Abstract
Objective
The objective of this systematic review is to summarize the effects of ivermectin for the prevention and treatment of patients with COVID-19 and to assess inconsistencies in results from individual studies with focus on risk of bias due to methodological limitations.
Methods
We searched the L.OVE platform through July 6, 2021 and included randomized trials (RCTs) comparing ivermectin to standard or other active treatments. We conducted random-effects pairwise meta-analysis, assessed the certainty of evidence using the GRADE approach and performed sensitivity analysis excluding trials with risk of bias.
Results
We included 29 RCTs which enrolled 5592 cases. Overall, the certainty of the evidence was very low to low suggesting that ivermectin may result in important benefits. However, after excluding trials classified as “high risk” or “some concerns” in the risk of bias assessment, most estimates of effect changed substantially: Compared to standard of care, low certainty evidence suggests that ivermectin may not reduce mortality (RD 7 fewer per 1000) nor mechanical ventilation (RD 6 more per 1000), and moderate certainty evidence shows that it probably does not increase symptom resolution or improvement (RD 14 more per 1000) nor viral clearance (RD 12 fewer per 1000).
Conclusion
Ivermectin may not improve clinically important outcomes in patients with COVID-19 and its effects as a prophylactic intervention in exposed individuals are uncertain. Previous reports concluding important benefits associated with ivermectin are based on potentially biased results reported by studies with substantial methodological limitations. Further research is needed.
Keywords: COVID-19, SARS-CoV-2, Coronavirus Infections, Systematic review, ivermectin, bias
What is new?
-
•
We found substantial differences in the results of studies with or without important methodological limitations.
-
•
Ivermectin's suggested benefits are mainly based on potentially biased results.
-
•
There is substantial uncertainty on ivermectin's effects for patients with COVID-19 or exposed to SARS-COV-2 and further research is needed.
1. Introduction
There is an urgent need to expand the evidence base on interventions for the prevention and treatment of COVID-19, an infection caused by SARS-CoV-2 that has the potential of progression into pneumonia, multi-organ failure and death [1]. The COVID-19 pandemic has seen a rapid increase in the number of studies testing potential therapeutic options, raising concerns about the quality and lack of scientific integrity, and also about the spread of this information, leading to the so-called “infodemic” [2,3]. According to the World Health Organization (WHO) international registry of clinical trials platform (ICTRP) [4], hundreds of potential interventions are being assessed in more than 10,000 clinical trials and observational studies.
Many drugs including ivermectin, were repurposed for the treatment of COVID-19, most often based on biological plausibility, in vitro research, or pathophysiological considerations. Ivermectin is a successful broad-spectrum anti-parasitic, included in WHO essential medicines list used to treat several neglected tropical diseases [5]. It emerged as a potential treatment for COVID-19 in mid-2020, following an in vitro study demonstrating its anti-viral properties [6].
Multiple systematic reviews have assessed the benefits and harm of ivermectin for COVID-19 patients with inconsistent findings and conclusions [7]. Although some organizations and groups have argued strongly in favor of implementing ivermectin for treatment and/or prevention of COVID-19 [8], current key clinical practice guidelines recommend against its use outside the context of clinical trials [9], [10], [11], [12].
Reasons for these major discrepancies are probably related to different evidence analytical and/or interpretation approaches. Assessing the risk of bias is one of the pillars of any systematic review and has proven to be essential for evidence interpretation in the present pandemic context where results of studies with major methodological limitations have led to erroneous conclusions, waste of resources and patients’ exposure to potentially harmful interventions [3,13,14]. Nevertheless, most available systematic reviews on ivermectin for COVID-19 have not appropriately assessed risk of bias as a potential explanation for inconsistency between trial results. Therefore, this systematic review aims to summarize the best available evidence on ivermectin for prevention and treatment of COVID-19 patients and explore potential explanations for heterogeneity in RCTs results with focus on studies methodological limitations.
2. Methods
This systematic review report is consistent with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [15].
2.1. Protocol registration
This systematic review is part of a larger project that aims to conduct multiple systematic reviews for different questions relevant to COVID-19. The protocol stating the shared objectives and methodology of these reviews was published elsewhere [16].
2.2. Search strategy
We systematically searched in the Living OVerview of Evidence (L.OVE; https://app.iloveevidence.com/covid19) platform, for studies on Ivermectin for COVID-19. L.OVE platform is a system that maps PICO (Patient– Intervention–Comparison–Outcome) questions to a repository developed by Epistemonikos Foundation and is the search platform for the Pan American Health Organization (PAHO) living systematic review of potential therapeutics for COVID-19.7 The search terms and databases covered are described on the L.OVE platform methods section available at: https://app.iloveevidence.com/covid19/methods. The repository that feeds the L.OVE platform was developed and is maintained through the automated and manual screening of multiple databases, trial registries, preprint servers and other sources. The last version of the methods, the total number of sources screened, and a living flow diagram and report of the project is updated regularly on the website. The searches cover the period from inception date of each database. We last searched the platform on July 6, 2021. There were no restrictions applied to the language or publication status.
2.3. Study selection
Two reviewers (A.I and G.R) working independently and in duplicate, performed study selection, including screening of titles and abstracts and of potentially eligible full-text articles. Reviewers resolved disagreements by discussion.
We included randomized controlled trials (RCTs) that recruited adults with suspected, probable, or confirmed COVID-19, or that were exposed to SARS-COV-2, comparing systemic ivermectin alone or in combination with other drugs, against placebo, standard care or other interventions, and reported on clinical important outcomes (see “Outcomes of interest” below). We included trials regardless of publication status (peer reviewed, in press, or preprint) or language. No restrictions were applied based on severity of COVID-19 illness, setting in which the trial was conducted (e.g., outpatient, inpatient, critical), dose administered or duration of treatment. We excluded studies in which inhaled ivermectin was used as intervention.
2.4. Data extraction
For each eligible trial one reviewer (A.I) extracted data using a standardized, pilot-tested data extraction form. The reviewer collected information on trial characteristics (trial registration, publication status, study status, design), participant characteristics (country, age, sex, comorbidities, and severity), and outcomes of interest. Extracted data was confirmed by a second reviewer (F.T). Discrepancies were resolved through discussion
2.5. Outcomes of interest
We selected clinically important outcomes considering published prioritization exercises performed in the context of different clinical practice guidelines [9,11]. We included all-cause mortality and invasive mechanical ventilation as critical outcomes, and symptom resolution or improvement, hospitalizations, viral clearance, symptomatic infection, and severe adverse events as important outcomes. For symptom resolution or improvement, we considered the proportion of patients with complete resolution of symptoms, or the proportion of patients discharged from hospital or the proportion of patients with important symptom improvement as reported by investigators. For viral clearance we considered the proportion of patients with negative PCR test. For severe adverse events we used the definition implemented by the investigators.
