Abstract
Background
Diabetes and hypertension disparities are pronounced among South Asians. There is regional variation in the prevalence of diabetes and hypertension in the US, but it is unknown whether there is variation among South Asians living in the US. The objective of this study was to compare the burden of diabetes and hypertension between South Asian patients receiving care in the health systems of two US cities.
Methods
Cross-sectional analyses were performed using electronic health records (EHR) for 90,137 South Asians receiving care at New York University Langone in New York City (NYC) and 28,868 South Asians receiving care at Emory University (Atlanta). Diabetes was defined as having 2 + encounters with a diagnosis of diabetes, having a diabetes medication prescribed (excluding Acarbose/Metformin), or having 2 + abnormal A1C levels (≥ 6.5%) and 1 + encounter with a diagnosis of diabetes. Hypertension was defined as having 3 + BP readings of systolic BP ≥ 130 mmHg or diastolic BP ≥ 80 mmHg, 2 + encounters with a diagnosis of hypertension, or having an anti-hypertensive medication prescribed.
Results
Among South Asian patients at these two large, private health systems, age-adjusted diabetes burden was 10.7% in NYC compared to 6.7% in Atlanta. Age-adjusted hypertension burden was 20.9% in NYC compared to 24.7% in Atlanta. In Atlanta, 75.6% of those with diabetes had comorbid hypertension compared to 46.2% in NYC.
Conclusions
These findings suggest differences by region and sex in diabetes and hypertension risk. Additionally, these results call for better characterization of race/ethnicity in EHRs to identify ethnic subgroup variation, as well as intervention studies to reduce lifestyle exposures that underlie the elevated risk for type 2 diabetes and hypertension development in South Asians.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13098-021-00766-w.
Keywords: Hypertension, Diabetes, South Asian, Co-morbidity, Electronic health record
Background
Over two-thirds of United States (US) adults with diabetes have hypertension, and half are not meeting blood pressure (BP) goals despite having antihypertensive treatment [1]. Hypertension is associated with a two times greater risk for cardiovascular disease events and mortality among adults with diabetes [2]. Diabetes disparities are pronounced among South Asians compared to other race/ethnic groups in the United States [3]. National studies have found US South Asians have the highest age-adjusted burden of diabetes (23%) compared to whites (6%), Chinese Americans (13%), Latinos (17%), and African Americans (18%) [4]. Further, among South Asians with diabetes, 72% also have co-morbid hypertension [5].
The purpose of this cross-sectional analysis is to compare diabetes and hypertension burden among South Asians receiving care at two large academic medical centers in New York City (NYC) and Atlanta. Understanding disparities across these populations may help disentangle issues related to environmental factors, health services, and/or immigration by region. These data will provide the foundation for discussion regarding similarities and differences between the populations across sites to inform the development of intervention strategies to reduce the burden of diabetes complications among South Asians in the US. These findings will facilitate bidirectional future research efforts to identify and address disparities across the populations. Given the rapid growth of South Asians in these regions and their high burden of diabetes and hypertension, a critical need exists to tailor, translate, and disseminate evidence-based interventions to maximize impact in ameliorating co-morbid cardiovascular disease disparities [6].
Methods
Study population
We utilized electronic health record (EHR) data on the Epic platform at New York University (NYU) Langone Health and the Cerner platform at Emory University to measure patient burden of chronic diseases. Eligibility criteria of patients at each of the sites were: (1) South Asian ethnicity (identified by common South Asian surnames, race/ethnicity as listed in the EHR, or language preference); (2) 18 + years of age or older; and (3) was seen by a primary care physician at NYU Langone between 2014 and 2019 or Emory between 2014 and 2017. Individuals were excluded if they were pregnant at the time of visit. This study was approved by the Institutional Review Board at NYU Grossman School of Medicine.
