Abstract
Enterococcus cecorum (formerly Streptococcus cecorum), originally isolated from poultry intestines, has rarely been encountered in human diseases. A 60-year-old man with liver cirrhosis and hepatocellular carcinoma developed peritonitis on the seventh day of his hospitalization. Cultures of one blood sample and one ascites fluid sample obtained on that day both grew E. cecorum. The patient received intravenous cefoxitin therapy and initially responded well. Unfortunately, another episode of peritonitis associated with septic shock developed 24 days after the start of treatment, and culture of one blood specimen yielded the same organism. The isolates were identified by the conventional biochemical tests, the API Rapid ID 32 Strep system, and the API ZYM system (both systems from bioMerieux, Marcy L'Etoile, France) and were further confirmed by cellular fatty acid chromatography and 16S rRNA gene partial sequencing. The identical biotype, antibiotype, and random amplified polymorphic DNA pattern of the three isolates documented the long-term persistence of this organism in the patient. To the best of our knowledge, this is the first clinical description of recurrent bacteremic peritonitis caused by E. cecorum.
CASE REPORT
A 60-year-old man was admitted to the hospital on 1 December 1998 because of black-colored stool, oliguria, jaundice, and disorientation of 1 week's duration. The patient had had hepatitis B virus-related liver cirrhosis diagnosed 20 years earlier. During his hospitalization, bleeding of esophageal and gastric varices, decompensated liver cirrhosis with ascites, and hepatic encephalopathy were diagnosed. He received supportive treatment, and his general condition improved. However, fever and abdominal pain developed on 19 December 1998. The patient was treated with intravenous cefoxitin (2 g every 8 h) under the suspicion of spontaneous bacterial peritonitis. Examinations of the ascites fluid specimen revealed a white blood cell count of 4,000/mm3 with 85% neutrophils. Bacterial cultures of one blood sample in BACTEC 6A aerobic culture bottles (Becton Dickinson, Sparks, Md.) and one ascites fluid (turbid) specimen collected on 19 December both yielded Enterococcus species (isolates A and B) after 2 days of incubation. The fever subsided and the abdominal pain resolved 2 days after the initiation of treatment. The two Enterococcus isolates were susceptible to penicillin (MICs, 0.094 μg/ml) as determined by the Etest (AB BIODISK, Solna, Sweden). The patient received cefoxitin treatment for a total of 14 days.
Unfortunately, fever, abdominal pain with shifting dullness, increasing abdominal girth, and tarry stool were noticed on 10 January 1999. One set of blood cultures (grown in BACTEC 6A aerobic bottles) revealed the same Enterococcus species; however, an ascites fluid culture was negative for the organism. The patient received intravenous ceftizoxime (2 g every 12 h) therapy. The patient's clinical condition deteriorated and was complicated by acute renal failure, jaundice, and intractable shock. Though the antibiotics were shifted to vancomycin (1 g per day) and meropenem (500 mg per day) because of the intractable septic shock, the patient died on the 53rd hospital day.
Microbiology.
The three isolates grew well on Trypticase soy agar supplemented with 5% sheep blood (BBL Microbiology Systems, Cockeysville, Md.) in 5% CO2 and in ambient air at 35°C. They were catalase-negative and gram-positive cocci. The isolates formed smooth, gray, and convex colonies with slight α-hemolysis on Trypticase soy agar supplemented with 5% sheep blood. These isolates did not grow on agar containing 5% NaCl and were unable to grow on bile esculin agar (BBL Microbiology Systems) in ambient air or 5% CO2 within 2 days, but scanty growth on both agar plates was identified after 3 to 5 days of incubation. Group D antigen reactions (Oxoid, Unipath Limited, Basingstoke, Hampshire, England) of the three isolates were negative. They did not hydrolyze pyrrolidony-β-naphthylamide but were able to hydrolyze leucine-β-naphthylamide. Further phenotypic identification of these three isolates to the species level was done using the API Rapid ID 32 Strep system (bioMerieux, Marcy L'Etoile, France), and the API ZYM (bioMerieux Vitek, Inc.). Enterococcus cecorum ATCC 43198 was used as a control strain in this study.