2.6. Risk of Bias
Two reviewers (A.I and M.R) independently assessed the risk of bias of all included trials using the revised Cochrane Risk of Bias 2.0 tool for randomized trials (RoB 2) [17], focusing on randomization, allocation concealment, blinding, attrition, or other biases relevant to the estimates of effect. In assessing the domain “risk of bias arising from randomization process”, in addition to exploring the balance of baseline prognostic in individual trials, we assessed overall balance by constructing Forest plots. We assumed that lack of blinding was less likely to introduce bias to “mortality” and “mechanical ventilation” outcomes hence we assessed risk of bias separately for those two outcomes as follows. For “mortality” and “mechanical ventilation” outcomes we rated trials at high risk of bias overall if one or more domains were rated as “high risk of bias”, and as “some concerns” if no domains were rated as “high risk of bias” and “Risk of bias arising from randomization process” and/or “Risk of bias due to missing outcome data” and/or “Risk of bias in selection of reported results” domains were classified as “some concerns”. The remaining trials were rated as “low risk of bias”. For other outcomes, we rated trials at high risk of bias overall if one or more domains were rated as “high risk of bias”, as “some concerns” if no domains were rated as “high risk of bias” and one or more domains were rated as “some concerns”, and low risk of bias overall if all domains were rated as “low risk of bias”. Reviewers resolved discrepancies by discussion.
2.7. Data synthesis
We summarized the effect of interventions on selected outcomes using relative risks (RRs) and corresponding 95% confidence intervals (95%CIs). We conducted frequentist random-effects pairwise meta-analyses using the R package “meta” in RStudio Version 1.4.1103 [18]. For the primary analysis, we assumed that interventions used in some trials as active comparators (hydroxychloroquine and lopinavir-ritonavir), are not related to important effects in patients with COVID-19 [9]. We considered those interventions as standard of care and performed sensitivity analysis to assess the robustness of results (see subgroup and sensitivity analyses).
2.8. Certainty of the evidence
We assessed the certainty of evidence using the grading of recommendations assessment, development, and evaluation (GRADE) approach [19]. Two methodologists with experience in using GRADE rated each domain for each comparison separately and resolved discrepancies by consensus. We rated the certainty for each comparison and outcome as high, moderate, low, or very low, based on considerations of risk of bias, inconsistency, indirectness, publication bias, and imprecision. We made judgments of imprecision using a minimally contextualised approach with the null effect as a threshold. This minimally contextualised approach considers whether the 95%CI includes the null effect, or, when the point estimate is close to the null effect, whether the 95%CI lies within the boundaries of small but important benefit and harm that corresponds to every outcome assessed [20,21]. To define severe or very severe imprecision we considered if the 95%CI included not only the null effect, but important benefits and harms. We used MAGIC authoring and publication platform (https://app.magicapp.org/) to generate the tables summarizing our findings. We calculated the absolute risks and risk differences from the RRs (and their 95%CIs) and the median risk in the control groups of studies reporting on severe patients for “mortality” and “mechanical ventilation” outcomes. For the remaining outcomes we used RRs (and their 95%CIs) and the median risk in the control groups of all analysed trials.
To communicate our findings and conclusions using statements we followed published guidance [22].
2.9. Subgroup and sensitivity analyses
To assess if overall estimates of effects could be influenced by trials reporting potentially biased results, we performed sensitivity analysis excluding trials categorized as “high risk of bias” and “some concerns”. We expected smaller effects after excluding those trials. In addition, as there is high certainty evidence on the lack of efficacy of some interventions for the treatment of patients with COVID-19 such as hydroxychloroquine and Lopinavir-Ritonavir [9], for the primary analysis, we considered those interventions as a part of the standard of care. However, we performed sensitivity analyses excluding trials in which hydroxychloroquine or Lopinavir-Ritonavir were used as comparators. We performed subgroup analysis based on intervention implemented and baseline disease severity, we expected larger effects in trials in which ivermectin was implemented in combination with other interventions and in patients with less severe disease.
2.10. Update of this systematic review
An artificial intelligence algorithm deployed in the COVID-19 topic of the L.OVE platform (https://app.iloveevidence.com/covid19) will provide instant notification of articles with a high likelihood of eligibility. These will be screened by paired reviewers iteratively who will also conduct data extraction and updates of the estimates of effects and certainty of the evidence. We will consider resubmission to a journal if there is a substantial modification of the effect estimate or certainty of the evidence for ivermectin, at the discretion of the reviewer team.
3. Results
The search strategy identified 680 potentially eligible records, of which 29 RCTs (reported in 78 references) were included. We identified two additional studies which we decided not to include. One was reported as a cluster randomized trial but methods and results were poorly reported and not consistent with a RCT [23]. The other was mentioned in a published review[ 24] but we were unable to obtain the full text [25]. We intended to contact the authors of these and other three included studies[ 26, 27, 28] for further methodological details by email, but only one responded [28]. On July 14, 2021, one of the included studies was retracted from the preprint server due to research misconduct concerns that are being investigated [29]. As the primary aim of our review was to assess the influence of potentially biased results on ivermectin's effects interpretation, we decided not to exclude it. The selection process is described by the PRISMA flow diagram in S1 Figure. The list of excluded studies is available upon request.
3.1. Trial characteristics
There was a total of 5592 patients from 29 RCTs, [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54] in which ivermectin was compared against standard of care or other treatments (S1 Table). Twenty trials were published in peer reviewed journals and nine were only published as preprints. One trial reported the results of three different cohorts, one of severe patients, one of mild patients and one of exposed persons, we therefore analyzed each cohort as a different trial [29]. The sample size ranged from 24 to 1342, with 2830 assigned to Ivermectin and 2483 assigned to control. Eighteen trials included patients with mild to moderate COVID-19 [26,[28], [29], [30],[32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], three studies included patients with severe to critical COVID-19 [29,46,47], six studies included patients with mild to critical disease [27,48,49,50,51,52], and four studies included non-infected patients exposed to SARS-COV-2 [29,31,53,54].
Ivermectin administered dose varied from 12 mg once to 400 μgm/kg once a day for 4 days. Ivermectin alone was used in most trials but five in which the intervention implemented was a combination of ivermectin with doxycycline [26,32,34,48], or iota-Carrageenan [54]. Comparator was standard of care with or without placebo in most trials. Active comparators included hydroxychloroquine or cloroquine [29,47], hydroxychloroquine plus azythomicin [32], lopinavir-ritnonavir[50] and vitamin C [31].
3.2. Risk of bias
The risk of bias assessment of the 29 included trials is summarized in (Table 1) . For mortality and mechanical ventilation, our assessment resulted in high risk of bias for four RCT (including the study retracted due to misconduct concerns) [29], some concerns for two and low risk of bias for seven RCTs. For all remaining outcomes, our assessment resulted in high risk of bias for thirteen RCTs, some concerns for nine and low risk of bias for seven RCTs. Most trials did not provide enough information to assess baseline differences between arms. Overall assessment of baseline prognostic factors suggested that ischemic heart disease was less frequent in patients assigned to ivermectin (S2 Figure). A detailed description of the trials’ methodological limitations is provided in a supplementary table (S2 Table).