Metric definitions
Identifying South Asian patients
We derived a list of 12,907 South Asian surnames by compiling a list of names from Social Security Administration data [7], death certificate data [8], and prior studies conducted among South Asians [9, 10]. For race/ethnicity as listed in the EHR, selection criteria included Asian Indian, Bangladeshi, Pakistani, or Sri Lankan. For preferred language, selection criteria included Bengali, Gujarati, Hindi, Kannada, Kashmiri, Malayalam, Nepali, Pakistani, Punjabi, Sindhi, Sinhalese, Tamil, or Urdu.
Rule-based algorithms developed for EHR were used to define diabetes and hypertension, as fully described elsewhere [11, 12]. Briefly, burden estimates were defined by combining: ICD-9-CM and ICD-10-CM diagnostic codes, lab results or vitals, or relevant medications (Additional file 1: Table S1). Biologically implausible measurements for vitals and labs, such as systolic BP outside the range of 140–250 mmHg or diastolic BP outside the range of 90–150 mmHg, were excluded from these algorithms.
Sensitivity analyses
Among NYC data, sensitivity analyses were conducted to restrict the sample to those identified as Asian Indian, Bangladeshi, Pakistani, or Sri Lankan race/ethnicity using the standard fields in the EHR. Modifications tested were based on variations in definitions seen in the literature and hypothesized concerns with data quality.
Statistical analyses
Proportion of individuals with diabetes, hypertension, and comorbid diabetes and hypertension were estimated by site and sex and were age-adjusted using direct standardization with the 2000 census as the standard population [13]. Participant characteristics were stratified by sex and compared by site using likelihood ratio χ2 tests. Analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC).
Results
The sample included 90,137 individuals receiving care at NYU Langone in NYC, and 28,868 individuals receiving care at Emory University in Atlanta (Table 1). Mean age was 48.8 (SD 17.4) years among women and 50.1 (SD 17.5) years among men in NYC compared to a mean age of 55.6 (SD 19.4) years among Atlanta women and 58.4 (SD 19.5) years among Atlanta men. Two-thirds of each sample had private insurance.
Table 1.
South Asian patient population by study site, 2014–2019*
| Characteristic | Women | Men | ||
|---|---|---|---|---|
| Atlanta (n = 14,916) | New York City (n = 50,815) | Atlanta (n = 11,825) | New York City (n = 39,322) | |
| South Asian inclusion criteria, n (%) | ||||
| South Asian surname | 14,916 (100) | 43,520 (85.6) | 11,825 (100) | 34,085 (86.7) |
| South Asian race/ethnicity | 15,730 (31.0) | 13,393 (34.1) | ||
| South Asian language | 2408 (4.7) | 1424 (3.6) | ||
| South Asian surname and race/ethnicity | 8995 (17.7) | 8452 (21.5) | ||
| South Asian surname and language | 1952 (3.8) | 1191 (3.0) | ||
| South Asian surname, language, and race/ethnicity | 1200 (2.4) | 749 (1.9) | ||
| 1000 (2.0) | 647 (1.6) | |||
| Age, mean (SD) | 56.9 (18.3) | 48.8 (17.4) | 60.2 (17.5) | 49.0 (17.0) |
| Race/ethnicity, n (%) | ||||