The reaction profiles generated by the API Rapid ID 32 Strep system (27176707110) and the API ZYM system for the three isolates and the control strain were identical. The following characteristics suggested our three isolates belonged to group IV E. cecorum: they had positive reactions for sorbitol, raffinose, sucrose, β-glucuronidase, and alkaline phosphatase and negative reactions for mannitol, sorbose, arginine, and arabinose (7).
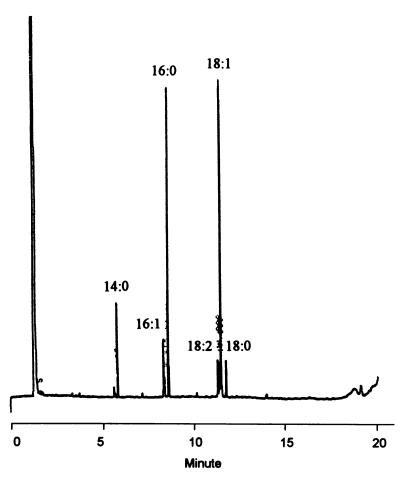
Cellular fatty acid analysis of the isolates was performed as previously described (10). Six major cellular fatty acids were found: octadecenoic acid (18:1), hexadecanoic acid (16:0), tetradecanoic acid (14:0), hexadecenoic acid (16:1), cis-9,12-octadecadienoic acid (18:2), and octadecanoic acid (18:0). The four isolates had identical cellular fatty acid profiles (Fig. 1).
FIG. 1.
Gas chromatogram of cellular fatty acid methyl esters of E. cecorum. The designations of the fatty acid peaks refer to the number of carbon atoms (number before the colon) and the number of double bonds (number after the colon) (Microbial Identification System; Microbial ID Inc., Newark, Del.).
Further identification of the isolates to species level was performed by 16S rRNA gene partial sequencing using a pair of universal primers, DG74 and RW01, as described previously (8). The amplification products were sequenced after being cloned into plasmids using a TA cloning kit. Following thermal cycling of the sequencing reactions with fluorescent dye-labeled primers or terminators, the nucleotide sequence was determined by an autosequencer (Perkin-Elmer, Applied Biosystem Division, Foster City, Calif.). A BLAST search was performed to compare the sequence of the clinical isolate with those in the GenBank and Ribosomal Database Project databases. The closest match observed was with E. cecorum.
MICs of the 11 antimicrobial agents for the three isolates (isolates A, B, and C) and the control strain (isolate D) of E. cecorum were determined by the agar dilution method using unsupplemented Mueller-Hinton agar (BBL Microbiology Systems) as described by the National Committee for Clinical Laboratory Standards (11). Staphylococcus aureus ATCC 29213 and Enterococcus faecalis ATCC 29212 were used as control strains in each set of the tests. The three isolates recovered from the patient were susceptible to all the antimicrobial agents tested and had identical antibiotypes, which were different from that of the control strain (Table 1). The β-lactamase activities, determined by means of a chromogenic cephalosporin assay (cefinase disk; BBL Microbiology Systems), of the three isolates were negative.
TABLE 1.
In vitro susceptibilities of E. cecorum isolates
| Agent | MIC (μg/ml) for E. cecorum isolate(s)
|
|
|---|---|---|
| A, B, and Ca | ATCC 43198 | |
| Penicillin | 0.12 | 0.12 |
| Vancomycin | 1 | 1 |
| Teicoplanin | 0.25 | 0.25 |
| Gentamicin | 4 | 4 |
| Rifampin | 0.03 | 0.03 |
| Ciprofloxacin | 4 | 1 |
| Trovafloxacin | 0.03 | 0.03 |
| Moxifloxacin | 0.06 | 0.06 |
| Quinupristin-dalfopristin | 1 | 1 |
| Linezolid | 1 | 0.5 |
Clinical isolates.
