Abstract
Objective
Patients with rheumatoid arthritis (RA) often suffer from bone complications due to persistent joint inflammation, especially incident fracture. Nowadays, Chinese herbal medicines (CHMs) have provided safe and effective therapy for treating skeletal conditions, but it is unclear whether CHMs can prevent fracture onset among RA individuals. This study aimed to determine the association between the use of CHMs and the risk of fracture among them.
Methods
This retrospective, population-based study retrieved administrative health data from the Taiwan National Health Insurance (NHI) database to identify patients with newly diagnosed RA between 2000 and 2009. Of the 6178 incident RA patients, 2495 matched pairs of CHMs users and non-CHMs users were identified by propensity score matching. Enrollees with hip fractures prior to RA onset were excluded. Included subjects were followed until the end of 2013. Incidence and adjusted hazard ratios (HR) of new-onset bone fracture in the multivariable Cox proportional hazard model were measured with 95% confidence interval (CI).
Results
Fracture incidence was lower in CHMs users than in the comparison cohort (26.91 vs 32.94 per 1000 person-years, respectively), with an adjusted HR of 0.82 (95% CI: 0.73–0.92). Subjects receiving CHMs for more than 2 years had a much lower risk of fracture onset by more than 50%. Some CHMs prescriptions (Yan Hu Suo, Bei Mu, Da Huang, Dang Shen, Fu-Zi, Shu-Jing-Huo-Xue-Tang, Dang-Gui-Nian-Tong-Tang, Jia-Wei-Xiao-Yao-San, Gan-Lu-Yin, and Gui-Zhi-Shao-Yao-Zhi-Mu-Tang) were associated with reduced fracture risk.
Conclusion
Adding CHMs to routine treatment was found to be related to lower fracture risk in RA patients.
Keywords: rheumatoid arthritis, fracture, Chinese herbal medicines, risk
Introduction
Rheumatoid arthritis (RA), a relatively common autoimmune disorder characterized by swelling, tenderness, and damage to synovial joints, affects 1% of adults globally.1 Findings from recent studies showed that individuals diagnosed with RA may experience a higher risk of overall fracture, of which vertebral and hip fractures were the most common fracture sites.2,3 Vertebral fractures may progressively exacerbate back pain and limit physical activity in RA patients.3 Notably, prior research has shown a widening gap in mortality rates between RA individuals with the concurrent hip fracture and the general population.4,5 The estimated excess mortality rate after hip fracture ranged from 8.4% to 36% in the first year.6 Additionally, Kwon et al reported that RA patients with concurrent osteoporotic fracture had a 30% greater risk of death from all causes than the general population.7 Faced with the alarming clinical manifestations, it is imperative to prevent or treat osteoporotic fracture when managing RA patients.2,8
Over the past decade, Chinese herbal medicines (CHMs), as a time-honored medical science, have been regarded as a commonly used complementary therapy for patients with RA.9–11 Previous studies have inferred that by abating the nuclear factor kappa beta (NF-κB) signaling, several Chinese herbs, including Bei-Mu and Da-Huang, may be beneficial in regulating pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6).12 It is noteworthy that these inflammatory mediators have been shown to moderate the production of bone marrow, leading to hypo-development of the skeleton, as well as brittleness of bone, thereby increasing susceptibility to fracture after RA onset.13,14 Given that there is growing evidence of abnormal inflammatory parameters in the link between RA and fracture, the application of CHMs might be considered in providing a care regimen for preventing or delaying the incidence of fracture among people with RA.
Despite the interest in CHMs, only a few studies have reported the characteristics of CHMs usage patterns in RA patients.9,11 To the best of our knowledge, no previous studies have directly determined an association between CHMs use and osteo-protective influence among RA patients. This study, therefore, aimed to corroborate the association, over time, between CHMs use and incident osteoporotic fracture among RA patients, using a nationwide medical claims database representative of the Taiwanese population.
Methods
Sources of Data
The Longitudinal Health Insurance Database (LHID) was utilized as the data source in this retrospective cohort study. LHID is a sub-dataset of the National Health Insurance Research Database (NHIRD) of Taiwan, made up of one million randomly sampled people with over 10 years of follow-up. It has also been reported that these recruited individuals have similar age and sex distribution to the general population of Taiwan because the Bureau of National Health Insurance (NHI) performed a multistage stratified systematic sampling method.15 This database compiled (i) demographic information of enrollees; (ii) health insurance claims data; (iii) diagnostic codes; (iv) contracted pharmacies; and (v) medical examination information on persons under the NHI program. This study was conducted in accordance with the Helsinki Declaration, and was approved by the local institutional review board and ethics committee of Buddhist Dalin Tzu Chi Hospital (No. B10004021-1).
Definition of Subjects and Variables
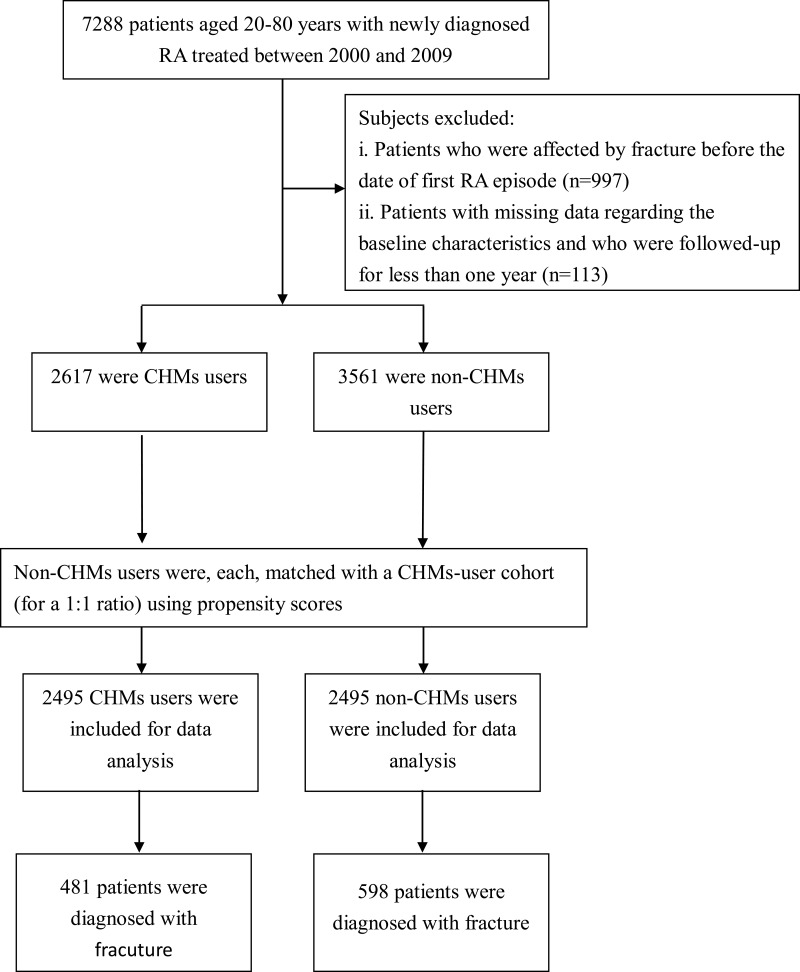
In this exploration, the diagnostic codes were based on the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) in the diagnosis field. As shown in Figure 1, patients who sought ambulatory health care or inpatient services between 2000 and 2009 due to newly diagnosed RA (ICD-9-CM code: 714.0) were initially identified. These patients were further linked to the catastrophic illness registry to ensure the validity of their diagnoses. The catastrophic illness certificate is granted based on formal diagnoses issued by physicians, and includes schizophrenia, mood disorders, autoimmune disorders, and cancer. The index date was considered the date when RA patients gained approval for catastrophic illness registration. Only those 20–80 years of age were included. Patients with a diagnosis of fracture prior to the first RA diagnosis (n = 997), and those who were not followed for one year after RA (n = 113) were excluded. Patients were classified as having fracture if they had at least three ambulatory visits for treatment or if they had been hospitalized due to bone fracture, as defined by the use of ICD-9-CM codes 800-829. Finally, a total of 6178 patients with RA were included.