Table 1.
Risk of bias of included trials
| Study | Risk-of-bias arising from randomization process | Risk-of-bias due to deviations from the intended interventions | Risk-of-bias due to misssing outcome data | Risk-of-bias in measurement of the outcome | Risk-of-bias in selection of the reported result | Overall Risk-of-bias judgement |
|
|---|---|---|---|---|---|---|---|
| Mortality and Invasive mechanical ventilation | Symptom resolution or improvement, hospitalization, infection, viral cleareance and adverse events | ||||||
| Shouman et al. [53] | High | Some Concerns | Low | Some Concerns | Low | - | High |
| Chowdhury et al. [32] | High | Some Concerns | Low | Some Concerns | Low | - | High |
| Podder et al. [33] | High | Some Concerns | Low | Some Concerns | Low | - | High |
| Hashim et al. [48] | High | Some Concerns | Low | Some Concerns | Low | High | High |
| Elgazzar et al. [29] | High | Some Concerns | Low | Some Concerns | Low | High | High |
| Krolewiecki et al. [35] | Low | Some Concerns | Low | Some Concerns | Low | Low | Some concerns |
| Niaee et al. [49] | High | Some Concerns | Low | Some Concerns | Low | High | High |
| Ahmed et al. [26] | High | Low | Low | Low | Some concerns | - | High |
| Chaccour et al. [30] | Low | Low | Low | Low | Low | - | Low |
| Chachar et al. [36] | Some Concerns | Some Concerns | Low | Some Concerns | Low | - | Some concerns |
| Babalola et al. [50] | Low | Some Concerns | Low | Some Concerns | Low | - | Some concerns |
| Kirti et al. [37] | Low | Low | Low | Low | Low | Low | Low |
| Chahla et al. [54] | High | Some Concerns | Low | Some Concerns | Low | - | High |
| Mohan et al. [38] | Low | Low | Low | Low | Low | - | Low |
| Shahbaznejad et al. [51] | Low | Low | Low | Low | Low | Low | Low |
| Samaha et al. [39] | High | Some Concerns | Low | Some Concerns | Low | - | High |
| Bukhari et al. [40] | High | Some Concerns | Low | Some Concerns | Low | - | High |
| Okumus et al. [46] | High | Some Concerns | Low | Some Concerns | Low | High | High |
| Beltran et al. [27] | Some Concerns | Low | Low | Low | Low | Some concerns | Some concerns |
| López-Medina et al. [41] | Low | Low | Low | Low | Low | Low | Low |
| Bermejo Galan et al. [47] | Low | Low | Low | Low | Low | Low | Low |
| Pott-Junior et al. [52] | Low | Some Concerns | Low | Some Concerns | Low | - | Some concerns |
| Kishoria et al. [42] | Low | Some Concerns | Low | Some Concerns | Low | - | Some concerns |
| Seet et al. [31] | Low | Some Concerns | Low | Some Concerns | Low | - | High |
| Mahmud et al. [33] | Low | Low | Some Concerns | Low | Low | Some concerns | Some concerns |
| Abd-Elsalam et al. [43] | Low | Some Concerns | Low | Some Concerns | Low | Low | Some concerns |
| Biber et al. [44] | Some Concerns | Low | Some Concerns | Low | Low | - | Some concerns |
| Faisal et al. [45] | High | Some Concerns | Low | Some Concerns | Low | - | High |
| Vallejos et al. [28] | Low | Low | Low | Low | Low | Low | Low |
3.3. Effects on assessed outcomes
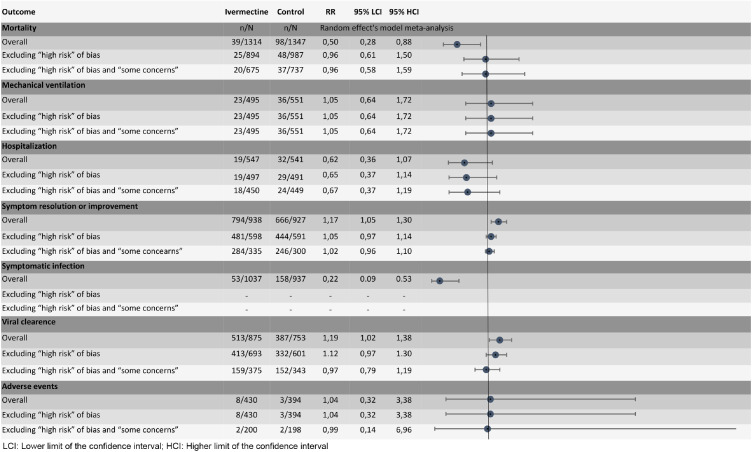
Table 2. and Fig. 1. provide a summary of finding for all assessed outcomes.
Table 2.
