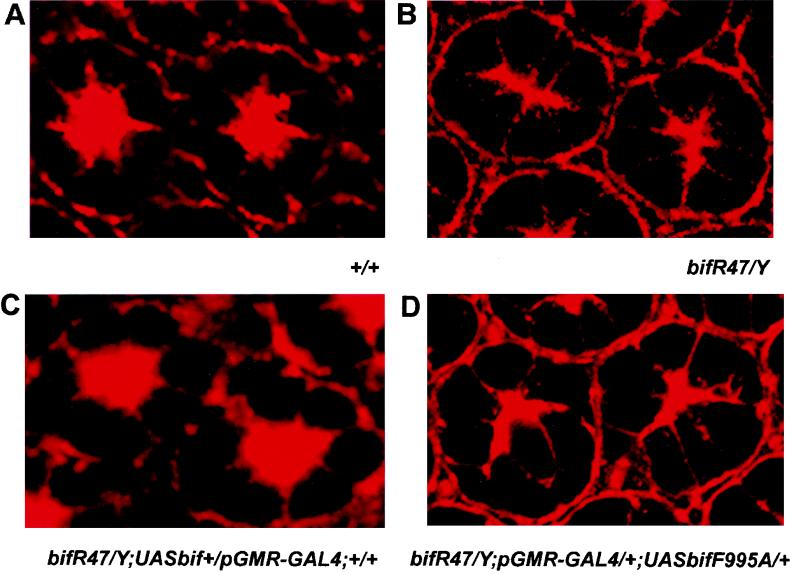
At the subcellular level, bif mutations affect F-actin localization, causing an abnormal pattern of F-actin distribution (5). In wt ommatidia from 55-h pupal eye imaginal discs, F-actin is localized in a starlike pattern at the center of each ommatidium, with intense localization at the microvillar tips of the rhabdomeres facing the central space (31) (Fig. 7A). In bif null ommatidia of the same stage, the starlike pattern of F-actin becomes abnormal, with an elongated and fused central region and decreased spacing in the center of the eye (Fig. 7B). The pGMR-GAL4-mediated expression of UAS-bif+ can rescue this defect, whereas expression of UAS-bifF995A cannot (Fig. 7C and D).
FIG. 7.
Rescue of the abnormal F-actin localization pattern of bif pupal eye discs. (A to D) Each panel shows two ommatidia from a 55-h pupal eye disc stained with tetramethyl rhodamine isothiocyanate-labeled phalloidin. (A) A wt F-actin staining pattern reveals the organization of rhabdomeres in the center of the eye. (B) A homozygous bifR47 mutant shows disorganized F-actin staining and merged rhabdomeres in the center of the eye, which is seen at a frequency of 92% in the 100 ommatidia scored in pupae. (C) Rescue of the bif pupal eye disc phenotype by pGMR-driven expression of a bif+ transgene. The expression of the wt UAS-bif transgene caused the defective F-actin staining to revert to near-wt patterns, with only 16% of the ommatidia retaining the mutant phenotype (n = 100). (D) Expression of the bifF995A mediated by the pGMR-GAL4 driver fails to rescue the defective F-actin distribution pattern associated with bifR47 allele, as 86% of the ommatidia scored retain the mutant phenotype (n = 100).
That the rescue of the various bif mutant phenotypes by expression of UAS-bif+ driven by pGMR-GAL4 is not always complete could be attributed to several reasons. There are several alternatively spliced transcripts produced by the bif locus and more than one isoform could be required to show complete rescue; it could also be due to differences between wt and pGMR-GAL4-driven levels and/or patterns of protein expression in the eye. Overall the levels of phenotypic rescue seen with the wt bif transgene are high, whereas the bifF995A mutant transgene, which encodes a protein that cannot bind PP1 in vitro shows no obvious rescue (see legends for Fig. 6 and 7). Therefore, the in vivo results are consistent with the in vitro data and demonstrate that an intact in vitro PP1-binding site in Bif is essential for its function in vivo.