Figure 1.
Flowchart showing the method of selecting and following study subjects.
The frequency of visits to physicians who practice Traditional Chinese Medicine was used to determine whether the enrollees had ever used CHMs treatment. Among the eligible patients, those receiving CHMs to treat RA for more than 30 days were deemed CHMs users (n = 2617), whereas those treated for 30 days or less were considered non-CHMs users. To remove the confounding effects of sociodemographic variables and coexisting medical conditions on the relationship between CHMs treatment and fracture in RA patients, we used a matching procedure with propensity scores to select CHMs treatment and non-treatment controls. The multivariate logistic regression model was utilized to estimate propensity score value, namely a probability index, ranging between 0 and 1, derived from the influence of all observed characteristics (listed at Table 1). Patients were matched by propensity score, using one-to-one nearest neighbor matching within 0.2 caliper distance, in which pairs of user and nonuser groups were formed, such that matched subjects had similar values of the propensity scores. Additionally, the index date for the follow-up period was the date of CHMs treatment initiation or, for non-CHMs users, the date of the initial RA onset, corrected by immortal time bias.16 Each subject was followed from the index date to the end of 2013 or was censored. The follow-up time, in person-years (PYs), was calculated until the date of newly diagnosed fracture onset or until being censored due to death, withdrawal from the insurance system, or loss to follow-up.
Table 1.
Demographic Data and Selected Comorbidities of Study Subjects
| Variables | Total Group | Non-CHMs Users | CHMs Users | Standardized Difference |
|---|---|---|---|---|
| N =2495(%) | N =2495(%) | |||
| Age (yr) | 0.041 | |||
| ≤50 | 2209(44.3) | 1079(43.2) | 1130(45.3) | |
| >50 | 2781(55.7) | 1416(56.8) | 1365(54.7) | |
| Mean (SD b) | 52.36(13.21) | 52.54(13.45) | 52.19(12.88) | 0.024 |
| Sex | 0.007 | |||
| Female | 3698(74.1) | 1845(73.9) | 1853(74.3) | |
| Male | 1292(25.9) | 650(26.1) | 642(25.7) | |
| Monthly income | 0.008 | |||
| Low | 2144(43.0) | 1072(43.0) | 1072(43.0) | |
| Median | 2640(52.9) | 1322(53.0) | 1318(52.8) | |
| High | 206(4.1) | 101(4.0) | 105(4.2) | |
| Residential area | 0.008 | |||
| Urban | 2869(57.5) | 1435(57.5) | 1434(57.5) | |
| Suburban | 770(15.4) | 388(15.6) | 382(15.3) | |
| Rural | 1351(27.1) | 672(26.9) | 679(27.2) | |
| Comorbidity | ||||
| Hypertension | 1325(26.6) | 675(27.1) | 650(26.1) | 0.021 |
| Diabetes | 620(12.4) | 316(12.7) | 304(12.2) | 0.014 |
| Heart disease | 793(15.9) | 405(16.2) | 388(15.6) | 0.016 |
| Chronic kidney disease | 49(1.0) | 23(0.9) | 26(1.0) | 0.012 |
| Cancer | 131(2.6) | 60(2.4) | 71(2.8) | 0.023 |
| Alcohol dependence syndrome | 9(0.2) | 4(0.2) | 5(0.2) | 0.009 |
| Tobacco use | 4(0.1) | 2(0.1) | 2(0.1) | 0.009 |
| Follow-up time (mean, median) | 7.22(7.24) | 7.28(7.20) | 7.16(7.26) |
Measurements of Covariates
Covariates assessed included baseline age, sex, monthly income, the urbanization level of enrollee’s residential area, former comorbidities and medication usage. Regarding monthly income, we used the premium category as a proxy to divide participants into four groups: ≤ New Taiwan Dollar (NTD) 17,800, NTD 17,881–43,900, and ≥ NTD $43,901. As to the urbanization level, it was calculated according to a published scheme that classified 359 communities in Taiwan into seven stratums, with a higher level indicating a higher degree of urbanization. The classification scheme included the population density, proportion of persons with a college-level education or higher, proportion of elderly residents, proportion of agricultural workers, and number of physicians per 100,000 population. In this work, the urbanization degree was grouped into three strata: urban (levels 1–2), suburban (levels 3–4), and rural (levels 5–7).17 Meanwhile, the recorded clinical characteristics of baseline comorbidities, included hypertension (ICD-9-CM 401–405), diabetes mellitus (DM; ICD-9-CM 250), heart disease (ICD-9-CM 410–429), chronic kidney disease (CKD; ICD-9-CM 585), cancer (ICD-9-CM 140–208), tobacco use (ICD-9-CM 305.1) and alcohol dependence syndrome (ICD-9-CM 303); all comorbidities were based on the medical records of each included patient assessed at one year prior to the initial cohort entry.