Summary of findings table
| Outcome Timeframe | Study results and measurements | Absolute effect estimates |
Certainty of the Evidence (Quality of evidence) |
Plain text summary | |
|---|---|---|---|---|---|
| SOC | Ivermectin | ||||
| Mortality (Overall) |
Relative risk: 0.50 (CI 95% 0.28 – 0.88) Based on data from 2661 patients in 12 studies Follow up: median 30 days |
183 per 1000 |
92 per 1000 |
Low Due to serious risk of bias and serious inconsistencya,b |
Ivermectin may reduce mortality |
| Difference: 91 fewer per 1000 (CI 95% 132 fewer – 22 fewer) |
|||||
| Mortality (excluding “some concerns” and “high risk of bias” trials) |
Relative risk: 0.96 (CI 95% 0.58 – 1.59) Based on data from 1412 patients in 6 studies Follow up: median 25.5 days |
183 per 1000 |
176 per 1000 |
Low Due to very serious imprecisionc |
Ivermectin may have little or no difference on mortality |
| Difference: 7 fewer per 1000 (CI 95% 71 fewer – 92 more) |
|||||
| Mechanical ventilation (overall - all studies classified as low risk of bias) |
Relative risk: 1.05 (CI 95% 0.64 – 1.72) Based on data from 1046 patients in 6 studies Follow up: median 30 days |
119 per 1000 |
125 per 1000 |
Low Due to very serious imprecisionc |
Ivermectin may have little or no difference on mechanical ventilation |
| Difference: 6 more per 1000 (CI 95% 43 fewer – 86 more) |
|||||
| Symptom resolution or improvement (overall) |
Relative risk: 1.17 (CI 95% 1.05 – 1.3) Based on data from 1865 patients in 11 studies Follow up: median 10 days |
714 per 1000 |
835 per 1000 |
Low Due to serious risk of bias and serious inconsistencya,b |
Ivermectin may increase symptom resolution or improvement |
| Difference: 121 more per 1000 (CI 95% 36 more – 214 more) |
|||||
| Symptom resolution or improvement (excluding “some concerns” and “high risk of bias” trials) |
Relative risk: 1.02 (CI 95% 0.96 – 1.1) Based on data from 635 patients in 3 studies Follow up: median 14 days |
714 per 1000 |
728 per 1000 |
Moderate Due to serious imprecisiond |
Ivermectin probably has little or no difference on symptom resolution or improvement |
| Difference: 14 more per 1000 (CI 95% 29 fewer – 71 more) |
|||||
| Hospitalization (overall) |
Relative risk: 0.62 (CI 95% 0.36 – 1.07) Based on data from 1088 patients in 4 studies Follow up: median 17.5 days |
54 per 1000 |
35 per 1000 |
Low Due to very serious imprecisione |
Ivermectin may reduce hospitalizations |
| Difference: 21 fewer per 1000 (CI 95% 35 fewer – 4 more) |
|||||
| Hospitalization (excluding some concerns and high risk of bias studies) |
Relative risk: 0.67 (CI 95% 0.37 – 1.19) Based on data from 899 patients in 2 study Follow up: median 25.5 days |
54 per 1000 |
36 per 1000 |
Low Due to very serious imprecisione |
Ivermectin may reduce hospitalizations |
| Difference: 18 fewer per 1000 (CI 95% 34 fewer – 8 more) |
|||||
| Symptomatic infection (overall - all studies classified as high risk of bias)14 |
Relative risk: 0.22 (CI 95% 0.09 – 0.53) Based on data from 1974 patients in 4 studies Follow up: median 21 days |
159 per 1000 |
35 per 1000 |
Low Due to very serious risk of biasa |
Ivermectin may decrease symptomatic infection |
| Difference: 124 fewer per 1000 (CI 95% 145 fewer – 75 fewer) |
|||||
| Viral clearance (overall) |
Relative risk: 1.19 (CI 95% 1.02 – 1.38) Based on data from 1628 patients in 13 studies Follow up: median 6 days |
400 per 1000 |
476 per 1000 |
Low Due to serious risk of bias and serious inconsistencya,b |
Ivermectin may increase viral clearance |
| Difference: 76 more per 1000 (CI 95% 8 more – 152 more) |
|||||
| Viral clearance (excluding “some concerns” and “high risk of bias” trials) |
Relative risk: 0.97 (CI 95% 0.79 v 1.19) Based on data from 718 patients in 3 studies Follow up: median 5 days |
400 per 1000 |
388 per 1000 |
Moderate Due to very serious imprecisiond |
Ivermectin probably has little or no difference on viral clearance |
| Difference: 12 fewer per 1000 (CI 95% 84 fewer – 76 more) |
|||||
| Severe adverse events (overall) |
Relative risk: 1.04 (CI 95% 0.32 – 3.38) Based on data from 824 patients in 4 studies Follow up: median 29 days |
5 per 1000 |
5 per 1000 |
Very low Due to serious risk of bias and very serious imprecisiona,c |
We are uncertain whether ivermectin increases or decreases severe adverse events |
| Difference: 0 fewer per 1000 (CI 95% 3 fewer – 12 more) |
|||||
| Severe adverse events (excluding “some concerns” and “high risk of bias” trials) |
Relative risk: 0.99 (CI 95% 0.14 – 6.96) Based on data from 398 patients in 1 study Follow up: median 21 days |
5 per 1000 |
5 per 1000 |
Very low Due to extremely serious imprecisionf |
We are uncertain whether ivermectin increases or decreases severe adverse events |
| Difference: 0 fewer per 1000 (CI 95% 4 fewer – 30 more) |
|||||
Inconsistency: Serious. The confidence interval of some of the studies do not overlap with those of most included studies.
Imprecision: Very serious. 95%CI includes important benefits and harms.
Imprecision: Serious. 95%CI includes important benefits.
Imprecision: Very serious. 95%CI includes absence of benefits and low number of events.
Imprecision: Extremely serious. 95%CI includes important benefits and harms and very low number of events.
Fig. 1.
Results of primary analysis and sensitivity analysis excluding trials with significant methodological limitations.
3.4. Mortality
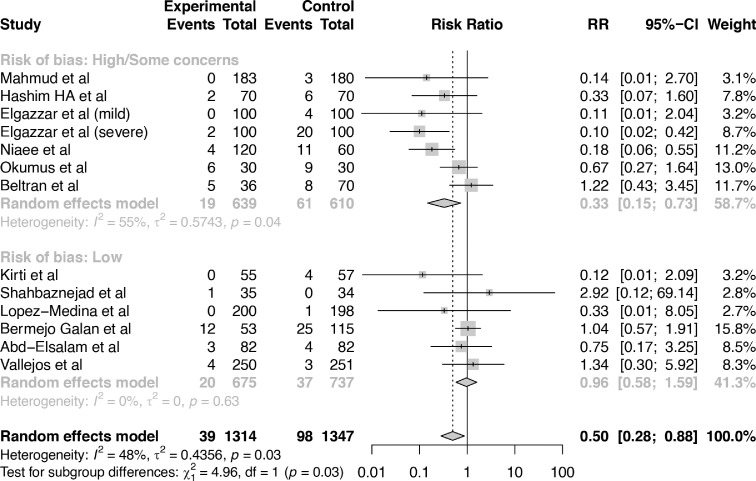
Twelve trials with 2661 patients reported on mortality [27,28,29,34,37,41,43,[46], [47], [48], [49],51]. Ivermectin may reduce mortality (RR 0.50, 95% CI:0.28 to 0.88; RD 91 fewer per 1,000 participants, 95% CI: 132 fewer to 22 fewer). The certainty of the evidence was low because of serious risk of bias and serious inconsistency (I2 48%). Sensitivity analysis excluding six trials classified as “some concerns” or “high risk of bias” showed that ivermectin may not reduce mortality (RR 0.96, 95% CI: 0.58 to 1.59; RD 7 fewer per 1,000 participants, 95% CI: 71 fewer to 92 more) (Fig. 2).
Fig. 2.
Comparison: ivermectin vs. Standard of care; Outcome: mortality; Analysis: subgroups by risk of bias classification.
3.5. Mechanical ventilation
Six trials with 1046 patients, classified as “low risk of bias”, reported on mechanical ventilation [28,35,37,43,47,51]. Ivermectin may not reduce the requirement of mechanical ventilation (RR 1.05, 95% CI:0.64 to 1.72; RD 6 more per 1,000 participants, 95% CI: 43 fewer to 86 more) (S3 Figure in S1 Appendix). The certainty of the evidence was low because of very serious imprecision.