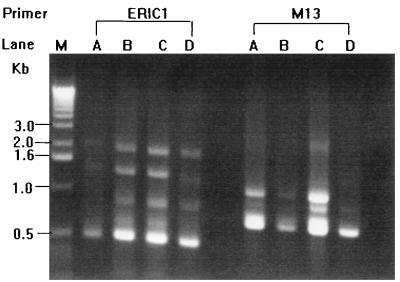
Random amplified polymorphic DNA (RAPD) patterns generated by arbitrarily primed PCR (APPCR) were obtained, and the results were interpreted as previously described (10). Two oligonucleotide primers, M13 (5′-GAGGGTGGCGGTTCT-3′) and ERIC1 (5′-GTGAATCCCCAGGAGCTTACAT-3′), were used. The three isolates recovered from the patient had identical RAPD patterns, which were different from those of the control strain (Fig. 2).
FIG. 2.
RAPD patterns of the four isolates of E. cecorum generated by APPCR using two primers, ERIC1 and M13. Lanes: M, molecular size marker; A to C, E. cecorum isolates from the patient; D, E. cecorum ATCC 43198.
Enterococcus cecorum was first isolated from chicken intestines and was formerly described as Streptococcus cecorum by Devriese et al. in 1983 (6). Since then, this species has been identified as part of the intestinal microflora of many animal hosts, not limited to poultry (4–7). The first human infection was reported in a malnourished adult patient with severe septicemia in 1997 (9). We describe a patient with liver cirrhosis and hepatocellular carcinoma who had recurrent bacteremic peritonitis due to this organism.
Three important points were demonstrated in the present study. First, this is the first clinical description of primary peritonitis and bacteremia due to E. cecorum. Second, the phenotypic and chemotaxonomic traits of our E. cecorum isolates (delayed growth on both 5% NaCl agar and bile esculin agar, negative pyrrolidony-β-naphthylamide reaction, devoid of demonstrable group D antigen, and absence of a cellular fatty acid, C19:0cyc11,12) were unique to those displayed by more frequently isolated enterococcal species (1, 7, 9). Third, using the biotyping, antibiotyping, and molecular typing methods, we documented that this organism can persist over the long term in the bloodstream or peritoneum and cause recurrent infection.
The portal of entry of this organism and the cause for recurrent infection in our patient were obscure. Greub et al. suggested that E. cecorum could colonize in the cutaneous lesions of a person who might have acquired the organism from domestic animals and then reach the bloodstream, possibly via placement of an intravenous catheter (9). Whether our patient had previous exposure to any poultry or domestic animals before admission was not known. This organism has never been reported to inhabit the human gastrointestinal tract or skin (7, 9). In our patient, the seeding of this organism into the peritoneum might have been secondary to catheter-related bacteremia or could have occurred via diseased intestinal mucosa where the organism had already been existing. However, stool cultures for the recovery of E. cecorum were not performed and cultures of the removed catheter could not yield the organism. The use of cefoxitin, a well-known suboptimal agent for treating severe enterococcal infection (11), during the first bacteremic episode might partly have contributed to the recurrence of the bacteremia.
Our E. cecorum isolates had biochemical reactions, including those in the API Rapid ID 32 Strep and API ZYM systems, identical with those of the isolate recovered by Greub et al. and also identical with that of the control strain (9). In addition to the biochemical identification test, sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of whole-cell proteins and reverse transcriptase sequencing of 16S rRNA are useful for accurate identification of E. cecorum (9, 12). The commercially available enterococcal nucleic acid probe (AccuProbe; Gen-Probe, San Diego, Calif.) for culture confirmation was reported to be 100% accurate in identifying Enterococcus species, but a negative reaction of the assay was documented in E. cecorum isolates (2, 7, 9). Although not valuable for species identification, pulsed-field gel electrophoresis has been superior for the interpretation of interstrain relationships (3). However, as described in our previous study (10), RAPD patterns generated by APPCR also appeared to be useful for strain discrimination. The present report further demonstrates that cellular fatty acid analysis is also a useful tool for the identification of E. cecorum.