Statistical Analysis
Data were analyzed using SAS version 9.3 software (SAS Institute Inc, Cary, NC, USA). The differences in distributions of sociodemographic data and comorbidities between the two groups were compared using standardized difference, which allowed to assess the balance of measured variables between CHMs and non-CHMs users in the matched sample, since assessment of balance in baseline variables between treated and untreated subjects must be performed by the methods that were not subject to the influence of sample size. A standardized difference of 0.1 or more was considered indicative of imbalance.18 Thereafter, the adjusted hazard ratio (HR) was calculated using Cox proportional hazards regression analysis with 95% confidence interval of risk of fracture in association with CHMs use, after adjusting for confounders reported at baseline. To further test the robustness of the relationship of CHMs use with fracture risk, CHMs users were divided into three subgroups: the first group used CHMs for 31–365 days, the second group used CHMs for 366–730 days, and the third group used CHMs treatment for more than 730 days. The Kaplan–Meier method was employed to estimate the cumulative risk of fracture in each group, and the differences across groups were assessed using the Log rank test. The proportional hazards assumption was examined by plotting the log (−log [survival function]) versus the log (survival time). A P < 0.05 was considered to be statistically significant.
Sensitivity analyses were performed to evaluate whether the association found was robust after considering the severity of RA. First, we only included RA patients who reported no comorbidities. Second, given the unavailability of RA severity index in this investigation, we substituted prescription of biological agents, used for six months or longer, as a surrogate variable for RA severity in the regression model. These agents included adalimumab, etanercept, infliximab, rituximab and tocilizumab.
Results
Distributions of demographic characteristics and comorbidities for the two groups are shown in Table 1. Of the total enrollees, 2495 were CHMs users and 2495 were the 1:1 propensity-score-matched controls. After the matching procedure, no significant differences were found between the two groups in age, sex, monthly income, residential area and comorbidities, indicating comparability of the two groups.
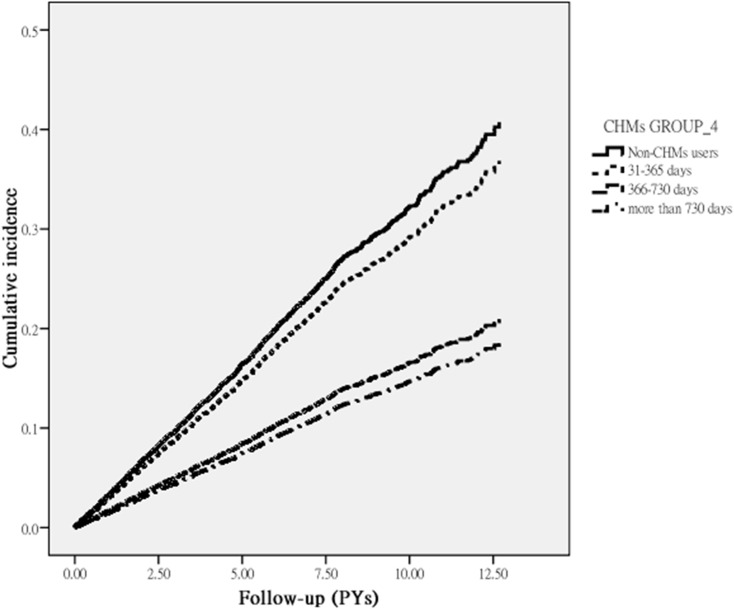
Among all eligible RA subjects, a total of 1079 first episodes of fracture occurred over an observation period of 36024.29 PYs in 4990 patients. CHMs users had a significantly lower incidence rate of fracture at 26.91 per 1000 PYs, compared with 32.94 per 1000 PYs for the non-CHMs users, with the adjusted HR of 0.82 (95% CI 0.73–0.92) (Table 2). Also, those receiving CHMs for more than 730 days experienced a 53% lower risk of fracture than those who did not receive CHMs. Figure 2 illustrates the Kaplan–Meier estimates of survival by days of CHMs use with a Log rank test of P < 0.001, which supports a statistically significant difference in the fracture rate across three groups of CHMs users.
Table 2.
Risk of Fracture for RA Patients with and without CHMs Use
| Patient Group | N | Events | PYs | Incidence | Crude HR (95% CI) | Adjusted HR* (95% CI) |
|---|---|---|---|---|---|---|
| Non-CHMs users | 2495 | 598 | 18156.35 | 32.94 | 1 | 1 |
| CHMs users | 2495 | 481 | 17867.94 | 26.91 | 0.82 (0.72–0.92) | 0.82 (0.73–0.92) |
| CHMs use within 31–365 days | 2053 | 421 | 14137.70 | 29.78 | 0.90 (0.80–1.02) | 0.92 (0.81–1.03) |
| CHMs use for 366–730 days | 255 | 35 | 2063.66 | 16.96 | 0.51 (0.37–0.72) | 0.54 (0.39–0.76) |
| CHMs use for more than 730 days | 187 | 25 | 1666.58 | 15.00 | 0.46 (0.31–0.68) | 0.47 (0.32–0.70) |
Note: *Model adjusted for sex, age, urbanization level, monthly income, and comorbidities.
Figure 2.
Cumulative incidence of fracture among RA subjects with and without use of CHMs (Log rank test, p<0.001).
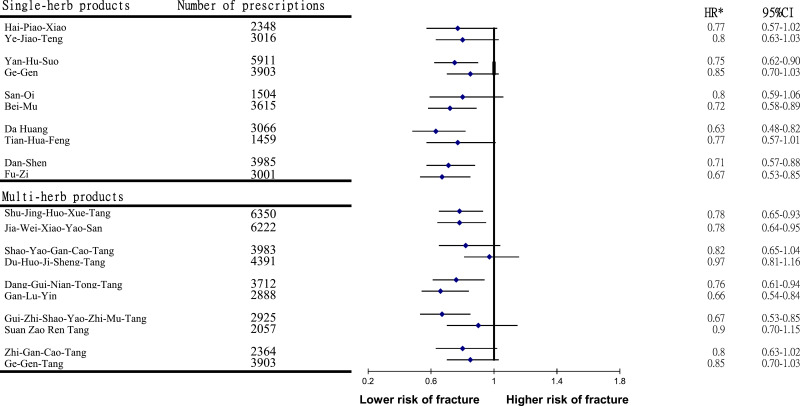
Table 3 shows the results stratified by age and sex. Collectively, a more beneficial effect of CHMs was detected among younger subjects. A multivariate stratified analysis further verified that the benefit of CHMs therapy in reducing the incidence of fracture was more predominant among females, with an adjusted HR of 0.80 (95% CI 0.71–0.92) (Table 3). The 10 most commonly prescribed herbal formulae for those with RA are summarized in Table 4. Among them, the prescriptions of Yan Hu Suo, Bei Mu, Da Huang, Dang Shen, Fu-Zi, Shu-Jing-Huo-Xue-Tang, Dang-Gui-Nian-Tong-Tang, Jia-Wei-Xiao-Yao-San, Gan-Lu-Yin, and Gui-Zhi-Shao-Yao-Zhi-Mu-Tang were related to the significant reduction of fracture risk (Figure 3).