3.6. Symptom resolution or improvement
Eleven trials with 1865 patients reported on symptom resolution or improvement [27,29,32,34,[36], [37], [38],41,42,45,46]. Ivermectin may increase symptom resolution or improvement (RR 1.17, 95% CI:1.05 to 1.30); RD 121 more per 1,000 participants, 95% CI: 36 more to 214 more). The certainty of the evidence was low because of serious risk of bias and serious inconsistency (I2 79%). Sensitivity analysis excluding eight trials classified as “some concerns” or “high risk of bias” showed that ivermectin probably does not increase symptom resolution or improvement (RR 1.02, 95% CI:0.96 to 1.10; RD 14 more per 1,000 participants, 95% CI: 29 fewer to 71 more) (S4 Figure in S1 Appendix).
3.7. Hospitalization
Four trials with 1088 patients reported on hospitalization[28,39,41,44]. Ivermectin may decrease hospitalizations (RR 0.62, 95% CI:0.36 to 1.07); RD 21 fewer per 1,000 participants, 95% CI: 35 fewer to 4 more). The certainty of the evidence was low because of very serious imprecision. Sensitivity analysis excluding two trials classified as “some concerns” or “high risk of bias” showed that ivermectin may decrease hospitalizations (RR 0.67, 95% CI:0.37 to 1.19); RD 18 fewer per 1,000 participants, 95% CI: 34 fewer to 10 more) (S5 Figure in S1 Appendix).
3.8. Symptomatic infection in exposed persons
Four trials including 1974 patients, classified as “high risk of bias”, reported on symptomatic infection [29,31,53,54]. Ivermectin may reduce symptomatic infection (RR 0.22, 95% CI:0.09 to 0.53); RD 124 fewer per 1,000 participants, 95% CI: 145 fewer to 75 fewer) (S6 Figure in S1 Appendix). The certainty of the evidence was low because of very serious risk of bias.
3.9. Viral clearance
Thirteen trials with 1628 patients reported on viral clearance [26,28,32,33,34,37,38,40,42,44,46,50,52]. Ivermectin may increase viral clearance (RR 1.19, 95% CI: 1.02 to 1.38); RD 76 more per 1,000 participants, 95% CI: 8 more to 152 more). The certainty of the evidence was low because of serious risk of bias and serious inconsistency (I2 56%). Sensitivity analysis excluding ten trials classified as “some concerns” or “high risk of bias” showed that ivermectin probably does not increase viral clearance (RR 0.97, 95% CI: 0.79 to 1.19); RD 12 fewer per 1,000 participants, 95% CI: 84 fewer to 76 more) (S7 Figure in S1 Appendix).
3.10. Severe adverse events
Four trials with 824 patients reported on severe adverse events [34,35,41,52]. It is uncertain if Ivermectin increases or decreases severe adverse events (RR 1.04, 95% CI: 0.32 to 3.38); RD 0 more per 1,000 participants, 95% CI: 3 fewer to 12 more). The certainty of the evidence was very low because of serious risk of bias and very serious imprecision. Sensitivity analysis excluding ten trials classified as “some concerns” or “high risk of bias” showed that it is uncertain if ivermectin increases or decreases severe adverse events (RR 0.99, 95% CI: 0.14 to 6.96); RD 0 more per 1,000 participants, 95% CI: 4 fewer to 30 more) (S8 Figure in S1 Appendix).
3.11. Additional analysis
Subgroup and sensitivity analysis did not suggest differential effects according to baseline disease severity, or when ivermectin was administered in combination with other interventions, or when it was compared against hydroxychloroquine or lopinavir-ritonavir, or when different outcome measurements time frames were used (S9 to S30 Figures in S2 Appendix). Visual inspection of the funnel plot for mortality suggested possible publication bias, (S31 Figure in S2 Appendix) however egger's test was not statistically significant (P = 0.13). Visual inspection of funnel plots for symptom resolution or improvement and viral clearance did not suggest publication bias, egger's test results P = 0.48 and P = 0.25 respectively (S32 and S33 Figures in S2 Appendix).
4. Discussion
This systematic review and meta-analysis provide a comprehensive overview of the available evidence on ivermectin for prevention and treatment of COVID-19. Overall, the body of evidence suggests that ivermectin may reduce mortality, may increase symptom resolution or improvement, may decrease hospitalizations, may increase viral clearance, and may decrease symptomatic infection in exposed individuals. However most trials have serious methodological limitations including lack of allocation concealment and lack of blinding, and reported results varied remarkably from striking benefits to null effects. GRADE assessment resulted in low or very low certainty of the evidence for all the outcomes, due to risk of bias, inconsistency, and imprecision. Visual inspection of funnel plot constructed for mortality outcome suggest possible publication bias which rises additional concerns about the certainty of the evidence on ivermectin's effects.
After excluding trials with significant methodological limitations inconsistency disappeared and results changed substantially. We found low certainty, due to imprecision, that ivermectin may not reduce mortality, nor reduce invasive mechanical ventilation, and moderate certainty evidence that ivermectin probably does not increase viral clearance or symptom resolution or improvement. Regarding hospitalizations, results did not change importantly suggesting that ivermectin may modestly reduce hospitalizations. However, certainty of the evidence remained low due to very serious imprecision. It is uncertain if ivermectin reduces or increases symptomatic infections in exposed individuals or increases severe adverse events as no trials classified as “low risk of bias” were identified, or the certainty of the evidence was very low.
Our systematic review has several strengths. The search strategy was comprehensive with explicit eligibility criteria, and no restrictions on language or publication status. We used a validated tool for risk of bias assessment and performed a thorough assessment providing details of trial limitations and potential important imbalances in baseline participant characteristics. We assessed the certainty of the evidence using the GRADE approach and interpreted the results considering absolute rather than relative effects.
Reporting was poor for a substantial number of included trials. For risk of bias assessment, we adopted a conservative approach and rated as low risk of bias only those trials for which it was clearly reported that no significant methodological limitations existed. Hence, we may have inappropriately classified some well executed trials as “some concerns” or “high risk of bias” due to their suboptimal reporting methods. Although for some trials we intended to contact the authors for clarification, most did not answer.