| American Indian or Alaska Native | 298 (2.0) | 317 (0.6) | 271 (2.3) | 228 (0.6) |
| Asian | 3088 (20.7) | 18,607 (36.5) | 2786 (23.6) | 15,375 (39.1) |
| Black or African American | 3488 (23.4) | 4187 (8.2) | 1872 (15.8) | 2509 (6.4) |
| Hispanic or Latino | 4 (0.03) | n/a | 3 (0.03) | n/a |
| Native Hawaiian or Other Pacific Islander | 129 (0.9) | 296 (0.6) | 82 (0.7) | 171 (0.4) |
| White | 4661 (31.2) | 9268 (18.2) | 3526 (29.8) | 6472 (16.5) |
| Other | 141 (0.9) | 9298 (18.3) | 135 (1.1) | 7766 (19.8) |
| Unknown | 3107 (20.8) | 8813 (17.4) | 3150 (26.6) | 6761 (17.2) |
| Language spoken | ||||
| Bengali | 102 (0.7) | 1005 (2.0) | 53 (0.4) | 728 (1.9) |
| English | 11,400 (76.4) | 44,658 (87.9) | 8764 (74.1) | 35,404 (90.0) |
| Gujarati | 89 (0.6) | 80 (0.2) | 56 (0.5) | 37 (0.09) |
| Hindi | 155 (1.0) | 443 (0.9) | 109 (0.9) | 242 (0.6) |
| Kannada | 0 (0.0) | 1 (0.01) | 0 (0.0) | 0 (0.0) |
| Malayalam | 6 (0.04) | 35 (0.07) | 4 (0.03) | 17 (0.04) |
| Nepali | 65 (0.4) | 89 (0.18) | 51 (0.4) | 73 (0.2) |
| Punjabi | 13 (0.09) | 144 (0.28) | 0 (0.0) | 95 (0.24) |
| Tamil | n/a | 17 (0.03) | n/a | 9 (0.02) |
| Urdu | 68 (0.5) | 772 (1.5) | 32 (0.3) | 333 (0.9) |
| Other | 449 (3.0) | 3569 (7.0) | 1122 (9.5) | 2382 (6.0) |
| Not recorded | 2569 (17.2) | 0 (0.0) | 2458 (20.8) | 0 (0.0) |
| Insurance status | ||||
| Medicare | 3326 (22.3) | 6048 (11.9) | 2742 (23.2) | 5017 (12.8) |
| Medicaid | 932 (6.2) | 9817 (19.3) | 502 (4.2) | 6783 (17.3) |
| Private Insurance (HMO, PPO, POS, Indemnity, EPO, Managed Care) | 9915 (66.5) | 33,159 (65.3) | 7838 (66.3) | 25,556 (65.0) |
| Other (self pay, workers comp, no fault, child health plus) | 743 (5.0) | 465 (0.9) | 743 (6.3) | 760 (1.9) |
| Missing | n/a | 1326 (2.6) | n/a | 1206 (3.1) |
| Smoke cigarettes, n (%) | ||||
| Yes | 765 (5.1) | 3470 (6.8) | 1113 (9.4) | 6546 (16.7) |
| No | 11,274 (75.6) | 46,768 (92.0) | 8597 (57.6) | 32,277 (82.1) |
| Missing/NA | 2877 (19.3) | 577 (1.1) | 2115 (14.2) | 499 (1.3) |
*All variables were collected using electronic health record
Using surnames as the criteria to identify South Asians, the most commonly reported first race/ethnicity in the NYC sample was Asian Indian (25.5% among women and 27.7% among men). Using the same list of surnames to select the Atlanta sample, Asian was the fourth most commonly reported first race/ethnicity (20.3% among women and 22.9% among men) after Caucasian, African American, and Unknown.
After age-adjustment, one-fifth (19.4% of women and 23.1% of men) of NYC patients had a hypertension diagnosis, while approximately a quarter of Atlanta patients had a hypertension diagnosis (23.3% of women and 26.5% of men) (Table 2). Age-adjusted diabetes burden was 9.2% among women and 12.6% among men in NYC, compared to 5.7% among women and 8.0% among men in Atlanta (Table 3). P-values for all statistical comparisons by location and sex were significant (p < 0.001). In Atlanta, 75.6% of those with diabetes had comorbid hypertension compared to 46.2% in NYC. Figure 1 underscores the large proportion of those with diabetes and co-morbid hypertension.
Table 2.