Table 3.
Incidence and Fracture Risk for RA Patients with and without CHMs Use Stratified by Sex and Age
| Variables | Non- CHMs Users | CHMs Users | Crude HR (95% CI) | Adjusted HR (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Fracture Event | PYs | Incidence | Fracture Event | PY | Incidence | |||
| Sex | ||||||||
| Female | 477 | 13105.75 | 36.40 | 369 | 13074.36 | 28.22 | 0.77 (0.67–0.88) | 0.80Õ (0.71–0.92) |
| Male | 121 | 5050.60 | 23.96 | 112 | 4793.57 | 23.36 | 0.97 (0.75–1.25) | 0.93Õ (0.72–1.20) |
| Age | ||||||||
| ≤ 50 | 186 | 8212.65 | 22.65 | 145 | 8572.22 | 16.92 | 0.75 (0.60–0.93) | 0.72* (0.58–0.88) |
| >50 | 412 | 9943.70 | 41.43 | 336 | 9295.72 | 36.15 | 0.87 (0.75–1.00) | 0.88* (0.76–1.02) |
Notes: ÕModel adjusted for age, urbanization level, monthly income, and comorbidities. *Model adjusted for sex, urbanization level, monthly income, and comorbidities.
Table 4.
The Top Ten Single Chinese Herbs and Chinese Herbal Formula Used Among the Studying Participants
| CHMs Name | Ingredients or Generic Name |
|---|---|
| Single-herb products | |
| Hai-Piao-Xiao | Sepiella maindroni de Rochebrune |
| Ye-Jiao-Teng | Polygonum multiflorum Thunb. |
| Yan-Hu-Suo | Corydalis yanhusuo (Y.H.Chou & Chun C.Hsu) W.T.Wang ex Z.Y.Su & C.Y.Wu |
| Ge-Gen | Pueraria lobata (Willd.) Ohwi |
| San-Qi | Panax notoginseng (Burkill) F.H.Chen |
| Bei-Mu | Fritillaria thunbergii Miq. |
| Da-Huang | Rheum palmatum L. |
| Tian-Hua-Fen | Trichosanthes kirilowii Maxim. |
| Dan-Shen | Salvia miltiorrhiza Bunge |
| Fu-Zi | Aconitum carmichaeli var. carmichaeli |
| Multi-herb products | |
| Shu-Jing-Huo-Xue-Tang | Bai-Shao (Radix Paeoniae Alba; Paeonia lactiflora Pall.), Dang-Gui (Radix Angelicae Sinensis; Angelica sinensis (Oliv.) Diels), Chuan-Xiong (Rhizoma Chuanxiong; Ligusticum striatum DC.), Di-Huang (Radix Rehmanniae; Rehmannia glutinosa (Gaertn.) DC.), Tao-Ren (Semen Persicae; Prunus persica (L.) Batsch), Cang-Zhu (Rhizoma Atractylodis; Atractylodes lancea (Thunb.) DC.), Fu-Ling (Poria; Wolfiporia cocos (Schw.) Ryv. and Cilbn.), Niu-Xi (Radix Cyathulae; Cyathula officinalis K.C. Kuan), Wei-Ling-Xian (Radix Clematidis; Clematis chinensis Osbeck), Han-Fang-Ji (Radix Stephaniae Tetrandrae; Stephania tetrandra S.Moore), Qiang Huo (Rhizoma et Radix Notopterygii; Notopterygium incisum K.C.Ting ex H.T.Chang), Fang-Feng (Radix Saposhnikoviae; Saposhnikovia divaricata (Turez.) Schischk.), Long-Dan-Cao (Radix Gentianae; Gentiana scabra Bunge), Bai-Zhi (Radix Angelicae Dahuricae; Angelica dahurica (Hoffm.) Benth. and Hook.f. ex Franch. and Sav.), Chen-Pi (Pericarpium Citri Reticulatae; Citrus reticulata Blanco), Gan-Cao (Radix Glycyrrhizae; Glycyrrhiza uralensis Fisch.), Sheng-Jiang (Rhizoma Zingiberis Recens; Zingiber officinale Roscoe) |
| Jia-Wei-Xiao-Yao-San | Dang-Gui (Radix Angelicae Sinensis; Angelica sinensis (Oliv.) Diels), Bai-Shao (Radix Paeoniae Alba; Paeonia lactiflora Pall.), Fu-Ling (Poria; Wolfiporia cocos (Schw.) Ryv. and Cilbn.), Bai-Zhu (Rhizoma Atractylodis Macrocephalae; Atractylodes macrocephala Koidz.), Chai-Hu (Radix Bupleuri; Bupleurum chinense DC.), Mu-Dan-Pi (Cortex Moutan; Paeonia × suffruticosa Andrews), Zhi-Zi (Fructus Gardeniae; Gardenia jasminoides J.Ellis), Gan-Cao (Radix Glycyrrhizae; Glycyrrhiza uralensis Fisch.), Bo-He (Herba Menthae Haplocalycis; Mentha alaica Boriss.), Sheng-Jiang (Rhizoma Zingiberis Recens; Zingiber officinale Roscoe) |
| Shao-Yao-Gan-Cao-Tang | Bai-Shao (Radix Paeoniae Alba; Paeonia lactiflora Pall.), Gan-Cao (Radix Glycyrrhizae; Glycyrrhiza uralensis Fisch.) |