DISCUSSION
We have identified the D. melanogaster protein Bif as a PP1-binding protein using the yeast two-hybrid system. Although human PP1γ1 was used to perform the screen, the high degree of sequence identity between mammalian PP1c and D. melanogaster PP1c should allow identification of true positives. Supporting this view are previous studies using the same approach, which identified D. melanogaster I-2 and a related testis-specific protein, I-t, as PP1-binding proteins (22, 25). The interaction of Bif with PP1 is likely to reflect an in vivo interaction for the following reasons. We have shown that the carboxy-terminal 474 amino acids of Bif identified in the two-hybrid screen interact with both mammalian PP1γ1 and D. melanogaster PP1-87B in vitro, as judged by several different techniques. This portion of Bif contains a consensus PP1-binding site, RVQF, and mutation of F995 within this motif blocks interaction with both mammalian PP1c and D. melanogaster PP1c in vitro. The analogous F residue in the PP1 consensus binding site of other regulatory subunits has been shown to make important hydrophobic contacts with PP1c that are essential for binding (18). Immunoprecipitation of endogenous Bif from adult and embryonic D. melanogaster extracts coprecipitates PP1c, as judged by both immunoblotting and protein phosphatase assays. In addition, interaction of PP1c with bacterially expressed and endogenous Bif can be efficiently disrupted by a synthetic peptide that is known to disrupt interaction of other PP1-binding proteins with PP1c (18, 22, 23, 29).
A role for bif in the development of the eye was previously described through the isolation and examination of mutations of the bif locus (5). Null bif mutants exhibited a rough eye phenotype at the morphological level, disorganized rhabdomeres in the ommatidia at the cellular level, and alterations in the actin cytoskeleton at the subcellular level. In order to see whether the binding of PP1 to Bif was important for its function, we decided to compare the effects of transforming a bif null mutant line with wt Bif and Bif mutated in the PP1-binding site at F995. Expression of wt bif transgenes resulted in significant rescue of the rough eye defects, rhabdomeral organization, and actin cytoskeleton abnormalities. The reason why rescue is not always complete may be that the level of Bif expression differs from the wt level; similarly, we cannot rule out the possibility that the transgenes used for transformation do not contain sites that would allow alternative splicing and the production of transcripts that encode distinct Bif proteins. Nevertheless, clear restoration towards the wt morphology for all of the phenotypes associated with bif loss of function is seen when the wt transgene is expressed in bif mutants. In contrast, expression of transgenes encoding the Bif F995A mutant protein, which disrupts binding to PP1, was unable to rescue any aspects of the mutant phenotype, even though the expression level of the transcript was similar to that in the wt rescue. These results indicate that the PP1-Bif interaction is critical for the rescue (and therefore function). Although it is possible that the F995A mutant causes a conformational change in Bif, this is unlikely because some weak binding of BifF995A to PP1 is observed, consistent with residues surrounding the PP1-binding motif still being in the correct orientation to contribute their normal interactions. The latter in the presence of F995 are likely to account for the very tight binding of wt Bif to PP1 that is observed.
The studies presented here indicate that the normal morphology of the adult eye is dependent on the interaction of Bif with PP1 and suggest that a major function of Bif is to target PP1c to a specific subcellular location to regulate the normal developmental pattern of the eye. At the molecular level, the organization of the actin cytoskeleton is dependent on the Bif-PP1 interaction, suggesting that PP1 may influence actin movement or operate in a pathway that regulates actin distribution within the cell. Although the actin cytoskeleton is a highly ordered structure, it is very dynamic, undergoing changes that affect cell shape, motility, and adhesion. Bif does not possess a known actin-binding motif, but its subcellular location within the eye is consistent with its playing a role in actin function and possibly binding to some component of the actin cytoskeleton (5). Recently two novel actin-binding proteins found in mammalian neurons, neurabin I and II, have been shown to bind PP1c (1, 32, 33, 37). Neurabin I was highly concentrated at the synapse of developed neurons and in the lamellopodia of the growth cone during the development of neurons, suggesting that it is required in synapse function and formation (34). Suppression of endogenous neurabin I expression with antisense oligonucleotides in hippocampal neurons inhibited neurite outgrowth. Neurabin II is ubiquitously expressed but is enriched in the postsynaptic density fraction of the brain (37). The presence of a PDZ domain that might bind to a transmembrane protein and their localization make it likely that neurabins I and II bind at the plasma membrane. The neurabins have therefore been suggested to serve as linkers between the actin cytoskeleton and the plasma membrane at cadherin-based cell-cell adhesion sites (37) and to localize PP1c to the plasma membrane in dendritic spines of neurons, where the complexes may modulate synaptic transmission (1). Both neurabins I and II show F-actin cross-linking activity, as do the α-actinin–spectrin family of actin-binding proteins. However, the actin-binding sites on the neurabins are distinct from other known actin-binding sites. Bif does not appear to be a Drosophila homologue of the mammalian neurabins, because it has no sequence similarity to these proteins and its tissue localization is distinct, In addition, there is a gene (CG16757) located at 62E6-8 in the Drosophila genome that encodes a putative neurabin homologue. However, the Bif-PP1c complex may serve functions in the photoreceptor cells of the eye analogous to those of the neurabin-PP1c complexes in other tissues, transmitting or modulating signals, possibly from cell-cell contacts, which cause a rearrangement of the actin cytoskeleton. This suggests that PP1c may play a general role in modulating the actin cytoskeleton.