Outcome* comparisons by study site
| Outcome | Atlanta, n (%**) | New York City, n (%)** | p-value*** |
|---|---|---|---|
| Diabetes | 2447 (6.7) | 11,542 (10.7) | < 0.0001 |
| Hypertension | 8233 (24.7) | 20,773 (20.9) | < 0.0001 |
| Proportion of those with diabetes having comorbid hypertension | 1849 (75.6) | 5335 (46.2) | < 0.0001 |
*EHR definitions described in Additional file 1: Table S1
**Age adjusted burden using the 2000 Census as the reference population
***p-values ≤ 0.0001 using chi-squared tests
Table 3.
Outcome* comparisons by sex and study site
| Outcome | Women | Men | p-value for women*** | p-value for men*** | ||
|---|---|---|---|---|---|---|
| Atlanta (n = 14,916) | New York City (n = 50,815) | Atlanta (n = 11,825) | New York City (n = 39,322) | |||
| n (%)** | n (%)** | n(%)** | n(%)** | |||
| Diabetes | 1119 (5.7) | 5383 (9.2) | 1328 (8.0) | 6158 (12.6) | < 0.0001 | < 0.0001 |
| Hypertension | 4285 (23.3) | 11,232 (19.4) | 3948 (26.5) | 10,036 (23.1) | < 0.0001 | < 0.0001 |
| Proportion of those with diabetes having comorbid hypertension | 852 (76.1) | 2510 (46.6) | 997 (75.1) | 2825 (45.9) | < 0.0001 | < 0.0001 |
*EHR definitions described in Additional file 1: Table S1
**Age adjusted burden using the 2000 Census as the reference population
***p-values ≤ 0.0001 using chi-squared tests
Fig. 1.
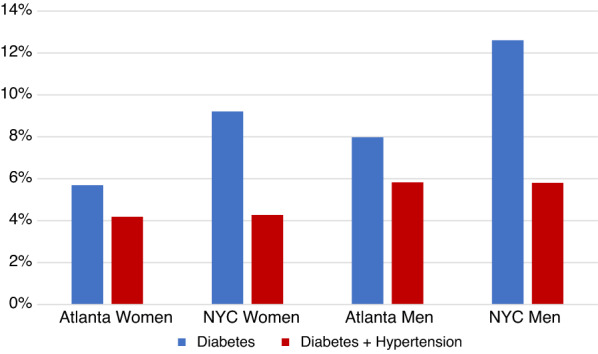
Age-adjusted proportion with diabetes and co-morbid hypertension by sex and region
Discussion
Diabetes burden in the US South Asian community is high, with national and regional data revealing the highest burden of diagnosed diabetes among Asian Indians compared to other Asian groups [14] and compared to non-Hispanic whites [15–19],20, 21. Research also indicates that South Asians living in the US have a higher burden of hypertension compared to some other race/ethnic populations [22, 23]. Our study found significant differences in the burden of diagnosed diabetes and hypertension in South Asians receiving care in NYC compared to Atlanta. Specifically, there was a higher burden of diagnosed diabetes in NYC compared to Atlanta (10.7% compared to 6.7% respectively). The burden of diabetes amongst South Asians in NYC and Atlanta reported in our study is lower than that reported in previous research. Prior community health surveys (NYC Community Health Resources and Needs Assessment 2013–2016 and NYC Community Health Survey 2013–2017) demonstrated that Asian Indians in NYC have a tremendous burden of self-reported diabetes (21%) [3]. Additionally, the burden of self-reported diabetes diagnoses among South Asians of normal weight (using adjusted-BMI guidelines for Asians) in NYC is more than triple the rates of diabetes among non-Hispanic whites of normal weight (10.2% vs 2.9%, respectively) [24]. Furthermore, in a community-based survey of Asian Indians in Atlanta, the self-reported burden of diabetes was 18.3%, nearly four times as high as non-Hispanic whites and twice as high as Hispanics [25]. Among those who reported diabetes, there was a > 3.5 odds of having co-morbid hypertension. This population further had higher burden of stroke (2.77%) compared to whites (2.12%) [26].