Multiple systematic reviews assessed ivermectin for COVID-19 [7]. Most of these reviews were already outdated at the time of writing this manuscript [55]. We did not identify studies included in other reviews that were not captured in our search strategy. Only five reviews incorporated a substantial proportion of the studies assessed in our review including a recently published systematic review by the Cochrane collaboration in which the authors excluded studies with high risk of bias or that compared ivermectin against other active interventions [24,[56], [57], [58], [59]. In agreement with our findings, all these reviews concluded that most of the studies assessing ivermectin for COVID-19 have considerable methodological limitations, and three judged the certainty of the evidence as low to very low for all outcomes [56,57] or not robust enough to justify ivermectin's use [58]. The authors of one systematic review concluded that ivermectin “may have a role in decreasing mortality in mildly/moderately ill COVID-19 patients” although they graded the certainty on ivermectin's effect on mortality as very low [59]. Bryant el at. graded the certainty of the evidence as low or very low for all outcomes except mortality for which they report moderate certainty in important mortality reduction. In contrast to our analysis, they reached this conclusion by not downgrading the certainty of the evidence for inconsistency even though they reported there was significant, not fully explained, heterogeneity in studies’ results. In addition, for mortality outcome, they report a sensitivity analysis excluding high risk of bias studies which, in contrast to our findings, did not result in different estimates of effect from the primary analysis. This can be explained by the fact that the authors did not exclude a relevant number of studies with important methodological limitations, that they classified as “unclear” risk of bias [24].
Due to the excessive amount of rapidly published research on COVID-19, often referred to as an “infodemic” [2,3], the scientific community has already faced a similar scenario to the one described for ivermectin in the present review. Small studies with significant methodological limitations suggested benefits for steroids, lopinavir-ritonavir, interferon β-1a and convalescent plasma among others [60], [61], [62], [63]. However, those potential benefits were seldom confirmed and mostly discarded by well-designed adequately powered studies [64], [65], [66], [67]. The limitations in the body of evidence on ivermectin for COVID-19 does not allow to reach firm conclusions, however the results of our analysis highlight that most of current suggested benefits of ivermectin are based on potentially biased estimates reported by studies with significant methodological limitations. Further research is needed to confirm or reject the effects of ivermectin on patient important outcomes.
There is an urgent need for high quality research both in health emergencies and in health relevant priorities in non-emergency settings. Those involved in evidence production should prioritize quality over quantity and speed to provide trustworthy information that is useful for decision-making. Although countries have capacities to conduct trials, and there exist global standards of quality assurance in clinical trials [68], [69], [70], a global coordinating mechanism is needed to streamline and harmonize research findings on an international scale.
5. Conclusions
Ivermectin may not improve clinically important outcomes in patients with COVID-19 and its effects as a prophylactic intervention in exposed individuals are uncertain. Previous reports concluding important benefits associated with ivermectin are based on potentially biased results reported by studies with substantial methodological limitations. Further research is needed.
Acknowledgments
We would like to thank Victoria Stanford for contributing to the writing of the final version of the manuscript.
CRediT authorship contribution statement
Ariel Izcovich: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization, Supervision. Sasha Peiris: Data curation, Writing – original draft, Writing – review & editing. Martín Ragusa: Investigation, Data curation, Writing – review & editing, Visualization. Fernando Tortosa: Investigation, Data curation, Writing – review & editing, Visualization. Gabriel Rada: . Sylvain Aldighieri: Writing – review & editing, Project administration, Funding acquisition. Ludovic Reveiz: Methodology, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Footnotes
Conflict of interest: The authors have declared that no competing interests exist. Authors hold sole responsibility for the views expressed in the manuscript, which may not necessarily reflect the opinion or policy of the Pan American Health Organization.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jclinepi.2021.12.018.
Appendix. Supplementary materials
References
- 1.Hu Y, Sun J, Dai Z, Deng H, Li X, Huang Q, et al. Prevalence and severity of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The lancet infectious diseases null. The COVID-19 infodemic. Lancet Infect Dis. 2020;20:875. doi: 10.1016/S1473-3099(20)30565-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearson H. How COVID broke the evidence pipeline. Nature. 2021;593:182–185. doi: 10.1038/d41586-021-01246-x. [DOI] [PubMed] [Google Scholar]
- 4.WHO international registry of clinical trials platform (ICTRP). Available at: https://www.who.int/clinical-trials-registry-platform. Accessed June 27, 2021.
- 5.WHO essentials medicine list. Available at: https://list.essentialmeds.org/medicines/58. Accessed on June 27, 2021.
- 6.Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.COVID-19 L.OVE platform (Epistemonikos Foundation). Ivermectin for COVID-19. Available at: https://app.iloveevidence.com/loves/5e6fdb9669c00e4ac072701d?question_domain=5b1dcd8ae611de7ae84e8f14&population=5e7fce7e3d05156b5f5e032a&intervention=5e89364e3d05155262cbab44&classification=systematic-review&studify=true). Accessed June 26, 2021.
- 8.British ivermectin recommendation development (BIRD). Available at: https://bird-group.org. Accessed on June 27, 2021.
- 9.WHO. Therapeutics and COVID-19: living guideline. Available at: https://app.magicapp.org/#/guideline/nBkO1E. Accessed on June 27, 2021.
- 10.IDSA guidelines on the treatment and management of patients with COVID-19. Available at: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/#toc-14. Accessed on June 27, 2021.
- 11.National COVID-19 clinical evidence task force. Caring for people with COVID-19. Available at: https://covid19evidence.net.au/. Accessed on June 27, 2021.
- 12.Pan American Health Organization. Guidelines for Care of Critically Ill Adult Patients with COVID-19 in the Americas. Available at: https://iris.paho.org/handle/10665.2/53895. Accessed on June 27, 2021.
- 13.Raynaud M, Zhang H, Louis K, Goutaudier V, Wang J, Dubourg Q, et al. COVID-19-related medical research: a meta-research and critical appraisal. BMC Med Res Methodol. 2021;21:1. doi: 10.1186/s12874-020-01190-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bull-Otterson L, Gray EB, Budnitz DS, Strosnider HM, Schieber LZ, Courtney J, et al. Hydroxychloroquine and chloroquine prescribing patterns by provider specialty following initial reports of potential benefit for COVID-19 Treatment — United States, January–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1210–1215. doi: 10.15585/mmwr.mm6935a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rada G, Verdugo-Paiva F, Ávila C, Morel-Marambio M, Bravo-Jeria R, Pesce F, et al. Evidence synthesis relevant to COVID-19: a protocol for multiple systematic reviews and overviews of systematic reviews. Medwave. 2020;20:e7868. doi: 10.5867/medwave.2020.03.7867. [DOI] [PubMed] [Google Scholar]
- 17.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 18.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Cognit Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, et al. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017;87:4–13. doi: 10.1016/j.jclinepi.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng L, Brignardello-Petersen R, Hultcrantz M, Siemieniuk RA, Santesso N, Traversy G, et al. GRADE guidelines 32: GRADE offers guidance on choosing targets of GRADE certainty of evidence ratings. J Clin Epidemiol. 2021;137:163–175. doi: 10.1016/j.jclinepi.2021.03.026. [DOI] [PubMed] [Google Scholar]
- 22.Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol. 2020;119:126–135. doi: 10.1016/j.jclinepi.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Chahla RE, Ruiz LM, Mena T, Brepe Y, Terranova P, Ortega ES, et al. Cluster randomised trials - ivermectin repurposing for COVID-19 treatment of outpatients with mild disease in primary health care centers. Res Sq. 2021 https://www.researchsquare.com/article/rs-495945/v1 Published online May 6 Available at: [Google Scholar]
- 24.Bryant A, Lawrie TA, Dowswell T, Fordham E, Mitchell S, Hill S, et al. Ivermectin for prevention and treatment of COVID-19 infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines. Am J Ther. 2021;28(4):e434–e460. doi: 10.1097/MJT.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petkov S. Multicenter, Randomized, Double-Blind, Placebo-Controlled Study Investigating Efficacy, Safety and Tolerability of Ivermectin HUVE-19 in Patients with Proven SARS-CoV-2 Infection (Covid-19) and Manifested Clinical Symptoms. 2021. Available at: https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-002091-12/BG. Accessed June 27, 2021.