We have shown that Bif inhibits the phosphorylase phosphatase activity of PP1c, similarly to a number of other PP1c-binding proteins, such as the myosin binding subunits (27) and 53BP2 (21). Neurabins I and II also inhibit the phosphorylase phosphatase activity of PP1c (1, 32, 33). Neurabin I has been shown to be phosphorylated in vitro by protein kinase A (PKA), which decreases its binding to PP1c (33). In addition, mutation of the phosphorylatable serine to glutamic acid reduces the inhibitory activity of neurabin, suggesting that the complex participates in a cyclic AMP-PKA signaling mechanism. Bif has several potential Ser/Thr phosphorylation sites; in the vicinity of the PP1-binding motif, the carboxy-terminal sequence of Bif, RRSSTIM, could serve as a potential phosphorylation site for PKA (as well as for a number of other kinases). Phosphorylation of Bif could affect PP1c binding and/or activity, allowing the Bif-PP1c complex to modulate signaling processes. Alternately or in addition, the Bif-PP1c complex might be required to dephosphorylate proteins associated with actin. Actin-binding proteins can bind to actin monomers, cross-link actin filaments into bundles or gels, sever actin filaments, or cap their growing ends. Some, such as myosin II (39) and cofilin (30), are known to undergo phosphorylation. Dephosphorylation of such proteins, possibly by PP1c complexes, may effect a redistribution of the actin cytoskeleton.
ACKNOWLEDGMENTS
We thank Heinrich Horstmann and Ng Chee Peng for assistance with electron microscopy and Steve Elledge for the yeast two-hybrid system. The work was supported by the Medical Research Council, London, United Kingdom, and the Institute of Molecular and Cell Biology, Singapore.
N. R. Helps and K. Babu made equally important contributions to this study. N. R. Helps performed the two-hybrid screen and analyzed the PP1-Bif interaction. K. Babu produced and examined the bif transgenic flies.