Similarly, we found differences in the burden of hypertension between South Asians receiving health care in NYC compared to Atlanta, with those in Atlanta having a higher burden of 24.7% compared to 20.9% in NYC. The burden of hypertension reported in our study is lower than that found in a representative survey of both diagnosed and undiagnosed hypertension in NYC, which found a burden of hypertension in South Asians of 43% [23].
Nationwide, 21.4% of people with diabetes are undiagnosed [27]. Differential access to care may partially explain the higher burden of diabetes in NYC versus Atlanta, as New York has expanded their Medicaid program to cover all people with household incomes below 133% of the federal poverty level, while Georgia has not. The lower burden of diabetes and hypertension amongst South Asians in our study compared to previous findings could be due to the fact that our data was collected from two large, private hospital systems, which provide patients with more consistent access to healthcare and may therefore make it more likely for patients to receive preventative care. It is interesting to note that there were significant differences in the burden of diabetes and hypertension between South Asians in the two regions of the US and additional research is necessary to assess differences in regional risk factors that could contribute to this disparity. In addition, a considerable number of South Asians in our study (n = 7184) had a diagnosis of comorbid diabetes and hypertension. Thus, scalable and translatable interventions that promote diabetes management and hypertension control in this population may have significant potential for public health impact and reducing disparities across the US and South Asia.
We acknowledge several limitations. One limitation of this analysis to consider is misclassification error of South Asian origin based on surnames. Prior work has suggested positive predictive values of surname lists ranging from 74 to 91% for Indian surnames in the US [7] and 89.3% among South Asians in Canada [8]. We conducted sensitivity analyses limiting the sample to those reporting South Asian race/ethnicity in the NYC sample, and diabetes burden was 11.2%, suggesting risk may be underestimated in these analyses. The cross-sectional nature of these analyses preclude us from examining change over time, but they provide an initial snapshot of regional differences in diabetes burden by region from the perspective of two academic health centers. Selection bias is another key limitation of our analyses. Prior work has demonstrated the study sample is more economically privileged compared to NYC as a whole due to the nature of the NYU patient population [11], and the patient population at Emory is also a selected sample. Several other limitations of using EHR data in clinical research have been previously noted [28–30], such as differences in procedures for documenting care across systems that could contribute to systematic differences in disease estimates across sites. Finally, we were unable to obtain data on potentially informative covariates such as obesity (BMI was frequently missing in the Atlanta data), diet, physical activity, immigration history, and socioeconomic status, thus limiting the range of analyses we were able to conduct.
Asian Americans currently compose 5% of the US population and approximately 32% of the immigrants entering the country [31]. The US Census Bureau projects that by 2060, the number of Asian Americans nationally will grow to over 39 million, approximately 9.3% of the US population [32, 33]. In NYC, the South Asian community grew by 49% from 2000 to 2010 (216,179 to 323,675, respectively). South Asians also make up the largest Asian American subgroup in the Atlanta metro area. Across South Asian groups, a significant portion of the community live in poverty (ranging from 17% of Asian Indians to 32% of Bangladeshis), have limited English proficiency impacting access to care (ranging from 25% of Asian Indians to 53% of Bangladeshis), and have poor access to culturally appropriate community resources [34–37].
Conclusions
This work highlights the need for health systems to collect more accurate demographic data on patients they care for to improve our population health. Currently, disaggregated data by race/ethnicity on disease prevalence are not available for all states; for example, diabetes prevalence among Asians is unavailable for Georgia [38]. Even so, we found evidence that two key chronic conditions—hypertension and diabetes—represents a significant burden of disease among South Asians in NYC and Atlanta. There is also a need for interventions tailored for South Asian subgroups that can reduce the burden of hypertension and diabetes. For example, a community health worker intervention that was successful in NYC is currently being tested among South Asians in Atlanta [39], and further dissemination of the intervention could reduce diabetes and hypertension burden among South Asians in the US.
Supplementary Information
Additional file 1: Table S1. Outcome definitions and list of ICD-10 codes and medications.