| Du-Huo-Ji-Sheng-Tang | Du-Huo (Radix Angelica Pubescentis; Angelica pubescens Maxim.), Xi–Xin (Herba cum Radix Asari; Asarum sieboldii Miq.), Fang-Feng (Radix Saposhnikoviae; Saposhnikovia divaricata (Turcz.) Schischk.), Qin-Jiao (Radix Gentianae Macrophyllae; Gentiana macrophylla Pall.), Sang-Ji-Sheng (Herba Taxilli; Taxillus chinensis (DC.) Danser), Du-Zhong (Cortex Eucommiae; Eucommia ulmoides Oliv.), Niu-Xi (Radix Cyathulae; Cyathula officinalis K.C. Kuan), Rou-Gui (Cortex Cinnamomi; Cinnamomum cassia (L.) J.Presl), Dang-Gui (Radix Angelicae Sinensis; Angelica sinensis (Oliv.) Diels), Chuan-Xiong (Rhizoma Chuanxiong; Ligusticum striatum DC.), Di-Huang (Radix Rehmanniae; Rehmannia glutinosa (Gaertn.) DC.), Bai-Shao (Radix Paeoniae Alba; Paeonia lactiflora Pall.), Ren-Shen (Radix Ginseng; Panax ginseng C.A.Mey.), Fu-Ling (Poria; Wolfiporia cocos (Schw.) Ryv. and Cilbn.), Gan-Cao (Radix Glycyrrhizae; Glycyrrhiza uralensis Fisch.) |
| Dang-Gui-Nian-Tong-Tang | Qiang-Huo (Rhizoma et Radix Notopterygii; Notopterygium incisum K.C.Ting ex H.T.Chang), Fang-Feng (Radix Saposhnikoviae; Saposhnikovia divaricata (Turcz.) Schischk.), Sheng-Ma (Rhizoma Cimicifugae; Cimicifuga foetida L.), Ge-Gen (Radix Puerariae; Pueraria lobata (Willd.) Ohwi), Bai-Zhu (Rhizoma Atractylodis Macrocephalae; Atractylodes macrocephala Koidz.), Dang-Gui (Radix Angelicae Sinensis; Angelica sinensis (Oliv.) Diels), Cang-Zhu (Rhizoma Atractylodis; Atractylodes lancea (Thunb.) DC.), Gan-Cao (Radix Glycyrrhizae; Glycyrrhiza uralensis Fisch.), Ku-Shen (Radix Sophorae Flavescentis; Sophora flavescens Aiton), Huang-Qin (Radix Scutellariae; Scutellaria baicalensis Georgi), Zhi-Mu (Rhizoma Anemarrhenae; Anemarrhena asphodeloides Bunge), Yin-Chen-Hao (Herba Artemisiae Scopariae; Artemisia capillaris Thunb.), Zhu-Ling (Polyporus; Polyporus umbellatus (Pers) Fries), Ze-Xie (Rhizoma Alismatis; Alisma orientale (Sam.) Juz.) |
| Gan-Lu-Ying | Di-Huang (Radix Rehmanniae; Rehmannia glutinosa (Gaertn.) DC.), Shi-Hu (Herba Dendrobii; Dendrobium loddigesii Rolfe), Tian-Men-Dong (Radix Asparagi; Asparagus cochinchinensis (Lour.) Merr.), Mai-Men-Dong (Radix Ophiopogonis; Ophiopogon japonicus (Thunb.) Ker Gawl.), Huang-Qin (Radix Scutellariae; Scutellaria baicalensis Georgi), Yin-Chen-Hao (Herba Artemisiae Scopariae; Artemisia capillaris Thunb.), Zhi-Ke (Fructus Aurantii; Citrus × aurantium L.), Pi-Pa-Ye (Folium Eriobotryae; Eriobotrya japonica (Thunb.) Lindl.), Gan-Cao (Radix Glycyrrhizae; Glycyrrhiza uralensis Fisch.) |
| Gui-Zhi-Shao-Yao-Zhi-Mu-Tang | Gui-Zhi (Ramulus Cinnamomi; Cinnamomum cassia (L.) J.Presl), Ma-Huang (Herba Ephedrae; Ephedra sinica Stapf), Fu-Zi (Radix Aconiti Lateralis; Aconitum carmichaeli var. carmichaeli), Zhi-Mu (Rhizoma Anemarrhenae; Anemarrhena asphodeloides Bunge), Bai-Shao (Radix Paeoniae Alba; Paeonia lactiflora Pall.), Bai-Zhu (Rhizoma Atractylodis Macrocephalae; Atractylodes macrocephala Koidz.), Fang-Feng (Radix Saposhnikoviae; Saposhnikovia divaricata (Turcz.) Schischk.), Sheng-Jiang (Rhizoma Zingiberis Recens; Zingiber officinale Roscoe), Gan-Cao (Radix Glycyrrhizae; Glycyrrhiza uralensis Fisch.) |
| Suan-Zao-Ren-Tang | Suan-Zao-Ren (Semen Zizyphi Spinosae; Ziziphus jujuba var. spinosa (Bunge) Hu ex H.F.Chow), Fu-Ling (Poria; Wolfiporia cocos (Schw.) Ryv. and Cilbn.), Zhi-Mu (Rhizoma Anemarrhenae; Anemarrhena asphodeloides Bunge), Chuan-Xiong (Rhizoma Chuanxiong; Ligusticum striatum DC.), Gan-Cao (Radix Glycyrrhizae; Glycyrrhiza uralensis Fisch.) |
| Zhi-Gan-Cao-Tang | Gan-Cao (Radix Glycyrrhizae; Glycyrrhiza uralensis Fisch.), Ren-Shen (Radix Ginseng; Panax ginseng C.A.Mey.), Gui-Zhi (Ramulus Cinnamomi; Cinnamomum cassia (L.) J.Presl), Di-Huang (Radix Rehmanniae; Rehmannia glutinosa (Gaertn.) DC.), Mai-Men-Dong (Radix Ophiopogonis; Ophiopogon japonicus (Thunb.) Ker Gawl.), E- Jiao (Colla Corii Asini; Equus asinus L.), Huo-Ma-Ren (Semen Cannabis; Cannabis sativa L.), Sheng-Jiang (Rhizoma Zingiberis Recens; Zingiber officinale Roscoe), Da-Zao (Fructus Jujubae; Ziziphus jujuba Mill.) |
| Ge-Gen-Tang | Ge-Gen (Radix Puerariae; Pueraria lobata (Willd.) Ohwi), Ma-Huang (Herba Ephedrae; Ephedra sinica Stapf), Gui-Zhi (Ramulus Cinnamomi; Cinnamomum cassia (L.) J.Presl), Bai-Shao (Radix Paeoniae Alba; Paeonia lactiflora Pall.), Sheng-Jiang (Rhizoma Zingiberis Recens; Zingiber officinale Roscoe), Da-Zao (Fructus Jujubae; Ziziphus jujuba Mill.), Gan Cao (Radix Glycyrrhizae; Glycyrrhiza uralensis Fisch.) |
Figure 3.