REFERENCES
- 1.Allen P B, Kwon Y-G, Nairn A C, Greengard P. Isolation and characterization of PNUTS, a putative protein phosphatase 1 nuclear targeting subunit. Proc Natl Acad Sci USA. 1998;273:4089–4095. doi: 10.1074/jbc.273.7.4089. [DOI] [PubMed] [Google Scholar]
- 2.Alphey L, Parker L, Hawcroft G, Guo Y, Kaiser K, Morgan G. KLP38B: a mitotic kinesin-related protein that binds PP1. J Cell Biol. 1997;138:395–409. doi: 10.1083/jcb.138.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arikawa K, Hicks J L, Williams D S. Identification of actin filaments in the rhadhnomeral microvilli of Drosophila photoreceptors. J Cell Biol. 1990;110:1993–1998. doi: 10.1083/jcb.110.6.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Axton J M, Dombrádi V, Cohen P T W, Glover D M. One of the protein phosphatase 1 isoenzymes in Drosophila is essential for mitosis. Cell. 1990;63:33–46. doi: 10.1016/0092-8674(90)90286-n. [DOI] [PubMed] [Google Scholar]
- 5.Bahri S M, Yang X, Chia W. The Drosophila bifocal gene encodes a novel protein which colocalizes with actin and is necessary for photoreceptor morphogenesis. Mol Cell Biol. 1997;17:5521–5529. doi: 10.1128/mcb.17.9.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baksa K, Morawietz H, Dombrádi V, Axton J M, Taubert H, Szabó G, Török I, Udvardy A, Gyurlovics H, Szöör B, Glover D M, Reuter G, Gausz J. Mutations in the protein phosphatase 1 gene at 87B can differentially affect suppression of position-effect variegation and mitosis in Drosophila melanogaster. Genetics. 1993;135:117–125. doi: 10.1093/genetics/135.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett D, Szöör B, Alphey L. The chaperone-like properties of mammalian inhibitor-2 conserved in a Drosophila homologue. Biochemistry. 1999;38:16276–16282. doi: 10.1021/bi9917028. [DOI] [PubMed] [Google Scholar]
- 8.Bollen M, Stalmans W. The structure, role and regulation of type I protein phosphatases. Crit Rev Biochem Mol Biol. 1992;27:227–281. doi: 10.3109/10409239209082564. [DOI] [PubMed] [Google Scholar]
- 9.Brand A H, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 10.Cagan R L, Ready D F. The emergence of order in the Drosophila pupal retina. Dev Biol. 1989;136:346–362. doi: 10.1016/0012-1606(89)90261-3. [DOI] [PubMed] [Google Scholar]
- 11.Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- 12.Cohen P, Alemany S, Hemmings B A, Resink T J, Stralfors P, Tung H Y L. Protein phosphatase-1 and protein phosphatase-2A from rabbit skeletal muscle. Methods Enzymol. 1988;159:390–408. doi: 10.1016/0076-6879(88)59039-0. [DOI] [PubMed] [Google Scholar]
- 13.Dombrádi V, Axton J M, Barker H M, Cohen P T W. Protein phosphatase 1 activity in Drosophila mutants with abnormalities in mitosis and chromosome condensation. FEBS Lett. 1990;275:39–43. doi: 10.1016/0014-5793(90)81434-p. [DOI] [PubMed] [Google Scholar]
- 14.Dombrd́i V, Axton J M, Brewis N D, da Cruz e Silva E F, Alphey L, Cohen P T W. Drosophila contains three genes that encode distinct isoforms of protein phosphatase 1. Eur J Biochem. 1990;194:739–745. doi: 10.1111/j.1432-1033.1990.tb19464.x. [DOI] [PubMed] [Google Scholar]
- 15.Dombrádi V, Cohen P T W. Protein phosphorylation is involved in the regulation of chromatin condensation during interphase. FEBS Lett. 1992;312:21–26. doi: 10.1016/0014-5793(92)81402-8. [DOI] [PubMed] [Google Scholar]
- 16.Dombrádi V, Mann D J, Saunders R D C, Cohen P T W. Cloning of the fourth functional gene for protein phosphatase 1 in Drosophila melanogaster from its chromosomal localisation. Eur J Biochem. 1993;212:177–183. doi: 10.1111/j.1432-1033.1993.tb17648.x. [DOI] [PubMed] [Google Scholar]