Acknowledgements
We would like to gratefully acknowledge the contributions of Drs. Islam, Lauderdale, and Shah for contributing South Asian surnames to make this analysis possible. We also appreciate Agnes Park’s assistance with compiling the surnames into a master list, and Victoria Lam’s contributions in generating the NYC EHR data.
Abbreviations
- BP
Blood pressure
- EHR
Electronic health record
- NYC
New York City
- NYU
New York University
- SD
Standard deviation
- US
United States
Authors' contributions
NI, MS, and JMB conceived of the study. NI and JMB developed the study protocol and the overall research plan. JMB, JC, and SC performed the data analysis. JMB wrote the manuscript. All authors assisted with editing the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the Centers for Disease Control and Prevention (grant U48DP001904) and the National Institutes of Health (Grant 3U54MD00053817S1). JMB’s time is partially supported by the National Institute of Diabetes and Digestive Kidney Diseases (Grant R01DK127916). NI’s time is partially supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (Grants R01DK110048-01A1, R18DK110740 and P30 DK111022R01DK11048), National Institute on Minority Health and Health Disparities (Grant U54MD000538), National Heart, Lung, and Blood Institute (Grant 1UG3HL151310), and National Center for Advancing Translational Science (Grant UL1TR001445). JH’s time is partially supported by the National Library of Medicine (Grant 5K01LM012924). MS’s time is partially supported by the National Institute on Minority Health and Health Disparities (K23 MD015088). UPG was funded in part by the National Institute of Diabetes and Digestive and Kidney Diseases grant number P30DK111024.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board at NYU Grossman School of Medicine.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Muntner P, Whelton PK, Woodward M, Carey RM. A comparison of the 2017 American College of Cardiology/American Heart Association Blood Pressure Guideline and the 2017 American Diabetes Association Diabetes and Hypertension Position Statement for US adults with diabetes. Diabetes Care. 2018;41(11):2322–2329. doi: 10.2337/dc18-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk. J Am Coll Cardiol. 2014;63(25):2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wyatt LC, Russo R, Kranick J, Elfassy T, Kwon SC, Wong JA, et al. Health Atlas for Asian Americans, Native Hawaiians, and Pacific Islanders: a comprehensive look at AA and NH&PI health in the U.S. 2021. https://med.nyu.edu/departments-institutes/population-health/divisions-sections-centers/healthbehavior/sites/default/files/pdf/csaah-health-atlas.pdf. Accessed 6 Dec 2021.
- 4.Kanaya AM, Herrington D, Vittinghoff E, Ewing SK, Liu K, Blaha MJ, et al. Understanding the high prevalence of diabetes in US south Asians compared with four racial/ethnic groups: the MASALA and MESA studies. Diabetes Care. 2014;37(6):1621–1628. doi: 10.2337/dc13-2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanaya AM, Wassel CL, Mathur D, Stewart A, Herrington D, Budoff MJ, et al. Prevalence and correlates of diabetes in South Asian Indians in the United States: findings from the metabolic syndrome and atherosclerosis in South Asians living in America study and the multi-ethnic study of atherosclerosis. Metab Syndr Relat Disord. 2010;8(2):157–164. doi: 10.1089/met.2009.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng YJ, Imperatore G, Geiss LS, Saydah SH, Albright AL, Ali MK, et al. Trends and disparities in cardiovascular mortality among US adults with and without self-reported diabetes, 1988–2015. Diabetes Care. 2018;41(11):2306–2315. doi: 10.2337/dc18-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lauderdale D, Kestenbaum B. Asian American ethnic identification by surname. Popul Res Policy Rev. 2000;19:283–300. doi: 10.1023/A:1026582308352. [DOI] [Google Scholar]