Risk of fracture in relation to the top ten most-used single-herb and multi-herb products among studying participants. *Model adjusted for age, sex, urbanization level, monthly income, and comorbidities.
In the first sensitivity analysis, while we only recruited the RA patients who reported no comorbidities for data analysis, with 1509 in the CHMs use group and 1505 in the non-CHMs use group, respectively. In this context, the CHMs use was still associated with a lower incidence rate of fracture, with an adjusted HR of 0.74 (95% CI 0.63–0.88). In the second sensitivity analysis, 56.7% (1415/2495) of the CHMs users and 55.3% (1380/2495) of the non-CHMs users ever received biological agents for six months or longer. After adding this factor to the multivariate model, we found that CHMs use was still associated with a lower incidence rate of fracture, with an adjusted HR of 0.81 (95% CI 0.74–0.93).
Discussion
This is the first population-based cohort study to identify the relationship between CHMs use and the occurrence of incident osteoporotic fracture in RA patients based on a nationwide health research database. To objectively evaluate the association between CHMs and the risk of fracture in RA patients, we conducted 1:1 propensity score matching for age, sex, monthly income, residential area, comorbidities and follow-up time to ensure that the CHMs group was commensurate with the non-CHMs group. Results obtained by this study of RA patients showed that, compared with patients who did not use CHMs therapy, the fracture risk in RA patients treated with CHMs was lower by 20%. Of note, those receiving CHMs for more than two years had a substantial reduced risk of fracture. The dose-dependent response suggests causation between CHMs use and reduction of fracture risk. Proposed mechanisms by which the prescribed herbal products protect against incident osteoporotic fracture may involve the regulation of inflammatory response,12 and increasing the bone mineral density via regulating the mitogen-activated protein kinase (MAPK) signaling pathway;19 both of these possible mechanisms appear to be beneficial in attenuating osteoporosis to prevent following fragility fracture.
Moreover, regardless of gender, younger patients benefited more from CHMs treatments than older patients in terms of risk prevention of fracture risk. This suggests that younger people who may experience fewer coexisting medical conditions, have a more positive attitude toward their medical conditions, or have better psychosocial and coping resources to rely upon than do older patients.20 These characteristics in younger adults may superimpose the protective effect of CHMs on bone health. Additionally, females benefited more from CHMs treatment than males. Previous studies demonstrated that estrogen was one of the main hormones that influenced the growth cycle and reabsorption of bone by inhibiting the activation of osteoclastogenesis and mesenchymal cell differentiation,21–23 which were known to induce bone fragility.
Further, the present study pointed out the specific CHMs products that were likely to be associated with reduced risk of fracture for RA patients. Among the commonly used single-product CHMs to treat RA, five herbs were observed to lower the risk of fracture. First, the use of Yan-Hu-Suo was associated with a 25% reduced risk of fracture. Traditionally, Yan-Hu-Suo was used to relieve pain caused by stagnation of blood and Qi (energy). A recent study showed that l-tetrahydropalmatine, a major compound purified from this formula, significantly suppresses the receptor activator of nuclear factor κB ligand (RANKL)-activated NF-κB and MAPK pathway.24 This pathway involves the inhibition of osteoclastogenesis and regulation of osteoblast differentiation and osteogenic formation.25
The present study also showed that use of Bei-Mu and Da-Huang may lessen the risk of fracture among RA subjects. These prescriptions were previously shown to have a pronounced anti-arthritic effect by inhibiting the production of inflammatory mediators, such as TNF-a, IL-6 and IL-8.26,27 Furthermore, through the induction of matrix metalloproteinase (MMPs) generations and osteoclast activation, these inflammatory mediators were shown to moderate the production of bone marrow, resulting in hypo-development of the skeleton and bone brittleness,13,14 thereby increasing susceptibility to fracture.
Another herbal product shown to be effective in lessening fracture risk is Dan-Shen. One recent in vivo study indicated that Dan-Shen may possess an anti-osteoporosis effect by suppressing trabecular bone loss and osteoclastogenesis.28 Meanwhile, Dan-Shen significantly regulates cytokine secretion and reduces oxidative stress,29 and accordingly, promotes bone regeneration and lessens the chance of inflammation-induced osteoporosis. In addition, the use of Fu-Zi demonstrated positive therapeutic effects against risk of fracture. Using a rodent model, Kim and colleagues found that Fu-Zi markedly promoted the proliferation rate of mouse bone marrow mesenchymal stem cells up to 122.24% compared to untreated cells,30 which may implicate the possible mechanism responsible for the positive effect of Fu-Zi observed in the present study.