- 17.Durfee T, Becherer K, Chen P-L, Yeh S-H, Yang Y, Kilburn A E, Lee W-H, Elledge S J. The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev. 1993;7:555–569. doi: 10.1101/gad.7.4.555. [DOI] [PubMed] [Google Scholar]
- 18.Egloff M-P, Johnson F, Moorhead G, Cohen P T W, Cohen P, Barford D. Structural basis for the recognition of regulatory subunits by the catalytic subunit of protein phosphatase 1. EMBO J. 1997;16:1876–1887. doi: 10.1093/emboj/16.8.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 20.Hay B A, Wolff T, Rubin G M. Expression of baculovirus P35 prevents cell death in Drosophila. Development. 1994;120:2121–2129. doi: 10.1242/dev.120.8.2121. [DOI] [PubMed] [Google Scholar]
- 21.Helps N R, Barker H M, Elledge S J, Cohen P T W. Protein phosphatase 1 interacts with p53BP2, a protein which binds to the tumour suppressor p53. FEBS Lett. 1995;377:295–300. doi: 10.1016/0014-5793(95)01347-4. [DOI] [PubMed] [Google Scholar]
- 22.Helps N R, Cohen P T W. Drosophila melanogaster protein phosphatase inhibitor-2: identification of a site important for PP1 inhibition. FEBS Lett. 1999;463:72–76. doi: 10.1016/s0014-5793(99)01573-2. [DOI] [PubMed] [Google Scholar]
- 23.Helps N R, Luo X, Barker H M, Cohen P T W. NIMA related kinase 2 (Nek2), a cell cycle-regulated protein kinase localized to centrosomes, is complexed to protein phosphatase 1. Biochem J. 2000;349:509–518. doi: 10.1042/0264-6021:3490509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helps N R, Street A J, Elledge S J, Cohen P T W. Cloning of the complete coding region for human protein phosphatase inhibitor 2 using the two hybrid system and expression of inhibitor 2 in E. coli. FEBS Lett. 1994;340:93–98. doi: 10.1016/0014-5793(94)80179-7. [DOI] [PubMed] [Google Scholar]
- 25.Helps N R, Vergidou C, Gaskell T, Cohen P T W. Characterisation of a novel Drosophila melanogaster testis specific PP1 inhibitor related to mammalian inhibitor-2: identification of the site of interaction with PP1. FEBS Lett. 1998;438:131–136. doi: 10.1016/s0014-5793(98)01286-1. [DOI] [PubMed] [Google Scholar]
- 26.Hubbard M J, Cohen P. On target with a new mechanism for the regulation of protein phosphorylation. Trends Biochem Sci. 1993;18:172–177. doi: 10.1016/0968-0004(93)90109-z. [DOI] [PubMed] [Google Scholar]
- 27.Johnson D F, Moorhead G, Caudwell F B, Cohen P, Chen Y H, Chen M X, Cohen P T W. Identification of the protein phosphatase-1-binding domains on the glycogen and myofibrillar targetting subunits. Eur J Biochem. 1996;239:317–325. doi: 10.1111/j.1432-1033.1996.0317u.x. [DOI] [PubMed] [Google Scholar]
- 28.Kimmel B E, Heberlein U, Rubin G M. The homeo domain protein rough is expressed in a subset of cells in the developing Drosophila eye where it can specify photoreceptor cell subtype. Genes Dev. 1994;4:712–727. doi: 10.1101/gad.4.5.712. [DOI] [PubMed] [Google Scholar]
- 29.Kreivi J-P, Trinkle-Mulcahy L, Lyon C E, Morrice N A, Cohen P, Lamond A I. Purification and characterisation of p99, a nuclear modulator of protein phosphatase 1 activity. FEBS Lett. 1997;420:57–62. doi: 10.1016/s0014-5793(97)01485-3. [DOI] [PubMed] [Google Scholar]
- 30.Lawler S. Regulation of actin dynamics: the LIM kinase connection. Curr Biol. 1999;9:R800–R802. doi: 10.1016/s0960-9822(99)80493-x. [DOI] [PubMed] [Google Scholar]
- 31.Longley R L J, Ready D F. Integrins and the development of three-dimensional structure of the Drosophila compound eye. Dev Biol. 1995;171:19. doi: 10.1006/dbio.1995.1292. [DOI] [PubMed] [Google Scholar]
- 32.MacMillan L B, Bass M A, Cheng N, Howard E F, Tamura M, Strack S, Wadzinski B E, Colbran R J. Brain actin-associated protein phosphatase 1 holoenzymes containing spinophilin, neurabin and selected catalytic subunit isoforms. J Biol Chem. 1999;274:35845–35854. doi: 10.1074/jbc.274.50.35845. [DOI] [PubMed] [Google Scholar]