- 8.Shah BR, Chiu M, Amin S, Ramani M, Sadry S, Tu JV. Surname lists to identify South Asian and Chinese ethnicity from secondary data in Ontario, Canada: a validation study. BMC Med Res Methodol. 2010;10:42. doi: 10.1186/1471-2288-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim S, Wyatt LC, Mammen S, Zanowiak JM, Mohaimin S, Goldfeld KS, et al. The DREAM Initiative: study protocol for a randomized controlled trial testing an integrated electronic health record and community health worker intervention to promote weight loss among South Asian patients at risk for diabetes. Trials. 2019;20(1):635. doi: 10.1186/s13063-019-3711-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez PM, Zanowiak J, Goldfeld K, Wyka K, Masoud A, Beane S, et al. Protocol for project IMPACT (improving millions hearts for provider and community transformation): a quasi-experimental evaluation of an integrated electronic health record and community health worker intervention study to improve hypertension management among South Asian patients. BMC Health Serv Res. 2017;17(1):810. doi: 10.1186/s12913-017-2767-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldman JM, Conderino S, Islam NS, Thorpe LE. Subgroup variation and neighborhood social gradients-an analysis of hypertension and diabetes among Asian patients (New York City, 2014–2017) J Racial Ethn Health Disparities. 2021;8(1):256–263. doi: 10.1007/s40615-020-00779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avramovic S, Alemi F, Kanchi R, Lopez PM, Hayes RB, Thorpe LE, et al. US veterans administration diabetes risk (VADR) national cohort: cohort profile. BMJ Open. 2020;10(12):e039489. doi: 10.1136/bmjopen-2020-039489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.RJ K, Schoenborn CA. Age adjustment using the 2000 projected U.S. population. Healthy people statistical notes, Hyattsville, MD: National Center for Health Statistics; 2001. [PubMed]
- 14.Narayan KM, Aviles-Santa L, Oza-Frank R, Pandey M, Curb JD, McNeely M, et al. Report of a national heart, lung, and blood institute workshop: heterogeneity in cardiometabolic risk in Asian Americans in the US opportunities for research. J Am Coll Cardiol. 2010;55(10):966–973. doi: 10.1016/j.jacc.2009.07.075. [DOI] [PubMed] [Google Scholar]
- 15.Lee JW, Brancati FL, Yeh HC. Trends in the prevalence of type 2 diabetes in Asians versus whites: results from the United States National Health Interview Survey, 1997–2008. Diabetes Care. 2011;34(2):353–357. doi: 10.2337/dc10-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye J, Rust G, Baltrus P, Daniels E. Cardiovascular risk factors among Asian Americans: results from a National Health Survey. Ann Epidemiol. 2009;19(10):718–723. doi: 10.1016/j.annepidem.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shih M, Du Y, Lightstone AS, Simon PA, Wang MC. Stemming the tide: rising diabetes prevalence and ethnic subgroup variation among Asians in Los Angeles County. Prev Med. 2014;63:90–95. doi: 10.1016/j.ypmed.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 18.King GL, McNeely MJ, Thorpe LE, Mau ML, Ko J, Liu LL, et al. Understanding and addressing unique needs of diabetes in Asian Americans, native Hawaiians, and Pacific Islanders. Diabetes Care. 2012;35(5):1181–1188. doi: 10.2337/dc12-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the united states, 1988–2012. JAMA. 2015;314(10):1021–1029. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 20.Islam NS, Wyatt LC, Kapadia SB, Rey MJ, Trinh-Shevrin C, Kwon SC. Diabetes and associated risk factors among Asian American subgroups in New York City. Diabetes Care. 2013;36(1):e5. doi: 10.2337/dc12-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thorpe LE, Upadhyay UD, Chamany S, Garg R, Mandel-Ricci J, Kellerman S, et al. Prevalence and control of diabetes and impaired fasting glucose in New York City. Diabetes Care. 2009;32(1):57–62. doi: 10.2337/dc08-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel J, Mehta A, Rifai MA, Blaha MJ, Nasir K, McEvoy JW, et al. Hypertension guidelines and coronary artery calcification among South Asians: results from MASALA and MESA. Am J Prev Cardiol. 