With regard to the commonly prescribed multi-herb products, Jia-Wei-Xiao-Yao-San, Gan-Lu-Yin, and Shu-Jing-Huo-Xue-Tang were all found to reduce the risk of incident fracture in RA patients. Both human and animal studies have disclosed that these remedies decreased the level of IL-6 or TNF-α via the suppression of nuclear factor kappa beta (NF-κB) activation.31–33 In addition to being a crucial transcription mediator regulating the induction of the inflammatory response, NF-κB was considered a potent mediator of inflammatory osteolysis, thereby contributing to enhanced osteoclastogenesis to exacerbate bone loss. Accordingly, numerous studies have exploited NF-κB as a target for bone therapies in addition to mitigating the effect of inflammatory disorders.34
Findings of the present study also indicated that the prescription of Dang-Gui-Nian-Tong-Tang diminished vulnerability to fracture. The extract of Angelica sinensis, a major ingredient purified from Dang-Gui-Nian-Tong-Tang, has been shown to elevate bone turnover markers and increase bone mineral density, indicating bone cell proliferation.35,36 Echoing a previous report, the present study also revealed that Gui-Zhi-Shao-Yao- Zhi-Mu-Tang is one of the commonly used decoctions for RA.9 The decoction produced protective effects on fracture risk, possibly through influencing the calcification of bone by osteoblast reproduction and reducing the inflammatory mediator levels that were associated with synovial inflammation and bone destruction.37
Although the findings of the present study have an appreciable level of clinical and research significance, several limitations should be considered. First, given the detection of a positive association between CHMs use and lower risk of fracture, the use of retrospective analysis of cross-sectional data usually limits the inference of causality. Also, retrospective cohort design may have misclassification bias that can influence measurement, just as in the use of ICD-9-CM diagnostic codes only. To minimize this bias, we enrolled only persons with new-onset fracture or RA, and only after the patients had at least 3 outpatient visits, reporting consistent diagnoses or at least one inpatient admission. The clarification of the diagnosis of RA was further verified by the catastrophic illness certificate. Meanwhile, it should also be noted that the NHI of Taiwan randomly reviews the charts and audits medical charges to verify the accuracy of claims.15 Second, in the LHID database detailed information of family history, educational level, and laboratory data were not accessible in LHID database, which may have restricted our capacity to accurately determine patients’ health status. Thus, research addressing these variables is worthy of further investigation. Third, since a reliable index of RA severity was unavailable from the LHID, and failure to control for this factor may bias the findings, we performed two sensitivity analyses to mitigate this concern. First, we limited the analysis to only those patients with no comorbidities. Second, we added to the analysis prescription of biological agents, taken for six months or longer, which is a common surrogate used for RA severity in the administrative claims data.11,38 These sensitivity analyses revealed that disease severity was not likely to alter the veracity of our findings that adding CHMs to conventional therapy reduced the risk of fracture in RA patients. Fourth, due to the use of a cohort study design, rather than a randomized controlled trial, inferences regarding the beneficial effect of CHMs on reducing fracture risk are likely limited due to unmeasured or unknown confounders. Therefore, caution should be taken when interpreting the findings. A randomized controlled trial is, therefore, recommended to clearly determine the efficacy of these CHMs, as well as the mechanisms that underlie their successful application. These limitations notwithstanding this study also possessed several strengths. One strength was that this nationwide register-based study had the opportunity to fully access records of hospital diagnoses and prescription claims; minimal risk of selection bias and attrition during follow-up. The study also sampled a large population of both men and women, which provided sufficient power to conduct detailed analyses, especially given the relatively low incidence of RA in the population.
Conclusions
This is the largest population-based cohort study based on Taiwan’s national health database designed to determine the association between the use of CHMs and the risk of incident fracture in RA patients. Longer duration of incorporating CHMs into routine treatment for RA patients was found to significantly reduce the risk of fracture by 53%. Results of this study may serve as a reference to help healthcare providers when planning and implementing therapeutic interventions that seek to improve the overall health of patients with RA. Further prospective randomized trials are recommended to clarify whether the association revealed in this study supports a causal link.
Acknowledgments
All authors declare that there are no conflicts of interest. This study is based, in part, on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health and managed by the National Health Research Institutes, Taiwan. The interpretation and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health, or the National Health Research Institutes. HHL, HL, YJC, and CHL contributed equally to this work. NSL is the co-corresponding author of this paper (q12015@tzuchi.com.tw).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval for the version to be published; and agreed to be accountable for all aspects of the work.
Disclosure
The authors have no conflicts of interest to declare.
References
- 1.Cross M, Smith E, Hoy D, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1316–1322. doi: 10.1136/annrheumdis-2013-204627 [DOI] [PubMed] [Google Scholar]
- 2.Jin S, Hsieh E, Peng L, et al. Incidence of fractures among patients with rheumatoid arthritis: a systematic review and meta-analysis. Osteoporos Int. 2018;29:1263–1275. doi: 10.1007/s00198-018-4473-1 [DOI] [PubMed] [Google Scholar]
- 3.Chen B, Cheng G, Wang H, Feng Y. Increased risk of vertebral fracture in patients with rheumatoid arthritis: a meta-analysis. Medicine. 2016;95:e5262. doi: 10.1097/MD.0000000000005262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin YC, Li YH, Chang CH, et al. Rheumatoid arthritis patients with hip fracture: a nationwide study. Osteoporos Int. 2015;26:811–817. doi: 10.1007/s00198-014-2968-y [DOI] [PubMed] [Google Scholar]
- 5.Zhang C, Feng J, Wang S, et al. Incidence of and trends in hip fracture among adults in urban China: a nationwide retrospective cohort study. PLoS Med. 2020;17:e1003180. doi: 10.1371/journal.pmed.1003180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi ST, Kwon SR, Jung JY, et al. Prevalence and fracture risk of osteoporosis in patients with rheumatoid arthritis: a multicenter comparative study of the FRAX and WHO criteria. J Clin Med. 2018;7:507. doi: 10.3390/jcm7120507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwon HY, Kim HH, Sung YK, Ha YC. Incidence and mortality of osteoporotic fracture in rheumatoid arthritis in South Korea using nationwide claims data. J Bone Metab. 2019;26:97–104. doi: 10.11005/jbm.2019.26.2.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staa TPV, Geusens P, Bijlsma JWJ, Leufkens HGM, Cooper C. Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:3104–3112. doi: 10.1002/art.22117 [DOI] [PubMed] [Google Scholar]
- 9.Huang MC, Pai FT, Lin CC, et al. Characteristics of traditional Chinese medicine use in patients with rheumatoid arthritis in Taiwan: a nationwide population-based study. J Ethnopharmacol. 2015;176:9–16. doi: 10.1016/j.jep.2015.10.024 [DOI] [PubMed] [Google Scholar]
- 10.Daily JW, Zhang T, Cao S, Park S. Efficacy and safety of GuiZhi-ShaoYao-ZhiMu decoction for treating rheumatoid arthritis: a systematic review and meta-analysis of randomized clinical trials. J Altern Complement Med. 2017;23:756–770. doi: 10.1089/acm.2017.0098 [DOI] [PubMed] [Google Scholar]
- 11.Li HH, Livneh H, Yeh CC, et al. Association between use of Chinese herbal medicine and depression risk in patients with rheumatoid arthritis: a nationwide retrospective cohort study. Int J Rheum Dis. 2019;22:986–994. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Huang H, Zhang Y, et al. Anti-inflammatory effect of tetrahydrocoptisine from Corydalis impatiens is a function of possible inhibition of TNF-α, IL-6 and NO production in lipopolysaccharide-stimulated peritoneal macrophages through inhibiting NF-κB activation and MAPK pathway. Eur J Pharmacol. 2013;715:62–71. doi: 10.1016/j.ejphar.2013.06.017 [DOI] [PubMed] [Google Scholar]
- 13.Wang K, Zhang D, Liu Y, et al. Traditional Chinese medicine formula Bi-Qi capsule alleviates rheumatoid arthritis-induced inflammation, synovial hyperplasia, and cartilage destruction in rats. Arthritis Res Ther. 2018;20:43. doi: 10.1186/s13075-018-1547-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang SJ, Yue W, Rahman K, et al. Mechanism of treatment of kidney deficiency and osteoporosis is similar by traditional Chinese medicine. Curr Pharm Des. 2016;22:312–320. doi: 10.2174/1381612822666151112150346 [DOI] [PubMed] [Google Scholar]
- 15.National health insurance database. LHID; 2000. Available from: https://nhird.nhri.org.tw/en/Data_Subsets.html. Accessed March 03, 2021.