In conclusion, this report described a case of E. cecorum peritonitis and bacteremia in a patient with liver cirrhosis and emphasized the recurrent nature of this organism in human diseases. To accurately recognize this organism, clinical microbiologists should be alert to the unusual phenotypic traits of this enterococcal species.
REFERENCES
- 1.Bosley S G, Wallace P L, Moss C W, Steigerwalt A G, Brenner D J, Swenson J M, Hebert G A, Facklam R R. Phenotypic characterization, cellular fatty acid composition, and DNA relatedness of aerococci and comparison to related genera. J Clin Microbiol. 1990;28:416–421. doi: 10.1128/jcm.28.3.416-421.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daly J A, Clifton N L, Seskin K C, Gooch W M., III Use of rapid, nonradioactive DNA probes in culture confirmation tests to detect Streptococcus agalactiae, Haemophilus influenzae, and Enterococcus spp. from pediatric patients with significant infections. J Clin Microbiol. 1991;29:80–82. doi: 10.1128/jcm.29.1.80-82.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Descheemaker P, Lammens C, Pot B, Vandamme P, Goosens H. Evaluation of arbitrarily primed PCR analysis and pulsed-field gel electrophoresis of large DNA fragments for the identification of enterococci important in human medicine. Int J Syst Bacteriol. 1997;47:555–561. doi: 10.1099/00207713-47-2-555. [DOI] [PubMed] [Google Scholar]
- 4.Devriese L A, Hommez J, Pot B, Haesebrouck F. Identification and composition of the streptococcal and enterococcal flora of tonsils, intestines and faeces of pigs. J Appl Bacteriol. 1994;77:31–36. doi: 10.1111/j.1365-2672.1994.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 5.Devriese L A, Ceyssens K, Haesebrouck F. Characteristics of Enterococcus cecorum strains from the intestines of different animal sources. Lett Appl Microbiol. 1991;12:137–139. [Google Scholar]
- 6.Devriese L A, Dutta G N, Farrow J A E, van de Kerckhove A, Phillips B A. Streptococcus cecorum, a new species isolated from chickens. Int J Syst Bacteriol. 1983;33:772–776. [Google Scholar]
- 7.Facklam R R, Sahm D F, Teixeira L M. Enterococcus. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: American Society for Microbiology; 1999. pp. 297–305. [Google Scholar]
- 8.Greisen K, Loeffelholz M, Purohit A, Leong D. PCR primers and probes for the 16S rRNA gene of most species of pathogenic bacteria, including bacteria found in cerebrospinal fluid. J Clin Microbiol. 1994;32:335–351. doi: 10.1128/jcm.32.2.335-351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greub G, Devriese L A, Pot B, Dominguez J, Bille J. Enterococcus cecorum septicemia in a malnourished adult patient. Eur J Clin Microbiol Infect Dis. 1997;16:594–598. doi: 10.1007/BF02447923. [DOI] [PubMed] [Google Scholar]
- 10.Hsueh P R, Teng L J, Pan H J, Chen Y C, Wang L H, Chang S H, Ho S W, Luh K T. Emergence of vancomycin-resistant enterococci at a university hospital in Taiwan: persistence of multiple species and multiple clones. Infect Control Hosp Epidemiol. 1999;20:828–833. doi: 10.1086/501592. [DOI] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. Performance standard for antimicrobial susceptibility testing. Ninth informational supplement. NCCLS document M100-S9. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1999. [Google Scholar]
- 12.Williams A M, Farrow J A E, Collins M D. Reverse transcriptase sequencing of 16S ribosomal RNA from Streptococcus cecorum. Lett Appl Microbiol. 1989;8:185–189. [Google Scholar]