2021 doi: 10.1016/j.ajpc.2021.100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fei K, Rodriguez-Lopez JS, Ramos M, Islam N, Trinh-Shevrin C, Yi SS, et al. Racial and ethnic subgroup disparities in hypertension prevalence, New York City Health and Nutrition Examination Survey, 2013–2014. Prev Chronic Dis. 2017;14:E33. doi: 10.5888/pcd14.160478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta LS, Wu CC, Young S, Perlman SE. Prevalence of diabetes in New York City, 2002–2008: comparing foreign-born South Asians and other Asians with US-born whites, blacks, and Hispanics. Diabetes Care. 2011;34(8):1791–1793. doi: 10.2337/dc11-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venkataraman R, Nanda NC, Baweja G, Parikh N, Bhatia V. Prevalence of diabetes mellitus and related conditions in Asian Indians living in the United States. Am J Cardiol. 2004;94(7):977–980. doi: 10.1016/j.amjcard.2004.06.048. [DOI] [PubMed] [Google Scholar]
- 26.Baweja G, Nanda NC, Parikh N, Bhatia V, Venkataraman R. Prevalence of stroke and associated risk factors in Asian Indians living in the state of Georgia, United States of America. Am J Cardiol. 2004;93(2):267–269. doi: 10.1016/j.amjcard.2003.09.060. [DOI] [PubMed] [Google Scholar]
- 27.CDC. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2020.
- 28.Bower JK, Patel S, Rudy JE, Felix AS. Addressing bias in electronic health record-based surveillance of cardiovascular disease risk: finding the signal through the noise. Curr Epidemiol Rep. 2017;4(4):346–352. doi: 10.1007/s40471-017-0130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phelan M, Bhavsar NA, Goldstein BA. Illustrating informed presence bias in electronic health records data: how patient interactions with a health system can impact inference. EGEMS (Wash DC) 2017;5(1):22. doi: 10.5334/egems.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldstein BA, Bhavsar NA, Phelan M, Pencina MJ. Controlling for informed presence bias due to the number of health encounters in an electronic health record. Am J Epidemiol. 2016;184(11):847–855. doi: 10.1093/aje/kww112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.U.S. Census Bureau. American Fact Finder: 2011 American Community Survey 5 year estimates, selected characteristics of the native and foreign-born populations (S0501) 2015. https://factfinder.census.gov.
- 32.Asian/Pacific American Heritage Month: May 2011. Profile American: Facts for Features. 2011. 2011. http://www.census.gov/newsroom/releases/archives/facts_for_features_special_editions/cb11-ff06.html.
- 33.Colby SL, Ortman JM. Projections of the size and composition of the U.S. population: 2014 to 2060. Washington D.C.; 2015.
- 34.U.S. Census Bureau. Profile of general demographic characteristics: 2000 supplementary survey summary tables. Geographic area: United States 2004. www.factfinder.com.
- 35.Asian American Federation. Profile of New York City’s Pakistani Americans: 2013 Edition. 2013.
- 36.Asian American Federation. Profile of New York City’s Indian Americans: 2013 Edition. 2013.
- 37.Asian American Federation. Profile of New York City’s Bangladeshi Americans: 2013 Edition. 2013.
- 38.CDC. Diabetes Data and Statistics. https://gis.cdc.gov/grasp/diabetes/DiabetesAtlas.html#.
- 39.Beasley JM, Shah M, Wyatt LC, Zanowiak J, Trinh-Shevrin C, Islam NS. A community health worker-led intervention to improve blood pressure control in an immigrant community with comorbid diabetes: data from two randomized, controlled trials conducted in 2011–2019. Am J Public Health. 2021;111(6):1040–1044. doi: 10.2105/AJPH.2021.306216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Outcome definitions and list of ICD-10 codes and medications.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