- 26.Ahmed S, Karim MM, Ross AG, Mohammad SH, Clemens JD, Sumiya MK, et al. A five day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.11.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beltran Gonzalez JL, González Gámez M, Mendoza Enciso EA, Esparza Maldonado RJ, Hernández Palacios D, Dueñas Campos S, et al. Efficacy and safety of convalescent plasma and intravenous immunoglobulin in critically ill COVID-19 patients. a controlled clinical trial. MedRxiv. 2021 http://medrxiv.org/lookup/doi/10.1101/2021.03.28.21254507 published online March 21Available at: [Google Scholar]
- 28.Vallejos J, Zoni R, Bangher M, Villamandos S, Bobadilla A, Plano F, et al. Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial. BMC Infect Dis. 2021;21:635. doi: 10.1186/s12879-021-06348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elgazzar A, Hany B, Youssef SA, Hany B, Hafez M, Moussa H. Efficacy and safety of ivermectin for treatment and prophylaxis of COVID-19 Pandemic. Res Sq. 2020 https://www.researchsquare.com/article/rs-100956/v1 published online December 28, 2020Available at: [Google Scholar]
- 30.Chaccour C, Casellas A, Blanco-Di Matteo A, Pineda I, Fernandez-Montero A, Ruiz-Castillo P, et al. The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: A pilot, double-blind, placebo-controlled, randomized clinical trial. EClinicalMedicine. 2021;32 doi: 10.1016/j.eclinm.2020.100720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seet RCS, Quek AML, Ooi DSQ, Sengupta S, Lakshminarasappa SR, Koo CY, et al. Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: an open-label randomized trial. Int J Infect Dis. 2021;106:314–322. doi: 10.1016/j.ijid.2021.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chowdhury ATMM, Shahbaz M, Karim MR, Islam J, Guo D, He S. A randomized trial of ivermectin-doxycycline and hydroxychloroquine-azithromycin therapy on COVID19 patients. Res Sq. 2020 https://www.researchsquare.com/article/rs-38896/v1 published online July 24Available at: [Google Scholar]
- 33.Podder C, Chowdhury N, Sina M, Haque W. Outcome of ivermectin treated mild to moderate COVID-19 cases: a single-centre, open-label, randomised controlled study. IMC J Med Sci. 2020;14:002. [Google Scholar]
- 34.Mahmud R, MdM Rahman, Alam I, Ahmed KGU, Kabir AK, Sayeed SK, et al. Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial. J Int Med Res. 2021;49 doi: 10.1177/03000605211013550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krolewiecki A, Lifschitz A, Moragas M, Travacio M, Valentini R, Alonso DF, et al. Antiviral effect of high-dose ivermectin in adults with COVID-19: a proof-of-concept randomized trial. EclinicalMedicine. 2021;37 doi: 10.1016/j.eclinm.2021.100959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chachar AZ, Khan KA, Asif M, Tanveer K, Khaqan A, Basri R, et al. Effectiveness of Ivermectin in SARS-CoV-2/COVID-19 Patients. Int J Sci. 2020;9:31–35. [Google Scholar]
- 37.Kirti R, Roy R, Pattadar C, Raj R, Agarwal N, Biswas B, et al. Ivermectin as a potential treatment for mild to moderate COVID-19: a double blind randomized placebo-controlled trial. MedRxiv. 2021 doi: 10.18433/jpps32105. http://medrxiv.org/lookup/doi/10.1101/2021.01.05.21249310 published online January 9Available at: [DOI] [PubMed] [Google Scholar]
- 38.Mohan A, Tiwari P, Suri T, Mittal S, Patel A, Jain A, et al. Ivermectin in mild and moderate COVID-19 (RIVET-COV): a randomized, placebo-controlled trial. Res Sq. 2021 doi: 10.1016/j.jiac.2021.08.021. https://www.researchsquare.com/article/rs-191648/v1 published online February 2Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samaha AA, Mouawia H, Fawaz M, Hassan H, Salami A, Bazzal AA, et al. Effects of a single dose of ivermectin on viral and clinical outcomes in asymptomatic SARS-CoV-2 infected subjects: a pilot clinical trial in lebanon. Viruses. 2021;13:989. doi: 10.3390/v13060989. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Bukhari KHS, Asghar A, Perveen N, Hayat, A, Mangat SA, Butt KR, et al. Efficacy of Ivermectin in COVID-19 patients with mild to moderate disease. MedRxiv. 2021 http://medrxiv.org/lookup/doi/10.1101/2021.02.02.21250840 published online February 5. Available at: [Google Scholar]
- 41.López-Medina E, López P, Hurtado IC, Dávalos DM, Ramirez O, Martínez E, et al. Effect of Ivermectin on Time to Resolution of Symptoms Among Adults With Mild COVID-19: A Randomized Clinical Trial. JAMA. 2021;325(14):1426–1435. doi: 10.1001/jama.2021.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kishoria N, Mathur SL, Parmar V, Kaur RJ, Agarwal H, Parihar BS, et al. Ivermectin as adjuvant to hydroxychloroquine in patients resistent to standard treatment for SARS-CoV-2: Results of an open-label randomized clinical study. PIJR. 2020;9(8):1–4. [Google Scholar]
- 43.Abd-Elsalam S, Noor RA, Badawi R, Khalaf M, Esmail ES, Soliman S, et al. Clinical study evaluating the efficacy of ivermectin in COVID-19 treatment: a randomized controlled study. J Med Virol. 2021;43(10):27122. doi: 10.1002/jmv.27122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biber A, Mandelboim M, Harmelin G, Lev D, Ram L, Shaham A, et al. Favorable outcome on viral load and culture viability using Ivermectin in early treatment of non-hospitalized patients with mild COVID-19 – A double-blind, randomized placebo-controlled trial. MedRxiv. 2021 doi: 10.1016/j.ijid.2022.07.003. http://medrxiv.org/lookup/doi/10.1101/2021.05.31.21258081 published online May 31. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faisal R, Shah SFA, Hussain M. Potential use of azithromycin alone and in combination with ivermectin in fighting against the symptoms of COVID-19. TPMJ. 2021;28:737–741. [Google Scholar]