- 16.Shariff SZ, Cuerden MS, Jain AK, Garg AX. The secret of immortal time bias in epidemiologic studies. J Am Soc Nephrol. 2008;19:841–843. doi: 10.1681/ASN.2007121354 [DOI] [PubMed] [Google Scholar]
- 17.Liu CY, Hung YT, Chuang YL, et al. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J Health Manag. 2006;4:1–22. [Google Scholar]
- 18.Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med. 2008;27:2037–2049. doi: 10.1002/sim.3150 [DOI] [PubMed] [Google Scholar]
- 19.Lee K, Seo I, Choi MH, Jeong D. Roles of mitogen-activated protein kinases in osteoclast biology. Int J Mol Sci. 2018;19:3004. doi: 10.3390/ijms19103004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shih CC, Liao CC, Su YC, Tsai CC, Lin JG. Gender differences in traditional Chinese medicine use among adults in Taiwan. PLoS One. 2012;7:e32540. doi: 10.1371/journal.pone.0032540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srivastava S, Toraldo G, Weitzmann MN, Cenci S, Ross FP, Pacifici R. Estrogen decreases osteoclast formation by down-regulating receptor activator of NF-κB ligand (RANKL)-induced JNK activation. J Biol Chem. 2001;276:8836–8840. doi: 10.1074/jbc.M010764200 [DOI] [PubMed] [Google Scholar]
- 22.Faienza MF, Ventura A, Marzano F, Cavallo L. Postmenopausal osteoporosis: the role of immune system cells. Clin Dev Immunol. 2013;2013:575936. doi: 10.1155/2013/575936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valenti MT, Dalle Carbonare L, Mottes M. Osteogenic differentiation in healthy and pathological conditions. Int J Mol Sci. 2017;18:41. doi: 10.3390/ijms18010041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhi X, Wang L, Chen H, et al. l‐tetrahydropalmatine suppresses osteoclastogenesis in vivo and in vitro via blocking RANK‐TRAF6 interactions and inhibiting NF‐κB and MAPK pathways. J Cell Mol Med. 2020;24:785–798. doi: 10.1111/jcmm.14790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao X. RANKL-RANK signaling regulates osteoblast differentiation and bone formation. Bone Res. 2018;6:35. doi: 10.1038/s41413-018-0040-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shrimali D, Shanmugam MK, Kumar AP, et al. Targeted abrogation of diverse signal transduction cascades by emodin for the treatment of inflammatory disorders and cancer. Cancer Lett. 2013;341:139–149. doi: 10.1016/j.canlet.2013.08.023 [DOI] [PubMed] [Google Scholar]
- 27.Li H, Hung A, Li M, Yang AWH. Fritillariae Thunbergii Bulbus: traditional uses, phytochemistry, pharmacodynamics, pharmacokinetics and toxicity. Int J Mol Sci. 2019;20:1667. doi: 10.3390/ijms20071667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SR, Jeon H, Kwon JE, et al. Anti-osteoporotic effects of Salvia miltiorrhiza Bunge EtOH extract both in ovariectomized and naturally menopausal mouse models. J Ethnopharmacol. 2020;258:112874. doi: 10.1016/j.jep.2020.112874 [DOI] [PubMed] [Google Scholar]
- 29.Gao H, Sun W, Zhao J, et al. Tanshinones and diethyl blechnics with anti-inflammatory and anti-cancer activities from Salvia miltiorrhiza Bunge (Danshen). Sci Rep. 2016;6:33720. doi: 10.1038/srep33720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim DR, Kim HY, Park JK, Park SK, Chang MS. Aconiti lateralis preparata radix activates the proliferation of mouse bone marrow mesenchymal stem cells and induces osteogenic lineage differentiation through the bone morphogenetic protein-2/smad-dependent runx2 pathway. Evid Based Complement Alternat Med. 2013;2013:586741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yasui T, Yamada M, Uemura H, et al. Changes in circulating cytokine levels in midlife women with psychological symptoms with selective serotonin reuptake inhibitor and Japanese traditional medicine. Maturitas. 2009;62:146–152. doi: 10.1016/j.maturitas.2008.12.007 [DOI] [PubMed] [Google Scholar]
- 32.Chen YH, Luo R, Lei SS, et al. Anti-inflammatory effect of Ganluyin, a Chinese classic prescription, in chronic pharyngitis rat model. BMC Complement Med Ther. 2020;20:265. doi: 10.1186/s12906-020-03057-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo RB, Wang GF, Zhao AP, Gu J, Sun XL, Hu G. Paeoniflorin protects against ischemia-induced brain damages in rats via inhibiting MAPKs/NF-IoB-mediated inflammatory responses. PLoS One. 2012;7:e49701. doi: 10.1371/journal.pone.0049701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abu-Amer Y. NF-κB signaling and bone resorption. Osteoporos Int. 2013;24:2377–2386. doi: 10.1007/s00198-013-2313-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Q, Populo SM, Zhang J, Yang G, Kodama H. Effect of Angelica sinensis on the proliferation of human bone cells. Clin Chim Acta. 2002;324:89–97. doi: 10.1016/S0009-8981(02)00210-3 [DOI] [PubMed] [Google Scholar]
- 36.Lim DW, Kim YT. Anti-osteoporotic effects of Angelica sinensis (Oliv.) Diels extract on ovariectomized rats and its oral toxicity in rats. Nutrients. 2014;6:4362–4372. doi: 10.3390/nu6104362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo Q, Mao X, Zhang Y, et al. Guizhi-Shaoyao-Zhimu decoction attenuates rheumatoid arthritis partially by reversing inflammation-immune system imbalance. J Transl Med. 2016;14:1–16. doi: 10.1186/s12967-016-0921-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen WJ, Livneh H, Hsu CH, et al. The relationship of acupuncture use to the endometriosis risk in females with rheumatoid arthritis: real-world evidence from population-based health claims. Front Med. 2021;7:601606. doi: 10.3389/fmed.2020.601606 [DOI] [PMC free article] [PubMed] [Google Scholar]