- 33.McAvoy T, Allen P B, Obaishi H, Nakanishi H, Takai Y, Greengard P, Nairn A C, Hemmings H C. Regulation of neurabin I interaction with protein phosphatase 1 by phosphorylation. Biochemistry. 1999;38:12943–12949. doi: 10.1021/bi991227d. [DOI] [PubMed] [Google Scholar]
- 34.Nakanishi H, Obaishi H, Satoh A, Wada M, Mandai K, Satoh K, Nishioka H, Matsuura Y, Mizoguchi A, Takai Y. Neurabin: a novel neural tissue-specific actin filament-binding protein involved in neurite formation. J Cell Biol. 1997;139:951–961. doi: 10.1083/jcb.139.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raghavan S, Williams I, Aslam H, Thomas D, Morgan G, Turner J, Fernandes J, Vijayraghavan K, Alphey L. Protein phosphatase 1β is required for the maintenance of muscle attachments. Curr Biol. 2000;10:269–272. doi: 10.1016/s0960-9822(00)00364-x. [DOI] [PubMed] [Google Scholar]
- 36.Ready D F, Hanson T E, Benzer S. Development of the Drosophila retina, a neurocrystalline lattice. Dev Biol. 1976;53:217–240. doi: 10.1016/0012-1606(76)90225-6. [DOI] [PubMed] [Google Scholar]
- 37.Satoh A, Nakanishi H, Obaishi H, Wada M, Takashashi K, Satosh K, Hiron K, Nishioka H, Hata Y, Mizoguchi A, Takai Y. Neurabin-II/spinophilin. An actin filament-binding protein with one PDZ domain localized at cadherin-based cell-cell adhesion sites. J Biol Chem. 1998;273:3470–3475. doi: 10.1074/jbc.273.6.3470. [DOI] [PubMed] [Google Scholar]
- 38.Shenolikar S. Protein serine/threonine phosphatases—new avenues for cell regulation. Annu Rev Cell Biol. 1994;10:55–86. doi: 10.1146/annurev.cb.10.110194.000415. [DOI] [PubMed] [Google Scholar]
- 39.Tan J, L, Ravid S, Spudich J A. Control of non-muscle myosins by phosphorylation. Annu Rev Biochem. 1992;61:721–759. doi: 10.1146/annurev.bi.61.070192.003445. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka J, Ito M, Feng J, Ichikawa K, Hamaguchi T, Nakamura M, Hartshorne D J, Nakano T. Interaction of myosin phosphatase target subunit 1 with the catalytic subunit of type 1 protein phosphatase. Biochemistry. 1998;37:16697–16703. doi: 10.1021/bi980782x. [DOI] [PubMed] [Google Scholar]
- 41.Tomlinson A, Ready D F. Neuronal differentiation in the Drosophila ommatidium. Dev Biol. 1987;120:366–376. doi: 10.1016/0012-1606(87)90239-9. [DOI] [PubMed] [Google Scholar]
- 42.Trinkle-Mulcahy L, Ajuh P, Prescott A, Claverie-Martin F, Cohen S, Lamond A I, Cohen P. Nuclear organisation of NIPP1, a regulatory subunit of protein phosphatase 1 that associates with pre-mRNA splicing factors. J Cell Sci. 1999;112:157–168. doi: 10.1242/jcs.112.2.157. [DOI] [PubMed] [Google Scholar]
- 43.Wera S, Hemmings B A. Serine/threonine protein phosphatases. Biochem J. 1995;311:17–29. doi: 10.1042/bj3110017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Westphal R S, Tavalin S J, Lin J W, Alto N M, Fraser I D C, Langeberg L K, Sheng M, Scott J D. Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science. 1999;285:93–96. doi: 10.1126/science.285.5424.93. [DOI] [PubMed] [Google Scholar]
- 45.Wolff T, Ready D F. Pattern formation in Drosophila retina. In: Bate M, Martinez-Arias A, editors. The development of Drosophila melanogaster. Vol. 2. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1993. pp. 1277–1325. [Google Scholar]
- 46.Wolff T, Ready D F. The beginning of pattern formation in the Drosophila compound eye: the morphogenetic furrow and the second mitotic wave. Development. 1991;113:841–850. doi: 10.1242/dev.113.3.841. [DOI] [PubMed] [Google Scholar]
- 47.Zhao S, Lee E Y C. A protein phosphatase-1-binding motif identified by the panning of a random peptide display library. J Biol Chem. 1997;272:28368–28372. doi: 10.1074/jbc.272.45.28368. [DOI] [PubMed] [Google Scholar]