- 46.Okumuş N, Demirtürk N, Çetinkaya RA, Güner R, Avcı IY, Orhan S, et al. Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients. BMC Infect Dis. 2021;21:411. doi: 10.1186/s12879-021-06104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bermejo Galan LE, dos, Santos NM, Asato MS, Vieira Araújo J, Lima Moreira A, Marques Araújo AM, et al. Phase 2 randomized study on chloroquine, hydroxychloroquine or ivermectin in hospitalized patients with severe manifestations of SARS-CoV-2 infection. Pathog GlobHealth. 2021;8:1–8. doi: 10.1080/20477724.2021.1890887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hashim HA, Maulood MF, Rasheed AM, Fatak DF, Kabah KK, Abdulamir AS. Controlled randomized clinical trial on using Ivermectin with Doxycycline for treating COVID-19 patients in Baghdad, Iraq. MedRvix. 2020 http://medrxiv.org/lookup/doi/10.1101/2020.10.26.20219345 published online October 27. Available at: [Google Scholar]
- 49.Niaee MS, Gheibi N, Namdar P, Zolghadr L, Javadi A, Karampour A, et al. Ivermectin as an adjunct treatment for hospitalized adult COVID-19 patients: a randomized multi-center clinical trial [Internet] Research Square. 2020 https://www.researchsquare.com/article/rs-109670/v1 published online November 24Available at: [Google Scholar]
- 50.Babalola OE, Bode CO, Ajayi AA, et al. Ivermectin shows clinical benefits in mild to moderate COVID19: a randomized controlled double-blind, dose-response study in Lagos. QJM: Int J Med. 2021:hcab035. doi: 10.1093/qjmed/hcab035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shahbaznejad L, Davoudi A, Eslami G, Markowitz JS, Navaeifar MR, Hosseinzadeh F, et al. Effect of ivermectin on COVID-19: a multicenter double-blind randomized controlled clinical trial. Clin Ther. 2021;43(6) doi: 10.1016/j.clinthera.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pott-Junior H, Bastos Paoliello MM, de Queiroz Constantino Miguel A, Constantino Miguel A, Ferreira da Cunha A, Melo Freire CC, et al. Use of ivermectin in the treatment of Covid-19: a pilot trial. Toxicol Rep. 2021;8 [Google Scholar]
- 53.Shoumann WM, Hegazy AA, Nafae RM, et al. Use of ivermectin as a potential chemoprophylaxis for COVID-19 in egypt: a randomised clinical trial. JCDR. 2021;15:27–32. [Google Scholar]
- 54.Chahla RE, Medina Ruiz L, Ortega ES, Morales MF, Barreiro F, George A, et al. A randomized trial - intensive treatment based in ivermectin and iota-carrageenan as pre-exposure prophylaxis for COVID- 19 in healthcare agents. MedRxiv. 2021 doi: 10.1097/MJT.0000000000001433. http://medrxiv.org/lookup/doi/10.1101/2021.03.26.21254398 published online March 30Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rada G. Ivermectin for COVID-19 (Matrix of evidence, Epistemonikos). Available at: http://www.epistemonikos.org/matrixes/601138e77aaac854cf94ac1f. Accessed June 27, 2021.
- 56.Popp M, Stegemann M, Metzendorf M-I, Gould S, Kranke P, Meybohm P, et al. Cochrane Database of Systematic Reviews. Cochrane Haematology Group, Cochrane Infectious Diseases Group; 2021. Ivermectin for preventing and treating COVID-19.http://doi.wiley.com/10.1002/14651858.CD015017.pub2 published online Jul 28 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.COVID-NMA. Available at: https://covid-nma.com. Accessed June 27, 2021.
- 58.Hill A, Abdulamir A, Ahmed S, Asghar A, Babalola OE, Basri R, et al. Meta-analysis of randomized trials of ivermectin to treat SARS-CoV-2 infection. Res Sq. 2021 https://www.researchsquare.com/article/rs-148845/v1 published online January 19Available at: [Google Scholar]
- 59.Karale S, Bansal V, Makadia J, Tayyeb M, Khan H, Ghanta SS, et al. A meta-analysis of mortality, need for ICU admission, use of mechanical ventilation and adverse effects with ivermectin use in COVID-19 Patients. MedRxiv. 2021 http://medrxiv.org/lookup/doi/10.1101/2021.04.30.21256415 published online May 4. Available at: [Google Scholar]
- 60.investigators GLUCOCOVID, Corral-Gudino L, Bahamonde A, Arnaiz-Revillas F, Gómez-Barquero J, Abadía-Otero J, et al. Methylprednisolone in adults hospitalized with COVID-19 pneumonia: an open-label randomized trial (GLUCOCOVID) Wien Klin Wochenschr. 2021;133:303–311. doi: 10.1007/s00508-020-01805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. NEJM. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davoudi-Monfared E, Rahmani H, Khalili H, Hajiabdolbaghi M, Salehi M, Abbasian L, et al. A randomized clinical trial of the efficacy and safety of interferon β-1a in treatment of severe COVID-19. Antimicrob Agents Chemother. 2020;64(9) doi: 10.1128/AAC.01061-20. https://journals.asm.org/doi/10.1128/AAC.01061-20 published online August 20Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Avendaño-Solà C, Ramos-Martínez A, Muñez-Rubio E, Ruiz-Antorán B, de Molina RM, Torres F, et al. Convalescent plasma for COVID-19: a multicenter, randomized clinical trial. MedRxiv. 2020 http://medrxiv.org/lookup/doi/10.1101/2020.08.26.20182444 published online September 29Available at: [Google Scholar]
- 64.The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. NEJM 2021;384:693–704. [DOI] [PMC free article] [PubMed]
- 65.RECOVERY collaborative group. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. The Lancet 2021;397:2049–2/59. [DOI] [PMC free article] [PubMed]
- 66.Horby PW, Mafham M, Bell JL, Linsell L, Staplin N, Emberson JR, et al. Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. The Lancet. 2020;396:1345–1352. doi: 10.1016/S0140-6736(20)32013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.WHO solidarity trial consortium. repurposed antiviral drugs for Covid-19 — Interim WHO Solidarity Trial Results. NEJM 2021;384:497–511. [DOI] [PMC free article] [PubMed]
- 68.WHO. Handbook for good clinical research practice (GCP). Available at: https://www.who.int/medicines/areas/quality_safety/safety_efficacy/gcp1.pdf. Accessed June 27, 2021.
- 69.FDA. Good Clinical practice (GCP). Available at: https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/good-clinical-practice. Accessed June 27, 2021.
- 70.ISO 14155:2011. Clinical investigation of medical devices for human subjects — Good clinical practice. Available at: https://www.iso.org/standard/45557.html. Accessed June 27, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.